Abstract
Background
Hypoxemia is a common adverse event during propofol sedation for gastrointestinal endoscopy. Maintaining a sufficiently slow infusion rate is known to reduce respiratory depression caused by propofol, but achieving this consistently is challenging due to variations in manual techniques. This study evaluated the use of a narrow-bore extension tube to administer propofol, which passively slows infusion, to reduce hypoxemia during gastroscopy sedation.
Methods
A randomized controlled trial was conducted among patients undergoing propofol sedation for gastroscopy. Participants were assigned to either the narrow-bore extension tube group, which restricts infusion rates, or the control group receiving a standard-bore extension tube with unrestricted infusion rates. The primary outcome was the incidence of hypoxemia (SpO2 < 90%), with secondary outcomes including severe hypoxemia (SpO2 < 90% for ≥ 60 s or SpO2 < 75%), time to achieve deep sedation, total propofol dosage, and the incidence of pain on propofol injection (POPI). Subgroup analyses by age and BMI were performed.
Results
The incidence of hypoxemia was significantly lower in the narrow-bore extension tube (11.7% vs. 27.0%, p = 0.001), and there was a corresponding decrease in severe hypoxemia (7.3% vs. 19.7%, p = 0.003). The median time to achieve deep sedation was longer in the narrow-bore group (70 vs. 54 s; p < 0.001). The greatest reduction in hypoxemia was observed in overweight patients (BMI 25–28 kg/m2, p = 0.029).
Conclusions
Using a narrow-bore extension tube to control propofol infusion rates significantly reduces hypoxemia during gastroscopy sedation. This cost-effective strategy is especially beneficial for higher-risk patients, such as those who are overweight.
Trial registration
The clinical trial was registered on Chinese Clinical Trial Register on December 16, 2024 (ChiCTR2400094042).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03283-9.
Keywords: Gastrointestinal endoscopy, Hypoxemia, Infusion rate control, Propofol, Sedation
Background
Propofol, a rapid-onset intravenous anesthetic, is widely utilized for sedation during endoscopic procedures owing to its brief action duration and swift recovery profile [1, 2]. However, the administration of large bolus doses or elevated infusion rates can lead to respiratory depression, a key factor in the development of hypoxemia, which may cause a rapid decline in arterial oxygen saturation [3, 4]. This risk is particularly pronounced in vulnerable populations, such as the elderly and individuals with obesity, who are more prone to respiratory complications during sedation [5, 6].
To mitigate the risk of hypoxemia, the slow and controlled administration of propofol is crucial. Empirical evidence has established a significant correlation between the rate of propofol infusion and respiratory depression, with slower infusion rates being associated with a reduced likelihood of hypoxemia [7, 8]. A key study suggests that an optimal infusion rate of 1000 mL/h for propofol significantly reduces respiratory complications during ambulatory upper gastrointestinal endoscopy [9]. Despite these recommendations, propofol is often administered manually in clinical settings, which can lead to instances of overly rapid infusion. This is particularly pronounced in high-volume endoscopy centers, where workflow optimization for efficiency may inadvertently compromise the precision of drug delivery, prioritizing speed over accuracy.
During a serendipitous observation in our clinical practice, we noted that narrow-bore extension tubes—typically utilized for infusion pump administration—imposed mechanical resistance when manually administering propofol. This resistance inherently modulates injection pressure, decelerating the infusion rate and potentially reducing rapid drug delivery linked to respiratory depression. Our intervention addresses a critical gap in manual sedation workflows: the lack of standardized tools to enforce slow infusion rates without requiring additional equipment or specialized training. Based on this finding, we hypothesize that using narrow-bore extension tubes for manual propofol administration can reduce the risk of hypoxemia during gastroscopy sedation, thereby enhancing patient safety. Thus, this study aims to evaluate the efficacy of narrow-bore extension tubes in achieving controlled propofol delivery and reducing the incidence of hypoxemia during sedated gastroscopy.
Methods
Study design
This randomized controlled trial was conducted at the Digestive Endoscopy Center of Shanghai Changhai Hospital from December 17, 2024 to January 17, 2025. The study protocol was approved by the Shanghai Changhai Hospital Medical Ethics Committee (CHEC2024-408) and registered in the China Clinical Trial Registry on December 16, 2024 (ChiCTR2400094042). It adhered to the principles of the Declaration of Helsinki and complied with Good Clinical Practice guidelines.
Participants
Eligibility criteria
Inclusion criteria: Adults aged 18 years or older scheduled for diagnostic sedated gastroscopy, with an American Society of Anesthesiologists Physical Status (ASA-PS) classification of I-III, and who provided written informed consent.
Exclusion criteria: Severe hepatic or renal dysfunction, known allergies to propofol, pregnancy or breastfeeding, history of substance abuse, and presence of chronic respiratory diseases such as chronic obstructive pulmonary disease or asthma.
Intervention
Participants were randomly assigned to one of two groups:
Intervention group (Group Narrow): Propofol was administered via a narrow-bore infusion extension tube (inner diameter: 2.03 mm, length: 150 cm) supplied by Shenzhen Shunmei Medical Co., Ltd.
Control group (Group Standard): Propofol was administered via a standard-bore extension tube (inner diameter: 3.05 mm, length: 150 cm) from the same manufacturer.
Figure 1a and b illustrates two extension tubes with different diameters and their distinctions.
Fig. 1.
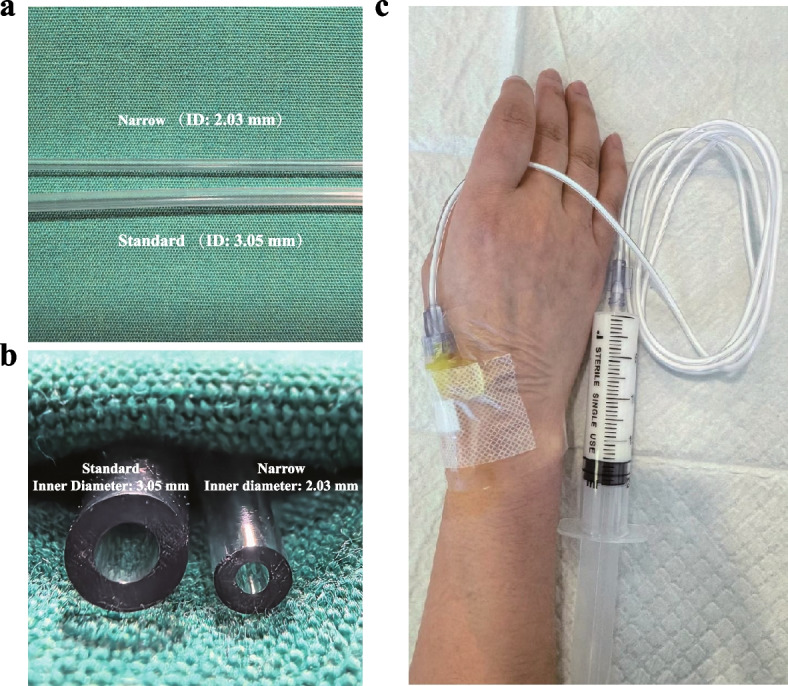
Comparison of standard-bore (inner diameter: 3.05 mm, length: 150 cm) and narrow-bore (inner diameter: 2.03 mm, length: 150 cm) extension tubes. a A side-by-side comparison of the tube connectors. b A top-down view of the tubes’ cross-sections. c Route of administration of propofol
All injections were administered by two experienced anesthesiologists following the same sedation protocol. Manual injection speed was not mechanically standardized but reflected real-world clinical practice. The narrow-bore tube’s intrinsic resistance served to passively modulate injection pressure. It can be anticipated that with the use of a narrow-bore extension tube, the actual infusion rate of propofol would be lower than that with a standard extension tube, due to increased resistance within the tube under the same force. This study aimed to simulate the use of different extension tubes as they would be used in real clinical practice.
Sample size calculation
A pilot study with 100 patients revealed hypoxemia incidences of 25% in Group Standard and 10% in Group Narrow. Using PASS 21.0.3 software, sample size calculations were conducted assuming a statistical power of 0.90, a significance level (α) of 0.05, and a 5% dropout rate. The analysis indicated that a total of 274 participants, with 137 allocated to each group, were necessary to achieve adequate power for the study.
Randomization and blinding
Patients were randomly assigned to one of the two groups in a 1:1 ratio using computer-generated random numbers. Odd numbers were assigned to Group Narrow, while even numbers were assigned to Group Standard. The study was designed as a single-blind trial. Participants were unaware of their group allocation (use of narrow-bore or standard extension tubes). Due to the nature of the procedure (the anesthesiologist needed to connect and use specific extension tubes), the anesthesiologist who administered sedation could not be blinded. However, to reduce assessment bias, all postoperative follow-ups, data collection (including recording vital signs data from monitors, determination of adverse events), and statistical analysis were conducted by researchers who were unaware of the group assignments in this study. All outcome assessors received training and were clearly informed of their blind status before the study began.
Sedation protocol
Pre-procedure preparation: Patients were instructed to fast for at least 8 h prior to the procedure. Patients were positioned in the left lateral decubitus position, and dorsal hand veins were cannulated with a 24G intravenous catheter (INTROCAN SAFETY® 3, 0.7 × 19 mm). Following the insertion of the intravenous catheter, 20 mL prefilled syringes containing propofol were connected to the infusion extension tubes designated for each study group. These infusion extension tubes were then attached to the intravenous catheter (Fig. 1c). Continuous monitoring of electrocardiography (ECG), noninvasive blood pressure (NIBP), and oxygen saturation (SpO2) was initiated throughout the procedure. Oxygen was administered at a rate of 4 L/min via nasal cannula, and baseline vital signs were recorded.
Propofol administration: The anesthesiologist administered an initial dose of 1.5–2.5 mg/kg propofol to achieve deep sedation, defined by a Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score of less than 3 [10]. When patients showed no significant movement in response to a jaw lift maneuver, the endoscopist could insert the endoscope. Additional boluses of 30 mg propofol were administered as required for patients whose sedation was deemed inadequate during the procedure.
Airway management: Airway interventions were systematically executed upon hypoxemia emergence, following a structured escalation protocol: If SpO₂ dropped below 90% for up to 10 s, the anesthesiologist paused propofol administration, increased oxygen flow, and performed a jaw lift to ensure airway patency. If SpO₂ remained below 90% for more than 10 s, modified manual chest compression were initiated to assist respiration [11]. If SpO₂ below 75%, the endoscopist immediately halted the procedure, withdrew the endoscope, and delivered oxygen via a face mask with positive pressure. In cases of persistent hypoxemia unresponsive to these measures, advanced airway management, including the use of a laryngeal mask airway or endotracheal intubation as clinically indicated, was initiated. This stepwise approach prioritized patient safety, with interventions escalating based on the severity of desaturation to minimize hypoxemia-related complications.
Data collection
Data collection was conducted by independent researchers and encompassed several key parameters: time to achieve deep sedation, lowest SpO2, propofol induction dose, total propofol dose, and procedure duration.
Adverse events, including hypoxemia, hypotension, and airway interventions, were meticulously documented. Following the procedure, patients were transferred to the post-anesthesia care unit and were queried about any pain on propofol injection (POPI) upon regaining consciousness.
Outcomes
The primary outcome measure was hypoxemia, defined as SpO2 < 90% for less than 60 s. Secondary outcome measures included severe hypoxemia (SpO2 < 90% for ≥ 60 s or SpO2 < 75%), time to achieve deep sedation, lowest SpO2, total propofol dosage, and the incidence of POPI.
Statistical analyses
Statistical analyses were performed using R version 4.2.2 and MSTATA software (www.mstata.com). Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range, IQR) depending on the normality of their distribution, which was assessed using the Shapiro–Wilk test and visual inspection of Q-Q plots. For normally distributed variables, Student’s t-test was applied; otherwise, the Mann–Whitney U test was used. Categorical variables were presented as numbers and frequencies. Comparisons between categorical variables were conducted using the chi-square test or Fisher’s exact test as appropriate. A p-value of < 0.05 was considered statistically significant, and for subgroup analyses, an FDR-adjusted p-value threshold was applied.
Propensity score matching (PSM) was employed to minimize baseline differences between the two groups. Matching variables included BMI and STOP-BANG score, as these are well-known risk factors for hypoxemia during sedation [12–14]. The STOP-BANG Score assesses eight dichotomous parameters: Snoring, Tiredness, Observed apnea, high Blood pressure, BMI > 35 kg/m2, Age > 50 years, Neck circumference > 40 cm, and male Gender. A score ≥ 3 indicates intermediate-to-high OSA risk, which may exacerbate hypoxemia during sedation [15]. A 1:1 nearest-neighbor matching algorithm without replacement was used, with a caliper width of 0.2 standard deviations of the logit of the propensity score. The balance between groups was evaluated using standardized mean differences (SMD), and an SMD of less than 0.2 was considered indicative of adequate balance. To assess the robustness of the results, a sensitivity analysis was conducted using a narrower caliper width (0.1), and results were consistent with the primary analysis.
Subgroup analyses were performed based on age and BMI categories. Age was stratified into two groups: non-elderly (less than 60 years) and elderly (60 years or older). BMI was categorized into three groups: normal weight (18–25 kg/m2), overweight (25–28 kg/m2), and obese (28 kg/m2 or higher). These stratifications allowed for the evaluation of the intervention's effects across different demographic and physiological profiles. Patients with missing data in key grouping or baseline variables were excluded from subgroup analyses to ensure the validity of the results.
Results
The study initially enrolled 278 patients. Four patients were subsequently excluded: two due to a history of alcohol abuse and asthma, and two who declined to participate. Ultimately, 274 patients who met the inclusion criteria successfully completed the sedated gastroscopy (Fig. 2).
Fig. 2.
CONSORT flow diagram
Baseline characteristics were similar between Group Standard and Group Narrow in terms of sex, age, height, and Modified Mallampati Score. However, there were significant differences in weight and BMI, with Group Standard having higher values (71.0 ± 13.0 kg and 25.1 ± 3.5 kg/m2) compared to Group Narrow (68.0 ± 13.0 kg and 24.2 ± 3.6 kg/m2, p = 0.028 and p = 0.039, respectively). Additionally, a higher proportion of participants in Group Standard had a history of hypertension (69.8%) compared to Group Narrow (55.2%) (Table 1).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Group standard, n = 1371 | Group narrow, n = 1371 | p-value2 |
|---|---|---|---|
| Sex | > 0.999 | ||
| Male | 82 (59.9%) | 82 (59.9%) | |
| Female | 55 (40.1%) | 55 (40.1%) | |
| Age (y) | 53 (42, 60) | 51 (42, 61) | 0.935 |
| ASA-PS | 0.053 | ||
| I | 60 (43.8%) | 76 (55.5%) | |
| II | 77 (56.2%) | 61 (44.5%) | |
| Height (m) | 1.7 (1.6, 1.7) | 1.6 (1.6, 1.7) | 0.286 |
| Weight (kg) | 71.0 ± 13.0 | 68.0 ± 13.0 | 0.028* |
| BMI (kg/m2) | 25.1 ± 3.5 | 24.2 ± 3.6 | 0.039* |
| Modified Mallampati Score | 0.869 | ||
| I | 46 (33.6%) | 45 (32.8%) | |
| II | 47 (34.3%) | 50 (36.5%) | |
| III | 35 (25.5%) | 36 (26.3%) | |
| IV | 9 (6.6%) | 6 (4.4%) | |
| STOP-BANG Score | 2 (1, 3) | 2 (1, 2) | 0.041* |
| Comorbidity | 0.397 | ||
| Hypertension | 37 (69.8%) | 16 (55.2%) | |
| DM | 10 (18.9%) | 9 (31.0%) | |
| Hypertension, DM | 6 (11.3%) | 4 (13.8%) |
ASA-PS American Society of Anesthesiologists physical status, BMI Body mass index, DM Diabetic mellitus
1n (%); Median (IQR); Mean ± SD
2Pearson’s Chi-squared test; Wilcoxon rank sum test; Welch Two Sample t-test; Fisher’s exact test
* p < 0.05
Outcomes
Hypoxemia occurred at a significantly higher rate in Group Standard (27.0%) compared to Group Narrow (11.7%, p = 0.001). Similarly, the incidence of severe hypoxemia, defined as SpO2 < 90% for ≥ 60 s or SpO2 < 75%, was significantly higher in Group Standard (19.7%) than in Group Narrow (7.3%, p = 0.003).
The median time to achieve deep sedation was significantly shorter in Group Standard with a median of 54 s compared to Group Narrow at 70 s (p < 0.001). The induction dose of propofol was significantly higher in Group Standard (150.0–175.0 mg) than in Group Narrow (140.0–160.0 mg, p = 0.012). This trend was also observed for the total propofol dose, which was higher in Group Standard (200.0–250.0 mg) compared to Group Narrow (150.0–210.0 mg, p < 0.001).
The lowest SpO2 were slightly but significantly lower in Group Standard (96%) compared to Group Narrow (97%, p = 0.007). Airway interventions were more frequently required in Group Standard, with jaw lift performed in 54.1% patients (20/37) and mask ventilation in 32.4% patients (12/37). In contrast, modified manual chest compression was more commonly required in Group Narrow, with 37.5% patients (6/16) undergoing this intervention. The overall rate of airway interventions was significantly higher in Group Standard (p = 0.039).
There was no significant difference in the incidence of POPI between the two groups, 19.7% of patients in the Narrow-bore group experienced POPI, compared to 14.6% in the Standard-bore group (p = 0.262). The duration of the gastroscopy procedure was similar between Group Standard (6.1 min) and Group Narrow (5.7 min, p = 0.156). Table 2 provides a comprehensive summary of the outcomes for both groups.
Table 2.
Comparative outcomes between narrow-bore and standard extension tubes: primary analysis and propensity-matched sensitivity evaluation
| Outcome | Primary | p-value2 | PSM adjusted | p-value2 | ||
|---|---|---|---|---|---|---|
| Standard, n = 1371 | Narrow, n = 1371 | Standard, n = 1201 | Narrow, n = 1201 | |||
| Hypoxemia | 37 (27.0%) | 16 (11.7%) | 0.001 | 28 (23.3%) | 13 (10.8%) | 0.010 |
| Severe hypoxemia | 27 (19.7%) | 10 (7.3%) | 0.003 | 19 (15.8%) | 8 (6.7%) | 0.025 |
| Time to achieve deep sedation (s) | 54.0 (46.0, 65.0) | 70.0 (63.0, 81.0) | < 0.001 | 54.0 (45.0, 65.0) | 70.0 (62.0, 80.0) | < 0.001 |
| Induction dose of propofol (mg) | 150.0 (150.0, 175.0) | 150.0 (140.0, 160.0) | 0.012 | 150.0 (150.0, 175.0) | 150.0 (138.0, 160.0) | 0.040 |
| Total dose of propofol (mg) | 200.0 (200.0, 250.0) | 200.0 (150.0, 210.0) | < 0.001 | 200.0 (180.0, 250.0) | 190 (150.0, 203.0) | < 0.001 |
| POPI | 20 (14.6%) | 27 (19.7%) | 0.262 | 19 (15.8%) | 24 (20.0%) | 0.400 |
| Lowest SpO2 (%) | 96 (89, 98) | 97 (93, 99) | 0.007 | 96 (91, 98) | 97 (93, 99) | 0.049 |
| Airway interventions | 0.039 | 0.076 | ||||
| Jaw lift | 20 (54.1%) | 3 (18.8%) | 17 (60.7%) | 3 (23.1%) | ||
| Modified Manual Chest Compression | 5 (13.5%) | 6 (37.5%) | 4 (14.3%) | 5 (38.5%) | ||
| Mask ventilation | 12 (32.4%) | 7 (43.8%) | 7 (25.0%) | 5 (38.5%) | ||
| Procedure duration (min) | 6. 1 (5.1, 8.2) | 5.7 (4.3, 7.4) | 0.156 | 6.1 (5.0, 8.2) | 5.6 (4.3, 7.4) | 0.188 |
POPI pain on propofol injection
1n (%); Median (IQR)
2Pearson’s Chi-squared test; Wilcoxon rank sum test; Fisher’s exact test
Post hoc analyses
Following PSM, which reduced baseline imbalances in covariates such as weight, BMI, and STOP-BANG scores, further post hoc analyses were conducted to explore the effect of narrow-bore infusion tube across various subgroups. The matching process yielded 240 matched pairs, with a significant reduction in SMDs post-matching, indicating improved covariate balance (Tables S1, see Additional file 1).
The primary outcome analysis after matching showed that the incidence of hypoxemia remained significantly lower in Group Narrow compared to Group Standard (23% vs. 11%; p = 0.01). Secondary outcomes, including total propofol dose and lowest SpO2, also showed consistent trends favoring Group Narrow (Table 2).
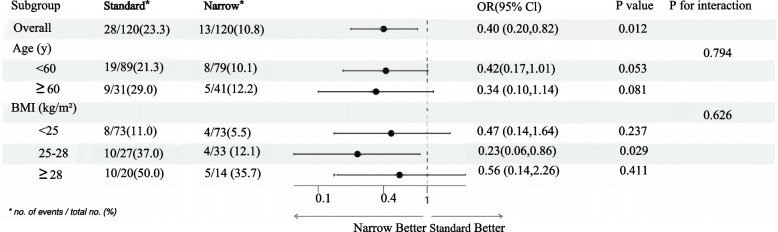
Subgroup analyses were performed to assess the differential effects of the narrow-bore tube based on patient characteristics including age and BMI. The overall analysis revealed a significant reduction in hypoxemia with the narrow-bore tube compared to the standard-bore tube, with an odds ratio (OR) of 0.40 [95% confidence interval (CI), 0.20–0.82], p = 0.012.
Age Subgroup: Patients under 60 years old showed a trend towards lower hypoxemia rates with the narrow-bore tube, with an OR of 0.42 [95% CI, 0.17–1.01], p = 0.053, which was not statistically significant. For patients aged 60 and above, the OR was 0.34 [95% CI, 0.10–1.14], p = 0.081, also not reaching statistical significance.
BMI Subgroup: The most notable reduction in hypoxemia was observed in the overweight patient group (25 kg/m2 ≤ BMI < 28 kg/m2), with an OR of 0.23 [95% CI, 0.06–0.86], p = 0.029, indicating a significant benefit of the narrow-bore tube in this subgroup. In contrast, no significant effects were observed in patients with a BMI below 25 kg/m2 (OR 0.47 [95% CI, 0.14–1.64], p = 0.237) or those who were obese (BMI ≥ 28 kg/m2, OR 0.56 [95% CI, 0.14–2.26], P = 0.411).
The P values for interaction in age and BMI subgroups were 0.794 and 0.626, respectively, suggesting no significant interaction effects between the type of tube and these patient characteristics on hypoxemia rates. These findings underscore the potential of narrow-bore tubes to reduce hypoxemia, particularly in overweight patients, and warrant further investigation into personalized sedation strategies (Fig. 3).
Fig. 3.
Forest map for post-hoc subgroup analysis
Sensitivity analyses
Sensitivity analyses, which involved adjusting the caliper width to 0.1 and applying multifactorial logistic regression, confirmed the robustness of the results. (Table S2 and S3, see Additional file 1) These additional analyses reinforced the conclusion that the Narrow-bore infusion tube significantly reduces the incidence of hypoxemia in certain high-risk subgroups, with particular efficacy in overweight patients.
Discussion
To our knowledge, this study is the first to systematically examine the impact of infusion tube diameter on sedation-related outcomes, presenting a simple yet effective intervention to address a common complication in propofol sedation. Our results demonstrate that the use of a narrow-bore infusion tube significantly reduces the incidence of hypoxemia during propofol sedation for gastroscopy, with a particular benefit observed in overweight patients (BMI 25–28 kg/m2). The slower infusion rate in the narrow-bore tube group, which resulted in longer induction times and reduced total propofol doses, is consistent with previous studies [7, 8, 16]. The significantly higher lowest SpO2 in the narrow-bore group further support the hypothesis that a gradual onset of sedation lowers the risk of over-sedation and respiratory complications.
Hypoxemia remains a prevalent issue during gastrointestinal endoscopy sedation [5, 12, 17, 18]. While interventions such as high-flow oxygen and nasal masks can manage hypoxemia, these approaches generally require specialized equipment [19–22]. In contrast, the narrow-bore infusion tube offers a preventive solution by optimizing propofol infusion rates from the outset, reducing the likelihood of respiratory depression and enhancing patient safety. This cost-effective method can be easily incorporated into routine clinical practice, particularly for patients at higher risk of hypoxemia.
The rate and method of propofol administration are critical determinants of sedation safety. Rapid bolus injections can lead to rapid increases in drug concentration, potentially overwhelming respiratory control mechanisms and precipitating hypoxemia [7]. Conversely, slower infusion rates, as achieved with the narrow-bore infusion tube, provide a more controlled sedation onset, allowing clinicians to adjust dosing to minimize respiratory risks [8, 16]. Our findings confirm that the narrow-bore infusion tube reduces the incidence of hypoxemia, highlights the importance of infusion rate control in ensuring patient safety.
Interestingly, our data suggest that overweight patients (BMI 25–28 kg/m2) benefited most from the narrow-bore infusion tube in terms of hypoxemia reduction, rather than those who are classified as obese (BMI ≥ 28 kg/m2). One possible explanation is that overweight patients have a lower respiratory reserve compared to normal-weight individuals, but they are not as severely compromised as obese patients. This intermediate risk level may make them more susceptible to rapid changes in sedation, while also making them more responsive to controlled infusion strategies [21]. While subgroup analyses by BMI categories (normal weight, overweight, obese) revealed a trend toward greater hypoxemia risk reduction in overweight participants (BMI 25–28 kg/m2; adjusted OR = 0.23, 95% CI: 0.06–0.86), no statistically significant interaction was observed between BMI categories and treatment effect (p-interaction = 0.626). These findings should be interpreted as exploratory post hoc observations rather than prespecified outcomes. The absence of significant effect modification highlights the need for prospective validation in stratified cohorts, particularly given the potential confounding from unmeasured variables such as neck circumference or upper airway anatomy.
While the Narrow-bore infusion tube was effective in reducing hypoxemia, it unexpectedly increased the incidence of POPI. This increase in pain is likely due to the prolonged infusion time, which increases the duration of contact between propofol and the venous endothelium, thereby enhancing its irritant effects [23, 24]. Although slower infusion rates may reduce hypoxemia, they may also elevate the risk of POPI. Pre-treatment with lidocaine or the use of other analgesics could help alleviate this discomfort and improve patient tolerance [25].
This study has several limitations. First, due to the relatively small number of outpatient patients aged 75 and older, we were unable to include this population in our age subgroup analysis. As a result, the effectiveness of our intervention in this elderly group remains unknown. Second, being a single-center study, the generalizability of our findings may be limited by local practices, such as preoxygenation protocols and variations in clinician experience. While all injections were administered by two experienced anesthesiologists following a standardized protocol (initial propofol dose 1.5–2.5 mg/kg), subtle differences in manual injection force could theoretically influence infusion rates. However, the narrow-bore tube’s mechanical resistance inherently restricts flow rate according to Poiseuille’s law, reducing dependency on operator skill. This was validated by the narrow-bore group’s significantly longer time to deep sedation (70 vs. 54 s) and 20–25% lower total propofol dosage, confirming consistent rate control. While equivalent pharmacodynamic effects might theoretically be achieved by slow manual injection using standard tubes, such consistency is difficult to maintain in practice. The narrow-bore tube’s value lies in its passive standardization of infusion rates, reducing reliance on operator skill. Third, despite employing PSM to minimize bias related to BMI and STOP-BANG scores, residual confounding from unmeasured variables (e.g., dynamic hemodynamic changes or preoperative hydration status) remains possible. Fourth, the lack of continuous hemodynamic monitoring may underestimate transient hypotension, particularly in elderly patients. Finally, direct measurement of injection speed was not performed, though the narrow-bore tube’s passive flow restriction was validated by clinical outcomes (hypoxemia reduction). Future studies may use infusion pumps to compare controlled rates or model pharmacokinetic profiles. Future multicenter trials with larger cohorts, standardized protocols for injection timing, and continuous hemodynamic monitoring—particularly in high-risk populations like the elderly—are needed to validate these findings. Future research should aim to balance the reduction in hypoxemia with effective management of POPI. Investigating alternative tube designs, infusion techniques, or adjunctive medications like lidocaine could help maintain the respiratory benefits of the Narrow-bore infusion tube while minimizing patient discomfort. Additionally, assessing long-term outcomes, including recovery times and overall patient satisfaction, will be important in determining the broader clinical utility of this approach in various sedation contexts.
Conclusions
This study demonstrates that using a narrow-bore infusion tube to control propofol infusion rates significantly reduces hypoxemia during gastroscopy sedation. Unlike other interventions, this approach physically limits the infusion rate, making it a simple and easily implementable strategy. It offers a cost-effective and accessible solution for enhancing patient safety, particularly for high-risk populations. However, addressing pain on propofol injection remains important, highlighting the need for a balanced approach to sedation management.
Supplementary Information
Acknowledgements
During the preparation of this work the authors used ChatGPT 3.5 in order to improve the readability and language of the manuscript. After using this tool, the authors reviewed and edited the content and take full responsibility for the content of the published article.
Abbreviations
- ASA-PS
American Society of Anesthesiologists physical status
- BMI
Body Mass Index
- ECG
Electrocardiogram
- IQR
Interquartile Range
- MI
Manual Infusion
- MOAA/S
Modified Observer’s Assessment of Alertness/Sedation
- NIBP
Noninvasive Blood Pressure
- POPI
Pain on propofol injection
- PSM
Propensity Score Matching
- SMD
Standardized Mean Difference
Authors’ contributions
JL, DDY, and XHF conceived and designed the study. DDY, SY, and YYW collected data. JL and LLB performed statistical analyses. JL, DDY and YYW drafted the manuscript. XHF and LLB revised the manuscript. JL, DDY, and YYW contributed equally to this work. XHF and LLB assumed direct responsibility for the manuscript. All authors approved the final submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Shanghai Changhai Hospital Medical Ethics Committee on December 2, 2024 (CHEC2024-408). Written informed consent was obtained from all participants.
Consent for publication
No personal data was involved in this clinical trial.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Lu, Didi Yang and Yueying Wang contributed equally to this work.
Contributor Information
Xiaohua Fan, Email: fxh95007@126.com.
Lulong Bo, Email: bartbo@smmu.edu.cn.
References
- 1.Zhou S, Zhu Z, Dai W, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127(1):56–64. 10.1016/j.bja.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Dumonceau JM, Riphaus A, Aparicio JR, European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline, et al. Non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol. 2010;27(12):1016–30. 10.1097/EJA.0b013e32834136bf. [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa T, Suzuki H. Propofol for gastrointestinal endoscopy. United Eur Gastroenterol J. 2018;6(6):801–5. 10.1177/2050640618767594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex DK. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther. 2006;24(2):163–71. 10.1111/j.1365-2036.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- 5.Lv LL, Zhang MM. Up-to-date literature review and issues of sedation during digestive endoscopy. Wideochir Inne Tech Maloinwazyjne. 2023;18(3):418–35. 10.5114/wiitm.2023.127854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadeer MA, Rocio Lopez A, Dumot JA, Vargo JJ. Risk factors for hypoxemia during ambulatory gastrointestinal endoscopy in ASA I-II patients. Dig Dis Sci. 2009;54(5):1035–40. 10.1007/s10620-008-0452-2. [DOI] [PubMed] [Google Scholar]
- 7.Stokes DN, Hutton P. Rate-dependent induction phenomena with propofol: implications for the relative potency of intravenous anesthetics. Anesth Analg. 1991;72(5):578–83. 10.1213/00000539-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Peacock JE, Lewis RP, Reilly CS, Nimmo WS. Effect of different rates of infusion of propofol for induction of anaesthesia in elderly patients. Br J Anaesth. 1990;65(3):346–52. 10.1093/bja/65.3.346. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Zhou Q, Xiao W, Zhou H. Determination of the appropriate propofol infusion rate for outpatient upper gastrointestinal endoscopy-a randomized prospective study. BMC Gastroenterol. 2016;16:49. 10.1186/s12876-016-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qadeer MA, Vargo JJ, Patel S, et al. Bispectral index monitoring of conscious sedation with the combination of meperidine and midazolam during endoscopy. Clin Gastroenterol Hepatol. 2008;6(1):102–8. 10.1016/j.cgh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wei J, Shen N, et al. Modified manual chest compression for prevention and treatment of respiratory depression in patients under deep sedation during upper gastrointestinal endoscopy: two randomized controlled trials. Anesth Analg. 2023;137(4):859–69. 10.1213/ANE.0000000000006447. [DOI] [PubMed] [Google Scholar]
- 12.Behrens A, Kreuzmayr A, Manner H, et al. Acute sedation-associated complications in GI endoscopy (ProSed 2 study): results from the prospective multicentre electronic registry of sedation-associated complications. Gut. 2019;68(3):445. 10.1136/gutjnl-2015-311037. [DOI] [PubMed] [Google Scholar]
- 13.Fang Z, Zou D, Xiong W, et al. Dynamic prediction of hypoxemia risk at different time points based on preoperative and intraoperative features: machine learning applications in outpatients undergoing esophagogastroduodenoscopy. Ann Med. 2023;55(1):1156–67. 10.1080/07853890.2023.2187878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laffin AE, Kendale SM, Huncke TK. Severity and duration of hypoxemia during outpatient endoscopy in obese patients: a retrospective cohort study. Can J Anaesth. 2020;67(9):1182–9. 10.1007/s12630-020-01737-x. [DOI] [PubMed] [Google Scholar]
- 15.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107(5):1543–63. 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 16.Blum J, Kochs E, Forster N, Schneider G. The influence of injection rate on the hypnotic effect of propofol during anesthesia: a randomized trial. PLoS Clin Trials. 2006;1(3): e17. 10.1371/journal.pctr.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137(4):1229–37. 10.1053/j.gastro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Frieling T, Heise J, Kreysel C, Kuhlen R, Schepke M. Sedation-associated complications in endoscopy–prospective multicentre survey of 191142 patients. Z Gastroenterol. 2013;51(6):568–72. 10.1055/s-0032-1330441. [DOI] [PubMed] [Google Scholar]
- 19.Xu R, Li Z, Jiang X, et al. Effect of supraglottic jet oxygenation and ventilation on hypoxemia in patients undergoing endoscopic surgery with sedation: a meta-analysis of randomized controlled trials. J Clin Anesth. 2024;97: 111559. 10.1016/j.jclinane.2024.111559. [DOI] [PubMed] [Google Scholar]
- 20.Chen DX, Yang H, Wu XP, et al. Comparison of a nasal mask and traditional nasal cannula during intravenous anesthesia for gastroscopy procedures: a randomized controlled trial. Anesth Analg. 2022;134(3):615–23. 10.1213/ANE.0000000000005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nay MA, Fromont L, Eugene A, et al. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). Br J Anaesth. 2021;127(1):133–42. 10.1016/j.bja.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 22.King AB, Alvis BD, Hester D, Taylor S, Higgins M. Randomized trial of a novel double lumen nasopharyngeal catheter versus traditional nasal cannula during total intravenous anesthesia for gastrointestinal procedures. J Clin Anesth. 2017;38:52–6. 10.1016/j.jclinane.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu T, Inomata S, Kihara S, Toyooka H, Brimacombe JR. Rapid injection reduces pain on injection with propofol. Eur J Anaesthesiol. 2005;22(5):394–6. 10.1017/s0265021505230673. [DOI] [PubMed] [Google Scholar]
- 24.Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43(6):492–4. 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 25.Euasobhon P, Dej-Arkom S, Siriussawakul A, et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev. 2016; CD007874. 10.1002/14651858.CD007874.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.