Abstract
The biokinetics of cell growth of Lactobacillus amylovorus DCE 471 and bacteriocin production by this strain were investigated as a function of the temperatures (28 to 44°C) and pH values (pH 4.2 to 6.4) that are characteristic of a sourdough fermentation process. The influence of temperature and pH on microbial behavior is described by using a successfully validated predictive model.
Sourdough fermentation is a cereal fermentation performed in a variety of technologies based on wheat or rye flour as the raw material (14). Type II doughs are used to produce bakery preproducts that are souring supplements or aroma carriers. The fermentations of these doughs are characterized by elevated temperatures (up to 37°C), shorter fermentation times (15 to 20 h), and higher water contents compared to traditional, type I sourdough fermentations. Also, Soudanese Kisra sourdoughs, made from sorghum flour, are produced at high temperatures (30 to 40°C). From an industrial point of view, type II sourdough fermentations are of increasing interest, but they require faster acidification with no effect on the baking and sensorial properties of the bread. While the growth requirements of the lactobacilli and yeasts involved with respect to pH, temperature, and organic acids (4), as well as the metabolic interactions between these microorganisms (5), have been well studied in sourdoughs, there is no information about the use of strong homofermentative acidifiers, such as Lactobacillus amylovorus, as novel starter cultures for type II sourdough and Kisra fermentations. Together with the flour amylases, L. amylovorus contributes to the breakdown of the starch from the cereal to form the maltose that serves as an energy source for the sourdough lactic acid bacteria, and this bacterium may also enhance the acidification process and play an important role in the inhibitory action of the sourdough end product. Also, production of bacteriocins that are low-molecular-mass peptides with narrow inhibitory spectra restricted to closely related strains may be useful for improving competition among the natural bacterial sourdough flora in favor of particular strains. Such production could be a criterion for selection of more competitive starters for implementation and stability of sourdough processes (13).
The objective of this study was to assess the suitability in sourdough fermentations of the bacteriocin-producing organism L. amylovorus DCE 471, a strong and fast acidifier with a competitive advantage (1, 2, 3). Our analysis was performed by using a basic sourdough simulation medium (SSM) and a predictive model which describes the influence on microbial behavior of the temperature and pH during sourdough fermentations.
L. amylovorus DCE 471, which was isolated from corn steep liquor and produces the bacteriocin amylovorin L471 (2), was used throughout this study. Lactobacillus delbrueckii subsp. bulgaricus LMG 6901T was used as an indicator organism to determine levels of amylovorin L471 activity (3). The strains were maintained and propagated and agar media were prepared as described previously (2).
A series of fermentations were performed in basic SSM containing (per liter) 10 g of tryptone (Oxoid, Basingstoke, United Kingdom), 12 g of yeast extract (Merck, Darmstadt, Germany), 5 g of Lab-Lemco (Oxoid), 0.5 g of cysteine-HCl, 0.2 g of MgSO4 · 7H2O, 0.05 g of MnSO4 · H2O, 2 g of KH2PO4, and 1 ml of Tween 80, as well as 10 g of maltose plus 10 g of fructose as energy sources. Lab-Lemco was added to avoid biphasic fermentation kinetics (unpublished results). Fermentations were carried out as described elsewhere (8). For preparation of the inoculum, 10 ml of SSM was inoculated with 0.5 ml of a freshly prepared L. amylovorus DCE 471 culture and incubated for 12 h at 37°C. This preculture was added to 90 ml of SSM. After 12 h of growth at 37°C, the second preculture was used to inoculate the fermentor. During the first experiments, the pH was maintained at 5.4 while fermentations were carried out at 28, 31, 34, 37, 40, and 44°C. In a second series of experiments, fermentations were carried out at a constant temperature of 37°C and at constant pH values of 4.2, 4.6, 5.0, 5.4, 5.8, and 6.4. To check the reproducibility, one fermentation (37°C, pH 5.4) was performed in triplicate, and the standard deviation was calculated. For validation of the model, two additional fermentations were performed, one at 35°C and a constant pH of 5.5 and the other at 42°C and a constant pH of 4.5.
Samples were withdrawn aseptically from the fermentor to determine biomass (as cell dry mass [CDM]), soluble bacteriocin activity (as AU), lactic acid concentration, and residual maltose, fructose, and glucose concentrations as described elsewhere (8). The standard deviations for the CDM, maltose, fructose, and lactic acid measurements were 0.11, 0.04, 0.035, and 0.02 g · liter−1, respectively.
Incubation experiments at the 100-ml scale were carried out at various temperatures and pH values to gain additional information about the growth of L. amylovorus DCE 471 cells at intermediate temperatures and pH values and to determine the growth limits of this organism.
Primary model development was performed as described by Leroy and De Vuyst (8), except that cell growth was modeled with a modification of the equation for logistic growth, maltose consumption and fructose consumption were modeled separately by using the maintenance energy model, and lactic acid production was calculated based on the consumption of both maltose and fructose (Table 1). Secondary modeling of the biokinetic parameters was performed as described by Leroy and De Vuyst (9); the γ-functions for temperature (γT and pH (γpH) that were used to calculate the maximum specific growth rate (μmax) were based on the equations of Rosso (11, 12). It was assumed that there was no interaction between temperature and pH, so that μmax = (μmax)opt × γT × γpH. Where appropriate, the quadratic correlation coefficient (r2) is given below.
TABLE 1.
Equations used for primary model development
| Model | Equationa |
|---|---|
| Cell growth | dX/dt = [μmax × (1 − X/Xmax)n − α] × X when t > λ |
| Sugar consumption (maltose) | dM/dt = −1/YX/M × dX/dt − mM × X |
| Sugar consumption (fructose) | dF/dt = −1/YX/F × dX/dt − mF × X |
| Lactic acid production | dL/dt = −1/YL/S × (dM/dt + dF/dt) |
| Bacteriocin production | dB/dt = kB × dX/dt − kinact × X × B |
Abbreviations: X, biomass concentration (in grams of CDM per liter); Xmax, maximum attainable biomass concentration (in grams of CDM per liter); μmax, maximum specific growth rate (per hour); t, time (in hours); λ, duration of the lag phase (in hours); n, inhibition exponent; α, specific rate of death (per hour); M, residual maltose concentration (in grams of maltose per liter); F, residual fructose concentration (in grams of fructose per liter); mM, maintenance coefficient for maltose (in grams of maltose per gram of CDM per hour); mF, maintenance coefficient for fructose (in grams of fructose per gram of CDM per hour); YX/M, cell yield coefficient for maltose (in grams of CDM per gram of maltose); YX/F, cell yield coefficient for fructose (in grams of CDM per gram of fructose); L, amount of lactic acid produced (in grams of lactic acid per liter); YL/M, yield coefficient for lactic acid production based on maltose (in grams of lactic acid per gram of maltose); YL/F, yield coefficient for lactic acid production based on fructose (in grams of lactic acid per gram of fructose); B, soluble bacteriocin activity (in arbitrary units [AU] per liter); kB, specific bacteriocin production (in AU per gram of CDM); kinact, apparent rate of bacteriocin inactivation (in liters per gram of CDM per hour).
As previously observed with MRS medium (1, 2,7), the primary metabolite kinetics of amylovorin L471 production, followed by apparent inactivation of the bacteriocin mainly due to adsorption, were confirmed with SSM cultures. Maltose and fructose were consumed simultaneously and converted into lactic acid (data not shown).
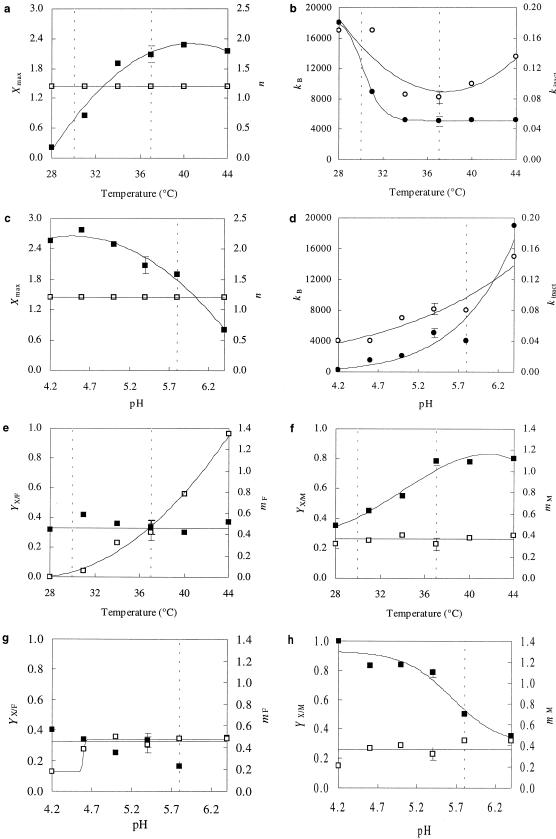
At the temperatures used for manufacturing type I sourdoughs (<30°C), growth was slow (μmax at constant pH 5.4 < 0.25 h−1), and the bacteriocin activity was low. At the temperatures used for manufacturing type II sourdoughs (between 30 and 37°C), growth was suboptimal (0.25 h−1 < μmax at constant pH 5.4 < 0.64 h−1). Both specific amylovorin L471 production (kB) and volumetric amylovorin L471 activity in the supernatant (Bmax) were high at these temperatures. The maximum Bmax values were observed at 37°C (5,500 kAU liter−1), 40°C (5,300 kAU liter−1), and 44°C (5,100 kAU liter−1) (results not shown). Lejeune et al. (7) obtained kB values of 3,500 and 4,000 kAU g of CDM−1 when they grew L. amylovorus DCE 471 in MRS medium at a constant pH of 5.0 and at fermentation temperatures of 37 and 45°C, respectively. These values are lower than those obtained in SSM at a constant pH of 5.4, but the effect of temperature was also limited in this temperature range. Since the maximum attainable biomass concentration (Xmax) varied only slightly in this temperature range (Fig. 1a), kB followed the same trend as Bmax, and the average kB value was 5,100 kAU g of CDM−1 (Fig. 1b). At 34°C, Xmax was lower (Fig. 1a). Also, Bmax (4,300 kAU liter−1) was lower at 34°C, resulting in a kB value comparable to that found at 37 to 44°C (Fig. 1b). At lower temperatures, Bmax was significantly lower (2,800 kAU liter−1 at 31°C and 1,900 kAU liter−1 at 28°C). Also, Xmax was markedly lower (Fig. 1a). Thus, it appears that L. amylovorus DCE 471 makes better use of the complex energy and nitrogen source at 37 to 44°C than at 34°C. Since kB was very high at the lower temperatures (Fig. 1b), especially 28°C, the low level of bacteriocin activity in the supernatant was due to the small amount of biomass formed (Fig. 1a) and the high apparent rate of bacteriocin inactivation (kinact) (Fig. 1b). The maximum cell yield was obtained at pH 4.2 to 5.0 (Fig. 1c). Thus, L. amylovorus DCE 471 growth is close to the optimum value (pH 5.4) at the starting pH for sourdough fermentations (around pH 5.8). At the end of sourdough fermentations (pH near 3.8), L. amylovorus DCE 471 still grows, but it grows slowly. Specific bacteriocin production increased with increasing pH, especially at pH values greater than 5.0 (Fig. 1d). Both kB and Bmax were highest at the pH that corresponded to the pH at the onset of sourdough fermentation (between pH 5.0 and 5.8). In accordance with the findings of De Vuyst et al. for growth and bacteriocin production by L. amylovorus DCE 471 in MRS medium at 37°C when constant pH values in the range from pH 4.5 to 7.0 were used, Bmax was high at pH 5.0, 5.4, and 5.8 (2). In the pH range from pH 4.2 to 6.4, kinact increased with increasing pH (Fig. 1d), which can be explained by increased adsorption of the bacteriocin molecules to the cells at higher pH values (15).
FIG. 1.
(a, b, e, and f) Influence of temperature at a constant pH of 5.4 on L. amylovorus DCE 471 in SSM. (a) Biomass production. Symbols: ▪, Xmax (in grams of CDM per liter); □, n. (b) Bacteriocin production. Symbols: •, kB (in kilo-arbitrary units [kAU] per gram of CDM); ○, kinact (in liters per gram of CDM per hour). (e) Fructose metabolism. Symbols: ▪, YX/F (in grams of CDM per gram of fructose); □, mF (in grams of fructose per gram of CDM per hour). (f) Maltose metabolism. Symbols: ▪, YX/M (in grams of CDM per gram of maltose); □, mM (in grams of maltose per gram of CDM per hour). (c, d, g, and h) Influence of pH at a constant temperature of 37°C on L. amylovorus DCE 471 in SSM. (c) Biomass production. Symbols: ▪, Xmax (in grams of CDM per liter); □, n. (d) Bacteriocin production. Symbols: •, kB (in kAU per gram of CDM); ○, kinact (in liters per gram of CDM per hour). (g) Fructose metabolism. Symbols: ▪, YX/F (in grams of CDM per gram of fructose); □, mF (in grams of fructose per gram of CDM per hour). (h) Maltose metabolism. Symbols: ▪, YX/M (in grams of CDM per gram of maltose); □, mM (in grams of maltose per gram of CDM per hour). Symbols indicate experimental values; the lines were drawn by using the model.
Both maltose and fructose were used for conversion into biomass and for maintenance. The cell yield coefficient for maltose (YX/M) increased as the temperature increased, but the effect leveled off at higher temperatures (Fig. 1f). A decrease in YX/M was observed as the pH increased, especially at higher pH values (Fig. 1h). By contrast, the fermentation temperature did not affect the cell yield coefficient for fructose (YX/F) (Fig. 1e). Hence, maltose was preferred over fructose for conversion into biomass at higher temperatures and at lower pH values. The maintenance coefficient for fructose (mF) also increased as the temperature increased, but the effect became more pronounced at higher temperatures (Fig. 1e). mF followed roughly the same trend as μmax. When the cells grew faster, the energy needed for maintenance was higher. This can be explained by the fact that cell maintenance (e.g., maintenance of the proton gradients across the cell membrane and turnover of macromolecules such as mRNA) is strongly growth dependent (10). Also, mF, but not the maintenance coefficient for maltose (mM), was affected by the fermentation pH; mF increased as the pH increased, and it was constant at pH values greater than pH 5.0 (Fig. 1g and h). Hence, fructose was the preferred energy source for maintenance, especially at temperatures greater than 34°C, possibly because it is converted faster by L. amylovorus DCE 471 than maltose at higher temperatures. Interestingly, in a sourdough fermentation maltose utilization results in biomass formation by lactic acid bacteria, whereas yeasts metabolize glucose and fructose that are present or generated in the flour (6). This contributes to the competitiveness of L. amylovorus in sourdoughs and even improves competitiveness, since high bacteriocin activity levels coincided with fast maltose consumption. This may be explained physiologically and ecologically since maltose is the most important energy source for L. amylovorus in a cereal environment.
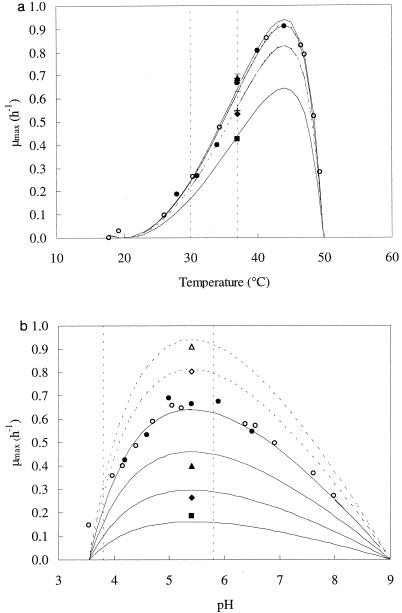
Figure 2a shows the relationship between temperature and μmax as calculated by using the secondary modeling approach. The optimum, minimum, and maximum temperatures for cell growth were 44, 20, and 49.8°C, respectively, illustrating the thermophilic character of L. amylovorus DCE 471. The activation energy was calculated to be 84.7 kJ mol−1 (Arrhenius plot not shown). At 37°C, the optimum, minimum, and maximum pH values for growth of L. amylovorus DCE 471 were 5.4, 3.55, and 9.0, respectively (Fig. 2b).
FIG. 2.
(a) Influence of temperature at a constant pH of 4.2 (▪), 4.6 (⧫), 5.0 (▴), 5.4 (• and ○), 5.8 (×, dotted line), or 6.4 (+, dotted line) on the μmax of L. amylovorus DCE 471 in SSM. (b) Influence of pH at a constant temperature of 28°C (▪), 31°C (⧫), 34°C (▴), 37°C (• and ○), 40°C (⋄, dotted line), or 44°C (▵, dotted line) on the μmax of L. amylovorus DCE 471 in SSM. Symbols indicate experimental values; the open symbols for both pH 5.4 and 37°C indicate the results of small-scale incubation experiments. The solid lines were drawn by using the model.
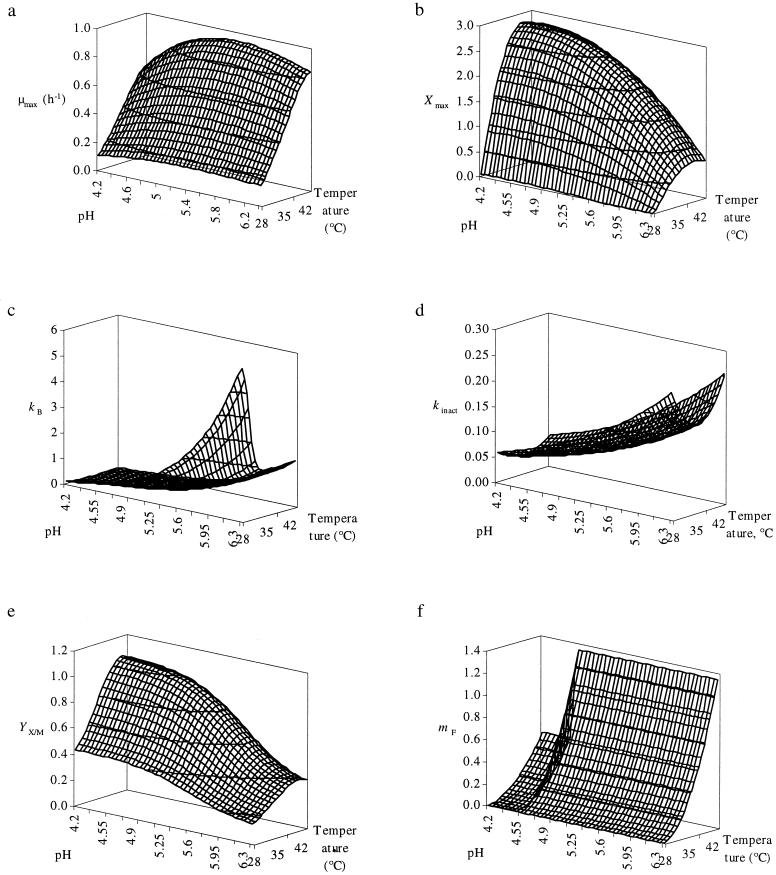
The predictive models, prepared for all biokinetic parameters derived from the primary model, are shown in Fig. 3. The correlation of the models (secondary modeling) with the experimental values (primary modeling) was satisfactory. The r2 values for μmax, YX/M, mF, Xmax, Bmax, kB, and kinact were 0.969, 0.886, 0.992, 0.968, 0.924, and 0.611, respectively. Validation of this predictive model with two additional fermentations was successful, as estimated values corresponded well with experimental measurements (data not shown). Hence, this modeling approach allows us to predict both cell growth and bacteriocin production by L. amylovorus DCE 471 in SSM for any combination of temperature between 28 and 44°C and pH between pH 4.2 and 6.4.
FIG. 3.
Surface model showing the influence of pH and temperature on μmax (per hour) (a), Xmax (in grams of CDM per liter) (b), kB (in kAU per gram of CDM) (c), kinact (in liters per gram of CDM per hour) (d), YX/M (in grams of CDM per gram of maltose) (e), and mF (in grams of fructose per gram of CDM per hour) (f).
In this paper, we show that the temperature and pH conditions that prevail during type II sourdough fermentations correspond to the range of conditions for good growth and bacteriocin production by L. amylovorus DCE 471. Furthermore, the use of L. amylovorus DCE 471 in a competitive environment contributes to proper and fast acidification of the cereal medium. Therefore, L. amylovorus DCE 471 may be a good organism to use together with a heterofermentative sourdough strain. Although SSM differs from the real sourdough environment, our findings should help us to predict and understand the behavior of this strain in a sourdough environment.
Acknowledgments
We acknowledge the financial support provided by the Institute for the Encouragement of Innovation through Science and Technology in Flanders (IWT), particularly by the STWW project “Functionality of Novel Starter Cultures in Traditional Fermentation Processes.” Also, the financial support provided by the Research Council of Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, and different food companies is greatly appreciated.
Vincent Schrijvers is acknowledged for his technical assistance.
REFERENCES
- 1.Callewaert, R., H. Holo, B. Devreese, J. Van Beeumen, I. Nes, and L. De Vuyst. 1999. Characterization and production of amylovorin L471, a bacteriocin purified from Lactobacillus amylovorus DCE 471 by a novel three-step method. Microbiology 145:2559-2568. [DOI] [PubMed] [Google Scholar]
- 2.De Vuyst, L., R. Callewaert, and K. Crabbé. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst, L., R. Callewaert, and B. Pot. 1996. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large-scale isolation of its bacteriocin amylovorin L471. Syst. Appl. Microbiol. 19:9-20. [Google Scholar]
- 4.Gänzle, M. G., M. Ehrmann, and W. P. Hammes. 1998. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl. Environ. Microbiol. 64:2616-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gobbetti, M. 1998. The sourdough microflora: interactions of lactic acid bacteria and yeasts. Trends Food Sci. Technol. 9:267-274. [Google Scholar]
- 6.Gobbetti, M., A. Corsetti, and J. Rossi. 1996. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of carbohydrates. Appl. Microbiol. Biotechnol. 41:456-460. [DOI] [PubMed] [Google Scholar]
- 7.Lejeune, R., R. Callewaert, K. Crabbé, and L. De Vuyst. 1998. Modeling the growth and bacteriocin production by Lactobacillus amylovorus DCE 471 in batch cultivation. J. Appl. Microbiol. 84:159-168. [Google Scholar]
- 8.Leroy, F., and L. De Vuyst. 1999. Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leroy, F., and L. De Vuyst. 1999. The presence of salt and a curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen, J., K. Nikolajsen, and J. Villadsen. 1991. Structured modelling of a microbial system. II. Experimental verification of a structured lactic acid fermentation model. Biotechnol. Bioeng. 38:11-23. [DOI] [PubMed] [Google Scholar]
- 11.Rosso, L., J. R. Lobry, and J. P. Flandrois. 1993. An unexpected correlation between cardinal temperatures of microbial growth highlighted by a new model. J. Theor. Biol. 162:447-463. [DOI] [PubMed] [Google Scholar]
- 12.Rosso, L., J. R. Lobry, S. Bajard, and J. P. Flandrois. 1995. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl. Environ. Microbiol. 61:610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todorov, S., B. Onno, O. Sorokine, J. M. Chobert, I. Ivanova, and X. Dousset. 1999. Detection and characterization of a novel antibacterial substance produced by Lactobacillus plantarum ST 31 isolated from sourdough. Int. J. Food Microbiol. 48:167-177. [DOI] [PubMed] [Google Scholar]
- 14.Vogel, R. F., R. Knorr, M. R. A. Müller, U. Steudel, M. G. Gänzle, and M. Ehrmann. 1999. Non-dairy lactic fermentations: the cereal world. Antonie Leeuwenhoek 76:403-411. [PubMed] [Google Scholar]
- 15.Yang, R., M. C. Johnson, and B. Ray. 1992. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 58:3355-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]