Abstract
Introduction
Legionella is the second cause of community-acquired pneumonia in Intensive Care Unit (ICU) patients. The aim of this study was to describe the epidemiology and outcome in patients with Legionella pneumonia (LP) in French ICUs.
Methods
A multi-center, retrospective, observational study in 12 French ICUs was performed between January 2014 and December 2019.
Results
LP was diagnosed in 162 patients during the study period. Invasive mechanical ventilation was required in 95 patients (58%), 73 (45%) of whom had acute respiratory distress syndrome (ARDS). Most of these patients were treated with a combination of antibiotics (128, patients; 79%). The most common combination consisted in a fluoroquinolone and a macrolide (118 patients). Median length of stay in an ICU was 11 [5; 11] days. At 28 days, 19 (12%) out of the 162 patients had not survived. In multivariate analyses, age (Incidence risk Ratio: IRR, 1.07; 95% CI, 1.01; 1.14) and a high Sequential Organ Failure Assessment (SOFA) score in the first 48 h (IRR, 1.47; 95% CI, 1.09; 2) were significantly associated with mortality.
Conclusion
In this French multicentric cohort, the LP prognosis in ICUs was apparently more favorable than in the literature, possibly because of the timely and improved LP management in ICUs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-025-00173-z.
Keywords: Legionella pneumophila, Intensive Care Unit, Antibiotherapy, Septic shock
Introduction
Legionnaire’s Disease is an infection caused by Legionella, a gram-negative intracellular bacillus, and is a frequent cause of community acquired-pneumonia in hospitalized patients [1, 2]. Legionella pneumophila is the most frequent species. Legionella is ubiquitous and can proliferate in water environments at temperatures between 20 and 45°C. Contamination occurs throughout aerosol inhalation or aspiration of water containing this microorganism. While several serotypes exist, serotype 1 has been associated with 82% of Legionella pneumonia (LP) cases in Europe [3]. Diagnosis is based on the usual diagnostic criteria for pneumonia combined with a microbiological criterion (culture, polymerase chain reaction or urinary antigen test) confirming presence of the Legionella species [4].
Since 2017, incidence in Europe has been increasing year after year, with 2.4 cases per 100,000 persons in 2021 [3]. Legionella represents one of the three most common causes of severe CAP ICUs [5–8]. Indeed, from 20 to 40% of patients with LP are admitted to the latter [6, 9, 10] with mortality rates ranging from 28 to 41% [10–14]. Despite the frequency and lethality of this disease, few prospective studies have been published, and all of them are descriptive. There have been no randomized trials concerning LP in ICUs.
National and international guidelines recommend antibiotic therapy with macrolides, quinolones or rifampicin [15–17]. In France, guidelines recommend monotherapy except for severe forms entailing ICU hospitalization or for immunocompromised patients to whom a combination of antibiotics can be administered [18]. Treatment usually lasts from 7 to 14 days and can continue for up to 21 days face to severe forms necessitating ICU admission, or in treatment of immunocompromised patients [18]. At present, LP in ICUs is usually treated with combination therapy, but the existing guidelines are based on retrospective studies without randomized trials [19–21].
Although this disease is the second cause of community-acquired pneumonia in ICUs [5], few studies have focused on the subject. We have carried out this study in order to describe the epidemiology and outcomes of LP in French ICUs.
The primary endpoint was 28-day survival, while the secondary objectives were length of stay in ICU and ventilator-free days.
Methods
Study design and participants
Between January 2014 and December 2019, we performed a multi-center retrospective analysis in 12 different French ICUs (tertiary university hospitals or general hospitals). All adults admitted to an ICU with a Legionella pneumonia diagnosis were included. Nosocomial legionella defined by hospital water supply contamination was excluded [1]. Cases were identified through a combination of pathology laboratory information systems and hospital coding records.
Definition
Community-acquired pneumonia is defined as an acute illness with cough and at least one of the following: new focal chest signs supported by chest imagery, fever > 4 days or dyspnea/tachypnea, without another obvious cause [15].
Legionella pneumonia diagnosis is defined according to the criteria of the European Center for Disease Prevention and Control as pneumonia with at least one the following bacteriological criteria [4]: (i) isolation of Legionella species from respiratory secretions or any normally sterile site; (ii) detection of Legionella pneumophila antigen in urine; (iii) significant increase in specific antibody level to Legionella pneumophila serogroup 1 in paired serum samples.
A diagnosis with polymerase chain reaction from respiratory secretions (sent to microbial laboratory) was accepted.
Acute Respiratory Distress Syndrome (ARDS) is defined by the Berlin definition [22] with respiratory symptoms for less than one-week, bilateral opacities, bilateral failure not fully explained by cardiac failure and PaO2/FiO2 < ≤ 300mmHg with PEEP ≥ 5cmH2O.
SOFA (Sequential Organ Failure Assessment) score maximum H0-H48 corresponds to the maximum SOFA score between SOFA at baseline and SOFA at H48. This variable is linear.
Acute respiratory failure is defined by respiratory signs with hypoxemia.
Bicarbonates correspond to bicarbonates at admission on arterial blood gas. Cytolysis is defined by ALAT higher than 50UI/l during the first 48 h. Jaundice is defined by total bilirubin greater than 30micromol/l during the first 48 h, and hyponatremia by natremia under 135mmol/l at baseline. Leukopenia is defined by leukocytes inferior to 4G/l and hyperleukocytosis by leukocytes higher than 10G/l. ‘Smokers’ is defined as current smokers, and ‘alcohol drinkers’ is defined as current alcohol consumers.
Data collection
For all included patients, patient demographics comorbidities and predisposing factors to LP were recorded, as were clinical manifestations, biological, specifically targeted therapies, other therapeutic management during ICU stay, and evolutive clinical data at days 28 and 60.
Data collection was completed by each investigation center on a case report form. The inspired oxygen fraction of patients on conventional oxygen therapy was calculated using the formula: (21% + oxygen flow rate in L/min x 3) [23].
The radiological focus was analyzed by each investigation center from chest radiograph or thoracic scanner.
Outcomes
The primary outcome was survival at 28 days. The secondary outcomes were length of stay in ICU and ventilator-free days [24]. Length of stay in ICU was defined as length of stay among survivors. Ventilator-free days were defined as the number of days from successful weaning to day 60.
Statistical analysis
Descriptive analysis of the population was presented using frequencies and percentages for qualitative data and median and interquartile range (IQR) for quantitative data.
Univariate and multivariate analyses were performed using the Cox proportional hazards regression models for survival at day 28, stratified by center. For all multivariable regression models, variables with p < 0.2 in the univariate models or clinical relevance were introduced using a stepwise selection method.
Negative binomial regression was used for secondary outcomes, stratified by center (Length of stay in ICU and ventilator-free days at D-60).
Multiple imputation was used for variables with missing values used in the multivariate model. Fifteen imputed datasets were created to account for variables with 15% or less of missing values (Table S19).
A p-value < 0.05 was considered significant. Statistical analyses were performed using SAS 9.4 software© (SAS Institute, Cary, NC, USA).
Ethical considerations
Written information about the study was provided to patients and their relatives though a letter sent directly to the patients’ last known residence before enrolment, following the French Reference Methodology MR-004. Their data were included in the study if they did not fail to respond or if they died. All data were anonymized prior to analysis. The study was approved by the Ethics Committee of the French Intensive Care Society (SRLF, Société de Réanimation de la Langue Française, CE 22–040).
Results
Demographic characteristics (Table 1)
Table 1.
Baseline characteristics on ICU admission (n = 162)
| Variables | Median [Q1; Q3] or number (Percentage) | |
|---|---|---|
| Age (years) | 61 | [53–72] |
| Male gender | 120 | (74.9) |
| BMI (kg/m2) | 25.5 | [22.7–30.4] |
| Diabetes | 21 | (15.9) |
| Alcohol | 52 | (32.1) |
| Smoker | 79 | (48.8) |
| Neoplasia | 18 | (11.1) |
| Hemopathy | 11 | (6.8) |
| Chemotherapy | 9 | (5.6) |
| Immunosuppressors | 8 | (4.9) |
| Immunotherapy | 5 | (3.1) |
| Corticosteroids | 6 | (3.7) |
| COPD | 16 | (9.9) |
| Chronic respiratory failure | 8 | (4.9) |
| Cardiomyopathy | 32 | (19.8) |
| Cirrhosis | 4 | (2.6) |
| Median days symptoms/hospitalization | 5 | [3–7] |
| Fever | 143 | (88.8) |
| Cough | 87 | (54) |
| Digestif disorders | 37 | (23) |
| Acute respiratory failure | 135 | (83.3) |
| PaO2/FiO2 < 100 | 30 | (19.2) |
| PaO2/FiO2 between 100 and 200 | 69 | (44.2) |
| PaO2/FiO2 between 200 and 300 | 28 | (17.9) |
| PaO2/FiO2 > 300 | 29 | (18.6) |
| Septic shock | 37 | (22.8) |
| Confusion | 54 | (33.3) |
| SOFA | 5 | [3–8] |
| Glasgow score | 15 | [14–15] |
| Coma (Glasgow score < 8) | 4 | (2.5) |
| Cytolysis | 96 | (60.4) |
| Jaundice | 18 | (11.4) |
| Hyponatremia | 95 | (59) |
| Leukopenia | 8 | (4.9) |
| Hyperleukocytosis | 117 | (72.2) |
| Bicarbonates (mmol/l) | 22 | [19–24] |
Data are presented as No (%) or median (Q1-Q3) unless otherwise indicated
BMI Body Mass Index
COPD Chronic Obstructive Pulmonary Disease
During the inclusion period, 162 patients with community-acquired LP were enrolled from 12 French ICUs. 87 patients (54%) were directly admitted to an ICU while 75 (46%) had previously been admitted to another department with a median stay of 2 days [1–3].
The median age was 61 years, and 120 patients (74.9%) were male. 79 patients (48.8%) were smokers, 52 (32.1%) were alcohol drinkers. 32 (19.8%) had cardiomyopathy, 21 (15.9%) had diabetes, 18 (11/1%) had neoplasia and 16 (9.9%) had Chronic Obstructive Pulmonary Disease (COPD) while only 28 patients (17.3%) had immunodepression or hemopathy. The median time between symptom onset and hospital admission was 5 days [3–7].
Infection characteristics (Table 1)
Fever at admission affected 143 patients (88.8%) while 135 patients (83.3%) had suffered from acute respiratory failure, and 54 patients (33.3%) had mental confusion. 37 patients (22.8%) were in septic shock on admission. Median SOFA was 5 [3–8].
Hyponatremia was present for 95 patients (59%) and hepatic cytolysis for 96 patients (60.4%).
A large number of types of radiological focus were observed (Table 2).
Table 2.
Radiological focus (n = 162)
| Radiological focus | Study population (n = 162) | |
|---|---|---|
| Unilateral alveolar | 62 | (39.5) |
| Bilateral alveolar | 22 | (14.1) |
| Unilateral alveolar-interstitial | 12 | (7.6) |
| Bilateral alveolar-interstitial | 46 | (29.3) |
| Unilateral interstitial | 2 | (1.3) |
| Bilateral interstitial | 13 | (8.3) |
Data are presented as No (%)
Diagnosis of Legionella
Legionella diagnosis was made by Urinary Antigen Test for 144 patients (88.9%). 2 patients were diagnosed by Legionella serology, including one post-mortem (Table 3). The diagnostic samples were obtained by bronchoalveolar lavage (BAL), broncho-aspiration or sputum, and analyzed by culture in 8 patients (4.9%) and by BAL or nasal polymerase chain reaction (PCR) in 7 patients (4.9%). Median time between hospitalization and diagnosis was 1 day [0–2].
Table 3.
Diagnosis method (n = 162)
| Diagnosis method | Study population (n = 162) | |
|---|---|---|
| Legionella UAT | 144 | (88.9) |
| BA or BAL culture | 7 | (4.3) |
| Sputum culture | 1 | (0.6) |
| BAL PCR | 5 | (3.7) |
| Nasal PCR | 2 | (1.2) |
| Serology | 2 | (1.2) |
Data are presented as No (%)
UAT Urinary Antigen Test
BA broncho-aspiration
BAL bronchoalveolar lavage
PCR polymerase chain reaction
Antibiotic treatment
Median time from hospital admission to administration of antibiotic against Legionella introduction was 0 days [0–1]; 156 patients (96.3%) received at least one antibiotic against Legionella on t admission to an ICU.
One hundred twenty-eight patients (79%) were treated with combination therapy. The most commonly used combination therapy was fluoroquinolone and macrolide (118 patients), (Table S1). Median combination therapy duration was 10 days [7–15], followed by mono-therapy for 67 patients. 33 patients (20.4%) received monotherapy only, 22 a fluoroquinolone and 11 a macrolide. Median antibiotic duration was 18 days [14–21]. One patient died on the day of ICU admission, before antibiotic treatment could be administered.
Time course of disease (Table 4)
Table 4.
Outcomes, length of stay and therapeutics (n = 162)
| Variables | Median or percentage | |
|---|---|---|
| SOFA at 48 h | 5 | [3–8] |
| No Ventilation | 33 | (20.4) |
| Non-invasive ventilation | 9 | (5.6) |
| Median days | 4 | [3–7] |
| High-flow nasal oxygen therapy | 21 | (13) |
| Median days | 4 | [2–5] |
| NIV and HFOT | 4 | (2.5) |
| Median days | 1.5 | [1-2.5] |
| Invasive ventilation | 95 | (58.6) |
| Median days | 10 | [6–17] |
| Invasive ventilation at admission (n = 95) | 79 | (83.2) |
| Invasive ventilation after 24 h (n = 95) | 16 | (16.8) |
| Median days ventilation/admission | 2.5 | [2-3.5] |
| Acute respiratory distress syndrome | 73 | (45.1) |
| Paralysis for ARDS (n = 73) | 65 | (85) |
| Prone position for ARDS (n = 73) | 39 | (53.4) |
| Extracorporeal membrane oxygenation | 8 | (4.9) |
| Vasopressors | 84 | (51.9) |
| Renal replacement therapy | 27 | (16.7) |
| Median days | 6.5 | [3–12] |
| Combination therapy antibiotics | 128 | (79.1) |
| Median days | 10 | [7–15] |
| Mono-therapy antibiotic | 33 | (20.4) |
| Antibiotherapy median days | 18 | [14–21] |
| No antibiotics | 1 | (0.6) |
| No antibiotics at ICU admission | 6 | |
| Median days antibiotic/hospitalization | 0 | [0–1] |
| Median days antibiotic/ICU | 0 | [0–0] |
| No antibiotics at ICU admission | 6 | (3.7) |
| Median days diagnosis/hospitalization | 1 | [0–2] |
| Median days of ICU | 11 | [5–19] |
| Median days of hospitalization | 17 | [10–29] |
| 28-day mortality | 19 | (11.7) |
| ICU mortality | 21 | (13) |
| Hospital mortality | 22 | (13.6) |
Data are presented as No (%) or median (Q1-Q3) unless otherwise indicate
NIV a non-invasive ventilation
HFOT high-flow nasal oxygen therapy
ARDS acute respiratory distress syndrome
Extracorporeal membrane oxygenation corresponds to veno-venous extracorporeal membrane oxygenation
Invasive ventilation was used for 95 patients (58.6%), of whom 79 were intubated during the first 24 h after ICU admission. 16 patients were intubated later in their hospital course, with a median of 2.5 [2-3.5] days between ICU admission and intubation. 73 patients (76.8%) had acute respiratory distress syndrome according to the Berlin definition [21]. Median invasive ventilation duration was 10 days [6–17]. Among ARDS patients, 65 received neuromuscular blocking agents and 39 were placed in a prone position.
Only 9 patients (5.6%) received non-invasive ventilation (NIV), 21 (13%) required high-flow nasal oxygen therapy (HFOT) only and 4 (2.5%) required a combination of NIV and HFOT. Veno-venous extracorporeal membrane oxygenation was performed in 8 patients.
Median ICU stay duration was 11 days [5–19], and the median hospital stay duration after ICU admission was 17 days [10–29].
Survival analysis
At 28 days, 19 patients (11.7%) had not survived, and were analyzed in the univariate and multivariate analysis. 2 other patients died after 28 days and before ICU discharge. Univariate analyses are available in the Supplementary Appendix (Table S2).
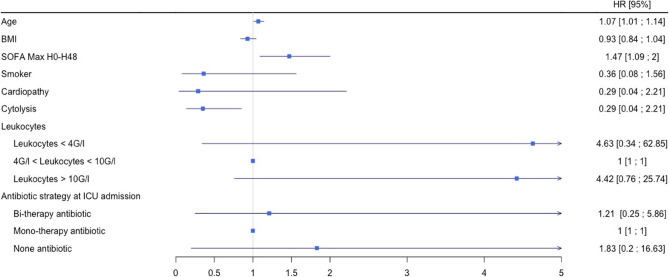
In multivariable analysis (Table S3 Fig. 1), age (HR, 1.07; 95% CI, 1.01–1.14) and a high SOFA (Sequential Organ Failure Assessment) score during the first 48 h (HR, 1.47; 95% CI, 1.09-2) were significantly associated with mortality (Table S3 and Fig. 1). No comorbidities were found to be correlated with increased mortality. Among biologic parameters, cytolysis was a protective factor (HR, 0.35; 95% CI, 0.14–0.85). Lack of antibiotics at ICUs admission was not associated with mortality.
Fig. 1.
Risk factors for mortality at D-28 after admission to ICU for LP (n = 19)
Length of stay in an ICU
Length of stay was analyzed for 141 patients who were discharged alive from an ICU. Univariate analyses are available in the Supplementary Appendix (Table S4).
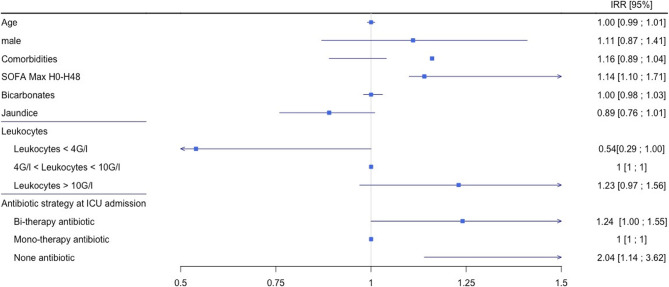
In multivariable analysis (Table S5, Fig. 2), only the number of comorbidities (Incidence Risk ratio: IRR, 1.17: 95% CI, 1.05–1.29), high SOFA score during the first 48 h (IRR, 1.14; 95% CI, 1.10–1.17) and no antibiotics on ICU admission (IRR, 2.04; 95% CI, 1.14–3.62) were significantly associated with ICU length of stay.
Fig. 2.
Multivariate analysis of length of stay in ICU (n = 141)
Ventilator-free days at 60 days
Ventilator-free days were analyzed for 95 patients having received invasive ventilation. Univariate analyses are available in the Supplementary Appendix (Table S6).
In multivariate analysis (Table S7, Fig. 3), only the high SOFA score during the first 48 h was significantly associated with ventilator-free days (IRR, 0.95; 95% CI, 0.91–0.99).
Fig. 3.
Multivariate analysis of ventilator-free days at 60 days (n = 93)
Discussion
This was a multi-center study aimed at determining mortality incidence in patients admitted to an ICU with Legionella pneumonia. It represents the second largest cohort of LP patients admitted to ICUs [9]. The mortality rate in our cohort was 11.7%, with age and SOFA score representing significant risk factors. The antibiotic therapy employed (fluoroquinolone, rifampicin or macrolide vs. any combination of two antibiotics) was not associated with mortality.
In our cohort, the epidemiological characteristics (age, sex, comorbidities) did not differ from those observed in previous studies [10–14]. Patients were mainly men in their sixties. Immunosuppression was not a constant factor. Many patients were smokers and/or alcohol users. These are the same epidemiological characteristics as those found in than other community-acquired pneumonia patients hospitalized in ICUs, except for alcohol, which was particularly prevalent in LP cases [25]. Since radiological focus was not specific, it cannot be used as a reason to suspect or rule out Legionella pneumonia. Surprisingly, more than 50% of patients showed an alveolar focus. Acute respiratory distress syndrome was frequent in our cohort, but less frequent than in the Cecchini et al. retrospective cohort [14], in which 69% of patients presented with ARDS. However, the Cecchini cohort used the previous definition of ARDS, which was modified in 2012. Heterogeneous definition may partially explain the conflicting results. Invasive ventilation was less frequent in our cohort than in other studies [11–14]; since our study is more recent, new oxygenation methods such as high-flow nasal oxygen therapy were used. A decrease in systematic intubation for hypoxemic pneumonia might also help to explain the difference.
Despite current expertise on Legionella pneumonia, according to general cohort studies the mortality rate is 4 to 9.3% [6, 26, 27] and from 28 to 41% s in ICU patients [10–12, 14]. Interestingly, in our cohort the mortality rate was low, which is probably due to two reasons: firstly, the diagnosis was made quickly because the Legionella Urinary Antigen Test (UAT) is recommended in severe community-acquired pneumonia [15, 16], and multiplex Polymerase Chain Reaction (PCR), which provides a rapid and sensitive diagnostic for pneumonia, is increasingly used in intensive care [28, 29]. Indeed, among five other cohorts tasked with analyzing LP in ICUs, only three used UAT as a diagnosis criterion. Secondly, appropriate antibiotic therapy started early due to the fact that an antibiotic acting on Legionella is recommended in cases of hospitalized community-acquired pneumonia [15–17], and some studies have suggested that delayed diagnosis is associated with mortality [12, 21, 30, 31]. In this study, we did not find a difference in mortality between combination or mono-therapy, nor did we find any difference related to delayed antibiotic therapy. A recent retrospective study demonstrated that a fluoroquinolone-based antimicrobial regimen in the early course of management was associated with lower ICU mortality [14]. Several reasons can explain the absence of similar results in our cohort. Firstly, appropriate initial therapy was implemented very quickly, as recommended by the latest guidelines on combination therapy with beta-lactam plus macrolide [17]. Secondly, most patients received a combination therapy antibiotic. Thirdly and interestingly, most patients received fluoroquinolones. Our hypothesis is that the improved and timely management of Legionella pneumonia in French ICUs can explain the absence of increased mortality in situations involving antibiotic therapy.
Length of stay and ventilator-free days were significantly associated with the high SOFA score recorded during the first 48 h. These results are consistent with severity of illness. It is noteworthy that the absence of antibiotics prior to ICU admission was associated with a prolonged length of stay. The rapidity of diagnostic tests likely accounts for the absence of observed associations with mortality. It is imperative to exercise caution when interpreting these results, given the limited number of patients included in the study who were without antibiotics against legionella pneumonia on ICU admission.
It should be acknowledged that our study is subject to a number of limitations. Firstly, it was a retrospective study, which is inherently susceptible to bias. Nevertheless, the cohort comprised a substantial number of centers and patients. Moreover, we strove to limit the number of missing data by using imputation models (Table S8). However, due to the very low mortality rate, the rarity of the event studied may have influenced the results. Unfortunately, some data concerning the management of mechanical ventilation were lacking, which may have influenced the outcomes.
A paucity of data exists regarding the antibiotic’s safety, particularly in regard to mono versus combination therapy. It is reasonable to hypothesize that combination therapy may have a greater number of undesirable effects, particularly in relation to cardiac function [32]. It would therefore be necessary to conduct a similar study with a larger prospective cohort in order to validate these data.
Conclusion
In this French multicentric cohort, the prognosis of Legionella pneumonia in ICU was more favorable than in the literature, probably because of improved and more timely management of Legionella pneumonia in the participating ICUs. Of note, the antibiotic strategy employed did not seem to modify mortality.
Supplementary Information
Acknowledgements
We thank Jefrey Arsham, an American translator for reviewing and editing this revised manuscript.
Abbreviations
- ALAT
Alanine Transaminase
- ASAT
aspartate aminotransferase
- ARDS
Acute Respiratory Distress Syndrome
- BAL
Bronchoalveolar Lavage
- COPD
Chronic Obstructive Pulmonary Disease
- CPK
phosphokinase creatinine
- HFOT
Hight-Flow nasal Oxygen Therapy
- HR
Hazard ratio
- ICU
Intensive Care Unit
- IQR
Interquartile Range
- IRR
Incidence Risk Ratio
- LP
Legionella pneumonia
- NIV
Non-Invasive Ventilation
- PCR
Polymerase Chain Reaction
- SOFA
Sequential Organ Failure Assessment
- UAT
Urinary Antigen Test
Authors' contributions
A.D carried out the study design and wrote the manuscript. N.T helped with the design. L.M.G carried out the statistical analysis of data. All authors contributed their patient data and validated the manuscript.
Funding
we did not receive funding for this article and no author has a conflict of interest for this publication.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Eligible patients were informed about the study by letter from each participating center. Their data were included in the study if they did not object. The study was approved by the Ethics Committee of the French Intensive Care Society (SRLF, Société de Réanimation de la Langue Française, CE 22–040).
Consent for publication
All authors consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stout JE, Yu VL, Legionellosis. N Engl J Med. 1997;337(10):682–7. [DOI] [PubMed] [Google Scholar]
- 2.Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, Kumar K, et al. Epidemiology and clinical management of legionnaires’ disease. Lancet Infect Dis. 2014;14(10):1011–21. [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. Legionnaires’ disease. In: ECDC. Annual Epidemiological Report for 2021. Stockholm: ECDC; 2023. Accessed: https://www.ecdc.europa.eu/sites/default/files/documents/legionnaires-disease-annual-epidemiological-report-2021.pdf.
- 4.Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions (Text with EEA relevance). Accessed: https://www.legislation.gov.uk/eudn/2018/945.
- 5.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–9. [DOI] [PubMed] [Google Scholar]
- 6.Viasus D, Di Yacovo S, Garcia-Vidal C, Verdaguer R, Manresa F, Dorca J, et al. Community-acquired Legionella pneumophila pneumonia: a single-center experience with 214 hospitalized sporadic cases over 15 years. Med (Baltim). 2013;92(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodhead M. Community-acquired pneumonia in europe: causative pathogens and resistance patterns. Eur Respir J Suppl. 2002;36:s20–7. [DOI] [PubMed] [Google Scholar]
- 8.Cillóniz C, Torres A, Niederman M, van der Eerden M, Chalmers J, Welte T, et al. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016;42(9):1374–86. [DOI] [PubMed] [Google Scholar]
- 9.Rello J, Allam C, Riuz-Spinelli A, Jarraud S. Severe legionnaires’disease. Ann Intensive Care. 2024;14(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gacouin A, Le Tulzo Y, Lavoue S, Camus C, Hoff J, Bassen R, et al. Severe pneumonia due to Legionella pneumophila: prognostic factors, impact of delayed appropriate antimicrobial therapy. Intensive Care Med. 2002;28(6):686–91. [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Gattarello S, Souto J, Sole-Violan J, Valles J, Peredo R, et al. Community-acquired Legionella pneumonia in the intensive care unit: impact on survival of combined antibiotic therapy. Med Intensiva. 2013;37(5):320–6. [DOI] [PubMed] [Google Scholar]
- 12.Falcone M, Russo A, Tiseo G, Cesaretti M, Guarracino F, Menichetti F. Predictors of intensive care unit admission in patients with Legionella pneumonia: role of the time to appropriate antibiotic therapy. Infection. 2021;49(2):321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Ebiary M, Sarmiento X, Torres A, Nogué S, Mesalles E, Bodí M, et al. Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med. 1997;156(5):1467–72. [DOI] [PubMed] [Google Scholar]
- 14.Cecchini J, Tuffet S, Sonneville R, Fartoukh M, Mayaux J, Roux D et al (2017) Antimicrobial strategy for severe community-acquired legionnaires’ disease: a multicentre retrospective observational study. J Antimicrob Chemother. 72(5):1502–9 [DOI] [PubMed] [Google Scholar]
- 15.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metlay JP, Waterer Gw L, Ac, Anzueto A, Brozec J, Crothers K, et al. Diagnosis and treatment of adults with Community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Loeches Torres A, Nagavci B, Aliberti, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023;49(6):615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afssaps. Traitement antibiotique de la légionellose chez l’adulte. Actualisation, juin 2011 [Antibiotic treatment of Legionnaires' disease in adults. Actualisation, June 2011]. French. Accessed: https://www.infectiologie.com/UserFiles/File/spilf/recos/2011-afssaps-map-legionellose.pdf.
- 19.Varner TR, Bookstaver PB, Rudisill CN, Albrecht H. Role of rifampin-based combination therapy for severe community-acquired Legionella pneumophila pneumonia. Ann Pharmacother. 2011;45(7–8):967–76. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Yanagihara K, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, et al. The clinical efficacy of fluoroquinolone and macrolide combination therapy compared with single-agent therapy against community-acquired pneumonia caused by Legionella pneumophila. J Infect. 2009;59(3):222–4. [DOI] [PubMed] [Google Scholar]
- 21.Gershengorn HB, Keene A, Dzierba AL, Wunsch H. The association of antibiotic treatment regimen and hospital mortality in patients hospitalized with Legionella pneumonia. Clin Infect Dis. 2015;60(11):e66–79. [DOI] [PubMed] [Google Scholar]
- 22.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. [DOI] [PubMed]
- 23.Coudroy R, Frat JP, Girault C, Thille W (2020) Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask. Thorax. 75(9):805–7 [DOI] [PubMed] [Google Scholar]
- 24.Bodet-Contentin L, Frasca D, Tavernier E, Feuillet F, Foucher Y, Giraudeau B. Ventilator-Free day outcomes can be misleading. Crit Care Med. 2018;46(3):425–9. [DOI] [PubMed] [Google Scholar]
- 25.Vallés J, Mesalles E, Mariscal D, del Mar Fernández M, Peña R, Jiménez JL, et al. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003;29(11):1981–8. [DOI] [PubMed] [Google Scholar]
- 26.Romay-Lema E, Corredoira-Sánchez J, Ventura-Valcárcel P, Iñiguez-Vázquez I, García Pais M-J, García-Garrote F et al (2018) Community acquired pneumonia by Legionella pneumophila: Study of 136 cases. Med Clin (Barc). 151(7):265–9 [DOI] [PubMed] [Google Scholar]
- 27.Chidiac C, Che D, Pires-Cronenberger S, Jarraud S, Campèse C, Bissery A, et al. Factors associated with hospital mortality in community-acquired legionellosis in France. Eur Respir J. 2012;39(4):963–70. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Ruan SY, Pan SC, Lee TF, Chien JY, Hsueh PR. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect. 2019;52(6):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monard C, Pehlivan J, Auger G, Alviset S, Tran Dinh A, Duquaire P, et al. Multicenter evaluation of a syndromic rapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit Care Lond Engl. 2020;24(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allgaier J, Lagu T, Haessler S, Imrey PB, Deshpande A, Guo N, et al. Risk factors, management, and outcomes of Legionella pneumonia in a large, nationally representative sample. Chest. 2021;159(5):1782–92. [DOI] [PubMed] [Google Scholar]
- 31.Heath C, Di G, Df L. Delay in appropriate therapy of Legionella pneumonia associated with increased mortality. Eur J Clin Microbiol Infect Dis. 1996;15(4):286–90. [DOI] [PubMed] [Google Scholar]
- 32.Abo-Salem E, Fowler JC, Attari M, Cox CD, Perez-Verdia A, Panakkath R, et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther. 2014;32(1):19–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.