Abstract
Objective:
Evaluate benefits and harms of prophylactic intraoperative methylprednisolone in subpopulations undergoing infant heart surgery.
Design:
Subpopulation analyses of The Steroids to Reduce Systemic Inflammation after Infant Heart Surgery trial, a double blind randomized placebo-controlled trial.
Setting:
24 congenital heart centers.
Patients:
Infants (< 1 year old) undergoing heart surgery with cardiopulmonary bypass. Patients stratified by Society of Thoracic Surgeons–European Association for Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) Mortality Category, age, gestational age, and presence of chromosomal or syndromic diagnosis (CSD).
Interventions:
Methylprednisolone (30mg/kg) versus placebo administered into cardiopulmonary bypass pump-priming fluid.
Measurements and Main Results:
Six postoperative outcomes: steroid use, acute kidney injury (AKI), thrombosis, infections, prolonged mechanical ventilation, peak blood glucose levels, and insulin exposure. 1200 patients received methylprednisolone or placebo. Beneficial effects associated with methylprednisolone included reduced use of postoperative hydrocortisone in neonates (OR 0.39[0.25-0.60]), both STAT Category groups (1-3: OR 0.64[0.46-0.89]; 4-5: OR 0.57[0.34-0.97]), term infants (OR 0.63[0.47-0.83]), and those without CSD (OR 0.63[0.46-0.86]). Methylprednisolone was associated with lower thrombosis occurrence among neonates (OR 0.37 [0.16-0.87]) and term infants (OR 0.38 [0.19-0.75]). Adverse associations included increased thrombosis among premature infants (P=0.005), increased AKI among neonates (OR 1.55 [1.02-2.37]) and those following STAT Category 1-3 operations (OR 1.34 [1.02-1.75]), and increased peak blood glucose levels and insulin exposure (all subgroups, P<0.001). No increase in overall infection or reduction in prolonged mechanical ventilation with methylprednisolone.
Conclusion:
Both beneficial and adverse associations were observed with prophylactic methylprednisolone. Reduction in postoperative hydrocortisone administration and absence of increased infection rates are arguments favoring prophylactic methylprednisolone use. Methylprednisolone was associated with increased peak blood glucose levels and a neutral to harmful association with odds of AKI. These data suggest certain subpopulations may benefit from prophylactic intraoperative methylprednisolone without significant harm.
Trial Registration:
NCT03229538, STeroids to REduce Systemic Inflammation After Infant Heart Surgery (STRESS)
Keywords: congenital heart disease, cardiac surgery, critical care, methylprednisolone
Introduction
Congenital heart disease (CHD) surgery carries significant morbidity, particularly during the operative admission.(1) Multiple interventions to mitigate risk have been studied, with varying results.(2–10) One avenue of interest has been the use of perioperative glucocorticoids to reduce systemic inflammation following cardiopulmonary bypass.(2–7) The Steroids to Reduce Systemic Inflammation after Infant Heart Surgery (STRESS) trial randomized 1200 infants undergoing cardiac surgery with cardiopulmonary bypass to receive prophylactic intraoperative methylprednisolone versus placebo.(2) The primary analysis showed no difference between placebo and methylprednisolone groups in the occurrence of the hierarchical composite outcome, which included death, transplant, and thirteen postoperative complications. We sought to evaluate benefits and harms associated with prophylactic intraoperative methylprednisolone in select subpopulations with respect to postsurgical sequelae and adverse events commonly associated with steroids.
Materials and Methods
Parent Study
The STRESS trial was a prospective, multicenter, double-blind, randomized, placebo-controlled trial within an existing registry with enrollment from 24 CHD centers across the United States (NCT03229538).(2) The study protocol was approved by the Vanderbilt University institutional review board (IRB #170859; Steroids to Reduce Systemic Inflammation after Infant Heart Surgery, 6/20/2017) and each participating site.(11) Procedures were in accordance with the ethical standards of the Helsinki Declaration of 1975. Informed consent was obtained for each patient as part of the enrollment procedure. Block randomization was performed by site to ensure equal allocation at each participating center. Infants were randomized to receive methylprednisolone (30mg/kg) or placebo. The study drug was administered into the cardiopulmonary-bypass priming fluid.
Subgroups and Outcomes
STRESS participants were neonates and infants (< 1 year-old) undergoing heart surgery with cardiopulmonary bypass. For the purposes of these analyses, they were categorized based on Society of Thoracic Surgeons–European Association for Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) Mortality Category(12) (Categories 1-3 and 4-5), age (neonate [0-30 days] and non-neonate [31-365 days]), premature birth (gestational age at birth < and ≥ 37 weeks) and any chromosomal or syndromic diagnosis (CSD) as defined by the 2016 Society of Thoracic Surgeons (STS) model specifications. Individuals with corrected gestational age < 37 weeks at time of surgery were excluded from enrollment. Thus, prematurity dichotomization is based on birth gestational age and not reflective of corrected gestational age at time of surgery. Missing data were handled by multiple imputation for stratifying variables (STAT Category n=3, prematurity status n=3, and CSD n=1).
The parent manuscript demonstrated a benefit for the primary composite outcome, a hierarchical composite outcome including death, transplant, and thirteen postoperative complications, within certain subgroups; therefore, we elected to target specific outcomes for subgroup analyses. Outcomes of interest, within these analyses, were defined in the STRESS trial study protocol and included postoperative steroid administration within 72 hours following surgery, acute kidney injury (AKI), prolonged postoperative mechanical ventilatory support, postoperative infectious complication, postoperative thrombosis, peak blood glucose levels within 72 hours following surgery, and postoperative insulin use within 24 hours after surgery. Steroid use following surgery was defined as having received hydrocortisone, dexamethasone, or methylprednisolone (excluding intraoperative study drug) within 72 hours after operation. Administered steroid doses were recorded as indexed for weight. AKI was defined as serum creatinine > 1.5x baseline or >= 0.3mg/dL increase above baseline (stage I), serum creatinine >= 2.0 - < 3x baseline (stage II), and serum creatinine >=3x baseline (stage III). Prolonged mechanical ventilatory support was defined as > 7 days postoperatively. Pneumonia, sepsis, deep wound infections, mediastinitis, and endocarditis were collapsed into a single overall infection outcome. Thrombosis occurring in central or peripheral veins, arteries, pulmonary arteries, systemic artery-to-pulmonary artery shunts were collated into a composite thrombosis outcome. Definitions for these outcomes were consistent with the STS registry. Peak blood glucose levels (< 72 hours after surgery) and postoperative insulin use (<24 hours after surgery) were recorded. Preoperative renal dysfunction was defined as serum creatinine >=2x baseline or need for dialysis and was adjusted for in the secondary analysis of AKI.
Statistical Methods
Stratified analyses were performed to assess the association between methylprednisolone and outcomes of interest. The four stratifications carried out were by 1) STAT Mortality Category (1-3 and 4-5), 2) age at operation (0-30 days and 31-365 days), 3) birth gestational age (< and ≥ 37 weeks), and 4) presence of CSD. The primary outcomes of interest were use of postoperative steroids, AKI, thrombosis, infections, prolonged mechanical ventilation, peak postoperative blood glucose levels, and insulin administration. Secondary analyses were performed to test for associations between prophylactic intraoperative methylprednisolone and 1) cumulative dose of hydrocortisone and 2) severity of AKI (stage I-III).
Pearson’s X2 and Wilcoxon rank-sum test were used to compare categorical and continuous variables respectively. Logistic regression was used to calculate odds ratio and 95% confidence intervals for binary outcomes of interest. Ordered logistical regression was performed to test the association between methylprednisolone administration with 1) severity of AKI and 2) cumulative hydrocortisone dose.
An odds ratio for postoperative hydrocortisone exposure adjusting for STAT Mortality Category, weight, and cardiopulmonary bypass time was calculated. STAT Mortality Category and cardiopulmonary bypass time were adjusted for in steroid dosing analysis, as doses were indexed for weight. Previous adult studies have demonstrated an association between hyperglycemia and AKI(13,14); therefore, an adjusted analysis for methylprednisolone and AKI when controlling for peak blood glucose levels and preoperative renal dysfunction was performed. A two-tailed P-value < 0.05 was considered significant. Given the exploratory nature of these subpopulation analyses, the authors elected not to perform correction for multiple comparisons. Statistical analyses were completed with Stata version 16 (Stata Corporation, College Station, Texas, USA).
Results
Study Population
The 1200 patients who received placebo or methylprednisolone as assigned were included in these analyses (eFigure 1 in Supplement 1). Balance between the treatment and placebo arms was maintained for each subgroup. Demographic and baseline clinical features are shown in eTable 1 in Supplement 1.
Postoperative Steroids
In the entire cohort, treatment with methylprednisolone was associated with reduction in both overall postoperative steroid use (OR 0.73 [0.58-0.93], P=0.012) and postoperative hydrocortisone use (OR 0.62 [0.47-0.81], P<0.001). Use of any postoperative steroid was reduced in patients receiving methylprednisolone compared to placebo for those undergoing STAT Category 4-5 operations (OR 0.55 95% CI [0.31-0.99], P=0.045), neonates (OR 0.39 [0.23-0.65], P<0.001), those of term gestational age (OR 0.67 [0.52-0.88], P=0.003), and those without CSD (OR 0.72 [0.54-0.97], P=0.029; Figures 1, 2, 3, and 4, eTable 2–5 in Supplement 1). Hydrocortisone (295/479 [62%]) and dexamethasone (196/479 [41%]) were the most common postoperative steroids with few receiving methylprednisolone (21/479 [4%]); 33 (7%) patients had dual steroid administration. Postoperative hydrocortisone use was lower, with treatment versus placebo, in both STAT Mortality Category groups (1-3: OR 0.64 [0.46-0.89], P=0.007; 4-5: OR 0.57 [0.34-0.97], P=0.036), neonates (OR 0.39 [0.25-0.60], P<0.001), term gestational age infants (OR 0.63 [0.47-0.83], P=0.001), and those without CSD (OR 0.63 [0.46-0.86], P=0.004; Figure 1–4). When adjusting for STAT Mortality Category, weight, and cardiopulmonary bypass time, the aforementioned groups retained their statistical significance and those of premature gestational age and those with chromosomal or syndromic diagnoses also had lower odds of hydrocortisone exposure (eTable 6 in Supplement 1). Cumulative postoperative hydrocortisone dose (mg/kg) was lower with intraoperative methylprednisolone compared to placebo among neonates (β= −3.00 [−4.30 to −1.69], P<0.001) and those undergoing STAT Mortality Category 4-5 operations (β= −2.86 [−4.60 to −1.12], P=0.001). Several additional subgroups showed modest decreases in postoperative hydrocortisone doses with intraoperative methylprednisolone (eTable 7 in Supplement 1). Descriptive data regarding center specific hydrocortisone use is included in eTable 8 in Supplement 1. Postoperative dexamethasone use did not differ between placebo and methylprednisolone groups for any of the eight subgroups (eTable 2–5 in Supplement 1). Overall cumulative dose of any steroid in prednisolone equivalents, including intraoperative methylprednisolone and any postoperative steroid dose within 72 hours following operation, was increased with the treatment arm (β=1.10 [0.54-1.66] mg/kg, P <0.001). Neonates (β=0.46 [−0.76 to 1.68]) and those undergoing STAT Category 4-5 operations (β=0.29 [−1.56 to 2.14]) showed no significant change in cumulative steroid dose with intraoperative methylprednisolone compared to placebo, while other subgroups did show an increase (eTable 9 in Supplement 1).
Figure 1: Outcomes for STAT Mortality Categories.

Univariate unadjusted odds ratio for the postoperative event listed on y-axis for respective subgroup. Odds ratio > 1 reflects an increase in event given prophylactic intraoperative methylprednisolone. **Indicates statistical significance (P<0.05). MV, mechanical ventilation.
Figure 2: Outcomes for Age Categories.

Univariate unadjusted odds ratio for the postoperative event listed on y-axis for respective subgroup. Odds ratio > 1 reflects an increase in event given prophylactic intraoperative methylprednisolone. **Indicates statistical significance (P<0.05). MV, mechanical ventilation.
Figure 3: Outcomes for Birth Gestational Age Categories.
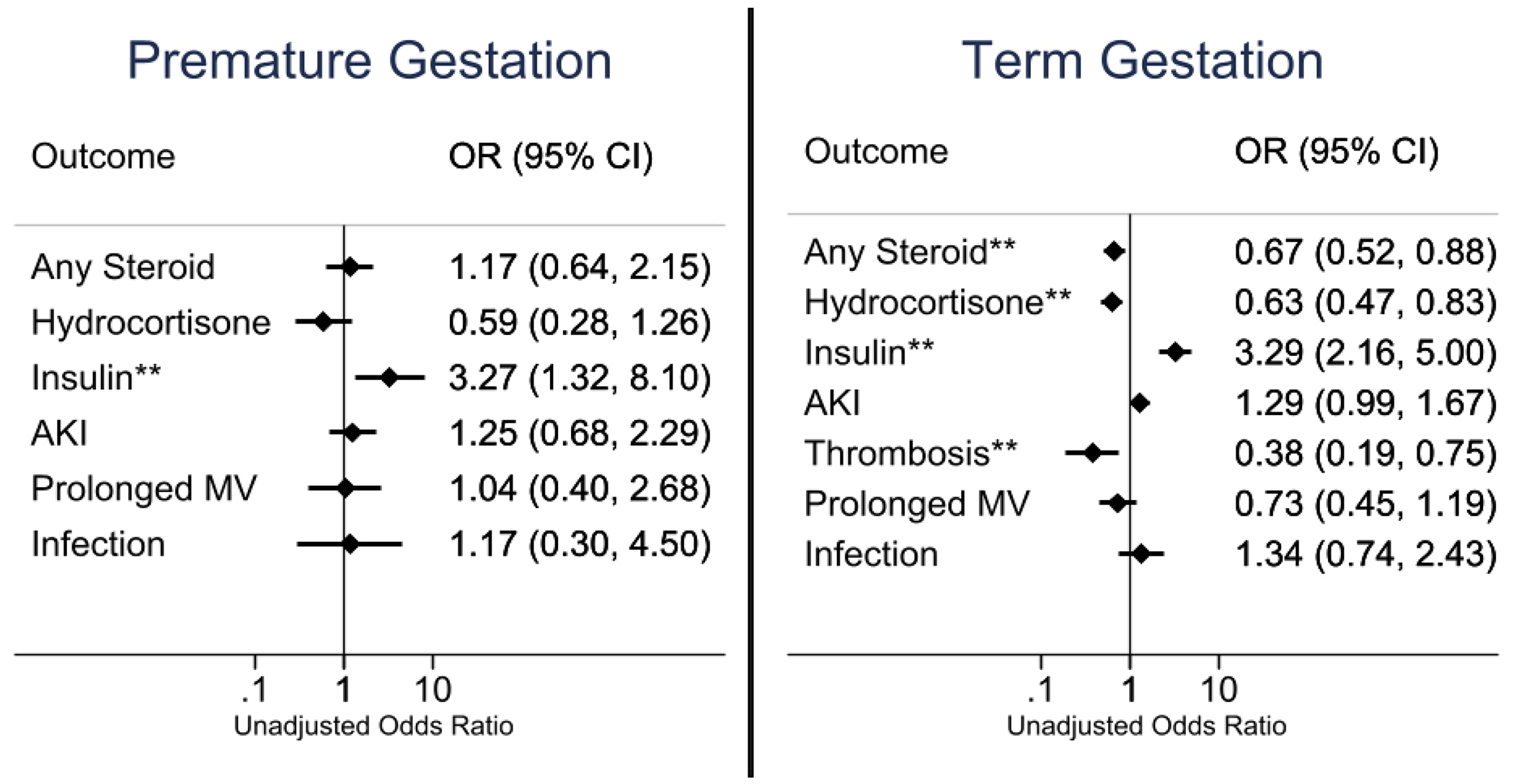
Univariate unadjusted odds ratio for the postoperative event listed on y-axis for respective subgroup. Odds ratio > 1 reflects an increase in event given prophylactic intraoperative methylprednisolone. The odds ratio for thrombosis in premature gestational age infants could not be calculated as there were no events in the placebo group. **Indicates statistical significance (P<0.05). MV, mechanical ventilation.
Figure 4: Outcomes for Presence and Absence of a Chromosomal or Syndromic Diagnosis.

Univariate unadjusted odds ratio for the postoperative event listed on y-axis for respective subgroup. Odds ratio > 1 reflects an increase in event given prophylactic intraoperative methylprednisolone. **Indicates statistical significance (P<0.05). MV, mechanical ventilation.
Postoperative Sequalae
Prophylactic intraoperative methylprednisolone treatment was associated with a higher peak glucose level (P<0.001) and an increase in insulin exposure (P<0.01; Figure 1–4, eTable 2–5 in Supplement 1) for all groups. Methylprednisolone was associated with AKI in those undergoing STAT Mortality Category 1-3 operations (OR 1.34 [1.02-1.75], P=0.036) and in neonates (OR 1.55 [1.02-2.37], P=0.039), but not associated with severity of AKI for any group (eTable 10 in Supplement 1). After adjusting for preoperative renal dysfunction and peak postoperative blood glucose levels, the association between prophylactic methylprednisolone and presence of AKI following STAT Mortality Category 1-3 operations (OR 1.12 [0.84-1.50], P=0.397) and among neonates (OR 1.40 [0.89-2.18], P=0.143) was no longer significant. In assessment of the entire cohort, peak blood glucose associated with presence of AKI (AKI: median 209 [169-265] vs. no-AKI: 185 [154-231] mg/dL, mean difference 26 [18-35] mg/dL, P<0.001), but not with AKI severity (1: 215 [170-267], 2: 202 [169-249], 3: 225 [165-305] mg/dL, OR 1.000 [0.998 to 1.002] P=0.983). Among those who were not treated with insulin, peak blood glucose remained higher in the treatment arm compared to placebo (174 [148-213]mg/dL vs. 198 [165-246], mean difference 29 [33 to 36] mg/dL, P<0.001), but there was no difference in presence or severity of AKI (OR 1.05 [0.96 to 1.60]; eTable 11 in Supplement 1).
Prophylactic methylprednisolone had mixed associations with rates of thrombosis (Figure 1–4). There were 54 thrombotic events in 51 patients; central (n=29) and peripheral (n=21) venous thromboses were the most common with arterial thrombi rare (n=4). There was a reduction in thrombosis for neonates (OR 0.37 [0.16-0.87], P=0.018) and those of term gestational age (OR 0.38 [0.19-0.75], P=0.004), but an increase in thrombosis for premature gestational age infants (8/100 vs. 0/93, P=0.005). All thrombotic events in the premature subgroup were in central or peripheral veins. There was no reduction in mechanical ventilation for any group with methylprednisolone prophylaxis (Figure 1–4). There was no difference in overall infections between the methylprednisolone and placebo arms for any group (Figure 1–4). Among patients randomized to intraoperative methylprednisolone, receiving additional postoperative steroids did not significantly increase the odds of a postoperative infectious complication (OR 2.03 [0.97-4.37], P=0.061).
Discussion
In these subgroup analyses of the STRESS trial, prophylactic intraoperative methylprednisolone demonstrated both benefits and adverse events for infants undergoing heart surgery with cardiopulmonary bypass. For several subgroups, prophylactic intraoperative methylprednisolone was associated with a reduction in postoperative steroids, and in particular hydrocortisone. Adverse events observed in the primary analysis included higher peak glucose levels and secondarily increased insulin exposure in the treatment arm for all groups and increased AKI in neonates as well as those undergoing STAT Mortality Category 1-3 operations. There was a mixed association observed on thrombosis occurrence. Notably, there was no significant increase in overall infections with the methylprednisolone group across any of the eight subpopulations.
The association between prophylactic intraoperative methylprednisolone and reduced use of postoperative steroids, specifically hydrocortisone, may suggest a lower incidence of postoperative low cardiac output syndrome (LCOS). The precise interpretation of the term LCOS varies across institutions, as does the threshold for intervention and preferred management strategies. Thus, a uniform metric was unavailable for inclusion in these analyses. However, LCOS occurs in up to 25% of patients following CHD surgery. Postoperative steroids, most commonly hydrocortisone, are used as treatment.(15,16) The authors acknowledge the challenge in designating LCOS, particularly in a multicenter study where practice pattern variation could influence incidence. The authors propose the timeframe (< 72 hours after operation) and clinical milieu would suggest hydrocortisone is being used to treat hemodynamic instability. Thus, the authors hypothesize the reduction in postoperative hydrocortisone represents improved postoperative hemodynamics. Within the entire cohort, prophylactic methylprednisolone was strongly associated with a reduction in hydrocortisone exposure postoperatively (OR 0.62 [0.47-0.81]) suggesting it is unlikely that this represents a chance finding. The neonate group demonstrated the greatest reduction of postoperative hydrocortisone exposure (OR 0.39 [0.25-0.60]). Prior works have studied the benefits of perioperative glucocorticoids particularly in the neonatal CHD population.(3–5,7,8) Graham et al. (n=176) reported no difference in the occurrence of LCOS between perioperative methylprednisolone and placebo. The study did demonstrate a lower peak vasoactive inotropic score and a trend towards reduction in postoperative steroids (27% vs 40%) though not statistically significant in the methylprednisolone group.(3) Vasoactive inotropic score was not collected as part of the STRESS trial and thus, unavailable for our analyses. Prior investigation into single versus dual methylprednisolone dosage also yielded no difference in the incidence of LCOS.(4) In the present analyses, the STRESS cohort demonstrated reduction in hydrocortisone and overall steroid use in those with higher mortality risk (STAT 4-5) operations, term gestations, and no CSD. Among those undergoing STAT Category 1-3 operations, there was lower postoperative hydrocortisone use with methylprednisolone. Several subgroups also demonstrated a reduction in cumulative hydrocortisone dose with intraoperative methylprednisolone. The largest reduction observed in neonates and those undergoing STAT Category 4-5 operations. These two groups also showed no difference in cumulative steroid exposure, including treatment dose, within 72 hours following operation between treatment versus placebo, suggesting perioperative steroids, whether intra- or postoperative, are indicated in these groups. Furthermore, no significant difference was noted in postoperative dexamethasone administration, for any subgroup, suggesting the benefit of intraoperative methylprednisolone is most likely in postoperative hemodynamics.
Previous works have demonstrated contrasting evidence for overall infections with intraoperative steroid prophylaxis.(3,5,8) Our analyses demonstrated no increase in overall infections with methylprednisolone prophylaxis. Overall, the neutral effect of methylprednisolone on infections is encouraging for the use of prophylactic intraoperative methylprednisolone in infants undergoing cardiac surgery.
Postoperative insulin exposure and peak blood glucose levels were increased in the treatment arm for all subgroups, consistent with the parent manuscript.(2) Nadir glycemic levels were not available for these analyses to comment on the occurrence or severity of hypoglycemia. Mixed data exist on hyperglycemia and insulin requirement following perioperative glucocorticoid use.(3,5,17,18) Graham et al. did not demonstrate a difference in insulin exposure or peak blood glucose levels postoperatively.(3) Both studies used a similar dose and timing of methylprednisolone in the respective trials. The only apparent difference is the longer timeframe over which blood glucose levels were measured in the STRESS trial (72 vs 36 hours). Other pediatric and adult trials demonstrate findings consistent with our own.(5,17,18) Hyperglycemia following CHD surgery has mixed effects on clinical outcomes.(19–23) A randomized controlled trial previously assessed the benefit of tight glycemic control versus standard care following CHD surgery. Those in the intervention arm had no difference in postoperative infections, mortality, or length of stay and hypoglycemia was a rare occurrence.(19) The authors acknowledge this is in the setting of a clinical trial and prevention of hypoglycemia may be more challenging in practice; however, if insulin can be used without adverse events and transient hyperglycemia can be tolerated without worse clinical outcomes, concern for postoperative glycemic control may not be prohibitive to prophylactic steroid use.
In the unadjusted analysis, methylprednisolone was associated with increased AKI in neonates and those undergoing STAT Category 1-3 operations. After controlling for peak blood glucose and preoperative renal dysfunction, these associations were no longer significant. Additionally, peak blood glucose was significantly associated with AKI in the multivariable regression analysis. This suggests hyperglycemia, a sequela of steroid use, may be the driving force for developing AKI rather than steroid use itself. It must be noted that a universal postoperative insulin protocol was not in place during this trial. Thus, exposure and dosage of insulin was at the discretion of the clinical team. The secondary adjusted analysis demonstrated no difference in AKI presence or severity between treatment and placebo among those without insulin administration. Peak blood glucose levels were unsurprisingly higher in those receiving insulin. This information together suggests that in those with higher glucose levels, which met a non-standardized threshold for insulin administration, AKI was more common with methylprednisolone than placebo. Concurrently, among those with lower peak blood glucose levels, which were not treated with insulin, AKI incidence and severity were similar between the two arms of the study. As mentioned previously, tight prophylactic glycemic control rather than reactively treating significant hyperglycemia may mitigate the presence of AKI. Further investigation into this association and possible prevention through tighter glycemic control would be necessary to prove this hypothesis. Furthermore, the etiology of postoperative AKI is complex and may be related to additional features such as cardiopulmonary bypass induced inflammation, perioperative fluid shifts, and perioperative renal malperfusion. All of which are challenging to quantify and analytically adjust for.
Prematurity has been a well-established risk factor for postoperative complications.(24,25) Preterm neonates and infants did not demonstrate reduced overall steroid use in our cohort. The effect of prophylactic intraoperative methylprednisolone on infants with premature gestational ages is not well studied, as previous trials have excluded this population from enrollment.(3,5) In this study, prophylactic methylprednisolone use in premature infants, with corrected gestational age ≥ 37 weeks at time of surgery, was associated with an elevated incidence of postoperative thrombosis. There was a reduction in hydrocortisone use in the covariate-adjusted analysis. Individuals of term gestational age had a reduction in postoperative hydrocortisone use and thrombosis without an increase in postoperative complications with methylprednisolone. Reports from adult European registries suggest glucocorticoids are associated with increased rates of thrombosis with mechanistic investigations proposing increased levels of procoagulant factors and impaired fibrinolytic activity.(26–29) It remains challenging to decipher the contribution from underlying inflammatory disease versus steroid exposure for thrombosis development. The authors hypothesize that prematurity may be associated with inherent risk factors for thrombosis including smaller caliber vasculature, vasculature immaturity, and prior venous and arterial access. Overall, thrombosis detection rates align with previous CHD studies.(30–33) Those without CSD had a reduction in postoperative steroids including hydrocortisone without a difference in postoperative sequelae profile with methylprednisolone.
Overall limitations of this study are inherently similar to those outlined in the original STRESS trial manuscript, including use of registry data.(2) Given the multicenter collaboration of this work, variations in institutional practice patterns must be considered in the interpretation of these results. The majority of patients included underwent a lower STAT Mortality Category operation, perhaps incurring a lower risk for postoperative sequalae. Differences across participating centers in postoperative glucose monitoring and threshold for insulin administration could influence outcomes related to hyperglycemia. Protocols for intraoperative modified ultrafiltration and postoperative empiric vasoactive and inotropic support could impact the frequency of rescue hydrocortisone administration and other outcomes of interest. Furthermore, the decision to administer postoperative steroids was at the discretion of institutional practice. Collection of steroid dosing data beyond the initial 72 hours postoperatively would be interesting to evaluate if overall hospital steroid exposure is reduced with intraoperative steroids. Clinical metrics such as vasoactive inotropic scores were not available but would be prudent to include in future assessment of intraoperative steroids on postoperative hydrocortisone use. Data regarding the indication for postoperative steroid use were unavailable in this dataset, though national practice patterns suggest hydrocortisone is widely used to treat LCOS, while dexamethasone is most often used peri-extubation. Collection of vasoactive inotropic scores, mixed venous saturation (SvO2), cardiac index, and clinical indication for steroid use may provide further granularity to assess the occurrence of LCOS in future studies. Urine output data was not integrated into the definition of AKI for this study and would be prudent to include in future explorations. Postoperative arterial and venous line placement was at the discretion of institutional protocols. Particular line locations were not available in this dataset and may have contributed to rates of thrombosis. Protocols for thrombosis surveillance were not standardized between centers and may have affected detection rates of thrombosis.
Large datasets can produce small confidence intervals and thus, infer small clinical differences as being statistically significant. Furthermore, some outcomes of interest (e.g., thrombosis and infections) had low rates of occurrence, which should be factored into the interpretation of the reported findings. Low prevalence outcomes are susceptible to noise, which may affect statistical testing. The authors presented granular data, as available, to be transparent about composite outcomes analyzed. We encourage the reader to consider the statistical, but also clinical implications of our findings.
Conclusion
In these subgroup analyses of the STRESS randomized trial, which enrolled infants undergoing CHD surgery with cardiopulmonary bypass, prophylactic intraoperative methylprednisolone reduced the need for postoperative hydrocortisone, in several groups with the largest reduction seen in the neonatal and term gestational age groups. Increased postoperative peak blood glucose levels and insulin use were universal in all groups, while other adverse event profiles were mixed in these groups.
Supplementary Material
Key Points.
Question:
What are benefits and adverse events associated with prophylactic intraoperative methylprednisolone for subpopulations undergoing infant heart surgery?
Findings:
In this secondary analysis of a randomized clinical trial, neonates receiving prophylactic methylprednisolone were less likely to require postoperative hydrocortisone. There was no increase in overall infections with the treatment arm; however, methylprednisolone was associated with higher postoperative blood glucose levels in all subgroups.
Meaning:
Hydrocortisone is commonly used as a treatment for low cardiac output following cardiopulmonary bypass; therefore, the authors postulate lower odds of hydrocortisone exposure suggest improved postoperative hemodynamic stability with prophylactic intraoperative methylprednisolone.
Acknowledgments
The authors would like to acknowledge the effort across all sites which participated in the STRESS trial.
Funding
Supported by grants from the 2018 Burroughs Wellcome Fund Physician-Scientist Institutional Award to Vanderbilt University (1018894), the National Centers for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) (U01TR-001803-01 and U24TR-001608-03), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the NIH (U18FD-006298-02 and P50HD106446), the Trial Innovation Network (5U24TR001608-06, funded through NCATS), and the Pediatric Trials Network (HHSN275201800003I and HHSN27500001, funded through NICHD).
Copyright Form Disclosure:
Drs. Sunthankar, Hill, Jacobs, Li, Graham, Benscoter, Van Bergen, Scott, Anderson, and Kannankeril received support for article research from the National Institutes of Health (NIH). Dr. Hill’s institution received funding from the National Center for Advancing Translational Sciences (NCATS); He received funding from Bristol Myers Squibb and Johnson& Johnson. Dr. Jacobs’ institution received funding from the NCATS (U01TR001803). Drs. Van Bergen’s, Anderson’s, and Kannakeril’s institutions received funding from the NIH. Dr. Anderson’s institution received funding from the National Heart, Lung, and Blood Institute (R01HL140055) (UM1 4833193) and Autus Inc. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Non-Standard Abbreviations
- AKI
Acute kidney injury
- CHD
Congenital heart disease
- CSD
Chromosomal or syndromic diagnosis
- LCOS
Low cardiac output syndrome
- MV
Mechanical ventilation
- STAT
Society of Thoracic Surgeons–European Association for Cardio-Thoracic Surgery Congenital Heart Surgery
- STRESS
Steroids to Reduce Systemic Inflammation after Infant Heart Surgery
Footnotes
Conflicts of Interest:
Consultant for Autus Valve Technologies, Inc. (Anderson). Consultant for SpeialtyCare (J. Jacobs). For the remaining authors, no conflicts of interest are present.
Data Sharing
The deidentified patient data set will be provided upon reasonable request and approval by the STRESS investigators. Inquiries can be sent to jerry.kirchner@duke.edu.
References
- 1.Jacobs JP, Mayer JE Jr., Pasquali SK et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg 2019;107:691–704. [DOI] [PubMed] [Google Scholar]
- 2.Hill KD, Kannankeril PJ, Jacobs JP et al. Methylprednisolone for Heart Surgery in Infants - A Randomized, Controlled Trial. N Engl J Med 2022;387:2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham EM, Martin RH, Buckley JR et al. Corticosteroid Therapy in Neonates Undergoing Cardiopulmonary Bypass: Randomized Controlled Trial. J Am Coll Cardiol 2019;74:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham EM, Atz AM, Butts RJ et al. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. J Thorac Cardiovasc Surg 2011;142:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomivorotov V, Kornilov I, Boboshko V et al. Effect of Intraoperative Dexamethasone on Major Complications and Mortality Among Infants Undergoing Cardiac Surgery: The DECISION Randomized Clinical Trial. JAMA 2020;323:2485–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert SM, Borasino S, Dabal RJ, Cleveland DC, Hock KM, Alten JA. Postoperative Hydrocortisone Infusion Reduces the Prevalence of Low Cardiac Output Syndrome After Neonatal Cardiopulmonary Bypass. Pediatr Crit Care Med 2015;16:629–36. [DOI] [PubMed] [Google Scholar]
- 7.Suominen PK, Keski-Nisula J, Ojala T et al. Stress-Dose Corticosteroid Versus Placebo in Neonatal Cardiac Operations: A Randomized Controlled Trial. Ann Thorac Surg 2017;104:1378–1385. [DOI] [PubMed] [Google Scholar]
- 8.Pasquali SK, Hall M, Li JS et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation 2010;122:2123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons KS, Schlapbach LJ, Horton SB et al. Statistical analysis plan for the NITric oxide during cardiopulmonary bypass to improve Recovery in Infants with Congenital heart defects (NITRIC) trial. Crit Care Resusc 2021;23:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman TM, Wernovsky G, Atz AM et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. Am Heart J 2002;143:15–21. [DOI] [PubMed] [Google Scholar]
- 11.Hill KD, Baldwin HS, Bichel DP et al. Rationale and design of the STeroids to REduce Systemic inflammation after infant heart Surgery (STRESS) trial. Am Heart J 2020;220:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs ML, Jacobs JP, Thibault D et al. Updating an Empirically Based Tool for Analyzing Congenital Heart Surgery Mortality. World J Pediatr Congenit Heart Surg 2021;12:246–281. [DOI] [PubMed] [Google Scholar]
- 13.Kourek C, Georgopoulou M, Kolovou K et al. Intensive Care Unit Hyperglycemia After Cardiac Surgery: Risk Factors and Clinical Outcomes. J Cardiothorac Vasc Anesth 2024;38:162–169. [DOI] [PubMed] [Google Scholar]
- 14.Barmanray RD, Kyi M, Worth LJ et al. Hyperglycemia in hospital: an independent marker of infection, acute kidney injury & stroke for hospital inpatients. J Clin Endocrinol Metab 2024. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman TM, Wernovsky G, Atz AM et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107:996–1002. [DOI] [PubMed] [Google Scholar]
- 16.Flores S, FitzGerald MR, Iliopoulos I et al. An International Survey of Corticosteroid Use for the Management of Low Cardiac Output Syndrome. Pediatr Crit Care Med 2017;18:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieleman JM, Nierich AP, Rosseel PM et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 2012;308:1761–7. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock RP, Devereaux PJ, Teoh KH et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386:1243–1253. [DOI] [PubMed] [Google Scholar]
- 19.Agus MS, Steil GM, Wypij D et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med 2012;367:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballweg JA, Wernovsky G, Ittenbach RF et al. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg 2007;84:2052–8. [DOI] [PubMed] [Google Scholar]
- 21.Polito A, Thiagarajan RR, Laussen PC et al. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation 2008;118:2235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadhwani A, Asaro LA, Goldberg C et al. Impact of Tight Glycemic Control on Neurodevelopmental Outcomes at 1 Year of Age for Children with Congenital Heart Disease: A Randomized Controlled Trial. J Pediatr 2016;174:193–198 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates AR, Dyke PC 2nd, Taeed R et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med 2006;7:351–5. [DOI] [PubMed] [Google Scholar]
- 24.Costello JM, Polito A, Brown DW et al. Birth before 39 weeks’ gestation is associated with worse outcomes in neonates with heart disease. Pediatrics 2010;126:277–84. [DOI] [PubMed] [Google Scholar]
- 25.Costello JM, Pasquali SK, Jacobs JP et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation 2014;129:2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squizzato A, Gerdes VE, Ageno W, Buller HR. The coagulation system in endocrine disorders: a narrative review. Intern Emerg Med 2007;2:76–83. [DOI] [PubMed] [Google Scholar]
- 27.Johannesdottir SA, Horvath-Puho E, Dekkers OM et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52. [DOI] [PubMed] [Google Scholar]
- 28.Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–43. [DOI] [PubMed] [Google Scholar]
- 29.van Zaane B, Nur E, Squizzato A et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost 2010;8:2483–93. [DOI] [PubMed] [Google Scholar]
- 30.Sunthankar SD, Moore RP, Byrne DW et al. Assessment of the CLOT (children’s likelihood of thrombosis) real-time risk prediction model of hospital-associated venous thromboembolism in children with congenital heart disease. Am Heart J 2024;272:37–47. [DOI] [PubMed] [Google Scholar]
- 31.Abdelghani E, Cua CL, Giver J, Rodriguez V. Thrombosis Prevention and Anticoagulation Management in the Pediatric Patient with Congenital Heart Disease. Cardiol Ther 2021;10:325–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giglia TM, Witmer C, Procaccini DE, Byrnes JW. Pediatric Cardiac Intensive Care Society 2014 Consensus Statement: Pharmacotherapies in Cardiac Critical Care Anticoagulation and Thrombolysis. Pediatr Crit Care Med 2016;17:S77–88. [DOI] [PubMed] [Google Scholar]
- 33.Manlhiot C, Menjak IB, Brandao LR et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation 2011;124:1511–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified patient data set will be provided upon reasonable request and approval by the STRESS investigators. Inquiries can be sent to jerry.kirchner@duke.edu.


