Abstract
In the global nitrogen cycle, bacterial denitrification is recognized as the only quantitatively important process that converts fixed nitrogen to atmospheric nitrogen gas, N2, thereby influencing many aspects of ecosystem function and global biogeochemistry. However, we have found that a process novel to the marine nitrogen cycle, anaerobic oxidation of ammonium coupled to nitrate reduction, contributes substantially to N2 production in marine sediments. Incubations with 15N-labeled nitrate or ammonium demonstrated that during this process, N2 is formed through one-to-one pairing of nitrogen from nitrate and ammonium, which clearly separates the process from denitrification. Nitrite, which accumulated transiently, was likely the oxidant for ammonium, and the process is thus similar to the anammox process known from wastewater bioreactors. Anaerobic ammonium oxidation accounted for 24 and 67% of the total N2 production at two typical continental shelf sites, whereas it was detectable but insignificant relative to denitrification in a eutrophic coastal bay. However, rates of anaerobic ammonium oxidation were higher in the coastal sediment than at the deepest site and the variability in the relative contribution to N2 production between sites was related to large differences in rates of denitrification. Thus, the relative importance of anaerobic ammonium oxidation and denitrification in N2 production appears to be regulated by the availability of their reduced substrates. By shunting nitrogen directly from ammonium to N2, anaerobic ammonium oxidation promotes the removal of fixed nitrogen in the oceans. The process can explain ammonium deficiencies in anoxic waters and sediments, and it may contribute significantly to oceanic nitrogen budgets.
The global nitrogen cycle is characterized by the maintenance of a small pool of fixed, or combined, nitrogen in continuous exchange with the huge reservoir of atmospheric dinitrogen, N2. Microbial nitrogen fixation and industrial nitrogen fixation are the most important processes that convert N2 to fixed nitrogen, while bacterial denitrification, i.e., anaerobic reduction of nitrate to dinitrogen coupled to the oxidation of organic matter or reduced iron or sulfur species (40, 48), is recognized as the only significant process that regenerates N2 (18, 33). It is the balance between these source and sink terms that controls the global inventory of fixed nitrogen. Because of the frequent role of nitrogen as a limiting nutrient for primary production (47), processes that affect the availability of fixed nitrogen are important regulators of ecosystem function and global biogeochemistry (8, 33). For example, denitrification counteracts the effects of eutrophication in coastal waters (37), and on a global scale, glacial to interglacial variations in denitrification rates have been suggested to explain changes in nitrogen availability in the oceans, thereby forcing global rates of net primary production and concentrations of atmospheric CO2 (7, 39).
In current marine nitrogen budgets, bacterial denitrification is the prevailing sink for nitrogen and most denitrification takes place in the seafloor (7, 13, 27). Here, nitrogen for denitrification is delivered mainly through a combination of ammonification, the degradative release of ammonium from organic material, and nitrification, the oxidation of ammonium via nitrite to nitrate (3, 37). The overall regulation of this ammonification-nitrification-denitrification pathway is relatively complex due to the involvement of both aerobic and anaerobic microbial processes (37), and consequently, the relative importance of N2 as a product depends strongly on the environmental conditions (27).
There are indications, however, that ammonium may be oxidized anaerobically in marine sediments with either nitrate or manganese oxides as the oxidant (1, 2, 12, 17, 25, 36). By shortcutting the pathway to N2, such reactions could support a more efficient conversion of fixed nitrogen than can be achieved through the well-established sequence of reactions. Ammonium oxidation coupled to manganese reduction has been suggested to occur in continental shelf sediments either through a direct reaction to N2 (25) or through oxidation to nitrate followed by denitrification (1, 17). Neither of these processes has been demonstrated directly in natural sediments, however, and they could not be detected in anoxic, manganese oxide-rich sediment with a sensitive 15N-labeling technique (44).
Ammonium oxidation coupled to nitrate reduction has been indicated by deficiencies of ammonium relative to other products of organic matter mineralization in anoxic, nitrate-containing pore waters of deep-sea sediments, and was hypothesized to have the following stoichiometry (2, 12, 36):
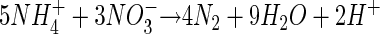 |
(1) |
A possible mechanism explaining these observations has been provided by the discovery in a wastewater bioreactor of bacterially catalyzed ammonium oxidation with nitrite in the anammox process (28, 46):
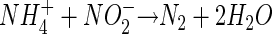 |
(2) |
Concomitant organotrophic nitrate reduction is the source of the nitrite, and N2 forms through the pairing of one nitrogen atom from ammonium with one from nitrite (46). The anammox reaction supports autotrophic growth in so far uncultured bacteria from the order Planctomycetales (34, 41; reviewed in references 20, 21, and 24). While this process and organisms performing it have been described from a second wastewater treatment facility (34), there are, to our knowledge, no reports of their occurrence or activity in natural environments.
Here we provide the first quantification of anaerobic ammonium oxidation in natural environments. We used nitrogen-15-labeled compounds to investigate the role of nitrate in ammonium oxidation in sediments from the Baltic-North Sea transition. These sites were chosen because their biogeochemistry has been described in detail and because they are representative of a wide range of continental shelf sediments.
MATERIALS AND METHODS
Sediment cores were collected from three sites with minimal disturbance of the surface by using a multiple corer (Skagerrak) or by divers (Aarhus Bay) and kept at bottom water temperature (Table 1). Sediment biogeochemistry and microbial processes have been studied in detail at all three sites (6, 22, 23, 43-45).
TABLE 1.
Characteristics of freshly collected sediments
| Location | Water depth (m) | Bottom water temp (°C) | O2 penetration deptha (cm) | NO3− penetration depthb (cm) | Max NO3− concnc (μM) |
|---|---|---|---|---|---|
| Aarhus Bay station 6 | 16 | 6.5 | 0.3 ± 0.1 | 1.2 | 19 |
| Skagerrak S6 | 380 | 6 | 0.6 ± 0.3 | 1.5-3 | 15 |
| Skagerrak S9 | 695 | 5.5 | 1.7 ± 0.6 | 2-4 | 35 |
Mean ± standard deviation; n = 3 or 4.
Range of two or three profiles.
Highest NO3− concentrations observed in pore water profiles. The maxima were typically located near the depth of oxygen depletion.
Incubations.
The depth of oxygen penetration was determined with oxygen microsensors (30), and the nitrate distribution in the pore water was analyzed after centrifugation of sediment sectioned into 0.3- to 0.5-cm depth intervals. Based on these determinations, sediment to be used in incubations was subsampled from the zone of nitrate consumption, i.e., from around the oxic-anoxic interface to the depth of nitrate depletion (Table 1), with all handling taking place in an anoxic glove bag in a room held at bottom water temperature. Incubations, pore water extraction, and analyses followed the procedures described in reference 44. In brief, sediment was incubated unamended or after addition of combinations of Na15NO3, 15NH4Cl (both >99.5% 15N), and their unlabeled analogues at 50 to 200 nmol/cm3 from ∼100 mM stock solutions. The sediment was incubated with no headspace in portions of, typically, 200 cm3 in gas-tight plastic bags stored in an N2 atmosphere (16) and sampled in the anoxic glove bag. For pore water extraction, sediment from the bags was loaded into 15-ml polypropylene centrifuge tubes that were filled completely and centrifuged. After centrifugation, 2 ml of the supernatant for N2 analysis was transferred through a butyl rubber septum to a 6.6-ml glass vial (Exetainer; Labco, High Wycombe, United Kingdom) that contained 50 μl of 50% ZnCl2 as an inhibitor of microbial activity and had previously been flushed with He or Ar. After N2 analysis, these samples were also used for determination of the isotopic composition of NO3−. The remaining supernatant was filtered through a 0.45-μm-pore-size cellulose acetate filter and stored frozen for analysis of NH4+, NO2−, NO3−, and the isotopic composition of NO2− plus NO3−. Additional incubations (Skagerrak S9 September 2000 and Aarhus Bay) were made by distribution of 9-ml portions of sediment into 12.6-ml Exetainers in which the headspace was flushed with He. These vials were sampled after vigorous shaking followed by centrifugation. For N2 analysis, a 2-ml sample was withdrawn from the headspace and transferred to a 6.6-ml Exetainer filled with He-degassed water while excess water was drained off through a hypodermic needle. The pore water supernatant was filtered for other analyses as described above. The two incubation procedures produced similar results.
Analysis.
Concentrations of 29N2 and 30N2 were determined by isotope ratio mass spectrometry (Robo-Prep-G+ in line with TracerMass; Europa Scientific, Crewe, United Kingdom) and calculated as excess above their natural abundances (44). Concentrations were corrected for an analytical recovery of 50 to 80%. The N isotope composition of NH4+ was analyzed after conversion to N2 with hypobromite (31), and the isotopic composition of NO3− plus NO2− was determined after conversion to NO with acidic V3+ (5), followed by reduction to N2 on hot Cu in line with the mass spectrometer. Nitrite concentrations were determined spectrophotometrically (14), and NO3− plus NO2− was determined through chemiluminescence after reduction to NO (5). Ammonium was determined by flow injection analysis with conductivity detection (15) or spectrophotometrically by the salicylate-hypochlorite method (4). To determine changes in the total inventory of NH4+ in the sediment, soluble NH4+ concentrations were multiplied by 1 + KNH4, where KNH4 is the adsorption coefficient for NH4+, determined from the initial recovery of added 15NH4+ in the pore water (44).
Calculations.
Two approaches were used to quantify the pathways of N2 production. In incubations with added 15NO3− and unlabeled NH4+, calculations were based on the accumulation of 29N2 and 30N2. Consistent with the results of the 15NH4+ experiments (see below), we assumed that N2 produced through anaerobic ammonium oxidation consisted of one nitrogen atom from NO3− and one from NH4+. Thus, in incubations with 15NO3−, the process produced 28N2 and 29N2 only, and this at a ratio corresponding to the labeling of the NO3− pool, as follows:
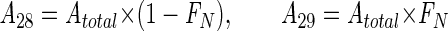 |
(3) |
where Am represents the production of N2 of mass m through anaerobic ammonium oxidation, Atotal = A28 + A29, and FN is the fraction of 15N in NO3− (typically, ∼0.95). Denitrification was assumed to produce 28N2, 29N2, and 30N2 through random isotope pairing (29) as follows:
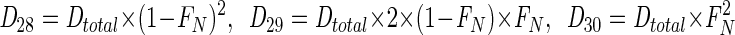 |
(4) |
where Dm denotes production of N2 through denitrification. With Pm representing the total, measured production of N2 of mass m, it follows from equations 3 and 4 and D30=P30 that
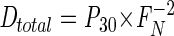 |
(5) |
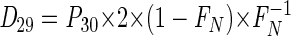 |
(6) |
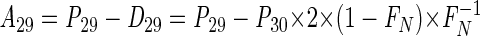 |
(7) |
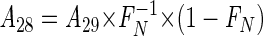 |
(8) |
and, by addition of equations 7 and 8,
 |
(9) |
In incubations with 15NH4+, anaerobic ammonium oxidation was quantified as follows from the consumption of 15NH4+: Atotal = −Δ[15NH4+]corr × FA−1, where Δ[15NH4+]corr is the change in the 15NH4+ total sediment concentration corrected for the consumption due to assimilation as determined in separate incubations and FA is the fraction of 15N in NH4+. Nitrate consumption coupled to ammonium oxidation was assumed to be equivalent to Atotal, and N2 production from denitrification was calculated as follows from the excess consumption of NO3− plus NO2−: Dtotal = 0.5 × (−Δ[NOx−] − Atotal), where Δ[NOx−] is the change in the concentration of NO3− plus NO2−. In Aarhus Bay, ammonium oxidation was too small for accurate quantification by this approach in incubations with 15NH4+. Instead, the process was quantified from 15N-N2 production and related to denitrification as determined in parallel incubations with 15NO3−.
The relative contribution of anaerobic ammonium oxidation to N2 production in each experiment was calculated based on three or more samplings during the incubation. At Skagerrak S9, rates of the process were determined from linear accumulations over several samplings while rates for Skagerrak S6 and Aarhus Bay are minimum estimates because all of the nitrate and nitrite was typically consumed at the second sampling in time course experiments, allowing only a two-point determination.
RESULTS AND DISCUSSION
Detection and stoichiometry.
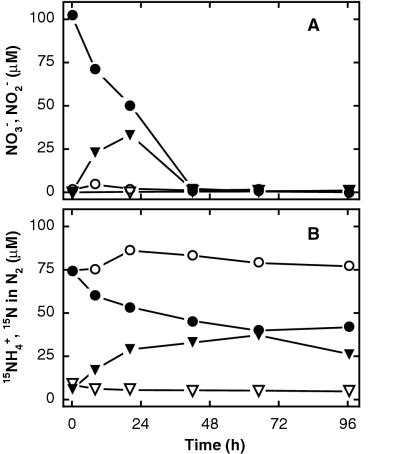
Nitrate-dependent ammonium oxidation was observed at all three sites. Thus, when 15N-ammonium and unlabeled nitrate were added to the sediment, 15N-ammonium and nitrate were consumed and 15N-labeled N2 was produced without delay, while in control incubations without added nitrate there was no production of 15N-labeled N2 after the rapid initial depletion of the native nitrate pool (Fig. 1 and 2). Nitrate, although present in concentrations of >10 μM in freshly collected sediment (Table 3), was consumed during the initial handling of the sediment for the control incubations and was depleted before completion of the first sampling (Fig. 1 and data not shown). In these controls, small amounts of 15N-labeled N2 that had already accumulated at the first sampling after the addition of 15N-ammonium indicated that ammonium oxidation was also coupled with the consumption of the small native nitrate pool (Fig. 1 and 2). A slight consumption of 15N-ammonium in the absence of nitrate could be attributed to bacterial nitrogen assimilation (3). Nitrite accumulated transiently during nitrate consumption, and production of 15N-labeled N2 and enhanced 15N-ammonium consumption continued until nitrate and nitrite were depleted (Fig. 1).
FIG. 1.
Anaerobic ammonium oxidation coupled to nitrate reduction in anoxic marine sediment from Skagerrak station S9 amended with 15N-ammonium and unlabeled nitrate (filled symbols) compared to a control with 15N-ammonium only (open symbols). Panels: A, time course of concentrations of nitrate (circles) and nitrite (triangles); B, time course of concentrations of 15N-ammonium (circles) and 15N in N2 (triangles). Examples are from May 2000, 1- to 3-cm depth, and are representative of three experiments.
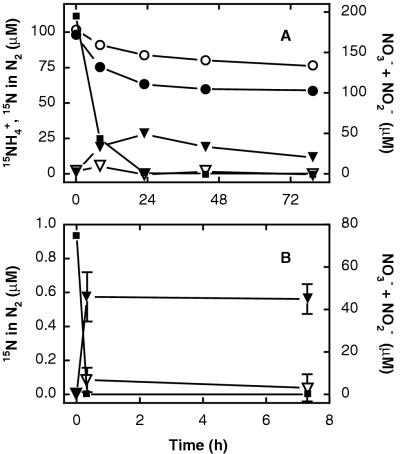
FIG. 2.
Anaerobic ammonium oxidation coupled to nitrate reduction in anoxic marine sediments amended with 15N-ammonium and unlabeled nitrate (filled symbols), compared to controls with 15N-ammonium only (open symbols), from Skagerrak station S6 (A) and Aarhus Bay (B). Time courses of the combined concentrations of nitrate and nitrite (squares, right axis), 15N-ammonium (circles, left axis, Skagerrak S6 only), and 15N in N2 (triangles, left axis). Nitrate and nitrite concentrations are only shown for nitrate-amended incubations. In incubations without nitrate addition, endogenous nitrate and nitrite were depleted at the first sampling time. Examples are from 1- to 4-cm and 0.8- to 1.3-cm depths at Skagerrak S6 and Aarhus Bay, respectively. Results in panel A are representative of three experiments, and the error bars in panel B represent the standard deviations of three parallel incubations.
Incubations with 15N labeling of ammonium, nitrate, or both revealed that during nitrate-dependent ammonium oxidation, nitrogen originating from ammonium was paired with nitrogen originating from nitrate. Thus, in incubations with 15N-ammonium and unlabeled nitrate, more than 97% of the 15N transformed to N2 was paired with 14N to form N2 with a molecular weight of 29 (29N2) (Fig. 3A). The 15N percentage in ammonium decreased during the experiments due to dilution with 14N from ammonification (3, 44), but ammonium was labeled with more than 50% 15N during most of the reaction, so with random pairing of ammonium-N into N2, less than 50% of the transformed 15N would have been combined with 14N to form 29N2 (29, 46). In combination with the nitrate dependence of ammonium oxidation, this indicated that nitrate was the source of 14N in 29N2.
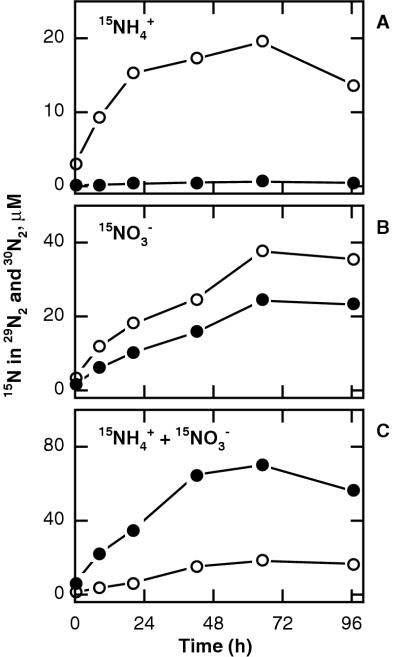
FIG. 3.
Accumulation of 15N in 29N2 (open circles) and 30N2 (filled circles) during parallel anoxic incubations of marine sediment amended with 15N-ammonium and unlabeled nitrate (same experiment as Fig. 1) (A), 15N-nitrate (B), and 15N-ammonium and 15N-nitrate (C). Percentages of labeling with 15N-ammonium and 15N-nitrate were 30 to 80% and 90%, respectively. During all experiments, unlabeled ammonium was generated through ammonification. Examples are from May 2000, Skagerrak S9, 1- to 3-cm depth, and are representative of two or more replicates.
Similarly, in incubations with 15N labeling of nitrate by more than 90% and no labeling of ammonium, up to 70% of the 15N-nitrate converted to N2 was found in 29N2 (Fig. 3B). This distribution deviated strongly from that expected during conventional denitrification, which under these conditions would produce less than 10% 29N2 through random isotope pairing (equation 4; reference 29), and the distribution indicated that most of the 14N in 29N2 originated from ammonium generated through ammonification. A lesser production of 30N2 in the 15N-nitrate experiments revealed that denitrification proceeded simultaneously with the anaerobic ammonium oxidation. Further confirmation of the pairing of nitrogen from nitrate and ammonium was obtained through 15N labeling of both nitrate and ammonium (Fig. 3C), which resulted in the highest yield of 30N2. In this case, the lesser production of 29N2 resulted mainly from the reaction of endogenous 14N-ammonium with 15N-nitrate.
The transformations of nitrate and ammonium to N2 are accompanied by oxidation state changes of −5 and +3, respectively (equation 1). Thus, the observed one-to-one combination of nitrogen from these two sources in N2 requires more reducing power than is delivered by ammonium and is not in agreement with the stoichiometry previously proposed for anaerobic ammonium oxidation in marine sediments (equation 1). Instead, the observed patterns of isotope pairing were similar to those reported for the anammox process (equation 2), including the slight production of 30N2 from 15N-ammonium (Fig. 3A) (46). Transient nitrite accumulation was observed in the incubations at both Skagerrak sites (Fig. 1), and it is likely that nitrite was the oxidant for ammonium while the reduction of nitrate to nitrite was coupled to the oxidation of organic matter in the sediments, in analogy to the transformations occurring in the wastewater reactor that supports anammox (41, 46). We are aware of no other processes that may occur in marine sediments and can explain our results well (see also reference 46). At Aarhus Bay, all nitrate and nitrite was consumed at the first sampling only 20 min after nitrate had been added (Fig. 2) and nitrite production could therefore not be demonstrated. Hence, we cannot exclude the possibility that anaerobic ammonium oxidation at this site was limited by low nitrite concentrations.
Further investigations are needed to determine whether organisms related to the known anaerobic ammonium oxidizers carry out anaerobic ammonium oxidation in the marine environment. Critical conditions for the anammox process in the wastewater reactor include a temperature of at least 20°C (42) and a cell density of the active bacterium of 1010 to 1011/cm3 (41). In the sediments explored here, anaerobic ammonium oxidation proceeded at 6 to 7°C and total bacterial numbers were about 109/cm3, which is typical of fine-grained marine sediments (32, 35). These differences suggest that different organisms are active in the two systems. Bacteria related to the anammox planctomycetes, but of unknown physiology, have been detected in natural environments, including marine sediments (24, 34), and are potential candidates for anaerobic ammonium oxidation in the seafloor.
Quantitative significance.
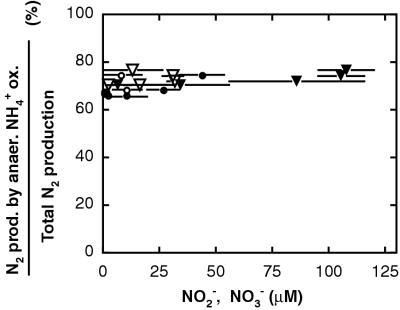
Rates of anaerobic ammonium oxidation and denitrification were calculated either based on the different patterns of isotope pairing observed during the two processes or from the measured consumption of 15N-ammonium (see Materials and Methods). The relative importance of the two processes showed no dependence on the concentration of either nitrate or nitrite as these changed during the incubations (Fig. 4). Hence, we expect that the elevated nitrate and nitrite concentrations during our experiments did not enhance the importance of ammonium oxidation with nitrate. Likewise, similar contributions of the two processes were observed in experiments in which ammonium was added and those in which ammonium was only available from ammonification in the sediment (Fig. 5).
FIG. 4.
Relative contribution of anaerobic ammonium oxidation to total N2 production (anaerobic ammonium oxidation plus denitrification) as a function of the concentration of nitrate (filled symbols) or nitrite (open symbols) during two parallel experiments from Skagerrak S9 with the initial addition of 15N-nitrate to ∼50 nmol cm−3 (circles) and ∼100 nmol cm−3 (triangles). The different nitrate concentrations correspond to the consumption of the initial amendment, while nitrite concentrations reflect accumulation followed by depletion (Fig. 1). The contribution of anaerobic ammonium oxidation was calculated for each period between two consecutive samplings and is plotted as a function of the average concentration of nitrate or nitrite during the same period. Error bars indicate the concentration change between the consecutive samplings.
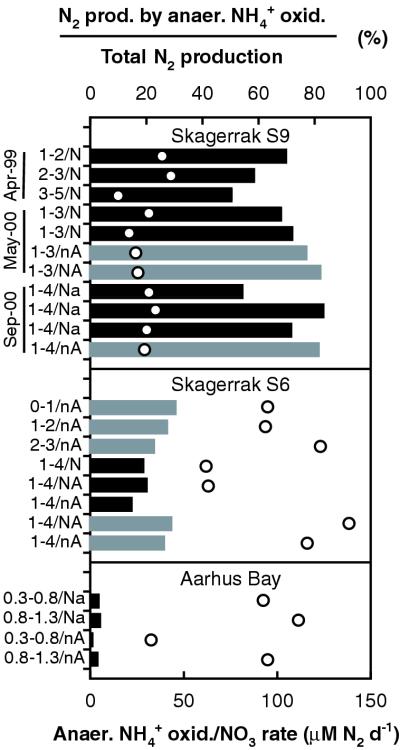
FIG. 5.
Summary of experiments from three locations. Columns and upper axis: relative contribution of anaerobic ammonium oxidation to N2 production calculated from isotope pairing (black) or 15NH4+ consumption (gray). Circles and lower axis: rate of N2 production through coupled ammonium oxidation-nitrate reduction. Conditions for each experiment summarized next to each column include the sediment depth interval (centimeters), the combination of nitrate and ammonium added (n and N, nitrate; a and A, ammonium; uppercase, 15N labeled; lowercase, unlabeled), and the time of sampling at Skagerrak S9. Sediment from Skagerrak S6 and Aarhus Bay was sampled in autumn 2000.
Anaerobic ammonium oxidation was an important process in the offshore sediments, accounting for, on average, 24% of the total N2 production at Skagerrak S6 and 67% of the total N2 production at Skagerrak S9 (Fig. 5). In the coastal bay, the contribution was only 2% and denitrification completely dominated N2 production. The results were reproducible at each site, including three visits to Skagerrak S9 over a 1.5-year interval. The strong decrease in the relative significance of anaerobic ammonium oxidation from Skagerrak S9 over S6 to Aarhus Bay occurred in spite of a concurrent increase in the rate of the process (Fig. 5); i.e., the decrease resulted from large differences in the volume-specific rate of denitrification. The variation in denitrification rates can be explained by differences in the availability of substrates for the denitrifying bacteria. Thus, rates of organic carbon mineralization in the surface sediment, reflecting organic substrate availability, vary similarly among the three sites (6, 23, 43, 44). The variation in the demand for electron acceptors between the sediments was also reflected in the oxygen penetration depth (Table 1). In addition to organic matter, ferrous iron and, possibly, iron sulfides may serve as substrates for denitrification (40). Consistent with this, our denitrification rates varied in parallel with concentrations of ferrous iron and solid reduced sulfur species in the surface sediment of the three sites (6, 44). Since the reduced inorganic substrates are themselves products of organic carbon oxidation (6), the extent to which they accumulate in the sediment will depend on the intensity of this process. Thus, although the relative significance of the potential substrates for denitrification is not known, the reactivity of organic matter can be used as a general key to substrate availability.
At Skagerrak S9, denitrifiers may have been further limited by competition for substrates from manganese-reducing bacteria, the activity of which is stimulated by a high manganese oxide content of the sediment (6, 44). Ammonium, however, is not a substrate for manganese reduction at this site (44). Thus, anaerobic ammonium oxidation may be particularly favored over denitrification as a sink for nitrate in manganese oxide-rich sediments. However, high concentrations of manganese oxides are not required for substantial contributions from the process, as demonstrated at S6, where manganese oxide concentrations are low (6).
The source of ammonium in the sediments is the degradation of organic matter (3). At all three sites, the average rates of anaerobic ammonium oxidation (S9, 30 μM day−1; S6, 99 μM day−1; Aarhus Bay, 83 μM day−1; Fig. 1) were higher than the rates of ammonium accumulation in the pore water measured in anoxic, nitrate-free incubations of sediment from the same depth intervals as studied here: 5 to 21 μM day−1 at Skagerrak S9 (44), 23 to 57 μM day−1 at Skagerrak S6 (6), and 15 to 32 μM day−1 at Aarhus Bay (B. Thamdrup, unpublished results). Thus, when nitrate or nitrite is available, anaerobic ammonium oxidation has the potential to consume a large fraction of the ammonium that is produced in the sediments.
Biogeochemical considerations.
In terms of the rates of benthic respiration and the associated redox zonation, sites S6 and S9 together delineate a range of conditions representative of wide areas of the seafloor on the continental shelves and upper slopes (e.g., see references 26 and 43). Based on a good correlation between rates of benthic respiration and N2 production in the North Atlantic (38), we also expect representative N2 fluxes from these sediments. Using the availability of reactive organic matter as a key to the relative importance of denitrification and coupled ammonium oxidation-nitrate reduction, as discussed above, we consequently expect the new process to contribute substantially to N2 production in such areas. The process may indeed be even more widespread, since it can explain the ammonium deficiencies observed in the anoxic, nitrate-containing zone of hemipelagic and pelagic sediments (2, 12, 36), and it is likely to contribute to similar deficiencies in oxygen-deficient waters such as those of the Black Sea (9, 10).
The anaerobic oxidation of ammonium to N2, as described here, adds a shunt to the conventional sequence of nitrogen transformations in sediments, which facilitates the removal of fixed nitrogen as N2. In current models of benthic nitrogen transformations, the ammonification-nitrification-denitrification pathway from organic nitrogen to N2 involves the production of both ammonium and nitrate, either of which may escape the sediment (3, 27). With coupled ammonium oxidation-nitrate reduction in function, ammonium may be consumed in the anoxic zone of the sediment, thus reducing its chance of escape. Furthermore, the process produces twice as much N2 as does denitrification per molecule of nitrate or nitrite consumed, which augments N2 production in sediments in which nitrification rates limit the supply of nitrate and nitrite. Thus, the process may help explain the very efficient conversion of ammonium to N2, which is typically inferred for shelf sediments, and which has hitherto been attributed solely to tight coupling of nitrification and denitrification (11, 19, 37).
Denitrification in shelf sediments constitutes one of the largest sinks for fixed nitrogen in current marine budgets (7, 27). Our results suggest that anaerobic ammonium oxidation could be of comparable importance. Budgets of fixed nitrogen in the oceans suggest a current imbalance due to an excess of denitrification over source terms (8). Because of the role of nitrogen as a limiting nutrient, the disparity has potential implications for primary production and thereby for oceanic CO2 uptake. Our finding of an additional sink for fixed nitrogen appears to further exacerbate this imbalance. Global denitrification rates are, however, very loosely constrained (7, 8, 13, 27). Hence, in future revisions of global marine N2 production, coupled ammonium oxidation-nitrate reduction should be included specifically and the regulatory characteristics of the process should be considered in studies of the dynamics of the nitrogen cycle.
Acknowledgments
We thank the masters, crews, and scientific parties of the cruises onboard R/V Arne Tiselius, R/V Gunnar Thorson, and R/V Dana for assistance during sampling. We thank Egon B. Frandsen, Anna Haxen, Tanja Quottrup, Lilian Salling, and Peter Søholt for technical assistance; Søren Rysgaard and Lars Peter Nielsen for fruitful discussions; and Don Canfield and two anonymous reviewers for comments on the manuscript.
Cruises were sponsored by the EU Large Scale Facility at Kristineberg Marine Research Station and by the Danish National Research Council. Bo Thamdrup was supported by the Danish National Research Foundation through the Danish Center for Earth System Science.
REFERENCES
- 1.Aller, R. C. 1990. Bioturbation and manganese cycling in hemipelagic sediments. Philos. Trans. R. Soc. Lond. A Math. Phys. Sci. 331:51-58. [Google Scholar]
- 2.Bender, M., R. Jahnke, R. Weiss, W. Martin, D. T. Heggie, J. Orchardo, and T. Sowers. 1989. Organic carbon oxidation and benthic nitrogen and silica dynamics in San Clemente Basin, a continental borderland site. Geochim. Cosmochim. Acta 53:685-697. [Google Scholar]
- 3.Blackburn, T. H., and K. Henriksen. 1983. Nitrogen cycling in different types of sediments from Danish waters. Limnol. Oceanogr. 28:477-493. [Google Scholar]
- 4.Bower, C. E., and T. Holm-Hansen. 1980. A salicylate-hypochlorite method for determining ammonia in seawater. Can. J. Fish. Aquat. Sci. 37:794-798. [Google Scholar]
- 5.Braman, R. S., and S. A. Hendrix. 1989. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium(III) reduction with chemiluminescence detection. Anal. Chem. 61:2715-2718. [DOI] [PubMed] [Google Scholar]
- 6.Canfield, D. E., B. B. Jørgensen, H. Fossing, R. Glud, J. Gundersen, N. B. Ramsing, B. Thamdrup, J. W. Hansen, L. P. Nielsen, and P. O. J. Hall. 1993. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 113:27-40. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, J. P. 1994. Carbon export from continental shelves, denitrification and atmospheric carbon dioxide. Cont. Shelf Res. 14:547-576. [Google Scholar]
- 8.Codispoti, L. A. 1995. Is the ocean loosing nitrate? Nature 376:724. [Google Scholar]
- 9.Codispoti, L. A., and J. P. Christensen. 1985. Nitrification, denitrification and nitrous oxide cycling in the eastern tropical South Pacific Ocean. Mar. Chem. 16:277-300. [Google Scholar]
- 10.Codispoti, L. A., G. E. Friederich, J. W. Murray, and C. M. Sakamoto. 1991. Chemical variability in the Black Sea: implications of continuous vertical profiles that penetrated the oxic/anoxic interface. Deep-Sea Res. I 38(Suppl. 2A):S691-S710. [Google Scholar]
- 11.Devol, A. H. 1991. Direct measurements of nitrogen gas fluxes from continental shelf sediments. Nature 349:319-321. [Google Scholar]
- 12.Emerson, S., R. Jahnke, M. Bender, P. Froelich, G. Klinkhammer, C. Bowser, and G. Setlock. 1980. Early diagenesis in sediments from the Eastern Equatorial Pacific. I. Pore water nutrient and carbonate results. Earth Planet. Sci. Lett. 49:57-80. [Google Scholar]
- 13.Galloway, J. N., W. H. Schlesinger, H. Levy, I. I., A. Michaels, and J. L. Schnoor. 1995. Nitrogen fixation: anthropogenic enhancement-environmental response. Global Biogeochem. Cycles 9:235-252. [Google Scholar]
- 14.Grasshoff, K. 1983. Determination of nitrite, p. 139-142. In K. Grasshoff, M. Ehrhardt, and K. Kremling (ed.), Methods of seawater analysis, 2nd ed. Verlag Chemie, Weinheim, Germany.
- 15.Hall, P. O. J., and R. C. Aller. 1992. Rapid small-volume flow injection analysis for ∑CO2 and NH4+ in marine and freshwaters. Limnol. Oceanogr. 37:1113-1119. [Google Scholar]
- 16.Hansen, J. W., B. Thamdrup, and B. B. Jørgensen. 2000. Anoxic incubation of sediment in gas-tight plastic bags: a method for biogeochemical process studies. Mar. Ecol. Prog. Ser. 208:273-280. [Google Scholar]
- 17.Hulth, S., R. C. Aller, and F. Gilbert. 1999. Coupled anoxic nitrification/manganese reduction in marine sediments. Geochim. Cosmochim. Acta 63:49-66. [Google Scholar]
- 18.Jaffe, D. A. 2000. The nitrogen cycle, p. 322-342. In M. C. Jacobson, R. J. Charlson, H. Rodhe, and G. H. Orians (ed.), Earth system science. Academic Press, San Diego, Calif.
- 19.Jenkins, M. C., and W. M. Kemp. 1984. The coupling of nitrification and denitrification in two estuarine sediments. Limnol. Oceanogr. 29:609-619. [Google Scholar]
- 20.Jetten, M. S. M., S. Logemann, G. Muyzer, L. A. Robertson, S. De Vries, M. C. M. Van Loosdrecht, and J. G. Kuenen. 1997. Novel principles in the microbial conversion of nitrogen compounds. Antonie van Leeuwenhoek 71:75-93. [DOI] [PubMed] [Google Scholar]
- 21.Jetten, M. S. M., M. Strous, K. T. van de Pas-Schoonen, J. Schalk, U. G. J. M. van Dongen, A. A. van de Graaf, S. Logemann, G. Muyzer, M. C. M. van Loosdrecht, and J. G. Kuenen. 1999. The anaerobic oxidation of ammonium. FEMS Microbiol. Rev. 22:421-437. [DOI] [PubMed] [Google Scholar]
- 22.Jørgensen, B. B. 1996. Case study—Aarhus Bay, p. 137-154. In K. Richardson and B. B. Jørgensen (ed.), Eutrophication in coastal marine ecosystems, vol. 52. American Geophysical Union, Washington, D.C. [Google Scholar]
- 23.Jørgensen, B. B. 1996. Material flux in the sediment, p. 115-135. In K. Richardson and B. B. Jørgensen (ed.), Eutrophication in coastal marine ecosystems, vol. 52. American Geophysical Union, Washington. D.C. [Google Scholar]
- 24.Kuenen, J. G., and M. S. M. Jetten. 2001. Extraordinary anaerobic ammonium-oxidizing bacteria. ASM News 67:456-463. [Google Scholar]
- 25.Luther, G. W., III, B. Sundby, B. L. Lewis, and P. J. Brendel. 1997. Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim. Cosmochim. Acta 61:4043-4052. [Google Scholar]
- 26.Middelburg, J. J., K. Soetaert, and P. M. J. Herman. 1997. Empirical relationships for use in global diagenetic models. Deep-Sea Res. I 44:327-344. [Google Scholar]
- 27.Middelburg, J. J., K. Soetaert, P. M. J. Herman, and C. H. R. Heip. 1996. Denitrification in marine sediments: a model study. Global Biogeochem. Cycles 10:661-673. [Google Scholar]
- 28.Mulder, A., A. A. van de Graaf, L. A. Robertson, and J. G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16:177-184. [Google Scholar]
- 29.Nielsen, L. P. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Ecol. 86:357-362. [Google Scholar]
- 30.Revsbech, N. P. 1989. An oxygen electrode with a guard cathode. Limnol. Oceanogr. 34:474-478. [Google Scholar]
- 31.Risgaard-Petersen, N., S. Rysgaard, and N. P. Revsbech. 1995. Combined microdiffusion-hypobromite oxidation method for determining nitrogen-15 isotope in ammonium. Soil Sci. Soc. Am. J. 59:1077-1080. [Google Scholar]
- 32.Sahm, K., B. J. MacGregor, B. B. Jørgensen, and D. A. Stahl. 1999. Sulphate reduction and vertical distribution of sulphate-reducing bacteria quantified by rRNA slot-blot hybridization in a coastal marine sediment. Environ. Microbiol. 1:65-74. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger, W. H. 1997. Biogeochemistry, 2nd ed. Academic Press, San Diego, Calif.
- 34.Schmid, M., U. Twachtmann, M. Klein, M. Strous, S. Juretschko, M. Jetten, J. W. Metzger, K.-H. Schleifer, and M. Wagner. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23:93-106. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt, J. L., J. W. Deming, P. A. Jumars, and R. G. Keil. 1998. Constancy of bacterial abundance in surficial marine sediments. Limnol. Oceanogr. 53:976-982. [Google Scholar]
- 36.Schultz, H. D., A. Dahmke, U. Schinzel, K. Wallmann, and M. Zabel. 1994. Early diagenetic processes, fluxes, and reaction rates in sediments of the South Atlantic. Geochim. Cosmochim. Acta 58:2041-2060. [Google Scholar]
- 37.Seitzinger, S. P. 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol. Oceanogr. 33:702-724. [Google Scholar]
- 38.Seitzinger, S. P., and A. E. Giblin. 1996. Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry 35:235-260. [Google Scholar]
- 39.Shaffer, G. 1990. A non-linear climate oscillator controlled by biogeochemical cycling in the ocean: an alternative model of quaternary ice age cycles. Clim. Dyn. 4:127-143. [Google Scholar]
- 40.Straub, K. L., M. Benz, B. Schink, and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strous, M., J. A. Fuerst, E. H. M. Kramer, S. Logemann, G. Muyzer, K. T. Van de Pas-Scoonen, R. Webb, J. G. Kuenen, and M. S. M. Jette. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446-449. [DOI] [PubMed] [Google Scholar]
- 42.Strous, M., J. G. Kuenen, and M. Jetten. 1999. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 65:3248-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thamdrup, B. 2000. Microbial manganese and iron reduction in aquatic sediments. Adv. Microb. Ecol. 16:41-84. [Google Scholar]
- 44.Thamdrup, B., and T. Dalsgaard. 2000. The fate of ammonium in anoxic manganese oxide-rich marine sediment. Geochim. Cosmochim. Acta 64:4157-4164. [Google Scholar]
- 45.Thamdrup, B., H. Fossing, and B. B. Jørgensen. 1994. Manganese, iron, and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim. Cosmochim. Acta 58:5115-5129. [Google Scholar]
- 46.Van de Graaf, A. A., A. Mulder, P. De Bruijn, M. S. M. Jetten, L. A. Robertson, and J. G. Kuenen. 1995. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61:1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitousek, P. M., and R. W. Howarth. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87-115. [Google Scholar]
- 48.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Ecol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]