Abstract
The vast majority of flowering plants depend on animal pollinators for sexual reproduction. These plants usually provide a reward, such as nectar and/or pollen, to their pollinators, and floral scent is often key to attract them. Some plants, however, do not provide any such reward, though they advertise one. Even though it is well known that such a food-deceptive pollination strategy is particularly common in orchids, the role of floral scent in attracting pollinators in such systems is often poorly understood. In this study, we compared the floral scent of the Eurasian deceptive lady’s slipper orchid Cypripedium calceolus with six co-flowering rewarding species visited by the same pollinators. Cypripedium calceolus produced more floral scent compounds than the co-flowering rewarding species together and differed in the floral scent composition from them. However, C. calceolus shared at least one compound with each co-flowering rewarding species, including widespread and less widespread compounds among flower scents, and had more compounds in common with the co-flowering rewarding species than they had with each other. Several compounds of C. calceolus, such as the aliphatic compounds 1-octanol, octyl acetate, and decyl acetate, did not occur in co-flowering plants but are known as pheromones of pollinating bees. Together, our results suggest that C. calceolus not only emits compounds that are generally common among flowering plants and attractive to many pollinators but specifically imitates floral scent compounds of multiple co-flowering plant species/pheromones of bees. These findings provide valuable insights into the ecology and evolution of floral scent in deceptive pollination systems in orchids.
Keywords: volatile organic compounds, deceptive pollination, co-flowering community, generalized food-deception, mimicry, orchid
1. Introduction
The vast majority of angiosperms rely on animal pollinators, primarily insects, for sexual reproduction (Ollerton et al., 2011). Most of these plants provide rewards, such as pollen and nectar (Endress, 1996), which they typically advertise through visual and olfactory cues (Chittka and Raine, 2006). However, not all plant species that signal a reward actually provide one (Johnson and Schiestl, 2016). Such deceptive pollination systems are particularly common in orchids (Orchidaceae), where approximately a third of all species studied do not produce floral rewards (van der Pijl and Dodson, 1966; Dafni, 1984; Nilsson, 1992). Most of the deceptive orchids are food deceptive (60% of the deceptive species) or sexually deceptive (38%) and exploit the food- or mate-seeking behavior, respectively, of their pollinators (Ackerman et al., 2023). In sexually deceptive orchids, the mechanisms of deception by highly specific floral shapes, structures, and especially scents reminiscent of relevant traits of female insects are well studied (Johnson and Schiestl, 2016; Peakall, 2023; Slavković and Bendahmane, 2025), whereas in food-deceptive systems, there are large gaps in the understanding of which floral traits shape these interactions (Johnson and Schiestl, 2016).
There are two main strategies in food-deceptive orchids (Jersáková et al., 2009; Schiestl and Schlüter, 2009). One is “Batesian floral mimicry” in which the deceptive species mimics a specific model species in one or more floral traits to attract the nectar- or pollen-seeking pollinators of the model species such as bees, beetles, and flies (Dafni, 1984; Roy and Widmer, 1999; van der Cingel, 2001). Previous studies have highlighted the importance of visual similarity between models and mimics in explaining the evolutionary drivers of specialized food mimicry (Peter and Johnson, 2008; Jersáková et al., 2012). For example, in the South African orchid Disia pulchra, which is pollinated by long-proboscid tabanid flies, artificial flowers with the same color spectra as the model species, but without scent, successfully attracted the pollinators (Jersáková et al., 2012). The role of floral scent in such Batesian mimicry systems seems to be less important (but see Scaccabarozzi et al., 2025), at least in short-distance attraction. However, it has been hypothesized that floral scent plays a more important role in long-distance attraction in systems such as between the orchid Orchis israelitica and its model, the lily Bellevalia flexuosa (Galizia et al., 2005). In the other, more common food-deceptive strategy, which is “generalized food deception”, pollinators are most likely deceived by floral traits widespread in rewarding flowers (Johnson and Schiestl, 2016). As an intermediate between Batesian floral mimicry and generalized food deception, guild mimicry has been suggested, in which the deceptive species imitates guilds of co-flowering species. Such guild mimicry has recently been suggested for Traunsteinera globosa (Jersáková et al., 2016).
A plant species with a yet unknown deceptive pollination strategy is the charismatic and widespread Eurasian lady slipper orchid Cypripedium calceolus. It has a bright yellow color and some widespread floral scent compounds, such as linalool and benzaldehyde (Nilsson, 1979; Braunschmid et al., 2017), that point to a generalized food-deceptive strategy. However, the flowers also release compounds less widespread among floral scents, such as (Z)-3-nonenyl acetate and lilac alcohol (Braunschmid et al., 2017), and even compounds described as pheromones of some of its pollinators, such as decyl acetate (Tengö and Bergström, 1977; Nilsson, 1979), rather indicating mimicry of specific plants and/or insects. The compounds attractive to the pollinators (mainly bees, but also hoverflies) (Braunschmid et al., 2021) need, however, to be determined. Also, the floral scents of co-flowering rewarding species of C. calceolus remain to be characterized, and the extent to which the floral scent of C. calceolus resembles the scents of these plants remains to be assessed (Schiestl, 2005; Jersáková et al., 2006).
In this study, we assessed the similarity of the floral scent of C. calceolus to co-flowering rewarding plant species in a population in the Bavarian Alps, Germany. Specifically, we asked (1) which floral scent compounds were shared between C. calceolus and each of the co-flowering plant species as well as which compounds the co-flowering plant species shared among each other and (2) how similar they were in their relative scent composition. If C. calceolus shared compounds generally widespread among floral scents with its co-flowering rewarding plant species, it would point to generalized food deception. If, however, it imitated the scent of specific co-flowering rewarding species, mimicry would more likely be involved.
2. Materials and methods
2.1. Study species
Cypripedium calceolus L. is a terrestrial, perennial orchid. Its distribution range is the boreal and temperate zones of Europe and Asia, and it grows in a variety of habitats, such as open to medium-shaded deciduous and coniferous forests, and alpine meadows and rubble, but predominantly on calcareous soil (Cribb, 1997). It flowers from May to July (in May/June in our focal population). Cypripedium calceolus has a plant height of up to 60 cm and the largest and most conspicuous flowers among European orchids. The inflorescence consists of one to two, rarely more, flowers. The yellow, 3- to 4-cm-long shoe-shaped labellum acts as a semi-trap. Once trapped, the pollinators can only escape through a posterior opening where they come into contact with reproductive organs (Cribb, 1997; Kull, 1999; Braunschmid et al., 2017). The remaining two petals and the three sepals have a lanceolate shape and a purple-brown color. The pollen grains are aggregated in a sticky smear. The flowers produce an apple- or apricot-like scent. Previous studies have shown that floral scent differs among regions and populations (Braunschmid et al., 2017, 2021). Cypripedium calceolus can reproduce vegetatively via horizontal rhizomes, is self-compatible, but relies on small insect pollinators for successful pollination (Nilsson, 1979 and references therein). The primary pollinators are various solitary bees, such as Lasioglossum spp. (L. bavaricum, the L. calceatum/L. albipes species complex, L. fratellum, L. fulvicorne, L. leucozonium, L. morio, L. quadrinotatum) and Andrena spp. (A. bicolor, A. cineraria, A. fucata, A. haemorrhoa, A. helvola, A. jacobi [=A. carantonica], A. nigroaenea, A. praecox, A. tibialis), and also hoverflies (Eristalis rupium, Pipiza austriaca, Platycheirus albimanus) (Nilsson, 1979; Braunschmid et al., 2017), which are most likely attracted by a combination of visual and olfactory cues (Daumann, 1968; Nilsson, 1979; Bergström et al., 1992; Braunschmid et al., 2017). Capsules contain several thousands of dust seeds that are wind dispersed (Kull, 1999).
2.2. Study site
The study was carried out in a C. calceolus population on the shore of the mountain lake Königssee, Berchtesgaden National Park, Bavaria, Germany. It is one of the populations with the highest number of individuals (1,000–2,000 shoots) and the population with the highest number of scent compounds and the highest scent emission of the four populations included in the study by Braunschmid et al. (2017). At this site, C. calceolus grows in a wet grassland patch near the lake shore, surrounded by very light forests of Salix spp. and Picea abies, and is mainly pollinated by the Lasioglossum calceatum/L. albipes species complex but also other solitary bees and some hoverflies (Braunschmid et al., 2017).
2.3. Assessment of co-flowering plant community
The co-flowering rewarding community was assessed and defined as all species in the vicinity (radius of 10 m) of flowering C. calceolus individuals that are known (e.g., Müller, 1881; Westrich, 2019; preliminary own observations) to be visited by insects (species, co-generics) that have been observed as flower visitors/pollinators of C. calceolus (Braunschmid et al., 2017, 2021). This resulted in eight species belonging to five families: Leontodon incanus L. (pollinators: e.g., Halictus rubicundus, H. tumulorum, the Lasioglossum calceatum/L. albipes species complex, L. leucozonium), Hieracium bifidum Kit. ex Hornem. (e.g., the Lasioglossum calceatum/L. albipes species complex), and Bellidiastrum michelii Cass. (e.g., Halictus sp., Nomada sp.) of the Asteraceae; Dryas octopetala L. (e.g., Lasioglossum albipes, L. morio) and Potentilla erecta (L.) Raeusch (Lasioglossum sp.) of the Rosaceae; Hippocrepis comosa L. (Lasioglossum calceatum) of the Fabaceae; Globularia cordifolia L. (Halictus sp. and/or Lasioglossum sp.) of the Plantaginaceae; and Primula farinosa L. (Halictus sp. and/or Lasioglossum sp.) of the Primulaceae ( Figure 1 ).
Figure 1.
Photos of the pollination units of Cypripedium calceolus (a) and the six co-flowering rewarding species that emitted any detectable scent and were included in the statistical analyses: Hieracium bifidum (b), Hippocrepis comosa (c), Leontodon incanus (d), Primula farinosa (e), Globularia cordifolia (f), and Dryas octopetala (g). White bars next to each pollination unit represent 1 cm.
2.4. Floral scent collection and analysis
The scent data on 14 individuals of C. calceolus of the study population were from Braunschmid et al. (2017), who collected floral scent from one flower per individual in May 2014 using dynamic headspace. We collected the floral scent of the eight co-flowering rewarding species using the same approach as Braunschmid et al. (2017). All our sampling took place, as for C. calceolus (Braunschmid et al., 2017), between 10:00 and 16:00. Floral scent was collected from five individuals each of L. incanus, D. octopetala, H. comosa, G. cordifolia, P. farinosa, and P. erecta, from seven individuals of H. bifidum, and from three individuals of B. michelii. Samples were collected in 2023 for all individuals except for two H. bifidum individuals and three G. cordifolia individuals, which were collected in 2015. For scent collection, one inflorescence (five inflorescences for P. erecta) was bagged with a polyethylene oven bag (10 × 30 cm; Toppits, Germany), and the scent was trapped for 30 min (5 min in case of P. farinosa) directly after bagging on an adsorbent tube (quartz glass tube: length 25 mm; inner diameter 2 mm) filled with 1.5 mg each of Carbotrap B (mesh 20–40, Supelco, Germany) and Tenax TA (mesh 60–80; Supelco, Germany). For scent collection, a rotary vane pump G 12/01 EB (Gardner Denver, Germany) with a flow of 200 ml/min was used. Negative controls were obtained by conducting the same procedure but for empty oven bags (n = 3) and two leaf samples per species.
Samples were stored in a freezer (−20°C) and analyzed within 2 weeks after collection with a gas chromatograph coupled to a mass spectrometer (GC/MS-QP2010 Ultra, Shimadzu Corporation, Japan), and to a thermal desorption (TD) unit (TD-20, Shimadzu, Japan), and equipped with a ZB-5 fused silica column (5% phenyl polydimethylsiloxane; 60-m long, inner diameter 0.25 mm, film thickness 0.25 μm, Phenomenex, USA), which was the same setup used by Braunschmid et al. (2017). Using the same settings as Braunschmid et al. (2017), samples were desorbed at 250°C for 15 min (flow: 25 ml/min) and cryofocused on a cold trap at −20°C in the TD-20, before they were transferred to the GC (cold trap heated to 250°C, transfer line from TD-20 to GC set to 260°C). Samples were run at a column flow (carrier gas: helium) of 1.5 ml/min. GC oven temperature started at 40°C, then increased by 6°C per min to 250°C, and was held for 1 min. The MS interface was set at 260°C and the ion source at 200°C. Mass spectra were taken at 70 eV (in EI mode) from m/z 30 to 350. The GC/MS data were processed using GCMSolution Version 4.11 software (Shimadzu Corporation, Japan). Compounds were tentatively identified by matches with the NIST 11, Wiley 9, FFNSC 2, Essential Oils, and Robert P. Adams 2007 mass spectral and retention index data bases and were confirmed by comparing mass spectra and retention times with those of authentic standards available in the stock collections of the Plant Ecology lab at the University of Salzburg. Known amounts of monoterpenes, aliphatics, and aromatics were injected into the GC/MS system, and mean peak areas of these compounds were used to calculate the total absolute amount of scent in our samples (see Dötterl et al., 2005). For analysis, we then calculated the scent emitted per pollination unit per hour. The pollination unit was an inflorescence for all co-flowering species except for D. octopetala, in which it was a single flower.
In all samples of P. erecta and B. michelii as well as in one sample of L. incanus, no flower-specific scent compounds were detected. Thus, P. erecta and B. michelii and the scentless sample of L. incanus were excluded from all statistical analyses and visualizations.
2.5. Statistical analyses
Data were processed and visualized using the statistical software program R (version 4.0.3; R Core Team, 2020) unless described otherwise.
We analyzed differences in the number of compounds emitted in two ways. At the level of individuals, we run a Kruskal–Wallis rank sum test in the base package in R followed by a Dunn’s test of multiple comparisons in the R package FSA (Ogle et al., 2021) with species as factor to assess whether the number of compounds emitted per sample differed among species.
We visualized shared and non-shared floral scent compounds between C. calceolus and the co-flowering species by generating a chord diagram based on binary data (presence/absence) using the R package circlize (Gu et al., 2014). Differences in the number of compounds shared between C. calceolus and the rewarding co-flowering plants and among the co-flowering plants were analyzed with a Mann–Whitney U-test in the base package in R.
Differences in relative amounts of each scent compound were visualized with non-metric multidimensional scaling (NMDS) based on the Bray–Curtis similarities of relative amounts of each scent compound using the vegan (Oksanen et al., 2020) and ggplot2 (Wickham, 2016) R packages and statistically analyzed using a permutational multivariate analysis of variance (PERMANOVA) in PRIMER 6.1.15 (Clarke and Gogley, 2006) with PERMANOVA+ for PRIMER 1.0.5 (Anderson et al., 2008) based on Bray–Curtis similarities, with species as fixed factor, and using 9,999 permutations. Differences among species in multivariate dispersion in scent composition were assessed using a permutational analysis of multivariate dispersion (PERMDISP) in PRIMER 6.1.15 (Clarke and Gogley, 2006) with PERMANOVA+ for PRIMER 1.0.5 (Anderson et al., 2008), again based on Bray–Curtis similarities, species as factor, and 9,999 permutations.
3. Results
The total amount of scent emitted per pollination unit varied among species ( Table 1 ; Supplementary Table S1 ). With a mean emission rate of 156 ng/h, C. calceolus had an intermediate scent emission compared to that of the six co-flowering species. It was in the same order of magnitude as the mean scent emission rate of H. bifidum and P. farinosa, whereas the mean scent emission rate was almost 10 times higher in H. comosa and less than half the amount in D. octopetala, L. incanus, and G. cordifolia ( Table 1 ).
Table 1.
Sample size (# individuals), number of compounds across all samples of a species (# compounds), and mean (minimum–maximum) total amout of scent trapped (ng/h per pollination unit) and relative amount (%) of floral scent compounds of Cypripedium calceolus and the six co-flowering rewarding species Hieracium bifidum, Leontodon incanus, Globularia cordifolia, Primula farinosa, Dryas octopetala, and Hippocrepis comosa.
| RI | Trait | Hieracium bifidum | Leontodon incanus | Globularia cordifolia | Primula farinosa | Dryas octopetala | Hippocrepis comosa | Cypripedium calceolus |
|---|---|---|---|---|---|---|---|---|
| # individuals | 7 | 4 | 5 | 5 | 5 | 5 | 14 | |
| # compounds | 14 | 2 | 3 | 9 | 11 | 23 | 67 | |
| Total amount of scent trapped (ng/h per pollination unit) | 187 (52–544) | 22 (14–31) | 62 (14–124) | 221 (31–672) | 35 (1–81) | 1,533 (224–2,191) | 156 (34–652) | |
| Relative amounts (%) | ||||||||
| Aliphatic compounds | ||||||||
| 855 | (Z)-3-Hexen-1-ol* | – | – | – | – | – | – | 1 (0–4) |
| 866 | 1-Hexanol* | – | – | – | – | – | – | tr (tr–1) |
| 902 | Heptanal* | – | – | – | – | – | – | 2 (tr–10) |
| 913 | Pentyl acetate | – | – | – | – | – | – | tr (0–1) |
| 1,006 | (Z)-3-Hexenyl acetate* | – | – | – | – | – | – | 3 (1–11) |
| 1,011 | Hexyl acetate* | – | – | – | – | – | – | 4 (2–9) |
| 1,070 | 1-Octanol* | – | – | – | – | – | – | 1 (tr–3) |
| 1,111 | Heptyl acetate* | – | – | – | – | – | – | 1 (1–2) |
| 1,122 | 3-Octyl acetate | – | – | – | – | – | – | tr (0–tr) |
| 1,129 | Octyl formate | – | – | – | – | – | – | tr (0–tr) |
| 1,162 | Octanoic acid* | – | – | – | – | – | – | tr (0–1) |
| 1,200 | (Z)- or (E)-2-Octenyl acetate | – | – | – | – | – | – | tr (0–tr) |
| 1,210 | Octyl acetate* | – | – | – | – | – | – | 32 (1–46) |
| 1,272 | 1-Decanol* | – | – | – | – | – | – | tr (0–1) |
| 1,295 | (Z)-3-Nonenyl acetate | – | – | – | – | – | – | tr (tr–1) |
| 1,309 | Nonyl acetate | – | – | – | – | – | – | tr (tr–2) |
| 1,409 | Decyl acetate* | – | – | – | – | – | – | 8 (tr–17) |
| 1,475 | 1-Dodecanol* | – | – | – | – | – | – | tr (0–2) |
| 1,608 | Dodecyl acetate | – | – | – | – | – | – | tr (0–1) |
| 1,808 | Tetradecyl acetate* | – | – | – | – | – | – | tr (0–tr) |
| RI | Trait | Hieracium bifidum | Leontodon incanus | Globularia cordifolia | Primula farinosa | Dryas octopetala | Hippocrepis comosa | Cypripedium calceolus |
| Aromatic compounds | ||||||||
| 966 | Benzaldehyde* | 58 (34–100) | 69 (0–100) | – | – | – | – | 4 (tr–29) |
| 1,025 | p-Methylanisole* | – | – | – | tr (0–1) | – | – | tr (0–tr) |
| 1,037 | Benzyl alcohol* | 25 (0–64) | 31 (0–100) | – | 16 (0–46) | – | – | 1 (0–6) |
| 1,048 | Phenylacetaldehyde* | 5 (0–15) | – | – | – | – | – | tr (0–tr) |
| 1,074 | p-Cresol* | – | – | – | 18 (0–48) | – | – | tr (0–2) |
| 1,082 | Benzyl formate | – | – | – | – | – | – | tr (0–tr) |
| 1,095 | Guaiacol* | – | – | 5 (1–16) | – | – | – | – |
| 1,120 | 2-Phenylethanol* | 1 (0–2) | – | – | – | – | – | tr (0–1) |
| 1,148 | 1,2-Dimethoxybenzene* | – | – | 77 (57–96) | – | – | – | – |
| 1,168 | Benzyl acetate* | – | – | – | – | – | – | tr (0–2) |
| 1,188 | p-Creosol | – | – | – | 1 (0–2) | – | – | – |
| 1,205 | Methyl salicylate* | – | – | – | – | – | 1 (0–2) | tr (0–1) |
| 1,262 | 2-Phenylethyl acetate* | – | – | – | – | – | – | 1 (0–3) |
| 1,366 | Eugenol* | tr (0–tr) | – | 17 (3–37) | – | – | – | tr (0–tr) |
| 1,385 | Methyl-2-hydroxy-3-phenylpropionate* | – | – | – | – | – | 27 (21–34) | – |
| 1,669 | cf. 1,4-Dimethylindanyl acetate | – | – | – | – | – | – | tr (0–2) |
| Terpenoids | ||||||||
| 987 | 6-Methyl-5-hepten-2-one* | – | – | – | – | – | – | 2 (0–3) |
| 993 | β-Myrcene* | – | – | – | – | – | – | tr (0–1) |
| 1,018 | Pinocarvone* | – | – | – | – | – | – | tr (0–tr) |
| 1,039 | (Z)-β-Ocimene* | – | – | – | – | – | tr (0–1) | tr (0–tr) |
| 1,045 | Lavender lactone* | – | – | – | – | 24 (2–100) | – | tr (0–1) |
| 1,050 | (E)-β-Ocimene* | – | – | – | – | – | 8 (7–9) | tr (0–1) |
| 1,056 | (Z)-Arbusculone | – | – | – | – | 9 (0–20) | – | – |
| 1,074 | (E)-Arbusculone | – | – | – | – | 5 (0–16) | – | – |
| 1,078 | (Z)-Linalool oxide furanoid* | – | – | – | – | – | – | tr (0–tr) |
| 1,094 | (E)-Linalool oxide furanoid* | tr (0–1) | – | – | – | – | – | 1 (tr–2) |
| 1,103 | Linalool* | – | – | – | – | – | 1 (0–2) | 30 (14–63) |
| 1,132 | allo-Ocimene* | – | – | – | – | – | tr (tr–tr) | tr (0–tr) |
| 1,137 | Epoxyoxoisophorone* | – | – | – | 1 (tr–1) | – | – | tr (0–tr) |
| 1,140 | neoallo-Ocimene* | – | – | – | – | – | tr (0–tr) | – |
| 1,144 | (E)-Ocimene epoxide* | – | – | – | – | – | tr (tr–tr) | – |
| 1,148 | Lilac aldehyde A* | – | – | – | – | 13 (0–20) | – | – |
| 1,150 | 4-Oxoisophorone* | – | – | – | 60 (24–97) | – | – | 2 (0–5) |
| 1,157 | Lilac aldehyde B+C* | – | – | – | – | 32 (0–58) | – | – |
| 1,172 | Lilac aldehyde D* | – | – | – | – | 8 (0–15) | – | – |
| 1,173 | Dihydrooxoisophorone | – | – | – | 1 (0–4) | – | – | – |
| 1,176 | (Z)-Linalool oxide pyranoid* | – | – | – | – | – | – | tr (0–tr) |
| 1,180 | (E)-Linalool oxide pyranoid* | 1 (0–2) | – | – | – | – | – | tr (tr–tr) |
| 1,216 | 4-Methyleneisophorone | – | – | – | 2 (tr–5) | – | – | – |
| 1,219 | Lilac alcohol B+C* | – | – | – | – | 2 (0–3) | – | tr (0–tr) |
| 1,233 | Lilac alcohol D* | – | – | – | – | 1 (0–2) | – | tr (0–tr) |
| 1,233 | Nerol* | – | – | – | – | – | – | tr (0–tr) |
| 1,257 | Geraniol* | – | – | – | – | – | – | tr (0–tr) |
| 1,292 | (E)-Linalool oxide acetate pyranoid | – | – | – | – | – | – | tr (0–tr) |
| 1,348–1,363 | Lilac alcohol formate A-D | – | – | – | – | – | – | tr (0–1) |
| 1,349 | 8-Oxolinalool | – | – | – | – | 4 (0–7) | – | – |
| 1,384 | Geranyl acetate* | – | – | – | – | – | – | tr (0–tr) |
| 1,395 | α-Copaene* | 2 (0–7) | – | – | – | – | – | – |
| 1,407 | β-Isocomene | tr (0–1) | – | – | – | – | – | – |
| 1,444 | β-Caryophyllene* | – | – | – | – | – | tr (0–tr) | – |
| 1,462 | (E)-β-Farnesene* | – | – | – | – | – | – | tr (0–tr) |
| 1,498 | (Z,E)-α-Farnesene | – | – | – | – | – | – | tr (0–tr) |
| 1,513 | (E,E)-α-Farnesene* | – | – | – | – | – | – | tr (0–1) |
| C5-branched chain compounds | ||||||||
| 876 | Isoamyl acetate* | – | – | – | – | – | – | tr (0–tr) |
| Nitrogen-containing compounds | ||||||||
| 1,228 | 2-Aminobenzaldehyde* | – | – | – | – | – | 2 (2–3) | – |
| 1,305 | Indole* | – | – | – | – | – | 18 (13–27) | tr (0–tr) |
| 1,422 | N-Formyl-2-aminobenzaldehyde | – | – | – | – | – | 12 (4–23) | – |
| Miscellaneous cyclic compounds | ||||||||
| 1,390 | (E)-Jasmone | – | – | – | – | – | tr (0–tr) | – |
| 1,415 | (Z)-Jasmone* | – | – | – | – | – | 4 (2–6) | – |
| Unknown compounds | ||||||||
| 1,295 | m/z: 106, 135, 77, 79, 107 | – | – | – | – | – | 23 (10–43) | – |
| Other unknown compounds pooled(25) | 7 (0–30)5 | – | – | tr (0–1)1 | 3 (0–5)2 | 3 (1–6)8 | tr (0–1)9 | |
The compounds are sorted by compound class and retention index (RI). The number of individuals, the number of compounds emitted, and the total absolute amount are also given. Values >5% are highlighted in bold. The superscribed numbers for the "other unknown compounds pooled" give the number of compounds that were pooled across all species and separately for each species.
*Compound identification verified through authentic standard; tr, values <0.5% but >0%; - compound not detected.
In total, 105 scent compounds belonging to seven compound classes were detected across all samples of C. calceolus and the six co-flowering species ( Table 1 ; Supplementary Table S2 ). The number of compounds per scent sample differed among species (Kruskal–Wallis rank sum test: χ 2 6 = 38.05, p < 0.001) with C. calceolus emitting significantly more compounds than all other species except H. comosa ( Figure 2 ). Across all C. calceolus samples, 67 compounds belonging to six compound classes were detected—20 aliphatic compounds, 12 aromatic compounds, 24 terpenoids, one C5-branched chain compound, one nitrogen-containing compound, and nine unknown compounds—with the two aliphatic compounds octyl acetate (mean relative amount: 32%) and decyl acetate (8%) and the terpenoid linalool (30%) having a mean relative amount of at least 5% ( Table 1 ). These were more scent compounds than we detected across all samples of the six co-flowering species together (58 compounds) ( Table 1 ). The number of compounds emitted in the co-flowering species ranged from 2 to 23 ( Table 1 ). The samples of L. incanus and G. cordifolia contained only aromatic compounds, two in L. incanus (benzaldehyde with a mean relative amount of 69%, benzyl alcohol: 31%) and three in G. cordifolia (1,2-dimethoxybenzene: 77%, eugenol: 17%, and guaiacol: 5%) ( Table 1 ). The samples of D. octopetala comprised 11 compounds, 9 terpenoids, and 2 unknown compounds, and were dominated by 4 isomers of lilac aldehyde, together accounting for 53% of the total scent emission, followed by lavender lactone (24%) and (Z)-arbusculone (9%) ( Table 1 ). The samples of P. farinosa and H. bifidum comprised aromatic compounds, terpenoids, and unknown compounds ( Table 1 ). Nine compounds were detected in the samples of P. farinosa, mainly 4-oxoisophorone (60%), p-cresol (18%), and benzyl alcohol (16%), and the samples of H. bifidum contained 14 compounds among which benzaldehyde (58%) and benzyl alcohol (25%) were the dominant compounds ( Table 1 ). The samples of H. comosa contained 23 compounds belonging to five compound classes (aromatic compounds, terpenoids, nitrogen-containing compounds, miscellaneous cyclic compounds, and unknown compounds), with the scent being dominated by methyl-2-hydroxy-3-phenylpropionate (27%), an unknown compound (23%), and indole (18%).
Figure 2.

Number of compounds per sample among Cypripedium calceolus (circles) and six co-flowering rewarding species (diamonds). Each symbol represents an individual. Boxplots are shown for each species and indicate the median, the first and third quartiles, and maximum and minimum values. Individuals and boxplots are color-coded according to species. Different lowercase letters at the top of the graph indicate statistically significant differences in the number of scent compounds recorded (Dunn’s test).
Overall, C. calceolus shared, with a median of 3.5 scent compounds, significantly more compounds with the co-flowering rewarding plants than the co-flowering plant species shared among each other (median 0; Mann–Whitney U-test: Z n1 = 6, n2 = 15 = 3.31, p < 0.001; Figure 3 ). Not a single floral scent compound was found occurring in all of the six co-flowering species, and only three substances were present in more than one species: benzyl alcohol was found in three species (H. bifidum, L. incanus and P. farinosa), benzaldehyde in two species (H. bifidum and L. incanus), and eugenol in two species (H. bifidum and G. cordifolia). Cypripedium calceolus, in contrast, shared 20 floral scent compounds (30% of the total of 67 compounds that were found across all C. calceolus samples) with the co-flowering community ( Table 1 ; Figure 4 ). Whereas most of them were shared with only one other species, the scent compound benzyl alcohol was shared with three and the compounds benzaldehyde and eugenol with two co-flowering species. Overall, C. calceolus shared seven compounds (the most compounds) with H. bifidum, six compounds each with H. comosa and P. farinosa, two compounds each with D. octopetala and L. incanus, and one compound with G. cordifolia. The shared compounds all belonged to the class of aromatic compounds, terpenoids, and nitrogen-containing compounds. Interestingly, 20 of the 67 compounds in C. calceolus were aliphatic compounds, but no aliphatic compounds were detected in the co-flowering plant species studied.
Figure 3.

Number of shared compounds between Cypripedium calceolus with the six co-flowering rewarding species (“C. calceolus with co-flowering”) and the six co-flowering rewarding species with each other (“Co-flowering with co-flowering”). Each symbol represents a pairwise species comparison. Boxplots indicate the median, the first and third quartiles, and maximum and minimum values.
Figure 4.
Chord diagram showing Cypripedium calceolus and the six co-flowering rewarding species as well as each floral scent compound by a separate segment. The ribbons link the species to the scent compounds. Species and species-specific compounds are color-coded, whereas compounds shared between C. calceolus and one or more co-flowering species are given in black. Compounds are grouped by compound classes, which are highlighted by different line styles between the compound name and the compound segment as indicated at the bottom of the figure. Compounds that have been shown to be physiologically active in antennae of pollinators of C. calceolus in a previous study by Braunschmid et al. (2017) are indicated by “*”. *1EAD activity has been shown for the stereoisomer (E)-2-octenly acetate (Braunschmid et al., 2017), *2EAD activity has been indicated for pooled A–C stereoisomers without discriminating among the single stereoisomers by Braunschmid et al. (2017).
One of the main compounds of C. calceolus, linalool (30%), was shared with H. comosa in which it contributed 1% to the floral bouquet. Contrary to that, eight other compounds, which occur only in small amounts (<4%) in C. calceolus, were found to be among the main compounds in another species: 4-oxoisophorone in P. farinosa (60%); benzaldehyde in L. incanus (55%) and H. bifidum (58%); benzyl alcohol in H. bifidum (25%), L. incanus (25%), and P. farinosa (16%); lavender lactone in D. octopetala (24%); indole in H. comosa (18%); p-cresol in P. farinosa (18%); eugenol in G. cordifolia (17%); and (E)-β-ocimene in H. comosa (8%). Other shared substances, which occurred both in C. calceolus and in its co-flowering species only in small amounts (≤5%), were 2-phenylethanol, phenylacetaldehyde, eugenol, (E)-linalool oxide furanoid, and (E)-linalool oxide pyranoid for H. bifidum; methyl salicylate, (Z)-β-ocimene, and allo-ocimene for H. comosa; lilac alcohol B+C for D. octopetala; and epoxyoxoisophorone, p-methylanisole, and one unknown substance for P. farinosa.
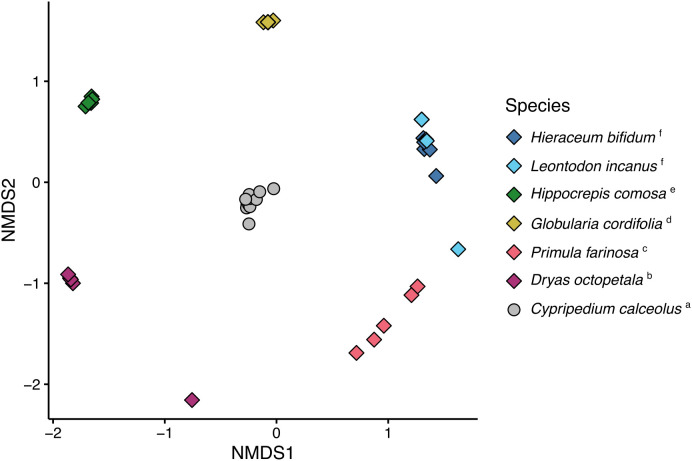
At the semiquantitative level, too, floral scent differed among species (PERMANOVA: pseudo-F 6,38 = 25.91, p < 0.001) with all pairwise species comparisons being statistically significant except for H. bifidum and L. incanus ( Figure 5 ). Multivariate dispersion of floral scent, however, did not differ among species (PERMDISP: F 6,38 = 2.10, p = 0.290). The scent of C. calceolus showed a relatively unique scent profile, but at the same time shared numerous floral compounds with its co-flowering plant community, so that, based on the dissimilarities of the floral scent bouquets, all C. calceolus individuals grouped in the center, and the co-flowering species spread in a circular pattern around C. calceolus and grouped according to species, except L. incanus and H. bifidum, which partially overlapped with each other ( Figure 5 ).
Figure 5.
Non-metric multidimensional scaling (NMDS), based on pairwise Bray–Curtis similarities, used to visualize semi-quantitative (dis)similarities among floral bouquets of Cypripedium calceolus (circles) and six co-flowering rewarding species (diamonds). Each symbol represents an individual, and individuals are color-coded according to species. The 2D stress of the NMDS was 0.04. Different lowercase letters at the end of the species names indicate statistically significant differences in scent composition (pairwise PERMANOVA).
4. Discussion
Our study in the food-deceptive orchid C. calceolus on floral scent imitation of the co-flowering rewarding plant community showed that C. calceolus shared scent compounds with all co-flowering species that emitted detectable floral scent and that (potentially) share pollinators with C. calceolus. Almost one-third of the 67 floral scent compounds of C. calceolus were also found in at least one of the co-flowering species, among them compounds generally widespread among floral scents but also less widespread compounds (Knudsen et al., 2006). Eight of the shared compounds have previously been shown in physiological measurements to elicit an antennal response in bees and hoverflies that pollinate C. calceolus (Braunschmid et al., 2017). Moreover, C. calceolus shared more compounds with the co-flowering rewarding plant community than the co-flowering species shared among each other. Together, these results indicate that (food-)deceptive orchids may not only emit compounds that are common among flowering plants and attractive to many pollinators but also specifically mimic floral scents of multiple co-flowering plant species.
Many food-deceptive orchids emit floral scent (e.g., in Cephalanthera rubra: Nilsson, 1983; Anacamptis morio: Nilsson, 1984; Epidendrum ciliare: Moya and Ackerman, 1993; Tolumnia variegata: Ackerman et al., 1997; Orchis mascula, O. pauciflora, and their hybrid O. × colemanii: Salzmann et al., 2007a; Dactylorhiza romana: Salzmann and Schiestl, 2007; and Traunsteineria globosa: Jersáková et al., 2016), with 11–49 compounds per species. Interestingly, with 67 compounds in our study population, C. calceolus emits more than these generalized food-deceptive orchids. This indicates that floral scent of C. calceolus might have some additional functions, by imitating specific models, than floral scent has in other food-deceptive species.
Our assessment of the floral scent of co-flowering rewarding species of C. calceolus helps to better understand such additional functions. For the four co-flowering species, L. incanus, H. comosa, G. cordifolia, and D. octopetala, floral scent has, to our knowledge, not been described before. For the remaining two co-flowering rewarding species, floral scent has previously been studied, and similar floral scent compounds to the ones we encountered had been found, but some compounds were different (P. farinosa: Gaskett et al., 2005; H. bifidum: Feulner et al., 2011). Such differences could partially arise through the usage of (slightly) different scent collection and analysis methods but could also reflect phenotypic plasticity (e.g., Majetic et al., 2009) or be the result of geographical differences in selection, for example, imposed by pollinators as suggested in other plant species (e.g., Gross et al., 2016; Chapurlat et al., 2018).
Two recent studies in food-deceptive orchids have compared the scent profile, as well as other floral traits, to co-flowering rewarding species (Jersáková et al., 2006; Scaccabarozzi et al., 2025). Traunsteineria globosa has been found to share almost 70% of its floral scent compounds with co-flowering rewarding species of Knautia and Scabiosa (Dipsacaceae) and Valeriana (Caprifoliaceae) to which it closely resembles in floral color and in the compact inflorescences (Jersáková et al., 2006). As fly pollinators did not discriminate between T. globosa and its potential model species but bees and butterflies did, Jersáková et al. (2006) suggested that T. globosa quite closely mimics its potential model species in visual signals but that bees and butterflies are able to discriminate the deceptive orchid from the rewarding species based on the differences in scent. In two species of the orchid genus Thelymitra, the floral scent of these orchids is quite similar to that of one species each of the rewarding tinsel lilies (Calectasia spp.) that have been suggested to be model species because of high similarities in flower color and especially flower morphology. However, the scent of the orchids is also quite similar to that of other co-flowering rewarding species that differ in flower color and morphology from the Thelymitra species (Scaccabarozzi et al., 2025). In this system, it has been suggested that floral scent may reinforce the similarity in floral color and morphology (Scaccabarozzi et al., 2025). In contrast, C. calceolus strongly differed morphologically from co-flowering rewarding species, and the yellow color of the prominent lip of C. calceolus was, to the human eye, similar only to that of some of the co-flowering rewarding species in our study population (yellow flowers of H. bifidum, L. incanus, and H. comosa and the yellow center of the flowers of D. octopetala). The floral scent of C. calceolus, too, differed qualitatively and quantitatively from that of the six co-flowering rewarding species. Nevertheless, C. calceolus shared 30% of its compounds and at least one and up to seven compounds with each of its co-flowering rewarding species. Thus, sharing some compounds with a variety of co-flowering rewarding species might be advantageous for C. calceolus in attracting pollinators.
Generalized food deception and Batesian food source mimicry probably represent two extremes of a continuum, and Batesian food source mimicry is thought to have evolved from generalized food deception (Jersáková et al., 2009; Qu et al., 2023; D’Aria et al., 2024). In our study, we find support for both strategies. In support of a generalized food-deceptive pollination strategy is our finding that the floral scent of C. calceolus contained 8 [(E)-β-ocimene, β-myrcene, linalool, benzaldehyde, methyl salicylate, benzyl alcohol, 2-phenylethanol, 6-methyl-5-hepten-2-one] of the 12 compounds that have been identified to occur in more than half of the seed plant families (Knudsen et al., 2006) and shared all of these except two of them (myrcene, 6-methyl-5-hepten-2-one) with at least one co-flowering species. Other generalized food-deceptive plants (e.g., Dactylorhiza sambuccina, Anacamptis morio and A. pyramidalis, Caladenia longicauda, Orchis mascula and O. pauciflora, and Traunsteinera globosa) contain 4 to 9 of these 12 most widespread compounds, which is 12%–45% of their compounds, and the 8 widespread compounds in the floral scent of C. calceolus in our study population are also found in the floral scent of at least one of these other food-deceptive species (Nilsson, 1980, 1984; Andersson et al., 2002; Salzmann et al., 2006, 2007b, 2007a; Jersáková et al., 2016). In comparison, C. calceolus contains a relatively low proportion (12%) of the 12 most widespread compounds and, thus, might have a broader mimicry strategy than these other generalized food-deceptive species. Indeed, we also found support for more specific imitation of the floral scent of co-flowering rewarding species in C. calceolus. Cypripedium calceolus did not only overlap in six of the most widespread compounds with the co-flowering rewarding species in our study population but also in 14 other, rarer compounds. Interestingly, three of these compounds (p-cresol, indole, epoxyoxoisophorone) are exclusively found in samples of the northern Alps but not south of the Alps or in Scandinavia (Braunschmid et al., 2021), and two of these compounds (p-cresol, indole) are physiologically active in solitary bees and hoverflies (Braunschmid et al., 2017) and, thus, might reflect an adaptation to local differences in the pollinator and/or the co-flowering rewarding plant community. However, it has not yet been tested which of the physiologically active floral scent compounds in C. calceolus are involved in pollinator attraction, but several compounds have been shown to attract insect species of the same genus or closely related genera as the pollinators of C. calceolus. For example, benzaldehyde, 4-oxoisophorone, and the combination thereof attracts several hoverfly species and one Lasioglossum species (El-Sayed et al., 2018); eugenol attracts the hoverfly Eupeodes corollae (Li et al., 2020); β-ocimene attracts honeybees (Pecetti et al., 2002); compounds, such as phenylacetaldehyde, methyl salicylate, linalool oxide pyranoid, linalool, and 2-phenylethanol, attract hoverflies (Primante and Dötterl, 2010; reviewed in Dötterl and Gershenzon, 2023 and references therein); p-anisaldehyde and phenylacetaldehyde attracts Halictidae (e.g., Lasioglossum) (Meagher, 2002; Theis, 2006); and (E,E)-α-farnesene, linalool, methyl salicylate, and 2-phenylethanol attract Andrena vaga (which is, however, not a pollinator of C. calceolus) (Dötterl and Vereecken, 2010 and references therein). Thus, several of the floral scent compounds of C. calceolus, including some that C. calceolus shared with the co-flowering rewarding species, might indeed affect the behavior of C. calceolus pollinators and lure them to the rewardless flowers. Together, these findings indicate that C. calceolus may have a pollination strategy between generalized food deception and Batesian floral mimicry. It is probably closer to generalized food deception than the pollination strategy of T. globosa, which has been suggested to have a guild mimicry strategy, and the pollination strategy of the Thelymitra system, which is closest to Batesian food source mimicry.
Interestingly, C. calceolus in our study population contained 20 aliphatic compounds, two of which (octyl acetate, decyl acetate) constituted more than 5% of the total scent. Aliphatic compounds, in general, and those in the scent bouquet of C. calceolus, in particular, are neither among the most widespread floral scent compounds (see Knudsen et al., 2006) nor among the compounds we detected in the co-flowering rewarding species. Several of these compounds, however, are well known from species-specific cephalic secretions of female and male Andrena bees (Tengö and Bergström, 1977; El-Sayed, 2025). For example, cephalic secretions of Andrena haemorrhoa, A. jacobi (=A. carantonica), and A. nigroaenea, all known as pollinators of C. calceolus (Braunschmid et al., 2021), have, among others, 1-dodecanol (A. haemorrhoa, A. jacobi), decyl, dodecyl and tetradecyl acetate (A. haemorrhoa), 1-octanol and 1-decanol (A. jacobi), and octyl acetate (A. nigroaenea) in common with floral scents of the studied C. calceolus population. These compounds are male aggregation and sex pheromones of Andrena spp. and were already previously discussed as being potentially involved in the attraction of Andrena pollinators to C. calceolus (Nilsson, 1979). In the studied population of C. calceolus, as is true for other populations in the northern Alps, however, Andrena bees are only minor pollinators (Braunschmid et al., 2017, 2021), and from the main Lasioglossum pollinators, such aliphatic compounds are not known to occur as pheromones (El-Sayed, 2025).
Overall, our results indicate that C. calceolus has a pollination strategy intermediate between generalized food deception, Batesian floral mimicry, and Batesian pheromone mimicry, and this might explain why this species emits more compounds than other food-deceptive plants. While such a triple deceptive strategy seems to be specific for C. calceolus, intermediate pollination strategies between generalized food deception and Batesian floral mimicry have also been suggested for other food-deceptive orchids (Jersáková et al., 2016; D’Aria et al., 2024). The pollination strategy of C. calceolus might reflect an evolutionary transition from generalized food deception to Batesian floral mimicry. Alternatively, it could reflect an adaptation to the exposure to a variable pollinator assemblage across the wide distribution range of C. calceolus, and thus, depending on the locally available pollinators and co-flowering rewarding plants, the relative importance of the different deceptive strategies might differ among populations. The majority of the species of the genus Cypripedium have a food-deceptive strategy, but other pollination strategies have been suggested. For example, generalized food deception has been proposed in C. guttatum, where the similarities with co-flowering species in color are suggested to be the results of a diverse co-occurring flora rather than floral mimicry (Bänziger et al., 2005). Several other species are highly specialized in mimicking, for example, fly oviposition sites (Ren et al., 2011; Li et al., 2012). Another example is the mimicry of a specific food-rewarding model plant (Pedicularis schistostegia, Orobanchaceae), which has, so far, only been suggested for C. macranthos var. rebunense (Sugiura et al., 2001, 2002). Thus, C. calceolus and other congeners might be an interesting system to study the evolutionary transition from generalized food deception to specialized deception pollination strategies.
Acknowledgments
Many thanks to Doris Huber and other members of the National Park Berchtesgaden, Bavaria, Germany, for the accommodation and boat transfer as well as for the research permit. We also thank M. Varga for the assistance with Figure 4 .
Funding Statement
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded in whole or in part by the Austrian Science Fund (FWF) (10.55776/P32142).
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
CE: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. FE: Investigation, Writing – original draft, Writing – review & editing. RG: Investigation, Writing – review & editing. HB: Conceptualization, Writing – review & editing. KG: Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Validation. SD: Conceptualization, Writing – review & editing, Validation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1627890/full#supplementary-material
Total absolute amount of scent trapped [ng/h per pollination unit] separately for each sample of the six co-flowering rewarding species.
Relative amount [%] for all 105 floral scent compounds and the number of compounds emitted separately for each sample of the six co-flowering rewarding species and Cypripedium calceolus.
References
- Ackerman J. D., Meléndez-Ackerman E. J., Salguero-Faria J. (1997). Variation in pollinator abundance and selection on fragrance phenotypes in an epiphytic orchid. Am. J. Bot. 84, 1383–1390. doi: 10.2307/2446136, PMID: [DOI] [PubMed] [Google Scholar]
- Ackerman J. D., Phillips R. D., Tremblay R. L., Karremans A., Reiter N., Peter C. I., et al. (2023). Beyond the various contrivances by which orchids are pollinated: global patterns in orchid pollination biology. Bot. J. Linn. Soc 202, 295–324. doi: 10.1093/botlinnean/boac082 [DOI] [Google Scholar]
- Anderson M. J., Gogley R. N., Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to software and Statistical Methods (Plymouth, UK: PRIMER-E; ). [Google Scholar]
- Andersson S., Nilsson L. A., Groth I., Bergström G. (2002). Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Bot. J. Linn. Soc 140, 129–153. doi: 10.1046/j.1095-8339.2002.00068.x [DOI] [Google Scholar]
- Bänziger H., Sun H., Luo Y.-B. (2005). Pollination of a slippery lady slipper orchid in south-west China: Cypripedium guttatum (Orchidaceae). Bot. J. Linn. Soc 148, 251–264. doi: 10.1111/j.1095-8339.2005.00400.x [DOI] [Google Scholar]
- Bergström G., Birgersson G., Groth I., Anders Nilsson L. (1992). Floral fragrance disparity between three taxa of lady’s slipper Cypripedium calceolus (Orchidaceae). Phytochemistry 31, 2315–2319. doi: 10.1016/0031-9422(92)83271-Y [DOI] [Google Scholar]
- Braunschmid H., Guilhot R., Dötterl S. (2021). Floral scent and pollinators of Cypripedium calceolus L. at different latitudes. Diversity 13, 5. doi: 10.3390/d13010005 [DOI] [Google Scholar]
- Braunschmid H., Mükisch B., Rupp T., Schäffler I., Zito P., Birtele D., et al. (2017). Interpopulation variation in pollinators and floral scent of the lady’s-slipper orchid Cypripedium calceolus L. Arthropod-Plant Interact. 11, 363–379. doi: 10.1007/s11829-017-9512-x [DOI] [Google Scholar]
- Chapurlat E., Anderson J., Ågren J., Friberg M., Sletvold N. (2018). Diel pattern of floral scent emission matches the relative importance of diurnal and nocturnal pollinators in populations of Gymnadenia conopsea . Ann. Bot. 121, 711–721. doi: 10.1093/aob/mcx203, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L., Raine N. E. (2006). Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 9, 428–435. doi: 10.1016/j.pbi.2006.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- Clarke K. R., Gogley R. N. (2006). PRIMER v6: User manual/tutorial (Plymouth Routines in Multivariate Ecological Research) (Plymouth, UK: PRIMER-E; ). [Google Scholar]
- Cribb P. (1997). The genus Cypripedium (Portland, OR, USA: Timber Press; ). [Google Scholar]
- D’Aria A., Scopece G., Ciaschetti G., Guzzetti L., Scaccabarozzi D., Cozzolino S. (2024). The deceptive pollination of Anacamptis morio: generalised food deception or an initial step towards adaptive mimicry? Plant Ecol. Divers. 17, 123–134. doi: 10.1080/17550874.2024.2412589 [DOI] [Google Scholar]
- Dafni A. (1984). Mimicry and deception in pollination. Annu. Rev. Ecol. Syst. 15, 259–278. doi: 10.1146/annurev.es.15.110184.001355 [DOI] [Google Scholar]
- Daumann E. (1968). Zur Bestäubungsökologie von Cypripedium calceolus L. Österr. Bot. Z. 115, 434–446. doi: 10.1007/BF01456538 [DOI] [Google Scholar]
- Dötterl S., Gershenzon J. (2023). Chemistry, biosynthesis and biology of floral volatiles: roles in pollination and other functions. Nat. Prod. Rep. 40, 1901–1937. doi: 10.1039/D3NP00024A, PMID: [DOI] [PubMed] [Google Scholar]
- Dötterl S., Vereecken N. J. (2010). The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can. J. Zool. Can. Zool. 88, 668–697. doi: 10.1139/z10-031 [DOI] [Google Scholar]
- Dötterl S., Wolfe L. M., Jürgens A. (2005). Qualitative and quantitative analyses of flower scent in Silene latifolia . Phytochemistry 66, 203–213. doi: 10.1016/j.phytochem.2004.12.002, PMID: [DOI] [PubMed] [Google Scholar]
- El-Sayed A. M. (2025). The Pherobase: database of pheromones and semiochemicals. Available online at: http://www.pherobase.com (Accessed May 13, 2025).
- El-Sayed A. M., Sporle A., Colhoun K., Furlong J., White R., Suckling D. M. (2018). Scents in orchards: floral volatiles of four stone fruit crops and their attractiveness to pollinators. Chemoecology 28, 39–49. doi: 10.1007/s00049-018-0254-8 [DOI] [Google Scholar]
- Endress P. K. (1996). Diversity and evolutionary biology of tropical flowers (Cambridge: Cambridge University Press; ). [Google Scholar]
- Feulner M., Schuhwerk F., Dötterl S. (2011). Taxonomical value of inflorescence scent in Hieracium s. str. Biochem. Syst. Ecol. 39, 732–743. doi: 10.1016/j.bse.2011.06.012 [DOI] [Google Scholar]
- Galizia C. G., Kunze J., Gumbert A., Borg-Karlson A.-K., Sachse S., Markl C., et al. (2005). Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav. Ecol. 16, 159–168. doi: 10.1093/beheco/arh147 [DOI] [Google Scholar]
- Gaskett A. C., Conti E., Schiestl F. P. (2005). Floral odor variation in two heterostylous species of Primula . J. Chem. Ecol. 31, 1223–1228. doi: 10.1007/s10886-005-5351-9, PMID: [DOI] [PubMed] [Google Scholar]
- Gross K., Sun M., Schiestl F. P. (2016). Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PloS One 11. doi: 10.1371/journal.pone.0147975, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Gu L., Eils R., Schlesner M., Brors B. (2014). circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812. doi: 10.1093/bioinformatics/btu393, PMID: [DOI] [PubMed] [Google Scholar]
- Jersáková J., Johnson S. D., Jürgens A. (2009). “Deceptive behavior in plants. II. Food deception by plants: from generalized systems to specialized floral mimicry,” in Plant-environment interactions: from sensory plant biology to active plant behavior. Ed. Baluška F. (Springer, Berlin, Heidelberg: ), 223–246. doi: 10.1007/978-3-540-89230-4_12 [DOI] [Google Scholar]
- Jersáková J., Johnson S. D., Kindlmann P. (2006). Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. Camb. Philos. Soc 81, 219–235. doi: 10.1017/s1464793105006986, PMID: [DOI] [PubMed] [Google Scholar]
- Jersáková J., Jürgens A., Šmilauer P., Johnson S. D. (2012). The evolution of floral mimicry: identifying traits that visually attract pollinators. Funct. Ecol. 26, 1381–1389. doi: 10.1111/j.1365-2435.2012.02059.x [DOI] [Google Scholar]
- Jersáková J., Spaethe J., Streinzer M., Neumayer J., Paulus H., Dötterl S., et al. (2016). Does Traunsteinera globosa (the globe orchid) dupe its pollinators through generalized food deception or mimicry? Bot. J. Linn. Soc 180, 269–294. doi: 10.1111/boj.12364 [DOI] [Google Scholar]
- Johnson S. D., Schiestl F. P. (2016). Floral mimicry (Oxford, New York: Oxford University Press; ). [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J., Ståhl B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. doi: 10.1663/0006-8101(2006)72[1:dadofs]2.0.co;2 [DOI] [Google Scholar]
- Kull T. (1999). Cypripedium calceolus L. J. Ecol. 87, 913–924. doi: 10.1046/j.1365-2745.1999.00407.x [DOI] [Google Scholar]
- Li H.-M., Liu W.-B., Yang L.-L., Cao H.-Q., Pelosi P., Wang G.-R., et al. (2020). Aromatic volatiles and odorant receptor 25 mediate attraction of Eupeodes corollae to flowers. J. Agric. Food Chem. 68, 12212–12220. doi: 10.1021/acs.jafc.0c03854, PMID: [DOI] [PubMed] [Google Scholar]
- Li P., Pemberton R., Zheng G., Luo Y. (2012). Fly pollination in Cypripedium: a case study of sympatric C. sichuanense and C. micranthum . Bot. J. Linn. Soc 170, 50–58. doi: 10.1111/j.1095-8339.2012.01259.x [DOI] [Google Scholar]
- Majetic C. J., Raguso R. A., Ashman T.-L. (2009). Sources of floral scent variation: can environment define floral scent phenotype? Plant Signal. Behav. 4, 129–131. doi: 10.4161/psb.4.2.7628, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. L., Jr (2002). Trapping noctuid moths with synthetic floral volatile lures. Entomol. Exp. Appl. 103, 219–226. doi: 10.1046/j.1570-7458.2002.00977.x [DOI] [Google Scholar]
- Moya S., Ackerman J. D. (1993). Variation in the floral fragrance of Epidendrum ciliare (Orchidaceae). Nord. J. Bot. 13, 41–47. doi: 10.1111/j.1756-1051.1993.tb00009.x [DOI] [Google Scholar]
- Müller H. (1881). Alpenblumen, ihre Befruchtung durch Insekten und ihre Anpassungen an dieselben. Engelmann Wilhelm. Leipzig, Germany: [Google Scholar]
- Nilsson L. (1979). Anthecological studies on the ladys slipper, Cypripedium calceolus (Orchidaceae). Bot. Not. 132, 329–347. [Google Scholar]
- Nilsson L. A. (1980). The pollination ecology of Dactylorhiza sambucina (Orchidaceae). Bot. Not. 133, 367–385. [Google Scholar]
- Nilsson L. A. (1983). Mimesis of bellflower (Campanula) by the red helleborine orchid Cephalanthera rubra . Nature 305, 799–800. doi: 10.1038/305799a0 [DOI] [Google Scholar]
- Nilsson L. A. (1984). Anthecology of Orchis morio (Orchidaceae) at its outpost in the north. Nova Acta Regiae Soc Sci. Ups. VC 3, 167–179. [Google Scholar]
- Nilsson L. A. (1992). Orchid pollination biology. Trends Ecol. Evol. 7, 255–259. doi: 10.1016/0169-5347(92)90170-G, PMID: [DOI] [PubMed] [Google Scholar]
- Ogle D. H., Wheeler A. P., Dinno A. (2021). FSA: Fisheries stock analysis. Available online at: https://github.com/droglenc/FSA (Accessed March 19, 2025).
- Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2020). vegan: community ecology package. R package version 2.5-7. Available online at: https://CRAN.R-project.org/package=vegan (Accessed November 3, 2021).
- Ollerton J., Winfree R., Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x [DOI] [Google Scholar]
- Peakall R. (2023). Pollination by sexual deception. Curr. Biol. 33, R489–R496. doi: 10.1016/j.cub.2023.02.066, PMID: [DOI] [PubMed] [Google Scholar]
- Pecetti L., Tava A., Felicioli A., Pinzauti M., Piano E. (2002). Effect of three volatile compounds from lucerne flowers on their attractiveness towards pollinators. Bull. Insectol. 55, 21–27. [Google Scholar]
- Peter C. I., Johnson S. D. (2008). Mimics and magnets: The importance of color and ecological facilitation in floral deception. Ecology 89, 1583–1595. doi: 10.1890/07-1098.1, PMID: [DOI] [PubMed] [Google Scholar]
- Primante C., Dötterl S. (2010). A syrphid fly uses olfactory cues to find a non-yellow flower. J. Chem. Ecol. 36, 1207–1210. doi: 10.1007/s10886-010-9871-6, PMID: [DOI] [PubMed] [Google Scholar]
- Qu H., Heifetz A., Seifan M. (2023). Batesian mimicry or general food deception? An evolutionary game between plants for pollinator services. J. Theor. Biol. 575, 111609. doi: 10.1016/j.jtbi.2023.111609, PMID: [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing; ). Available online at: https://www.R-project.org/ (Accessed May 13, 2025). [Google Scholar]
- Ren Z.-X., Li D.-Z., Bernhardt P., Wang H. (2011). Flowers of Cypripedium fargesii (Orchidaceae) fool flat-footed flies (Platypezidae) by faking fungus-infected foliage. Proc. Natl. Acad. Sci. 108, 7478–7480. doi: 10.1073/pnas.1103384108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B. A., Widmer A. (1999). Floral mimicry: a fascinating yet poorly understood phenomenon. Trends Plant Sci. 4, 325–330. doi: 10.1016/s1360-1385(99)01445-4, PMID: [DOI] [PubMed] [Google Scholar]
- Salzmann C. C., Brown A., Schiestl F. P. (2006). Floral scent emission and pollination syndromes: Evolutionary changes from food to sexual deception. Int. J. Plant Sci. 167, 1197–1204. doi: 10.1086/508022 [DOI] [Google Scholar]
- Salzmann C. C., Cozzolino S., Schiestl F. P. (2007. a). Floral scent in food-deceptive orchids: Species specificity and sources of variability. Plant Biol. 9, 720–729. doi: 10.1055/s-2007-965614, PMID: [DOI] [PubMed] [Google Scholar]
- Salzmann C. C., Nardella A. M., Cozzolino S., Schiestl F. P. (2007. b). Variability in floral scent in rewarding and deceptive orchids: The signature of pollinator-imposed selection? Ann. Bot. 100, 757–765. doi: 10.1093/aob/mcm161, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann C. C., Schiestl F. P. (2007). Odour and colour polymorphism in the food-deceptive orchid Dactylorhiza romana . Plant Syst. Evol. 267, 37–45. doi: 10.1007/s00606-007-0560-z [DOI] [Google Scholar]
- Scaccabarozzi D., Flematti G. R., Brundrett M., Vallejo-Marín M., Lunau K., Gagliano M., et al. (2025). Nuances of floral mimicry in Queen of Sheba orchids. Annals of Botany, mcaf119. doi: 10.1093/aob/mcaf119, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl F. P. (2005). On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92, 255–264. doi: 10.1007/s00114-005-0636-y, PMID: [DOI] [PubMed] [Google Scholar]
- Schiestl F. P., Schlüter P. M. (2009). Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 54, 425–446. doi: 10.1146/annurev.ento.54.110807.090603, PMID: [DOI] [PubMed] [Google Scholar]
- Slavković F., Bendahmane A. (2025). Plants, Pollinators and Pheromones: promises and lies of semiochemicals. Plant, Cell & Environment. doi: 10.1111/pce.15670, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N., Fujie T., Inoue K., Kitamura K. (2001). Flowering phenology, pollination, and fruit set of Cypripedium macranthos var. rebunense, a threatened lady’s slipper (Orchidaceae). J. Plant Res. 114, 171–178. doi: 10.1007/PL00013980 [DOI] [Google Scholar]
- Sugiura N., Goubara M., Kitamura K., Inoue K. (2002). Bumblebee pollination of Cypripedium macranthos var. rebunense (Orchidaceae); a possible case of floral mimicry of Pedicularis schistostegia (Orobanchaceae). Plant Syst. Evol. 235, 189–195. doi: 10.1007/s00606-002-0229-6 [DOI] [Google Scholar]
- Tengö J., Bergström G. (1977). Comparative analyses of complex secretions from heads of Andrena bees (Hym., Apoidea). Comp. Biochem. Physiol. Part B Comp. Biochem. 57, 197–202. doi: 10.1016/0305-0491(77)90143-2 [DOI] [Google Scholar]
- Theis N. (2006). Fragrance of Canada thistle (Cirsium arvense) attracts both floral herbivores and pollinators. J. Chem. Ecol. 32, 917–927. doi: 10.1007/s10886-006-9051-x, PMID: [DOI] [PubMed] [Google Scholar]
- van der Cingel N. A. (2001). An atlas of orchid pollination: America, Africa, Asia and Australia. (A. A. Balkema, Rotterdam, Netherlands: ). [Google Scholar]
- van der Pijl L., Dodson C. H. (1966). Orchid flowers: their pollination and evolution (Coral Gables, Florida: The Fairchild Tropical Garden and the University of Miami Press; ). [Google Scholar]
- Westrich P. (2019). Die Wildbienen Deutschlands. (Stuttgart, Germany: Verlag Eugen Ulmer; ). Available online at: https://www.ulmer.de/usd-6076169/die-wildbienen-deutschlands-.html. [Google Scholar]
- Wickham H. (2016). ggplot2: Elegant graphics for data analysis (Cham: Springer International Publishing; ). doi: 10.1007/978-3-319-24277-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total absolute amount of scent trapped [ng/h per pollination unit] separately for each sample of the six co-flowering rewarding species.
Relative amount [%] for all 105 floral scent compounds and the number of compounds emitted separately for each sample of the six co-flowering rewarding species and Cypripedium calceolus.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.