Abstract
The response of Lactobacillus delbrueckii subsp. bulgaricus cells to heat stress was studied by use of a chemically defined medium. Two-dimensional electrophoresis (2-DE) analysis was used to correlate the kinetics of heat shock protein (HSP) induction with cell recovery from heat injury. We demonstrated that enhanced viability, observed after 10 min at 65°C, resulted from the overexpression of HSP and from mechanisms not linked to protein synthesis. In order to analyze the thermoadaptation mechanisms involved, thermoresistant variants were selected. These variants showed enhanced constitutive tolerance toward heat shock. However, contrary to the wild-type strain, these variants were poorly protected after osmotic or heat pretreatments. This result suggests that above a certain threshold, cells reach a maximum level of protection that cannot be easily exceeded. A comparison of protein patterns showed that the variants were able to induce more rapidly their adaptive mechanisms than the original strain. In particular, the variants were able to express constitutively more HSP, leading to the higher level of thermoprotection observed. This is the first report of the study by 2-DE of the heat stress response in L. delbrueckii subsp. bulgaricus.
Lactobacillus delbrueckii subsp. bulgaricus is one of the most important industrial lactic acid bacteria. This fact is mainly due to its use in yogurt and fermented milk for human consumption. However, the price of current commercial preparations restricts its use, mainly because of the high susceptibility of L. delbrueckii subsp. bulgaricus to preservation processes. For example, this species would be a very interesting probiotic (40) if the production cost of these sensitive bacteria could be decreased substantially. This goal could be achieved by increasing cell survival or by using a less deleterious production process. Direct spray drying of vegetative microbial cells is emerging (3) as an efficient and inexpensive preservation method with a high output and may lower this cost. Knowledge of the resistance of these bacteria to heat stress would be helpful for mastering cell viability during this dehydration treatment. Teixeira et al. (35) studied L. delbrueckii subsp. bulgaricus thermotolerance and clearly showed the successful application of such a preservation method to concentrated suspensions of these bacteria (34, 35). When cells were submitted to a heat pretreatment at 50°C or to a hyperosmotic pretreatment before lethal temperature challenge (65°C), the viability of the cells increased (13). Nevertheless, little is known of potential methods for enhancing cell survival and mechanisms used by the cell to protect itself from heat stress or to repair damage.
Two-dimensional electrophoresis (2-DE) of proteins is currently one of the highest-resolution analytical techniques available for the study of protein expression patterns (the promising genome-wide DNA arrays are not yet available for L. delbrueckii subsp. bulgaricus). New technological developments have improved reproducibility and enhanced quantitation of the evidenced proteins. Moreover, new computer analysis software allows easier analysis of the information. These techniques allow researchers to analyze the cellular responses of a variety of bacteria to different stresses. They have also been used to compare the responses of a particular species to different stimuli (9). These data give promising insight into the mechanisms of protection triggered by different stresses in bacteria. The first published study by 2-DE of the L. delbrueckii subsp. bulgaricus response to stress was carried out for acid stress (18). More than 700 protein spots were routinely detected with silver staining. Among these, three heat shock proteins (HSPs) (DnaK, GroES, and GroEL) were induced after 15 min at pH 4.75. Another study revealed the feasibility of 35S labeling experiments and 2-DE examination of L. delbrueckii subsp. bulgaricus proteins during lag phase in milk (26).
The results presented here highlight the crucial role of de novo protein synthesis for L. delbrueckii subsp. bulgaricus cell survival during heat stress. Nevertheless, heat adaptation also involves elements not linked to protein neosynthesis. Moreover, an easy method to obtain thermoresistant variants is presented.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
L. delbrueckii subsp. bulgaricus type strain ATCC 11842 and L. delbrueckii subsp. bulgaricus strain 546 (industrial yogurt starter; Rhodia-Food) were used. The latter strain was characterized phenotypically and by genomic fingerprinting as indicated below. Lactobacillus MRS medium (Difco Laboratories, Detroit, Mich.) was used as a rich growth medium (6), and MPL (5) was used as a defined minimal medium containing 5 g of d-glucose as a carbon source per liter. Liquid cultures were incubated at 37°C without stirring, and MRS agar plates were incubated anaerobically (AnaerocultA; E. Merck AG, Darmstadt, Germany) at 37°C. Cells from single colonies were inoculated into MRS broth medium and grown for 16 h at 37°C. They were then subcultured at a 1:50 (vol/vol) dilution in MPL medium supplemented with 5 g of d-glucose per liter and incubated for 10 h at 37°C without stirring. This MPL preculture was used to inoculate MPL working cultures. Cell growth was monitored by taking optical density measurements at 570 nm (OD570; model DU 7400 spectrophotometer; Beckman, Gagny, France).
Heat shock treatment and challenge.
Cells were harvested in the exponential or stationary phase by centrifugation (16,000 × g, 10 min) at room temperature. The pellet was resuspended in MPL medium at room temperature to reach a final OD570 of 1 unit, which corresponds to a population of 2 × 108 CFU/ml. Then, 200-μl samples were transferred to and kept in a water bath at the challenge temperature for 40 min. At various intervals, samples were immediately diluted in a sterile diluent containing 1 g of peptone and 8.5 g of NaCl per liter (pH 7.0) at room temperature and were inoculated in duplicate on MRS agar plates. The viable cell counts in these samples were determined after 24 h of anaerobic incubation at 37°C. The data are expressed as percent colony-forming ability after exposure to the challenge temperature, with the CFU at 0 min set to 100%.
Adaptation conditions (pretreatments).
Pretreatments were performed on exponential cells adjusted to an OD570 of 1 unit in MPL medium. Cells were exposed to a given stress agent under nonlethal conditions before exposure to a heat challenge. Different pretreatments were applied for 30 min. Cells were incubated at 50°C or at room temperature in MPL medium containing either 0.4 M NaCl or 0.88 M trehalose (same osmolarity). Then, samples were exposed to heat challenge at 65°C for 10 min. Viable cell numbers were determined on MRS agar plates.
For experiments testing the effect of the inhibition of protein synthesis on thermoadaptation, chloramphenicol was used at 10 μg/ml. This concentration is five times the MIC determined for this strain. Exponential cells were recovered at an OD570 of 1 unit in MPL medium containing chloramphenicol at 10 μg/ml and were incubated at room temperature for 20 min before pretreatment and heat challenge at 65°C for 40 min.
Thermoresistant variants of L. delbrueckii subsp. bulgaricus.
L. delbrueckii subsp. bulgaricus 546 cells grown in MPL medium were collected at an OD570 of 0.5 by centrifugation. The pellets were resuspended in new MPL medium to an OD570 of 2 units. Subfractions of 0.2 ml were incubated for 15 min at 65°C. Samples were immediately diluted in sterile diluent at room temperature and then poured in duplicate onto MRS agar plates. The viable cell numbers in these samples were determined after 24 h of anaerobic incubation at 37°C. Five colonies of survivors were cultivated in MRS medium and then cultivated twice in MPL medium. The last culture of each clone was submitted to a second thermal treatment corresponding to a heat shock of 20 min at 65°C. In the same way, at the end of the thermal treatment, five survivors of each clone were selected for additional thermal treatments. The next thermal treatments, at 65°C, were carried out for 25, 30, and 45 min. From each heat treatment, five survivors were selected for the next cycle. At the end of the five cycles, two variants were retained to determine their survival capacities. The viability of the variants was regularly tested throughout the study and fluctuated by less than 1 log unit. The characteristics of growth of the two variants (V5242 and V5221) and of wild-type strain 546 were examined by culturing on milk medium at 32, 37, and 43°C and measuring the impedance (30). The identities of the variants were checked phenotypically (carbohydrate fermentation patterns; API 50 CH plates; BioMerieux, Marcy l'Etoile, France) and by genomic fingerprinting.
Genomic DNA preparation, restriction digestion, and pulsed-field gel electrophoresis (PFGE).
For genomic fingerprinting, a high-molecular-weight L. delbrueckii subsp. bulgaricus genomic DNA preparation was prepared by the lysis method with an agarose block (20). Samples trapped in the agarose block were digested with 10 U of NotI enzyme (Eurogentec, Seraing, Belgium) for 4 h. Restriction fragments in the 1- to 150-kb range were resolved in a single run on a Bio-Rad (Ivry sur Seine, France) CHEF/DRII system. A constant voltage of 6 V/cm in a 1% (wt/vol) agarose gel by a single-phase procedure for 16 h with a pulse ramping of 2 s was used. Patterns were also obtained for several other L. delbrueckii subsp. bulgaricus strains, including DSM 20072 (from the collection of the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunsweig, Germany) and CIP 71-36 and CIP 53-165 (from the collection of the Pasteur Institute, Paris, France).
Radioactive labeling and whole-cell protein extraction.
Cells grown in MPL-0.5% glucose medium were harvested (8,000 × g, 5 min) at mid-log phase (OD570, 0.5) and resuspended to an OD570 of 1 unit in fresh MPL medium that contained 0.5% glucose and that was prewarmed to 37 or 65°C. One milliliter of cell suspension was labeled with 500 μCi of [35S]methionine-cysteine labeling mix (ICN Pharmaceuticals, Orsay, France) for 15 min at 37°C (control cells) or at 65°C (heat-stressed cells). The incorporation of labeled amino acids was stopped with 1 ml of stop solution (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM methionine, 1 mM cysteine, 1 mg of chloramphenicol per ml, 0.4 mM phenylmethylsulfoxyl fluoride [PMSF]). Washed cells were then resuspended in protoplasting solution (25 mM Tris-HCl [pH 7.5], 0.5 M sucrose, 5 mg of lysozyme per ml, 1 mg of chloramphenicol per ml, 0.4 mM PMSF) and incubated for 5 min at 37°C prior to centrifugation. The cell pellet was resuspended in 200 μl of lysis solution (50 mM Tris-HCl [pH 7.5], 0.3% lauryl sulfate sodium salt [SDS], 200 mM dithiothreitol [DTT], 0.4 mM PMSF) and immediately sonicated on ice by using a Vibra Cell sonicator (Bioblock Scientific, Illkirch, France) equipped with a tapered microtip (3 bursts of 1 min at 1-min intervals; power output, 2.5 arbitrary units). The lysate was brought to 95°C for 10 min to improve protein solubilization, and insoluble materials were removed by centrifugation. Four volumes of ice-cold acetone were added, and proteins were precipitated for 30 min on ice prior to centrifugation. The organic supernatant was discarded, and the protein pellet was passively air dried at room temperature for 1 h and then dissolved in isoelectric focusing (IEF) solution {7 M urea, 2 M thiourea, 25 mM DTT, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 2% immobilized pH gradient (IPG) buffer [Amersham Pharmacia Biotech (APB), Buckinghamshire, England]}.
IEF.
The first-dimensional separation was carried out on IPGs (4/7 Immobilin Dry Strips, 18 cm; APB) as described by Görg et al. (12) by using a Multiphor II electrophoresis unit (APB). The following voltage gradient was applied: from 0 to 50 V in 1 min; 50 V for 1 h; from 50 to 150 V in 0.02 h; 150 V for 1 h; from 150 to 300 V in 0.02 h; 300 V for 2 h; from 300 to 3,500 V in 5 h; and 3,500 V for 11 h. Protein samples (6 × 105 dpm) were loaded into cups at the anode end of each IEF gel. Equal amounts of radioactivity were loaded on the gels. After IEF, strips were equilibrated for 8 min in a solution containing 50 mM Tris (pH 6.2), 6 M urea, 30% (vol/vol) glycerol, 2% (wt/vol) SDS, and 0.3% (wt/vol) DTT, followed by a second bath with the same solution but with 4.5% (wt/vol) iodoacetamide instead of DTT.
SDS-polyacrylamide gel electrophoresis.
The second-dimensional separation was performed essentially as described by Laemmli (16) by using an IsoDalt apparatus (Hoeffer, San Francisco, Calif.) with a 14% acrylamide separating gel, without a stacking gel, and at a constant voltage (below 180 V). Large plate gels (200 by 250 by 1 mm) were used to improve resolution. Ten gels were run simultaneously in one tank to improve reproducibility. A low-molecular-weight electrophoresis calibration kit (APB) was used to provide protein molecular mass references (phosphorylase b, 94,000; bovine serum albumin, 67,000; ovalbumin, 43,000; carbonic anhydrase, 30,000; soybean trypsin inhibitor, 20,100; and α-lactalbumin, 14,400 Da). Gels were stained with Coomassie brilliant blue (PhastGel Blue R; APB) according to the manufacturer's indications and stored in a 20% ethanol solution at 4°C.
Analysis of protein spots on 2-DE gels.
Two-dimensional patterns of labeled polypeptides were acquired by using phosphor screens and a Storm Phosphorimager (APB). Data analysis was performed with Melanie II 2-D polyacrylamide gel electrophoresis software (release 2.2; Bio-Rad). Reference points (landmarks) were marked on images to align and match gels. After gel alignment and matching, pairs (spots present in several gels) could be highlighted. Differential analysis of control and stress gels was performed. Relative rates of synthesis (RRS) were determined by calculating the ratio of the radioactivity in a spot to the radioactivity in the entire gel. The induction factor for one spot was determined by calculating the ratio of the RRS in the gel to the RRS of the same spot in the reference gel.
N-terminal amino acid sequencing.
Cells from a 200-ml culture in MPL medium were lysed, and proteins were extracted with SDS as described above. The protein content of the extracts was determined by the method of Lowry et al. (21) with bovine serum albumin as a standard. For each micropreparative gel, 200 μg of whole-cell protein was acetone precipitated and separated by 2-DE. After Coomassie brilliant blue staining, corresponding spots from four gels were cut out and pooled. Gel pieces were rinsed extensively in 20% ethanol and crushed in 60 mM sodium acetate buffer (pH 8.5) containing 0.1% SDS and 20 mM DTT prior to passive extraction (14). After centrifugation, the extracts were transferred to a Prosorb sample preparation cartridge (Applied Biosystems, Foster City, Calif.), and protein was concentrated onto a polyvinylidene difluoride membrane according to the supplier's instructions. The membrane was then applied to an automatic Beckman/Porton LF3000 protein sequencer (Beckman Instruments, Inc., Fullerton, Calif.). Sequence homologies were searched with the FASTA program (24).
RESULTS
Study of L. delbrueckii subsp. bulgaricus survival during heat stress.
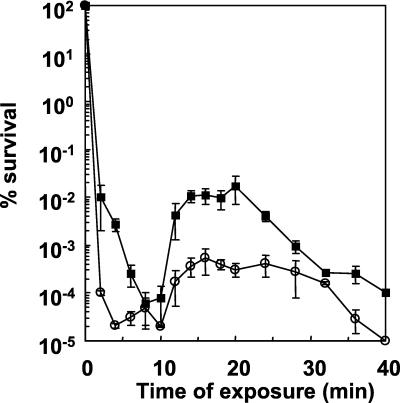
After having determined 65°C to be a lethal temperature for this bacterium, we monitored the viability of L. delbrueckii subsp. bulgaricus strain 546 at this temperature for 40 min in the absence and in the presence of a protein synthesis inhibitor. The viability kinetics showed unexpected profiles (Fig. 1). During the first 10 min at 65°C, the percentage of L. delbrueckii subsp. bulgaricus survivor numbers decreased rapidly to 8 × 10−5, but from 10 to 20 min of heat challenge, this percentage showed a sudden increase up to 1.7 × 10−2. Then, the viability decreased slowly until 50 min of heat challenge, when no more survivors were detected (data not shown). When protein synthesis was blocked by the addition of 10 μg of chloramphenicol per ml, the first decrease in surviving cells was quicker and more pronounced. After 10 min of challenge, an increase in viability was still observed, as in the presence of protein synthesis, but with a weaker amplitude.
FIG. 1.
Implication of protein synthesis in the viability of L. delbrueckii subsp. bulgaricus strain 546 during heat shock at 65°C. Cells in exponential phase were centrifuged, resuspended in MPL medium in the absence (▪) or in the presence (○) of 10 μg of chloramphenicol/ml, and incubated at room temperature for 20 min before heat shock at 65°C for the indicated times. Viability was monitored by plate counting during the challenge. Each point is the average of at least 11 independent experiments. Standard deviations are shown by bars.
Analysis of thermoresistant variants.
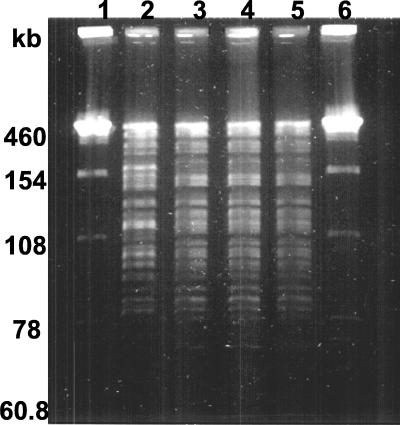
We selected thermoresistant variants as described in Materials and Methods to study the mechanisms involved in thermoadaptation. The behavior of two variants, V5221 and V5242, selected for their high viability after heat shock during selection (the percentages of survival of strains V5221 and V5242 in the last selection step at 65°C for 45 min were 4 and 2.5 log units higher, respectively, than that of strain 546), is presented below. Variants V5221 and V5242 had the same profiles of carbohydrate metabolism as L. delbrueckii subsp. bulgaricus 546 and, notably, the ability to hydrolyze the disaccharide trehalose, with is specific to strain 546. Moreover, PFGE clearly indicated the same profile for the original strain and the two variants, but this profile was different from that of strain ATTC 11842 (Fig. 2). The profiles of strains 546, V5221, and V5242 were identical.
FIG. 2.
PFGE patterns of NotI digests of genomic DNAs from L. delbrueckii subsp. bulgaricus strains. Lanes 1 and 6, molecular size standards; lane 2, strain ATCC 11842; lane 3, strain 546; lane 4, strain V5221; lane 5, strain V5242.
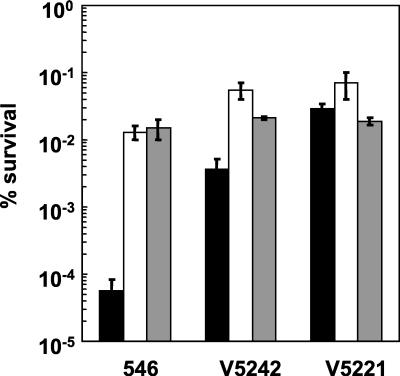
We next tested the effect on the variants of heat shock at 65°C for 10 min without and with thermal and osmotic pretreatments. Viability was compared with the results found for wild-type strain 546. Figure 3 shows that, upon heat shock, variants V5221 and V5242 showed better survival (up to 2.5 and 1.5 log units, respectively) than L. delbrueckii subsp. bulgaricus strain 546. The effect of both pretreatments on viability was greater for V5242 than for V5221, the thermal pretreatment being the most efficient; however, these pretreatments were still unable to increase cell viability by a factor of 250-fold, as for strain 546. Osmotic pretreatment was unable to increase strain V5221 viability in the presence of heat stress, in contrast to the results for strains V5242 and 546. The same osmotic effects were observed with trehalose and saccharose as with NaCl (data not shown).
FIG. 3.
Effects of heat and osmotic pretreatments on the thermotolerance of L. delbrueckii subsp. bulgaricus strains 546, V5221, and V5242. Exponential-phase cells were centrifuged, resuspended in MPL medium, and incubated for 10 min at 65°C without pretreatment (black columns), after treatment at 50°C for 30 min (white columns), or after treatment for 30 min with 0.4 mol of NaCl/liter (gray columns). Each point is the average of at least five independent experiments. Standard deviations are shown by bars.
Kinetic study of variant viability during heat shock.
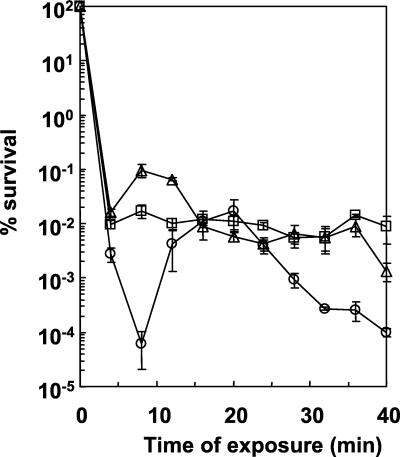
As we did for wild-type strain 546, we tested the effect on variant viability of the duration of heat shock at 65°C (Fig. 4). Both variants exhibited a survival rate profile different from that of wild-type strain 546. Indeed, although a 4-log-unit decrease occurred during the first 4 min, viability remained constant for both variants during the next 30 min; in contrast, the wild-type strain exhibited another decrease after 20 min.
FIG. 4.
Viability of L. delbrueckii subsp. bulgaricus strains 546 (○), V5221 (□), and V5242 (▵) during heat shock at 65°C. Exponential-phase cells were centrifuged, recovered in MPL medium, and incubated at 65°C. Each point is the average of at least five independent experiments. Standard deviations are shown by bars.
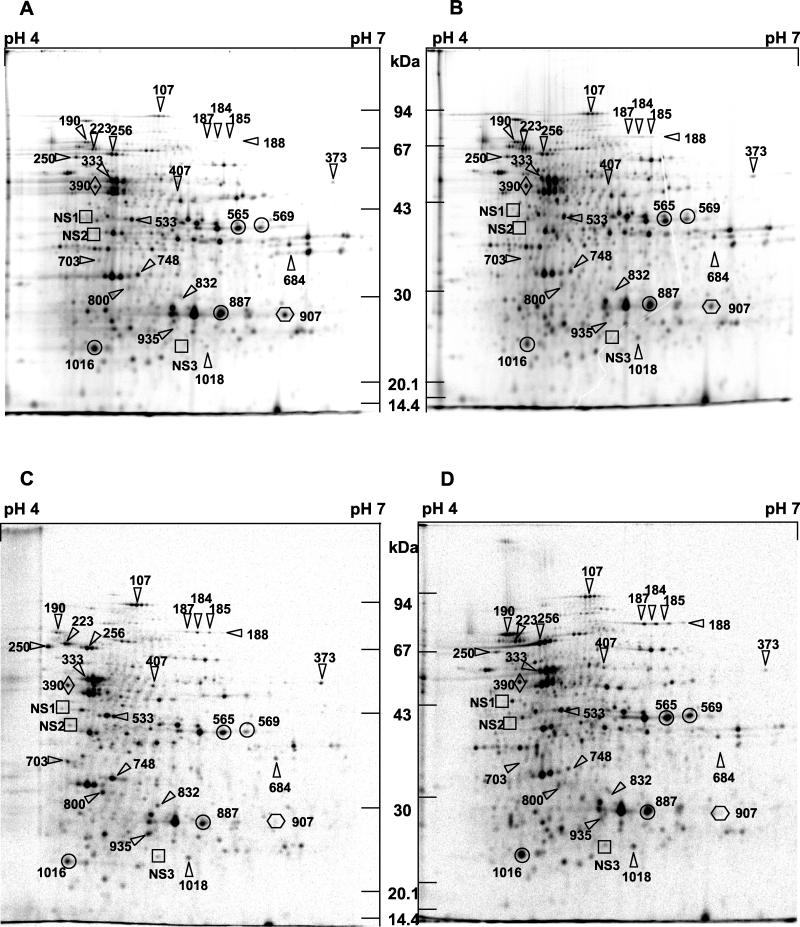
2-DE investigation of protein changes in wild-type and variant strains.
The proteins involved in the heat shock response were studied by 2-DE. Variant strain V5221 was chosen for this comparison because it was more viable under heat stress than variant strain V5242. The protein profiles in Fig. 5A show about 1,190 spots between 150 and 14 kDa, with pIs between 4 and 7. A comparison of the proteomic profiles of L. delbrueckii subsp. bulgaricus 546 and V5221 under standard conditions (Fig. 5A and B) revealed that at least one protein (NS3), which was constitutively expressed in strain V5221, was not detected in the wild-type strain. Moreover, two proteins in strain V5221 (107 and 223) showed expression levels up to 2- or 3.5-fold higher than those in strain 546. On the other hand, proteins 250 and 565 were underexpressed in strain V5221 compared with strain 546.
FIG. 5.
Protein patterns determined for L. delbrueckii subsp. bulgaricus strains 546 and V5221 by 2-DE imaging of [35S]methionine-labeled polypeptides from both strains in exponential growth phase. Cells were grown in MPL medium, centrifuged, and resuspended in MPL medium. (A and B) Control conditions for strains 546 and V5221, respectively. (C and D) Protein patterns after heat shock for strains 546 and V5221, respectively. Cells were grown and prepared in the same manner as for the controls, except that they were heated at 65°C for 15 min. Abscissa, pH range; ordinate, kilodaltons. Symbols: ▵, proteins induced in strain 546 after heat shock at 65°C for 15 min (HSPs); □, newly synthesized proteins (NS1, NS2, and NS3) in strain 546 after heat shock; ○, proteins up-regulated in strain V5221 and down-regulated in strain 546 after heat shock; , proteins down-regulated during thermal stress; ◊, protein remaining unchanged during thermal stress.
As shown in Fig. 5C and D, heat stress repressed 47% of the total proteins for strain V5221 and 60% for strain 546. Protein NS3, found in strain V5221 under control conditions, was newly synthesized in the wild-type strain in response to heat stress. In parallel, among the 200 spots selected by the Melanie software, we selected the 20 proteins up-regulated in response to thermal treatment and displaying induction factors of between 2- and 5.5-fold in strain 546. Furthermore, three proteins (NS1, NS2, and NS3) that were absent from the strain 546 reference pattern were detected after heat treatment of that strain. In comparison, some of the HSPs were poorly (or not) induced by heat stress in variant V5221 (for example, spots 250, 373, 748, and 800). Some heat-inducible polypeptides (684 and 703) were not even synthesized in the variant, either under control conditions or in response to thermal stress.
Interestingly, the levels of expression of several proteins induced in strain 546 after heat stress were comparable to the levels observed in strain V5221 under control conditions. Indeed, proteins 107, 223, and 333 showed expression levels that were up to 3.5-fold higher in strain V5221 than in strain 546 under both control and stress conditions. However, they were still inducible by heat stress. A series of HSPs were induced better in strain V5221 than in strain 546 (184, 185, 187, 190, and 256). In contrast, another series of polypeptides that were underexpressed in variant V5221 compared with strain 546 were heat inducible in the variant strain, while their synthesis was heat repressed in strain 546 (spots 565, 569, 887, and 1016 in circles in Fig. 5). These five spots were expressed at the same levels in heat-stressed 546 and in V5221 under control conditions.
Analysis of heat-induced proteins in strain 546.
Among the proteins up-regulated during thermal treatment, five were detected with Coomassie brilliant blue staining and subjected to N-terminal sequencing. Databases were screened and revealed homologies to known proteins. The results are shown on Table 1. Proteins 256 and 190 were unambiguously identified as the universal chaperones GroEL and DnaK and showed 100% identity with the corresponding 60- and 70-kDa HSPs from Bacillus halodurans (32) and Bacillus subtilis (39), respectively. The sequences matched the sequence immediately downstream of the initiation methionine. The sizes and isoelectric points were also consistent with those of these known HSPs. Protein 223 showed significant homology with Streptococcus salivarius phosphoenolpyruvate protein phosphotransferase PtsI, which is enzyme I of the phosphoenolpyruvate-dependent sugar phosphotransferase system (11). It also matched the N-terminal ends of PtsI proteins from various streptococci and from Streptomyces coelicolor. Protein 250 showed less marked homology with known proteins. The best score was obtained with B. subtilis YwsC (73% identity). This protein is described as a polyglutamate synthase involved in peptidoglycan biosynthesis. Interestingly, this sequence was identical to that of CapB, described as being involved in the biosynthesis of B. subtilis and Bacillus anthracis capsules (7). Protein 107 showed 86% identity with a Clostridium acetobutylicum protein whose function remains unknown (4); however, the matching protein displayed a different isoelectric point and a different molecular mass.
TABLE 1.
Sequences of heat-induced proteins from L. delbrueckii subsp. bulgaricus strain 546
| L. delbrueckii subsp. bulgaricus protein characteristics | Most closely matching protein |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spot | N-terminal sequence | pIa | kDaa | Protein | Microorganism | pI | kDa | % Identity | Accession no. in databasesb |
| 107 | NADYDSKEYLK | 5.3 | 91 | Unknown | Clostridium acetobutylicum | 4.4 | 18 | 86 | U38234 |
| 190 | SKVIGIDLGT | 4.7 | 72 | Dnak | Bacillus subtilis | 4.8 | 66 | 100 | P17820 |
| 223 | TETLXGIAXSDGVAV | 4.8 | 67 | PtsI | Streptococcus salivarius | 4.5 | 63 | 80 | P30299 |
| 250 | SANXKT(T)GKT(T)GEL | 4.6 | 64 | Ywsc | Bacillus subtilis | 5.3 | 44 | 73 | Q9AJM3 |
| 256 | AKDIKFSEDA | 4.9 | 66 | Groel | Bacillus halodurans | 4.8 | 57 | 100 | O50305 |
Determined from the 2-DE gels calibrated by using molecular mass and isoelectric point calibration kits (APB) and Melanie II software.
OWL database.
Kinetic analysis of protein synthesis during heat shock in strain 546.
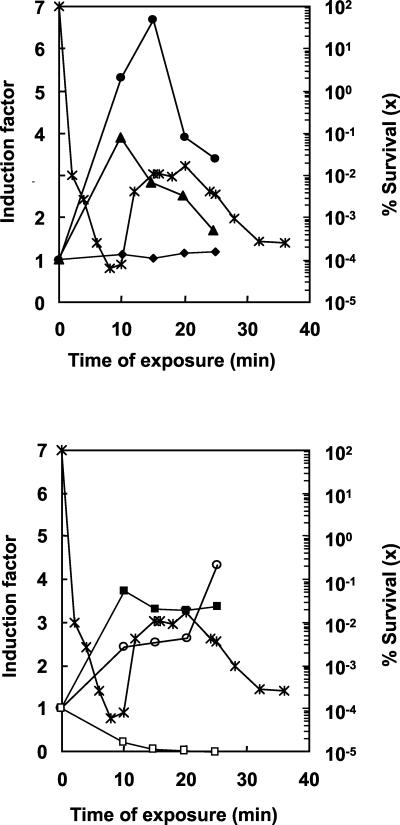
The surprising development of survival during the first half of the heat challenge led us to investigate relative rates of protein synthesis. Extracts of cells stressed at 65°C for 10, 15, 20, and 25 min in the presence of labeled methionine were separated by 2-DE, and the levels of expression of selected proteins were determined at each time. Figure 6 shows the correlation between the kinetics of viability and the induction of several proteins, taken as examples of typical behavior, during heat stress. Different types of kinetics were observed. Some HSPs showed maximum induction between 0 and 10 min (e.g., 250) and could be considered early HSPs. Some polypeptides showed maximum induction after 10, 15, 20, or 25 min of stress and then showed decreased expression (e.g., 184), while others were permanently heat induced (223). Some others showed a constant level of synthesis (e.g., 390), while others were instantaneously repressed (907). Finally, some polypeptides showed a slowly increasing rate of biosynthesis, corresponding to a late response to heat stress (e.g., 1018). However, the majority of proteins showed a maximum level of expression and a supposed maximum level of activity at between 10 and 20 min.
FIG. 6.
Variations in induction factors during heat stress. For each polypeptide, the induction factor (defined as the ratio of the relative rates of synthesis for stressed and control cells) was determined at 10, 15, 20, and 25 min of adaptation. Each point is the average of two independent experiments. Symbols: •, 184; ▴, 250; ⧫, 390; ▪, 223; ○, 1018; □, 907. Induction factors are plotted in arithmetic scale (left). The kinetics of viability established in Fig. 1 are represented in logarithmic scale (right) for comparison.
DISCUSSION
Surprising kinetics of viability of L. delbrueckii subsp. bulgaricus.
In a previous study (13), it was demonstrated that L. delbrueckii subsp. bulgaricus 546 acquired enhanced thermotolerance after moderate heat shock. This adaptation was supposed to depend on the synthesis of a widely conserved set of proteins called HSPs. During heat stress, cellular viability depends on the ability of cells to induce the necessary HSP to repair cell damage and to maintain metabolic activity. In this study, we confirmed that L. delbrueckii subsp. bulgaricus cells under heat stress induced complex adaptation mechanisms, including some fluctuations in proteins synthesis. During the first 10 min of challenge, protein synthesis was necessary to limit mortality. When the synthesis of these early HSPs was blocked, the decrease in viability was more dramatic. These results indicate that the involved proteins were quickly induced and slowed down the rate of cell death. Heat stress may have converted a large number of cells into a viable but nonculturable state, with such cells being incorrectly considered dead in our plating method.
The second aspect of the kinetics can be explained in the following manner. The cells needed at least 10 min to induce a second protective mechanism or to reach a protection level sufficient to counteract the deleterious effects of heat on the cell machinery. This mechanism induced adaptation proteins whose activities were responsible for the revival of viability observed after 10 min. Exposure to 65°C for up to 10 min induced a protective function(s) that probably allowed previously noncultivable bacteria to repair heat injury. That second event was represented by a larger number of colonies being recovered on plates and was responsible for the apparent increase in viability. These colonies appeared because of an increase in the viability of the cells due to the action of the thermoadaptation mechanism(s) against the heat stress in addition to the stress induced by the subsequent plating. This observation is in close agreement with the observed induction of the groESL operon in Lactobacillus johnsonii at 55°C, which required 30 min to attain maximum levels (38). For L. delbrueckii subsp. bulgaricus, the decrease in viability observed after 25 min corresponded to death caused by irreparable damage to the cells. Most of the published studies on microbial cell viability during heat stress have been carried out for only a few minutes. Only the initial and drastic L. delbrueckii subsp. bulgaricus cell death was reported, the elapsed time corresponding to 4 min of challenge (33). Even when the tests were longer, as described for Enterococcus faecalis and Rhodothermus obamensis (30 and 60 min, respectively), the curves always showed a constant decrease in cell viability (1, 31).
The slight revival observed in the absence of protein synthesis demonstrated that the thermoprotection was not based only on protein neosynthesis-dependent mechanisms. Preexisting proteins could be activated in response to a temperature shift, or other nonprotein molecules could be involved. Nevertheless, the hypothesis of an incomplete arrest of protein biosynthesis should be kept in mind, even with the use of chloramphenicol 20 min before heat stress at a concentration 5 times above the MIC. Moreover, as damaged proteins are believed to be one of the signals used to induce HSPs (28), incubation with chloramphenicol may disrupt internal housekeeping protease synthesis in cells. Then, the accumulation of abnormal polypeptides in the cytoplasm may lead to HSP activation (17). Nevertheless, the pleiotropic effects of chloramphenicol, comparable to those induced during the stringent response, were observed at higher concentrations of chloramphenicol (100 μg/ml) for Escherichia coli (29). Kusano et al. (15) demonstrated that in E. coli, chloramphenicol (1 to 10 μg/ml) induced the cold shock response known to decrease the expression of HSPs (36). So, on the basis of our study, we cannot rule out the possibility of the existence of a thermoadaptation mechanism not linked to the biosynthesis of proteins but rather able to activate a protective effect. Protein-independent adaptive responses may also confer cross-protection toward other stresses (8).
Behavior of thermoresistant variants.
Two thermoresistant variants were derived from wild-type strain 546 and were shown to be closely related to it by use of genomic fingerprinting. This method allows easy discrimination of L. delbrueckii subsp. bulgaricus strains from other strains in our collection, as already observed by Moschetti et al. (23), who calculated a discrimination index of 0.95 for this species. The profiles were identical; thus, no profound genetic modification that could have changed the profiles occurred.
Viability studies showed that these variants should display a more efficient protective mechanism. Thermotolerance was thus compared to that in the wild-type strain after different pretreatments. These pretreatments were selected on the basis of previous reports showing cross-protection and common responses between thermal and osmotic stimuli (10). The results showed that both variants were constitutively more tolerant to heat stress, while thermal and osmotic pretreatments were poorly protective, in contrast to the results for the wild-type strain. These results indicate first that variants with higher heat resistance can be easily selected in L. delbrueckii subsp. bulgaricus. The two strains selected in this study displayed higher levels of resistance. It is likely that many different heat resistance mechanisms are naturally present among the cell population. The selected population for each variant shared the other tested phenotypic parameters (sugar metabolism, morphology, and colony characteristics) with the wild-type strain (data not shown). The existence of such subpopulations within a strain has been frequently reported, especially among thermophilic lactobacilli. Resistance to lysozyme in Lactobacillus helveticus was found to be different between different cells of the same population. The resistance factor varied from 1 to 50. These observations were explained by the authors as the result of large variability in the phenotypic expression of resistance in each “cellular line” (37). Such stable variants, easily selectable with subcultures from the wild-type strain, were also described for exopolysaccharide production in L. delbrueckii subsp. bulgaricus (2). If the stability of resistance is high and if other interesting technological parameters are not altered, such easy and efficient treatments could be applied to obtain heat-resistant cells.
New techniques could be used to analyze such variants, such as multilocus typing, which analyzes several housekeeping genes involved in transcriptional or translational cell machinery, to highlight a high degree of genetic polymorphisms among strains of a species (22). In this study, the growth characteristics of the two variants in different types of milk and during yogurt production were indistinguishable from those of the wild-type strain (data not shown). However, data for our variants indicated that above a determined threshold, cells reached a maximum level of protection that could not be exceeded, whatever the pretreatment used. The variant selection was based on the ability to survive heat shock at 65°C over increasing periods of time. This selection favored variations in the protective mechanism(s) involved during such heat shock. If the selected variants did not show a remarkable increase in survival after pretreatments, then the cellular mechanisms triggered during the pretreatments would be identical or less efficient than those triggered during selection. The phenotypic characteristics observed in the variants were produced mainly by a constitutive state of heat shock response and to a minor extent by an improvement in the mechanisms used during pretreatment. This is the first time that such observations have been noted.
Effect of duration of heat shock on variant viability.
The decrease in viability was shown to be less drastic for the variants than for wild-type strain 546, especially during the first rapid decline. This difference in behavior between L. delbrueckii subsp. bulgaricus variants and wild-type strains showed that V5221 and V5242 were able to trigger their adaptive mechanisms more rapidly than the wild-type strain. This event may be associated with proteins showing more efficient activity or may result from a deregulated heat response.
Changes in protein expression in the wild-type strain and variant strain V5221.
Significant differences were observed between the wild-type strain and a variant strain. A subset of HSPs were constitutively overexpressed in the variant, while another subset was not synthesized to a greater degree, whatever the conditions used. In parallel, smaller protein synthesis reductions were observed in the variant strain. These observations are concordant with the higher constitutive thermotolerance of strain V5221. The expression profile for HSPs in the variant strain under standard conditions is similar to the profile for the heat-stressed cells of strain 546, suggesting that strain V5221 is constitutively heat adapted. Consistently, the smaller number of heat-repressed polypeptides in the variant would reflect better tolerance and less cell damage.
Heat-induced proteins in L. delbrueckii subsp. bulgaricus.
Thermal treatment was shown here to trigger the well-conserved heat stress response, involving GroeL and DnaK, in L. delbrueckii subsp. bulgaricus. Interestingly, these two chaperones were also induced during acid adaptation in the same bacterium (19). This finding reveals the overlap of the acid and thermal stress responses. Cell wall biosynthesis is deeply influenced by physicochemical external factors, especially in L. delbrueckii subsp. bulgaricus strains, for which many strange shapes were observed easily. The presence here of enzymes involved in peptidoglycan biosynthesis is therefore not surprising. Nevertheless, how the resulting modifications of the cell wall (thickness, reticulation, and so forth) could help the cell to survive better is still not clear. An enzyme (PtsI) of the sugar phosphotransferase transport system was also induced by heat in L. delbrueckii subsp. bulgaricus. Homologues of PtsI proteins were shown to be encoded within the RpoN regulon and may well play a role in transcriptional regulation in E. coli (25, 27). Thermal adaptation may thus involve transcription redirection by such a system in L. delbrueckii subsp. bulgaricus.
Kinetics of protein abundance during heat shock.
The kinetic analysis of protein synthesis rates during heat shock clearly showed distinct classes of polypeptides. Early, late, and constant HSPs were detected. In contrast, some cell proteins were repressed while others remained unchanged. These observations correlated with the viability kinetics observed, suggesting that the revival of viability was correlated with an increase in protein synthesis after 10 or 20 min of stress. The heat stress induced some neosynthesis and influenced the induction or activation of other HSPs.
In conclusion, modifications in the proteome after heat shock at 65°C showed that L. delbrueckii subsp. bulgaricus strain 546 activates a thermoadaptation mechanism (at least one) that involves HSPs in response to heat stress. This mechanism involves the synthesis of proteins during the first 10 min as well as later activity and neosynthesis of adaptation proteins responsible for the recovery observed after 10 min of exposure at 65°C. Nevertheless, a mechanism that does not involve protein synthesis cannot be ruled out. This pathway should require the activity of specific proteins and/or the action of other cellular components. We described an easy and rapid method for obtaining stable thermoresistant variants. The study of the kinetics of viability of L. delbrueckii subsp. bulgaricus strains 546 and V5221, the modifications of responses to heat and osmotic pretreatments for strain 546 and both variants, and the comparison of protein patterns demonstrated that a higher level of expression and constitutive expression of proteins could be responsible for the higher level of thermoprotection observed for the variant. Increasing knowledge of the heat resistance mechanisms of L. delbrueckii subsp. bulgaricus will lead to new opportunities to enhance them by appropriate technological treatments before application of cell preservation methods or by use of genetic engineering to build new food-grade starters. Studies are being performed to elucidate the mechanisms involved in thermoadaptation.
Acknowledgments
We thank Phil Neal for improving the English of the manuscript and S. Guezennec for carrying out PFGE experiments.
Part of this work was funded by Rhodia-Food and by grants from the MENRT in the program Aliment Demain (97.C.0262).
REFERENCES
- 1.Boutibonnes, P., J. C. Giard, A. Hartke, B. Thammavongs, and Y. Auffray. 1993. Characterisation of the heat shock response in Enterococcus faecalis. Antonie Leeuwenhoek 64:47-55. [DOI] [PubMed] [Google Scholar]
- 2.Bouzar, F., J. Cerning, and M. Demazeaud. 1996. Exopolysaccharide production in milk by Lactobacillus delbrueckii ssp. bulgaricus CNRZ 1187 and by two colonial variants. J. Dairy Sci. 79:205-211. [Google Scholar]
- 3.Boyaval, P., and P. Schuck. 1994. Le séchage des microorganismes par atomisation. Indust. Alim. Agric. 111:807-818. [Google Scholar]
- 4.Boynton, Z. L., G. N. Bennett, and F. B. Rudolph. 1996. Cloning, sequencing, and expression of genes encoding phosphotransacetylase and acetate kinase from Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 62:2758-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chervaux, C., S. D. Ehrlich, and E. Maguin. 2000. Physiological studies of Lactobacillus delbrueckii subsp. bulgaricus strains in a novel chemically defined medium. Appl. Environ. Microbiol. 66:5306-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Man, J. C., M. Rogosa, and E. Sharpe. 1960. A medium for the cultivation of the lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 7.Ezzell, J. W., and S. L. Welkos. 1999. The capsule of Bacillus anthracis: a review. J. Appl. Microbiol. 87:250.. [DOI] [PubMed] [Google Scholar]
- 8.Flahaut, S., A. Benachour, J. C. Giard, P. Boutibonnes, and Y. Auffray. 1996. Defence against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Arch. Microbiol. 165:317-324. [DOI] [PubMed] [Google Scholar]
- 9.Flahaut, S., A. Hartke, J. C. Giard, A. Benachour, P. Boutibonnes, and Y. Auffray. 1996. Relationship between stress response towards bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol. Lett. 138:49-54. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher, S. A., and L. N. Csonka. 1998. Characterization of the induction of increased thermotolerance by high osmolarity in Salmonella. Food Microbiol. 15:307-317. [Google Scholar]
- 11.Gagnon, G., C. Vadeboncoeur, R. C. Levesque, and M. Frenette. 1992. Cloning, sequencing and expression in Escherichia coli of the ptsI gene encoding enzyme I of the phosphoenolpyruvate:sugar phosphotransferase transport system from Streptococcus salivarius. Gene 121:71-78. [DOI] [PubMed] [Google Scholar]
- 12.Görg, A., W. Postel, J. Weser, S. Gunther, S. M. Strahler, S. M. Hanash, and L. Sommerlot. 1987. Elimination of point streaking on silver stained two-dimensional gels by addition of iodoacetamide to the eliquibration buffer. Electrophoresis 8:122-124. [Google Scholar]
- 13.Gouesbet, G., G. Jan, and P. Boyaval. 2001. Lactobacillus delbrueckii subsp. bulgaricus thermotolerance. Lait 81:301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurth, J., and W. Stoffel. 1990. A facile method for the isolation and preparation of proteins and peptides for sequence analysis in the picomolar range. Biol. Chem. Hoppe-Seyler 371:675-685. [PubMed] [Google Scholar]
- 15.Kusano, K., M. R. Waterman, M. Sakaguchi, T. Omura, and N. Kagawa. 1999. Protein synthesis inhibitors and ethanol selectively enhance heterologous expression of P450s and related proteins in Escherichia coli. Arch. Biochem. Biophys. 367:129-136. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim, E. M., T. Smokvina, S. D. Ehrlich, and E. Maguin. 1998. Réponse de Lactobacillus bulgaricus aux stress acides et sels biliaires, p. 181. In Proceedings of the 5th Symposium. French Society for Microbiology, Lille, France.
- 19.Lim, E. M., S. D. Ehrlich, and E. Maguin. 2000. Identification of stress-inducible proteins in Lactobacillus delbrueckii subsp. bulgaricus. Electrophoresis 21:2557-2561. [DOI] [PubMed] [Google Scholar]
- 20.Lortal, S., A. Rouault, S. Guezenec, and M. Gautier. 1997. Lactobacillus helveticus: strain typing and genome size estimation by pulsed field gel electrophoresis. Curr. Microbiol. 34:180-185. [DOI] [PubMed] [Google Scholar]
- 21.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 22.Mora, D., M. G. Fortina, C. Parini, D. Daffonchio, and P. L. Manachini. 2000. Genomic subpopulations within the species Pediococcus acidilactici detected by multilocus typing analysis: relationships between pediocin AcH/PA-1 producing and non-producing strains. Microbiology 146:2027-2038. [DOI] [PubMed] [Google Scholar]
- 23.Moschetti, G., G. Blaiotta, M. Aponte, G. Mauriello, F. Villani, and S. Coppola. 1997. Genotyping of Lactobacillus delbrueckii subsp. bulgaricus and determination of the number and forms of rrn operons in L. delbrueckii and its subspecies. Res. Microbiol. 148:501-510. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabus, R., J. Reizer, I. Paulsen, and M. H. J. Saier. 1999. Enzyme I(Ntr) from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J. Biol. Chem. 274:26185-26191. [DOI] [PubMed] [Google Scholar]
- 26.Rechinger, K. B., H. Siegumfeldt, I. Svendsen, and M. Jakobsen. 2000. “Early” protein synthesis of Lactobacillus delbrueckii ssp. bulgaricus in milk revealed by [35S]methionine labeling and two-dimensional gel electrophoresis. Electrophoresis 21:2660-2669. [DOI] [PubMed] [Google Scholar]
- 27.Reizer, J., A. Reizer, M. J. Merrick, G. Plunkett, D. J. Rose, and M. H. J. Saier. 1996. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene 181:103-108. [DOI] [PubMed] [Google Scholar]
- 28.Ruis, H., and C. Schuller. 1995. Stress signaling in yeast. Bioessays 17:959-965. [DOI] [PubMed] [Google Scholar]
- 29.Shen, V., and H. Bremer. 1977. Chloramphenicol-induced changes in the synthesis of ribosomal, transfer, and messenger ribonucleic acids in Escherichia coli B/r. J. Bacteriol. 130:1098-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silley, P., and S. Forsythe. 1996. Impedance microbiology--a rapid change for microbiologists. J. Appl. Bacteriol. 80:233-243. [DOI] [PubMed] [Google Scholar]
- 31.Takai, K., T. Nunoura, Y. Sako, and A. Uchida. 1998. Acquired thermotolerance and temperature-induced protein accumulation in the extremely thermophilic bacterium Rhodothermus obamensis. J. Bacteriol. 180:2770-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira, P., H. Castro, and R. Kirby. 1994. Inducible thermotolerance in Lactobacillus bulgaricus. Lett. Appl. Microbiol. 18:218-221. [Google Scholar]
- 34.Teixeira, P., H. Castro, and R. Kirby. 1995. Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J. Dairy Sci. 78:456-462. [Google Scholar]
- 35.Teixeira, P., H. Castro, F. X. Malcata, and R. Kirby. 1995. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray drying. J. Dairy Sci. 78:1025-1031. [Google Scholar]
- 36.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veaux, M., E. Neviani, G. Giraffa, and J. Hermier. 1991. Evidence of variability in the phenotypic expression of lysozyme resistance in Lactobacillus helveticus. Lait 7:75-85. [Google Scholar]
- 38.Walker, D. C., H. S. Girgis, and T. R. Klaenhammer. 1999. The groELS chaperone operon of Lactobacillus johnsonii. Appl. Environ. Microbiol. 65:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wetzstein, M., J. Dedio, and W. Schumann. 1990. Complete nucleotide sequence of the Bacillus subtilis dnaK gene. Nucleic Acids Res. 18:2172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollowski, I., G. Rechkemmer, and B. L. Pool-Zobel. 2001. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 73:451-455. [DOI] [PubMed] [Google Scholar]