Abstract
Neurosteroids synthesized within the central nervous system play essential roles in modulating neurotransmission, providing neuroprotection, regulating immune responses, influencing behavior and cognition, and mediating stress physiology. Despite their broad significance, the specific brain cell types capable of de novo steroid synthesis from cholesterol remain poorly defined. In this study, we analyzed single-cell transcriptomic data to map steroidogenic gene expression across cell populations in the murine brain, focusing on the de novo production of the neurosteroid pregnenolone. Our findings reveal that de novo steroidogenesis, as marked by Cyp11a1 expression, is predominantly confined to specific neuronal subtypes, particularly glutamatergic neurons of the intra- and extra-telencephalic regions and the corticothalamic layer. In contrast, Star expression, which is essential for mitochondrial cholesterol import, was more broadly distributed, occurring in both neuronal and non-neuronal cells—including oligodendrocytes, astrocytes, immune cells, and vascular cells. In these non-neuronal populations, Star was notably co-expressed with mitochondrial Cyp27a1, indicative of bile acid synthesis rather than neurosteroidogenesis. This distinction highlights that Star expression alone is not a reliable marker of de novo neurosteroidogenic capacity in the brain, as its functional significance depends on the broader enzymatic context in which it occurs. The resulting single-cell map of de novo neurosteroid biosynthetic capacity across brain regions, including modest sex-associated differences, provides a foundational framework for understanding neurosteroid signaling in distinct cell types and its relevance to brain physiology and pathophysiology.
Keywords: neuron, astrocyte, oligodendrocyte, microglia, pregnenolone, bile
Introduction
Neurosteroids, distinctively named for their synthesis within the nervous system (Baulieu 1981), have been associated with a wide array of physiologic and pathophysiologic processes (Paul & Purdy 1992; Mellon & Griffin 2002; Lloyd-Evans & Waller-Evans 2020). In contrast to endocrine effects of peripheral steroid hormones (McEwen 1991), neurosteroid effects are deemed to mediate local autocrine and paracrine processes that regulate brain function (Robel & Baulieu 1994). Unlike cells of the adrenal gland and gonads, steroidogenesis in the brain is not linked to dedicated steroidogenic cells, highlighting the specialized functional evolution of cell type- and steroid-specific in vivo mechanisms of action and physiologic function. Nevertheless, steroidogenic capability within the brain with precision of steroids being synthesized and involvement of specific cell types in the process remain largely uncharacterized. Additionally, the distinct biosynthetic pathways of neurosteroids have often been generalized in the literature, obscuring important nuances in their understanding and function.
Since the inception of the term “neurosteroids,” steroidogenesis in brain cells has been accurately attributed to either de novo production from cholesterol or intermediary synthesis from local or blood-borne precursors (Baulieu 1981). The first step in de novo steroid synthesis is the conversion of cholesterol to pregnenolone by CYP11A1, an enzyme locate on the matrix-facing side of the inner mitochondrial membrane as part of a ternary complex with adrenodoxin reductase and adrenodoxin (Hanukoglu & Jefcoate 1980). The potential for de novo neurosteroidogenesis was first observed in isolated oligodendrocyte mitochondria (Hu et al. 1987), and immunohistochemical detection of CYP11A1 provided early insight into brain regions capable of this process (Le Goascogne et al. 1987). However, cholesterol delivery to CYP11A1 has been identified as a rate-limiting step, requiring the action of the steroidogenic acute regulatory protein (STAR) (Clark et al. 1994). In situ hybridization studies have identified STAR expression in discrete brain regions, as supporting its potential role in de novo neurosteroidogenesis (Furukawa et al. 1998; King et al. 2002). Nevertheless, it is important to recognize that mitochondrial cholesterol import is also essential for the acidic pathway of bile acid synthesis via CYP27A1 (Oftebro et al. 1981), complicating the interpretation of STAR as a specific marker of neurosteroid biosynthesis.
This area has also been complicated by persistent misinformation arising from inaccurate representations of the mitochondrial translocator protein (TSPO) function [reviewed in (Selvaraj & Tu 2016)]. It is important to emphasize that TSPO is not involved in cholesterol import for steroidogenesis, as demonstrated by multiple independent studies (Banati et al. 2014; Morohaku et al. 2014; Tu et al. 2014; Wang et al. 2016). Pharmacological off-target effects (Tu et al. 2015) and fundamental mischaracterizations of TSPO’s role (Fan et al. 2015, 2018) have contributed to conclusions that are now recognized as artifactual and biologically unsupported, as detailed in recent evaluations (Selvaraj et al. 2016, 2020; Selvaraj & Stocco 2018).
In this study, we set out to pinpoint de novo neurosteroidogenesis to better understand its physiological signaling in distinct cell types of the murine brain. By analyzing a high-resolution single-cell transcriptomics dataset (Yao et al. 2023), we identify de novo neurosteroid biosynthetic gene expression across classified cell populations from various murine brain regions, and define their specific capability of de novo neurosteroid biosynthesis.
Materials and Methods
Dataset
Used in this study is the single cell dataset (Yao et al. 2023) generated by the BRAIN Initiative Cell Census Network, available through the Neuroscience Multiomic Data Archive (NeMO; https://data.nemoarchive.org/other/grant/aibs_internal/zeng/transcriptome/scell/). It includes single-cell RNA sequencing (scRNA-seq) and single-nucleus RNA sequencing (snRNA-seq) data obtained from 95 (v2) and 222 (v3) mice using next GEM technology (10xGenomics) for 4,041,289 brain cells. The samples were from various brain regions that were dissected and dissociated to single cells for sequencing. To ensure comprehensive neuronal profiling, fluorescence-activated cell sorting (FACS) sorting or isolating single nuclei were also incorporated to enrich for neuronal cell populations to address challenges in isolating neuronal cell types. Sequenced data were processed using the CellRanger v8.0 pipeline with the mouse reference transcriptome GRCm38 and vM23. The protocol used by the authors (Yao et al. 2023), included stringent quality control parameters such as the detection levels of 62 housekeeping genes, a quality control score assessing the integrity of cytoplasmic RNA, and the removal of cells with a doublet score greater than 0.3 that filtered out of 43% of cells from the 10xv3 dataset and 29% from the 10xv2 dataset, ensuring that only high-quality cells were retained for subsequent combination and analyses. Clusters (32 cell types) identified based on the number and significance of conserved differentially expressed genes was available in the metadata files for both 10xv2 and 10xv3 datasets and was incorporated in our analysis.
Datamining
Hierarchical data formats (HDF) that stored both expression values (Log2 CPM), cell, region, and quality control as multidimentional arrays were analyzed using a Linux platform. Unzipped HDF files from the 10 regions that were sequenced in 10Xv2 and 13 regions in 10Xv3 were read using an R toolkit for single cell genomics Seurat v5 (Hao et al. 2021, 2024) in the R statistical computing platform (https://www.r-project.org). The Seurat objects created contained the count matrix which represents the expression of all the genes across all the cell types, which was subsequently extracted and converted to data matrices. The Ensembl ID for the genes (Table S1) were separated in data files to extract the expression levels across all the different regions. Parameters of the sequenced cells including the brain region it was obtained from, cell-type characterization, sex and age of the animal it was derived from, the X and Y coordinates for clustering cell-types were integrated from the metadata. The different regions were subsequently concatenated into a master file that contained the expression and metadata information for all the sequenced cells. Data extraction, sorting and pivoting data were performed by using tidyr 1.3.1 (Wickham, H et al. 2024a) and dplyr 1.1.4 (Wickham, H et al. 2024b) packages in the R platform.
Cell type and regional nomenclature
The different regions in this dataset are identified as: cerebellum (CB), cortex caudal ganglionic eminence (CTX-CGE), cortex medial ganglionic eminence (CTX-MGE), cerebral nuclei-lateral ganglionic eminence (CNU-LGE), cerebral nuclei-anterior hypothalamic (CNY-HYa), dentate gyrus immature (DG-IMN), hypothalamus (HY), hypothalamus gonadotropin releasing hormone expressing (HY Gnrh1), hypothalamus medial mammillary nucleus (HY-MM), intratelencephalic-extratelencephalic (IT-ET), lateral septal complex (LSX), medial habenula-lateral habenula (MH-LH), midbrain (MB), medulla (MY), midbrain-hindbrain (MB-HB), near-projecting-cortico-thalamic layer (NP-CT), olfactory bulb-immature (OB-IMN), olfactory bulb-Cajal-Retzius (OB-CR), pineal (Pineal), pons (P), and thalamus (TH). The different cell types in this dataset are identified as: GABAergic neurons (GABA), glutamatergic neurons (Glut), dopaminergic neurons (Dopa), serotonergic neurons (Sero), astrocytes-ependymal cells (Astro-Epen), olfactory ensheathing cells (OEC), immune cells (Immune), oligodendrocyte lineage (OPC-Oligo), and vascular cells (Vascular).
Data filtering and calculation
Expression data for neuronal cell types: GABAergic neurons (CB, CGE, CNU, CNU-HYa, HY, LSX, MB, MGE, MY, P), glutamatergic neurons (CB, CNU-HYa, HY Gnrh1, HY, HY MM, IT-ET, MB, MH, MOB, MY, NP-CT-L6b, P, Pineal, TH, MOB-DG), dopaminergic neurons (MB), and serotonergic neurons (MB-HB) and non-neuronal cell types: astrocytes, olfactory ensheathing cells, immune cells, oligodendrocytes, and vascular cells) across different regions were classified. An exclusion cut-off of Log2CPM<1 was applied to all datapoints analyzed. For overall expression in each cell type, the ratio of cell numbers that show expression to total number of cells sequenced was obtained. For quantifying co-expression of both Star and Cyp11a1 or Star and Cyp27a1 in cells, double positive cells irrespective of individual expression ranges were filtered, and the ratio of double positive cells to total number of cells sequenced for each cell type were calculated. Sex-specific differences in gene expression were analyzed by extracting donor sex and gene expression data from metadata for 2,381,293 male and 1,782,372 female cells. For each gene, the percentage of cells expressing was calculated for each cell type, and male-to-female ratios were computed.
Data visualization
The t-distributed stochastic neighbor embedding (t-SNE) plots were constructed to visualize the distribution of cells with specific gene expressions. For this plot, we used the X and Y coordinates generated for the combined 10xv2 and 10xv3 datasets through nonlinear dimensionality reduction available from metadata files (Yao et al. 2023). Initially, a scatter plot was created using the X and Y coordinates of all sequenced cells to distinguish cell type and region information consistent with and reproducing the original data package (Yao et al. 2023). Subsequently, cells expressing individual genes of interest, colored based on expression levels, were layered on the map of all sequenced cells. This permitted visualization of the spatial distribution of cells expressing the specific genes compared to the total population of sequenced cells. For graphing the overall expression of specific genes across different cell types and sex-specific differences, the ratios computed for each cell type were visualized as percentages using the ggplot2 package in R (Wickham 2016).
Results
Cell map classification of the murine brain
Steroidogenic gene analysis was conducted using an available high-resolution transcriptomics and spatial cell type atlas of the murine brain (Yao et al. 2023). The single cell map of different cell types and regions in the murine brain was reconstructed (Figure 1A) to facilitate the exploration and interpretation of de novo steroidogenic gene expression. This comprehensive map categorizes brain cells into 34 distinct functional classes, including neuronal subtypes—GABAergic, glutamatergic, dopaminergic, and serotonergic neurons—and non-neuronal subtypes—astrocytes, ependymal cells, oligodendrocytes, immune cells (includes microglia), olfactory ensheathing cells, and vascular cells (Figure 1A–B). Each cell type is linked to specific brain regions, identified by prefixes: CB (cerebellum), CNU (cerebral nuclei), CT (corticothalamic layer), CTX (cortex), ET (extratelencephalic), HB (hindbrain), HY (hypothalamus), IT (intratelencephalic), LH (lateral habenula), LSX (lateral septal complex), MB (midbrain), MH (medial habenula), MY (medulla), OB (olfactory bulb), P (pons) and TH (thalamus). This classification provided a framework to analyze the regional and cell type-specific capacity for de novo steroidogenesis based on gene expression data.
Figure 1. Single cell map classification of the murine brain.

(A) A t-distributed stochastic neighbor embedding (t-SNE) plot visualizing single cell distribution patterns from the murine brain color coded based on marker-defined regional cell types. Plot is a reconstruction from the cell atlas of the whole mouse brain (Yao et al. 2023). (B) De novo steroidogenesis involves the synthesis of pregnenolone from precursor cholesterol. The process involves two key steps that are movement of cholesterol across the mitochondrial intermembrane space (IMS) mediated by the steroidogenic acute regulatory protein (STAR), and activity of the cytochrome P450 monooxygenase (CYP11A1) that catalyzes the side-chain hydroxylation and cleavage of cholesterol to pregnenolone.
Neurons contribute to de novo neurosteroid biosynthesis
Expression of Cyp11a1 indicates the capability of cells to convert cholesterol to the first steroid pregnenolone (Figure 1B). In the murine brain, distribution of Cyp11a1 was primarily skewed towards neuronal subpopulations (Glutamatergic and GABAergic neurons) (Figure 2A). The group of IT-ET Glutamatergic neurons showed the highest levels of Cyp11a1 expression, followed by NP-CT-L6b Glutamatergic neurons. However, the expression of Star, essential for mitochondrial cholesterol import, considered rate-limiting for providing substrate for CYP11A1 activity was observed in both neuronal and non-neuronal cell populations (Figure 2B). In non-neuronal cells higher levels of Star were detected in oligodendrocytes (OPC-Oligo) and astrocytes (Astro-Epen) that show little Cyp11a1 expression. We examined for specific co-expression of Star and Cyp11a1 for precisely identifying cells actively involved in de novo neurosteroid biosynthesis. Our analysis revealed that only 10.3% of Cyp11a1 expressing cells in neurons also expressed Star, with highest levels of co-expression observed in Glutamatergic neuron populations of IT-ET (61.1%) and NP-CT-L6b (21.8%) representing the predominant groups (Figure 2C). Although Cyp11a1-Star co-expression was detected in a small percentage of non-neuronal cells (astrocytes, oligodendrocytes, immune and vascular cells; 0.04%), the vast majority of Star expressing non-neuronal cells were without Cyp11a1, suggesting a functional feature distinct from de novo neurosteroid biosynthesis.
Figure 2. Neurons are the major contributors to de novo neurosteroid biosynthesis.
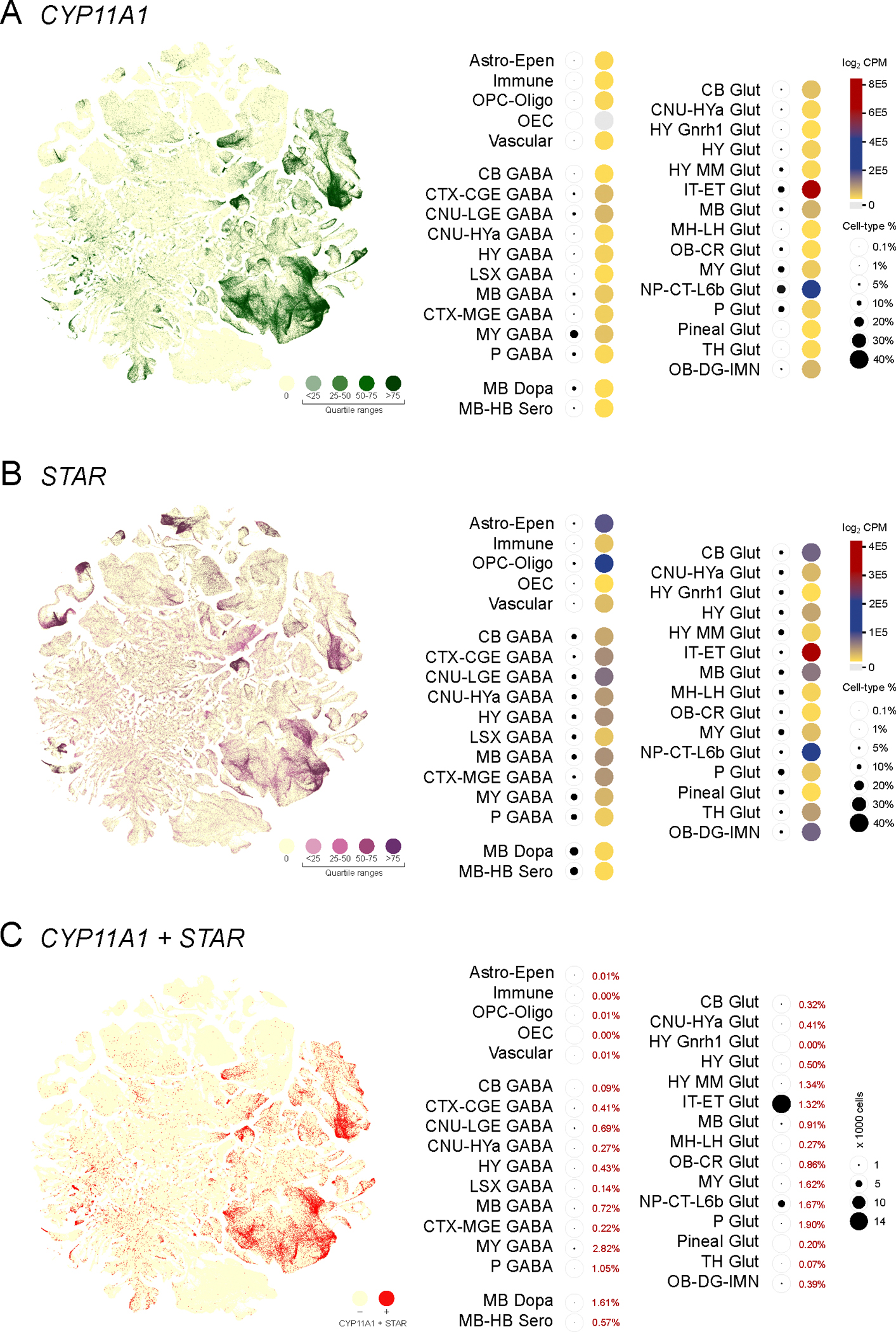
(A) Expression of Cyp11a1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges). Valuation of regional and cell type specific Cyp11a1 expression (heatmap with total expression log2CPM) and percentage of cell type-specific expression as indicators of pregnenolone production indicates that majority of de novo neurosteroidogenesis occurs in different types of glutamatergic and GABAergic neurons. The glutamatergic IT-ET neurons showed the highest levels of Cyp11a1 expression, followed by NP-CT-L6b neurons. (B) Expression of Star from single cells visualized in a cell type defined t-SNE plot (color range indicates quartiles) and heatmap. Valuation of regional and cell type specific Star as an essential step in steroidogenesis indicates a pattern inconsistent with Cyp11a1, encompassing both neuronal and non-neuronal cells. (C) A t-SNE plot visualizing co-expression of Cyp11a1 and Star (Red), highlights the frequency of co-expression correlated to the different neuronal cell populations.
Non-neuronal cells are active in the acidic pathway of bile acid synthesis
Expression of Cyp27a1 indicates the capability of cells to convert cholesterol to 27-hydroxycholesterol and 3β-hydroxy 5-cholestenoic acid, the first two sequential products in the acidic pathway of bile acid synthesis that also occurs within mitochondria (Figure 3A). In the murine brain, distribution of Cyp27a1 was primarily skewed towards non-neuronal cells, with abundant expression observed in oligodendrocytes (OPC-Oligo), immune cells (Immune) and astrocytes (Astro-Epen) (Figure 3B). Cell populations expressing Cyp27a1 and the levels of Cyp27a1 expressed were both higher in the non-neuronal cells. As activity of CYP27A1 also requires mitochondrial cholesterol import by STAR, we examined the population of cells that co-expressed Cyp27a1 and Star. Our findings indicated that co-expression of Cyp27a1 and Star was seen in at a higher rate in non-neuronal cells (5.6%) compared to neuronal cells (0.8%), specifically in oligodendrocytes (OPC-oligo; 88.3%) (Figure 3C). The next step of intermediary synthesis that results in the formation of 3β, 7⍺- dihydroxy 5-cholestenoic acid mediated by Cyp7b1 could be observed in both non-neuronal and neuronal cells (Figure S1A), with access to either local or circulating 3β-hydroxy 5-cholestenoic acid. Intermediary steroid synthesis that is also mediated by CYP7B1 is presented in ‘Single cell resolution of neurosteroidogenesis in the murine brain: intermediary biosynthesis’ (Koganti & Selvaraj 2025). We also identified that the classical or basic pathway for bile acid synthesis based on microsomal Cyp7a1 was not represented in brain cells (Figure S1B).
Figure 3. Non-neuronal glial cells appear active in bile acid synthesis.
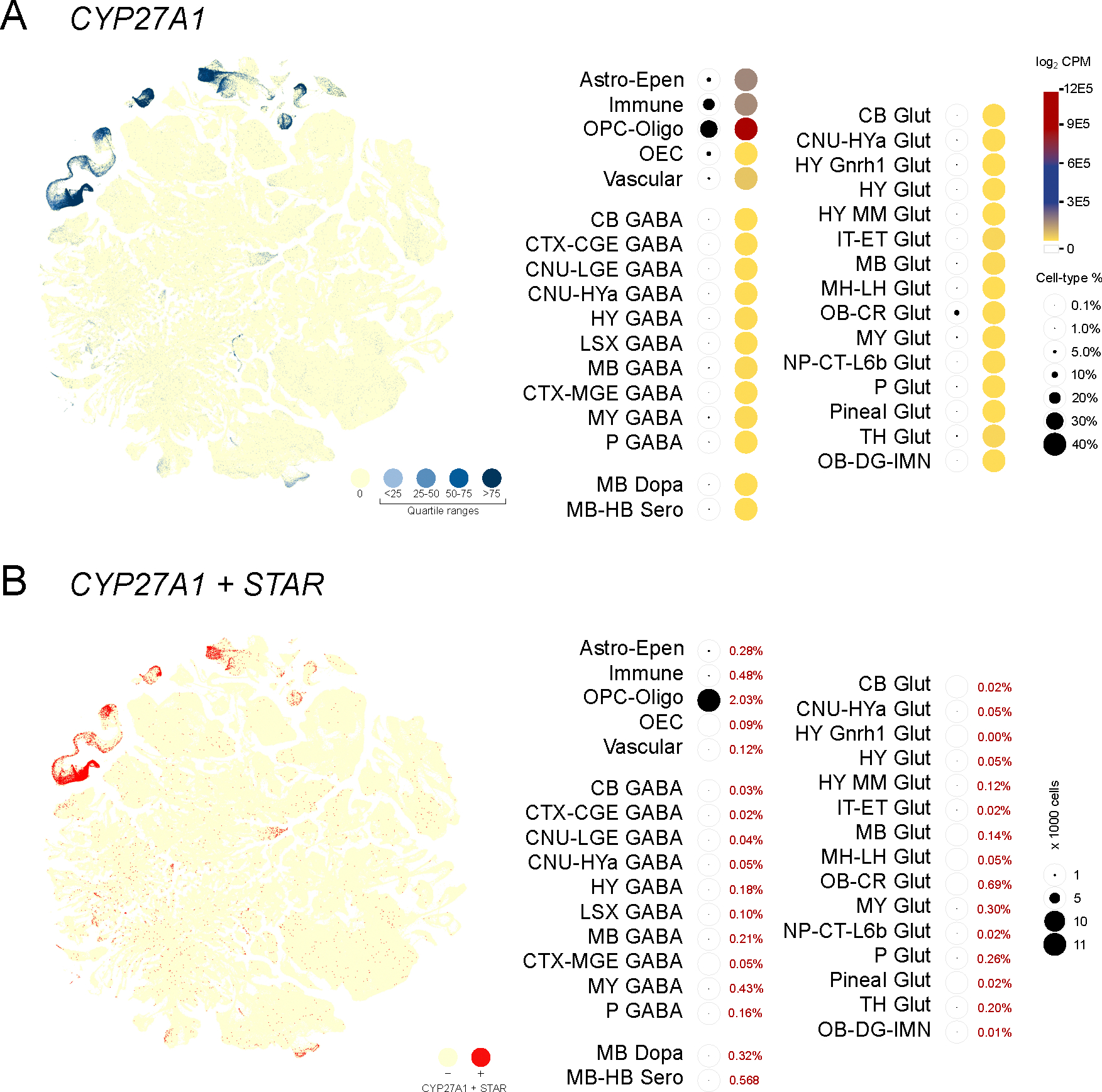
(A) Expression of Cyp27a1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges). Valuation of Cyp27a1 expression (heatmap with total expression log2CPM) and percentage of cell type-specific expression as indicators of the acidic pathway of bile acid production shows that majority of bile acid synthesis occurs in different non-neuronal glial cells: OPC-oligo>Astro-Epen>Immune>Vascular cells. (B) A t-SNE plot visualizing co-expression of Cyp27a1 and STAR (Red), indicates substantial co-expression correlated to glial cell populations.
Analysis of subgroups within non-neuronal cells
Among non-neuronal cells, Cyp11a1 was expressed only in 0.06% of cells, whereas Cyp27a1 was expressed in 5.16% of cells. Evaluating co-expression of Cyp11a1 and Star within the different non-neuronal cell lineages identified only 0.04% of cells, whereas Cyp27a1 and Star recorded a significantly higher rate of 87.47% of cells (Figure 4). In comparing Cyp11a1 and Cyp27a1 together with markers of OPCs (Pdgfra positive), and mature oligodendrocytes (Mog positive) showed that both OPCs and mature oligodendrocytes expressed Cyp27a1 and little Cyp11a1. Similarly, astrocytes populations (Gfap positive), immune cell populations that includes C1qa positive microglia and Pdgfrb positive pericytes all showed higher degrees of co-expression with Cyp27a1, but not with Cyp11a1 (Figure 4). These findings clearly indicated a substantially higher degree of bile acid biosynthetic capacity compared to an almost absent de novo steroidogenic capacity in these cells.
Figure 4. Non-neuronal cells are scarce contributors to de novo neurosteroid biosynthesis.
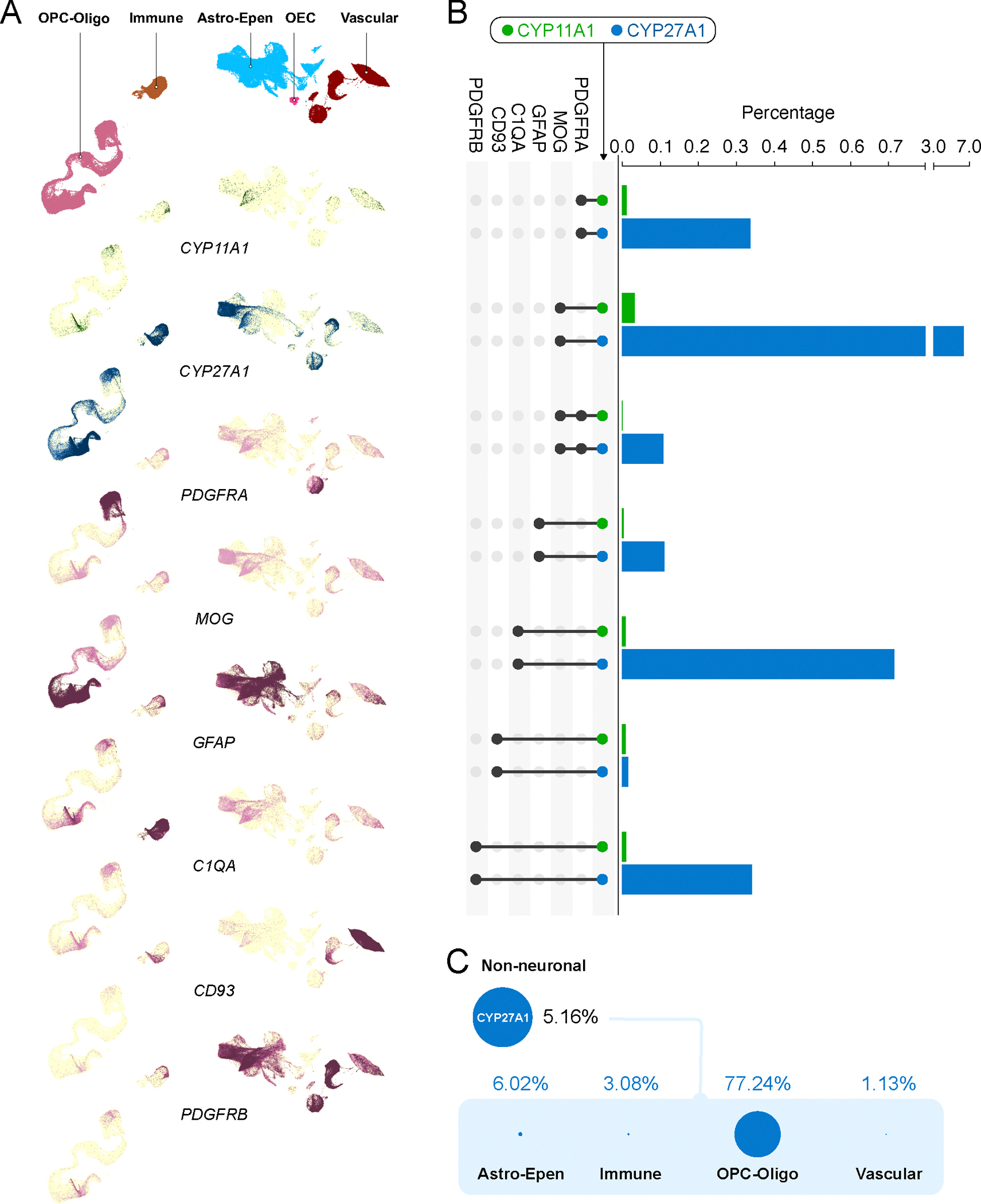
(A). Cropped t-SNE label plots showing OPC-Oligo, Immune, Astro-Epen and Vascular and t-SNE expression plots for Cyp11a1, Cyp27a1, Pdgfra, Mog, Gfap, C1qa, Cd93 and Pdgfrb (color range indicates quartiles). (B) Co-expression of Cyp11a1 and Cyp27a1 with different non-neuronal cells (based on markers) are presented as a percentage of the total population of non-neuronal cells. Evaluating Cyp11a1 and Cyp27a1 expression together with co-expression of different non-neuronal cell lineages indicated a higher degree of bile acid biosynthetic capacity and diminutive de novo neurosteroidogenic capacity in these cells. (C) Distribution of Cyp27a1 expressing non-neuronal cells were primarily associated with OPC-Oligo with minor populations of Astro-Epen, Immune and Vascular cells.
Subtle sex-bias in expression of STAR and CYP11A1
Comparing t-SNE plots and analysis of the male-to-female ratios of the percentage of cells expressing Cyp11a1 and Star permitted the identification of potential sex-specific biases. Male and female t-SNE plots for Cyp11a1 and Star did not reveal any distinctions in expression distribution. In the analysis of ratios among Cyp11a1 and Star expressing cell types and brain regions, the percentage of cells expressing Cyp11a1 and Star were found to deviate in male vs female for some cell types and brain regions (Figure S2). Notably, males had a higher percentage expression of Cyp11a1 in CB GABA, and to a lesser extent MB Dopa and P Glut, whereas females had a higher percentage expression in HY GABA and LSX GABA, and to a lesser extent HY Glut, HY MM Glut and MH-LH Glut. In non-neuronal cells males had a higher percentage of expression of Cyp11a1 in Immune and OPC-Oligo. As the percentages/ratios negate robustness of population size across the different cell types, the scale of the differences observed cannot be directly extrapolated as numerical or quantitative impact. Nevertheless, these data provided some evidence of potential sex-specific differences in de novo neurosteroidogenesis in some specific brain regions.
Discussion
A fundamental criterion in defining neurosteroidogenesis is the expression of steroidogenic enzymes within the central nervous system. Among these, STAR (Clark et al. 1994) and CYP11A1 (Hanukoglu & Jefcoate 1980) are critical components required for the de novo synthesis of pregnenolone—the first steroid produced in the steroidogenic pathway (Stocco & Clark 1996). In this study, we focus on identifying the specific cell types and associated brain regions that possess the capacity to initiate this de novo neurosteroid biosynthesis. The subsequent enzymatic steps that convert pregnenolone into downstream steroid products are classified as intermediary biosynthesis. These processes involve the utilization of pregnenolone and other metabolites by cells expressing the necessary downstream enzymes. Detailed analyses of intermediary neurosteroid synthesis across various brain regions and cell types are presented in a companion manuscript (Koganti & Selvaraj Under review).
Previous studies have regarded STAR expression in brain cells as a definitive marker of de novo neurosteroid biosynthesis (King et al. 2002). However, this interpretation warrants refinement, as STAR-mediated mitochondrial cholesterol import is also a critical step in the acidic pathway of bile acid synthesis, catalyzed by CYP27A1 (Oftebro et al. 1981). Consequently, STAR expression in the brain is not specific to neurosteroidogenesis and may instead reflect its role in an alternative cholesterol metabolic pathway. Our findings support this revised view: Star exhibits distinct co-expression patterns with Cyp11a1 and Cyp27a1 across different cell types, indicating that it contributes to both neurosteroid and bile acid synthesis in a cell type-specific manner. Notably, the predominance of Star expression in glial and vascular cells, where it co-localizes with Cyp27a1 but not Cyp11a1, suggests that its primary function in these populations is linked to bile acid synthesis. This reinterpretation is significant, as bile acids, beyond their classical role in gut lipid absorption, are increasingly recognized for their regulatory effects on cellular lipid and glucose metabolism (Zhang et al. 2006; Chiang & Ferrell 2019), pathways closely tied to neurodegenerative disease risk (Farooqui et al. 2012; Procaccini et al. 2016). The relevance of STAR in this non-steroidogenic context is further underscored by our observation that the canonical, non-mitochondrial STAR-independent bile acid synthesis pathway mediated by Cyp7a1 is undetectable in brain cells.
Finding that de novo steroidogenesis is predominantly restricted to neuronal cell types with minimal contribution from glial and vascular cells forces recalibration of physiological considerations linked to neurosteroidogenic responses and signaling in vivo. Prior work in this field have often emphasized the role of glial cells considering it a primary site of de novo neurosteroid biosynthesis (Hu et al. 1987; King et al. 2002), a context that has been overwhelmingly used to associate functional outcomes (Robel & Baulieu 1994). Our findings are in contrast to these reports. Moreover, antibody localization studies that have reported CYP11A1 expression restricted to myelinated regions in the brain white matter (Le Goascogne et al. 1987; Iwahashi et al. 1990), that are enriched in glial cells and do not contain neuronal cell bodies. This is not difficult to reconcile as immunostaining methods have limitations with regard to specificity, non-specific binding and false positives (Fritschy 2008), and has been demonstrated as such in this context (Furukawa et al. 1998; Kimoto et al. 2001). However, experiments in vitro have indicated rather robust de novo neurosteroid biosynthesis in olidodendrocytes after 3–6 weeks of culture (Hu et al. 1987; Jung-Testas et al. 1989). It remains to be examined if this is the result of an in vitro artifact or part of a broader response to stress (akin to pathologies resulting from inflammation or trauma). On this basis, it is plausible that pathology-associated de novo neurosteroidogenesis might be associated with a shift in cell type and/or regional expression patterns. Finding that a degree of sex bias exists for de novo steroidogenesis supports the possibility that some cell type responses might be associated with different physiological states (Takase et al. 1999; Inai et al. 2003). Added to de novo biosynthesis, interplay with intermediary biosynthesis of specific neurosteroids might be highly relevant to fully comprehend the cellular responses and potential effects of physiological changes and stressors.
In dedicated steroidogenic cells of the gonads and adrenal glands, STAR expression is induced by trophic stimulation, typically functioning as an acute, turn-on/turn-off process, which explains its designation as the steroidogenic “acute” regulatory protein (Clark et al. 1994). A similar induction model with a cyclic AMP-mediated pathway has been suggested for neurosteroid biosynthesis in brain slices (Barbaccia et al. 1992; Roscetti et al. 1994). Due to this possibility of episodic STAR-mediated de novo neurosteroid biosynthesis, interpretation that the expression of Cyp11a1 can be a reliable indicator of de novo neurosteroidogenesis is clearly supported. Given that neurosteroids can exert effects across membrane bilayers, it is plausible that tight regulation of de novo neurosteroid biosynthesis is required, potentially acting as a regulatory step for specific intermediary biosynthetic reactions, supporting regional autocrine or paracrine signaling and functional modulation.
Associating TSPO with de novo neurosteroidogenesis is fundamentally inaccurate (Selvaraj & Tu 2016). Our previous work explored the neuroprotective effects of TSPO-binding drugs, such as etifoxine (Daugherty et al. 2013), and like others (Rupprecht et al. 2010), we initially hypothesized that these effects might be due to increased neurosteroidogenesis. However, studies using Tspo-gene deleted models have revealed new mechanistic insights in conventional steroidogenic tissues (Tu et al. 2016; Koganti & Selvaraj 2020), and surprising findings in glial fibrillary acidic protein-positive (GFAP+) cells that suggest a potential pathogenic rather than protective role for TSPO (Daugherty et al. 2016). These reports underscore the importance of filtering out misleading pharmacological interpretations of TSPO function (Tu et al. 2015; Singh et al. 2020). Moreover, de novo neurosteroidogenesis should not be confused with intermediary synthesis, as might be possible with some neuropeptides (Do-Rego et al. 2001; Rego et al. 2007). While TSPO remains a therapeutic target in various contexts (Filiou et al. 2017), it is fair to note that the precise functional role of TSPO-binding drugs still remains largely unclear (Gavish & Veenman 2018). Recent analysis of neurosteroidgenesis in Tspo-gene deleted mice indicated no effects on both de novo and intermediary neurosteroid synthesis (Liere et al. 2023). In our findings, Tspo expression was largely restricted to non-neuronal cell types and not correlated with Cyp11a1. Predominant Tspo levels were observed in immune and vascular cells, with only scant expression in astrocytes and oligodendrocytes (Figure S3A).
Recently, it has been alleged that CYP1B1, a CYP1 family and aryl-hydrocarbon receptor inducible cytochrome P450 enzyme (Zhang et al. 1998; Shehin et al. 2000), may contribute to de novo pregnenolone biosynthesis in a manner analogous to CYP11A1 (Lin et al. 2023). However, this finding based primarily on pharmacological assays and approaches limited in scope, requires further validation. CYP1B1 is known for its metabolism of a wide range of xenobiotics such as 7-ethoxyresorufin, caffeine, theophylline, and diverse procarcinogens (Crespi et al. 1997; Shimada et al. 1997, 1998) and endobiotics such as testosterone, estradiol, retinoids, arachidonic acid and melatonin (Hayes et al. 1996; Choudhary et al. 2004; Ma et al. 2005). In our analysis, Cyp1b1 expression was found to be predominantly restricted to a specific subtype of vascular cells (Figure S3B). As such, this distribution does not support a role for CYP1B1 in de novo neurosteroidogenesis and does not impact our interpretations, even when considering such unsubstantiated functional speculation. Instead, the expression pattern aligns more convincingly with the established role of CYP1B1 in vascular biology, including its contribution to angiogenesis and neovascularization, as demonstrated in knockout mouse models (Tang et al. 2009, 2010).
In neurons, de novo synthesized pregnenolone may exert both autocrine and paracrine effects. Given that neuronal mitochondria are capable of trafficking to distal synaptic sites, it is plausible that de novo neurosteroid biosynthesis can occur precisely where these molecules are functionally required. This mirrors the established strategy of targeting energy supply to spatially defined cellular compartments—a mechanism already demonstrated for neuronal mitochondria (Rangaraju et al. 2019). Such spatial precision in neurosteroid production could underlie why de novo biosynthesis is largely confined to neurons and absent in non-neuronal cells.
Pregnenolone is a well-known, potent positive allosteric modulator of the GABAA receptor in the central nervous system (Lambert et al. 1995). Quantitative studies have shown that neurosteroids enhance both the frequency and duration of GABAA receptor channel opening (Twyman & Macdonald 1992). Enhancing inhibitory transmission through such mechanisms has been linked to the therapeutic effects of neurosteroids and their mimetics in treating anxiety, insomnia, pain, and seizures (MacKenzie & Maguire 2013). In glutamatergic neurons, pregnenolone sulfate has been shown to potentiate NMDA receptor currents while inhibiting AMPA and kainate receptor activity (Wu et al. 1991; Yaghoubi et al. 1998). Moreover, continuous de novo pregnenolone synthesis appears essential for maintaining homeostatic synaptic transmission in the hippocampal dentate gyrus (Tanaka & Sokabe 2012). In rodent models, intranasal administration of pregnenolone has been demonstrated to enhance aspects of learning and memory (Ducharme et al. 2010; Abdel-Hafiz et al. 2016). Collectively, these points of evidence link de novo neurosteroid biosynthesis to specialized neuronal functions and signaling.
For neuronal effects, pregnenolone is a long-known potent positive allosteric modulator of GABAA receptor in the CNS (Lambert et al. 1995). Direct quantitative evidence points to increases in the average GABAA receptor channel open duration and frequency with these steroids (Twyman & Macdonald 1992). Promoting inhibitory transmission has been associated with therapeutic interventions with steroids and mimetics to achieve anxiolytic, hypnotic, analgesic and anticonvulsant activities (MacKenzie & Maguire 2013). In glutamatergic neurons, effects of pregnenolone sulfate have been documented to augment NMDA (N-methyl-d-aspartate) receptor currents, whereas the effect appears inhibitory for AMPA and kainite receptors (Wu et al. 1991; Yaghoubi et al. 1998). It has been noted that continuous de novo synthesis of pregnenolone is required for homeostatic transmission at the hippocampal dentate gyrus (Tanaka & Sokabe 2012). In rodent models, intranasal delivery of pregnenolone has been shown to directly improve aspects of learning and memory (Ducharme et al. 2010; Abdel-Hafiz et al. 2016). All these points of evidence associate de novo neurosteroid biosynthesis to specific neuronal physiology.
In critical evaluation, the dataset used for this analysis (Yao et al. 2023), is not without limitations as in any large-scale analyses. Cell dissociation from dissected brain regions can incorporate bias, in that mature neuronal cell bodies can be relatively difficult to separate resulting in disproportional losses and variable RNA yields. Dissociation can also impact the proportions of representative cell types within different brain regions. Furthermore, the depth of sequencing can be variable both across different cell types and even within cell populations that might directly affect the rigor of co-expression analyses, particularly for genes that are at lower levels of expression. Some of these limitations were evident in our studies in that there was only a ~10% overlap in Cyp11a1 and Star expression. Although this can be argued as physiological in that Star is well known to be acutely regulated in transcription (Selvaraj et al. 2018), it could also the result of differences in sequencing depth of individual cells. Nevertheless, the abundance of 4,041,289 brain cells analyzed at population levels for different cell types make our overall conclusions regarding neurosteroidogenesis quite robust with weaknesses that only prevent refinement in interpreting narrow subpopulation expression structures.
In summary, glutamatergic neurons are the predominant source of de novo biosynthesized neurosteroids in the murine brain, accounting for 86.2% of all CYP11A1-positive cells (Figure 5). Together with GABAergic neurons (12.8%), they represent nearly 99% of CYP11A1-expressing cells. In contrast, non-neuronal cells constitute only a small fraction (0.89%) of CYP11A1-positive cells. These findings underscore the key role of neuronal subtypes, particularly glutamatergic neurons, in neurosteroid synthesis within the brain, while highlighting the limited contribution of non-neuronal cells to this process. Future studies could further explore how this localized biosynthetic capacity integrates with intermediary pathways and whether neuron-specific regulation of neurosteroidogenesis underlies responses to stress, disease, or pharmacological intervention.
Figure 5. Extent of involvement by brain cells towards de novo neurosteroid biosynthesis.

Of the total 4,041,289 single cell data points processed for this study, 6.74% were positive for Cyp11a1. These Cyp11a1 positive cells were >99% neuronal (6.68%) with little representation of <1% in non-neuronal cells (0.06%). Within neurons, Cyp11a1 positive glutamatergic neurons were much higher (5.81%) than GABAergic neurons (0.86%), with much lower representation from dopaminergic and serotonergic neurons (0.01% for each). Among non-neuronal cells, oligodendrocytes and their progenitors showed highest expression (0.03%), followed by astrocytes and ependymal cells (0.016%), vascular cells (0.11%) and immune cells (0.005%). These findings indicate that de novo neurosteroid biosynthesis is predominantly from glutamatergic neurons within neuronal populations of the murine brain.
Supplementary Material
Figure S1. Only the acidic pathway for bile acid synthesis is represented in brain cells. (A) Downstream of the CYP27A1 product 3β-hydroxy 5-cholestenoic acid, CYP7B1 catalyzes its intermediary conversion to 3β, 7⍺- dihydroxy 5-cholestenoic acid in the acidic pathway for bile acid synthesis. Expression of Cyp7b1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows the participation of a larger number of cell types that involve both neuronal and non-neuronal cells – that might use both local and circulating substrates for this intermediary synthesis. (B) Expression of microsomal Cyp7b1 is negligible to almost absent in all brain regions suggesting that the classical or basic pathway for bile acid synthesis is not active in the murine brain.
Figure S2. Sex differences in neuronal and non-neuronal de novo steroidogenesis. Expression of Cyp11b1 and Star from male and female cells as t-SNE plots did not reveal any significant shift in the patterns of expression (colors indicate zero and positives as four quartile ranges). Quantitatively delineating the expression of Cyp11b1 and Star as male and female cells indicated subtle sex-bias in expression levels. Of the Cyp11b1 and Star-expressing cell numbers, the ratios showed some level of deviation towards being higher in males for some cell types and regions, compared to being higher in females for others. Albeit at a low percentage, expression of Star in OEC and Pineal Glut was observed only in male cells (red encircled star).
Figure S3. Brain TSPO and CYP1B1 expression. (A) Expression of Tspo from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows distinctively higher populations and expression in OEC, Astro-Epen, Immune and OPC-Oligo. Extremely low scattered Tspo expression is also observed in some neuronal populations without any distinctive enrichments. (B) Expression of Cyp1b1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows distinctively higher enrichment in a sub-population of Vascular and OEC. Extremely low scattered expression is also observed in CTX-CGE GABA, Astro-Epen and OB-IMN.
Table S1. Genes names and Ensembl IDs for data extracted for mapping.
Acknowledgements
The authors would like to thank the Allen Institute for Brain Science and all its contributors for generating different datasets available for public, non-profit use.
Funding
Funding for this study was from the National Institutes of Health (Grant Number DK110059) to VS.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Data availability statement
All raw data analyzed in this study are available as part of the Allen Brain Map available for public, non-profit use (https://portal.brain-map.org/). All processed data presented in this study are available within the article and its Supplementary Materials.
References
- Abdel-Hafiz L, Chao OY, Huston JP, Nikolaus S, Spieler RE, de Souza Silva MA & Mattern C 2016. Promnestic effects of intranasally applied pregnenolone in rats. Neurobiology of Learning and Memory 133 185–195. (doi: 10.1016/j.nlm.2016.07.012) [DOI] [PubMed] [Google Scholar]
- Banati RB, Middleton RJ, Chan R, Hatty CR, Wai-Ying Kam W, Quin C, Graeber MB, Parmar A, Zahra D, Callaghan P, Fok S, Howell NR, Gregoire M, Szabo A, Pham T, Davis E, Liu GJ. 2014. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nature Communications 5. (doi: 10.1038/ncomms6452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Ambrosio C & Massotti M 1992. Cyclic AMP-dependent increase of steroidogenesis in brain cortical minces. European Journal of Pharmacology 219 485–486. (doi: 10.1016/0014-2999(92)90495-p) [DOI] [PubMed] [Google Scholar]
- Baulieu E-E 1981. Steroid hormones in the brain: Several mechanisms? In Steroid Hormone Regulation of the Brain, pp 3–14. Eds Fuxe K, Gustafsson J-Å & Wetterberg L. Pergamon. (doi: 10.1016/B978-0-08-026864-4.50007-4) [DOI] [Google Scholar]
- Chiang JYL & Ferrell JM 2019. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annual Review of Nutrition 39 175–200. (doi: 10.1146/annurev-nutr-082018-124344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M & Schenkman JB 2004. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1B1. Drug Metabolism and Disposition 32 840–847. (doi: 10.1124/dmd.32.8.840) [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR & Stocco DM 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). The Journal of Biological Chemistry 269 28314–28322. [PubMed] [Google Scholar]
- Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS & Sutter TR 1997. Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis 12 83–89. (doi: 10.1093/mutage/12.2.83) [DOI] [PubMed] [Google Scholar]
- Daugherty DJ, Selvaraj V, Chechneva OV, Liu XB, Pleasure DE & Deng W 2013. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med 5 891–903. (doi: 10.1002/emmm.201202124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty DJ, Chechneva O, Mayrhofer F & Deng W 2016. The hGFAP-driven conditional TSPO knockout is protective in a mouse model of multiple sclerosis. Scientific Reports 6. (doi: 10.1038/srep22556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Rego JL, Mensah-Nyagan AG, Beaujean D, Leprince J, Tonon MC, Luu-The V, Pelletier G & Vaudry H 2001. The octadecaneuropeptide ODN stimulates neurosteroid biosynthesis through activation of central-type benzodiazepine receptors. Journal of Neurochemistry 76 128–138. (doi: 10.1046/j.1471-4159.2001.00053.x) [DOI] [PubMed] [Google Scholar]
- Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML & Mattern C 2010. Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration. European Journal of Pharmacology 641 128. (doi: 10.1016/j.ejphar.2010.05.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M & Papadopoulos V 2015. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proceedings of the National Academy of Sciences of the United States of America 112 7261–7266. (doi: 10.1073/pnas.1502670112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wang K, Zirkin B & Papadopoulos V 2018. CRISPR/Cas9‒Mediated Tspo Gene Mutations Lead to Reduced Mitochondrial Membrane Potential and Steroid Formation in MA-10 Mouse Tumor Leydig Cells. Endocrinology 159 1130–1146. (doi: 10.1210/en.2017-03065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Farooqui T, Panza F & Frisardi V 2012. Metabolic syndrome as a risk factor for neurological disorders. Cellular and Molecular Life Sciences 69 741–762. (doi: 10.1007/s00018-011-0840-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiou MD, Banati RB & Graeber MB 2017. The 18-kDa Translocator Protein as a CNS Drug Target: Finding Our Way Through the Neuroinflammation Fog. CNS & Neurological Disorders Drug Targets 16 990–999. (doi: 10.2174/1871527316666171004125107) [DOI] [PubMed] [Google Scholar]
- Fritschy J-M 2008. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. European Journal of Neuroscience 28 2365–2370. (doi: 10.1111/j.1460-9568.2008.06552.x) [DOI] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T & Ichikawa Y 1998. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. Journal of Neurochemistry 71 2231–2238. (doi: 10.1046/j.1471-4159.1998.71062231.x) [DOI] [PubMed] [Google Scholar]
- Gavish M & Veenman L 2018. Regulation of Mitochondrial, Cellular, and Organismal Functions by TSPO. In Advances in Pharmacology, pp 103–136. Academic Press Inc. (doi: 10.1016/bs.apha.2017.09.004) [DOI] [PubMed] [Google Scholar]
- Hanukoglu I & Jefcoate CR 1980. Mitochondrial cytochrome P-450scc. Mechanism of electron transport by adrenodoxin. The Journal of Biological Chemistry 255 3057–3061. [DOI] [PubMed] [Google Scholar]
- Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R. 2021. Integrated analysis of multimodal single-cell data. Cell 184 3573–3587.e29. (doi: 10.1016/j.cell.2021.04.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Stuart T, Kowalski MH, Choudhary S, Hoffman P, Hartman A, Srivastava A, Molla G, Madad S, Fernandez-Granda C, Satija R. 2024. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nature Biotechnology 42 293–304. (doi: 10.1038/s41587-023-01767-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ & Sutter TR 1996. 17β-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proceedings of the National Academy of Sciences of the United States of America 93 9776–9781. (doi: 10.1073/pnas.93.18.9776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P & Baulieu EE 1987. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proceedings of the National Academy of Sciences of the United States of America 84 8215–8219. (doi: 10.1073/pnas.84.23.8215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inai Y, Nagai K, Ukena K, Oishi T & Tsutsui K 2003. Seasonal changes in neurosteroid concentrations in the amphibian brain and environmental factors regulating their changes. Brain Research 959 214–225. (doi: 10.1016/S0006-8993(02)03745-9) [DOI] [PubMed] [Google Scholar]
- Iwahashi K, Ozaki HS, Tsubaki M, Ohnishi J, Takeuchi Y & Ichikawa Y 1990. Studies of the immunohistochemical and biochemical localization of the cytochrome P-450scc-linked monooxygenase system in the adult rat brain. Biochimica Et Biophysica Acta 1035 182–189. (doi: 10.1016/0304-4165(90)90114-c) [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Hu ZY, Baulieu EE & Robel P 1989. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology 125 2083–2091. (doi: 10.1210/endo-125-4-2083) [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N & Kawato S 2001. Neurosteroid Synthesis by Cytochrome P450-Containing Systems Localized in the Rat Brain Hippocampal Neurons: N-Methyl-d-Aspartate and Calcium-Dependent Synthesis. Endocrinology 142 3578–3589. (doi: 10.1210/endo.142.8.8327) [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. 2002. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 22 10613–10620. (doi: 10.1523/JNEUROSCI.22-24-10613.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganti PP & Selvaraj V 2020. Lack of adrenal TSPO/PBR expression in hamsters reinforces correlation to triglyceride metabolism. Journal of Endocrinology. (doi: 10.1530/JOE-20-0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganti & Selvaraj 2025. Single cell resolution of neurosteroidogenesis in the murine brain: intermediary biosynthesis. J Endocrinol 265 e240333. ( 10.1530/JOE-24-0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C & Peters JA 1995. Neurosteroids and GABAA receptor function. Trends in Pharmacological Sciences 16 295–303. (doi: 10.1016/S0165-6147(00)89058-6) [DOI] [PubMed] [Google Scholar]
- Le Goascogne C, Robel P, Gouézou M, Sananes N, Baulieu E-E & Waterman M 1987. Neurosteroids: Cytochrome P-450 scc in Rat Brain. Science 237 1212–1215. (doi: 10.1126/science.3306919) [DOI] [PubMed] [Google Scholar]
- Liere P, Liu G-J, Pianos A, Middleton RJ, Banati RB & Akwa Y 2023. The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions. International Journal of Molecular Sciences 24 2474. (doi: 10.3390/ijms24032474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Cheung G, Zhang Z & Papadopoulos V 2023. Mitochondrial cytochrome P450 1B1 is involved in pregnenolone synthesis in human brain cells. Journal of Biological Chemistry 299. (doi: 10.1016/j.jbc.2023.105035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E & Waller-Evans H 2020. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays in Biochemistry 64 591–606. (doi: 10.1042/EBC20200043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Idle JR, Krausz KW & Gonzalez FJ 2005. Metabolism of melatonin by human cytochromes P450. Drug Metabolism and Disposition 33 489–494. (doi: 10.1124/dmd.104.002410) [DOI] [PubMed] [Google Scholar]
- MacKenzie G & Maguire J 2013. Neurosteroids and GABAergic signaling in health and disease. Biomolecular Concepts 4 29–42. (doi: 10.1515/bmc-2012-0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS 1991. Steroid hormones are multifunctional messengers to the brain. Trends in Endocrinology and Metabolism: TEM 2 62–67. (doi: 10.1016/1043-2760(91)90042-l) [DOI] [PubMed] [Google Scholar]
- Mellon SH & Griffin LD 2002. Neurosteroids: biochemistry and clinical significance. Trends in Endocrinology and Metabolism: TEM 13 35–43. (doi: 10.1016/s1043-2760(01)00503-3) [DOI] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W & Selvaraj V 2014. Translocator Protein/Peripheral Benzodiazepine Receptor Is Not Required for Steroid Hormone Biosynthesis. Endocrinology 155 89–97. (doi: 10.1210/en.2013-1556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftebro H, Saarem K, Björkhem I & Pedersen JI 1981. Side chain hydroxylation of C27-steroids and vitamin D3 by a cytochrome P-450 enzyme system isolated from human liver mitochondria. Journal of Lipid Research 22 1254–1264. [PubMed] [Google Scholar]
- Paul SM & Purdy RH 1992. Neuroactive steroids. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 6 2311–2322. [PubMed] [Google Scholar]
- Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V & Matarese G 2016. Role of metabolism in neurodegenerative disorders. Metabolism 65 1376–1390. (doi: 10.1016/j.metabol.2016.05.018) [DOI] [PubMed] [Google Scholar]
- Rangaraju V, Lauterbach M & Schuman EM 2019. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 176 73–84.e15. (doi: 10.1016/j.cell.2018.12.013) [DOI] [PubMed] [Google Scholar]
- Rego JLD, Leprince J, Luu-The V, Pelletier G, Tonon M-C & Vaudry H 2007. Structure-activity relationships of a series of analogs of the endozepine octadecaneuropeptide (ODN(11)(−)(18)) on neurosteroid biosynthesis by hypothalamic explants. Journal of Medicinal Chemistry 50 3070–3076. (doi: 10.1021/jm0610548) [DOI] [PubMed] [Google Scholar]
- Robel P & Baulieu E-E 1994. Neurosteroids: Biosynthesis and function. Trends in Endocrinology & Metabolism 5 1–8. (doi: 10.1016/1043-2760(94)90114-7) [DOI] [PubMed] [Google Scholar]
- Roscetti G, Ambrosio C, Trabucchi M, Massotti M & Barbaccia ML 1994. Modulatory mechanisms of cyclic AMP-stimulated steroid content in rat brain cortex. European Journal of Pharmacology 269 17–24. (doi: 10.1016/0922-4106(94)90021-3) [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D & Schumacher M 2010. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature Reviews Drug Discovery 9 971–988. (doi: 10.1038/nrd3295) [DOI] [PubMed] [Google Scholar]
- Selvaraj V & Stocco DM 2018. Letter to the Editor: Dubious Conclusions on TSPO Function. Endocrinology 159 2528–2529. (doi: 10.1210/en.2018-00052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V & Tu LN 2016. Current status and future perspectives: TSPO in steroid neuroendocrinology. J Endocrinol 231 R1–R30. (doi: 10.1530/JOE-16-0241) [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Tu LN & Stocco DM 2016. Crucial Role Reported for TSPO in Viability and Steroidogenesis is a Misconception. Commentary: Conditional Steroidogenic Cell-Targeted Deletion of TSPO Unveils a Crucial Role in Viability and Hormone-Dependent Steroid Formation. Front Endocrinol (Lausanne) 7 91. (doi: 10.3389/fendo.2016.00091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM & Clark BJ 2018. Current knowledge on the acute regulation of steroidogenesis. Biology of Reproduction 99 13–26. (doi: 10.1093/biolre/ioy102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Morohaku K, Koganti PP, Zhang J, He W, Quirk SM & Stocco DM 2020. Commentary: Amhr2-Cre-Mediated Global Tspo Knockout. Frontiers in Endocrinology 11. (doi: 10.3389/fendo.2020.00472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehin SE, Stephenson RO & Greenlee WF 2000. Transcriptional Regulation of the Human CYP1B1 Gene: Evidence for involvement of an aryl hydrocarbon receptor response element in constitutive expression. Journal of Biological Chemistry 275 6770–6776. (doi: 10.1074/jbc.275.10.6770) [DOI] [PubMed] [Google Scholar]
- Shimada T, Gillam EMJ, Sutter TR, Strickland PT, Guengerich FP & Yamazaki H 1997. Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metabolism and Disposition 25 617–622. [PubMed] [Google Scholar]
- Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP & Gillam EMJ 1998. Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Archives of Biochemistry and Biophysics 357 111–120. (doi: 10.1006/abbi.1998.0808) [DOI] [PubMed] [Google Scholar]
- Singh A, Dashnyam M, Chim B, Escobar TM, Dulcey AE, Hu X, Wilson KM, Koganti PP, Spinner CA, Xu X, Jadhav A, Southall N, Marugan J, Selvaraj V, Lazarevic V, Muljo SA, Ferrer M. 2020. Anxiolytic Drug FGIN-1–27 Ameliorates Autoimmunity by Metabolic Reprogramming of Pathogenic Th17 Cells. Scientific Reports 10. (doi: 10.1038/s41598-020-60610-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM & Clark BJ 1996. Regulation of the Acute Production of Steroids in Steroidogenic Cells*. Endocrine Reviews 17 221–244. (doi: 10.1210/edrv-17-3-221) [DOI] [PubMed] [Google Scholar]
- Takase M, Ukena K, Yamazaki T, Kominami S & Tsutsui K 1999. Pregnenolone, pregnenolone sulfate, and cytochrome P450 side-chain cleavage enzyme in the amphibian brain and their seasonal changes. Endocrinology 140 1936–1944. (doi: 10.1210/endo.140.4.6641) [DOI] [PubMed] [Google Scholar]
- Tanaka M & Sokabe M 2012. Continuous de novo synthesis of neurosteroids is required for normal synaptic transmission and plasticity in the dentate gyrus of the rat hippocampus. Neuropharmacology 62 2373–2387. (doi: 10.1016/j.neuropharm.2012.02.007) [DOI] [PubMed] [Google Scholar]
- Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR & Sheibani N 2009. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113 744–754. (doi: 10.1182/blood-2008-03-145219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Scheef EA, Gurel Z, Sorenson CM, Jefcoate CR & Sheibani N 2010. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. American Journal of Physiology-Cell Physiology 298 C665–C678. (doi: 10.1152/ajpcell.00153.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM & Selvaraj V 2014. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. Journal of Biological Chemistry 289 27444–27454. (doi: 10.1074/jbc.M114.578286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Zhao AH, Stocco DM & Selvaraj V 2015. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinology 156 1033–1039. (doi: 10.1210/en.2014-1707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Zhao AH, Hussein M, Stocco DM & Selvaraj V 2016. Translocator Protein (TSPO) Affects Mitochondrial Fatty Acid Oxidation in Steroidogenic Cells. Endocrinology 157 1110–1121. (doi: 10.1210/en.2015-1795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE & Macdonald RL 1992. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. The Journal of Physiology 456 215–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhai K, Xue Y, Yang J, Yang Q, Fu Y, Hu Y, Liu F, Wang W, Cui L, Chen H, Zhang J, He W. 2016. Global Deletion of TSPO Does Not Affect the Viability and Gene Expression Profile. PLOS ONE 11 e0167307. (doi: 10.1371/journal.pone.0167307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H 2016. Ggplot2. Cham: Springer International Publishing. (doi: 10.1007/978-3-319-24277-4) [DOI] [Google Scholar]
- Wickham H, Vaughan D, & Girlich M 2024a. tidyr: Tidy Messy Data. R package version 1.3.1. [Google Scholar]
- Wickham H, François R, Henry L, Müller K, & Vaughan D 2024b. dplyr: A Grammar of Data Manipulation. R package version 1.1.4. [Google Scholar]
- Wu FS, Gibbs TT & Farb DH 1991. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Molecular Pharmacology 40 333–336. [PubMed] [Google Scholar]
- Yaghoubi N, Malayev A, Russek SJ, Gibbs TT & Farb DH 1998. Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Research 803 153–160. (doi: 10.1016/S0006-8993(98)00644-1) [DOI] [PubMed] [Google Scholar]
- Yao Z, van Velthoven CTJ, Kunst M, Zhang M, McMillen D, Lee C, Jung W, Goldy J, Abdelhak A, Aitken M Baker K, Baker P, Barkan E, Bertagnolli D, Bhandiwad A, Bielstein C, Bishwakarma P, Campos J, Carey D, Casper T, Chakka AB, Chakrabarty R, Chavan S, Chen M, Clark M, Close J, Crichton K, Daniel S, DiValentin P, Dolbeare T, Ellingwood L, Fiabane E, Fliss T, Gee J, Gerstenberger J, Glandon A, Gloe J, Gould J, Gray J, Guilford N, Guzman J, Hirschstein D, Ho W, Hooper M, Huang M, Hupp M, Jin K, Kroll M, Lathia K, Leon A, Li S, Long B, Madigan Z, Malloy J, Malone J, Maltzer Z, Martin N, McCue R, McGinty R, Mei N, Melchor J, Meyerdierks E, Mollenkopf T, Moonsman S, Nguyen TN, Otto S, Pham T, Rimorin C, Ruiz A, Sanchez R, Sawyer L, Shapovalova N, Shepard N, Slaughterbeck C, Sulc J, Tieu M, Torkelson A, Tung H, Valera Cuevas N, Vance S, Wadhwani K, Ward K, Levi B, Farrell C, Young R, Staats B, Wang M-QM, Thompson CL, Mufti S, Pagan CM, Kruse L, Dee N, Sunkin SM, Esposito L, Hawrylycz MJ, Waters J, Ng L, Smith K, Tasic B, Zhuang X, Zeng H. 2023. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624 317–332. (doi: 10.1038/s41586-023-06812-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Savas Ü, Alexander DL & Jefcoate CR 1998. Characterization of the Mouse Cyp1B1 Gene: Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. Journal of Biological Chemistry 273 5174–5183. (doi: 10.1074/jbc.273.9.5174) [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM & Edwards PA 2006. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proceedings of the National Academy of Sciences 103 1006–1011. (doi: 10.1073/pnas.0506982103) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Only the acidic pathway for bile acid synthesis is represented in brain cells. (A) Downstream of the CYP27A1 product 3β-hydroxy 5-cholestenoic acid, CYP7B1 catalyzes its intermediary conversion to 3β, 7⍺- dihydroxy 5-cholestenoic acid in the acidic pathway for bile acid synthesis. Expression of Cyp7b1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows the participation of a larger number of cell types that involve both neuronal and non-neuronal cells – that might use both local and circulating substrates for this intermediary synthesis. (B) Expression of microsomal Cyp7b1 is negligible to almost absent in all brain regions suggesting that the classical or basic pathway for bile acid synthesis is not active in the murine brain.
Figure S2. Sex differences in neuronal and non-neuronal de novo steroidogenesis. Expression of Cyp11b1 and Star from male and female cells as t-SNE plots did not reveal any significant shift in the patterns of expression (colors indicate zero and positives as four quartile ranges). Quantitatively delineating the expression of Cyp11b1 and Star as male and female cells indicated subtle sex-bias in expression levels. Of the Cyp11b1 and Star-expressing cell numbers, the ratios showed some level of deviation towards being higher in males for some cell types and regions, compared to being higher in females for others. Albeit at a low percentage, expression of Star in OEC and Pineal Glut was observed only in male cells (red encircled star).
Figure S3. Brain TSPO and CYP1B1 expression. (A) Expression of Tspo from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows distinctively higher populations and expression in OEC, Astro-Epen, Immune and OPC-Oligo. Extremely low scattered Tspo expression is also observed in some neuronal populations without any distinctive enrichments. (B) Expression of Cyp1b1 from single cells visualized in a cell type defined t-SNE plot with colors that indicate zero and positives (as four quartile ranges), shows distinctively higher enrichment in a sub-population of Vascular and OEC. Extremely low scattered expression is also observed in CTX-CGE GABA, Astro-Epen and OB-IMN.
Table S1. Genes names and Ensembl IDs for data extracted for mapping.
Data Availability Statement
All raw data analyzed in this study are available as part of the Allen Brain Map available for public, non-profit use (https://portal.brain-map.org/). All processed data presented in this study are available within the article and its Supplementary Materials.


