Abstract
Pharmaceutical analysis is essential to drug development and quality assurance, ensuring that products meet stringent safety and efficacy standards. Quantitative solid-state NMR (qSSNMR) has become a key technique, enabling precise quantification and characterization of solid drug formulations. This mini-review highlights the evolution of qSSNMR, focusing on improvements in detection limits, resolution, and high-throughput capabilities. This review explores technical advancements and applications for analyzing complex pharmaceutical mixtures. While challenges remain for widespread adoption, efforts in automation, user-friendly software, and collaboration aim to address these.
Keywords: complex drug formulations, pharmaceutical analysis, qSSNMR, quantitative solid-state NMR, solid-state characterization
1 |. General Overview
Drug analysis is a fundamental aspect of the pharmaceutical industry, as these measurements are key assessments toward pharmaceutical products safety, efficacy, and quality. The rigorous evaluation of active pharmaceutical ingredients (APIs) and excipients is essential for regulatory compliance and to meet the expectations of healthcare providers and patients [1, 2]. Accurate drug analysis helps in identifying the chemical composition, purity, stability, and bioavailability of pharmaceutical formulations, which are critical factors influencing therapeutic outcomes [3–5].
To achieve these objectives, a variety of analytical tools are employed in pharmaceutical analysis [6]. Conventional techniques such as high-performance liquid chromatography (HPLC) [7], mass spectrometry (MS) [8], and solution-state nuclear magnetic resonance (NMR) [9] are widely adopted due to their ability to provide detailed information about the chemical and physical properties of drug substances. These methods are favored for their sensitivity, specificity, and reliability, allowing for the detection of impurities and the quantification of active ingredients even at trace levels [6]. However, the solvation process can induce significant changes in the drug’s physical and chemical properties, resulting in the loss of unique spectroscopic signatures that are essential for accurate identification and quantification of the drug [10, 11].
How can we revolutionize the way we approach drug analysis, especially for solid formulations? Solid-state nuclear magnetic resonance (SSNMR) stands at the forefront of pharmaceutical analysis, offering unparalleled insights into the structural and compositional intricacies of solid drug formulations [12–15]. Recently, SSNMR has gained prominence for its unique capability to analyze solid formulations directly, preserving the integrity of the information necessary for effective drug analysis [16]. SSNMR is particularly valuable for characterizing polymorphism, monitoring crystalline-amorphous transitions, and detecting low-level impurities in their native states [16]. The adoption of SSNMR is further driven by the need for more comprehensive and accurate assessments of complex pharmaceutical products.
2 |. Complex Drug Analysis and qSSNMR
As more complex drugs are being developed, the pharmaceutical industry faces increasing challenges in the characterization and quantification of APIs and excipients within complex solid and semi-solid formulations. As drug delivery systems, formulation compositions, and manufacturing processes grow more intricate, the demand for advanced analytical techniques capable of providing detailed insights into pharmaceutical materials has never been greater. Accurate and precise quantification is essential, as the precise measurement of APIs and excipients in solid formulations directly impacts the safety, efficacy, and quality of medications [17]. However, quantifying pharmaceutical materials in their native solid or semi-solid state remains a significant challenge, primarily due to the limited available techniques of solid-state analysis with the required accuracy and sensitivity for meaningful stability and quality assessments [18]. Furthermore, the complexity of multicomponent solid formulations, which include APIs, polymerics, excipients, and impurities, necessitates analytical techniques with sufficient chemical resolution and sensitivity to deconvolute these components effectively [19, 20].
Peaks of NMR spectra directly inform chemical identity and quantity. The practice of using solution NMR for quantitative drug analysis, that is, quantitative NMR (qNMR), started as early as the 1960s, for example, quantifying of aspirin, phenacetin, and caffeine in tablets [21] and later in the 1980s’ quantifying of dicyclomine in tablets [22, 23]. In recent years solution-state qNMR has been further developed for complex drug mixtures analysis like heparin [24] and pentosan [25], novel oligonucleotide therapeutics [26, 27], as well as complex drug products like paclitaxel in albumin nanoparticles [28] and difluprednate in nanoemulsion [29]. However, more complications arise for solid and semisolid dosage forms due to different microstructural forms, for example, crystalline versus amorphous [30, 31]. These formulation properties are termed Q3 quality attributes [32], representing the arrangement of matter or microstructural properties within a formulation [33]. Routine analytical tests for qualitative (Q1) and quantitative (Q2) analysis can be performed on extracts of drug products, which inevitably eliminates Q3 features. Therefore, non-invasive analytical methods would be ideal for Q3 characterization. Among all analytical tools, high-resolution SSNMR spectroscopy can be performed directly on drug dosage forms including (but not an exhaustive list) powders, tablets, crystals, gels, in their native crystalline or amorphous forms, leading to unambiguous interpretation of structural states of drug, excipients, the interactions between them and sometimes, distribution of drugs in each state [12–15]. This new Q3 SSNMR application differs from classic NMR usage toward chemical structure elucidation but on the challenging issue of microstructure state and their distribution.
3 |. History and Applications of qSSNMR
The evolution of quantitative SSNMR (qSSNMR) has been marked by significant milestones that have transformed its application in pharmaceutical science. Since its introduction to pharmaceutical science in the 1980s [37, 38], SSNMR has increasingly established itself as a robust, reliable, and quantitative tool for analyzing pharmaceutical materials from chemical to biological modalities [16, 23, 39]. Figure 1 illustrates the timeline of qSSNMR applications in pharmaceutical science, showing its evolution over each decade. The first qSSNMR study, conducted in 1990 by Suryanarayanan and coworkers, utilized 13C NMR to analyze carbamazepine anhydrates [40], marking the beginning of its use as a transformative analytical tool. Nearly two decades later, in 2007 19F NMR was employed for quantification [41, 42], offering distinct advantages owing to the four-fold higher gyro-magnetic ratio and 100-fold greater natural abundance of 19F compared with 13C, enabling better selectivity at natural abundance. The field saw significant advancements in the near 2020s as more nuclei of 1H, 31P, and 35Cl were explored for qSSNMR applications [43–46]. For example, 1H quantification in the solid state became more accessible with recent advancements in UF-MAS, which dramatically improved sensitivity and resolution by averaging out dipolar interactions [34, 46–48].
FIGURE 1 |.

Timeline of quantitative solid-state NMR (qSSNMR) applications in pharmaceutical science, with a forward outlook toward achieving lower limits of detection (LOD), higher resolution, and faster high-throughput (HT) capabilities. These advancements are driven by innovations in new and evolving applications within pharmaceutical analysis [16], ultrafast magic angle spinning (UF-MAS) [34], dynamic nuclear polarization (DNP) [35], and automated sample handling and experimental optimization techniques [36].
Among solid-state characterization techniques, SSNMR is often regarded as the gold standard, frequently used to calibrate other orthogonal methods [49, 50]. Compared with conventional analytical methods, qSSNMR offers distinct advantages and excels in several key areas of pharmaceutical analysis. SSNMR is particularly effective in characterizing polymorphism, crystalline-amorphous transitions, and detecting low-level components in both drug substances and drug products, without interference from excipients [16].
Building on its historical development, qSSNMR has found diverse applications in pharmaceutical analysis. Some representative qSSNMR applications are illustrated in Figure 2. Polymorphism, the attributes of a substance to exist in multiple crystalline or amorphous forms, can significantly influence a drug product’s dissolution, solubility, bioavailability, and stability. qSSNMR is a powerful tool to distinguish between polymorphic forms by leveraging their unique spectroscopic fingerprints and distinct relaxation behaviors, allowing researchers to identify and quantify various polymorphs and phases within a formulation (see Figure 2A), which are part of the Q3 quality attributes used for complex generic drug development [32]. The ability of qSSNMR to monitor crystalline-amorphous interconversions is crucial for understanding the stability of drug products during processing and storage. These phase transitions can significantly alter the physical and chemical properties of APIs, thereby affecting their performance and safety. By using qSSNMR, researchers can track these transitions in real time, providing valuable data for formulation development and quality control. Additionally, qSSNMR excels at detecting low-level components, such as impurities or degradation species within intact drug products, where traditional analytical techniques may require extensive drug product extraction. The sensitivity of qSSNMR allows for the identification and quantification of even trace components within complex matrices. For example, a recent study by Su and coworkers [53] shows that 19F qSSNMR enables the detection of a very low drug loading of 0.04% w/w (see Figure 2C). Moreover, 19F and 13C qSSNMR techniques have been developed to quantify amorphous APIs and polymer crystallization in extruded implants [54, 55]. This capability is necessary for ensuring the quality of pharmaceutical products, as even small amounts of impurities can have significant implications for patient health.
FIGURE 2 |.
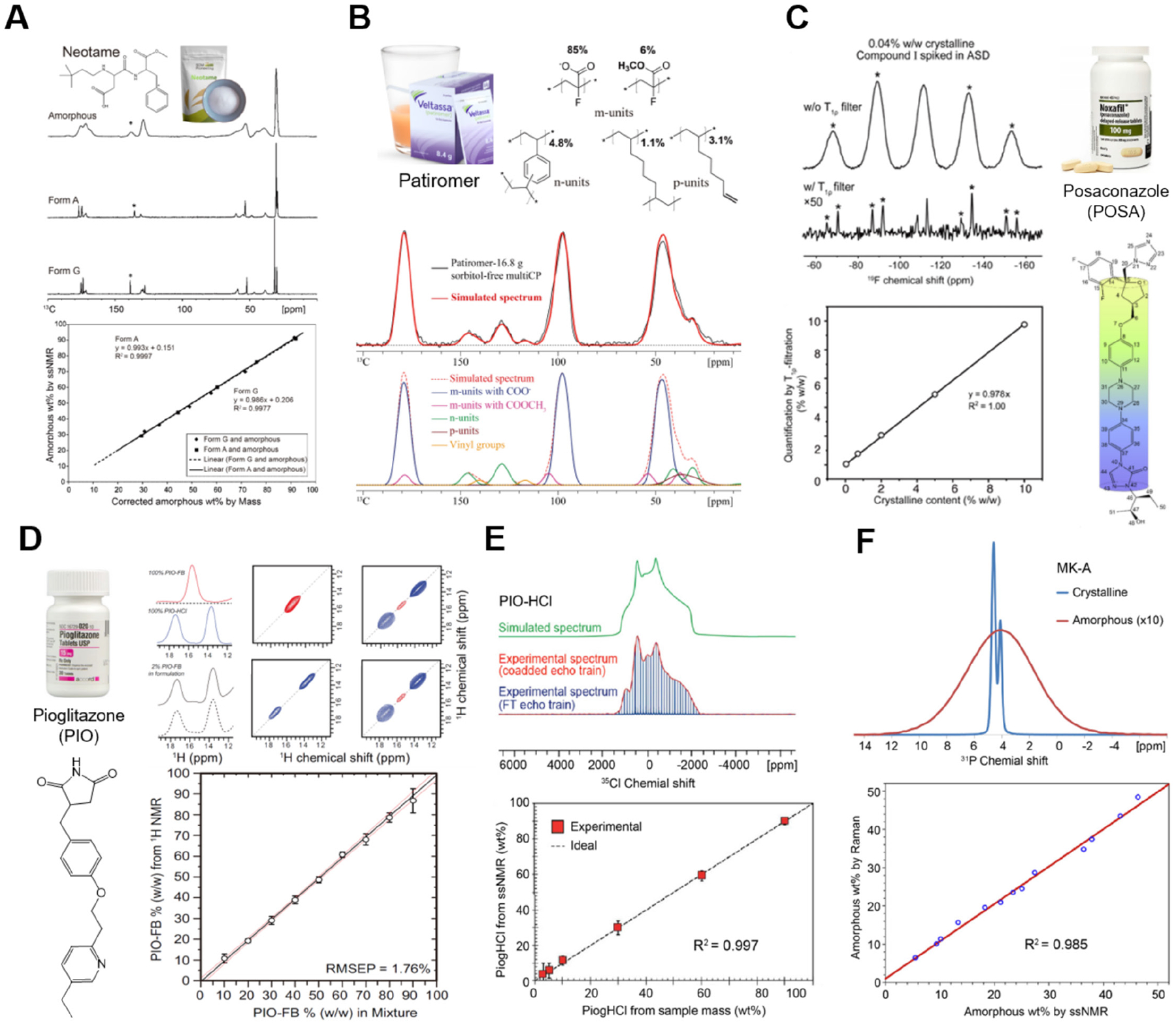
Representative applications of qSSNMR. (A) Quantification of crystalline neotame Forms A and G in mixtures with amorphous neotame. Below the spectra, the linear correlation between SSNMR results and the weight percent of neotame calculated by mass is illustrated [51]. (B) Full 13C spectral deconvolution and fitting of polymeric API patiromer with SSNMR quantification results of model monomer units shown above [52]. (C) Left: 1H-19F cross polarization (CP) spectra of a 0.04% w/w crystalline Compound I ASD sample with and without spin–lattice relaxation at rotating frame (T1ρ) filtration, along with a calibration plot quantifying 0.04%–10% w/w crystalline Compound I [53]. Right: Posaconazole drug and its chemical structure. (D) Left: Pioglitazone drug and its chemical structure. Right: one- and two-dimensional 1H-1H spectra of a multicomponent formulation with 2% w/w pioglitazone free base (PIO-FB) and 18% w/w PIO-HCl, along with the corresponding correlation curve of PIO-FB weight fraction under 60 kHz MAS [46]. (E) 35Cl static SSNMR spectrum (red and blue) of pure PIO-HCl and the corresponding calculated spectrum (green), with a plot of PIO-HCl weight fractions versus sample mass [45]. (F) 31P SSNMR of MK-A crystalline and amorphous reference standards. Below shows the linear correlation between results obtained by Raman and SSNMR [44]. Figures adapted with permission from American Chemical Society and Elsevier.
4 |. Recent Advancements in qSSNMR
More NMR technological advancements have greatly expanded the capabilities of qSSNMR, as illustrated in Figure 3. First, the development of advanced pulse programming techniques, such as spectral editing and relaxation filters [57, 60, 61], has enhanced the ability to distinguish between closely related species in complex mixtures. Additionally, the introduction of UF-MAS [34], which spins samples at 60 kHz or higher, has revolutionized both the sensitivity and resolution of SSNMR experiments. UF-MAS enables the rapid acquisition of high-resolution 1H NMR data, making it invaluable for high-throughput analysis in pharmaceutical applications. While UF-MAS enhances resolution and sensitivity, challenges such as rotor packing consistency, particularly for small-diameter rotors, and the maintenance of stable high-frequency spinning continue to pose barriers to fully automated workflows. Furthermore, the implementation of dynamic nuclear polarization (DNP) [58], which transfers polarization from electrons to target nuclei, has significantly broadened qSSNMR applications by enhancing sensitivity, even in pharmaceutical materials at natural abundance [62–64]. Another key advancement is the development and use of cryogenically cooled MAS SSNMR probes, or CryoProbes [59], which drastically reduce electronic noise and significantly improve the signal-to-noise ratio (S/N) of NMR spectra.
FIGURE 3 |.

Technological advancements in qSSNMR. (A) Three-spin coherence CH2 selection pulse sequence [56] (top) and its application (bottom) in a cellulose-derived pharmaceutical excipient, hydroxyl propyl methylcellulose acetate succinate. The CH2 selection highlights the OCH2 C6 while suppressing OCH [57]. (B) UF-MAS rotor loaded on a stage under optical microscopy (left) and its application (right) in pharmaceuticals. A comparison of 1D and 2D 1H NMR spectra for posaconazole was performed, highlighting the differences between spectra acquired under 40–110 kHz and those obtained under conventional 12 kHz MAS [31]. (C) Schematic illustration of 1H DNP (top left), DNP MAS unit (bottom left), and its application (right). DNP-enhanced 13C (top right) and 15N CP MAS spectra (bottom right) of natural abundance posaconazole deuterated vinyl acetate amorphous solid dispersions [58]. (D) Schematic illustration of CP MAS CryoProbe (left) and its applicati.n (right) in the quantitative analysis of posaconazole. Comparison of 1D 13C (top) and 15N (bottom) CP spectra of crystalline POSA Form I obtained using the CryoProbe (red) and a standard 4 mm probe (blue) [59]. Figures adapted with permission from American Chemical Society, Elsevier, and Wiley.
These technological innovations have transformed the landscape of pharmaceutical analysis by enabling researchers to obtain high-quality spectra. They further pave the way for high-throughput and automated qSSNMR (see Figure 4). The general workflow includes several key steps: (i) automated sample handling, which manages sample transfer, insertion, and ejection; (ii) data acquisition, where advanced techniques and pulse sequences improve resolution and sensitivity; and (iii) streamlined data analysis, providing quantitative results through reliable spectral interpretation, including spectral deconvolution, modeling, and fitting. Conceptually, high-throughput qSSNMR automation significantly reduces the time and labor traditionally associated with SSNMR, enhancing efficiency while improving data reliability and reproducibility. This makes qSSNMR more accessible for routine use in pharmaceutical laboratories.
FIGURE 4 |.

Conceptual and aspirational workflow of high-throughput qSSNMR automation for pharmaceutical analysis.
5 |. Challenges in qSSNMR
Despite its many advantages, qSSNMR has faced challenges in gaining widespread adoption in the pharmaceutical industry [16, 65]. Some of the primary barriers, aside from sensitivity, include (i) Sample preparation challenges: Preparing solid samples for analysis can be complex, for example, grinding may introduce artifacts or alter the compound’s solid forms. (ii) Data interpretation complexity: qSSNMR spectra are often difficult to interpret due to line broadening and peak overlaps, especially with complex molecules or mixtures, requiring specialized expertise and advanced data analysis techniques. (iii) High cost: The equipment and infrastructure needed for qSSNMR are expensive, limiting accessibility for small-scale pharmaceutical companies and research groups. (iv) Competition from other techniques: More widely adopted techniques, considered easier to use, often compete with qSSNMR in the pharmaceutical industry.
Nevertheless, there have been collective and growing efforts to overcome these barriers, especially for complex drug products where traditional methods could be less chemistry-specific or informative. For example, advancements in automation and user-friendly software for data collection and analysis are continually evolving [66]. Additionally, the increasing availability of training resources and collaborative platforms is helping to demystify qSSNMR and promote its broader adoption among pharmaceutical scientists. Furthermore, the integration of qSSNMR with other orthogonal analytical techniques, such as X-ray diffraction (XRD) [67, 68], Raman spectroscopy [69], and differential scanning calorimetry (DSC) [53], by leveraging the resolution, quantification level, and accessibility of each tool, offers exciting opportunities for the comprehensive characterization of pharmaceutical materials. By combining the strengths of these methods, researchers can gain a more holistic and accurate understanding of complex formulations, ultimately enhancing drug development processes.
qSSNMR remains in its early stages, taking advantage of external calibration methods and lacking standardized protocols, in comparison with qNMR. Looking ahead, qSSNMR in pharmaceutical analysis is promising in informing microstructural Q3 quality attributes of complex drug dosage forms. qSSNMR’s ability to provide detailed compositional and structural data positions it as a critical tool in this evolving analytical landscape. It is anticipated that qSSNMR analysis will extend beyond solid dosages of small molecule drugs to include biologics formulated in solid state [70], suspensions [71–73] or frozen solution [74–76]. For example, ssNMR has recently been used to quantify the mobile water [74] and various species of phosphate buffer ions [75] during the freezing process of biologics. Additionally, standardizing qSSNMR procedures will be essential for establishing it as a routine analytical technique in pharmaceutical laboratories. Collaborative efforts between academic, industrial, and regulatory institutes will be key to developing best practices and guidelines for qSSNMR applications [77, 78].
6 |. Conclusion
In conclusion, qSSNMR is emerging as a transformative analytical technique in the pharmaceutical industry, providing unique capabilities for characterizing and quantifying APIs and excipients in solid formulations. SSNMR technological advancements, along with its applications in key areas such as polymorphism, phase transitions, and low-level component detection, highlight the technique’s increasing importance in pharmaceutical analysis. qSSNMR not only enhances the accuracy of pharmaceutical analysis but may also play a pivotal role in ensuring the quality of medications. As the industry adopts automation and aims to improve the reliability and efficiency of analytical methods, qSSNMR is positioned to have a role in drug development, quality control, and quality assurance. By promoting collaboration and standardization across various sectors, we are excited about the potential to fully unlock the benefits of qSSNMR, ultimately contributing to the quality of drug products and patient outcomes.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer
This article reflects the views of the authors and should not be construed to represent US FDA’s views or policies. The authors declare the following competing financial interests: E.J.M. is a partial owner of Kansas Analytical Services, which provides solid-state NMR services to the pharmaceutical industry. E.J.M. is a partial owner of Spectral NMR Technologies, which provides specialized NMR equipment for non-destructive testing of pharmaceutical products. No data from either Kansas Analytical Services or Spectral NMR Technologies are presented.
Data Availability Statement
Scientific data included in this review article are available in the cited references.
References
- 1.Lee AY, Erdemir D, and Myerson AS, “Crystal Polymorphism in Chemical Process Development,” Annual Review of Chemical and Biomolecular Engineering 2, no. 1 (2011): 259–280. [DOI] [PubMed] [Google Scholar]
- 2.Narang AS, Desai D, and Badawy S, “Impact of Excipient Interactions on Solid Dosage Form Stability,” in Excipient Applications in Formulation Design and Drug Delivery (Springer International Publishing, 2015): 93–137. [Google Scholar]
- 3.Zhang GG, Law D, Schmitt EA, and Qiu Y, “Phase Transformation Considerations During Process Development and Manufacture of Solid Oral Dosage Forms,” Advanced Drug Delivery Reviews 56, no. 3 (2004): 371–390. [DOI] [PubMed] [Google Scholar]
- 4.Willart J and Descamps M, “Solid State Amorphization of Pharmaceuticals,” Molecular Pharmaceutics 5, no. 6 (2008): 905–920. [DOI] [PubMed] [Google Scholar]
- 5.Wegiel LA, Mauer LJ, Edgar KJ, and Taylor LS, “Crystallization of Amorphous Solid Dispersions of Resveratrol During Preparation and Storage—Impact of Different Polymers,” Journal of Pharmaceutical Sciences 102, no. 1 (2013): 171–184. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MR, AlOthman ZA, and Rahman N, “Analytical Techniques in Pharmaceutical Analysis: A Review,” Arabian Journal of Chemistry 10 (2017): S1409–S1421. [Google Scholar]
- 7.Bhardwaj SK, Dwivedia K, and Agarwala D, “A Review: HPLC Method Development and Validation,” International Journal of Analytical and Bioanalytical Chemistry 5, no. 4 (2015): 76–81. [Google Scholar]
- 8.Glish GL and Vachet RW, “The Basics of Mass Spectrometry in the Twenty-First Century,” Nature Reviews. Drug Discovery 2, no. 2 (2003): 140–150. [DOI] [PubMed] [Google Scholar]
- 9.Holzgrabe U, Deubner R, Schollmayer C, and Waibel B, “Quantitative NMR Spectroscopy—Applications in Drug Analysis,” Journal of Pharmaceutical and Biomedical Analysis 38, no. 5 (2005): 806–812. [DOI] [PubMed] [Google Scholar]
- 10.Chadha R and Bhandari S, “Drug–Excipient Compatibility Screening—Role of Thermoanalytical and Spectroscopic Techniques,” Journal of Pharmaceutical and Biomedical Analysis 87 (2014): 82–97. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M, Bhatia R, and Rawal RK, “Applications of Various Analytical Techniques in Quality Control of Pharmaceutical Excipients,” Journal of Pharmaceutical and Biomedical Analysis 157 (2018): 122–136. [DOI] [PubMed] [Google Scholar]
- 12.Harris RK, “Applications of Solid-State NMR to Pharmaceutical Polymorphism and Related Matters,” Journal of Pharmacy and Pharmacology 59, no. 2 (2007): 225–239. [DOI] [PubMed] [Google Scholar]
- 13.Berendt RT, Sperger DM, Munson EJ, and Isbester PK, “Solid-State NMR Spectroscopy in Pharmaceutical Research and Analysis,” TrAC - Trends in Analytical Chemistry 25, no. 10 (2006): 977–984. [Google Scholar]
- 14.Vogt FG, “Characterization of Pharmaceutical Compounds by Solid-State NMR,” eMagRes 4 (2015): 297. [Google Scholar]
- 15.Geppi M, Mollica G, Borsacchi S, and Veracini CA, “Solid-State NMR Studies of Pharmaceutical Systems,” Applied Spectroscopy Reviews 43, no. 3 (2008): 202–302. [Google Scholar]
- 16.Li M, Xu W, and Su Y, “Solid-State NMR Spectroscopy in Pharmaceutical Sciences,” TrAC - Trends in Analytical Chemistry 135 (2021): 116152. [Google Scholar]
- 17.Baghel S, Cathcart H, and O’Reilly NJ, “Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs,” Journal of Pharmaceutical Sciences 105, no. 9 (2016): 2527–2544. [DOI] [PubMed] [Google Scholar]
- 18.Newman AW and Byrn SR, “Solid-State Analysis of the Active Pharmaceutical Ingredient in Drug Products,” Drug Discovery Today 8, no. 19 (2003): 898–905. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson GA, Forbes RA, and Reutzel-Edens SM, “Characterization of the Solid State: Quantitative Issues,” Advanced Drug Delivery Reviews 48, no. 1 (2001): 67–90. [DOI] [PubMed] [Google Scholar]
- 20.Chasse T, Conway SL, Danzer GD, et al. , “Industry White Paper: Contemporary Opportunities and Challenges in Characterizing Crystallinity in Amorphous Solid Dispersions,” Journal of Pharmaceutical Sciences 111, no. 6 (2022): 1543–1555. [DOI] [PubMed] [Google Scholar]
- 21.Hollis DP, “Quantitative Analysis of Aspirin, Phenacetin, and Caffeine Mixtures by Nuclear Magnetic Resonance Spectrometry,” Analytical Chemistry 35, no. 11 (1963): 1682–1684. [Google Scholar]
- 22.Hanna GM, “Nuclear Magnetic Resonance Spectroscopic Determination of Dicyclomine Hydrochloride in Tablet, Capsule, and Injection Dosage Forms,” Journal of the Association of Official Analytical Chemists 67, no. 2 (1984): 222–224. [PubMed] [Google Scholar]
- 23.Jaki BU, Bzhelyansky A, and Pauli GF, “Quantitative NMR (qNMR) for Pharmaceutical Analysis: The Pioneering Work of George Hanna at the US FDA,” Magnetic Resonance in Chemistry 59, no. 1 (2021): 7–15. [DOI] [PubMed] [Google Scholar]
- 24.Keire DA, Trehy ML, Reepmeyer JC, et al. , “Analysis of Crude Heparin by 1H NMR, Capillary Electrophoresis, and Strong-Anion-Exchange-HPLC for Contamination by Over Sulfated Chondroitin Sulfate,” Journal of Pharmaceutical and Biomedical Analysis 51, no. 4 (2010): 921–926. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Dai W, Qian K, Scott B, and Chen K, “A Precise qNMR Method for the Rapid Quantification of Lot-To-Lot Variations in Multiple Quality Attributes of Pentosan Polysulfate Sodium,” AAPS Journal 25, no. 3 (2023): 50. [DOI] [PubMed] [Google Scholar]
- 26.Bjørstorp S and Malmstrøm J, “Quantitative 31P NMR Spectroscopy Platform Method for the Assay of Oligonucleotides as Pure Drug Substances and in Drug Product Formulations Using the Internal Standard Method,” Analytical Chemistry 96, no. 28 (2024): 11198–11204. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Chen F, Zhang D, Wang Y, Kozak D, and Chen K, “An Accurate and Fast 31P qNMR Assay Method for Oligonucleotide Therapeutics,” Analytical Chemistry 96, no. 42 (2024): 16514–16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh MS, Patil SM, Kozak D, et al. , “An NMR Protocol for In Vitro Paclitaxel Release From an Albumin-Bound Nanoparticle Formulation,” AAPS PharmSciTech 21 (2020): 1–8. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Park JH, Zheng J, Cai B, Keire DA, and Chen K, “Multiphase Drug Distribution and Exchange in Oil-in-Water Nanoemulsion Revealed by High-Resolution 19F qNMR,” Molecular Pharmaceutics 19, no. 7 (2022): 2142–2150. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Huang C, Li M, et al. , “Molecular Mechanism of Crystalline-to-Amorphous Conversion of Pharmaceutical Solids From 19F Magic Angle Spinning NMR,” Journal of Physical Chemistry B 124, no. 25 (2020): 5271–5283. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Tsutsumi Y, Huang C, et al. , “Molecular Packing of Pharmaceuticals Analyzed With Paramagnetic Relaxation Enhancement and Ultrafast Magic Angle Pinning NMR,” Physical Chemistry Chemical Physics 22, no. 23 (2020): 13160–13170. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration Physicochemical and Structural (Q3) Characterization of Topical Drug Products Submitted in ANDAs, (2022) [Google Scholar]
- 33.Chang R-K, Raw A, Lionberger R, and Yu L, “Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products,” AAPS Journal 15 (2013): 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiyama Y, Hou G, Agarwal V, Su Y, and Ramamoorthy A, “Ultrafast Magic Angle Spinning Solid-State NMR Spectroscopy: Advances in Methodology and Applications,” Chemical Reviews 123, no. 3 (2022): 918–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y, Andreas L, and Griffin RG, “Magic Angle Spinning NMR of Proteins: High-Frequency Dynamic Nuclear Polarization and (1) H Detection,” Annual Review of Biochemistry 84 (2015): 465–497, 10.1146/annurev-biochem-060614-034206. [DOI] [PubMed] [Google Scholar]
- 36.Johann C, Wegner S, Althoff G, and Struppe J, “Automation in Solid State NMR,” Journal of Magnetic Resonance 355 (2023): 107554, 10.1016/j.jmr.2023.107554. [DOI] [PubMed] [Google Scholar]
- 37.Balimann G, Groombridge CJ, Harris RK, Packer KJ, Say B, and Tanner SF, “Chemical Applications of High-Resolution 13C NMR Spectra for Solids,” Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences 299, no. 1452 (1981): 643–663. [Google Scholar]
- 38.Byrn S, Pfeiffer R, Ganey M, Hoiberg C, and Poochikian G, “Pharmaceutical Solids: A Strategic Approach to Regulatory Considerations,” Pharmaceutical Research 12 (1995): 945–954. [DOI] [PubMed] [Google Scholar]
- 39.Phyo P, Zhao X, Templeton AC, Xu W, Cheung JK, and Su YC, “Understanding Molecular Mechanisms of Biologics Drug Delivery and Stability From NMR Spectroscopy,” Advanced Drug Delivery Reviews 174 (2021): 1–29, 10.1016/j.addr.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Suryanarayanan R and Wiedmann TS, “Quantitation of the Relative Amounts of Anhydrous Carbamazepine (C15H12N2O) and Carbamazepine Dihydrate (C15H12N2O·2H2O) in a Mixture by Solid-State Nuclear Magnetic Resonance (NMR),” Pharmaceutical Research 7 (1990): 184–187. [DOI] [PubMed] [Google Scholar]
- 41.Farrer BT, Peresypkin A, and Wenslow RM, “Quantitation of Crystalline Material Within a Liquid Vehicle Using 1H/19F CP/MAS NMR,” Journal of Pharmaceutical Sciences 96, no. 2 (2007): 264–267. [DOI] [PubMed] [Google Scholar]
- 42.Du Y and Su Y, “(19)F Solid-State NMR Characterization of Pharmaceutical Solids,” Solid State Nuclear Magnetic Resonance 120 (2022): 101796, 10.1016/j.ssnmr.2022.101796. [DOI] [PubMed] [Google Scholar]
- 43.Maruyoshi K, Iuga D, Watts AE, Hughes CE, Harris KD, and Brown SP, “Assessing the Detection Limit of a Minority Solid-State Form of a Pharmaceutical by 1H Double-Quantum Magic-Angle Spinning Nuclear Magnetic Resonance Spectroscopy,” Journal of Pharmaceutical Sciences 106, no. 11 (2017): 3372–3377. [DOI] [PubMed] [Google Scholar]
- 44.Wabuyele BW, Sotthivirat S, Zhou GX, Ash J, and Dhareshwar SS, “Dispersive Raman Spectroscopy for Quantifying Amorphous Drug Content in Intact Tablets,” Journal of Pharmaceutical Sciences 106, no. 2 (2017): 579–588. [DOI] [PubMed] [Google Scholar]
- 45.Hirsh DA, Su Y, Nie H, et al. , “Quantifying Disproportionation in Pharmaceutical Formulations With 35Cl Solid-State NMR,” Molecular Pharmaceutics 15, no. 9 (2018): 4038–4048. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Lu X, Xu W, et al. , “Quantifying Pharmaceutical Formulations From Proton Detected Solid-State NMR Under Ultrafast Magic Angle Spinning,” Journal of Pharmaceutical Sciences 109, no. 10 (2020): 3045–3053. [DOI] [PubMed] [Google Scholar]
- 47.Wong YA, Aspers RL, Uusi-Penttilä M, and Kentgens AP, “Rapid Quantification of Pharmaceuticals via 1H Solid-State NMR Spectroscopy,” Analytical Chemistry 94, no. 48 (2022): 16667–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirsh DA, Wijesekara AV, Carnahan SL, et al. , “Rapid Characterization of Formulated Pharmaceuticals Using Fast MAS 1H Solid-State NMR Spectroscopy,” Molecular Pharmaceutics 16, no. 7 (2019): 3121–3132, 10.1021/acs.molpharmaceut.9b00343. [DOI] [PubMed] [Google Scholar]
- 49.Dong Y-D and Boyd BJ, “Applications of X-Ray Scattering in Pharmaceutical Science,” International Journal of Pharmaceutics 417, no. 1–2 (2011): 101–111. [DOI] [PubMed] [Google Scholar]
- 50.Strachan CJ, Rades T, Gordon KC, and Rantanen J, “Raman Spectroscopy for Quantitative Analysis of Pharmaceutical Solids,” Journal of Pharmacy and Pharmacology 59, no. 2 (2007): 179–192. [DOI] [PubMed] [Google Scholar]
- 51.Offerdahl TJ, Salsbury JS, Dong Z, et al. , “Quantitation of Crystalline and Amorphous Forms of Anhydrous Neotame Using 13C CPMAS NMR Spectroscopy,” Journal of Pharmaceutical Sciences 94, no. 12 (2005): 2591–2605. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Z, Su Y, and Schmidt-Rohr K, “Vinyl and Methyl-Ester Groups in the Insoluble Polymer Drug Patiromer Identified and Quantified by Solid-State NMR,” Journal of Pharmaceutical and Biomedical Analysis 246 (2024): 116228. [DOI] [PubMed] [Google Scholar]
- 53.Du Y, Phyo P, Li M, et al. , “Quantifying Micromolar Crystallinity in Pharmaceutical Materials Utilizing 19F Solid-State NMR,” Analytical Chemistry 94, no. 44 (2022): 15341–15349. [DOI] [PubMed] [Google Scholar]
- 54.Zhu XL, Punia A, Skomski D, et al. , “Insights Into Factors Affecting Ethylene-Vinyl Acetate Copolymer Crystallinity in Islatravir Implant,” Molecular Pharmaceutics 21, no. 4 (2024): 1933–1941, 10.1021/acs.molpharmaceut.3c01198. [DOI] [PubMed] [Google Scholar]
- 55.Skomski D, Liu Z, Su Y, et al. , “An Imaging Toolkit for Physical Characterization of Long-Acting Pharmaceutical Implants,” Journal of Pharmaceutical Sciences 109, no. 9 (2020): 2798–2811, 10.1016/j.xphs.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 56.Mao J-D and Schmidt-Rohr K, “Separation of Aromatic-Carbon 13C NMR Signals From Di-Oxygenated Alkyl Bands by a Chemical-Shift-Anisotropy Filter,” Solid State Nuclear Magnetic Resonance 26, no. 1 (2004): 36–45. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Z, Su Y, and Schmidt-Rohr K, “Corrected Solid-State 13C Nuclear Magnetic Resonance Peak Assignment and Side-Group Quantification of Hydroxypropyl Methylcellulose Acetyl Succinate Pharmaceutical Excipients,” Magnetic Resonance in Chemistry 61, no. 11 (2023): 595. [DOI] [PubMed] [Google Scholar]
- 58.Ni QZ, Yang F, Can TV, et al. , “In Situ Characterization of Pharmaceutical Formulations by Dynamic Nuclear Polarization Enhanced MAS NMR,” Journal of Physical Chemistry B 121, no. 34 (2017): 8132–8141, 10.1021/acs.jpcb.7b07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du Y, Struppe J, Perrone B, Hassan A, Codina A, and Su Y, “Efficient Analysis of Pharmaceutical Drug Substances and Products Using a Solid-State NMR CryoProbe,” Analyst 148, no. 4 (2023): 724–734. [DOI] [PubMed] [Google Scholar]
- 60.Petrova SP, Zheng Z, Heinze DA, et al. , “Gelation During Ring-Opening Reactions of Cellulosics With Cyclic Anhydrides: Phenomena and Mechanisms,” Biomacromolecules 25, no. 12 (2024): 7777–7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Z and Schmidt-Rohr K, “Phenolic Syringyl End Groups in 13C-Enriched Hardwoods Detected and Quantified by Solid-State NMR,” Solid State Nuclear Magnetic Resonance 133 (2024): 101947. [DOI] [PubMed] [Google Scholar]
- 62.Bertarello A, Berruyer P, Artelsmair M, et al. , “In-Cell Quantification of Drugs by Magic-Angle Spinning Dynamic Nuclear Polarization NMR,” Journal of the American Chemical Society 144, no. 15 (2022): 6734–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossini AJ, Zagdoun A, Hegner F, et al. , “Dynamic Nuclear Polarization NMR Spectroscopy of Microcrystalline Solids,” Journal of the American Chemical Society 134, no. 40 (2012): 16899–16908. [DOI] [PubMed] [Google Scholar]
- 64.Rossini AJ, Widdifield CM, Zagdoun A, et al. , “Dynamic Nuclear Polarization Enhanced NMR Spectroscopy for Pharmaceutical Formulations,” Journal of the American Chemical Society 136, no. 6 (2014): 2324–2334. [DOI] [PubMed] [Google Scholar]
- 65.Silva I. d. A. A., Bartalucci E, Bolm C, and Wiegand T, “Opportunities and Challenges in Applying Solid-State NMR Spectroscopy in Organic Mechanochemistry,” Advanced Materials 35, no. 52 (2023): 2304092. [DOI] [PubMed] [Google Scholar]
- 66.Borcik CG, DeZonia B, Ravula T, Harding BD, Garg R, and Rienstra CM, “OPTO: Automated Optimization for Solid-State NMR Spectroscopy,” Journal of the American Chemical Society 147 (2025): 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Z, Nie H, Benmore CJ, et al. , “Probing Molecular Packing of Amorphous Pharmaceutical Solids Using X-Ray Atomic Pair Distribution Function and Solid-State NMR,” Molecular Pharmaceutics 20, no. 11 (2023): 5763–5777, 10.1021/acs.molpharmaceut.3c00628. [DOI] [PubMed] [Google Scholar]
- 68.Correa-Soto C, Trasi NS, Schmitt PD, et al. , “Second Harmonic Generation Microscopy as a Tool for the Early Detection of Crystallization in Spray Dried Dispersions,” Journal of Pharmaceutical and Biomedical Analysis 146 (2017): 86–95. [DOI] [PubMed] [Google Scholar]
- 69.Francis AT, Nguyen TT, Lamm MS, et al. , “In Situ Stimulated Raman Scattering (SRS) Microscopy Study of the Dissolution of Sustained-Release Implant Formulation,” Molecular Pharmaceutics 15, no. 12 (2018): 5793–5801, 10.1021/acs.molpharmaceut.8b00965. [DOI] [PubMed] [Google Scholar]
- 70.Ling J, Du Y, Wuelfing WP, et al. , “Molecular Mechanisms for Stabilizing Biologics in the Solid State,” Journal of Pharmaceutical Sciences 114, no. 2 (2025): 736–765, 10.1016/j.xphs.2024.11.017. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Reichert P, Narasimhan C, et al. , “Investigating Crystalline Protein Suspension Formulations of Pembrolizumab From MAS NMR Spectroscopy,” Molecular Pharmaceutics 19, no. 3 (2022): 936–952, 10.1021/acs.molpharmaceut.1c00915. [DOI] [PubMed] [Google Scholar]
- 72.Banks D, Kempf JG, Du Y, et al. , “Investigation of Protein Therapeutics in Frozen Conditions Using DNP MAS NMR: A Study on Pembrolizumab,” Molecular Pharmaceutics 21, no. 12 (2024): 6363–6375, 10.1021/acs.molpharmaceut.4c00929. [DOI] [PubMed] [Google Scholar]
- 73.Larpent P, Codan L, Bothe JR, et al. , “Small-Angle X-Ray Scattering as a Powerful Tool for Phase and Crystallinity Assessment of Monoclonal Antibody Crystallites in Support of Batch Crystallization,” Molecular Pharmaceutics 21, no. 8 (2024): 4024–4037, 10.1021/acs.molpharmaceut.4c00418. [DOI] [PubMed] [Google Scholar]
- 74.Du Y and Su Y, “Quantification of Residual Water in Pharmaceutical Frozen Solutions via 1H Solid-State NMR,” Journal of Pharmaceutical Sciences 113, no. 8 (2024): 2405–2412, 10.1016/j.xphs.2024.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Du Y, Li J, Suryanarayanan R, and Su Y, “Probing Chemical Equilibrium in Frozen Sodium Phosphate Buffer Solution by 31P Solid-State NMR,” Journal of Physical Chemistry Letters 15, no. 21 (2024): 5714–5720, 10.1021/acs.jpclett.4c00877. [DOI] [PubMed] [Google Scholar]
- 76.Du Y, Li J, Xu W, et al. , “Solid-State NMR Spectroscopy to Probe State and Phase Transitions in Frozen Solutions,” Molecular Pharmaceutics 20, no. 12 (2023): 6380–6390, 10.1021/acs.molpharmaceut.3c00764. [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Rienstra CM, and Chen K, “Higher Order Structure Differences Among Insulin Crystalline Drugs Revealed by 2D Heteronuclear NMR,” ChemMedChem 19, no. 23 (2024): e202400340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brader M, Kim HA, Koo O, Nagapudi K, and Su Y, “Industrial Horizons in Pharmaceutical Science,” Molecular Pharmaceutics 21, no. 9 (2024): 4183–4188, 10.1021/acs.molpharmaceut.4c00544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Scientific data included in this review article are available in the cited references.


