Abstract
Antimicrobial resistance (AMR) poses a global threat to public health. While antibiotic overuse is a primary driver, emerging evidence suggests that non-antibiotic medications (NAMs) may also contribute. This concern is particularly relevant in residential aged care facilities (RACFs), where both NAMs and antibiotics are frequently used. We investigated whether nine commonly used NAMs in RACFs, including ibuprofen, diclofenac, acetaminophen, furosemide, metformin, atorvastatin, tramadol, temazepam, and pseudoephedrine at gut-relevant concentrations, enhance ciprofloxacin-induced mutagenesis in Escherichia coli. Our findings showed that ibuprofen and acetaminophen significantly increased mutation frequency and conferred high-level ciprofloxacin resistance. Whole-genome sequencing identified mutations in GyrA, MarR, and AcrR, with the latter two correlated with overexpression of AcrAB-TolC drug efflux pump. Co-exposure to two NAMs further elevated mutation rates and ciprofloxacin resistance levels. This study underscored the overlooked role of NAMs in driving AMR and highlighted the need to reassess polypharmacy risks in aged care settings.
Subject terms: Biochemistry, Microbiology
Introduction
The development and spread of antimicrobial-resistant organisms and resistance genes represent one of the greatest threats facing public health1. This is exemplified by current reports estimating that 4.95 million deaths were associated with antimicrobial resistance in 20192. It is well-known that the prevalent amplifier behind the development of antimicrobial resistance (AMR) is the overuse of antibiotics in clinical and agricultural settings3,4. However, in addition to antibiotics, the use of non-antibiotic medications (NAMs) such as diuretics, statins, proton-pump inhibitors, amongst others, have also been shown to contribute the rise of AMR5,6.
A recent report assessing antimicrobial properties of NAMs revealed that more than 200 commonly used drugs possessed antibiotic-like effects on gut bacteria7. Currently, the global pharmaceutical market is primarily made up of non-antibiotic drugs, which occupy 95% of the market8. The pharmaceutical market also continues to grow, experiencing significant growth during the last two years, with worldwide revenues totalling 1.48 trillion (US) dollars in 20229. The growth of this industry is not surprising, as the world’s population is ageing10, and this ageing population relies on an arsenal of medicines to manage chronic disease11, with some taking nine or more prescribed drugs per day, a phenomenon known as polypharmacy, and one which is increasing worldwide11–14. Older persons also represent a population with a high consumption of antibiotics, which are frequently overused in residential aged care facilities (RACFs) to manage urinary tract and respiratory infections15–19.
NAMs can have both a direct and/or indirect effect on the development of AMR. Some NAMs display direct antibacterial activity, such as statins20. Others, such as certain nonsteroidal anti-inflammatory drugs (NSAIDs), can promote the development of cross-resistance. This involves selecting for resistance mechanisms that concurrently confer resistance to antibiotics, with multidrug efflux pumps being a prime example21. Bacteria can also acquire additional resistance mechanisms by taking up a plasmid expressing resistance factors in a process termed transformation. Diclofenac is an example of a NAM that could increase the transformation efficiency with a resultant increase in acquired resistance6. Compounding these is the fact that some antibiotics like ciprofloxacin, a fluoroquinolone, and non-antibiotics like tramadol, a weak opioid, are not completely degraded in the body and end up in wastewater through the excretion of urine and/or stool22. Once there, these, and the metabolites of both antibiotics and non-antibiotic drugs, exert their combined effect and create a perfect environment for the development and dissemination of AMR.
To assess the propensity of NAMs to promote the development of AMR, we investigated nine medications; acetaminophen, ibuprofen, diclofenac, furosemide, atorvastatin calcium, metformin, pseudoephedrine, temazepam, and tramadol, which are commonly used by older people23,24. As these NAMs are frequently co-administered with antibiotics, mutation frequencies were determined in the presence of ciprofloxacin. Ciprofloxacin is not only a known inducer of mutations but is also used in the treatment of urinary tract infections (UTIs), which are one of the most common bacterial infections25,26. The frequent usage of ciprofloxacin in RACF settings has led to the emergence of ciprofloxacin resistance and ciprofloxacin-resistant Escherichia coli, which has posed a threat to residents living in RACFs27. The effect of the nine NAMs and combinations of two NAMs on the mutation frequencies was determined for two E. coli isolates, E. coli BW25113, a derivative of E. coli K-12, and E. coli 6146 that was isolated from a faecal sample of a retirement community resident. The antimicrobial susceptibilities of the NAM-induced mutants and the mechanisms underlying the observed resistance were assessed by whole genome sequencing. The role of drug efflux pumps, which can be over-expressed in response to stress and contribute to cross-resistance, was also further explored.
Results
NAM exposure increases fitness of E. coli in the presence of ciprofloxacin
To assess the propensity of commonly used non-antibiotics to induce bacterial resistance to antibiotics, six NAMs were chosen based on their frequent use among the elderly in RACFs28,29. Ciprofloxacin was used in this study as it is a known inducer of mutations30 with an increase in ciprofloxacin-resistant uropathogens representing a threat to residents living in aged care facilities27,31–33. Mutation frequencies were determined in E. coli BW25113 and E. coli 6146 (an antibiotic sensitive strain isolated from a retirement resident). The baseline antimicrobial susceptibility of the test strains, E. coli BW25113 and E. coli 6146, was determined and shown to be almost identical and wild type for the agents tested (Table 1). None of the NAMs assessed in this study displayed antimicrobial activity against these isolates, even at the highest concentration tested (512 μg/mL). To obtain a comprehensive set of antibiotic susceptibility data, erythromycin and novobiocin, to which E. coli strains are known to be resistant, were also included34.
Table 1.
MICs for E. coli BW25113 and E. coli 6146 against antibiotics and NAMs assessed in this study
| MIC (µg/mL) | ||||
|---|---|---|---|---|
| ECOFF* | E. coli BW25113 | E. coli 6146 | ||
| Antibiotics | Ciprofloxacin | 0.06 | 0.016 | 0.016 |
| Levofloxacin | 0.125 | 0.03 | 0.03 | |
| Minocycline | 4 | 2 | 2 | |
| Amoxicillin | 8 | 8 | 8 | |
| Ceftazidime | 0.5 | 0.5 | 0.5 | |
| Cefepime | 0.25 | 0.06 | 0.03 | |
| Meropenem | 0.06 | 0.03 | 0.03 | |
| Colistin | 2 | 1 | 2 | |
| Nitrofurantoin | 64 | 16 | 16 | |
| Trimethoprim-sulfamethoxazole | 0.5 | 0.5 | 0.5 | |
| Erythromycin | - | 128 | 128 | |
| Novobiocin | - | 256 | 64 | |
| Non-Antibiotics | Acetaminophen | - | >512 | >512 |
| Atorvastatin | - | >512 | >512 | |
| Diclofenac | - | >512 | >512 | |
| Furosemide | - | >512 | >512 | |
| Ibuprofen | - | >512 | >512 | |
| Metformin | - | >512 | >512 | |
| Pseudoephedrine | - | >512 | >512 | |
| Temazepam | - | >512 | >512 | |
| Tramadol | - | >512 | >512 | |
*ECOFF: epidemiological cut-off values. Source: https://mic.eucast.org/.
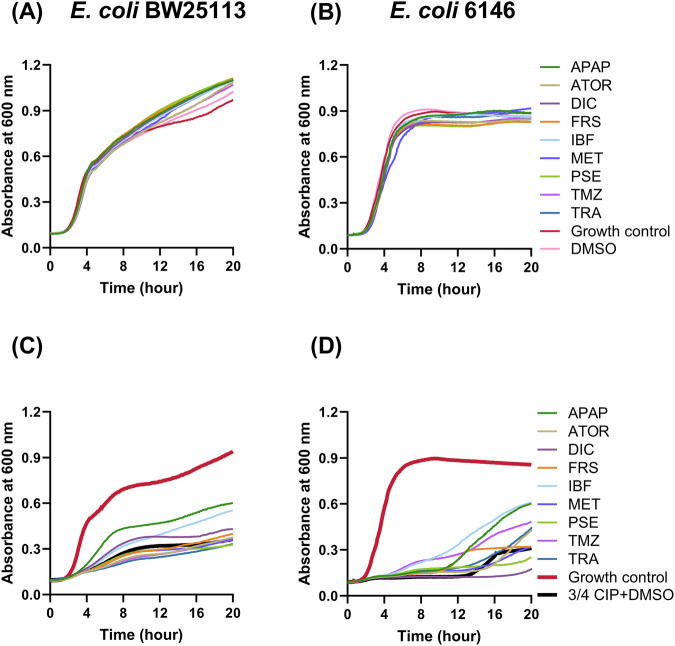
To investigate whether growth was affected by NAM exposure, the growth of E. coli BW25113 and E. coli 6146 was analysed in the presence of sub-inhibitory concentrations of ciprofloxacin and/or gut concentrations of the NAMs analysed in this study (Fig. 1). As expected, when compared to non-exposed cells the addition of NAMs alone did not impact the growth of E. coli cells (Fig. 1A, B). However, the growth rate and maximum cell density were dramatically affected at 3/4 the MIC of ciprofloxacin for both E. coli BW25113 and E. coli 6146 cells. Reduction of cell growth was observed from 0.457 ± 0.009 h−1 to 0.140 ± 0.006 h−1 and 0.443 ± 0.006 h−1 to 0.007 ± 0.001 h−1 for E. coli BW25113 and E. coli 6146 respectively (Fig. 1C, D). Additionally, the start of the exponential growth rate was delayed for E. coli 6146 cells in the presence of ciprofloxacin (Fig. 1D). While in the presence of ¾ the MIC of ciprofloxacin, diclofenac, ibuprofen, and acetaminophen increased the growth rates of E. coli BW25113 to 0.201 ± 0.004, 0.179 ± 0.006, and 0.2823 ± 0.013 h−1 respectively (Fig. 1C). The same three NAMs were also able to reduce the length of the lag phase (Fig. 1D). Collectively, certain NAMs increase the fitness and adaptability of E. coli under stress conditions such as growth in the presence of an antibiotic.
Fig. 1.
Growth profiles of E. coli BW25113 and 6146 in the presence of sub-inhibitory ciprofloxacin and NAMs. A, B The effect of individual NAMs on growth of E. coli BW25113 and E. coli 6146. C, D The effect of individual NAMs on the growth of E. coli BW25113 and E. coli 6146 in the presence of ¾ MIC ciprofloxacin. Growth control represented cells in the absence of NAMs and ciprofloxacin. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin, PSE pseudoephedrine, TMZ temazepam, TRA tramadol. Growth curves were performed four times on different days with different batches of cells. Representative traces from technical triplicates, selected based on the overall results, are shown here.
NAM exposure induces mutations within E. coli
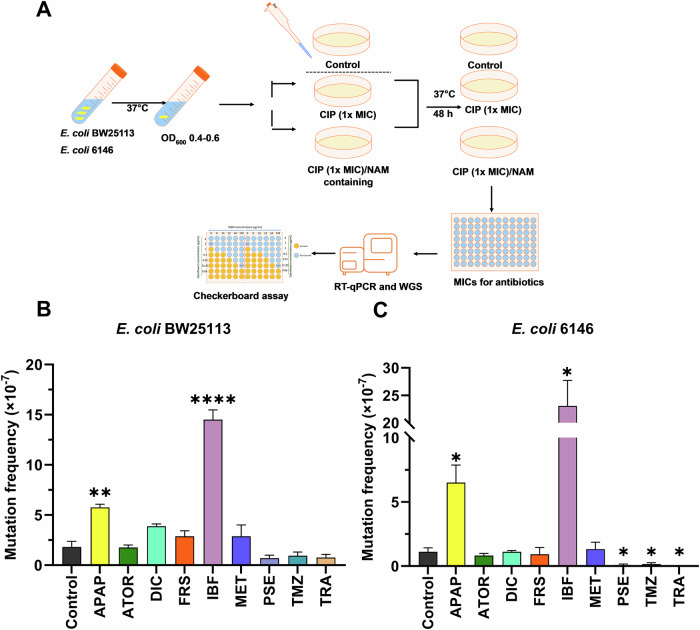
To investigate the potential of the NAMs assessed in this study to induce the development of AMR by mutation, E. coli BW25113 and 6146 cells were exposed to NAMs at the projected gut concentrations and ciprofloxacin at 1 x the MIC (0.015 µg/mL) and cultured for 48 hours (Fig. 2A)7. All assays were carried out on plates supplemented with 0.015 µg/ml ciprofloxacin (1x MIC), as no colonies (mutants) were observed at higher ciprofloxacin concentrations including 2x and 4x MIC.
Fig. 2. Mutation frequencies following exposure to ciprofloxacin and NAMs.
A Workflow used to determine the mutation frequencies of E. coli BW25113 and 6146 cells exposed to NAMs and ciprofloxacin. B, C The mutation frequency of E. coli BW25113 and E. coli 6146 were determined upon the exposure to NAMs at 216 μg/mL for acetaminophen (APAP), 6.6 μg/mL for atorvastatin (ATOR), 15.4 μg/mL for diclofenac (DIC), 13.2 μg/mL for furosemide (FRS), 99.8 μg/mL for ibuprofen (IBF), 194.6 μg/mL for metformin (MET), 40 μg/mL for pseudoephedrine (PSE), 3.3 μg/mL for temazepam (TMZ), and 33.3 μg/mL for tramadol (TRA) and ciprofloxacin (CIP) (0.015 μg/mL). Differences between mutants treated with ciprofloxacin/NAMs and the Control (ciprofloxacin with DMSO treated) were analysed by independent-sample t-tests and corrected by Bonferroni correction method, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data are expressed as mean ± SEM from four experiments done on different days with different batches of cells.
Higher mutation frequencies were observed for E. coli BW25113, with (1.45 ± 0.19) × 10-6 and (5.75 ± 0.64) × 10−7 cells exposed to ibuprofen (P < 0.0001) and acetaminophen (p < 0.01), respectively, compared to exposure to ciprofloxacin alone (Fig. 2). Similarly, a significant increase in mutation frequency was observed for E. coli 6146 following the exposure to acetaminophen (6.50 ± 2.73) × 10−7 and ibuprofen (2.30 ± 0.92) × 10−6. Remarkably, the mutation frequencies of pseudoephedrine, temazepam, and tramadol showed a significant decrease for E. coli 6146 relative to exposure to ciprofloxacin only. The mutation frequencies of the remaining NAMs assessed in this study, were not significantly different from that of the ciprofloxacin only treatment, although the mutation frequencies for E. coli BW25113 was higher than for the clinical strain when cells were exposed to ciprofloxacin in combination with diclofenac, atorvastatin, furosemide, and metformin (Fig. 2).
NAM exposure leads to elevated ciprofloxacin resistance in select isolates
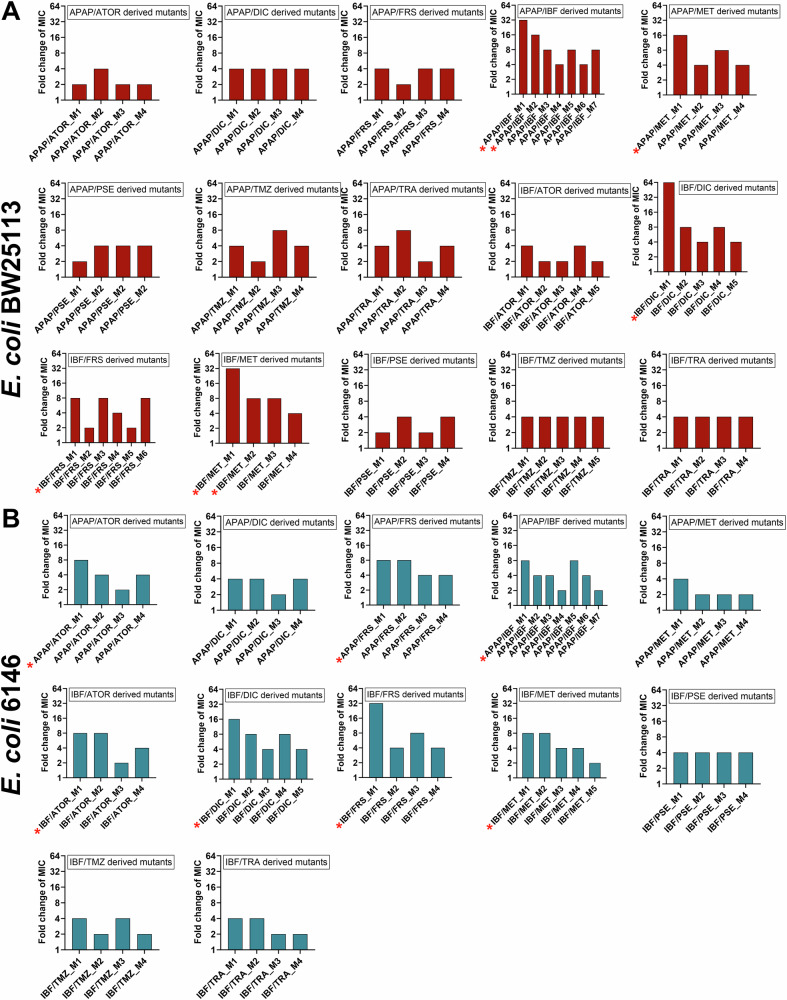
To investigate levels of acquired ciprofloxacin resistance, a representative number (√n + 1) of E. coli BW25113 and 6146 derived mutants were selected from each ciprofloxacin/NAM combination plate and the MIC of ciprofloxacin was determined (Fig. 3). As expected, E. coli BW25113 cells exposed to ciprofloxacin only developed resistance resulting in increased MICs of ciprofloxacin (2 to 8-fold increase in ciprofloxacin MIC as compared to WT E. coli). A comparable rise in ciprofloxacin resistance was also observed for NAM/ciprofloxacin derived mutants, with most E. coli BW25113-derived mutants displaying a 2 to 4-fold increase in MICs (Fig. 3A). However, two mutants, one exposed to atorvastatin/ciprofloxacin (BW_Ator_M1) and one exposed to ibuprofen/ciprofloxacin (BW_Ibf_M1) displayed higher levels of ciprofloxacin resistance with a MIC of 0.125 µg/mL (8-fold increase). Two other BW25113 E. coli derived mutants, acetaminophen/ciprofloxacin BW_Apap_M1 and BW_Apap_M2 displayed a 16 and 4-fold increase in ciprofloxacin resistance respectively. Interestingly, the ibuprofen/ciprofloxacin and acetaminophen/ciprofloxacin mutants not only displayed a high increase in ciprofloxacin MIC, but the mutation frequencies for these NAM/ciprofloxacin combinations were the only ones that were significantly higher than the mutation frequency of ciprofloxacin alone. Therefore, these mutants were selected for further assessment and their resistance profiles against an extended number of antibiotics determined.
Fig. 3. Fold change of MIC values for ciprofloxacin against NAM/ciprofloxacin mutants compared to WT.
A, B A representative number (√n + 1) of E. coli derived mutants from select NAM/ciprofloxacin plates were picked and the MIC of ciprofloxacin was determined for E coli BW25112 and E coli 6146. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin, PSE pseudoephedrine, TMZ temazepam, TRA tramadol. * Indicates mutants selected for further analysis.
Evaluation of the E. coli 6146 derived mutants and an examination of the ciprofloxacin MICs revealed a similar trend, with ciprofloxacin only exposed mutants showing a 4 to 8-fold increase in ciprofloxacin MIC as compared to the wild type. Mutants exposed to pseudoephedrine, temazepam, and tramadol/ciprofloxacin were not further investigated due to the limited number of colonies on plates. Notably, higher ciprofloxacin MIC values were observed for the E. coli 6146-derived mutants when compared to BW25113-derived mutants. The ciprofloxacin MIC for one mutant exposed to furosemide/ciprofloxacin (Frs_M1) increased to 0.5 µg/mL (32-fold increase in MIC), hence this mutant was selected for further assessment. Also selected for further analysis were mutants Ator_M4 (atorvastatin/ciprofloxacin exposed) and Dic_M4 (diclofenac/ciprofloxacin exposed) as these also displayed higher levels of ciprofloxacin resistance (MICs of 0.25 µg/mL) displaying 16-fold increase compared to WT E. coli 6146, as well and one acetaminophen/ciprofloxacin mutant (6146_Apap_M2) with a MIC of 0.125 µg/mL, showing an 8-fold increase ciprofloxacin resistance. Finally, as a larger mutation frequency was observed for cells following ibuprofen/ciprofloxacin exposure, a representative mutant, 6146_Ibf_M1, which displayed a MIC of 0.06 µg/mL (4-fold increase in MIC) was also assessed further.
NAMs exposure increases resistance to multiple antibiotics
Evaluation of selected ciprofloxacin resistant E. coli BW25113 and 6146-derived mutants against select antibiotics (Table S1), revealed elevated levofloxacin resistance for a large number of mutants. This is expected as the same resistance mechanisms are utilised by E. coli against these quinolones35. However, the fold increase in MIC values for ciprofloxacin or levofloxacin differed for the mutants assessed here, suggesting a slight variation in the mechanisms of resistance against these two compounds36. Of the compounds assessed here, in addition to increased ciprofloxacin and levofloxacin MICs, all BW25113-derived mutants except for BW_M1 and BW_Ator_M1 displayed a 4-fold increase in the MIC for minocycline. A 4-fold increase in the MIC for ceftazidime was also observed for BW_Apap_M2 and BW_Ator_M1.
Evaluation of resistance of the mutants of clinical isolate E. coli 6146 revealed higher MIC values of ciprofloxacin compared to the mutants derived from the E. coli BW25113 strain. For example, mutant Frs_M1 saw an increase of 32-fold for ciprofloxacin MIC and 16-fold for levofloxacin MIC. A 16-fold increase in ciprofloxacin MIC was also observed for mutants Dic_M4 and Ator_M4. These mutants also showed elevated levofloxacin resistance with an 8-fold increase in their levofloxacin MICs. In addition, elevated resistance against cefepime was observed in the M1 mutant, Apap_M1, Dic_M1, and Ator_M1, with a 4-fold increase in the MIC of cefepime. Comparatively, while mutants derived from E. coli 6146 displayed higher levels of resistance against ciprofloxacin, levofloxacin, and cefepime than the BW25113 strain, resistance to other antibiotics such as ceftazidime and minocycline was not observed. Although furosemide did not increase the mutation frequency, mutant 6146_Frs_M1 displayed a 32-fold increase in ciprofloxacin MIC, therefore, this mutant was selected for sequencing.
The combination of two NAMs increases fitness in the presence of sub-MIC concentration of ciprofloxacin
To further explore the effects of a combination of medications, mutation frequencies were evaluated in the presence of two NAMs and ciprofloxacin. As exposure of E. coli BW25113 and 6146 to gut concentrations of ibuprofen and acetaminophen individually significantly enhanced mutation frequencies, and as mutants derived from the exposure to these compounds in the presence of ciprofloxacin displayed elevated MIC values for ciprofloxacin and other classes of antibiotics, these two NAMs were chosen for further analysis, and their ability to induce mutations were assessed in the presence of an additional NAM.
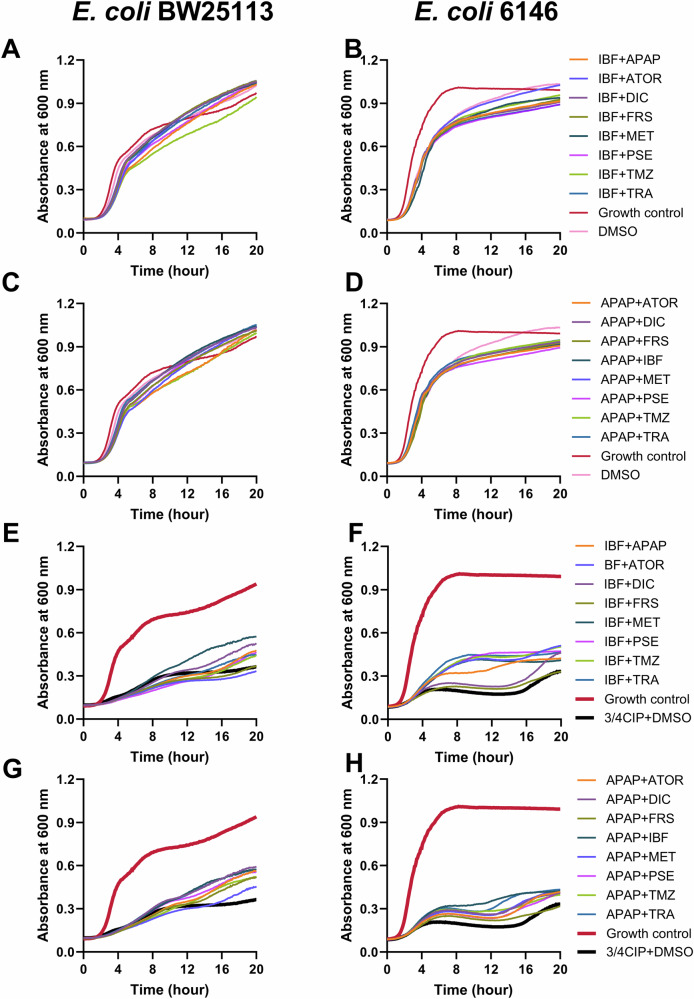
Bacterial growth was assessed in the presence of ibuprofen- and acetaminophen-based NAM combinations (Fig. 4). The growth of E. coli BW25113 was not significantly affected, while there was a modest effect on the growth of E. coli 6146 cells affected when exposed to two NAMs in the absence of ciprofloxacin. For E. coli BW25113, acetaminophen-based combinations, with the exceptions of APAP + TMZ and APAP + MET, increased the growth rate, whereas most ibuprofen-based combinations decreased the growth rate. For E. coli 6146, the addition of NAM combinations, especially for the ibuprofen-based NAM combinations (except for IBF + FRS), resulted in an increase in growth rate, maximum cell density and a reduction in the log phase delay compared to NAM-free control (3/4 CIP + DMSO). The results indicate that these NAM combinations could enhance the fitness and adaptability of these two strains.
Fig. 4. Growth profiles of E. coli BW25113 and E. coli 6146 in the presence of a sub-inhibitory inhibitory concentration of ciprofloxacin and 2 NAMs.
A–D The effect of 2 NAMs on growth of E. coli BW25113 and E. coli 6146. E–H The effect of 2 NAMS on the growth of E. coli BW25113 and E. coli 6146 in the presence of ¾ MIC ciprofloxacin. Growth control represented cells in the absence of NAMs and ciprofloxacin. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin, PSE pseudoephedrine, TMZ temazepam, TRA tramadol. Growth curves were performed four times on different days with different batches of cells. Representative traces based on the overall results are shown here.
The combination of two NAMs enhances the mutation frequency in the presence of ciprofloxacin
Assessment of mutation frequency following the exposure of cells to 2 NAMs and ciprofloxacin, revealed that the presence of acetaminophen combined with ibuprofen significantly promoted the number of E coli BW25113 (P < 0.05) and 6146 (P < 0.01) mutants compared to cells exposed to ciprofloxacin only (Fig. 5). The mutation frequency for E. coli exposed to acetaminophen and ibuprofen (1.40 ± 0.56 × 10−6 for BW25113 and 1.57 ± 0.51 × 10−6 for 6146) was similar and even lower to that of cells exposed to ibuprofen alone (1.45 ± 0.19 × 10−6 for BW25113 and 2.30 ± 0.92 × 10−6 for 6146). Notably, a high mutation frequency (P < 0.05) was also observed for BW25113 cells following the exposure to ibuprofen and temazepam. As for E. coli 6146, the exposure to atorvastatin, diclofenac (P < 0.01), frusemide, and metformin (P < 0.05) resulted in the significantly increased mutation frequency compared to the cells exposed to ciprofloxacin only treatment. In contrast, the remain NAMs with ibuprofen showed decreased mutation frequency, relative to that of cells exposed to ibuprofen.
Fig. 5. Mutation frequencies of E. coli BW25113 and E. coli 6146 following exposure to ciprofloxacin and NAM combinations.
A, B The mutation frequency following the exposure to two NAMs are indicated for E. coli BW25113 and E. coli 6146. Significant differences between mutants treated with additional NAMs and the control (ciprofloxacin treated only) were analysed by independent-sample t-tests and corrected by Bonferroni correction method, *P < 0.05, **P < 0.01, and ****P < 0.0001. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin, PSE pseudoephedrine, TMZ temazepam, TRA tramadol
NAM combinations promote ciprofloxacin induced resistance to produce ciprofloxacin resistant phenotypes
A representative number (√n + 1) of E. coli BW25113 and 6146 derived mutants were randomly picked from ciprofloxacin/NAMs combination plates and the MIC values for a panel of antibiotics were determined (Fig. 6). As expected, most mutants exposed to two NAMs/ciprofloxacin displayed higher levels of ciprofloxacin resistance, with a 2 to 4-fold increase in MICs compared to mutants exposed to ciprofloxacin and one NAM. In E. coli BW25113 derived mutants, two mutants, BW_Apap/Ibf_M1 and BW_Ibf/Met_M1, showed a 32-fold increase in ciprofloxacin resistance, with a MIC of 0.5 µg/mL. Notably, the mutant BW_Ibf/Dic exhibited the highest levels of ciprofloxacin resistance, with an MICs of 1 µg/mL, representing a 64-fold increase compared to the MIC value of the WT.
Fig. 6. Fold change of MIC values for ciprofloxacin against two NAMs/ciprofloxacin mutants compared to WT.
A, B A representative number (√n + 1) of E. coli derived mutants from select 2 NAMs/ciprofloxacin plates were picked and the MIC of ciprofloxacin was determined for E coli BW25112 and E coli 6146 derived mutants. Asterisks indicate mutants selected for further analysis. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin, PSE pseudoephedrine, TMZ temazepam, TRA tramadol
Concerning E. coli 6146 derived mutants, two mutants, 6146_Ibf/Frs_M1and 6146_Ibf/Dic_M1, exhibited higher ciprofloxacin resistance, with MICs of 0.5 µg/mL and 0.25 µg/mL, showing a 32- and 16- fold increase in ciprofloxacin MIC values, respectively. The following mutants were selected for further assessment, 6146_Apap/Ibf_M1, 6146_Apap/Frs_M1, 6146_Apap/Ator_M1, and 6146_Ibf/Met_M1 from E. coli 6146-derived mutants, as MICs of 0.125 µg/mL were observed.
Mutants with at least 8-fold increased ciprofloxacin resistance, representing each group of resistant isolates were selected and their resistance profiles against ten antibiotics were determined (Table S2). The mutants derived from exposure to two NAMs exhibited a higher level of ciprofloxacin resistance than those derived from exposure to a single NAM, with such mutants as BW_Ibf/Dic_M1 whose ciprofloxacin resistance increased up to 64-fold and mutants BW_Apap/Ibf_M1 and BW_Ibf/Met_M1 showing a 32-fold increase in ciprofloxacin MIC relative to wild-type E. coli BW25113. Similarly, the MIC values for levofloxacin, an antibiotic from the same class as ciprofloxacin, also increased by 4 to 16-fold for the NAM-derived mutants. In comparison, an increase in resistance against antibiotics from other classes was lower, with mutants BW_Apap/Met_M1, BW_Ibf/Frs_M1 and BW_Ibf/Met_M2 displaying an increase in MIC of 2 to 4-fold for minocycline, cefepime, ceftazidime and amoxicillin.
For E. coli 6146, mutants 6146_Ibf/Frs_M1 and 6146_Ibf/Dic_M1 manifested 32-fold and 16-fold increases in ciprofloxacin MIC values. These mutants also showed increased levofloxacin resistance, with a 16 and 8-fold increase in MIC, respectively. Finally, 4-fold increased cefepime resistance was observed in mutants 6146_Ibf/Apap_M1 and 6146_Apap/Ator_M1, with the latter also displaying increased minocycline resistance (4-fold increase in MICs).
Genomic analysis reveals multiple mutations in mutants exposed to NAMs and ciprofloxacin
To identify mutations that gave rise to ciprofloxacin resistance, mutants were selected for whole genome sequencing. The resistance determinants assessed were primarily focused on those involved in fluoroquinolone resistance, such as GyrA/B, ParC/E37,38, as well as on RND efflux pumps (AcrAB-TolC)39,40, and their regulators (local regulator AcrR and global regulator MarR)41, as these also play an important role in such resistance42. Finally, mutations within stress response proteins such as SoxS and SoxR were also assessed as these have previously been identified to play a role in the overexpression of efflux pumps43.
Several mutations were identified in the mutants assessed, with mutations and deletions observed in MarR, AcrR and GyrA (Table 2). For the BW25113-derived mutant M1, a unique substitution mutation was detected in SoxR (R20L). Previously described GyrA mutations, S83L, D87G and D87Y, which are reportedly associated with a strong influence on quinolone resistance36,44 were observed in five mutants. Of these, one mutant was derived from E. coli 6146 cells exposed to furosemide and four from cells exposed to ibuprofen in addition to one other NAM and ciprofloxacin. All efflux pump-associated mutations were primarily located in their regulators, AcrR and MarR. Among these, a substitution mutation of AcrR (T5N) was identified in three mutants: Ibf_Frs and Apap_Met_M1 from BW25113, and Ibf/Apap_M1from 6146. Meanwhile, unique deletions in the MarR regulator occurred among these mutants. Interestingly, many mutants exposed to two NAMs exhibited more than one mutation i.e., an increase of mutation rate (an estimate of the probability of a mutation occurring per cell per generation) as well as mutation frequency was observed.
Table 2.
Mutations, deletions and truncations of select proteins in E. coli BW25113 and E. coli 6146 derived mutants following the exposure to one and two NAMs and 1x MIC of ciprofloxacin
| Strains | Protein | Mutation(s)* | Fold increase in Cip MIC | |
|---|---|---|---|---|
| BW25113 derived | BW_M1 | SoxR | R20L | 8 |
| BW_Ibf_M1 | ND | ND | 8 | |
| BW_Apap_M1 | MarR | E136D, deletion – Y137 | 16 | |
| BW_Apap_M2 | MarR | 1 base pair deletion | 4 | |
| BW_Ator_M1 | MarR | deletions (L75, D76) | 8 | |
| BW_Ibf/Met_M1 | GyrA | D87Y | 32 | |
| BW_Ibf/Dic_M1 | GyrA | S83L | 64 | |
| BW_Ibf/Frs_M1 | MarR | L78R | 8 | |
| AcrR | T5N | |||
| BW_Apap/Ibf_M1 | GyrA | S83 deletion | 32 | |
| MarR | V84E | |||
| BW_Apap/Met_M1 | MarR | 13 base pair deletion | 16 | |
| AcrR | T5N | |||
| 6146 derived | 6146_M1 | MarR | 180 base pair deletion | 8 |
| 6146_Ibf_M1 | GyrA | 3 base pair deletion (S83 deletion) | 8 | |
| 6146_Frs_M1 | GyrA | S83L | 32 | |
| 6146_Ibf/Frs_M1 | GyrA | D87G | 32 | |
| MarR | 13 base pair deletion | |||
| 6146_Ibf/Apap_M1 | MarR | 2 base pair insertion | 8 | |
| AcrR | T5N |
ND No mutations determined, Cip ciprofloxacin, MIC Minimum Inhibitory Concentration, Ibf ibuprofen, Met metformin, Dic diclofenac, Frs furosemide.
Assessment of the role of efflux pumps in acquired resistance
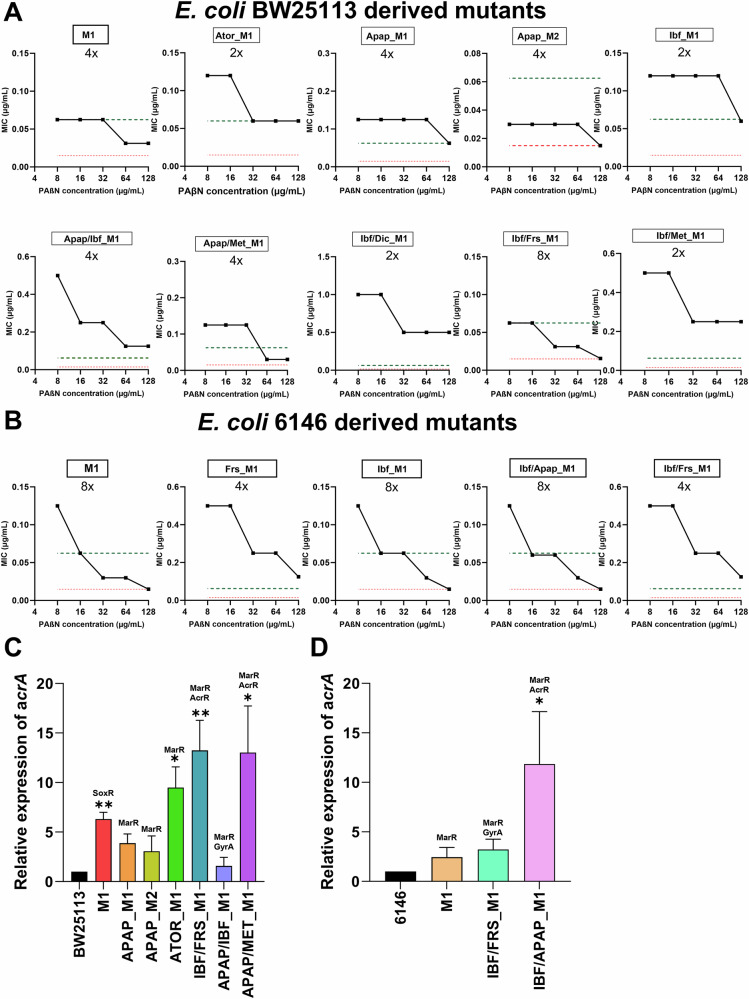
Given that many mutations found in the E. coli derived mutants were related to efflux pump regulators, the potential role of RND efflux pumps in the observed ciprofloxacin resistance was investigated. Checkerboard assays were carried out in the presence of PAβN, an efflux pump inhibitor45–47. In total, 15 mutants were assessed to determine the role of RND efflux pumps in resistance, with results revealing that the addition of PAβN partially reversed resistance (2-fold reduction) in four of the E. coli BW25113 derived mutants, with a further 5 mutants seeing a 4-fold reduction in ciprofloxacin MIC and one mutant, BW_Ibf/Frs_M1, displaying an 8-fold reduction in ciprofloxacin resistance (Fig. 7A). All BW25113 derived mutants displaying a 4 to 8-fold reduction in ciprofloxacin resistance were found to carry mutations in MarR or AcrR or both. Finally, the addition of PAβN to two BW25113-derived mutants, BW_Apap_M2 and BW_Ibf/Frs_M1, abolished ciprofloxacin resistance, indicating that the resistance observed is mediated by efflux through the RND-type efflux pumps.
Fig. 7. Evaluation of efflux pump-mediated antimicrobial resistance.
A, B Reversal of resistance in the presence of the efflux pump inhibitor PAβN for E. coli BW25113 and E. coli 6146 derived mutant isolates. The black lines correspond to the MICs in the presence of PAβN, and red and green dashed lines represent the MIC of wild type and average MIC of mutants exposed to ciprofloxacin only respectively. C, D The expression levels of the AcrAB-TolC efflux pump were assesses by RT-qPCR of the acrA gene in E. coli BW25113 and E coli 6146 derived mutants. The results presented are the mean ± SEM of three experiments with different batches of cells and RNA preparations. Significant differences between mutants and the WT strain were analysed by independent-sample t-tests and corrected by Bonferroni correction method, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. APAP Acetaminophen, ATOR atorvastatin calcium, CIP ciprofloxacin, DIC diclofenac, FRS furosemide, IBF ibuprofen, MET metformin
The addition of PAβN to E. coli 6146-derived mutants resulted in greater reductions in resistance, with mutants 6146_Frs_M1 and 6146_Ibf/Frs_M1 displaying 4-fold reductions in ciprofloxacin MIC, and an 8-fold reduction in resistance seen in the remaining three mutants (Fig. 7B). The addition of PAβN to three E. coli 6146-derived mutants reversed ciprofloxacin resistance to wild-type levels. Of these, 6146_Ibf_M1 was found to harbour a GyrA mutation (ΔS83), and no additional efflux pump associated mutations. As such, the reversal of resistance may be due to the transient upregulation of efflux pumps37,48, which was subsequently inhibited by the addition of PAβN.
RT-qPCR revealed AcrAB-TolC efflux pump over-expression
As numerous MarR and AcrR mutations were observed in many of the NAM-derived E. coli mutants and a reduction in MIC values was observed in the presence of the efflux pump inhibitor PAβN, RT-qPCR was carried out to assess the upregulation of the AcrAB-TolC efflux pump. A total of ten NAM derived E. coli mutants, seven E. coli BW25113 and three E. coli 6146 were selected for analysis (Fig. 7). A significant increase in the expression of the AcrAB efflux pump was observed in all the mutants showing a fold increase ranging from 2.44 to 13.02 compared to their wild-type strains, with the exception of the BW25113-derived mutant Apap/Ibf_M1. The BW25113-derived mutant Apap/Ibf_M1 showed a 4-fold reduction in ciprofloxacin MIC following the addition of PAβN and harboured a mutation in MarR (V84E), but the expression levels of AcrA only increased by 1.56-fold. Notably, the most substantial increase in AcrAB expression was observed in BW25113-derived mutants Ibf/Frs_M1 (P < 0.001), Apap/Met_M1 (P < 0.001), and 6146-derived mutant Ibf/Apap_M1 (P < 0.01), exhibiting 13.24-, 13.02- and 11.85-fold upregulation, respectively. Interestingly, these mutants harboured MarR as well as the AcrR (T5N) mutations. In addition, the acrA gene encoding efflux pump was significantly upregulated in mutant Aror_M1 containing a 2 bp deletion in MarR (P < 0.05).
Discussion
It has been well-established that antibiotics at sub-inhibitory concentrations could promote the development and spread of antibiotic resistance49,50. Similarly, NSAIDs, including acetaminophen and ibuprofen, have been shown to induce antibiotic resistance at high concentrations (5 – 10 mM) by increasing AcrAB-TolC efflux pump expression21. Frequent exposure to antidepressants such as fluoxetine, sertraline, and duloxetine could induce AMR through the acquisition of mutations51. However, little is known about the contribution of other NAMs to the emergence and spread of AMR and the effect of a range of NAMs have not been investigated at clinically relevant intestinal concentrations. Generally, the long-term consumption of medications among older persons leads to combined exposure to antibiotics like ciprofloxacin and NAMs in the range of mg/L in the gut microbiota7,52. Our findings highlight the potential for E. coli to develop mutations leading to AMR following exposure to NAMs and ciprofloxacin in RACFs where medications are widely used.
Overall, our results demonstrated that two commonly consumed pain relief medications (ibuprofen and acetaminophen), can significantly enhance antibiotic resistance via mutation at gut-relevant concentrations in E. coli strain BW25113 and E. coli 6146. This was evident in the increased number of mutants and the enhancement of maximum cell density. Other NAMs, such as diclofenac and furosemide, also increased mutation frequency when compared to the non-NAM control (ciprofloxacin with DMSO). However, other NAMs such as temazepam, tramadol, and pseudoephedrine had minimal impact on the mutation frequency for E. coli BW25113 and E. coli 6146. Although ciprofloxacin bioavailability is known to be significantly reduced when co-administered with compounds containing metallic cations or sucralfate53, no such interactions were observed with the NAMs used in this study. On the contrary, diclofenac increased ciprofloxacin bioavailability54, ruling out the possibility that its effect on mutation frequency was due to diminished ciprofloxacin bioavailability. Considering polypharmacy is common among the elderly in RACFs, the effects caused by ibuprofen and acetaminophen also warrant attention. Interestingly, we found that the combined exposure to ibuprofen and acetaminophen induced sa imilar mutation frequency to that of ibuprofen alone, rather than resulting in a cumulative increase in mutation frequency. However, the mutants resulting from exposure to two NAMs acquired exhibited higher levels of ciprofloxacin resistance.
In addition, E. coli BW25113 and 6146-derived mutants exhibited reduced susceptibility to multiple antibiotics (≥4-fold), particularly showing increased ciprofloxacin resistance, with MIC values rising to 32-fold for this agent. Among these antibiotics, β-lactam antibiotics (amoxicillin, ceftazidime, meropenem), as well as ciprofloxacin, levofloxacin, and minocycline are substrates of the AcrAB-TolC efflux pump, which can extrude multiple classes of antibiotics55,56. Therefore, increased resistance observed against these antibiotics suggests a potential link to this efflux pump. Furthermore, RT-qPCR analysis revealed the overexpression of AcrAB-TolC efflux pump, supporting a close relationship between the NAMs and efflux pump expression. In the early stages of antimicrobial resistance development, drug efflux pumps play a pivotal role by enabling bacteria to survive initial, sub-inhibitory concentrations of antibiotics. When a sensitive bacterial population is exposed to low levels of antibiotics, efflux pumps can transiently reduce intracellular drug concentrations, allowing cells to persist and adapt57. This survival window is critical: it provides time for the bacteria to activate stress responses and regulatory networks that upregulate efflux pump expression or other resistance mechanisms. These pumps act rapidly and non-specifically, making them one of the first lines of defence against antimicrobial agents58. The overexpression of drug efflux pumps leads to the repression of the DNA mismatch repair gene mutS, and a concomitant increase in mutation frequency59,60. Therefore, efflux pump overexpression not only supports survival but also facilitates the selection of stable genetic mutations that confer high-level resistance61. Thus, efflux pumps are not merely passive transporters but active participants in the adaptive landscape of bacterial resistance evolution. Hence, the ability of NAMs to induce higher activity of efflux pumps, even without causing any mutations, could have a considerable effect on AMR. In addition, in our study, although exposure to two NAMs did not induce higher mutation frequencies, mutants derived from two NAMs/ciprofloxacin combinations exhibited higher levels of resistance to multiple antibiotics compared to those exposed to a single NAM/ciprofloxacin combination because of the increase in mutation rate and the induction of multiple mutations per colony.
Whole genome sequencing was conducted to elucidate the mechanisms responsible for multiple antibiotic resistance in mutants. The analysis revealed that the combination of NAMs with ciprofloxacin can induce nonsynonymous single-nucleotide polymorphisms (SNPs) in regulator genes, leading to the efflux pump overexpression. In addition to mutations occurring in quinolone resistance determinant regions in topoisomerases genes such as gyrA, multiple mutations were also identified (deletions and substitution) in regulator genes, such as marR, acrR, and soxR which are regulators of the AcrAB-TolC system, highlighting the connection between antibiotics and efflux pumps50,62. MarR negatively regulates the transcription factor MarA, which in turn regulates the expression of multiple other proteins, including those of the AcrAB-TolC efflux pump. Therefore, the mutations in MarR resulted in increased expression of the marRAB operon, leading to upregulation of AcrAB-TolC efflux pump and reduced antibiotic susceptibility63. Mutant BW_APAP/IBF carrying MarR V84E and GyrA Δ83 not only conferred high ciprofloxacin resistance but also had a small increase in MICs to ceftazidime and minocycline. This was consistent with a previous study that clinical isolates carrying MarR V84E provided antibiotic resistance to different classes of antibiotics64. AcrR acts as a local repressor to prevent the overexpression of the AcrAB-TolC efflux pump. Strikingly, upon exposure to two NAMs/ciprofloxacin, an increase in the number of mutations was observed in certain E. coli isolates, with five mutants developing mutations in both MarR and AcrR or GyrA and MarR. Of these, the T5N mutation observed in AcrR occurred in three mutants, which showed reduced susceptibility to different classes of antibiotics. Also, RT-qPCR analysis showed mutants carrying the T5N substitution resulted in the highest level of AcrAB-TolC efflux pump expression. A previous study postulated that the T5N mutation observed in AcrR may render this regulator inactive or potentially destabilize it65, with the increased expression of the AcrAB-TolC efflux pump observed in this study, supporting this theory. Some global regulators, such as SoxS, are regulated by SoxR, whose activation by superoxides contributes to the expression of SoxS, which in turn upregulates the transcription of acrAB66,67. The R20L substitution observed in the BW_M1 mutant could account for the upregulation of AcrAB-TolC efflux pump, thereby leading to increased resistance to antibiotics.
Our findings based on phenotypic analysis revealed that only a few mutants fully reverse ciprofloxacin resistance to the original levels in the presence of PAβN, an efflux pump inhibitor. It has been shown that high levels of antibiotic resistance are not only attributed to increased expression of efflux pumps but may result from the accumulation of mutations in different targets41,42,68. In fluoroquinolone-resistant E. coli isolates, initial mutations in gyrA, such as S83L, which is the most common substitution are essential for the development of high-level fluoroquinolone resistance69. High level of fluoroquinolone resistance is a stepwise process and is often the result of accumulating amino-acid substitutions70. In this study, a combination of gyrA mutations (S83L and D87G/Y) along with mutations in efflux pump regulators contributed to the high levels of fluoroquinolone resistance. Microbes increase the efflux of antimicrobials to lower the intracellular concentration of antimicrobials and provide fitness, allowing them to survive longer in the presence of low concentrations of antibiotics than expected41,63. Additionally, mutations in clinical isolates tend to favour those that impose a small cost on fitness and confer small reduction in antibiotic susceptibility. This growth advantage allows the strain to gradually accumulate mutations, eventually evolving into high-level resistance41,63.
Some NAMs exhibit toxicity against E. coli, altering bacterial physiology, stress response and growth71. However, in the absence of antibiotics, long-term exposure to NAMs alone may not exert sufficient selective pressure to drive cross-resistance to antibiotics. This phenomenon changes when E. coli is exposed to both antibiotics and NAMs, with previous studies reporting a synergistic effect between them. For example, antidepressants such as sertraline in combination with tetracycline, and duloxetine with chloramphenicol, induce multiple antibiotic resistance in E. coli52,72. In our study, the coexistence of ciprofloxacin and NAMs leads to the evolution of cross-resistance to antibiotics. Given the widespread exposure to both NAMs and ciprofloxacin is common among residents in RACF, the development of AMR in E. coli in vivo appears plausible.
Polypharmacy not only increases the risk of adverse drug effects and likelihood of drug-drug interactions but also complicates the use of antibiotics. Notably, several commonly consumed pharmaceuticals like proton pump inhibitors (PPIs) and metformin have been shown to cause gut dysbiosis by altering the composition of the intestinal microbiota. The eradication of the majority of protective gut microbiota allows an environment conducive to overgrowth and colonization of harmful bacteria like Clostridium difficile5,73. In addition, it has been noted that, beyond most known antibiotics, the use of proton pump inhibitors (PPIs) also increases the risk of Clostridium difficile infections74. As expected, the incidence of Clostridium difficile infections doubled when PPIs were concurrently administered with fluoroquinolones75. Similarly, our study reveals that certain NAMs at intestinal-relevant concentrations can promote the AMR in the presence of ciprofloxacin. These findings highlight the urgent need to re-evaluate combinations between NAMs and antibiotics, particularly within complex polypharmacy regimens.
Collectively, this study provides evidence that medication used in pain relief, such as acetaminophen and ibuprofen, when combined with ciprofloxacin, not only significantly increases mutation frequency but also confer multiple antibiotic resistance. Other NAMs like diclofenac and furosemide also contribute to increased mutation frequency and high levels of fluoroquinolone resistance. Although the mutation frequency induced by two NAMs is not synergistic for mutation frequency increase, high-level ciprofloxacin resistance is observed among the mutants due to an increased mutation rate and subsequent accumulation of mutations. Older people such as those living in RACFs are taking numerous medications daily76 highlighting the need to consider medicine combinations when prescribing. This study also paves the way for investigating the effect of regimens comprising multiple medications (i.e., polypharmacy) on the development of AMR.
Methods
Bacterial strains and culture conditions
Bacterial strains used in this study included the E. coli BW25113 strain, a derivative of E. coli K-12 strain77,78, and E. coli 6146, an antibiotic-sensitive strain isolated from a faecal sample obtained from a retirement community in Adelaide, Australia. The project received ethical approval from the University of South Australia’s human research ethics committee to collect faecal samples from RACF and retirement residents (Application ID: 201882). Typically, strains were cultured in Luria-Bertani (LB) media (Difco®, Franklin Lakes, New Jersey) at 37 °C. The NAMs were dissolved in Dimethyl sulfoxide (DMSO), and antibiotics were dissolved in their recommended solvents. Nutrient agar (NA) was chosen as the medium for studies, with NA plates supplemented with ciprofloxacin and NAMs to assess mutation frequency.
Culture conditions and growth assays
Growth assays were carried out in a volume of 200 μL of the inoculum in cation-adjusted Mueller Hinton II Broth (BD, Australia). Briefly, cells standardised to OD600 of 0.015 were added to each well of a 96-well microtiter plate (Costar®) containing concentration ranges of ciprofloxacin (0, ½, and ¾ the MIC of ciprofloxacin) and each NAM at gut concentration7. The 96 well-plate was incubated for 20 hours at 37 °C in the EnSpire 2300 Multilabel Reader (PerkinElmer, USA). Absorbance at 600 nm was measured and recorded at 20 minutes time intervals.
Estimation of the concentration of NAMs in the intestine
The gut concentration of NAMs was calculated using a model based on Maier et al. 7. Briefly, this method assumes that an oral drug is dissolved in 300 mL of water, including water allowing to swallow the pill (240 mL) and the resting water (60 mL) in the small intestine. The model also assumes that 90% of oral drugs will be absorbed into the bloodstream, meaning that 10% of the drugs will remain in the intestinal area. Single doses will be obtained from the Defined Daily Dose (DDD) database WHO79.
| 1 |
Based on the above model, NAMs concentration used in the study were 216 μg/mL for acetaminophen, 15.4 μg/mL for diclofenac, 13.2 μg/mL for furosemide, 99.8 μg/mL for ibuprofen, 194.6 μg/mL for metformin, 6.6 μg/mL for atorvastatin, 40 μg/mL for pseudoephedrine, 3.3 μg/mL for temazepam, and 33.3 μg/mL for tramadol.
Antimicrobial susceptibility testing
Antimicrobials assessed in this study included ciprofloxacin, norfloxacin, levofloxacin, minocycline, cefepime, meropenem, erythromycin, and novobiocin, all obtained from Sigma Aldrich (Sydney, Australia). NAMs assessed included acetaminophen, furosemide, atorvastatin calcium, diclofenac, ibuprofen, and metformin were also used and were also obtained from Sigma Aldrich (Sydney, Australia).
Minimum inhibitory concentrations (MICs) were determined using the broth microdilution assay ISO 20776-1 as recommended by European Committee on Antimicrobial Susceptibility Testing80. Each strain was tested in triplicate.
Determination of mutation frequency
Mutation frequency was assessed using both the E. coli BW25113 (wild type) and E. coli 6146 (an antibiotic sensitive isolate from the faecal sample of a retirement resident). Overnight cultures grown at 37 °C in 5 mL LB broth were diluted (1:100) in LB and grown to mid-log phase (OD600 of 0.4–0.6) at 37 °C. A volume of 100 μL of each culture was spread plated onto NA plates containing 1x, 2x, and 4x the MIC of ciprofloxacin (0.015 to 0.06 μg/mL) and NAMs, at clinically relevant concentrations to select ciprofloxacin-resistant bacteria. Plates containing ciprofloxacin and the NAM solvent (DMSO) served as the ciprofloxacin only control. Cultures were incubated for 48 h at 37 °C7. The mutation frequency (number of mutants in the population) was calculated according to the following equation81:
| 2 |
To address plating bias, assays were carried out on four different days with different batches of cells. A representative number of E. coli-derived mutants, calculated as √n + 1 (where n is the number of colonies per plate) were randomly picked for ciprofloxacin MIC determination. The representative mutants displaying at least 4-fold increased ciprofloxacin resistance were selected for further analysis to avoid including strains whose MIC increase may have been due to MIC assay variation.
Checkerboard assays
To determine the effect of RND efflux pump-mediated resistance, checkerboard assays were carried out on ciprofloxacin-resistant isolates in the presence and absence of the efflux pump inhibitor Phenylalanine-arginine β-naphthylamide (PAβN) as described previously82–84. Ciprofloxacin was diluted vertically down the 96-well microtiter plate (Costar®) (2-fold serial dilutions), and PAβN was serially diluted horizontally across the plate (2-fold serial dilutions). Inoculum of 1.5 × 106 CFU/mL was added to each well and plates were incubated at 37 °C for 18 hours, shaking at 120 rpm. The MICs in the presence of PAβN were used to compare the fold change in MICs in the absence of PaβN.
DNA extraction, whole genome sequencing and data processing
Genomic DNA was extracted using the NucleoSpin® Microbial DNA extraction kit (MACHEREY-NAGEL, Germany) according to manufacturer’s instruction. The concentration and purify of DNA (A260/A280, 1.8–2.0) were assessed using the Cytation 5® (Bio-Tek®, Winooski, USA) plate reader and the quality of the DNA analysed by agarose gel electrophoresis. All samples were then submitted to the Australian Genome Research Facility (AGRF) for library preparation and paired-end sequencing were performed on the Illumina Hi-Seq platform.
Raw 150 base paired-end sequencing reads were assembled and annotated using the TORMES pipeline (v.1.3)85. To assess possible mutations, draft genomes of E. coli-derived mutant were screened against the wild-type using BRESEQ86. Amino acids of target wild-type proteins were aligned to mutant amino acid sequences using CLUSTAL O v.1.2.487. Sequence variations were visualized using ESPript v.3.088.
RNA extraction, Reverse-Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was carried out to quantify the transcriptional level of acrA, which encodes for the AcrA membrane fusion protein of the AcrAB-TolC multidrug efflux pump. Isolates displaying over 4-fold increased ciprofloxacin resistance (MIC of ciprofloxacin ≥ 0.015 μg/mL) were selected for RT-qPCR analysis to assess the potential role of efflux pumps in this resistance.
Overnight cultures inoculated with single colonies were diluted (1:100) in LB broth and grown until mid-log phase (A600 of 0.5) at 37 °C. The total RNA was extracted as previously described42. Briefly, 1 mL of cultures were centrifugated, resuspended in Trizol and chloroform, and then further purified using the NucleoSpin®RNA extraction kit (MACHEREY-NAGEL, Germany) followed by rDNase digestion which was performed to remove DNA during this period. The concentration and purity of total RNA were quantified through Cytation 5® (Bio-Tek®, Winooski, USA).
All RT-qPCR reactions were performed using a magnetic induction cycler (Bio Molecular Systems, Australia) and the KAPA SYBR® FAST qPCR Master Mix kit (Kapa Biosystems, USA). The housekeeping gene, recA, was used to normalise the transcriptional levels of acrA, the target gene. The primer sequences include AcrA_F 5′-GATCCAGCCACCTATCAGGC-3′; AcrA_R 5′-ACGCTTGACCGTCAGTTGAT-3′; RecA_F 5′- TTAAACAGGCCGAATTCCAG-3′; RecA_R 5′-CCGCTTTCTCAATCAGCTTC-3′. Cycling parameters were as follows: reverse transcription at 42 °C for 10 min, inactivation at 95 °C for 3 min, and 40 cycles of PCR at 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 15 s. No template control (NTC) and no reverse-transcriptase control (NRT) were established, and the qPCR reaction samples carried out in triplicate.
Statistical analysis
Graph Prism v9. was used for all data analysis. Significant differences were assessed using an independent-sample t-tests and corrected by the Bonferroni correction method, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. All experiments were conducted in triplicate.
Supplementary information
Acknowledgements
We are grateful to the aged care provider and Mr Barry Lowe for sample collection and lists of commonly used drugs in the RACFs, and to A/Prof Janet Slugget (UniSA) for advice on polypharmacy and the use of medications in RACFs in Australia. We also thank Dr. Lex Leong (SA Pathology) for providing whole-genome sequencing. Project funding for this study is provided through the Medical Research Future (MRFF, GN1152556) to HV and JT. CH is the recipient of an RTPi scholarship.
Author contributions
H.C: Writing – original draft, Writing – review & editing, Methodology, Investigation, Formal analysis, Validation, Visualization, Data curation, S.A.S.: Conceptualization, Writing – original draft, Writing – review & editing, Methodology, Investigation, Formal analysis, Supervision, Validation, Data curation, J.T.: Writing – review & editing H.V.: Conceptualization, Methodology, Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision.
Data availability
Sequencing has been deposited in the NCBI BioProject database PRJNA1281333.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s44259-025-00144-w.
References
- 1.WHO Global action plan on antimicrobial resistance. Microbe Mag.10, 354–355 (2015). [Google Scholar]
- 2.Murray, J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet399, 629–655 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousham, E. K., Unicomb, L. & Islam, M. A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: integrating behavioural, epidemiological and One Health approaches. Proc. R. Soc. B: Biol. Sci.285, 20180332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin, M. J., Thottathil, S. E., & Newman, T. B. Antibiotics overuse in animal agriculture: a call to action for health care providers. In (Vol. 105, pp. 2409–2410): American Public Health Association. (2015) [DOI] [PMC free article] [PubMed]
- 5.Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut65, 740–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang et al. Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation. ISME J.14, 2179–2196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature555, 623–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamad, B. The antibiotics market. Nat. Rev. Drug Discov.9, 675–676 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Mikulic, M. Global pharmaceutical industry - statistics & facts. Retrieved from https://www.statista.com/topics/1764/global-pharmaceutical-industry/#topicOverview. (2024).
- 10.Christensen, K., Doblhammer, G., Rau, R. & Vaupel, J. W. Ageing populations: the challenges ahead. Lancet374, 1196–1208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sluggett, J. K., Inacio, M. C. & Caughey, G. E. Medication management in long-term care: using evidence generated from real-world data to effect policy change in the Australian setting. Am. J. Epidemiol.193, 1645–1649 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wastesson, J. W., Morin, L., Tan, E. C. & Johnell, K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin. Drug Saf.17, 1185–1196 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Dagli, R. J. & Sharma, A. Polypharmacy: a global risk factor for elderly people. J. Int Oral. Health6, i–ii (2014). [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, K. N. et al. What is the best definition of polypharmacy for predicting falls, hospitalizations, and mortality in long-term care facilities?. J. Am. Med. Dir. Assoc.22, 470–471 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Sluggett, J. K. et al. National trends in antibiotic use in Australian residential aged care facilities, 2005–2016. Clin. Infect. Dis.72, 2167–2174 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Nicolle, L. E. et al. Antimicrobial use in long-term-care facilities. Infect. Control Hosp. Epidemiol.21, 537–545 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Fleming, A., Bradley, C., Cullinan, S. & Byrne, S. Antibiotic prescribing in long-term care facilities: a meta-synthesis of qualitative research. Drugs Aging32, 295–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher, D. et al. Surveillance of Antimicrobial Use in Long-Term Care Facilities: An Antimicrobial Mapping Survey. J. Am. Med. Dir. Assoc.25, 105144 (2024). [DOI] [PubMed] [Google Scholar]
- 19.ACSQHC. Antimicrobial use in the community: 2023. Retrieved from Australian Commission on Safety and Quality in Health Care: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/antimicrobial-use-community-2023 (2024).
- 20.Ko, H. H. T., Lareu, R. R., Dix, B. R. & Hughes, J. D. Statins: antimicrobial resistance breakers or makers?. PeerJ5, e3952 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma, T. et al. Non-steroidal anti-inflammatory drugs, acetaminophen and ibuprofen, induce phenotypic antibiotic resistance in Escherichia coli: roles of marA and acrB. FEMS Microbiol. Lett.365, fny251 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Bírošová, L., Lépesová, K., Grabic, R. & Mackuľak, T. Non-antimicrobial pharmaceuticals can affect the development of antibiotic resistance in hospital wastewater. Environ. Sci. Pollut. Res.27, 13501–13511 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Roughead, E. E. et al. Effect of an ongoing pharmacist service to reduce medicine-induced deterioration and adverse reactions in aged-care facilities (nursing homes): a multicentre, randomised controlled trial (the ReMInDAR trial). Age ageing51, afac092 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheme, P. B. PBS Expenditure and Prescriptions Report 1 July 2023 to 30 June 2024. Canberra: Australian Government Department of Health, 2020–2021. (2025)
- 25.Breidenstein, E. B., Bains, M. & Hancock, R. E. Involvement of the lon protease in the SOS response triggered by ciprofloxacin in Pseudomonas aeruginosa PAO1. Antimicrob. agents Chemother.56, 2879–2887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blondeau, J. M. Current issues in the management of urinary tract infections: extended-release ciprofloxacin as a novel treatment option. Drugs64, 611–628 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Fasugba, O., Gardner, A., Mitchell, B. G. & Mnatzaganian, G. Ciprofloxacin resistance in community-and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect. Dis.15, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juurlink, D. N. et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA289, 1652–1658 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Kalisch Ellett, L. M. et al. Medication-related hospital admissions in aged care residents. Australas. J. Ageing40, e323–e331 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Martinez, J. et al. Correlation of quinolone resistance levels and differences in basal and quinolone-induced expression from three qnrA-containing plasmids. Clin. Microbiol. Infect.12, 440–445 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Mulder, M. et al. Risk factors for resistance to ciprofloxacin in community-acquired urinary tract infections due to Escherichia coli in an elderly population. J. Antimicrob. Chemother.72, 281–289 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Sapula, S. A. et al. The scope of antimicrobial resistance in residential aged care facilities determined through analysis of Escherichia coli and the total wastewater resistome. Microbiol. Spectr.11, e00731–00723 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart, R. et al. Antibiotic use and misuse in residential aged care facilities. Intern. Med. J.42, 1145–1149 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Visvalingam, J., Palaniappan, K. & Holley, R. A. In vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control79, 288–291 (2017). [Google Scholar]
- 35.Morgan-Linnell, S. K., Becnel Boyd, L., Steffen, D. & Zechiedrich, L. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. agents Chemother.53, 235–241 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu, Y. et al. Specific patterns of gyr A mutations determine the resistance difference to ciprofloxacin and levofloxacin in Klebsiella pneumoniae and Escherichia coli. BMC Infect. Dis.13, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arzanlou, M., Chai, W. C. & Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem.61, 49–59 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Redgrave, L. S., Sutton, S. B., Webber, M. A. & Piddock, L. J. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol.22, 438–445 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Lomovskaya, O. & Bostian, K. A. Practical applications and feasibility of efflux pump inhibitors in the clinic—a vision for applied use. Biochem. Pharmacol.71, 910–918 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Venter, H., Mowla, R., Ohene-Agyei, T. & Ma, S. RND-type drug efflux pumps from Gram-negative bacteria: molecular mechanism and inhibition. Front. Microbiol.6, 377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piddock, L. J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev.19, 382–402 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amsalu, A. et al. Efflux pump-driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated from different ecological niches: a case study in the development of multidrug resistance in environmental hotspots. Microorganisms8, 1647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koutsolioutsou, A., Pena-Llopis, S. & Demple, B. Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates. Antimicrob. agents Chemother.49, 2746–2752 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagel, S., Hüllen, V., Wiedemann, B. & Heisig, P. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob. Agents Chemother.43, 868–875 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lomovskaya, O. et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother.45, 105–116 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venter, H. Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci. Rep.39, BSR20180474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinana, A. D., Vargiu, A. V., May, T. & Nikaido, H. Aminoacyl β-naphthylamides as substrates and modulators of AcrB multidrug efflux pump. Proc. Natl. Acad. Sci.113, 1405–1410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernando-Amado, S. et al. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist. Updates28, 13–27 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Gullberg, E. et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog.7, e1002158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz-García, F. et al. Low ciprofloxacin concentrations select multidrug-resistant mutants overproducing efflux pumps in clinical isolates of Pseudomonas aeruginosa. Microbiol. Spectr.10, e00723–00722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, Y. et al. Antidepressants can induce mutation and enhance persistence toward multiple antibiotics. Proc. Natl. Acad. Sci.120, e2208344120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, D. et al. Combined exposure to non-antibiotic pharmaceutics and antibiotics in the gut synergistically promote the development of multi-drug-resistance in Escherichia coli. Gut Microbes14, 2018901 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Issa, M. M. et al. Effects of paracetamol on the pharmacokinetics of ciprofloxacin in plasma using a microbiological assay. Clin. Drug Investig.27, 463–467 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Iqbal, Z., Khan, A., Naz, A., Khan, J. A. & Khan, G. S. Pharmacokinetic Interaction of Ciprofloxacin with Diclofenac. (2009). [DOI] [PubMed]
- 55.Kobylka, J. et al. AcrB: a mean, keen, drug efflux machine. Ann. N. Y. Acad. Sci.1459, 38–68 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Du, D. et al. Structure of the AcrAB-TolC multidrug efflux pump. Nature509, 512–515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du, D., Venter, H., Pos, K. M., & Luisi, B. F. The machinery and mechanism of multidrug efflux in gram-negative bacteria. Caister Academic Press, (2013).
- 58.Du, D. et al. Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol.16, 523–539 (2018). [DOI] [PubMed] [Google Scholar]
- 59.El Meouche, I. & Dunlop, M. J. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science362, 686–690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol.21, 280–295 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Grimsey, E. M. et al. Overexpression of RamA, which regulates production of the multidrug resistance efflux pump AcrAB-TolC, increases mutation rate and influences drug resistance phenotype. Antimicrob. agents Chemother.64, e02460–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bialek-Davenet, S. et al. In vitro selection of ramR and soxR mutants overexpressing efflux systems by fluoroquinolones as well as cefoxitin in Klebsiella pneumoniae. Antimicrob. agents Chemother.55, 2795–2802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Praski Alzrigat, L., Huseby, D. L., Brandis, G. & Hughes, D. Fitness cost constrains the spectrum of marR mutations in ciprofloxacin-resistant Escherichia coli. J. Antimicrob. Chemother.72, 3016–3024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ching, C. & Zaman, M. H. Development and selection of low-level multi-drug resistance over an extended range of sub-inhibitory ciprofloxacin concentrations in Escherichia coli. Sci. Rep.10, 8754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerken, H. & Misra, R. Genetic evidence for functional interactions between TolC and AcrA proteins of a major antibiotic efflux pump of Escherichia coli. Mol. Microbiol.54, 620–631 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Pérez, A. et al. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob. agents Chemother.56, 6256–6266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aly, S. A., Boothe, D. M. & Suh, S.-J. A novel alanine to serine substitution mutation in SoxS induces overexpression of efflux pumps and contributes to multidrug resistance in clinical Escherichia coli isolates. J. Antimicrob. Chemother.70, 2228–2233 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Moon, D. C. et al. Emergence of a new mutation and its accumulation in the topoisomerase IV gene confers high levels of resistance to fluoroquinolones in Escherichia coli isolates. Int. J. Antimicrob. agents35, 76–79 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Johnning, A. et al. Resistance mutations in gyrA and parC are common in Escherichia communities of both fluoroquinolone-polluted and uncontaminated aquatic environments. Front. Microbiol.6, 1355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkey, P. M. Mechanisms of quinolone action and microbial response. J. Antimicrob. Chemother.51, 29–35 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Hall, R. J. et al. Non-antibiotic pharmaceuticals are toxic against Escherichia coli with no evolution of cross-resistance to antibiotics. npj Antimicrob. Resist.2, 11 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li, L. et al. Insight into synergetic mechanisms of tetracycline and the selective serotonin reuptake inhibitor, sertraline, in a tetracycline-resistant strain of Escherichia coli. J. Antibiot.70, 944–953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theodorakis, N. et al. Antibiotic resistance in the elderly: mechanisms, risk factors, and solutions. Microorganisms12, 1978 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tawam, D. et al. The positive association between proton pump inhibitors and Clostridium difficile infection. Innov. Pharm. 12, 10.24926/iip.v24912i24921.23439 (2021). [DOI] [PMC free article] [PubMed]
- 75.Gordon, D. et al. Incidence of Clostridium difficile infection in patients receiving high-risk antibiotics with or without a proton pump inhibitor. J. Hosp. Infect.92, 173–177 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Chen, E. Y. et al. Medication regimen complexity in 8 Australian residential aged care facilities: impact of age, length of stay, comorbidity, frailty, and dependence in activities of daily living. Clin. Interven. Aging14, 1783–1795 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grenier, F., Matteau, D., Baby, V., & Rodrigue, S. Complete genome sequence of Escherichia coli BW25113. Genome announcements, 2, 10.1128/genomea.01038-01014 (2014). [DOI] [PMC free article] [PubMed]
- 78.Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci.97, 6640–6645 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment. (1996).
- 80.Eucast, D. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect.6, 509–515 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Jin, M. et al. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ. Int.120, 421–430 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Pisoni, L. A. et al. Combined structure-and ligand-based approach for the identification of inhibitors of AcrAB-TolC in Escherichia coli. ACS Infect. Dis.9, 2504–2522 (2023). [DOI] [PubMed] [Google Scholar]
- 83.Blaikie, J. M. et al. Resistome analysis of Klebsiella pneumoniae Complex from residential aged care facilities demonstrates intra-facility clonal spread of multidrug-resistant isolates. Microorganisms12, 751 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohene-Agyei, T., Mowla, R., Rahman, T. & Venter, H. Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiologyopen3, 885–896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quijada, N. M., Rodríguez-Lázaro, D., Eiros, J. M. & Hernández, M. TORMES: an automated pipeline for whole bacterial genome analysis. Bioinformatics35, 4207–4212 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Barrick, J. E. et al. Identifying structural variation in haploid microbial genomes from short-read resequencing data using breseq. BMC Genomics15, 1–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sievers, F. & Higgins, D. G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci.27, 135–145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res.42, W320–W324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing has been deposited in the NCBI BioProject database PRJNA1281333.