Abstract
Histone deacetylases (HDACs) are epigenetic regulators frequently altered in cancer. Here we report that overexpression of HDAC1/2 occurs in Hepatocellular Carcinoma (HCC) patients, correlating with poor prognosis. We show that romidepsin, a class-I HDAC inhibitor, elicits a combinatorial perturbation of distinct molecular processes in HCC cells, altering lipid composition, mitotic spindle machinery, and levels of cell cycle/survival signals. Collectively, these alterations lead HCC cells to a vulnerable state, conferring dependency to receptor tyrosine kinase (RTK) signalling support. The cytostatic effects of romidepsin alone is converted into cytotoxicity by the RTK inhibitor cabozantinib in HCC models. We document that romidepsin+cabozantibib confers an immune-stimulatory profile in Alb-R26Met mouse models, with direct effects on primary human dendritic cell maturation in vitro. Our findings put forward the intricate crosstalk between epigenetics, metabolism, and immune response in cancer. The broad action of romidepsin on distinct cellular functions highlights its therapeutic potential for HCC treatment.
Subject terms: Targeted therapies, Hepatocellular carcinoma, Lipidomics
Targeting histone deacetylases (HDACs) alone has shown limited success in solid tumours. Here, authors report that the HDAC1/2 inhibitor romidepsin confers responsiveness to receptor tyrosine kinase inhibitors, with enhanced therapeutic effects in models of hepatocellular carcinoma, leading to tumour regression and an immune-stimulatory profile.
Introduction
The reversibility of epigenetic changes makes it possible to recondition the biological properties of cancer cells and their microenvironment, offering exceptional therapeutic opportunities. This can be achieved using epigenetic drugs, proved to be effective on cancer cells, with some of them already in clinical trials as anticancer treatments1,2. The increased expression and activity of histone deacetylases (HDACs) are among the most common epigenetic dysregulations found in cancer, and considered as key events in the development and progression of tumours3,4. The predominant function of HDACs is to regulate the levels of lysine acetylation in histone tails and other cellular proteins by erasing the acetyl group, as opposed to histone acetyltransferases (HATs). Loss of the acetyl group in histones leads to chromatin compaction, thus impacting gene transcription. In cancer, this event contributes to tumour suppressor gene silencing, modifying a set of genes collectively favouring oncogenic properties. The relevance of dysregulated HDACs in cancer is strengthened by the promising effects of HDAC inhibitors (HDACi) for anticancer treatments5–7. Some FDA-approved HDACi are used to treat haematological cancers8.
For solid tumours, the exploitation of HDACi for anticancer treatment is under preclinical and clinical investigation8–10. Such therapeutic opportunity is challenged by the fact that different HDACs are overexpressed in distinct cancer types11. This may imply a specificity in the alteration of HDAC subsets according to the type of cancer, and possibly even between patient subgroups. In such a scenario, targeting different HDACs may not be equivalent, since cancer cells may have selective vulnerabilities to specific HDAC blockage. Additionally, the specificity in HDAC targeting using selective HDACi could maximize effects on cancer cells, while minimizing side effects using pan-HDACi. As for most of the treatment options, HDACi as monotherapy have had limited success in clinical trials, particularly in solid tumours. Still, they provide opportunities to design treatments in combination with other anticancer agents to achieve additive/synergistic therapies12. Such potential combinatorial settings may enhance efficacy, while reducing toxicity and limiting the emergence of secondary resistance to treatment.
We explored the functionality of HDACs and vulnerability to their blockage in the context of hepatocellular carcinoma (HCC), the most frequent type of liver cancer, which keeps challenging researchers and oncologists for aggressiveness and resistance to available therapies13–15. HCC is truly exceptional among cancers, as illustrated by an expression pattern distinct from seventeen different cancer types analysed that substantially overlap16. This is consistent with the observation that treatments effective in other cancers have largely failed when applied to HCC17. The multi-receptor tyrosine kinase (RTK) inhibitors (RTKi), sorafenib, lenvatinib, cabozantinib, and regorafenib, have been for years the only treatment options for HCC patients, with an overall response rate of less than 10% (except for lenvatinib that reaches 18.8%)18, with some variations according to clinical trials, patient cohorts, and type of inhibitors used. Immune checkpoint inhibitors have been recently introduced in HCC clinical treatment, with an overall response rate between 15 to 20%. Still, in the first-line setting, immune checkpoint inhibitors (e.g., nivolumab as PD-1 blockage) did not improve the overall survival in randomised trials compared with sorafenib19. Combinatorial therapies lead to about 30% of overall response rate, with a remarkable 8% complete responses18. Based on the EASL Clinical Practice Guideline, RTKi are currently recommended as first-line therapy in case of contraindication to PD-L1 + VEGFR blockage, and as second-line therapy18. Thus, the use of therapies predominantly targeting the tumour microenvironment and the lack of cancer cell-directed therapy other than RTKi, limits considerably HCC treatment choices. The possibility to enhance the effectiveness of RTKi, and to switch RTKi cytostatic into cytotoxic effects, ideally in combination with FDA-approved anticancer agents, is among the desired therapeutic options.
The complexity of HCC disease resides in its extraordinary heterogeneity of molecular alterations, among other factors. Besides genetic mutations, epigenetic alterations have been extensively documented in HCC and some of them have been reported to directly impact HCC at the initiation phases, during evolution towards aggressiveness, and confer resistance to treatments. It is thought that epigenetic dynamics in liver function and disease are particularly important as the organ is constantly challenged to adapt to a variety of environmental changes, including diet, metabolites, circadian cues, microbiota, toxins, and infections. Collectively, while influencing regenerative processes of the liver, these stimuli render the epigenome particularly vulnerable and represent risk factors leading to HCC20–22. As for other cancer types, epigenetic alterations in liver cancer provide new putative biomarkers and therapeutic opportunities23–26.
Because of the functionality of HDAC mechanisms in HCC, strategies designed to target them with available agents have been explored in vitro and/or in preclinical studies24,27–29, as for other cancer types30,31. Based on promising outcomes, three clinical trials have been conducted to assess the effectiveness of HDACi in HCC patients, either alone32 or in combination with sorafenib33,34. Besides some encouraging data, the therapeutic benefit of HDACi must be evaluated together with other backbone treatments to highlight the best possible effects for HCC. Moreover, thorough evaluations are needed to identify the most promising combinatorial options among several available, although not equivalent, HDACi and RTKi agents.
In the present study, by analysing eight independent HCC cohorts, we illustrate a consistent upregulation of HDAC1 and HDAC2 in a large proportion of patients, associated with decreased overall survival. We explored the treatment effectiveness of romidepsin, a specific inhibitor of HDAC1 and HDAC2 (belonging to class-I HDACs), alone and in combination with RTKi. Romidepsin (Istodax®) is used in the clinic for the treatment of cutaneous T-cell lymphoma. Moreover, nearly 97 clinical studies are identified with romidepsin (https://clinicaltrials.gov/ct2/results?cond=Cancer&term=romidepsin&cntry=&state=&city=&dist), including 24 active phase trials for oncogenic indications with combined treatments for breast cancer (NCT02393794), pancreatic cancer (NCT04257448), prostate cancer (NCT00106418), solid tumours with liver dysfunction, lymphoma/leukaemia (NCT01638533), breast cancer, lymphoma (NCT03432741), besides lymphoma or myeloma (20 trials). Mechanistically, we provide collective evidence that romidepsin confers vulnerability of HCC cells by perturbing survival and cell cycle signals, lipid metabolism regulators, and the mitotic spindle machinery. Such a romidepsin-driven vulnerable state confers RTK signalling dependency to HCC.
Results
HDAC1 and HDAC2 are overexpressed in a subset of human HCC patients and high levels correlate with a worse prognosis
There are four classes of HDACs in humans, subdivided according to their expression, cellular location, structure, and mechanism of action3. Focussing on class-I HDACs, which include HDAC1, HDAC2, HDAC3, and HDAC8, we analysed their expression levels in HCC patients by revisiting five independent cohort’s databases: TCGA-LIHC, LICA-FR, LIRI-JP, GSE144269, and GSE14520. We found that both HDAC1 and HDAC2 are overexpressed in HCC as compared to normal liver samples in all five cohorts (Fig. 1a–e). The group of patients with high levels of HDAC1 or HDAC2 shows a significant decrease in overall survival and disease-free survival as compared to the low-expressing patient group, indicating that HDAC1 or HDAC2 upregulation correlates with worse prognosis (Fig. 1a, b). HDAC8 is upregulated in HCC tissue, whereas HDAC3 is either up- or down-regulated according to the analysed cohort (Fig. S1a–e). We found no significant correlation between HDAC3 and HDAC8 levels with overall survival or disease-free interval in TCGA-LIHC, and an inverse correlation of HDAC3 in the GSE14520 cohort (Fig. S1a, b). Interestingly, patients with concurrent high levels of HDAC1 and HDAC2 have poor clinical outcomes, highlighting the detrimental effect of high HDAC1/HDAC2 expression levels, both singly and together (Fig. 1f).
Fig. 1. Class-I HDAC expression levels in human HCC patients and correlation with overall survival and disease-free interval.
a, b HDAC1/2 gene expression (left) and curves showing the overall survival and disease-free survival (right), expressed as percentage of HCC patients with high (red) versus low (blue) HDAC1 and HDAC2 levels, in TCGA-LIHC (a; N (C) = 50, N(T) = 371, HDAC1 p < 0.0001, HDAC2 p = 0.0333) and GSE14520 (GPL571 and GPL3921; b; N (C) = 241, N(T) = 247, HDAC1 p < 0.0001, HDAC2 p < 0.0001) cohorts. c–e Boxplot and paired dot plot of HDAC1 and HDAC2 levels in LICA-FR (c; N (C) = 226, N(T) = 161, HDAC1 p < 0.0001, HDAC2 p < 0.0001), LIRI-JP (d; N (C) = 202, N(T) = 243, HDAC1 p < 0.0001, HDAC2 p < 0.0001), and GSE144269 (e; N (C) = 70, N(T) = 70, HDAC1 p < 0.0001, HDAC2 p < 0.0001) human HCC patient cohorts. f Curves showing overall survival (left) and disease-free survival (right) of human HCC patients (n = 247), expressed in percentages, with high (red) versus low (blue) levels of HDAC1/2 (top), and with high-HDAC1/low-HDAC2 (green) versus high-HDAC2/low-HDAC1 (orange; bottom). g mRNA levels of HDAC1 and HDAC2 in a cohort of HCC patients from the STORM clinical trial (GSE109211, n = 140) treated with sorafenib. h HDAC1 and HDAC2 levels expressed as fold change (FC) of FFPE samples from HCC-St.Joseph's cohort (n = 10). i HDAC1/2 expression in the HCC-NatCom cohort35, classified by Edmonson histological grading stages (n = 132). j Curves reporting the survival of patients with high (red) versus low (blue) HDAC1 or HDAC2 levels in the HCC-NatCom cohort (n = 132). k, l Dot plots showing levels of protein expression (k) and phosphorylation of different serine residues (l) of HDAC1 and HDAC2 in control (n = 33) and tumour (n = 186) samples from patient biopsies (HCC-NatCom cohort). a–f, k Dot-plot was analysed with two-tailed non-paired (a, b, c, d, k) or paired (e), Mann-Whitney. a, b, f, j Kaplan Meir was analysed with Logrank and/or Grehan-Breslow, exact p values indicated in the figures. g, i Two-tailed non-paired Kruskal-Wallis followed by Dunn’s multiple comparison. significance: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Box plots are represented as min-max, bars represent SEM, and notches represent median levels. C: Control liver tissue; T: tumour tissue. Source data are provided as a Source Data file.
Next, we analysed the expression of the class-I HDACs in a cohort of 140 HCC patients (GSE109211) enrolled in the STORM trial. The study was divided into two arms, with patients receiving as adjuvant treatment either placebo (73 patients) or sorafenib (67 patients). The end-point of the trial was the recurrence-free survival that was taken into account to segregate patients into responders and non-responders. We observed that patients classified as sorafenib responders, elicit significantly lower levels of both HDAC1/2 as compared to sorafenib non-responders (Fig. 1g). This was not the case for HDAC3 (Fig. S1f), confirming that lower levels of HDAC1/2 are associated to a better prognosis. We also observed that, in the placebo group, patients with lower levels of HDAC1/2 are associated to a better prognosis (Fig. 1g). Such correlation between HDAC1/2 levels and prognosis is noteworthy also considering that this turned out to be a negative trial in which no benefit to adjuvant sorafenib was observed. Results corroborate that HCC patients with lower levels of HDAC1/2 have a better clinical outcome, and that there is no correlation with the therapeutic effect of sorafenib.
We experimentally extended these studies on a human HCC patient cohort from the St. Joseph Hospital (HCC-St.Joseph cohort; Marseille, France) and found HDAC1 and/or HDAC2 mRNA upregulation in 8/10 patients (Fig. 1h, S1g; clinical data corresponding to these patients are reported in Fig. S1h). We further analysed transcriptomics data of the HCC-NatCom human patient cohort35 and found HDAC1 and HDAC2 overexpression in tumour biopsies compared with controls (healthy liver). Notably, HDAC1/2 levels significantly increase with Edmonson stages (higher levels of HDAC1/2 when histology determines an undifferentiated HCC, and hence more aggressive; Fig. 1i). Again, this is not the case for HDAC3 or HDAC8, for which there is an inverse trend (Fig. S1i). Supporting the correlation with aggressiveness, we also found significantly higher levels of HDAC1 and HDAC2 in patients with metastasis (Fig. S1j). Moreover, HCC patients from this cohort with high HDAC2 levels exhibit a lower overall survival compared with the low levels group (Fig. 1j, S1k), corroborating the above findings obtained from public databases. We further extended these studies by analysing proteomics data from the same HCC-NatCom patient cohort and found upregulated HDAC1 and HDAC2 protein levels in HCC patients compared with controls (Fig. 1k). Notably, several HDAC1/2 Serine residues related to their activation are phosphorylated in HCC patients compared with controls (Fig. 1l), which are indicative of HDAC1 and HDAC2 activity/function36–41. Altogether, these findings show high HDAC1 and HDAC2 levels in a large proportion of HCC patients from eight independent human cohorts, supporting the potential interest of targeting specifically HDAC1/2 in HCC.
Using the cBioportal, we next analysed gene alterations, including copy number and mutations, in a total of 1287 patients provided from 9 different studies and found alterations in only 2% of HCC patients (30/1287; HDAC1 in 0.9%; HDAC2 in 1.1%; HDAC3 in 0.3%; HDAC8 in 0.4%; Fig. S2a). Missense mutations are more frequent for HDAC1, HDAC2, and HDAC8, whereas heterogeneous mutations, including truncating, in-frame, and fusion, are present in HDAC3 (Fig. S2b, c). We observed that class-I HDACs have a lower number of mutations compared to other HDACs (Fig. S3a, b). When analysing the survival and disease-free survival of patients with at least one mutation among any HDAC gene versus patients without mutations, we observed worse outcomes with mutations (Fig. S3c). Overall, results indicate that genetic mutations of class-I HDACs are not prevalent in HCC tissues, in contrast to other cancer types in which somatic mutations correlate with loss of activity, rendering cancer cells more resistant to HDACi42. Moreover, all mutations found in HDACs were variants with unknown significance (VUS), but no putative mutations (drivers) were reported.
Finally, using the FunGeST Liver Cell Lines Database (https://lccl.zucmanlab.com/hcc/molecularFeatures/rnaExpression?index=1), we analysed gene expression levels of different HDACs in a panel of 34 human HCC cell lines. We found HDAC1 and HDAC2 consistently overexpressed in all HCC cell lines, whereas the other HDAC members are only slightly expressed, with levels changing in different cell lines (Fig. 2a). Together, these findings illustrate a consistent increase in the expression levels of HDAC1 and HDAC2 in human HCC patients, which correlates with worst prognosis.
Fig. 2. Response of human HCC cell lines to HDAC versus BET blockage.
a Heatmap showing the gene expression values for all HDACs (the class is specified on the left) in 32 human HCC cell lines, analysing data at http://zucmanlab.com/. b Heatmap showing the drug response of 32 human HCC cell lines to clinically relevant RTKi (orange) and epigenetic drugs (green), expressed as GI50, extracted from http://zucmanlab.com/. c Dot plot reporting drug response expressed as GI50 of the indicated human HCC cell lines to RTKi currently used in the clinic for HCC treatment (cabozantinib, lenvatinib, sorafenib, regorafenib). Data are presented as mean values +/- SEM. d Heatmap reporting cell viability after 48 h treatment with HDACi (romidepsin, EDOS-101, ACY957, panobinostat) and BETi (JQ1, OTX-015, mivebresib) at the indicated concentrations. Data are expressed as means of at least three independent experiments (scatter dot-plots are reported in Fig. S6 with corresponding statistics). Viability percentages are reported using a blue (high)-to-red (low) colour code (the scale depicted on the right is used as a reference in all viability studies). e Western blots of the reported proteins in the indicated human HCC cell lines non-treated (NT) and treated with romidepsin (0.03 µM) for 8 h and 24 h. Quantifications are reported in Fig. S7 with corresponding independent experiments and statistics. f Western blots displaying expression of the indicated proteins in JHH5 and HLE cells non-treated and treated with mivebresib (1 µM) for 8 h and 24 h (one independent experiment). Source data are provided as a Source Data file.
Romidepsin reduces the viability of a panel of human HCC cell lines and alters the expression levels of cell cycle and survival regulators
Over the last years, the identification of chemical compounds targeting epigenetic signals has increased their potential use as anticancer agents. This includes HDAC and bromodomain and extra-terminal motif (BET) inhibitors (Fig. S4a–c). Particularly for HDACi, a large number of clinical trials in different cancer types have been performed, and few in the context of HCC (Fig. S4c). By revisiting data available on https://lccl.zucmanlab.com/hcc/drugs in relation to cell sensitivity to RTKi and to the epigenetic drugs tested, we found that the majority of cells are resistant to both single treatments (Fig. 2b–c). We observed no correlation with expression levels of MET, FGFR4, and VEGFR2 in HCC cell lines when analysed in relation to mild sensitivity to RTKi (Fig. S5a, b). On the basis of the above data from human HCC patients, we tested the effectiveness of agents targeting HDAC1 and HDAC2 on five human HCC cell lines with distinct molecular features and RTK expression levels (Hep3B, HLE, Huh7, JHH5, SNU449). Specifically, cells were exposed to romidepsin (IC50 inhibitor profile: 36 nM for HDAC1, 47 nM for HDAC2, 510 nM for HDAC4, 1400 nM for HDAC6; reported pharmacologically active for HDAC1/2), EDO-S101 (IC50 inhibitor profile: 9 nM for HDAC1 and HDAC2, 25 nM for HDAC3, 107 nM for HDAC8, 6 nM for HDAC6, 72 nM for HDAC10), and ACY-957 (IC50 inhibitor profile: 7 nM for HDAC1, 18 nM for HDAC2, 1300 nM for HDAC3). We found a dose-dependent reduction of cell viability, and a striking superiority of romidepsin as compared to EDO-S101 and ACY-957 on all tested HCC cells (Fig. 2d, S6). Reduced cell viability was observed as well with panobinostat, a pan HDAC inhibitor, although at higher doses to reach comparable effects with romidepsin (Fig. 2d, S6). Inhibition of HDAC is known to prevent deacetylation of lysine residues on histone tails (besides other proteins), allowing their recognition by BET proteins43. We therefore explored the effects of BET inhibitors (BETi: JQ1, OTX-015, mivebresib) and found no major effects on HCC cell viability (maximal response of about 50% reduction in Hep3B cells only at highest doses; Fig. 2d, S6). Biochemical studies revealed a consistent downregulation of MYC and of its targets Cyclin D1 (regulating cell cycle G1/S transition), Survivin (an antiapoptotic signal), as well as an upregulation of p21CDKN1a (p21; an inhibitor of cell cycle progression) in HCC cells treated with romidepsin (Fig. 2e, S7). Note that MET levels were unaffected (JHH5, Huh7) or reduced only at 24 h (Hep3B, HLE), whereas FGFR4 levels were slightly down- (Hep3B, JHH5, Huh7) or up- (HLE) regulated. This may indicate that cells maintain responsiveness to drugs targeting their function. These changes were not observed in the presence of BETi (Fig. 2f), despite the reported capability of both HDACi and BETi to alter MYC levels44,45. Thus, weak biochemical effects of BETi in the tested HCC cell lines correlate with moderate effects on cell viability. Together, these results show that romidepsin reduces the viability of HCC cells, accompanied by alteration of cell cycle/survival signals, with a range of responses varying among the cell lines we tested.
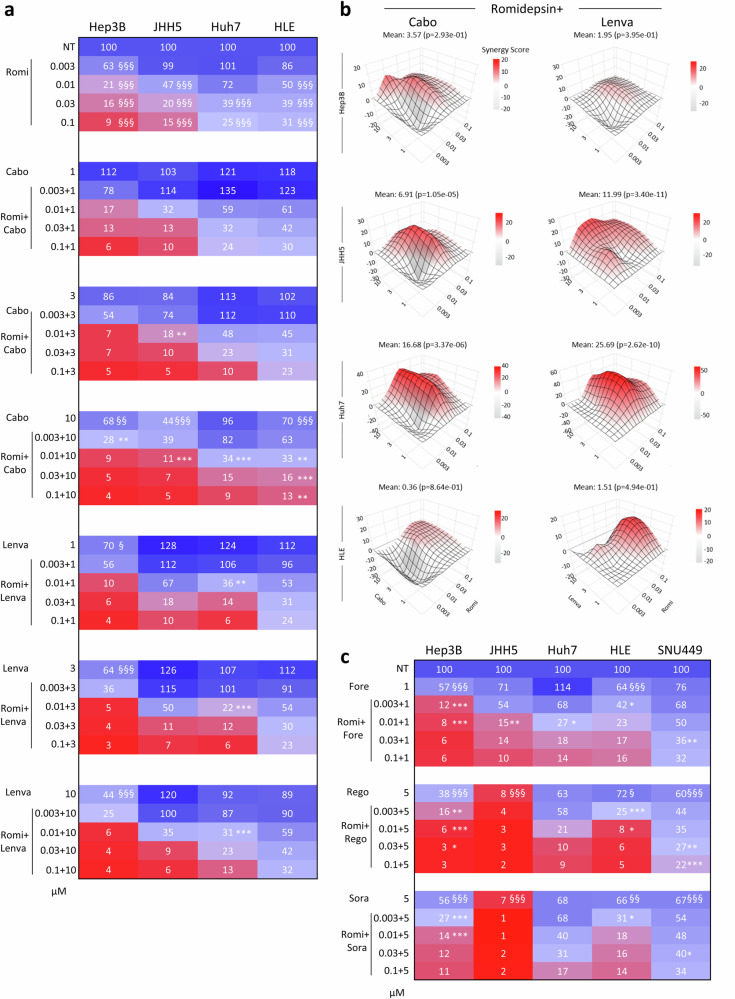
Romidepsin effects on human HCC cell viability are potentiated by cabozantinib, lenvatinib, regorafenib, and sorafenib
Previous studies have reported that reduced HCC cell viability by HDAC targeting (using pan-HDACi such as vorinostat, resminostat, or panobinostat) is potentiated by sorafenib46–48. However, the effect of romidepsin has not been explored in relation to the four RTKi used in the clinic for HCC treatment: cabozantinib, lenvatinib, regorafenib, and sorafenib. We therefore used the above human HCC cell lines to assess whether romidepsin effects are potentiated by RTKi, despite the fact that these cell lines are resistant to RTKi treatment alone. Both cabozantinib and lenvatinib alone elicit marginal effects on the viability of HCC cells (Fig. 3a, S8). Romidepsin effects are potentiated by cabozantinib in all tested cell lines, with SNU449 cells being most resistant to the combined treatment as observed with romidepsin alone (Fig. 3a, S8–9). Notably, cabozantinib reduces cell viability combined with low and intermediate doses of romidepsin (Fig. 3a, S8). Similarly, lenvatinib exhibits strong effects on cell viability in combination with romidepsin at low and intermediate doses, with HLE cells being less sensitive (Fig. 3a, S8). Combined treatments of romidepsin with cabozantinib or lenvatinib are synergistic at specific doses, as shown by the Bliss score method (Fig. 3b, Supplementary Table 1). We also tested the combinatorial effects of romidepsin with foretinib, another RTKi used in clinical trials, which inhibits MET among other targets. Again, combined romidepsin plus foretinib treatment reduces cell viability compared with romidepsin alone (Fig. 3c, S9–10). Reduced cell viability is comparable to that obtained with cabozantinib, with Hep3B and JHH5 being more sensitive than Huh7, HLE, and SNU449 being more resistant. A similar behaviour in cell response was found combining romidepsin with regorafenib or sorafenib (Fig. 3c, S9–10). Together, these results indicate that romidepsin confers vulnerability of human HCC cells to RTKi used in the clinic for HCC treatment.
Fig. 3. Romidepsin sensitises human HCC cells to RTKi.
a Heatmap reporting effects on viability of cells exposed to romidepsin (Romi), cabozantinib (Cabo) or lenvatinib (Lenva), alone or in combination, at the indicated doses (µM) for 48 h. Cells were treated with the indicated inhibitors, alone and in combinations by adding the drugs at the same time (24 h after seeding). Data are the mean of three independent experiments. Scatter dot plots are shown in Fig. S8 with corresponding statistics, two-tailed one-way ANOVA followed by Tukey multiple comparison; p < 0.0001 for all cell lines. b Synergy maps for the indicated drug combinations reported in (a). c Heatmap showing the effects on viability of cells following combined treatments of romidepsin with foretinib (Fore), regorafenib (Rego), or sorafenib (Sora) for 48 h. Data are the mean of three independent experiments (full heatmap including romidepsin and cabozantinib treatment is reported in Fig. S9, scatter dot plots are shown in Fig. S10 with corresponding statistics, two-tailed one-way ANOVA followed by Tukey multiple comparison; p < 0.0001 for all cell lines). Significance: *,§: p < 0.05; **,§§: p < 0.01; ***,§§§: p < 0.001. § indicates single treatments versus controls; * indicates combined treatments versus their respective single treatments. Source data are provided as a Source Data file.
Romidepsin leads to alterations of lipid metabolism pathways and their associated transcriptional regulators
We next explored the underlying mechanisms of action of romidepsin associated with the acquisition of cell vulnerability to RTK targeting, by analysing protein changes through an unbiased proteomic approach. As RTKi, we focused on cabozantinib for: a) its (partial) effects on HCC cell lines we used, shown in Fig. 2b; b) its more pronounced effects when combined with romidepsin, shown in Fig. 3a; c) its broad effects on distinct RTKs compared to lenvatinib or regorafenib, which action in HCC is predominantly associated with FGFR targeting. Huh7 cells were non-treated or treated with romidepsin, cabozantinib, or combined romidepsin+cabozantinib (RomiCabo) for 24 h. This time point was chosen in relation to biochemical changes observed in romidepsin-treated cells (Fig. 2e), limiting possible signalling/cellular alterations at later time points that can be secondary to the reduced viability. We found 797 proteins with significant differential abundance in romidepsin, 550 proteins in cabozantinib, and 838 proteins in RomiCabo compared with non-treated cells (Fig. S11a-b, Supplementary Data 1–2). Cross comparison of differential protein abundance among conditions revealed 350 proteins commonly altered in only romidepsin and RomiCabo treated cells, which define a common landscape of protein changes occurring in both conditions (Fig. S11b). Instead, 64 proteins are commonly altered in only cabozantinib versus romidepsin, and 69 in only cabozantinib versus RomiCabo (Fig. S11b, Supplementary Data 1-2). Moreover, 233 proteins are specifically abundant in the RomiCabo treated cells (Fig. S11b, Supplementary Data 1–2).
According to GO_Biological process database, several signalling and cellular processes are specifically enriched in each treatment condition (Fig. 4a, Supplementary Data 3–10). Among top-ranked enriched pathways in cells treated with romidepsin that emerged from our proteomic studies, we intriguingly found several related to mitotic spindle organization and to lipid metabolism, including regulators of fatty acid, triglycerides, cholesterol, and ceramides (Fig. 4a, b). Notably, romidepsin or cabozantinib exhibits a rather complementary switch of lipid regulator patterns, and RomiCabo is very similar to romidepsin alone despite specific changes likely resulting from the contribution of cabozantinib (Fig. 4a, b). Results are indicative of a lipid metabolism proteome switch in treated cells compared with the non-treated condition. This is accompanied by changes of proteins related to membrane organization, indicative that alterations in membrane lipid composition may affect membrane receptor/protein signalling (Fig. S11c).
Fig. 4. Romidepsin perturbs lipid metabolism in human HCC cells.
a Top panels: graphs reporting top-15 most significant enriched pathways according to GO_Biological_Process analyses in Huh7 cells treated with romidepsin (Romi), cabozantinib (Cabo), and RomiCabo versus non-treated cells (NT). Bottom panels: graphs reporting top-20 enrichments based on GO_Biological_Process analysis, with up-regulated (left) and down-regulated (right) pathways in the indicated conditions (RomiCabo versus cabozantinib-treated Huh7 cells). Pathways related to the mitotic spindle and lipid metabolism are highlighted in green and orange, respectively. Complete proteomics data are in Supplementary Data 1-10. b Heatmap reporting z-score values of proteins related to fatty acids and triglycerides, cholesterol, and ceramides metabolic pathways in the indicated conditions (R: romidepsin; C: cabozantinib; R + C: RomiCabo). a, b A two-tailed heteroscedastic t-test was performed between each condition. Proteins with p values < 0.05 were considered as significant; n = 3 independent biological experiments. c, d Heatmap representation of unsupervised hierarchical clustering and principal component analysis (PCA) plots from lipidomic analysis of ceramides (c) and lysophospholipids (d) in Huh7 and Hep3B cells. e Triacylglycerols dot-plots for association of carbon atoms (fatty acid chain length) and double-bonds (saturation) from lipidomic analysis. Each lipid is represented by a circle and the colour of the circle correlates with the condition association direction (red: positive, blue: negative). The size of the circle correlates with the statistical significance (larger circles represent smaller p-values). c–e Multivariate (PCA, Hierarchical heatmap); n = 4 independent biological experiments. Source data are provided as a Source Data file.
Results prompted us to carry out an in-depth analysis of changes in lipid classes and species linked to romidepsin treatment by performing lipidomic studies in the same experimental conditions employed for proteomics, using two human HCC cell lines: Huh7 and Hep3B. Unsupervised clustering and principal component analysis (PCA) of samples based on the determined lipid landscape show that each treatment condition forms a distinct cluster (Fig. S11d, Supplementary Data 11–16). Moreover, romidepsin and RomiCabo clusters are closer compared with non-treated or cabozantinib clusters (Fig. S11d). The use of the two HCC cell lines allowed us to identify major and consistent changes in three lipid classes: ceramides, lysophospholipids, and triglycerides (Fig. 4c–e). Effects on other lipid classes are likely linked to the distinct cellular/molecular context of the two cell lines (Fig. S12a–e). Specifically, in romidepsin and RomiCabo treated cells, we found a striking increase in ceramide species, the products of sphingolipid (SL) hydrolysis, with a paralleled decrease in SL species (Fig. 4c, S12b). Concerning the lysophospholipid class, romidepsin and cabozantinib each individually contributes to the increase in lysophosphatidyl-ethanolamine (LPE) and -choline (LPC), leading to an additive effect in RomiCabo condition (Fig. 4d). These most common lysophospholipids, resulting from the Phospholipase A2 (PLA) driven phospholipid hydrolysis, may participate to the fatty acid remodelling of membrane phospholipids or act as bioactive lipid mediators in and outside cells. Changes in phospholipids show the variability in lipid composition within this family depending on the treatment and the cell type (Fig. S12a). Moreover, the abundance of cholesteryl esters is also altered in treated cells, with a decrease in RomiCabo condition, although with changes according to cell type (Fig. S12c). Regarding the glycerolipid class, a very distinct pattern of di- and tri-glycerides (DG and TG, respectively) was found in romidepsin and cabozantinib versus untreated cells, with a predominant romidepsin or cabozantinib pattern in the RomiCabo combination, according to the cell line (Hep3B and Huh7 cells, respectively; Fig. 4e, S12d–e). Of notice, cabozantinib induces the most extreme shift in TG and fatty acid composition, with a marked and concomitant increase of long chain and unsaturated fatty acids (Fig. 4e, S12e). This is maintained in RomiCabo condition, indicating that cells favour the incorporation of unsaturated long-chain fatty acids into TG to prevent lipid peroxidation caused by oxidative stress, and hence ferroptosis. We therefore assessed oxidative stress by measuring reactive oxygen species (ROS) by flow cytometry using MitoSOX superoxide indicators and Mitotracker in live cells. We observed increased ROS species in RomiCabo conditions compared with non-treated cells (Fig. S12f). This result is consistent with the increased breakdown of phospholipids into lysophospholipids and free arachidonic acids, from which lipid mediators contributing to oxidative stress (i.e. eicosanoids) are synthetized. Collectively, these lipidomic results highlight specific changes of distinct lipid classes and species in HCC cells exposed to romidepsin or cabozantinib alone versus their combination.
Among regulators of lipid metabolism genes are the liver X receptor (LXR) and the farnesoid X receptors (FXR), two nuclear receptors that form heterodimers with retinoid X receptor (RXR) and bind respectively to LXR- or FXR-response elements in promoter region of target genes49,50. Transcriptional activities of LXR and FXR are induced in response to elevated intracellular levels of cholesterol derivatives or bile acids, respectively51. Notably, LXR/RXR and FXR/RXR activation is among top ranked pathways we found in the IPA analysis of proteomics data in treated versus non-treated HCC cells (Fig. 5a, Supplementary Data 3–5). Indeed, we found that HCC treated cells are characterised by a higher z-score of proteins related to LXR/RXR and FXR/RXR pathways (Fig. 5b). Intriguingly, romidepsin and RomiCabo share a similar set of proteins, whereas different from those found in cabozantinib alone (Fig. 5b, Supplementary Data 3–5). HDAC1/2 are part of the repressor complex of LXR/RXR and FXR/RXR, as de-acetylation of target genes represses transcriptional activation52. We therefore explored whether romidepsin triggers LXR transcriptional activity using an LXR response-element upstream of luciferase. We found significantly high LXR transcriptional activity in cells either treated with romidepsin alone or with RomiCabo (Fig. 5c, d). We further assessed the implication of LXR and FXR on the regulation of some of their target genes by RT-qPCR. In romidepsin or RomiCabo-treated cells, we found an upregulation of SREBF1 and MLXIPL (two master regulators of fatty acid and cholesterol metabolism), CD36/FAT (promoting long-chain fatty acid uptake), and CYP7A1 (a transporter of bile acid), but not of SCD1 (an enzyme involved in the desaturation of de novo synthetized fatty acids; Fig. 5e, f). We additionally found a downregulation of ACACA and FASN (implicated in the de novo fatty acid synthesis; Fig. 5g). These results indicate that there is a distinct regulation of LXR and FXR target genes by romidepsin in HCC cells. We further explored the implications of gene regulation by LXR versus FXR using chemical agents. We found that SREBF1 upregulation by an LXR agonist (GSK3987; not by an FXR agonist: FXR Agonist-3) is further potentiated by romidepsin (Fig. 5e). Romidepsin-triggered SREBF1 upregulation was impaired by an LXR antagonist (GSK2033; Fig. 5e). Instead, MLXIPL and CD36/FAT are upregulated by an FXR, but not LXR, agonist (Fig. 5e). Together, these findings indicate that romidepsin treatment (alone or in combination with cabozantinib) leads to a rather specific perturbation of FXR and LXR-induced target gene set involved in lipid pathway regulation (Fig. 5h).
Fig. 5. Romidepsin perturbs the expression of lipid regulators through LXR and FXR pathways.
a Top-15 most significant enriched pathways according to IPA analyses from the proteome of Huh7 cells treated with romidepsin (Romi), cabozantinib (Cabo), or RomiCabo versus non-treated cells (NT). Pathways related to LXR/RXR and FXR/RXR are highlighted in blue. b z-score from the proteome for proteins related to LXR/RXR (green) or FXR/RXR (red); some proteins have overlapping upstream regulators LXR and FXR. (a, b) A two-tailed heteroscedastic t-test was performed between each condition. Protein with p values < 0.05 were considered as significant; n = 3 independent biological experiments. c Scheme illustrating the explored mechanism of action of romidepsin in relation to LXR/RXR and FXR/RXR pathways and lipid metabolism regulation. d LXR luciferase reporter assay in non-treated and treated Huh7 cells. Luciferase activity is reported as arbitrary units (a.u). An LXR agonist (GSK3987) was used as a positive control (n = 3 independent biological experiments; p < 0.0001). e mRNA expression by RT-qPCR (expressed as RQ) of SREBF1, MLXIPL, CD36, and SCD1 in romidepsin (R), cabozantinib (C), or RomiCabo versus non-treated Huh7 cells. On the right of each graph are values corresponding to cells after treatment with FXR agonist (FXR agonist 3), LXR agonist (GSK3987), or LXR antagonist (GSK2033), either alone or in combination with romidepsin and RomiCabo (at least four independent experiments; SREBF1, MLXIPL, CD36, and SCD1 p < 0.0001). f, g mRNA expression by RT-qPCR of CYP7A1 (n = 11 independent biological experiments, p = 0.0077) (f), ACACA (n = 7 independent biological experiments, p = 0.0043), and FASN (n = 4 independent biological experiments, p < 0.0001) (g) in the indicated conditions. h Representations of different transcriptional expression outcomes in LXR and/or FXR pathways after treatment with romidepsin. d–g two-tailed unpaired one-way ANOVA followed by Tukey’s (d, e) or Dunnet’s (f, g) multiple comparison. Significance: *,$,#,%,£p < 0.05; **,$$,##,%%,££p < 0.01; ***,$$$,###,%%%,£££p < 0.001. * is comparison versus NT or as indicated by the statistical lines; $ versus romi; # versus cabo; % versus RomiCabo; £ versus FXR agonist+Romi. Schemes in c and h were performed with public open source Servier Medical Art, licensed under CC BY 4.0. Source data are provided as a Source Data file.
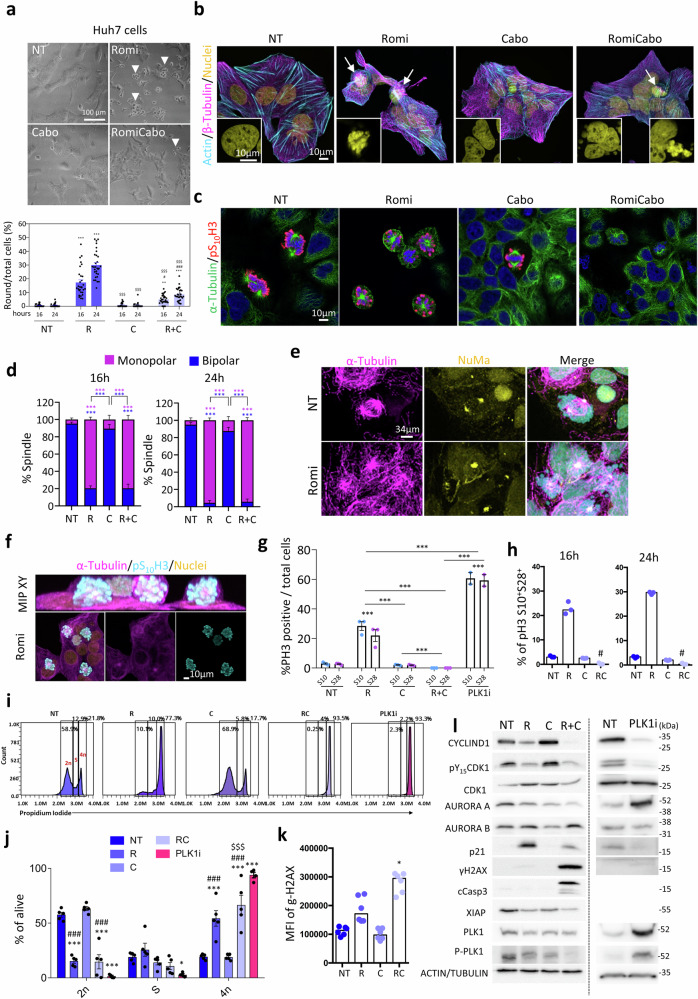
Romidepsin alters mitotic spindle organisation, leading to cell cycle alteration and failed mitosis
As mentioned above, another top-ranked enriched pathway in cells treated with romidepsin (alone or with cabozantinib) is mitotic spindle and nuclear membrane regulation/assembly (Fig. 4a, Supplementary Data 6-8). By analysing our proteomic data with the spindle proteome reported in53, we collectively found about 500 proteins significantly deregulated (Fig. S13a, Supplementary Data 17–18). Intriguingly, we observed that in romidepsin treated cells, there was a progressive increase over time of round birefringent cells, which were stably adherent on the top of morphologically normal cells (Fig. 6a, S13b–f). These round cells were neither present in non-treated or cabozantinib-treated conditions (Fig. 6a, S13b–f). Instead, in RomiCabo conditions we observed the appearance of cells debris over time, beside a proportion of round cells (Fig. 6a, S13b–f). We performed a series of immunofluorescence analyses to characterise this round cell shape in Huh7 cells. In romidepsin cultures, we found a large proportion of round cells with cortical Actin and β-Tubulin, particularly those on the upper Z label plan (Fig. 6b, S14). Mitotic cells in culture conditions are often rounded, with cortical actin and tubulin filaments. Consistently, these round cells are characterised by regularly dotted nuclei, possibly corresponding to chromosomes in mitosis rather than irregular dots - fragmented - nuclei, which instead are observed in RomiCabo conditions (Fig. 6b, S14). We further explored the population of round cells in the romidepsin condition by immunostaining with anti-α-Tubulin, a microtubule protein that is part of the dynamic mitotic spindle machinery implicated in chromosome segregation. α-Tubulin staining shows aberrant mitotic spindle appearance in cells treated with romidepsin, with monopolar instead of bipolar spindle (Fig. 6c, d). Monopolar spindles were also found in RomiCabo, but not in cabozantinib or non-treated cells (Fig. 6c, d). Anti-NuMA immunostaining, which labels the position of centrosomes in mitotic cells, confirmed the presence of aberrant spindles in cells treated with romidepsin alone or in combination with cabozantinib, with a higher proportion of monopolar than bipolar spindles (Fig. 6e, S15a). We intriguingly found that in romidepsin condition, round cells on the upper Z label plan are phosphoS10Histone H3 (pS10H3) positive (Fig. 6f). We further explore this aspect by quantifying the proportion of cells positive for pS10H3 and pS28H3, which sequential phosphorylation occurs in early G2 and early mitosis, respectively54. Immunofluorescence analyses highlighted a 30% percent of pS10H3 and pS28H3 positive cells in RomiCabo-treated cells compared with cabozantinib or non-treated cells (Fig. 6g, S15b), which corresponds to the percentage of round cells reported in Fig. 6a. Instead, in RomiCabo condition cells are neither positive for pS10H3 and pS28H3 (Fig. 6g, S15b). Results are confirmed by flow cytometry studies, showing around 30% of double-positive pS10H3 and pS28H3 cells in romidepsin compared to non-treated or cabozantinib conditions, and very few double-positive cells following RomiCabo treatment (Fig. 6h, S15c). To get insights into the population of double-negative pH3 cells in RomiCabo condition, we evaluated the DNA content with Propidium Iodide by flow cytometry. The DNA profiles show a striking shift from 2n to 4n in romidepsin and RomiCabo conditions compared to non-treated and cabozantinib treated cells (Fig. 6i, j). Results were further confirmed in Hep3B and JHH5 cells (Fig. S16a). To further understand the cell state of these double-negative pH3 with double-DNA content (4n) in RomiCabo-treated condition, we investigated the presence of a potential DNA damage preventing the entry in G2-M. By flow-cytometry analysis of γH2AX, a well-known sensor of DNA damage, we observed significant increase in the median fluorescence intensity (MFI) in RomiCabo-treated cells (Fig. 6k; S16b for Hep3B and JHH5 cells). It is known that once DNA damage is sensed, the repair proteins are recruited leading to two possible outcomes: either the DNA repair process is successful, allowing cells to progress in G2/M; or the DNA repair process fails and cells exit the cycle towards apoptosis. Through biochemical analyses, in RomiCabo-treated condition we confirmed induction of γH2AX and found as well cleaved-Caspase3, accompanied by decreased levels of the anti-apoptotic XIAP (Fig. 6l, S17–23a). Moreover, we observed alterations of several cell cycle regulators: a reduction in CYCLIN D1, the pY15CDK1/CDK1 ratio, and Aurora A, along with an upregulation of p21 (Fig. 6l, S17–23a). Immunofluorescence analysis confirmed increased number of cleaved-Caspase3 positive cells in RomiCabo condition (Fig. S23b). Furthermore, a pan-Caspase inhibitor (Z-VAD-FMK), but not a ferroptosis inhibitor (ferrostatin-1), significantly rescues cell death caused by RomiCabo (Fig. S23c). Collectively, these results indicate that whereas romidepsin-treated cells go through a G2/M arrest, RomiCabo-treated cells exit the cell cycle after DNA duplication and go through apoptosis. Hence, the addition of cabozantinib converts a cytostatic effect of romidepsin into cytotoxicity.
Fig. 6. Romidepsin treatment causes mitotic spindle defects and perturbs cell cycle progression in human HCC Huh7 cells.
a Representative bright-field images of Huh7 cells non-treated (NT), treated with romidepsin (Romi), cabozantinib (Cabo), or RomiCabo for 24 h. White arrowheads indicate round cells. Percentage of round cells (normalized over total cell number, n = 3 independent biological experiments; p < 0.0001)). b Immunostaining of Huh7 cells after 24 h of treatment: Actin (cyan), β-Tubulin (magenta), Nuclei (yellow; n = 3 independent biological experiments). White arrows indicate round cells. c, d Immunostaining of monopolar and bipolar spindles with α-Tubulin (green), pS10H3 (red), nuclei (blue), (n = 3 independent biological experiments; p < 0.0001). e Immunostaining of aberrant spindles with NuMa (yellow) and α-Tubulin (magenta; n = 3 independent biological experiments). f Maximal intensity projection (MIP) in XY plan of Huh7 cells treated for 24 h with romidepsin: α-Tubulin (magenta), pS10H3 (cyan), nuclei (yellow), reported as representative 3D reconstruction. g pS10H3 (S10) and pS28H3 (S28) positive cells at 24 h in non-treated (NT), romidepsin (R), cabozantinib (C), RomiCabo (R + C), and PLK1i conditions, normalised over total cell number (DAPI; n = 3 independent biological experiments; p < 0.0001). h Percentage of double-positive pS10H3 and pS28H3 alive cells after 16 h (left; n = 3 independent biological experiments; p = 0.0017) and 24 h (right; n = 3 independent biological experiments; p = 0.0017) of indicated treatments, measured by flow cytometry. i, j Representative cytometry profiles (i) of the DNA content (2n, S, 4n) of alive cells after 24 h, with Propidium Iodide and (j) quantification among alive cells (n = 5 independent biological experiments; p < 0.0001). k Median fluorescence intensity (MFI) of γH2AX among live cells after 24 h by flow cytometry (n = 6 independent biological experiments; p < 0.0001). l Western blots of reported signalling proteins (quantifications are shown in Figs. S17–18, 23 with the corresponding statistics, n = 3 independent biological experiments). Statistical analysis was performed with one-way ANOVA (a, g) or two-way ANOVA (d), followed by Tukey multiple comparison (a, d, g). Paired two-tailed Friedman followed by Dunn’s multiple comparison (h, k). Unpaired two-tailed two-way ANOVA followed by Dunnet’s multiple comparison (j). In (g, i, j) PLK1i condition was used as positive control of mitotic arrest. Levels of significance: *,$,#p ≤ 0.05; **,$$,##p ≤ 0.01; ***,$$$,###p ≤ 0.001. Bars represent SEM and notches in dot-plots represent mean levels. *indicates the significance between treated versus non-treated cells; $is versus romidepsin; #is versus cabozantinib. Source data are provided as a Source Data file.
Recent reports started uncovering an intricated link between lipid composition, cell shape, and mitosis55–57. The complementary effects we observed in romidepsin treated cells could result from an independent perturbation of lipids and of cell cycle process, or the result of a predominant effect on one of these processes that consequently perturbs the other. To provide insights, we reasoned to compare the action of romidepsin with that of an agent blocking mitosis, like PLK1 inhibitor (PLK1i; GSK461364). By exposing Huh7 cells to PLK1i, we observed the formation of round cells, increased pS10H3 and pS28H3 positive cells, high percentage of cells with 4n DNA content, and decreased CYCLIND1 levels and pY15CDK/CDK1 ratio (Fig. 6g, 6i-j, 6l, S18, S21). We then performed additional lipidomic analyses on Huh7 and Hep3B cells and found that the lipid pattern is completely different in cells exposed to romidepsin versus PLK1i (Fig. S24a). This is further illustrated by comparing the profiles of ceramides, sphingolipids, lysophospholipids, phospholipids, diacylglycerols, triglycerides, and cholesterol, as shown by the differential lipid abundance illustrated by the heatmaps and PCA projections (Fig. S24b–e, S25a–d), despite variable changes between cell lines. We therefore conclude that romidepsin acts independently on both regulators of lipid metabolism and of cell cycle progression.
Effects of combined romidepsin plus cabozantinib treatment on patient-derived HCC tumoroids and in the Alb-R26Met cancer mouse model recapitulating HCC heterogeneity
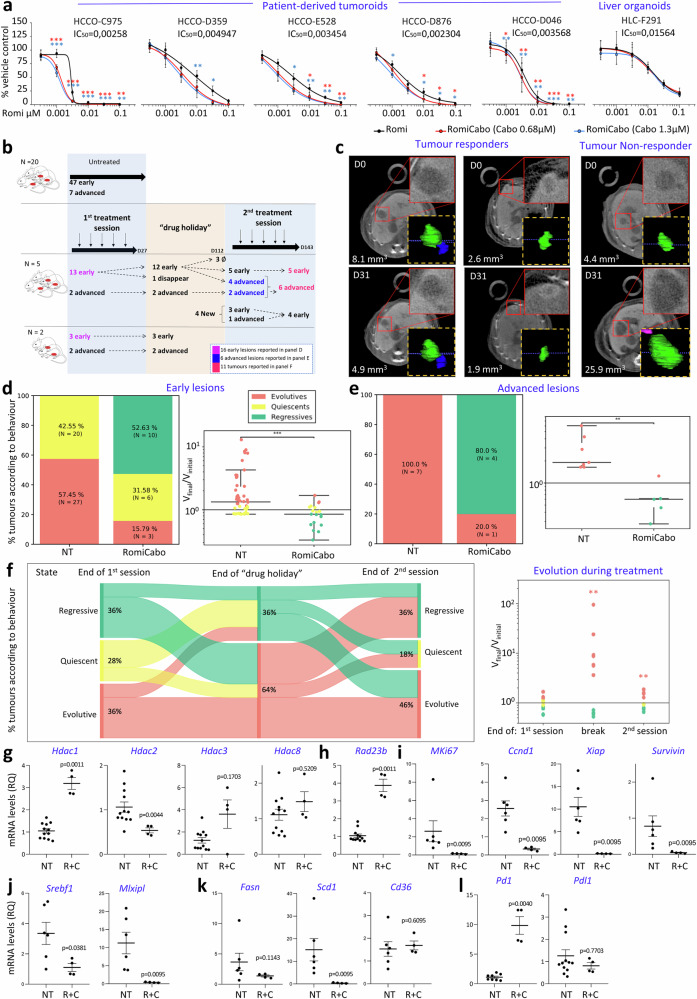
We next assessed the therapeutic relevance of romidepsin plus cabozantinib treatment using a panel of patient-derived human HCC tumoroids, previously reported to be resistant to RTKi58. All tumoroids we tested were responsive to romidepsin treatment, with a range of doses varying among tumoroids (Fig. 7a). Furthermore, we found that cabozantinib potentiated romidepsin effects at low doses in tumoroid HCCO-C975, while enhanced responsiveness in tumoroids requiring higher doses of romidepsin (e.g., viability of HCCO-D359 with 0.01 µM romidepsin alone versus combined with 1.3 µM cabozantinib: 52.76 vs 26.83 %; for HCCO-E528: 29.6 vs 13.43 %; for HCCO-D876: 23.57 vs 10.35 %; for HCCO-D046: 12.25 vs 4.02 %; Fig. 7a, S26, Supplementary Table 1). Romidepsin exerts some effects on liver organoids, used as controls, only at high doses, without any potentiation by cabozantinib (Fig. 7a). These results exemplify the sensitivity of HCC patient-derived tumoroids, but not of liver organoids, to romidepsin plus cabozantinib treatment.
Fig. 7. RomiCabo effects on patients derived tumoroids and on spontaneous tumours in the Alb-R26Met HCC mouse model.
a Viability of patient-derived tumoroids and liver organoids in romidepsin and RomiCabo treatments. The IC50 of RomiCabo is shown, all others are included in Supplementary Table 2 (p values in Supplementary Data 23). Unpaired t-test corrected with Benjamini-Hochberg, stars compare Romi versus RomiCabo (red and blue: 0.68 µM and 1.3 µM cabozantinib, respectively; n ≥ 4 independent biological experiments). Tumoroid viability upon cabozantinib alone is reported in Fig. S26a. b Protocol followed for in vivo study in Alb-R26Met mice. ∅ indicate lesions of a mouse that died during “drug holiday”. The scheme was performed with free open source (pngegg.com). c Examples of PC-CT imaged tumours, with 3D reconstruction, before and after treatment: responding (left) and non-responding (right). Red squares: high magnification tumours. d, e Left: percentage of tumours according to their behaviour (evolutive, quiescent, regressive). Right: ratio between final versus initial tumour volume (logarithmic scale). NT: non-treated tumours. Tumours are considered regressive if this ratio is less than 0.85, evolutive if greater than 1.15, and quiescent between these two thresholds. Unpaired two-tailed Mann-Whitney (d: p = 0.0013; e: p = 0.0041); number of independent samples is indicated. f Left: Sankey diagram represents lesions’ longitudinal behaviour at the end of the first session, of the “drug holiday”, and of the second session. Right: Ratio between final versus initial tumour volume (logarithmic scale) at the same time points as Sankey diagram. Unpaired two-tailed Mann-Whitney, p = 0.0061 (break) and p = 0.0025 (2nd session), number of independent samples indicated in b, d, e. g–l Expression levels of class-I Hdacs (g), Rad23b (h), Mki-67, Ccnd1 (CyclinD1), Xiap, Survivin (i), Srebf1, Mlxipl (j), Fasn, Scd1, Cd36 (k), Pd1, Pdl1 (l) in Alb-R26Met non-treated (NT) versus RomiCabo treated (R + C) tumours. RT-qPCR (as RQ) relative to NT tumours. g–l Unpaired two-tailed Mann-Whitney, exact p values are indicated (n ≥ 4 independent samples). Levels of significance: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Bars represent SEM and notches in dot-plots represent mean levels. Source data are provided as a Source Data file.
To explore in vivo the effects of RomiCabo treatment, we took advantage of a unique immunocompetent HCC model we have generated, the Alb-R26Met mice, which spontaneously develops liver tumours over time59,60. The Alb-R26Met model recapitulates resistance to RTKi used in the clinic59,61, therefore allowing to assess in vivo the possibility of combining them with romidepsin to overcome RTKi resistance. By recapitulating the molecular heterogeneity characterising HCC patients, the Alb-R26Met model allows assessing the effect of a given treatment on a cohort of tumours expected to have different vulnerabilities and hence behaving as either sensitive or resistant. We used the imaging methodology based on photon counting micro-computed tomography (PC-CT), which we previously developed and applied on the Alb-R26Met model60,62, to longitudinally assess treatment effects on early (eHCC; with a size ranging from 0.5 to 3 mm3) and advanced (aHCC; > 3 mm3) HCC (Fig. 7b). Examples of tumour response are shown in Fig. 7c. Strikingly, RomiCabo treatment led to a regression of about 53% (10/19) of early lesions, whereas 31% remained quiescent (6/19), and only 16% continued to evolve (3/19; Fig. 7d). This repartition in the behaviour of early lesions was never observed across all imaging studies we performed, in which none (0/47) had spontaneous regression, 43% (20/47) lesions were quiescent and 57% (27/47) were evolving (Fig. 7d, left). Concerning advanced tumours, those non-treated systematically grew in size over time (7/7), whereas in RomiCabo treated mice about 80% (4/5) regressed (Fig. 7e, left). Moreover, volume progression of early and advanced lesions treated with RomiCabo was significantly reduced compared with non-treated lesions (Fig. 7d–e, right).
Next, we longitudinally tracked the behaviour of 12 treated lesions during a “drug holiday” period (a break of the treatment for 85 days; Fig. 7b). The longitudinal behaviour of these lesions is illustrated with a Sankey diagram in Fig. 7f. After a “drug holiday”, 58% (7/12) of these lesions were evolving, and 42% (5/12) early lesions were still regressive with one of them completely disappeared (Fig. 7f). It is tempting to speculate that RomiCabo treatment imprinted an intrinsic response in this subset of regressing lesions, eliciting antitumoral effects even during “drug holidays”, leading to a management of lesions over time. After this “drug holiday” period, we started a second session of RomiCabo treatment for 32 days and followed lesion behaviour longitudinally (Fig. 7b). At the end of treatment, 36% (4/11) tumours regressed, indicative of their maintained sensitivity to RomiCabo treatment despite a “drug holiday” period. Moreover, 18% (2/11) tumours remained quiescent during treatment, whereas 46% (5/11) tumours continued to evolve (Fig. 7f, right), exemplifying a proportion of tumours (3/11) that were and remained resistant to RomiCabo treatment (Fig. 7f, right). Of notice, the entity of volume progression in RomiCabo treated advanced tumours was drastically reduced, despite their growth during drug holiday (Fig. 7f, right). Together, these outcomes illustrate the potency of RomiCabo treatment on a subset of tumours in the Alb-R26Met model. We evaluated the potential toxicity of treatments in a control cohort of mice in two ways. By collecting plasma for aspartate aminotransferase (AST) activity measurement by colorimetric assay, we found similar AST activity levels in all groups (Fig. S27a). Moreover, the follow-up of mouse weight showed variations lower than 4% in the different groups (Fig. S27b–c). Then, we analysed by RT-qPCR the expression levels of class-I Hdacs and found downregulated Hdac2, increased Hdac1, and unchanged Hdac3 or Hdac8 levels in RomiCabo treated compared with non-treated tumours (Fig. 7g). Strikingly, Rad23b, a biomarker of tumour sensitivity to HDACi correlating with good prognosis32, was significantly increased in RomiCabo-treated tumours (Fig. 7h). In accordance with proliferation and cell cycle perturbations observed upon RomiCabo treatment in vitro, we found by RT-qPCR in Alb-R26Met RomiCabo-treated tumours a significative decrease in Mki-67 (proliferation marker), Ccnd1 (CYCLIND1 gene), Xiap, and Survivin compared with non-treated tumours (Fig. 7i). We additionally observed by RT-qPCR that transcription factors related to lipid metabolism like Srebp1 or Mlxipl were significantly downregulated in RomiCabo-treated compared with non-treated tumours, as it is the case for their target genes Fasn and Scd1, also implicated in lipid pathways (Fig. 7j–k). Results show alteration of lipid regulators following RomiCabo treatment, despite differences likely due to short (24 h) versus long ( ~ 30 days) treatment for in vitro and in vivo studies, respectively. As first-line treatment for advanced HCC in the clinic is PDL1/PD1 targeting, we assessed whether RomiCabo treatment has an impact on their levels and found by RT-qPCR increases Pd1 levels in RomiCabo-treated tumours (Fig. 7l). Collectively, ex vivo and in vivo preclinical studies exemplify the effectiveness of RomiCabo on HCC treatment.
Romidepsin plus cabozantinib treatment leads to a remodelling of the tumour immune microenvironment in the Alb-R26Met liver cancer model
The effectiveness of RomiCabo treatment on liver tumour growth and on Pd1 levels in the Alb-R26Met model prompted us to analyse the immune profile within the tumour microenvironment of untreated versus treated mice. After imaging, untreated and treated mice were sacrificed and dissected tumours were processed to obtain dissociated cells for spectral cytometry analysis with an antibody panel dedicated to quantify immune cell infiltration: T lymphocytes (TL; CD4+ and CD8+), B lymphocytes (BL), Natural Killer TL (NK-TL) cells, NK cells, neutrophils, monocytes, macrophages, subtypes of Dendritic Cells such as conventional cDC1, cDC2, plasmacytoid DCs (pDC), and monocyte-derived DC (MoDC; Supplementary Data 19 S21). We found a significant increase in overall leukocyte infiltration in RomiCabo treated compared with untreated tumours, as illustrated by a higher percentage of CD45+ cells (Fig. 8a). Using unsupervised approach for cell clustering, a striking remodelling of the tumour immune infiltrate (CD45+ cells) was observed in RomiCabo treated tumours, as visualized in a uniform manifold approximation and projection (UMAP) of different immune cells (Fig. 8b, c). Then we used a hierarchical gating strategy to quantify the proportion of immune cell populations (Fig S28a). We found a significant increase of granulocytes, TL-CD4 + , NK-TL cells, MoDC and a significant reduction of BL and cDC2 proportion in RomiCabo-treated versus untreated tumours (Fig. 8d–f, S28b). Interestingly, we observed a significant increase of Molecule of Histocompatibility of class-II (MHC-II) at the surface of MoDC and cDC2 in RomiCabo-treated tumours, reflecting their enhanced maturation (Fig. 8f). Moreover, we quantified the expression of the co-inhibitory molecule PD1 and the activation marker CD44 at the surface of TLs. We found a striking reduction in the proportion of PD1+ among TL-CD4+ and TL-CD8+ together with a decrease in the level of PD1 at the surface of both populations in RomiCabo-treated tumours (Fig. 8g, h), indicative of decreased exhausted TLs. This was accompanied by increased percentages of CD44+ TL-CD4+ and TL-CD8+, and increased CD44 expression level at the surface of both TLs, in RomiCabo-treated tumours (Fig. 8g, h), indicative of increased activated LTs. As for TLs, NK-TLs express less levels of PD1 and increased levels of CD44 expression at their surface, in RomiCabo-treated tumours (Fig.8i).
Fig. 8. Remodelling of the immune landscape in Alb-R26Met tumours upon RomiCabo treatment.
a Dot plot reporting the percentage of CD45+ cells among total live single cells in the Alb-R26Met non-treated (NT) and RomiCabo-treated tumours. A dedicated antibody panel (Supplementary Data 19) was used to quantify the immune cell infiltration by spectral flow cytometry, and a representative gate strategy to identify immune cell population is detailed in Fig. S28). b, c Uniform Manifold Approximation and Projection (UMAP) of immune cell clusters in Alb-R26Met tumours, with clusters overlay of indicated immune cells in the legend (b) and as density of cells (c) in non-treated (NT) and RomiCabo-treated Alb-R26Met tumours. T lymphocytes (TL: CD4+ and CD8+), B lymphocytes (BL), Natural Killer TL (NK-TL) cells, NK cells, neutrophils, monocytes, macrophages, conventional Dendritic Cells (cDC1 and cDC2), monocyte-derived DC (MoDC), and plasmacytoid DCs (pDC). d Pie-chart representing the percentages of distinct immune cell types in non-treated and RomiCabo-treated tumours, based on their marker identification with the antibody panel. e Dot-plots comparing the percentage of neutrophils, macrophages, monocytes, MoDC (top), B lymphocyte, TL-CD4+ , TL-CD8+, NKT (bottom), among CD45+ population, in non-treated (NT) and RomiCabo-treated tumours. f Dot-plots reporting median fluorescence intensity (MFI) of MHC-II in MoDC (top) and cDC2 (bottom), among CD45+ population, in non-treated (NT) and RomiCabo-treated tumours. g–i Dot plot reporting the percentage and the MFI of PD1 or CD44 in the indicated immune cell types. j–l Dot-plots showing the mRNA expression levels of the reported immune-checkpoints in Alb-R26Met non-treated (NT) and RomiCabo-treated (R + C) bulk tumours. RT-qPCR values are expressed as RQ, relative to non-treated tumours. Statistical analysis was performed with unpaired two-tailed Mann-Whitney, exact p values are indicated, at least 10 (a–i) or 4 (j–l) independent samples were used. Bars represent SEM, and notches in dot-plots represent mean levels. Source data are provided as a Source Data file.
We expanded these analyses by following through RT-qPCR the expression levels of immune-checkpoints in RomiCabo versus untreated tumours. Interestingly, we found increased Icos and Icosl levels in RomiCabo-treated tumours (Fig. 8j), known as co-stimulatory signals. Moreover, beside increased levels of Pd1 (coherent with increased PD1 levels in CD45- cells; Fig. S28c, d), we found upregulation of Ctla4 and its ligand Cd80, Lgals9 (TIM3 interactor), and of Lag3, whereas no significant changes for Cd86 and Tim3 expression (Fig. 8k, l). Thus, RomiCabo treatment profoundly reshape the tumoral immune landscape towards an immune-stimulatory profile, in addition to the multiple effects elicited on cancer cells.
Based on these findings, we investigated whether RomiCabo treatment impacts human MoDC maturation, using a DC model based on in vitro differentiation of purified monocytes from healthy donor blood (Fig. 9a, S29a). After MoDC differentiation, cells were stimulated with lipopolysaccharide (LPS) to induce their activation, adding conditioned medium from untreated or RomiCabo-treated Huh7 cells. We then assessed MoDC maturation by flow cytometry. As expected, LPS induces a significant increase in the co-stimulatory molecules CD80, CD86, and MHC-II. Notably, the addition of conditioned medium from RomiCabo-treated HCC cell lines leads to a significant decrease in CD80 expression, without affecting CD86 or MHC-II levels on the MoDC surface, neither cell viability (Fig. 9b–d, S29b). We additionally found that this CD80 downregulation was a direct effect of RomiCabo on MoDCs, as CD80 levels decreased even when RomiCabo was added in the absence of conditioned medium from Huh7 cells (Fig. S29c). It has been reported that CD80 preferentially binds to CTLA-4 rather than CD28 on T cells, promoting regulatory T cell activation and leading to a more immunoregulatory environment compared to CD8663. Overall, results indicate that the in vivo efficacy of RomiCabo on tumours is likely related to a combinatorial action on cancer cells, while remodelling the tumour immune landscape towards an immune-stimulatory profile, including direct effects on MoDC.
Fig. 9. Effect of RomiCabo treatment on the maturation profile of LPS-activated human MoDCs.
a Scheme illustrating the strategy employed to generate and stimulate MoDCs from human peripheral blood mononuclear cells (PBMCs) of healthy donors. After differentiation, immature MoDCs were kept non-treated (NT), activated with LPS without or with conditioned medium for 18 h. Conditioned medium were obtained from Huh7 cells either non-treated (NT) or treated with RomiCabo for 24 h. Scheme was performed with public open source Servier Medical Art, licensed under CC BY 4.0. Gate strategy for MoDC analysis is detailed in Supplementary Fig. S29. b Histograms show a representative flow cytometry profile of the expression of CD80, CD86, or HLA-DR (MHC-II) molecules at the surface of MoDCs non-treated (NT, black), treated with LPS alone (LPS; red), or treated with LPS plus the indicated conditioned medium (LPS+Sup; blue). c, d Quantification of the median fluorescence intensity (MFI) of CD80, CD86, and HLA-DR (MHC-II; c), and the percentage of CD80 and CD86 positive cells (d) obtained by flow cytometry. MFI of CD80 and CD86 was corrected by fluorescent minus two (minus CD80 and CD86 antibodies). Each dot represents a distinct donor of MoDC. Statistical differences were assessed using Friedman one-way repeated measure analysis of variance by ranks, followed by Dunn’s multiple comparison test. Exact p values are as follows: CD80 (c: p < 0.0001; d: p < 0.0017), CD86 (c: p = 0.0006; d: p = 0.0017), HLA-DR (c: p = 0.0003). *p ≤ 0.05; **p ≤ 0.01. Source data are provided as a Source Data file.
Discussion
Epigenetic dysregulation in cancer receives increasing attention for its impact on tumorigenic traits and for the availability of therapeutically pertinent epidrugs. Some epidrugs are already in the clinic for the treatment of specific cancers, and others are being evaluated in multiple clinical trials. These agents may represent promising therapeutic opportunities particularly for complex and resistant cancer types like HCC. Among them are inhibitors of the HDAC family, composed by distinct members whose action varies according to the oncogenic context and the cancer type in which they operate12. HDAC blockage using specific versus pan-HDACi should therefore be carefully evaluated for anticancer therapies.
In the context of HCC, epigenetic alterations have been reported in both precancerous lesions and in HCC26,61,64. Changes in the expression levels of distinct HDACs have been observed and some correlate with disease progression27,46,65. The therapeutic benefit of targeting HDACs has been explored in the past using pan-HDACi24,66. Recently, major changes in epigenetic regulators correlating with a worse prognosis has been reported in hepatoblastoma, the most common liver paediatric cancer23. Furthermore, the NIH Clinical Proteomic Tumor Analysis Consortium (CPTAC) has lately launched a proteomic classification of HCC patients to search for potential therapeutic targets, and identified three sub-groups characterized by the enrichment of genes related to metabolism, immune, or proliferation pathways. Among signals characterizing the proliferation subgroup are HDAC2, CDK1, and PCNA67. In the present work, focusing on class-I HDACs, we report an upregulation of HDAC1/2 in a large fraction of HCC patients, correlating with decreased overall survival and disease-free survival. These findings drove us to discover that romidepsin, a clinically relevant inhibitor of HDAC1/2, is highly effective on in vitro, ex vivo, and in vivo HCC models. Results are strenghtened by recent studies we performed on a mouse HCC tumoroid panel recapitulating inter- and intra- heterogeneity for disease modelling and combinatorial drug discovery68. Romidepsin effects on HCC cells are superior to those elicited by two other HDAC1/2 blocking agents (EDO-S101 and ACY-957), possibly for its exceptional HDAC1/2 inhibitory efficacy and/or for its action on additional key targets not identified yet. Our proteomics analysis highlighted alterations of several regulators of distinct cellular and molecular pathways. We propose that the potent effects of romidepsin on HCC is linked to a combinatorial perturbation of distinct cellular/molecular processes. First, romidepsin alters lipid metabolism regulators, triggering distinct changes of lipid composition. Perturbation of lipid metabolism can severely impact multiple functions, such as cell proliferation and survival, inflammation, membrane organization, drug response and efflux69. It has been reported that local and/or overall changes in lipid composition and saturation of cell membrane alter: 1) the curvature of the membrane in processes related to vesicle trafficking, mitosis, and daughter cell separation; 2) membrane fluidity, thus impacting anchorage and recruitment of signalling mediators, including RTKs and xenobiotic transporter70–73. Moreover, lipid species such as ceramides and LPE with a conical shape favour membrane curvature. Additionally, ceramides elicit a pro-apoptotic action74, which is in agreement with increased apoptosis in RomiCabo conditions. Second, romidepsin drastically perturbs the spindle proteome, leading to defects in spindle organization and centrosome position during mitosis. Perturbed cell cycle progression is corroborated by a reduction of CyclinD1, p-CDK1, AurKA, and an increase of p21. Our experimental evidence indicates that romidepsin likely acts independently on lipid regulators and mitotic spindle. This is supported by our lipidomic studies showing distinct lipid profiles in cells treated with PLK1i versus romidepsin, despite both treatments trigger G2/M and cell cycle alterations. Nevertheless, it is likely that direct perturbations exerted by romidepsin on lipids and on cell cycle also reciprocally impact both cellular processes. This is consistent with an intricated link between lipid composition, cell shape, and mitosis evidenced by previous studies55–57.
Reports from the literature have linked HDACi to altered transcription factor activity, decreased anti-apoptotic and increased pro-apoptotic signals, deregulation of cell cycle regulators, ROS generation, disruption of chaperon protein function, and inhibition of DNA repair75. In addition to perturbed cell cycle regulators, we show that romidepsin alters the levels of several signals, including MYC, Survivin, and XIAP, shown in the literature to be essential in HCC. Thus, romidepsin dwindles a set of signals below a threshold level, while perturbing lipid metabolism and the mitotic spindle. Collectively, such context confers on HCC cells a vulnerable state, exacerbating their dependency on support pathways like those provided by RTKs. The dependency of romidepsin-treated HCC cells to RTK inputs is exemplified by a switch from cytostatic effects of romidepsin into cytotoxicity when combined with RTKi, even if cells do not respond to RTKi alone (Fig. 10). Thus, romidepsin could expand the range of HCC patients potentially benefiting from RTKi treatment. Nevertheless, we showed that different RTKis are not equivalent in their combinatorial action with romidepsin, as their potency varies among the cell lines we tested. It is tempting to speculate that the difference in the effects of distinct combinations may rely on multiple factors, including: a) the initial, although slight, sensitivity of HCC cells to each RTKi; b) the effects of romidepsin on the levels of each RTK (e.g., levels of FGFR4, but not of MET, are reduced in in romidepsin-treated JHH5 cells); c) the vulnerability conferred by romidepsin in relation to the HCC subtypes (e.g., Hep3B cells are sensitive, whereas SNU449 cells are more resistant). As for current clinical treatments, a remaining challenge is the prediction of HCC patients responding to HDACi combined with - an optimal - RTKi. Treatment evaluations with patients-derived tumoroids could be a pertinent strategy towards personalized medicine. Undeniably, the identification of predictive biomarkers for treatment response would be of tremendous help.
Fig. 10. Scheme summarizing the effect of romidepsin alone or in combination with cabozantinib.
Romidepsin (Romi): 1 perturbs cell cycle signals and reduces cell survival regulators, 2 alters lipid metabolism modulators, and 3 causes defects in the mitotic machinery. Collectively, these events lead to a cytostatic effect and confer HCC cells dependency on RTK signalling support, which is an exploitable vulnerability. Consequently, the combination of romidepsin with RTKi (indicated here with cabozantinib; Cabo) leads to cytotoxic effects. Moreover, RomiCabo induces an immune infiltration and a switch towards an immune stimulatory profile, contributing to tumours regression. NT: non-treated cells. Scheme was performed with public open source Servier Medical Art, licensed under CC BY 4.0. Source data are provided as a Source Data file.
Our evaluation of RomiCabo effects in a preclinical setting using the Alb-R26Met model, recapitulating the HCC heterogeneity and matching molecular and immune features of HCC patients26,59,60, revealed at least three relevant points to take into consideration. First, this combinatorial treatment triggers regression/quiescence of about 80% of both early and advanced tumours. Furthermore, for still evolving tumour, the increase in tumour volume over time was significantly reduced compared to non-treated tumours. The heterogeneity in the tumour response following RomiCabo treatment exemplifies the heterogeneity of HCC patients, a key feature recapitulated by the Alb-R26Met model. Future in vivo studies will clarify the effectiveness of romidepsin combined with other RTKi, and in distinct mouse genetic settings. Second, a proportion of tumours still responds to RomiCabo after a “drug holiday”, implying the possibility of managing responding tumours over time. It will be interesting to longitudinally assess the therapeutic benefit of pulses of treatments to optimally handle the disease while limiting the appearance/expansion of resistant clones. This is linked to an emerging concept proposing that minimal residual disease cells, also defined as drug-tolerant persister cells, can regenerate the tumour cell population, although most of the population remains sensitive to the initial therapy76. The influence of pulses versus constant treatments has been additionally reported, and mathematically modelled, in some studies77–79. In the absence of an eradication treatment, such scenario could offer the possibility to transform responding tumours into a chronic, manageable disease over time, while limiting the emergence of resistant cells and reducing drug side effects. Molecularly, in RomiCabo treated tumours we found a striking upregulation of Rad23b, previously reported to be positively correlated with HCC stabilisation in patients treated with belinostat (a pan-HDACi)32. Future clinical studies could determine whether zinc finger protein 64 (ZFP64) levels in blood cells might predict the response of HCC to RomiCabo treatment, as suggested for resminostat plus sorafenib33.
Despite the effects on HCC cells, RomiCabo treatment increases the immune infiltration in the tumour microenvironment and leads to a striking immune landscape remodelling. This includes a switch in the composition of immune cell type infiltrates: increased granulocytes, activated moDC and cDC2, concomitant to increased TL-CD4+ and NK-TLs, and changes in the activation state of both TLs and NK-TLs (reduced PD1+-exhausted and increased CD44+-activated cells). Furthermore, RomiCabo treated tumours have increased Icos/Icosl co-stimulatory signals, which are required for optimal anti-tumour response in immune-checkpoint inhibitor treated tumour-bearing mice80. We extended the relevance of our findings to human MoDC by showing a direct downregulation by RomiCabo of CD80, a potential mechanism contributing to the increased immunogenicity observed in vivo. This is supported by previous studies showing that CD80 levels are reduced in HDACi-treated cells81. Moreover, it has been reported that CD80 in DC is involved in maintenance of immune tolerance82, its blockage potentiates CD8+ TL83, and that CD80 preferentially binds to CTLA-4 rather than CD28 on T cells, promoting regulatory T cell activation, leading to a more immunoregulatory environment compared to CD8663. Thus, the immune-stimulatory profile we document in RomiCabo treated tumours is likely a combinatorial effect of the resulting cytotoxicity on cancer cells and of the direct action of drugs on immune cells. The upregulation of Ctla4/Cd80, Lgals9, and Lag3 in RomiCabo-treated tumours is indicative of possible strategies to further reinforce antitumoral effects with the use of ICIs blocking their action. This would require comparative studies of different ICI combinations with RomiCabo to identify the best treatment type, while analysing tumour and immune cells to desirably link type of response with signatures. Interestingly, a very recent study reported that the upregulation of HDAC1/2/3 correlates to ICI resistance in patients. The use of a specific class I HDAC inhibitor, CXD101, in a tumour mouse model, resensitized to ICI therapy through STAT-1 and IFN-γ pathways84. In our study, we demonstrate that romidepsin resensitizes HCC cells and tumours to RTKi, and that RomiCabo remodels the tumour immune landscape, which can be indicative of pertinent ICI treatment other than the traditional anti-PD1/PDL1 pathway.
In conclusion, our findings illustrate how RTKi, used for years in the clinic to treat HCC patients, are invaluable agents also for therapeutic combinations with romidepsin. Notwithstanding the lack of signatures to select patients, the benefit of immunotherapies is encouraging, although not potentiated when combined with RTKi. Among reasons, it is possible that their use as single agents to target cancer cells is not sufficient as mainly cytostatic. A potential benefit of combining pan-HDACi with immune checkpoint inhibitors has been suggested by a mouse model based on xenograft injection of HCC cells85, as well as in other cancer models86,87. In view of romidepsin effects we reported here, we think that combinatorial treatments of romidepsin with anticancer agents plus adequate immunotherapies deserve further evaluation to design pertinent clinical trials.
Methods
Ethics statement
All procedures involving the use of animals were performed in accordance with the European Community Council Directive of 22 September 2010 on the protection of animals used for experimental purposes (2010/63/EU). The experimental protocols were carried out in compliance with institutional Ethical Committee guidelines for animal research (comité d’éthique pour l’expérimentation animale – Comité d’éthique de Marseille) and in compliance with French law, under an agreement number E1305521, “Ministère de l’Enseignement Supérieur de la Recherche et de l’Innovation”. Mice were kept in a dedicated pathogen-free facility, with a light/dark cycle, and in cages with an enriched environment, and controlled temperature and humidity. Mice received Safe Complete Care Competence (SAFE A04) as complete aliment ad libitum and were housed in environmentally enriched cages under pathogen-free conditions. The project authorization of Maina laboratory relevant to this study is APAFIS #8214-2016121417291352.v5, delivered by the “Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation”. Concerning the HCC-St.Joseph cohort of FFPE samples, the ethical approval and informed patient consent was obtained.
Analysis of human RNA-seq and (phospho)proteomic data
The human RNA-seq and clinical data from LIHC-TCGA are available through UCSC Xenabrowser portal (https://xenabrowser.net/) (50 normal liver and 372 primary tumour samples, from which only 371 had survival information), both LICA-FR (161 tumour samples), and LIRI-JP (202 normal liver and 243 tumour samples) through ICGC Data Portal (https://dcc.icgc.org/). The data from GSE14520 (GPL571 and GPL3921; 239 normal liver and 247 tumour samples, without survival information for five) and GSE144269 (70 patients with paired normal liver and tumour samples) cohorts were downloaded from NCBI GEO data portal (https://www.ncbi.nlm.nih.gov/gds). The calculation of Log2 Fold Change (log2 tumour/control samples) was applied to each individual patient using as a control the mean of control samples (TCGA, GSE14520). For the LICA-FR cohort, as no normal samples are publicly available, we used instead the data available in GTEX portal (https://gtexportal.org/home/) for healthy liver samples (226 normal liver samples). For overall survival and disease-free interval, patients were segregated in groups using the median as a cut-off for patients expressing either high or low HDACs levels. Data from the STORM trial (GSE109211) included patients receiving as adjuvant treatment either placebo (73 patients) or sorafenib (67 patients). Proteome and phospho-proteome data from HCC patient biopsies (for proteome: 33 control and 186 tumour samples; for phospho-proteomics: 12 control and 72 tumour samples) are available in PRIDE (PXD025705 and PXD025836, respectively)35. Transcriptomics data from HCC biopsies (15 control and 155 tumour samples) from HCC-NatCom cohort is available upon request in EGA repository (EGAD00001008205).
Cell culture
HepG2 (ATCC HB-8065) cells were grown in EMEM (ThermoFisher Scientific). HLF (JCRB0405), Huh7 (JCRB0403), and Hep3B (ATCC HB-8065) cells were grown in DMEM (ThermoFisher Scientific). HLE (JCRB0404) and SNU449 (ATCC CRL-2234) cells were grown in RPMI (Gibco), while JHH5 cells were grown in William E medium (ThermoFisher Scientific). Unless differently indicated, all the media were supplemented with 10% foetal bovine serum, penicillin-streptomycin, and sodium pyruvate. JHH5, SNU449, and Huh7 cells were kindly provided by S. Rebouissou. All other cells were obtained from ATCC or JCRB without further authentication. Cells were cultured in an incubator at 37 °C and 5% CO2. All cells are routinely tested by PCR-based assay to verify that they are free of Mycoplasma contamination.
Patient-derived HCC tumoroid and organoid generation and culture
HCC tumour tissues for tumoroid generation were collected from patients undergoing diagnostic liver biopsy at the University Hospital Basel. Written informed consent was obtained from all patients, and the study was approved by the local ethics committee (protocol number EKNZ 2014-099). Tumoroids were generated as described previously58. Briefly, tumour tissue was dissociated and seeded into basement membrane extract type 2 (BME2, Bio-Techne). After BME2 polymerization, expansion medium (EM) was added. The EM composition is: Advanced DMEM/F-12 (ThermoFisher Scientific) supplemented with B-27 (ThermoFisher Scientific), N-2 (ThermoFisher Scientific), 10 mM Nicotinamide (Sigma), 1.25 mM N-Acetyl-L-cysteine (Sigma), 10 nM [Leu15]-Gastrin (Sigma), 10 μM Forskolin (Tocris), 5 μM A83-01 (Tocris), 50 ng/mL EGF (Peprotech), 100 ng/mL FGF10 (Peprotech), 25 ng/mL HGF (Peprotech), 10% RSpo1-conditioned medium (v/v, homemade). For each organoid and concentration, Romi was compared to ComboA (Cabo 0.68 µM) and ComboB (Cabo 1.3 µM). Python 3.11.5 and Scipy 1.15.2 were used to perform 8 unpaired t-tests (https://docs.scipy.org/doc/scipy/reference/generated/scipy.stats.ttest_ind.html) for each independent organoid. Correction was applied using Benjamini-Hochberg method (https://docs.scipy.org/doc/scipy/reference/generated/scipy.stats.false_discovery_control.html).
Drug treatment and cell viability assay
The drugs used on cell cultures are listed in Supplementary Data 20 (TargetMol). Cell viability (collectively cell survival and proliferation, as assessed using the Cell Counting Kit-8) was determined as previously reported59. Briefly, 10000 or 5000 cells/well (for studies in Figs. 2 and 3, respectively) were seeded in 150 µL volume of medium per well in 96 well plates in the presence of 10% serum. After 24 h, inhibitors were applied, either individually or in combination, at the indicated concentrations for the indicated time. The Cell Counting Kit-8 (CCK-8) colorimetric assay (TargetMol) was used to assess cell viability after 48 h. Colorimetric signals were measured with a spectrophotometer microplate reader (Berthold). Cell viability was normalized to non-treated (NT) cells. Data obtained from viability assays are the mean of at least three independent experiments performed in triplicate. Synergy map and bliss score were calculated using SynergyFinder (http://www.synergyfinder.org/#!/); score > 0 was considered as a synergic effect.
For drug sensitivity assays involving tumoroids, following dissociation into single cells using 0.25% Trypsin-EDTA (ThermoFisher Scientific), cells were plated at 1000 cells/well in 384-well microplates. After 3 days, drugs were added with a D300e digital dispenser (Tecan). Cell viability was measured after 5 days of treatment using the CellTiter-Glo 3D reagent (Promega). Results were normalized to vehicle control (DMSO) and curve fitting was performed using Prism v9 (GraphPad) software and nonlinear regression (four parameters model).
Western blots
Protein expression levels in HCC cell lysates of non-treated and treated cells (treatments are listed in Supplementary Data 20) were analysed by Western blot, using the EBM protein extraction buffer (20 mM Tris HCl pH 7.5, 150 mM NaCl, 5 mM EGTA, 5 mM EDTA, 10% glycerol, 1% Triton) supplemented by a cocktail of protease (5 µg/mL leupeptin, 5 µg/mL pepstatin, 2 µg/mL aprotinin, 5 mM PMSF) and phosphatase (10 mM NaF, 1 mM NaPP, 1 mM Na3VaO4, 10 mM β-glycero-phosphate) inhibitors, following previously described procedures88,89. FIJI software was used for quantification of Western blots. Full blots of gels, obtained by acquiring the ECL signal using the BioRad imager system, were merged with a picture of the membranes, and the corresponding Ponceau red stain are shown in Source Data File. The antibodies used for western blots are reported in Supplementary Data 21.
Immunocytochemical analyses on cultured cells
Human HCC cell lines were fixed and processed for immunofluorescent staining as previously reported61,90,91. List of antibodies used for immunostaining are reported in Supplementary Data 21. Briefly, after 12 h, 16 h, or 24 h treatment with romidepsin and cabozantinib, alone or in combination, Huh7 cells were fixed in 4% paraformaldehyde (PFA) for 30 min, then washed three times with PBS for 5-10 min. The fixed samples were then permeabilized with 0,3% TritonX-100 for 20 min, blocked with 4% BSA in PBS for 1 h. The blocking solution diluted to 1% BSA was used to dilute primary antibodies and samples were incubated for 2 h at RT in a humidified chamber. Cells were then washed with PBS and incubated with secondary antibodies (diluted 1:400) for 1 h at RT in a humidified chamber. After washes, cells were incubated with DAPI (5 µg/mL). For NuMa staining, cells were fixed and processed for immunofluorescent staining as previously reported92. Coverslips were then mounted using ProLong™ Gold Antifade Mountant (ThermoFisher Scientific: #P10144) and images were taken with a Zeiss LSM780 Confocal (63X objective).
Preparation of samples for proteomics studies
Huh7 cells, either non-treated or treated for 24 h with romidepsin, cabozantinib, or RomiCabo, were lysed using the RIPA protein extraction buffer (20 mM Tris HCl pH 7.5, 150 mM NaCl, 5 mM EGTA, 5 mM EDTA, 10% glycerol, 1% SDS) with the addition of proteases and phosphatases inhibitors (5 µg/mL leupeptin, 5 µg/mL pepstatin, 2 µg/mL aprotinin, 5 mM PMSF, 10 mM NaF, 1 mM NaPP, 1 mM Na3VaO4, 10 mM β-glycerophosphate). Lysates were then sonicated and after 30 min in ice they were centrifuged at 13000 g for 15 min (4 °C). Supernatants were collected and stored at -80 °C. Proteomic analyses were performed at BoRdeaux Institute of onCology (BRIC).
Label-free quantitative proteomics
Three independent biological replicates on total protein extracts from Huh7 cells treated for 24 h with romidepsin, cabozantinib or their combination were used for proteomic analysis. 10 µg of proteins were loaded on a 10% acrylamide SDS-PAGE gel and proteins were visualized by Colloidal Blue staining. Migration was stopped when samples had just entered the resolving gel and the unresolved region of the gel was cut into only one segment. The steps of sample preparation and protein digestion by the trypsin were performed as previously described93. Specifically, each SDS-PAGE band was cut into 1 mm×1 mm gel pieces. Gel pieces were discoloured in 25 mM ammonium bicarbonate (NH4HCO3), 50% Acetonitrile (ACN) and shrunk in ACN for 10 min. After ACN removal, gel pieces were dried at room temperature. Proteins were first reduced in 10 mM dithiothreitol, 100 mM NH4HCO3 for 60 min at 56 °C then alkylated in 100 mM iodoacetamide, 100 mM NH4HCO3 for 60 min at room temperature and shrunk in ACN for 10 min. After ACN removal, gel pieces were rehydrated with 50 mM NH4HCO3 for 10 min at room temperature. Before protein digestion, gel pieces were shrunk in ACN for 10 min and dried at room temperature. Proteins were digested by incubating each gel slice with 10 ng/µL of trypsin (V5111, Promega) in 40 mM NH4HCO3, rehydrated at 4 °C for 10 min, and finally incubated overnight at 37 °C. The resulting peptides were extracted from the gel by three steps: a first incubation in 40 mM NH4HCO3 for 15 min at room temperature and two incubations in 47.5% ACN, 5% formic acid for 15 min at room temperature. The three collected extractions were pooled with the initial digestion supernatant, dried in a SpeedVac, and resuspended with 0.1% formic acid for a final concentration of 0.02 µg/µL. NanoLC-MS/MS analysis were performed using an Ultimate 3000 RSLC Nano-UPHLC system (ThermoFisher Scientific) coupled to a nanospray Orbitrap Fusion™ Lumos™ Tribrid™ Mass Spectrometer (ThermoFisher Scientific). Each peptide extracts were loaded on a 300 µm ID x 5 mm PepMap C18 precolumn (ThermoFisher Scientific) at a flow rate of 10 µL/min. After a 3 min desalting step, peptides were separated on a 50 cm EasySpray column (75 µm ID, 2 µm C18 beads, 100 Å pore size, ThermoFisher Scientific: #ES903) with a 4-40% linear gradient of solvent B (0.1% formic acid in 80% ACN) in 115 min. The separation flow rate was set at 300 nL/min. The mass spectrometer operated in positive ion mode at a 1.9 kV needle voltage. Data was acquired using Xcalibur 4.4 software in a data-dependent mode. MS scans (m/z 375-1500) were recorded at a resolution of R = 120000 (@ m/z 200) and a standard AGC target, ions collected within 50 ms, followed by a top speed duty cycle of up to 3 seconds for MS/MS acquisition. Precursor ions (2 to 7 charge states) were isolated in the quadrupole with a mass window of 1.6 Th and fragmented with HCD@30% normalized collision energy. MS/MS data was acquired in the Orbitrap cell with a resolution of R = 30000 (@m/z 200), a standard AGC target and a maximum injection time in automatic mode. Selected precursors were excluded for 60 s. Protein identification was done in Proteome Discoverer 2.5. Mascot 2.5 algorithm was used for protein identification in batch mode by searching against a Uniprot Homo sapiens database (79 071 entries, release January, 2022). Two missed enzyme cleavages were allowed for the trypsin. Mass tolerances in MS and MS/MS were set to 10 ppm and 0.02 Da. Oxidation (M) and acetylation (K) were searched as dynamic modifications and carbamidomethylation (C) as static modification. Raw LC-MS/MS data were imported in Proline Web94 for feature detection, alignment, and quantification. Proteins identification was accepted only with at least 2 specific peptides with a pretty rank=1 and with a protein FDR value less than 1.0% calculated using the “decoy” option in Mascot. Label-free quantification of MS1 spectra by extracted ion chromatograms (XIC) was carried out with parameters indicated previously93. Protein ratios were median normalized. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD041166.
For proteome data analysis, the differential expression was calculated as the ratio of treated (romidepsin, cabozantinib, or the combined treatment RomiCabo) versus non-treated cells, and log2 transformed. Only significantly expressed proteins were considered (p value ≤ 0.05). For heatmap representation (Fig. S11a), z-score was calculated as: (mean (treated cells) – mean (non-treated cells))/standard-deviation of treated and non-treated cells (a top200 ranking of upregulated proteins of the proteome is reported in Supplementary Data 1). The list of differentially expressed proteins with the distinct treatments were confronted and the intersections is represented as a Venn Diagram (http://www.interactivenn.net/; Fig. S11b, Supplementary Data 2). The Ingenuity Pathway Analysis (IPA) was performed using the Qiagen software (Fig. S5a, Supplementary Data 3-5). For GO_Biological_Process (Fig. 4a, Supplementary Data 6-10) we represented the top-ranking pathways obtained through the platform: https://maayanlab.cloud/Enrichr/. For spindle proteome analysis, proteins over- or under-expressed after single or combined treatment in Huh7 cells were extracted from our proteomics data and compared with the spindle proteome list previously published53. Only significantly deregulated proteins found were represented in the heatmap shown in Fig. S11A.
Lipidomic analysis
2×106 Huh7 or Hep3B cells were seeded in 10 cm dish and the day after were treated with drugs (romidepsin, cabozantinib, or RomiCabo; each combination in four independent biological replicates). After 24 h, cells were washed with PBS, scraped and collected in 15 mL tubes for centrifugation at 2500 rpm for 5 min. Supernatant was discarded and pellet was snap frozen. In parallel, number of cells was counted with trypan blue and an aliquot was used to quantify the sample protein with Bradford method. Lipids were extracted according to a modified Bligh and Dyer protocol. 200 µL of water was added to the cell pellets. After vortexing (30 s), samples were transferred in a glass tube, then 4 µL of internal Standard deutered SPLASH Lipidomix was added. 500 µL of methanol was used to rinse the first tube and then transferred in the glass tube to which 250 µL of chloroform was finally added. The mixture was vortexed for 10 min before a new addition of 250 µL of water and 250 µL of chloroform. The mixture was vortexed again for 10 min and finally centrifuged (3000 rpm at 4 °C for 15 min). Then, 400 µL of the organic phase was collected in a new glass tube and dried under a stream of nitrogen. The dried extract was resuspended in 60 µL of isopropanol/methanol/water 50/30/20 and transferred in an injection vial. Reverse phase liquid chromatography was selected for separation with an UPLC system (Ultimate 3000, ThermoFisher Scientific). Lipid extracts were separated on an Accucore C18 150 ×2.1, 2.5 µm column (ThermoFisher Scientific) operated at 400 µL/min flow rate. The injection volume was 3 µL. Eluent solutions were ACN/H2O 50/50 (V/V) containing 10 mM ammonium formate and 0.1% formic acid (solvent A) and IPA/ACN/H2O 88/10/2 (V/V) containing 2 mM ammonium formate and 0.02% formic acid (solvent B). The step gradient of elution was in % B: 0.0 min, 35%; 4.0 min, 60%; 8.0 min, 70%; 16.0 min, 85%; 25.0 min, 97%. The UPLC system was coupled with a Q-exactive Mass Spectrometer (ThermoFisher Scientific); equipped with a heated electrospray ionization (HESI) probe. This spectrometer was controlled by Xcalibur software (version 4.1.31.9.) and operated in electrospray positive and negative mode. Reagents used were LC-MS grade: Acetonitrile (ACN) and isopropropanol (IPA; Carlo Erba); Chloroform (CHCl3; Merck); Methanol (MeOH; VWR); Formic acid Optima LC-MS quality (ThermoFisher Scientific); Ultrapure water (Purelab flex; Veolia Water); formate ammonium (99%; Acros Organics).
Data were acquired with dd-MS2 mode. MS spectra were acquired at a resolution of 70 000 (200 m/z) in a mass range of 250 − 1200 m/z with an AGC target 1e6 value of and a maximum injection time of 100 ms. 15 most intense precursor ions were selected and isolated with a window of 1 m/z and fragmented by HCD (Higher energy C-Trap Dissociation) with normalized collision energy (NCE) of 25 and 30 eV. MS/MS spectra were acquired in the ion trap with an AGC target 1e5 value, the resolution was set at 35 000 at 200 m/z combined with an injection time of 80 ms. Data were reprocessed using Lipid Search 4.2.21 (ThermoFisher). The product search mode was used and the identification was based on the accurate mass of precursor ions and MS2 spectral pattern. The parameters for the ion identification and alignment were set up as follow: ion precursor tolerance 5 ppm; ion product tolerance 5 ppm; ion and adducts searched [H + ], [NH4 + ] and [Na + ]; alignment retention time tolerance 0.4 min; ID quality filters A, B and C.
Lipidomic bioinformatic analysis
Bioinformatic analysis of the lipidomic profiling was performed using R (4.2.2). To normalize the LC-MS/MS peak areas, we selected metabolites that were detected in at least 50% of the samples. The remaining missing values were replaced by half of the minimum peak area measured in the data set. Next, the probabilistic quotient normalization (PQN) method95 was performed for data normalization, using the “non-treated” samples as reference. The final normalized values were log2 transformed. For computation of the z-scores, each lipid was centred by the reference samples’ mean and scaled by the reference samples’ standard deviation. For the differential analysis, t-tests were used to compare peaks between groups and fold changes were calculated for each lipid. A cut-off threshold of 0.05 for p-value and fold change of 1.5 was used to select the most significant lipids. The Benjamini-Hochberg method was used to correct p-value for multiple testing. Hierarchical clustering was performed on the log transformed normalized data (mean centred and scaled by standard deviation). Clustering was performed using the Ward method and Pearson’s correlation was used for distance calculation.
Reactive oxygen Species analysis by flow cytometry
Huh7 cells were seeded at a confluency of 1.2 × 105 cells per well in a 12 well-plate. After 24 h, cells were treated with drugs (romidepsin, cabozantinib, or RomiCabo; in quadruplicates). After 24 h, cells were washed with PBS and incubated with MitoSox (Invitrogen: #M36008, 3 µM) and MitoTracker (Invitrogen: #M7514, 3 µM) for 20 min at 37 °C and 5% CO2. Cells were then washed with PBS and detached with trypsin. After centrifugation at 600 rpm for 5 min, cells were resuspended in PBS and analysed by flow cytometry.
LXR luciferase reporter assay
Huh7 cells were seeded at a confluency of 0.4 × 106 cells per well in a 6 well-plate. After 24 h, cells were transfected with Lipofectamine LTX as per manufacturer’s instruction (ThermoFisher Scientific: #15338100) with a plasmid containing the sequence for the LXR response element coupled to luciferase (Addgene: #177622 LXRE Luc). After 24 h cells were detached and split into 24 well-plate at a confluency of 4 × 104. The day after, cells were treated with romidepsin, cabozantinib, RomiCabo, an LXR agonist alone or in combination with romidepsin (non-treated cells as negative control). After 24 h of treatment, cells were lysed and luciferin reagent was added following manufacturer’s instructions (Promega: #E1910). Luciferase activity was measured using a Polarstar Omega plate reader.
Cell cycle and DNA damage analysis by flow cytometry
Huh7, Hep3B, and JHH5 cells were seeded in a 10 cm dish, and after 24 h treated with drugs (romidepsin, cabozantinib, RomiCabo) or non-treated as control. Cells were detached at different time points (16 h, 24 h, and 48 h), centrifuged, washed twice with PBS, then resuspended in FACS buffer (PBS with 2% FBS and 2 mM EDTA). Live count percentage was verified with Biorad TC20 cell counter. Dead cells were stained with Zombie Acqua fixable viability dye (Biolegend: #423102) following manufacturer’s instructions, then permeabilized with 70% cold ethanol for 1 h on ice. Subsequently, cells were washed to rehydrate them and incubated with 0.5 µg/mL of anti-Ki67-Alexa Fluor 488 (eBiosciences, clone SolA15e: #16320994), or/and anti-phosphoS28Histone H3 (Miltenyi, clone REA379: #130-126-509) or anti-γH2AX (Ser139) (Biolegend, clone 2F3: #613420) for 20 min. Cell were washed with FACS buffer. 2 µg/mL of Propidium Iodide plus 10 µg/mL of RNAse was added and cells were immediately acquired in the spectral cytometer Aurora from Cytek, with the 5 L 16UV-16V-14B-10YG-8R configuration, and using spectro-flow software. Data were analysed by using FlowJo software v10.10.
Alb-R26Met mice
The generation of the R26stopMet mice (international nomenclature Gt(ROSA)26Sortm1(Actb-Met)Fmai) carrying a conditional mouse-human chimeric Met transgene inserted at the Rosa26 locus has been previously reported89,96,97. In the R26stopMet model, slightly enhanced wild-type MET levels are achieved following removal of the stop cassette using the Cre recombinase59. In the Alb-R26Met mice, with increased MET levels in the liver, tumours spontaneously form over time. Tumours recapitulate the most aggressive HCC patient subtype defined as “proliferative-progenitor”, primary resistance to drugs used in clinic, the molecular and immune heterogeneity of patients, and the temporal heterogeneity of tumour onset59,60,89. The Alb-R26Met HCC, as the “proliferative-progenitor” patients, is characterized by a striking enrichment in genes that are simultaneously overexpressed and hypermethylated in gene body CpG islands26,61. The mouse line expressing Cre recombinase under the Alb promoter (B6.Cg-Tg(Alb-cre)21Mgn/J) was obtained from the Jackson Laboratory. Alb-R26Met mice were generated by crossing the R26stopMet (in 129S2/SvPasOrlRj strain) and Alb-Cre (in C57BL/6JRj strain) mice26,59. Consequently, tumours formed in F1 C57:129 Sv Alb-R26Met mice, genotyped by PCR analysis of genomic DNA as previously reported89,96. Only male mice with an age of 53 to 75 weeks were used in these studies.
cDNA and quantitative RT-PCR analysis
cDNA was synthesized using a Reverse Transcription Kit (iScript Supermix, Bio-Rad: #1708841). PCR reactions were performed using SYBR GreenERqPCR SuperMix (ThermoFisher Scientific: #11761) and specific primers designed with PrimerBlast NCBI tool (Supplementary Data 22). Expression levels were quantified using the comparative Ct method (2−ΔΔCT method) with the housekeeping gene 36b4 (Rplp0) as a control for internal normalization, and results are expressed as RQ = 2^(−ΔΔCT).
In vivo evaluation of RomiCabo treatment in tumour-bearing Alb-R26Met mice using the PIXSCAN-FLI system for longitudinal in vivo imaging
The PIXSCAN-FLI is a micro Photon-Counting Computed Tomography (PC-CT) scanner that we previously reported60,62. Briefly, it is made of three main blocks whose characteristics are: a) the X-ray source block embedding an X-ray tube Microfocus L12161-07 (Hamamatsu Photonics K.K., Japan) with a tungsten anode; b) the animal block embedding a rotative motor for performing tomographic scans that can be also translated along the three axes; c) the X-ray hybrid pixel camera comprising more than 500 000, 500 µm thick, 130 µm x 130 µm silicon pixels. To perform imaging, we used a specific contrast agent, the ExiTronNano 12000 (Miltenyi Biotec©), which is specifically uptaken by macrophages and liver resident Kupffer cells, therefore leading to highly contrasted liver (and spleen). Mice were anesthetized with gas anaesthesia Isoflurane to process imaging and received a dose of 110 mGy per scan. Imaging data acquisition and processing were performed following the previously described procedure62. Alb-R26Met mice carrying liver tumours were treated every two days by oral gavage with cabozantinib (10 mg/kg; TargetMol) and every four days by intraperitoneal injection with romidepsin (1 mg/kg; TargetMol). Romidepsin was prepared in DMSO diluted in PBS and cabozantinib in DMSO diluted in water. After “drug holidays”, mice were treated every two days with both drugs. Mice were treated for 31 days. Mouse body weight was monitored 3 times per week. Imaging was performed every 10 days. The ethical maximum tumour volume accepted is 1500 mm³, and was not exceeded for this study. Livers and tumours were dissected and immediately frozen in dry ice for RT-qPCR or dissociated for spectral flow cytometry analyses.
Cell dissociation for spectral flow cytometry analysis
Dissected tumours were cut in small pieces and placed in a 2 mL eppendorf tube for cell dissociation. 1 mL of an enzymatic solution was added in each tube. The solution contained collagenase IV (0.75 mg/mL; Sigma Aldrich: #C1538-500MG) and DNAse I (25 µg/mL; Sigma Aldric: #11284932001) diluted in CO2-independent medium (ThermoFisher Scientific: #11580536), supplemented with 5% FBS and penicillin/streptomycin. After an incubation of 15-20 min of the samples in a water bath at 37 °C (pipetting up and down every 5 min to facilitate cell dissociation), the solution with dissociated cells was then filtered through 70 µm nylon strainers into a new 2 mL tube. The filter was washed with 1 mL of FACS buffer (48.8 mL of PBS, 1 mL of FBS, 200 µL of EDTA 0.5 M pH8). Dissociated cells were incubated 5 min on ice and centrifuged 5 min at 1000 rpm 4 °C. Cell pellet was resuspended in 1 mL of ACK red blood cell lysis buffer (ThermoFisher Scientific: #A1049201), incubated 1 min on ice, then centrifuged for 5 min at 1000 rpm 4 °C. The cell pellet was then resuspended in 500 µL of FACS buffer. Cell count and viability were verified with trypan blue using a Biorad Cell Counter (TC20), and samples maintained in ice before spectral cytometry analysis.
Spectral cytometry analysis
For all single cell suspensions from dissociated tumours, cells were washed and filtered, and Fc receptors were blocked (anti-CD16/CD32, Invitrogen). Cells were then incubated with the corresponding antibody panel (Supplementary Data 19) for 15 min at 4 °C in FACS buffer (PBS1 x 2 mm EDTA 2% FBS). Cells were washed twice and resuspended in FACS buffer plus 5 ng/mL of DAPI. Samples were acquired in the spectral cytometer Aurora from Cytek, with the 5 L 16UV-16V-14B-10YG-8R configuration, and using spectro-flow software. Supervised and unsupervised analysis were performed using OMIQ software. Clustering of filtered CD45+ cells was performed using FlowSOM method and UMAP was used to visualize the tumour immune compartment.
Total mRNA extraction from patient FFPE samples
FFPE patient samples from St Joseph Hospital (HCC-St.Joseph cohort; Marseille, France) were processed with a microtome whose surface was cleaned with DNAzap (Invitrogen), and 8-10 slices of 10 µm were recovered into a RNAse free tube. A total mRNA was extracted following manufacturer instructions of PureLink™ FFPE RNA Isolation kit (Invitrogen: #K156002). Quality and concentration of RNA were evaluated with Nanodrop (ThermoFisher Scientific).
Total mRNA extraction from cells or frozen tissue
Total mRNA from frozen tissues was isolated using the RNeasy Mini Kit (Qiagen), following the manufacturer’s instructions. DNase (Qiagen) treatment was included to eliminate genomic DNA. mRNA was extracted from frozen samples after homogenizing about 20 mg of tissue in the specific lysis buffer at 6300 rpm twice for 30 s using Precellys 24 (Bertin technologies), then the RNeasy Mini Kit (Qiagen) was used. Quality and concentration of RNA were evaluated with Nanodrop (ThermoFisher Scientific).
AST (aspartate aminotransferase) activity levels
Blood samples were collected after 20 days of treatments from a control mouse cohort in an EDTA-coated eppendorf tube, then plasma was obtained in the supernatant after centrifuging at 1200 rpm for 20 min at 4 °C. A colorimetric experiment was performed following the manufacturer’s instructions (Merck-Sigma: #MAK055). Briefly, 10 µL of plasma were used in each well and completed with the master reaction mix. Readings were performed at 37 °C each 5 min in the PolarStar Omega plate reader using A450 nm. Activity was calculated using the glutamate standards and the formula: AST activity ((A450 final-A450 initial)*Dilution factor)/((Tfinal-initial)*Volume). Time is expressed in minutes, and volume in milliliters; the final units of AST activity are milliunits/mL or U/L.
Purification of human peripheral blood mononuclear cells (PBMCs) from adult blood
Fresh blood samples were collected from healthy donors and obtained from the Établissement Français du Sang (EFS; Marseille, France). A contract has been established between EFS and the Centre de Recherche en Cancérologie de Marseille (CRCM), in conformity with national regulations and ethical guidelines. Written informed consent was obtained for each healthy donor. PBMCs were isolated by centrifugation on a Ficoll gradient (Ficoll-Paque PLUS, GE Healthcare Life Sciences: #GE17-1440-03).
Monocyte-derived dendritic cells (MoDC) generation and activation
Monocytes were selected from PBMCs using anti-CD14 antibody-coated magnetic beads and magnetic columns, according to the manufacturer’s instructions (MiltenyiBiotec: #130-050-201). To generate immature MoDCs, CD14+ cells were cultured for 5 days with IL-4 (50 ng/mL; PeproTech: #200-04-5UG) and GM-CSF (10 ng/mL; PeproTech: #300-03-5UG) in RPMI 1640 Medium, GlutaMAX (ThermoFisher Scientific) with 10% FCS. MoDCs were treated for 18 h with medium, LPS (100 ng/mL, LPS-EB Ultrapure, activates TLR4 only, ThermoFisher Scientific) alone, together with conditioned medium from untreated or RomiCabo-treated Huh7 for 24 h, or medium with or without RomiCabo (to test the direct effect of the drug). Differentiation and maturation profile of MoDC was assessed by using anti-CD14 APC-Vio770 (Miltenyi, clone REA599: #130-110-522), anti-CD1a-PE-Cy7 (Biolegend, clone HI149: #300122), anti-CD80-PE (BD Pharmingen, clone L307.4: #557227), anti-CD86-BV605 (BD Horizon, clone 2331: #562999), HLA-DR (Miltenyi, clone REA805: #130-111-788), and DAPI. Cells were processed with a spectral cytometer Aurora from Cytek, with the 5 L 16UV-16V-14B-10YG-8R configuration, and using spectro-flow software. Data were analysed by using FlowJo software v10.10.
Statistical analysis
All data were analysed using GraphPad Prism software (version-8 and version-9). Normality of the samples was assessed using the Shapiro-Kolmogorov test. For data showing normal distribution, the statistically significant differences between 2 groups of samples were estimated by applying an unpaired Student t-test, or one-way ANOVA (for more than two groups of samples) followed by multiple comparison Dunnet’s test (all samples compared to control), Tukey’s test (all samples compared). For samples with a non-Gaussian distribution of data, either the Mann-Whitney test (2 groups) or the Kruskal-Wallis test (more than 2 groups) was used. Results are expressed as the mean ± standard error of the mean; SEM; in dot plots, each dot corresponds to each analysed sample and notches correspond to the mean. For overall survival and disease-free interval, statistic was performed by Logrank and/or Grehan-Breslow tests. Statistical significance (p-values) was defined as not significant (ns): p > 0.05; *: p < 0.05; **: p < 0.01; ***: p < 0.001. Significance is indicated in figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
These results are in part based upon public data generated by TCGA Research Network: http://cancergenome.nih.gov/ or in public repositories including ICGC DataPortal, Firebrowse, and NCBI GEO. We thank: the MET’CONNECT structuring action funded by Canceropôle PACA for lipidomic analyses; all members of our labs and F. Helmbacher for helpful discussions; K. Wangensteen and V. Géli for extremely valuable feedback on the manuscript; D. Grégoire and G. Desandre for precious suggestions and help with experiments; S. Rebuissou for providing the JHH5, SNU449, and Huh7 cell lines; A.A. Raymond on the Oncoprot platform for running protein extracts for proteomic studies; G. Charras to share the protocol for NuMa staining; people at the CRCM core facilities for their essential technical support on mouse, cytometry, and microscopy. This work was financially supported by GEFLUC – Les Entreprises contre le Cancer, ARC (Association pour la Recherche sur le Cancer; PJA20181208172 and ARCPGA2023010005822_6352), FdF (Fondation de France; 2014_00051580 and 2016_00067080) to F.M; by INCa (2020-11/279/NI-KA) to F.S. and F.M.; with financial support from ITMO Cancer of Aviesan within the framework of the 2021-2030 Cancer Control Strategy, on funds administered by Inserm (21CD125-00) to F.M. and J.P.B; from AFEF (Association Française pour l’étude du Foie) and Canceropôle Provence-Alpes-Côte d’Azur – Institut Cancer Immunologie to C.S. This work received support from the French government under the France 2030 investment plan, as part of the Initiative d’ Excellence d’Aix-Marseille Université - A*MIDEX (AMX-19-IET-001). F.M. lab receives funding from Région Provence-Alpes-Côte d’Azur and Canceropôle Provence-Alpes-Côte d’Azur, SATT Sud-Est, and Ruban Rose Avenir. C.S. was supported by a Fondation pour la Recherche Médicale (FRM) fellowship. M.G. was supported by a Fondation de France (FdF) fellowship. F.C was supported by the 80 Prime project DePIcT of the Mission pour les Initiatives Transverses et Interdisciplinaires (MITI) of CNRS. The development of the PIXSCAN-FLI PC-CT prototype was partly supported by France Life Imaging (grant ANR-11-INBS-44-0006 from the French PIA (Programme d’Investissements d’Avenir) and by the Cancéropôle Provence-Alpes-Côte d’Azur. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
C.S.: performed computational work with human HCC databases and proteomics outcomes, metabolic studies, contributed to molecular and functional analysis, data analysis and interpretation; provided input on studies and contributed to write the manuscript. M.G.: performed Western blot analysis, the majority of in vitro experiments, data analysis and interpretation; provided input on studies and contributed to write the manuscript. F.C.: performed all the in vivo studies, including imaging analysis and processing, contributed to data analysis and interpretation; provided input on studies and on the manuscript. A.D.: performed spindle proteome analysis, immunostaining studies, data analysis and interpretation; provided input on studies and on the manuscript. P.M.V.: performed flow cytometry, immune cell experiments, data analysis and interpretation; provided input on studies and on the manuscript. M.M.: performed flow cytometry and data analysis. S.R.: generated all Alb-R26Met mouse cohort required for studies and contributed to in vivo studies. A. E.: contributed to computational work with human HCC databases. P.K.: performed computational work with lipidomic outcomes and data interpretation. D.D.: performed lipidomic analysis and contributed to data interpretation. L.K.: performed studies with patient-derived tumoroids. S.N.: designed and performed studies with patient-derived tumoroids and contributed to data interpretation. Y.B.: supervised and provided input on in vivo imaging analysis. M.D.: provided input on in vivo imaging analysis. S.P.: provided input on studies. G.B.: provided input on studies. J.W.D.: performed proteomic analysis. F.S.: supervised and provided input on proteomics analysis. M.H.H.: provided access to human HCC patient transcriptomic, proteomic datasets, and patient-derived tumoroids. S.V.: contributed to interpret proteomic data on metabolism and provided input on studies. X.A.: provided HCC samples and clinical data for RT-qPCR analysis. F.G.: contributed to interpreting proteomic and lipidomic outcomes; provided input on studies and contributed to writing the manuscript. J.P.B.: provided input on studies and on the manuscript. C.M.: supervised and provided input on in vivo imaging studies and on the manuscript. F.M.: designed the study, contributed to experimental work, analysed and interpreted data, ensured financial support, and wrote the manuscript. F.C and A.D equally contributed to this work.
Peer review
Peer review information
Nature Communications thanks Hai-long Piao and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All human transcriptomic and clinical data from the different cohorts used in this manuscript were retrieved from publicly available sources. All details and accession numbers are reported in the Materials and Methods section. (Phospho)Proteomic data from human HCC biopsies (HCC-NatCom cohort) were accessed upon request to Dr. M. Heim, EGAS00001005074 (EGA repository). The data generated from mass spectrometry of Huh7 cells proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository and are accessible with the dataset identifier PXD041166 through the link: https://www.ebi.ac.uk/pride/archive/projects/PXD041166. The data generated from lipidomic studies in Huh7 and Hep3B cells are deposited in the Metabolomics Workbench repository under the accession number 10.21228/M84P01. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Celia Sequera, Margherita Grattarola.
Contributor Information
Celia Sequera, Email: celia.SEQUERA-HURTADO@univ-amu.fr.
Flavio Maina, Email: flavio.maina@univ-amu.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62934-0.
References
- 1.Yu, X. et al. Cancer epigenetics: from laboratory studies and clinical trials to precision medicine. Cell Death Discov.10, 28 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Barrena, M. G., Arechederra, M., Colyn, L., Berasain, C. & Avila, M. A. Epigenetics in hepatocellular carcinoma development and therapy: The tip of the iceberg. JHEP Rep.2, 100167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberland, M., Montgomery, R. L. & Olson, E. N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet10, 32–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du, G., Yang, R., Qiu, J. & Xia, J. Multifaceted influence of histone deacetylases on dna damage repair: Implications for hepatocellular carcinoma. J. Clin. Transl. Hepatol.11, 231–243 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang, F. F., Hu, T., Liu, J. Q., Yu, X. Q. & Ma, L. Y. Histone deacetylases (HDACs) as the promising immunotherapeutic targets for hematologic cancer treatment. Eur. J. Med Chem.245, 114920 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Squarzoni, A., Scuteri, A. & Cavaletti, G. HDACi: The columbus’ egg in improving cancer treatment and reducing neurotoxicity? Cancers (Basel)14 (2022). 10.3390/cancers14215251 [DOI] [PMC free article] [PubMed]
- 7.Contreras-Sanzon, E. et al. Histone deacetylases modulate resistance to the therapy in lung cancer. Front Genet13, 960263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondarev, A. D. et al. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharm.87, 4577–4597 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Pramanik, S. D. et al. Potential of histone deacetylase inhibitors in the control and regulation of prostate, breast and ovarian cancer. Front Chem.10, 948217 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biersack, B., Nitzsche, B. & Hopfner, M. HDAC inhibitors with potential to overcome drug resistance in castration-resistant prostate cancer. Cancer Drug Resist5, 64–79 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wawruszak, A. et al. Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells. Cancers (Basel)1110.3390/cancers11020148 (2019). [DOI] [PMC free article] [PubMed]
- 12.Hontecillas-Prieto, L. et al. Synergistic enhancement of cancer therapy using hdac inhibitors: opportunity for clinical trials. Front Genet11, 578011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty, E. & Sarkar, D. Emerging therapies for hepatocellular carcinoma (HCC). Cancers (Basel)1410.3390/cancers14112798 (2022). [DOI] [PMC free article] [PubMed]
- 14.Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med380, 1450–1462 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Yang, J. D. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol.16, 589–604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlen, M.et al.A pathology atlas of the human cancer transcriptome. Science357, 10.1126/science.aan2507 (2017). [DOI] [PubMed]
- 17.Llovet, J. M. & Hernandez-Gea, V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res.20, 2072–2079 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Bruix, J., Chan, S. L., Galle, P. R., Rimassa, L. & Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol.75, 960–974 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Yau, T. et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol.23, 77–90 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Nagaraju, G. P., Dariya, B., Kasa, P., Peela, S. & El-Rayes, B. F. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol.86, 622–632 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Wilson, C. L., Mann, D. A. & Borthwick, L. A. Epigenetic reprogramming in liver fibrosis and cancer. Adv. Drug Deliv. Rev.121, 124–132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann, D. A. Epigenetics in liver disease. Hepatology60, 1418–1425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claveria-Cabello, A. et al. Identification and experimental validation of druggable epigenetic targets in hepatoblastoma. J. Hepatol.79, 989–1005 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Toh, T. B., Lim, J. J. & Chow, E. K. Epigenetics of hepatocellular carcinoma. Clin. Transl. Med8, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braghini, M. R. et al. Epigenetic remodelling in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res41, 107 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arechederra, M. et al. Hypermethylation of gene body CpG islands predicts high dosage of functional oncogenes in liver cancer. Nat. Commun.9, 3164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsilimigras, D. I., Ntanasis-Stathopoulos, I., Moris, D., Spartalis, E. & Pawlik, T. M. Histone deacetylase inhibitors in hepatocellular carcinoma: A therapeutic perspective. Surg. Oncol.27, 611–618 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Coradini, D. & Speranza, A. Histone deacetylase inhibitors for treatment of hepatocellular carcinoma. Acta Pharm. Sin.26, 1025–1033 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Garmpis, N. et al. Histone Deacetylase Inhibitors in the Treatment of Hepatocellular Carcinoma: Current Evidence and Future Opportunities. J. Pers. Med.11, 10.3390/jpm11030223 (2021). [DOI] [PMC free article] [PubMed]
- 30.Biersack, B., Polat, S. & Hopfner, M. Anticancer properties of chimeric HDAC and kinase inhibitors. Semin Cancer Biol.83, 472–486 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Suraweera, A., O’Byrne, K. J. & Richard, D. J. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: Achieving the full therapeutic potential of HDACi. Front Oncol.8, 92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo, W. et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J. Clin. Oncol.30, 3361–3367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitzer, M. et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma - The SHELTER study. J. Hepatol.65, 280–288 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Gordon, S. W. et al. Phase I study of sorafenib and vorinostat in advanced hepatocellular carcinoma. Am. J. Clin. Oncol.42, 649–654 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Ng, C. K. Y. et al. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat. Commun.13, 2436 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pflum, M. K., Tong, J. K., Lane, W. S. & Schreiber, S. L. Histone deacetylase 1 phosphorylation promotes enzymatic activity and complex formation. J. Biol. Chem.276, 47733–47741 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Loponte, S. et al. Dynamic phosphorylation of histone deacetylase 1 by aurora kinases during mitosis regulates zebrafish embryos development. Sci. Rep.6, 30213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai, S. C. & Seto, E. Regulation of histone deacetylase 2 by protein kinase CK2. J. Biol. Chem.277, 31826–31833 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Galasinski, S. C., Resing, K. A., Goodrich, J. A. & Ahn, N. G. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J. Biol. Chem.277, 19618–19626 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Adenuga, D. & Rahman, I. Protein kinase CK2-mediated phosphorylation of HDAC2 regulates co-repressor formation, deacetylase activity and acetylation of HDAC2 by cigarette smoke and aldehydes. Arch. Biochem Biophys.498, 62–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan, D. H. et al. Protein kinase CK2 regulates the dimerization of histone deacetylase 1 (HDAC1) and HDAC2 during mitosis. J. Biol. Chem.288, 16518–16528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ropero, S. et al. A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat. Genet38, 566–569 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Doroshow, D. B., Eder, J. P. & LoRusso, P. M. BET inhibitors: a novel epigenetic approach. Ann. Oncol.28, 1776–1787 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell146, 904–917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chueh, A. C., Tse, J. W., Togel, L. & Mariadason, J. M. Mechanisms of histone deacetylase inhibitor-regulated gene expression in cancer cells. Antioxid. Redox Signal23, 66–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lachenmayer, A. et al. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol.56, 1343–1350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu, F. T. et al. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappa B signaling. Int J. Oncol.45, 177–188 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Soukupova, J. et al. Resminostat induces changes in epithelial plasticity of hepatocellular carcinoma cells and sensitizes them to sorafenib-induced apoptosis. Oncotarget8, 110367–110379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, B. & Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol.14, 452–463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang, J. Y. L. & Ferrell, J. M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell Endocrinol.548, 111618 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalaany, N. Y. & Mangelsdorf, D. J. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev. Physiol.68, 159–191 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Hoang, J. J., Baron, S., Volle, D. H., Lobaccaro, J. M. & Trousson, A. Lipids, LXRs and prostate cancer: are HDACs a new link?. Biochem Pharm.86, 168–174 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Sauer, G. et al. Proteome analysis of the human mitotic spindle. Mol. Cell Proteom.4, 35–43 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Crosio, C. et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell Biol.22, 874–885 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atilla-Gokcumen, G. E. et al. Dividing cells regulate their lipid composition and localization. Cell156, 428–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koberlin, M. S. et al. A fast-acting lipid checkpoint in G1 prevents mitotic defects. Nat. Commun.15, 2441 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scaglia, N., Tyekucheva, S., Zadra, G., Photopoulos, C. & Loda, M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle13, 859–868 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nuciforo, S. et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep.24, 1363–1376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan, Y. N. et al. A phosphokinome-based screen uncovers new drug synergies for cancer driven by liver-specific gain of nononcogenic receptor tyrosine kinases. Hepatology66, 1644–1661 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Cannet, F. et al. Tracing specificity of immune landscape remodeling associated with distinct anticancer treatments. iScience28, 112071 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arechederra, M. et al. ADAMTSL5 is an epigenetically activated gene underlying tumorigenesis and drug resistance in hepatocellular carcinoma. J. Hepatol.74, 893–906 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Cassol, F. et al. Tracking dynamics of spontaneous tumors in mice using photon-counting computed tomography. iScience21, 68–83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pentcheva-Hoang, T., Egen, J. G., Wojnoonski, K. & Allison, J. P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity21, 401–413 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Lehmann, U. et al. Distinct methylation patterns of benign and malignant liver tumors revealed by quantitative methylation profiling. Clin. Cancer Res11, 3654–3660 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Ler, S. Y. et al. HDAC1 and HDAC2 independently predict mortality in hepatocellular carcinoma by a competing risk regression model in a southeast asian population. Oncol. Rep.34, 2238–2250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagaraju, G. P., Dariya, B., Kasa, P., Peela, S. & El-Rayes, B. F. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol10.1016/j.semcancer.2021.07.017 (2021). [DOI] [PubMed]
- 67.Yang, X. et al. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell42, 180–197 (2024). [DOI] [PubMed] [Google Scholar]
- 68.Grattarola M. et al. Establishment of a mouse hepatocellular carcinoma tumoroid panel recapitulating inter- and intra- heterogeneity for disease modelling and combinatorial drug discovery. Cell Communication and Signaling10.1186/s12964-025-02391-w (in press).
- 69.Huang, C. & Freter, C. Lipid metabolism, apoptosis and cancer therapy. Int J. Mol. Sci.16, 924–949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kunduri, G., Acharya, U. & Acharya, J. K. Lipid polarization during cytokinesis. Cells11, 10.3390/cells11243977 (2022). [DOI] [PMC free article] [PubMed]
- 71.Szlasa, W., Zendran, I., Zalesinska, A., Tarek, M. & Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr.52, 321–342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larsen, J. B. et al. Membrane curvature and lipid composition synergize to regulate N-Ras anchor recruitment. Biophys. J.113, 1269–1279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMahon, H. T. & Boucrot, E. Membrane curvature at a glance. J. Cell Sci.128, 1065–1070 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alizadeh, J. et al. Ceramides and ceramide synthases in cancer: Focus on apoptosis and autophagy. Eur. J. Cell Biol.102, 151337 (2023). [DOI] [PubMed] [Google Scholar]
- 75.Bose, P., Dai, Y. & Grant, S. Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharm. Ther.143, 323–336 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marine, J. C., Dawson, S. J. & Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer20, 743–756 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Foo, J. & Michor, F. Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput Biol.5, e1000557 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hastings, J. F. et al. Analysis of pulsed cisplatin signalling dynamics identifies effectors of resistance in lung adenocarcinoma. Elife9, 10.7554/eLife.53367 (2020). [DOI] [PMC free article] [PubMed]
- 79.Greene, J. M., Gevertz, J. L. & Sontag, E. D. Mathematical approach to differentiate spontaneous and induced evolution to drug resistance during cancer treatment. JCO Clin. Cancer Inf.3, 1–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu, T., He, Q. & Sharma, P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res71, 5445–5454 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Frikeche, J. et al. Impact of HDAC inhibitors on dendritic cell functions. Exp. Hematol.40, 783–791 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Sansom, D. M., Manzotti, C. N. & Zheng, Y. What’s the difference between CD80 and CD86?. Trends Immunol.24, 314–319 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Lang, T. J., Nguyen, P., Peach, R., Gause, W. C. & Via, C. S. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J. Immunol.168, 3786–3792 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Tu, Y. et al. Pharmacological activation of STAT1-GSDME pyroptotic circuitry reinforces epigenetic immunotherapy for hepatocellular carcinoma. Gut74, 613–627 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Llopiz, D. et al. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol. Immunother.68, 379–393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi, Y. et al. Romidepsin (FK228) regulates the expression of the immune checkpoint ligand PD-L1 and suppresses cellular immune functions in colon cancer. Cancer Immunol. Immunother.70, 61–73 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deng, R. et al. HDAC is indispensable for IFN-gamma-induced B7-H1 expression in gastric cancer. Clin. Epigenetics10, 153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furlan, A. et al. Met acts through Abl to regulate p53 transcriptional outcomes and cell survival in the developing liver. J. Hepatol.57, 1292–1298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan, Y. et al. Tissue-specific gain of RTK signalling uncovers selective cell vulnerability during embryogenesis. PLoS Genet11, e1005533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castellanet, O. et al. BCL-XL blockage in TNBC models confers vulnerability to inhibition of specific cell cycle regulators. Theranostics11, 9180–9197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lamballe, F. et al. Modeling heterogeneity of triple-negative breast cancer uncovers a novel combinatorial treatment overcoming primary drug resistance. Adv. Sci. (Weinh.)8, 2003049 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelkar, M. et al. Spindle reorientation in response to mechanical stress is an emergent property of the spindle positioning mechanisms. bioRxiv10.1101/2022.02.13.480269 (2022). [DOI] [PMC free article] [PubMed]
- 93.Campion, O. et al. LRP-1 Matricellular Receptor Involvement in Triple Negative Breast Cancer Tumor Angiogenesis. Biomedicines9, 10.3390/biomedicines9101430 (2021). [DOI] [PMC free article] [PubMed]
- 94.Bouyssie, D. et al. Proline: an efficient and user-friendly software suite for large-scale proteomics. Bioinformatics36, 3148–3155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem.78, 4281–4290 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Genestine, M. et al. Enhanced neuronal Met signalling levels in ALS mice delay disease onset. Cell Death Dis.2, e130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tonges, L. et al. Hepatocyte growth factor protects retinal ganglion cells by increasing neuronal survival and axonal regeneration in vitro and in vivo. J. Neurochem117, 892–903 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All human transcriptomic and clinical data from the different cohorts used in this manuscript were retrieved from publicly available sources. All details and accession numbers are reported in the Materials and Methods section. (Phospho)Proteomic data from human HCC biopsies (HCC-NatCom cohort) were accessed upon request to Dr. M. Heim, EGAS00001005074 (EGA repository). The data generated from mass spectrometry of Huh7 cells proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository and are accessible with the dataset identifier PXD041166 through the link: https://www.ebi.ac.uk/pride/archive/projects/PXD041166. The data generated from lipidomic studies in Huh7 and Hep3B cells are deposited in the Metabolomics Workbench repository under the accession number 10.21228/M84P01. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.