Abstract
Background
To evaluate the primary resistance factors to enfortumab vedotin (EV) monotherapy by comparing treatment outcomes between the early progressive disease (EPD) group and non-EPD group.
Methods
We retrospectively analyzed 121 patients with advanced urothelial carcinoma who received EV monotherapy across five institutions between 2019 and 2024. The patients were categorized into the EPD group (n = 34), defined by radiologically confirmed progressive disease within 3 months of EV initiation, and the non-EPD group (n = 87). The clinical parameters and oncological outcomes were compared between groups. The emergence of new metastatic lesions was defined as the detection of metastases in organs not previously identified as metastatic sites at baseline, during prior chemotherapy or immune checkpoint inhibitors (ICIs) before the initiation of EV.
Results
The median overall survival was significantly shorter in the EPD group than in the non-EPD group (6.5 vs. 19.9 months, p < 0.001). The EPD group had a significantly higher incidence of new metastatic lesions and a lower prevalence of normal Hb levels. Multivariate analysis identified low Hb and the presence of new metastatic lesions as independent predictors of EPD. Among patients with new metastases in the EPD group, an average of 74% of lesions emerged during ICI treatment and 75% involved multiple foci. Notably, more than 50% of these new lesions showed progression at the same sites following EV therapy.
Conclusions
Patients with low hemoglobin levels and new metastatic lesions before EV treatment may be at increased risk for EPD. For these patients, alternative treatment strategies should be considered before initiating EV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10147-025-02822-1.
Keywords: Early progressive disease, Enfortumab vedotin, Metastatic urothelial carcinoma, Primary resistance
Introduction
Urothelial carcinoma (UC) is among the most common malignancies, with bladder cancer accounting for approximately 95% of all UC cases [1]. Globally, bladder cancer was the ninth most frequently diagnosed cancer in 2022, with an estimated 614,000 new cases and 220,000 deaths reported [2]. Approximately 20% of patients are diagnosed with locally advanced or metastatic UC (la/mUC), necessitating systemic therapy such as platinum-based chemotherapy and immune checkpoint inhibitors (ICIs). However, most patients eventually develop disease progression and require additional lines of systemic treatment [3–5].
The advent of enfortumab vedotin (EV), an antibody–drug conjugate, has led to significant advances in the treatment of la/mUC following chemotherapy and ICI failure. EV has demonstrated promising clinical outcomes and has become a standard treatment option for previously treated UC. In the pivotal EV-301 trial, which formed the basis for regulatory approval, EV achieved a disease control rate of 71.9% and significantly improved overall survival (OS; median 12.88 vs. 8.97 months) and progression-free survival (PFS; median 5.55 vs. 3.71 months) compared with chemotherapy [6]. A Japanese subgroup analysis of the EV-301 trial also confirmed improved outcomes with EV in this population [7].
Despite its clinical benefits, a subset of patients’ experiences early progressive disease (EPD) shortly after the initiation of EV therapy, and the characteristics of these patients remain poorly understood. Additionally, while combination therapy with EV and pembrolizumab has recently been approved as a first-line treatment for treatment-naïve UC [8], monotherapy with EV still has clinical relevance. Such scenarios include disease recurrence following nivolumab plus gemcitabine–cisplatin therapy [9] or adjuvant nivolumab following neoadjuvant chemotherapy [10].
Erdafitinib, a fibroblast growth factor receptor (FGFR) inhibitor, has also emerged as a treatment option in this setting. The Phase III THOR trial demonstrated that erdafitinib significantly improved OS compared with chemotherapy in patients with FGFR alterations, including a Japanese cohort comparable with that in the EV-301 trial [11, 12]. As such, the choice between EV and erdafitinib for patients with ICI-refractory UC remains a matter of clinical consideration. Identifying predictors of EPD in patients treated with EV could provide valuable guidance in optimizing treatment selection.
In this multicenter retrospective study, we investigated factors associated with primary resistance to EV in patients with la/mUC to improve clinical decision-making in this patient population.
Patients and methods
Patient selection
We retrospectively analyzed 152 patients with locally advanced or metastatic UC (la/mUC) who were treated with EV following prior chemotherapy and ICIs at five institutions between December 2021 and March 2024. A flowchart of the patient selection process is shown in Fig. 1. Twenty-eight patients with incomplete clinical data and three patients who did not undergo initial evaluation within 3 months of EV initiation were excluded. Ultimately, 121 eligible patients were included in the study.
Fig. 1.
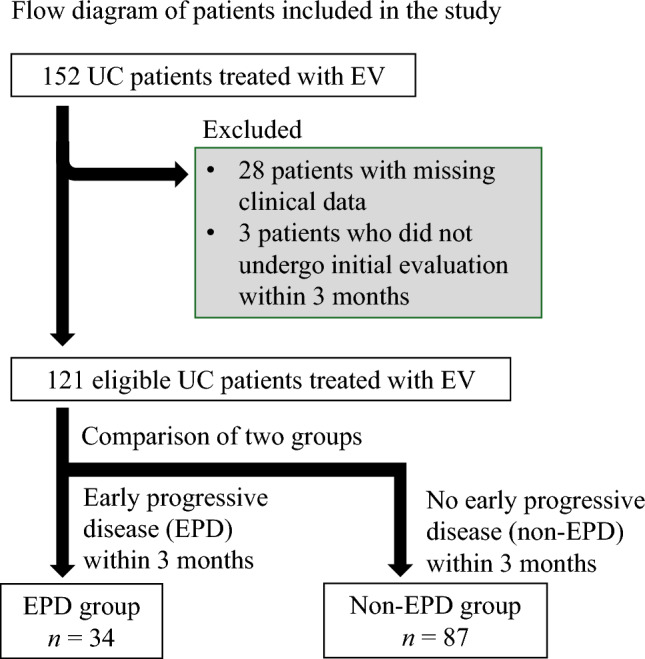
Flow diagram of patients included in the study. EV enfortumab vedotin, UC urothelial carcinoma
The patients were stratified into two groups based on disease progression within 3 months of initiating EV: the EPD group (n = 34), defined as clinical or radiographic progression at first evaluation, and the non-EPD group (n = 87), defined as absence of progression within the same timeframe.
The baseline demographic and clinical characteristics—including age, sex, performance status, tumor histology, prior treatment history, metastatic sites, and laboratory values prior to EV administration—were collected from electronic medical records. The emergence of new metastatic lesions was defined as the detection of metastases in organs not previously identified as metastatic sites at baseline, during prior chemotherapy or ICIs before the initiation of EV. Chemotherapy and ICI treatment included neoadjuvant and adjuvant settings, and EV administration was performed for recurrence within one year after neoadjuvant and adjuvant therapy in this study. In patients who received neoadjuvant chemotherapy followed by postoperative adjuvant ICI, the baseline for identifying new metastatic lesions was defined as the initiation of neoadjuvant chemotherapy.
This study was approved through a centralized ethical review conducted by the Institutional Review Board of the St. Marianna University [Approval No. 6261 (B46)]. This centralized approval was subsequently approved by our institution (Approval No. C-IRB2023-045) and all other participating sites, and conducted in accordance with the Declaration of Helsinki.
Treatment and response evaluation
As previously described [13], EV was administered at a dose of 1.25 mg/kg via intravenous infusion on Days 1, 8, and 15 of each 28-day cycle. The treatment decisions regarding the initial dose, interruptions, discontinuation, or dose reductions were made at the discretion of the attending physicians.
Although follow-up protocols varied by institution, the patients generally underwent computed tomography every 2–3 months. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [14]. Response categories included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). PD was also assigned to patients with clinical progression or cancer-related death as determined by the treating physician at the last follow-up. The disease control rate was defined as the proportion of patients achieving CR, PR, or SD. Adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events, version 5.0.
Statistical analysis
Progression-free survival and OS were calculated from the date of the initial EV dose using the Kaplan–Meier method. Survival differences between the EPD and non-EPD groups were compared using the log-rank test. Clinicopathological variables between groups were analyzed using the Wilcoxon signed-rank test or analysis of variance, as appropriate.
Cox proportional hazards models were used for univariate and multivariate analyses to identify risk factors associated with EPD. The variables included in the multivariate analysis were selected based on previous studies investigating prognostic factors for OS in la/mUC patients [15, 16]. Continuous variables, such as hemoglobin (Hb) and C-reactive protein (CRP) levels, were dichotomized using median values as cutoffs. In addition, Hb was dichotomized using median values as cutoffs for male and female.
All statistical analyses were performed using JMP software (SAS Institute Inc., Cary, North Carolina). The p value of <0.05 was considered statistically significant.
Results
The median patient age of EV initiation was 72 years (range: 29–92 years). The primary tumor site was the upper urinary tract in 65 (53.8%) patients, and 67 (55.4%) patients had undergone radical surgery prior to receiving EV. A total of 34 (28.1%) patients were classified in the EPD group. No patients in the EPD group discontinued EV due to adverse events or other reasons before progression was confirmed.
The median PFS and OS after EV initiation were 6.5 months (95% confidence interval [95% CI], 5.0–8.9 months) and 14.8 months (95% CI, 12.6–17.9 months), respectively (Fig. 2). The patients’ characteristics just before EV initiation of the EPD and non-EPD groups are compared in Tables 1 and 2. The EPD group had significantly lower Hb levels and a higher incidence of new metastatic lesions. Additionally, there were trends toward a higher frequency of bone metastases and elevated CRP levels in the EPD group. There were no significant differences in prior chemotherapy or ICI regimens between the groups.
Fig. 2.
Kaplan–Meier curves of a PFS and b OS in the overall patient cohort treated with EV. EV enfortumab vedotin, OS overall survival, PFS progression-free survival
Table 1.
Patient characteristics before the initiation of EV
| Variables | Overall (n = 121) | EPD vs. non-EPD | ||
|---|---|---|---|---|
| EPD (n = 34) | Non-EPD (n = 87) | p | ||
| Median age, year (range) | 72 (29–92) | 72 (29–85) | 73 (46–92) | 0.3014 |
| Gender male, n (%) | 84 (67.7%) | 25 (73.5%) | 59 (67.8%) | 0.5398 |
| ECOG PS, n (%) | ||||
| 0 | 82 (67.7%) | 23 (67.7%) | 59 (67.8%) | 0.5409 |
| 1 | 31 (25.7%) | 10 (29.4%) | 21 (24.1%) | |
| 2 | 8 (6.6%) | 1 (2.9%) | 7 (8.1%) | |
| Primary tumor location, n (%) | ||||
| Bladder | 56 (46.2%) | 15 (44.1%) | 41 (47.1%) | 0.7654 |
| Upper urinary tract | 65 (53.8%) | 19 (55.9%) | 46 (52.9%) | |
| Periods from first-line treatment to the initiation of EV treatment, median (range) months | 10.9 (1.3–50.6) | 11.2 (1.3–37.8) | 10.7 (1.6–50.6) | 0.4513 |
| Radical surgery (yes), n (%) | 67 (55.4%) | 18 (52.9%) | 49 (56.3%) | 0.7367 |
| Histology, n (%) | ||||
| Pure urothelial carcinoma | 89 (73.6%) | 23 (67.7%) | 66 (75.8%) | 0.6108 |
| Variant histology, n (%) | 22 (18.2%) | 8 (23.5%) | 14 (16.1%) | |
| Others/unknown | 10 (8.2%) | 3 (8.8%) | 7 (8.1%) | |
| Chemotherapy regimens | 0.8904 | |||
| GC/GCa | 106 (87.6%) | 29 (85.3%) | 77 (88.5%) | |
| MVAC | 6 (5%) | 2 (5.9%) | 4 (4.6%) | |
| Others | 9 (7.4%) | 3 (8.8%) | 6 (6.9%) | |
| Immunotherapy regimens | 0.6303 | |||
| Pembrolizumab | 80 (66.1%) | 24 (70.6%) | 56 (64.4%) | |
| Avelumab | 29 (24%) | 8 (23.5%) | 21 (24.1%) | |
| Others | 12 (9.9%) | 2 (5.9%) | 10 (11.5%) | |
| Total median tumor diameter before EV, mm (range) | 49 (13–202) | 59 (14–144.1) | 45.5 (13–202) | 0.4839 |
| Site of metastasis before EV, n (%)a | ||||
| Lymph node only (without internal metastasis) | 24 (19.8%) | 6 (17.6%) | 18 (20.7%) | 0.706 |
| Lung | 58 (47.9%) | 18 (50.0%) | 40 (45.5%) | 0.6903 |
| Liver | 21 (17.3%) | 5 (13.9%) | 16 (18.2%) | 0.6878 |
| Bone | 31 (27.2%) | 12 (36.1%) | 19 (22.7%) | 0.0844 |
| Peritoneal dissemination | 12 (9.9%) | 3 (8.8%) | 9 (10.2%) | 0.7871 |
| Local recurrence, n (%) | 21 (17.3%) | 9 (25.0%) | 12 (13.6%) | 0.1039 |
| The number of patients with the emergence of new metastatic lesions before EV, n (%) | 52 (42.9%) | 22 (64.7%) | 30 (34.5%) | 0.0025 |
ECOG PS Eastern Cooperative Oncology Group performance status, EPD early progressive disease, EV enfortumab vedotin, GC gemcitabine + cisplatin, GCa gemcitabine + carboplatin, MVAC methotrexate + vinblastine + doxorubicin + cisplatin
aIf multiple metastases were present, each site was counted separately
Table 2.
Laboratory data just before the initiation of EV
| Variables | Overall (n = 121) | EPD vs. non-EPD | ||
|---|---|---|---|---|
| Median (range) | EPD (n = 34) | Non-EPD (n = 87) | p | |
| WBC | 6400 (3200–28,520) | 6370 (3200–28,300) | 6395 (3300–28,520) | 0.7864 |
| Neutrophil | 4192 (2851–26,780) | 4331 (2380–25,158) | 4040 (1851–12,731) | 0.4262 |
| Lymphocyte | 1258 (224–5,217) | 1332 (380–2516) | 1177 (224–5217) | 0.6159 |
| Monocyte | 428 (28–1470) | 410 (186–935) | 428 (149–1470) | 0.6322 |
| Hb | ||||
| Male | 11.6 (7–15.4) | 10.5 (7–13.6) | 11.7 (7.9–15.4) | 0.0182 |
| Female | 11.2 (7.4–14.3) | |||
| PLT 10*3 | 251 (109–595) | 269 (147–589) | 248 (109–595) | 0.125 |
| LDH | 200.5 (97–1296) | 200 (97–1296) | 200 (118–861) | 0.6022 |
| Alb | 3.8 (1.6–4.6) | 3.7 (1.6–4.5) | 3.8 (2.3–4.6) | 0.4297 |
| CRP | 1.04 (0.03–14.32) | 2.27 (0.06–14.32) | 0.62 (0.03–13.57) | 0.0889 |
| NLR at baseline | 3.5 (0.7–42) | 3.6 (1.03–17.78) | 3.3 (0.71–42) | 0.4195 |
| LMR at baseline | 2.9 (0.3–47.1) | 2.9 (0.81–6.16) | 3.0 (0.28–47.14) | 0.5759 |
| PLR at baseline | 192.8 (49–2,151.7) | 207.6 (84.9–647.6) | 193.5 (49.1–2,151.7) | 0.3384 |
Alb albumin, CRP C-reactive protein, EPD early progressive disease, Hb hemoglobin, LDH lactate dehydrogenase, LMR lymphocyte/monocyte ratio, NLR neutrophil/lymphocyte ratio, PLT platelet, PLT/LMR platelet/lymphocyte ratio, WBC white blood cell
The median PFS was significantly shorter in the EPD group than in the non-EPD group (Supporting Information Figure S1: 1.7 vs. 10.7 months, p < 0.001). Similarly, the median OS was significantly shorter in the EPD group (Fig. 3: 6.5 vs. 19.9 months, p < 0.001).
Fig. 3.
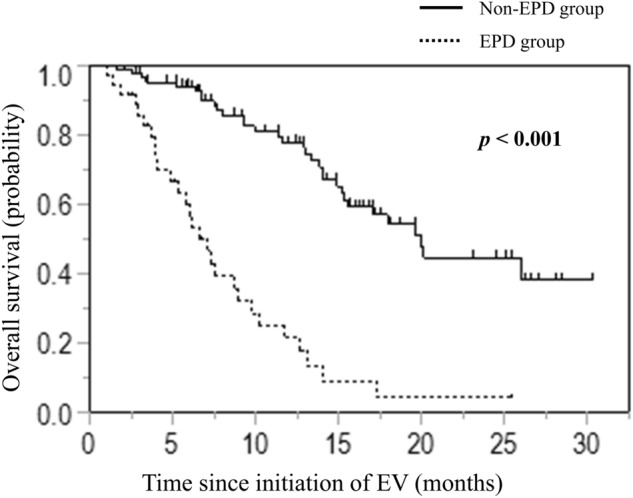
Kaplan–Meier curves of OS stratified by EPD and non-EPD groups treated with EV. EPD early progressive disease, EV enfortumab vedotin, OS overall survival
The univariate and multivariate analyses of risk factors associated with EPD are shown in Table 3. In univariate Cox regression analysis, the presence of new metastatic lesions and low Hb levels were significantly associated with EPD. In multivariate analysis, both factors remained independent predictors of EPD: emergence of new metastatic lesions (hazard ratio [HR] = 2.71; p = 0.0105) and low Hb (HR = 3.34; p = 0.0067).
Table 3.
Univariate and multivariate analyses for EPD
| Variables | Early-progression disease | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| ECOG PS, 1–2 vs. 0 | 1.15 | 0.56–2.37 | 0.689 | 1.16 | 0.51–2.64 | 0.7184 |
| Site of metastasis before EV | ||||||
| Liver, yes vs. no | 0.83 | 0.32–2.16 | 0.7087 | 0.93 | 0.34–2.52 | 0.8912 |
| Emergence of new metastatic lesions just before EV | 2.56 | 1.27–5.19 | 0.0069 | 2.71 | 1.26–5.82 | 0.0105 |
| Laboratory data median | ||||||
| Hb, low vs. high | 3.49 | 1.61–7.57 | 0.0015 | 3.34 | 1.39–7.99 | 0.0067 |
| CRP, high vs. low | 1.86 | 0.93–3.72 | 0.0793 | 1.03 | 0.43–2.18 | 0.9371 |
CRP c-relative protein, ECOG PS Eastern Cooperative Oncology Group performance status, EPD early progressive disease, EV enfortumab vedotin
Further analysis of new metastatic lesions in the EPD group is presented in Table 4. Among the 34 patients in the EPD group, 22 (64.7%) developed new metastatic lesions. The distribution of these new lesions—accounting for overlapping cases—was as follows: regional lymph nodes (n = 4), non-regional lymph nodes (n = 2), lungs (n = 8), liver (n = 4), bones (n = 8), and other sites (n = 2). Local recurrence was observed in six cases.
Table 4.
Details of the new metastatic lesion in EPD group
| Site of the new metastatic appearance | Total (na) | Treatment timing of the appearance | Occurrence as multiple lesions (n, %) | The number of subsequent PDs at those new metastatic sites after EV (n, %) | |
|---|---|---|---|---|---|
| Chemotherapy (n, %) | Immunotherapy (n, %) | ||||
| Regional lymph node | 4 | 2 (50%) | 2 (50%) | 4 (100%) | 2 (50%) |
| Extra-regional lymph node | 2 | 0 | 2 (100%) | 1 (50%) | 0 |
| Lung | 8 | 4 (50%) | 4 (50%) | 7 (87.5%) | 6 (75%) |
| Liver | 4 | 2 (50%) | 2 (50%) | 3 (75%) | 2 (50%) |
| Bone | 8 | 0 | 8 (100%) | 5 (62.5%) | 5 (62.5%) |
| Others | 2 | 0 | 2 (100%) | – | 1 (50%) |
| Local recurrence | 6 | 2 (33.3%) | 4 (66.7%) | – | 3 (50%) |
EV enfortumab vedotin, PD progressive disease
aMeasurable for each organ, including duplicate cases
We also assessed the timing of new metastatic lesion development by treatment phase: 10 cases occurred during chemotherapy and 24 during ICI therapy. Most new lesions emerged as multiple foci rather than solitary lesions.
Furthermore, we evaluated whether the sites of new metastatic lesions during prior therapy were also sites of PD after EV treatment. The rates of subsequent progression at these new metastatic sites following EV were 50% for regional lymph nodes (2/4), 75% for lungs (6/8), 50% for liver (2/4), and 62.5% for bones (5/8).
Discussion
This multicenter retrospective study demonstrated an association between EPD and poor prognosis in patients with locally advanced or metastatic UC (la/mUC) treated with EV after chemotherapy and ICIs. We identified two key predictors of EPD: the emergence of new metastatic lesions and low Hb levels. Notably, new metastatic lesions that developed before EV initiation were often refractory to subsequent EV treatment. To the best of our knowledge, this is the first report to focus on EPD in the context of EV therapy and to explore its predictive factors.
In real-world clinical practice, the observed median PFS and OS with EV treatment were 6.5 and 14.8 months, respectively. These values were slightly longer than those reported in the EV-301 trial [6]. Additionally, our results were comparable with outcomes reported in other Japanese prospective and retrospective EV studies [17–20], supporting the potential benefit of EV in real-world settings.
Low Hb is a component of the Bellmunt risk score, the only validated prognostic model for second-line therapy in UC [15, 16]. Therefore, the pretreatment Hb levels may serve as a simple yet valuable biomarker to identify patients who may benefit from EV.
We also found that the emergence of new metastatic lesions was significantly associated with EPD. Most new lesions developed during ICI therapy, rather than during chemotherapy. However, there were no significant differences in ICI regimens between the EPD and non-EPD groups, suggesting that the emergence of new metastatic lesions reflects treatment resistance regardless of the specific ICI agent used. Hara et al. [21] previously reported an association between the emergence of new lesions during pembrolizumab therapy and poor outcomes, possibly due to tumor heterogeneity. Based on these findings, we speculate that patients who develop new metastatic lesions during ICI therapy may follow an aggressive disease course in EPD group. On the other hand, the timing of new metastatic lesions in non-EPD groups also developed primarily during ICI therapy as well as EPD group. However, most of these new lesions appeared as solitary lesions rather than multiple foci. In addition, the incidence of subsequent progression at these sites after EV observed at the final evaluation was lower compared to EPD group (Supplemental Table 1). These differences suggest a distinct disease behavior and we hypothesize that underlying tumor heterogeneity may account for these results. These highlights the need to consider alternative treatment strategies, such as targeted therapy with erdafitinib, although its efficacy in patients with new metastatic lesions remains uncertain and warrants further investigation.
The emergence of new metastatic lesions may reflect resistance within the tumor microenvironment to both chemotherapy and ICIs. EV is an antibody–drug conjugate targeting Nectin-4, which is highly expressed in UC and has been proposed as a potential biomarker for EV efficacy [22, 23]. We hypothesize that new metastatic lesions may exhibit reduced or absent Nectin-4 expression, contributing to EPD. Previous studies have shown that Nectin-4 expression can decrease during metastatic evolution and may be absent in over one-third of patients with metastatic UC [24]. In our cohort, most new lesions progressed after EV administration within the same metastatic organ, supporting the possibility of Nectin-4 loss in these lesions. Nevertheless, further studies are needed to confirm the relationship between Nectin-4 expression and EV resistance.
It is also important to consider that EV resistance may result from mechanisms other than Nectin-4 downregulation [25]. Notably, in our study, the pre-existing lesions responded relatively well to EV, whereas newly developed lesions often did not, suggesting distinct resistance mechanisms. This supports the idea that tumor evolution leads to heterogeneity in treatment response.
Indeed, previous genomic analyses of metastatic UC have revealed substantial heterogeneity between primary tumors and metastatic lesions, indicating branched evolution during disease progression [26, 27]. Loriot et al. [27] reported that clinically actionable mutations—such as FGFR2/3 alterations (26%), PIK3CA mutations (13%), and ERBB2 and TSC1 mutations (7%)—were present across various metastatic sites with similar frequencies. These findings suggest that selecting targeted therapies based on molecular profiling may be a rational approach, particularly for patients at high risk of EPD.
Our study has several limitations. First, the retrospective design and limited sample size may have influenced the results. Nevertheless, our sample of 121 cases represents one of the largest cohorts to date and provides valuable real-world evidence from a multicenter setting. Second, due to its retrospective and multicenter design, imaging modalities and assessment intervals were not standardized across institutions. In particular, the use of unified imaging modalities, such as 18F-fluorodeoxyglucose positron emission tomography or enhanced CT, and the diagnostic criteria for liver and bone metastases varied, potentially introducing heterogeneity in metastatic evaluation. Therefore, a prospective study with standardized imaging protocols is needed to validate our findings in the future study. Third, Nectin-4 expression was not evaluated due to the practical challenges of obtaining biopsy specimens from multiple sites at specific treatment stages. Despite these limitations, our findings may inform future therapeutic strategies, including consideration of appropriate therapy for cases in which EPD is anticipated after EV. Although platinum rechallenge is considered an option, recent reports suggest that its efficacy after pembrolizumab or avelumab is limited when compared with EV [28, 29]. Thus, EV remains the preferred treatment in current clinical practice.
In conclusion, EPD is associated with poor outcomes in patients treated with EV. The presence of new metastatic lesions and low Hb levels before EV initiation are predictive of EPD. In such cases, individualized treatment strategies—including companion diagnostics and comprehensive genomic profiling—should be considered to optimize patient outcomes beyond EV therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all patients, their families, patient advocacy groups, clinicians, and staff for their involvement and contribution to the study.
Author contributions
Project development: D.I., E.K., and W.O. Data collection: D.I., N.H., G.K., R.Y., Y.S., and Y.E. Data analysis and interpretation: D.I. and W.O. Manuscript drafting: D.I. Writing—review and editing: N.H., R.Y., S.S., E.K., and W.O. Supervision: S.S., Y.K., E.K., and W.O.
Data availability
The data supporting the findings of and the datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
N. Hayakawa has received institutional research funding from MSD, Chugai Pharma, Astellas Pharma, Astra Zeneca, and Janssen Pharma; Y. Endo has received honoraria for Astellas Pharma, Merck BioPharma, Nippon Kayaku, Bristol Myers Squibb, Ono Pharma, and MSD; S. Shirotake has received honoraria for Ono Pharma, and Bristol Myers Squibb; E. Kikuchi has participated in consulting or advisory roles for Astellas Pharma, Astra Zeneca, Ferring Pharma, Merck BioPharma, Janssen Pharma, Chugai Pharma, and MSD and has participated in speakers bureaus for Astellas Pharma, Astra Zeneca, Merck BioPharma, Janssen Pharma, MSD, Bristol Myers Squibb, Chugai Pharma, and Nippon Kayaku, and has received institutional research funding from MSD, Chugai Pharma, Astellas Pharma, Astra Zeneca, and Janssen Pharma; W. Obara has received honoraria for Astellas Pharma, Takeda Pharma, Ono Pharma, Bristol Myers Squibb, Astra Zeneca, and MSD, and has received institutional research funding from Astellas Pharma, Chugai Pharma, and Janssen Pharma. All other authors declare no competing interests.
Approval of the Research Protocol by the Institutional Review Board
This study was approved through a centralized ethical review conducted by the Institutional Review Board of the St. Marianna University [Approval No. 6261 (B46)]. This centralized approval was subsequently approved by the institutional review board of Iwate Medical University (Approval No. C-IRB2023-045) and all other participating sites.
Animal studies
Not applicable.
Informed consent
Informed consent was obtained from participants or from the families of deceased patients using the opt-out method through publicly accessible websites.
Registry and registration number of the study/trial
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miyazaki J, Nishiyama H (2017) Epidemiology of urothelial carcinoma. Int J Urol 24:730–734. 10.1111/iju.13376 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74:229–263. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients withemogrobinladder cancer. J Clin Oncol 23:4602–4608. 10.1200/jco.2005.07.757 [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles T, Park SH, Voog E et al (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218–1230. 10.1056/nejmoa2002788 [DOI] [PubMed] [Google Scholar]
- 6.Powles T, Rosenberg JE, Sonpavde GP et al (2021) Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubara N, Yonese J, Kojima T et al (2023) An open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Med 12:2761–2771. 10.1002/cam4.5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T, Valderrama BP, Gupta S et al (2024) Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 390:875–888. 10.1056/NEJMoa2312117 [DOI] [PubMed] [Google Scholar]
- 9.van der Heijden MS, Sonpavde G, Powles T et al (2023) Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med 389(19):1778–1789. 10.1056/NEJMoa2309863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajorin DF, Witjes JA, Gschwend JE et al (2021) Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 384:2102–2114. 10.1056/NEJMoa2034442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loriot Y, Matsubara N, Park SH et al (2023) Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med 389:1961–1971. 10.1056/NEJMoa2308849 [DOI] [PubMed] [Google Scholar]
- 12.Matsubara N, Miura Y, Nishiyama H et al (2024) Phase 3 THOR Japanese subgroup analysis: erdafitinib in advanced or metastatic urothelial cancer and fibroblast growth factor receptor alterations. Int J Clin Oncol. 10.1007/s10147-024-02583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa N, Kikuchi E, Kaneko G et al (2024) Association between response to enfortumab vedotin and peripheral neuropathy in urothelial carcinoma patients: a multicenter retrospective study. Jpn J Clin Oncol 54(11):1194–1200. 10.1093/jjco/hyae082 [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, Choueiri TK, Fougeray R et al (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28:1850–1855. 10.1200/JCO.2009.25.4599 [DOI] [PubMed] [Google Scholar]
- 16.Bamias A, Merseburger A, Loriot Y et al (2023) New prognostic model in patients with advanced urothelial carcinoma treated with second-line immune checkpoint inhibitors. J Immunother Cancer 11:e005977. 10.1136/jitc-2022-005977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuokaya W, Koike Y, Yata Y et al (2024) Real world evidence of enfortumab vedotin in patients with advanced urothelial cancer: a multicenter observational study. Int J Urol 31:342–347. 10.1111/iju.15368 [DOI] [PubMed] [Google Scholar]
- 18.Taguchi S, Kawai T, Ambe Y et al (2023) Enfortumab vedotin versus platinum rechallenge in post-platinum, post-pembrolizumab advanced urothelial carcinoma: a multicenter propensity score-matched study. Int J Urol 30:1180–1186. 10.1111/iju.15300 [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Akatsuka J, Takeda H et al (2024) Real-world insights into efficacy and safety of enfortumab vedotin in Japanese patients with metastatic urothelial carcinoma: findings, considerations, and future directions. Curr Oncol 31(2):759–768. 10.3390/curroncol31020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taoka R, Kamada M, Izumi K et al (2024) Peripheral neuropathy and nerve electrophysiological changes with enfortumab vedotin in patients with advanced urothelial carcinoma: a prospective multicenter cohort study. Int J Clin Oncol 29:602–611. 10.21203/rs.3.rs-3564291/v1 [DOI] [PubMed] [Google Scholar]
- 21.Hara T, Teishima J, Okamura Y et al (2024) Appearance of new lesions associate with poor prognosis in pembrolizumab-treated urothelial carcinoma. Clin Genitourin Cancer 22:102236. 10.1016/j.clgc.2024.102236 [DOI] [PubMed] [Google Scholar]
- 22.Challita-Eid PM, Satpayev D, Yang P et al (2016) Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 76:3003–3013. 10.1158/0008-5472.CAN-15-1313 [DOI] [PubMed] [Google Scholar]
- 23.Klümper N, Eckstein M (2024) Biomarkers of response to anti-NECTIN4 antibody-drug conjugate enfortumab vedotin in urothelial cancer. Eur Urol Focus 10:224–226. 10.1016/j.euf.2024.04.001 [DOI] [PubMed] [Google Scholar]
- 24.Klümper N, Ralser DJ, Ellinger J et al (2023) Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res 29:1496–1505. 10.1158/1078-0432.CCR-22-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury R, Saleh K, Khalife N et al (2023) Mechanisms of resistance to antibody-drug conjugates. Int J Mol Sci 24:9674. 10.3390/ijms24119674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, Castro MAA, Gibb EA, Kanchi RS, Gordenin DA, Shukla SA, Sanchez-Vega F, Hansel DE, Czerniak BA, Reuter VE, Su X, de Sa Carvalho B, Chagas VS, Mungall KL, Sadeghi S, Pedamallu CS, Lu Y, Klimczak LJ, Zhang J, Choo C, Ojesina AI, Bullman S, Leraas KM, Lichtenberg TM, Wu CJ, Schultz N, Getz G, Meyerson M, Mills GB, McConkey DJ, TCGA Research Network, Weinstein JN, Kwiatkowski DJ, Lerner SP (2018) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171:540–556.e25. 10.1016/j.cell.2017.09.007. Erratum in: Cell 174:1033. 10.1016/j.cell.2018.07.036 [DOI] [PMC free article] [PubMed]
- 27.Loriot Y, Kamal M, Syx L et al (2024) The genomic and transcriptomic landscape of metastastic urothelial cancer. Nat Commun 15(1):8603. 10.1038/s41467-024-52915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura N, Miyake M, Takamatsu N et al (2025) Platinum-based chemotherapy rechallenge or enfortumab vedotin after maintenance avelumab or pembrolizumab for locally advanced or metastatic urothelial carcinoma. Bladder Cancer 11:23523735251317423. 10.1177/23523735251317423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchimoto T, Tsuchida S, Komura K et al (2024) Durable response to enfortumab vedotin compared to re-challenging chemotherapy in metastatic urothelial carcinoma after checkpoint inhibitors. Target Oncol 19(3):401–410. 10.1007/s11523-024-01047-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of and the datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.



