Abstract
Objective:
To evaluate whether abnormal sleep is associated with adverse outcomes for children who survived active provoked neonatal seizures, and their parents.
Study Design:
This 9-center study prospectively followed newborns with acute provoked seizures. When children reached age 5-years, parents completed the Children’s Sleep Habits Questionnaire (CSHQ), the Pediatric Sleep Questionnaire–Sleep Related Breathing Disorders (PSQ-SRBD) subscale, Vineland Adaptive Behavior Scales–3, and the Hospital Anxiety Depression Scale (HADS). Children also underwent the Wechsler Preschool and Primary Scale of Intelligence-IV (WPPSI-IV). Spearman correlations and multivariable analyses were used to evaluate risk factors for sleep problems.
Results:
The mean CSHQ score was 45±7; 77/118 (65%) scored above the healthy sleep threshold of 41. On the PSQ-SRBD, 32/119 (27%) screened positive for sleep-disordered breathing (SDB). SDB symptoms were more common among children with cerebral palsy (42% with vs. 22% without, p=0.03) and epilepsy (54% with vs. 24% without, p=0.02). Children with lower scores on the Vineland-3 (rho=−0.25, p=0.01) and WPPSI-IV (rho=−0.31, p=0.004) at age 5 years were more likely to have symptoms of SDB. Worse CSHQ and PSQ-SRBD scores were associated with higher parental anxiety (rho=0.28, p=0.002, and rho=0.34, p=0.0002, respectively) and depression scores on the HADS (rho=0.16, p=0.08, and rho=0.17, p=0.07, respectively).
Conclusions:
Two-thirds of early school-aged survivors of acute provoked neonatal seizures had parent-reported sleep abnormalities and one quarter screened positive for SDB. Early screening and effective treatment for sleep disorders could be an innovative, practice-changing approach to improve outcomes after neonatal seizures.
Introduction:
Children who survive acute provoked neonatal seizures are at risk for neurodevelopmental consequences that include epilepsy and cerebral palsy, as well as cognitive and behavioral challenges.(1) Novel interventions are needed to improve the developmental trajectories of these at-risk children.
Sleep problems and sleep disorders are common in childhood(2–4), yet clinicians and families may not realize that effective treatment is possible. Among healthy children, symptoms of sleep-disordered breathing (SDB) have been shown to predict subsequent development of hyperactivity and other behavior problems as much as 7 years later, even after SDB symptoms have resolved.(5, 6) Two randomized controlled trials showed robust impact of treatment for childhood SDB, even when mild, on subsequent neurobehavioral outcomes and quality of life.(7, 8) Indeed, the American Academy of Pediatrics recommends that pediatricians screen all children for obstructive sleep apnea syndrome (OSA), since it is a highly prevalent condition and “identification and treatment can result in alleviation of current symptoms, improved quality of life, prevention of sequelae, education of parents, and decreased health care utilization.”(9)
We hypothesize that the consequences of sleep disorders are heightened among children with acute provoked neonatal seizures since they are at elevated risk for neurobehavioral problems. We also hypothesize that children’s sleep problems are associated with parent well-being. If so, early screening and effective treatment could provide a novel mechanism to improve outcomes for these children and families. As a first step toward evaluating sleep as a modifiable risk factor for adverse neurobehavioral outcomes, we screened a multicenter, longitudinal cohort of 5-year-old survivors of neonatal seizures for sleep problems.
Methods:
Children born between July 2015 and March 2018 who were enrolled in a prospective multicenter cohort study (NCT02789176) of neonates with acute provoked seizures were invited to participate in a school-aged follow-up study (NCT04337697) which was conducted 2020–2024. Participants were treated at one of the nine centers of the Neonatal Seizure Registry.(10, 11) Each center has a level IV NICU and a level IV comprehensive pediatric epilepsy program that follows the 2011 American Clinical Neurophysiology Society guidelines for continuous electroencephalography (EEG) monitoring.18 The study questions, design, outcome measures and analyses were informed by a Parent Advisory Panel. The local institutional review board at each site approved the study, and parents provided written informed consent.
Inclusion criteria:
seizures due to an acute provoked etiology with onset at <44 weeks postmenstrual age, parents who were able to participate in English or Spanish (professional interpreters were available), and completion of the 24-month follow-up protocol.
Exclusion criteria:
transient cause of neonatal seizures (e.g., mild hypoglycemia, hyponatremia, hypocalcemia with normal neuroimaging), neonatal onset epilepsy syndromes, risk for adverse outcome independent of the neonatal seizures and underlying brain injury (e.g., inborn errors of metabolism, fetal infection, brain malformation), or death during the neonatal seizure admission.
When the children reached age 5 years, families completed validated instruments to measure sleep behaviors, risk for SDB, child development, and parent mental health. Parents either completed the surveys online or could opt to have a study coordinator read the questions verbatim and record their responses.
Sleep:
The Child Sleep Habits Questionnaire (CSHQ) is a widely-used, broad sleep assessment that has been applied successfully to children with diverse neurodevelopmental diagnoses.(12) The abnormal threshold score for CSHQ is 41 (higher scores are more abnormal). In a cohort of healthy school-aged children, 23% scored higher than 41.(13)
The Pediatric Sleep Questionnaire – Sleep-Related Breathing Disorders (PSQ-SRBD) Scale is the most commonly used symptom inventory for OSA, and reflects risk for polysomnogram-confirmed diagnoses.(14, 15) Multiple studies have identified the PSQ-SRBD as an effective instrument for assessment of childhood risk for OSA.(16, 17) The threshold score for PSQ-SRBD is 0.33 (higher scores are more abnormal). This screening test has ~85% sensitivity and specificity for polysomnogram-defined SDB in school-aged children.(14)
Development:
The Vineland Adaptive Behavior Scales, 3rd edition (Vineland-3) is a validated tool to assess adaptive behavior for people at risk for intellectual, developmental, and other disabilities.(18) Standard scores have a mean of 100 and standard deviation (SD) 15. We considered scores <70 to indicate functional impairment.
The Wechsler Preschool and Primary Scale of Intelligence, 4th edition, WPPSI-IV, measures cognitive development for children ages 2–6 years. Standard scores have a mean of 100 and SD 15.
Parent Mental Health:
The Hospital Anxiety and Depression Scale (HADS) is a well-validated measure of symptoms of anxiety and depression.(19) HADS has been used extensively to characterize the impact of NICU hospitalization(10, 20–22) or childhood illness(23, 24) on parent psychological well-being. On the HADS Anxiety and Depression subscales, scores of 8–10 are considered borderline abnormal and scores >10 are abnormal.
Epilepsy & Cerebral Palsy:
Epilepsy was defined as recurrent unprovoked seizures after the neonatal period(25) as determined through parent report and corroborated by medical record review. Similarly, cerebral palsy diagnosis and severity (Gross Motor Function Classification System, GMFCS(26)) were reported by parents and confirmed in the medical records.
Statistical Analyses:
Univariate statistics were used to describe the overall sample, as well as the subgroups of children with epilepsy or cerebral palsy. Spearman correlations, two-sample t-tests, and one-way ANOVA were calculated to assess relationships between clinical variables and sleep questionnaire results. Linear regression was used to test the association between child development (Vineland-3, WPSSI) and parent-reported child sleep problems (CSHQ, PSQ-SRBD).
Multivariable models were developed to test the hypothesis that children with more severe neurodevelopmental disabilities have more sleep problems. We adjusted for preterm birth (<37 weeks gestation), GMFCS at 24 months, epilepsy, Vineland-3, and WPPSI scores. Secondary analyses examined children with vs. without cerebral palsy given the hypothesis that abnormal tone associated with cerebral palsy may predispose children to OSA. These models were built using backwards stepwise selection, removing predictors with highest p-value until only predictors with p <0.05 were retained. To assess for independent associations between parents’ mental health and children’s sleep problems, multivariable models for parent mental health (HADS scores) included the child’s diagnosis of epilepsy, cerebral palsy, and impaired functional development (Vineland-3 < 70).
All analyses were completed using SAS 9.4 (Carey, NC) with a two-tailed p-value of 0.05 considered statistically significant.
Results:
A total of 188 children enrolled in the school-aged follow-up study, and the parents of 119 of the 158 (75%) with assessments at age 5 years completed sleep surveys. These 119 children form the cohort for the current report. Children included in the present analysis had similar clinical profiles to the full cohort in which they were originally enrolled (Supplemental Table 1). About half were boys (64/119, 54%), most were full term (103/119, 87%), and nearly half had hypoxic-ischemic encephalopathy as the etiology of their neonatal seizures (56/119, 47%; Table 1). The mean CSHQ score was 45±7 and 77/118 (65%) scored above the healthy threshold of 41 (one parent did not complete the CSHQ). On the PSQ-SRBD, 32/119 (27%) screened positive for SDB. CSHQ and PSQ-SRBD were positively correlated with one another (rho = 0.47, p <0.0001) and did not differ significantly by gestational age or neonatal seizure etiology (Table 1).
Table 1:
Associations between clinical and demographic characteristics and sleep scale scores for 119 five-year old survivors of acute provoked neonatal seizures
| Clinical Characteristics | N-119 | Association with CSHQ score | Association with PSQ-SRBD score |
|---|---|---|---|
| Neonatal Characteristics | |||
| Gestational age, weeks mean (SD) | 38.8 (2.9) |
rho* = −0.09 p = 0.34 |
rho = −0.002 p = 0.99 |
| Preterm birth, n (%) | 17 (14%) | p = 0.32 | p = 0.20 |
| Male sex, n (%) | 64 (54%) | p = 0.49 | p = 0.45 |
| Neonatal seizure etiology, n (%) Hypoxic Ischemic Encephalopathy Ischemic Stroke Hemorrhages Other |
56 (47%) 34 (29%) 17 (14%) 12 (10%) |
p = 0.80 | p = 0.58 |
| Outcome at age 2 years | |||
| Cerebral palsy, n (%) | 31 (26%) | p = 0.52 | p = 0.003 |
| Epilepsy, n (%) | 13 (11%) | p = 0.30 | p = 0.01 |
| Outcome at age 5 years | |||
| Vineland-3, mean (SD) | 90.4 (18.6) |
rho = −0.05 p = 0.59 |
rho = −0.25 p = 0.01 |
| WPPSI-IV, mean (SD) | 92.8 (23.7) |
rho = −0.12 p = 0.28 |
rho = −0.30 p = 0.004 |
rho derived from Spearman correlation
Abbreviations: CSHQ: Children’s Sleep Habits Questionnaire; PSQ-SRBD: Pediatric Sleep Questionnaire – Sleep-Related Breathing Disorders subscale; GMFCS: Gross Motor Function Classification System; Vineland-3: Vineland Adaptive Behavior Scales, 3rd edition; WPPSI-IV - Wechsler Preschool and Primary Scale of Intelligence, 4th edition
Univariate Analyses:
CSHQ scores were not significantly different among children with or without cerebral palsy or epilepsy (Figure 1). Abnormal PSQ-SRBD scores were more common among children with cerebral palsy (13/31, 42% with cerebral palsy vs. 19/87, 22% without, p=0.03) and epilepsy (7/13, 54% with epilepsy vs. 25/103, 24% without, p=0.02; Figure 2).
Figure 1:
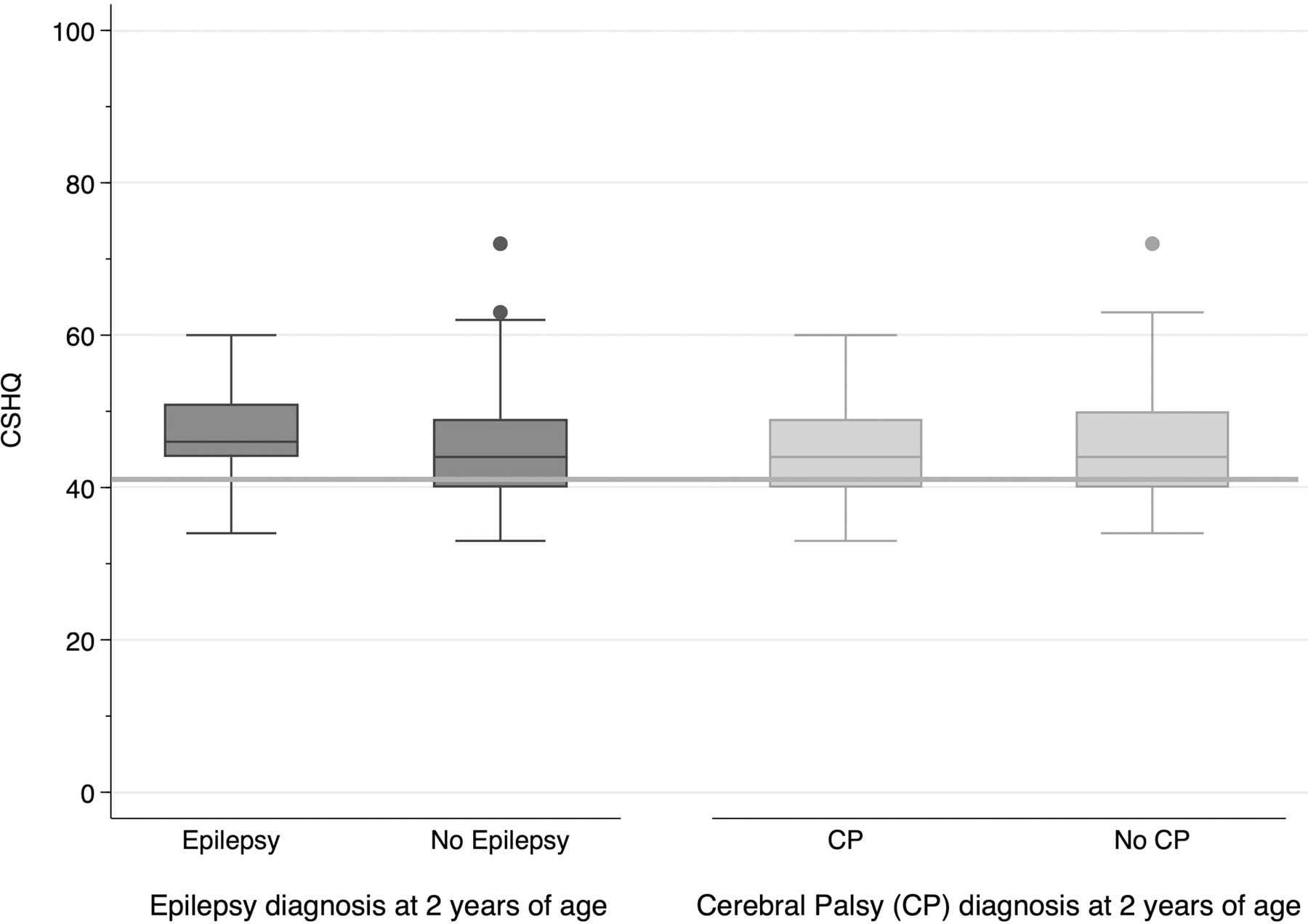
Among 118 survivors of neonatal seizures, 77/118 (65%) scored above the healthy sleep threshold of 41. Abnormal CSHQ scores were not different for children with epilepsy (11/13 with epilepsy vs. 66/105 without, p=0.3) or cerebral palsy (19/31 with cerebral palsy vs. 58/86 without, p=0.52).
Boxplot legend: middle line = median, box ends = 25th and 75th percentiles, whiskers = (25th or 75th percentile)*1.5xIQR, dots = outliers; horizontal line = abnormal CSHQ threshold score (41).
Figure 2:

Among 119 survivors of neonatal seizures, abnormal PSQ-SRBD scores were associated with epilepsy (7/13, 54% with epilepsy vs. 25/105, 24% without, p=0.02) and with cerebral palsy 13/31, 42% with cerebral palsy vs. 19/84, 22% without, p=0.03).
Boxplot legend: middle line = median, box ends = 25th and 75th percentiles, whiskers = (25th or 75th percentile)*1.5xIQR, dots = outliers; horizontal line = abnormal PSQ-SRBD threshold score (0.33).
Children with lower scores on the Vineland-3 (rho= −0.25, p=0.01) and WPPSI-IV (rho= −0.30, p=0.004) at age 5 years were more likely to have higher (more abnormal) PSQ-SRBD scores.
Worse (higher) CSHQ and PSQ-SRBD scores were both associated with parental symptoms of anxiety (rho=0.28, p=0.002, and rho=0.34, p=0.0002) and depression on the HADS (rho=0.16, p=0.08, and rho=0.17, p=0.07).
Multivariable models:
In the multivariable model, none of the neonatal or follow-up clinical variables were associated with CSHQ. However, children who were born preterm had significantly lower (better) PSQ-SRBD scores [model coefficient estimate −0.11 (standard error, SE, 0.05) p=0.02] and those who had Vineland <70 had significantly higher (worse) PSQ-SRBD scores (estimate 0.19, SE 0.05, p=0.0003).
In multivariable models of children with cerebral palsy, prematurity was associated with lower (better) CSHQ (estimate −8.7, SE 3.1, p=0.009) and PSQ-SRBD scores (estimate −0.2, SE 0.08, p=0.05). Epilepsy was associated with higher (worse) CSHQ scores (estimate 6.6, SE 2.6, p=0.02) and having a Vineland score <70 was associated with higher (worse) PSQ-SRBD scores (estimate 0.14, SE 0.07, p=0.04). Among children without cerebral palsy, neither the multivariable model for CSHQ nor the model for PSQ-SRBD had any significant clinical predictors.
In multivariable analyses (Supplemental Table 2), parental HADS-anxiety scores were associated with CSHQ and cerebral palsy, but not epilepsy or Vineland-3 <70. Parental HADS-anxiety scores were also associated with higher PSQ-SRBD scores, but neither cerebral palsy, nor epilepsy or Vineland-3 <70 contributed to the model. Parental HADS-depression scores were associated with CSHQ, but cerebral palsy, epilepsy, and Vineland <70 did not contribute to the model. We found no association between HADS-depression scores and PSQ-SRBD scores.
Discussion:
In this prospective, multicenter study, about two-thirds of 5-year-old survivors of acute provoked neonatal seizures had parent-reported sleep abnormalities and one quarter screened positive for symptoms of SDB. These results suggest that sleep disorders are far more common among children with a history of neonatal seizures than the healthy pediatric population. While epilepsy and cerebral palsy were associated with symptoms of SDB, only 14% of participating children carried either of these diagnoses; thus, many children without epilepsy or cerebral palsy also had parent-reported sleep problems.
Sleep disorders in infancy and childhood can contribute to adverse neurobehavioral outcomes and parental anxiety and depression.(27, 28) As sleep disorders are readily treatable, they may be modifiable risk factors for adverse child and parent outcomes such as those seen in survivors of acute provoked neonatal seizures. Thus, early screening and effective treatment for sleep disorders may be a novel approach to improve childhood outcomes after neonatal seizures. Treatment of sleep disorders in children can range from behavioral interventions to manage chronic insomnia disorder to supplemental oxygen, adenotonsillectomy, or positive airway pressure for sleep-disordered breathing.
The American Academy of Pediatrics technical report for diagnosis and management of OSA highlights that early diagnosis and treatment of SDB may improve long-term cognitive, social, and academic outcomes.(29) Emerging evidence suggests, for example, that early treatment of SDB in infants with trisomy 21 may result in improved cognitive development by age 3 years.(30) Conversely, there are risks of not treating children with SDB – including long-term consequences on cognitive development, executive function, and social interactions.(29) Given the elevated risk for abnormal cognitive and neurobehavioral outcomes among children with neonatal seizures and our finding that most of these children may have a sleep disorder by age 5 years, our study suggests an urgent need to (1) begin routinely screening such children for sleep disorders, and (2) conduct additional research to assess whether early diagnosis and treatment of sleep disorders can alter the trajectory of neurobehavioral outcomes in this patient population.
How early to begin screening for sleep problems remains an open question. Assessment at school age may miss early SDB which, though resolved, may have contributed to impaired brain development. A cohort study of children aged 2 to 13 years found that SDB symptoms at baseline, compared with four years later, were a stronger predictor of newly-emerged hyperactive behavior at study end.(5) Others reported that SDB symptoms as early as ages 6 or 18 months – even when the symptoms resolved by the time of assessments at older ages – were associated with 40 to 50% increased odds of behavior problems, including hyperactivity, at age 7 years.(6) These results suggest that harm to the developing brain caused by SDB may occur within the first two years, with substantive impact on behavior and development, only to emerge years later when these phenotypes typically become readily distinguishable from age-appropriate behavior.(6) Given these findings, combined with evidence that early treatment improves outcomes for infants with trisomy 21(30), future studies should evaluate screening paradigms to identify and treat sleep problems earlier.
Multiple factors likely contribute to an increased risk for sleep disorders among survivors of neonatal seizures. Abnormal tone, as manifest by cerebral palsy, may increase the risk for OSA.(31) Brain injury may also alter central regulation of respiratory drive and result in central sleep apnea or hypoventilation. Meanwhile, many sleep issues in the earliest years are behavioral. For example, chronic insomnia disorder (formerly subcategorized as sleep-onset association disorder and limit-setting disorder) often arises from remediable interactions between well-meaning parents and their children.(32, 33) Vulnerable child syndrome, commonly experienced after critical neonatal illness,(34) may exacerbate these behavioral sleep challenges. Appropriate screening, referral to sleep medicine clinicians, and definitive testing (e.g. with polysomnography) may result in effective treatment and mitigation of negative neurodevelopmental consequences.
Our study’s strengths include the longitudinal follow-up of children recruited as neonates at nine US children’s hospital, with careful characterization of the neonatal seizures, neurodevelopment, parent well-being, and symptoms of sleep disorders assessed with validated instruments. School-aged follow-up began in 2020 – during the COVID-19 pandemic; thus, due to stay-at-home orders, limitations on clinical research protocols at study centers, and other pandemic-related challenges, not all families in the original cohort were able or willing to return for testing. Yet, the clinical profiles of children who participated in the present analysis were very similar to those of the originally enrolled cohort (Supplemental Table 1). It is possible that the pandemic had an impact on the children’s sleep – an increase in parent-reported sleep problems was reported in the context of the pandemic.(35, 36) We did not enroll a healthy control group, as our study is focused on discerning detailed outcomes of children who experienced neonatal seizures.
Additional limitations include the small sample size in key subgroups – particularly post-neonatal epilepsy. Subgroup analyses are, therefore, subject to lack of precision. We did not confirm diagnoses of sleep disorders through chart review, formal sleep medicine evaluations, or polysomnography; rather, we tested the hypothesis that widely used screening surveys would identify many children who are at high risk for sleep disorders. Screening instruments do not obviate the need for diagnostic testing (e.g. polysomnography). Future work should include systematic screening for other SDB risk factors (e.g. obesity) and formal evaluations for behavioral sleep disorders and diagnostic testing for SDB and other sleep-related diagnoses. The parents of children with abnormal CSHQ and PSQ-SRBD scores often reported symptoms of anxiety and depressions on the HADS. It is possible that more anxious or depressed parents will more readily report problems on a validated survey; it is also possible that children’s abnormal sleep has direct impact on their parents’ mental health. The interactions between sleep, neurodevelopment and behavior, and parental mental health, are complex. Our results demonstrate associations among these domains; further work is needed to identify the causal pathways.
Conclusion:
This study’s results suggest that most 5-year-old children who had acute provoked neonatal seizures are at high risk for sleep disorders. Assessment and treatment for sleep disorders, starting at the earliest ages, for survivors of neonatal seizures could be highly impactful during childhood and, by extension, throughout the lifespan. Systematic screening, and referral of children with abnormal CSHQ and PSQ-SRDB scores for formal sleep evaluations and subsequent treatment, could be an innovative and practice-changing approach to optimizing long-term outcomes.
Supplementary Material
Acknowledgements:
The authors are tremendously grateful to the participating children and parents, as well as to the Neonatal Seizure Registry parent advisory panel partners and study coordinators, for their perseverance and dedication to addressing the research questions most important to the neonatal seizure community.
Funding/Support:
This study was supported by NIH (NS111166) and PCORI (1507-31187). The study sponsors did not have a role in study design, data collection, analysis or interpretation, writing of this report, or the decision to submit the paper for publication.
RAS receives a stipend for her role as President of the Pediatric Epilepsy Research Foundation; serves as a consultant for the Epilepsy Study Consortium, and receives royalties from UpToDate for authorship of topics related to neonatal seizures.
Abbreviations:
- CP
Cerebral palsy
- CSHQ
Children’s Sleep Habits Questionnaire
- EEG
Electroencephalography
- GMFCS
Gross Motor Function Classification System
- HADS
Hospital Anxiety Depression Scale
- OSA
Obstructive sleep apnea
- PSQ-SRBD
Pediatric Sleep Questionnaire–Sleep Related Breathing Disorders subscale
- SDB
Sleep-disordered breathing
- WPPSI-IV
Wechsler Preschool and Primary Scale of Intelligence-IV
Footnotes
Conflict of Interest Disclosures: The remaining authors have no conflicts of interest relevant to this article to disclose.
Clinical Trial Registration: NCT02789176 and NCT04337697
References:
- 1.Glass HC, Grinspan ZM, Shellhaas RA. Outcomes after acute symptomatic seizures in neonates. Semin Fetal Neonatal Med 2018;23(3):218–22. [DOI] [PubMed] [Google Scholar]
- 2.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr 2000;21(1):27–36. [DOI] [PubMed] [Google Scholar]
- 3.Sadeh A, Mindell JA, Luedtke K, Wiegand B. Sleep and sleep ecology in the first 3 years: a web-based study. J Sleep Res 2009;18(1):60–73. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5(2):242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28(7):885–90. [DOI] [PubMed] [Google Scholar]
- 6.Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129(4):e857–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. The New England journal of medicine. 2013;368(25):2366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Cook K, Chervin RD, Ishman S, Baldassari CM, Mitchell RB, et al. Adenotonsillectomy for Snoring and Mild Sleep Apnea in Children: A Randomized Clinical Trial. JAMA 2023;330(21):2084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–84. [DOI] [PubMed] [Google Scholar]
- 10.Franck LS, Shellhaas RA, Lemmon ME, Sturza J, Barnes M, Brogi T, et al. Parent Mental Health and Family Coping over Two Years after the Birth of a Child with Acute Neonatal Seizures. Children (Basel). 2021;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass HC, Soul JS, Chang T, Wusthoff CJ, Chu CJ, Massey SL, et al. Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures. JAMA neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J Dev Behav Pediatr 2008;29(2):82–8. [DOI] [PubMed] [Google Scholar]
- 13.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 14.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine. 2000;1:21–32. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133(3):216–22. [DOI] [PubMed] [Google Scholar]
- 16.Incerti Parenti S, Fiordelli A, Bartolucci ML, Martina S, D’Antò V, Alessandri-Bonetti G. Diagnostic accuracy of screening questionnaires for obstructive sleep apnea in children: A systematic review and meta-analysis. Sleep Med Rev 2021;57:101464. [DOI] [PubMed] [Google Scholar]
- 17.Michelet D, Julien-Marsollier F, Vacher T, Bellon M, Skhiri A, Bruneau B, et al. Accuracy of the sleep-related breathing disorder scale to diagnose obstructive sleep apnea in children: a meta-analysis. Sleep Med 2019;54:78–85. [DOI] [PubMed] [Google Scholar]
- 18.Price JA, Morris ZA, Costello S. The Application of Adaptive Behaviour Models: A Systematic Review. Behav Sci (Basel). 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 20.Pace CC, Spittle AJ, Molesworth CM, Lee KJ, Northam EA, Cheong JL, et al. Evolution of Depression and Anxiety Symptoms in Parents of Very Preterm Infants During the Newborn Period. JAMA pediatrics. 2016;170(9):863–70. [DOI] [PubMed] [Google Scholar]
- 21.Carter JD, Mulder RT, Frampton CM, Darlow BA. Infants admitted to a neonatal intensive care unit: parental psychological status at 9 months. Acta Paediatr 2007;96(9):1286–9. [DOI] [PubMed] [Google Scholar]
- 22.Eutrope J, Thierry A, Lempp F, Aupetit L, Saad S, Dodane C, et al. Emotional reactions of mothers facing premature births: study of 100 mother-infant dyads 32 gestational weeks. PloS one. 2014;9(8):e104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besier T, Born A, Henrich G, Hinz A, Quittner AL, Goldbeck L. Anxiety, depression, and life satisfaction in parents caring for children with cystic fibrosis. Pediatr Pulmonol 2011;46(7):672–82. [DOI] [PubMed] [Google Scholar]
- 24.Jones C, Reilly C. Parental anxiety in childhood epilepsy: A systematic review. Epilepsia. 2016;57(4):529–37. [DOI] [PubMed] [Google Scholar]
- 25.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. [DOI] [PubMed] [Google Scholar]
- 26.Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, et al. Validation of a model of gross motor function for children with cerebral palsy. Physical therapy. 2000;80(10):974–85. [PubMed] [Google Scholar]
- 27.Martin CA, Papadopoulos N, Chellew T, Rinehart NJ, Sciberras E. Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: Systematic review. Res Dev Disabil 2019;93:103463. [DOI] [PubMed] [Google Scholar]
- 28.Martin J, Hiscock H, Hardy P, Davey B, Wake M. Adverse associations of infant and child sleep problems and parent health: an Australian population study. Pediatrics. 2007;119(5):947–55. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–55. [DOI] [PubMed] [Google Scholar]
- 30.Fauroux B, Sacco S, Couloigner V, Amaddeo A, Ravel A, Prioux E, et al. Early detection and treatment of obstructive sleep apnoea in infants with Down syndrome: a prospective, non-randomised, controlled, interventional study. Lancet Reg Health Eur 2024;45:101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia J, Wical B, Wical W, Schaffer L, Wical T, Wendorf H, et al. Obstructive sleep apnea in children with cerebral palsy and epilepsy. Dev Med Child Neurol 2016;58(10):1057–62. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Sleep Medicine. The International Classification of Sleep Disorders – Third Edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2023. [Google Scholar]
- 33.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 2002;11(1):1–16. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz K Vulnerable Child Syndrome. Pediatr Rev 2019;40(6):313–5. [DOI] [PubMed] [Google Scholar]
- 35.Cai H, Chen P, Jin Y, Zhang Q, Cheung T, Ng CH, et al. Prevalence of sleep disturbances in children and adolescents during COVID-19 pandemic: a meta-analysis and systematic review of epidemiological surveys. Transl Psychiatry. 2024;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma M, Aggarwal S, Madaan P, Saini L, Bhutani M. Impact of COVID-19 pandemic on sleep in children and adolescents: a systematic review and meta-analysis. Sleep Med 2021;84:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


