Abstract
Rickets is a major public health concern globally. It results from impaired mineralization of the growing bone at its growth plate associated with abnormal calcium and phosphate metabolism. Among different classifications, nutritional deficiency is the commonest variety, and the genetic form of rickets has also been identified frequently in this genomic era. Treatment and management of rickets should be targeted as per type along with the pathogenesis of development of this condition. Management differs for each form of rickets and therapy requires distinct treatment, monitoring and follow-up schedule according to the pattern of response to therapy. The objective of this review is to summarize the treatment and management of different types of rickets in the light of recent guidelines and relevant literature.
Keywords: Vitamin D, Nutritional rickets, Refractory rickets, Management, Children
Introduction
Rickets is a disease of growing bone resulting from alteration of calcium and phosphate homeostasis causing impaired apoptosis of hypertrophied chondrocytes in the metaphyseal growth plate [1]. Nutritional rickets is a global health problem and discussed widely with non-communicable diseases now a days. It is more prevalent in low and middle-income countries, particularly in the Indian sub-continent, Africa and Middle East [2]. Rickets is mainly caused by deficiency and impaired metabolism of vitamin D, calcium and phosphate among children [3]. The prevalence of genetic causes has also increased over the recent era with the discovery of novel genes. Treatment of rickets should be targeted on the pathogenesis, which is strictly connected with the type of rickets [4]. There are two principal types of rickets which have been conventionally referred to as calcipenic and phosphopenic rickets [5]. Calcipenic rickets is mainly due to calcium and vitamin D deficiency as well as abnormality of vitamin D metabolism and function at its site of action [6]. Phosphopenic rickets can be sub-divided into two forms based on excessive renal phosphate wasting and reduced dietary intake. Excess renal loss of phosphate may either be mediated through excess fibroblast growth factor-23 (FGF23) or renal tubular defect [7]. The testing of vitamin D status and use of its supplement has increased substantially in recent years [8]. Treatment of rickets with vitamin D and oral calcium supplementation is the common practice in low-resource settings now a days. Despite strict adherence to this regime, if rickets fails to heal; refractory rickets must be considered. These forms of rickets are again subdivided into hypophosphatemic rickets and vitamin D-dependent rickets (VDDR) [7]. The discovery of a phosphaturic hormone, FGF23 together with detection of underlying genetic defects in many hereditary forms of rickets provide a landscape of understanding the pathogenesis and type-specific diagnosis of rickets. Progress continue in the field of research, and perhaps a human monoclonal antibody to FGF23, Burosumab is the ultimate frontier in the management of rickets till date [9].
Diagnosis of refractory rickets is challenging due to the unavailability of genetic testing everywhere despite of its paramount importance. Till date, substantial variability exists regarding Optimal vitamin D dosing, duration of supplementation and status of vitamin D across different guidelines. Provision of calcium and vitamin D enriched food in appropriate quantity during the period of rapid growth (infancy, childhood and adolescent) would be the best preventive strategy of nutritional rickets in low resource settings, this crucial issue is poorly addressed by the parents and physician as well because of ignorance. Dietary management is also a neglected part in CKD-MBD as more attention has been paid to medication and other management of CKD. Disparity also exists in access to vitamin D supplementation due to public health implication barrier in resource limited countries.
With a view to highlighting the therapeutic approach to various forms of rickets based on recent relevant literature and current recommendations as well as to raise awareness regarding judicious use of vitamin D supplementation among pediatricians, this burning issue has been focused on in this review.
Methodology
This article focuses on different types of rickets, their treatment and prevention of rickets among pediatric age groups. Relevant literatures were searched independently by two authors from the Google Scholar and PubMed databases. More emphasis was given to recent studies to report the updated information regarding this topic. Although we did not grade the strength of our work, this review presents a brief, but specific therapeutic approaches to the various types of rickets in the light of current guidelines.
Treatment of different forms of rickets
It is necessary to differentiate between hypocalcemic rickets and hypophosphatemic rickets based on main biochemical findings which will guide to choose the initial treatment approach. The presence of elevated alkaline Phosphatase (ALP) activity is more relevant in hypocalcemic rickets than hypophosphatemic rickets [10]. Serum PTH level is either normal or mildly elevated, whereas secondary hyperparathyroidism is the most striking biochemical sign in hypocalcemic rickets. Hypocalcemia is evident in both the form of rickets, but may be normal in hypophosphatemic rickets. Hypophosphatemia can be found in both the types, which is due to secondary hyperparathyroidism in hypocalcemic rickets [4]. TmP/GFR is another tool to distinguish dietary phosphate deficiency from renal phosphate wasting [11].
The therapy of rickets is based on vitamin D and calcium supplementation along with phosphate when necessary. Both vitamin D and calcium supplementation are mandatory in case of malabsorption. Vitamin D may have a role in calcium deficiency. Vitamin D with calcium may not be needed always, rather increases the risk of nephrocalcinosis and kidney stones [12].
Nutritional rickets
The treatment of nutritional rickets comprises of intensive phase followed by a maintenance phase. There exists a wide range of recommendations.
The US Endocrine society recommends 2000 IU/day of cholecalciferol for all age groups in the intensive phase followed by 400–600 IU/day in the maintenance phase. The National Osteoporosis Society of the United Kingdom recommends 3000 IU in infants ˂6 months old, 6000 IU in 6 months to 12 years old and 10,000 IU in 12–18 years old of cholecalciferol per day in intensive phase followed by 400–600 IU/day in maintenance phase [13]. Optimal dose of vitamin D supplementation varies as per different guidelines (Table 1).
Table 1.
Different guidelines for vitamin D supplementation [14]
| First Author & year |
Title | Type | Vitamin D S Status cut-off Values considered |
Vitamin D Toxicity level considered |
Population | Vitamin D supplementation |
|---|---|---|---|---|---|---|
|
Munns et al. 2016 [15] |
Global Consensus Recommendations on prevention and management of nutritional rickets |
Consensus statement from expert members of various international societies |
Sufficiency, > 50 nmoI/L Insufficiency,30–50 nmoI/L Deficiency, < 30nmoI/L |
> 250nmoI/L, with hypercalcemia, hypercalciuria, and suppressed PTH |
Children And adults |
Infants (0–12 months) 400 IU/day in the first year of life, Independent of their mode of feeding. Beyond 12 months of age 600 up to 2000IU/day (Minimum duration 12 weeks pregnant women 600 IU/d throughout pregnancy |
|
Braegger et al., 2013 [16] |
Vitamin D in the healthy European pediatric population |
Consensus Statement from members of the ESPHGAN Committee of nutrition |
Sufficiency > 50 nmoI/L Severe deficiency < 25 nmoI/L |
No agreement on a vitamin D toxicity threshold” | Children |
Infants (birth to 12 months) 400IU/day in the first year of life Beyond 12 months: > 600IU/day In children from identified risk groups |
|
Saggese et al. 2018 [17] |
Vitamin D in pediatric age: consensus of the Italian Pediatric Society and the Italian society of preventive and social paediatrics jointly with the Italian federation of pediatricians |
Consensus Statement From national Pediatric societies in Italy |
Sufficiency > 30 ng/ml (> 75 nmoI/L) Insufficiency 20-29ng/ml (50–74 nmoI /L) Deficiency < 20ng/ ml (< 50nmoI/L) Severe deficiency < 10ng/ml (< 25nmoI/L) |
Not Reported | Children |
preterm infants: weight > 1500 g: 400-800IU/day, Weight < 1500 g. : 200-400IU/day by enteral feeding 400IU/day in all newborn independent of the type of feeding subjects beyond 12 months of age: 600up to 1000 IU/day in children and adolescents with risk factor for Vitamin D deficiency. |
ESPGHAN (European society of pediatric gastroenterology, nutrition and hepatology)
Vitamin D supplementation in the form of cholecalciferol (vitamin D3) is preferable to ergocalciferol (vitamin D2) because of its longer half-life and higher efficacy. Minimum duration of 3 months is recommended for intensive phase [18].
Children with obesity and those are on treatment with anticonvulsants, glucocorticoids, and anti-retroviral medications may require higher doses of vitamin D to maintain their serum 25(OH)D concentrations in the sufficient range (as much as 6000 IU daily). Glucocorticoid inhibits vitamin- D at its receptor binding with retinoid X receptor (RXR), thereby inhibiting its absorption through the intestine. Anticonvulsants and anti-retroviral drugs enhance the catabolism of 25(OH)D into its inactive molecule [19]. Calcipenic nutritional rickets need calcium supplementation (Table 2).
Table 2.
Calcium treatment in patients with rickets with hypocalcemia [11]
| Condition | Calcium Salts |
Doses, mg/kg (ml/kg) |
Mode of administration |
|---|---|---|---|
|
Symptomatic Acute hypocalcemia |
Gluconate 10% (10 ml ≈ 90 mg elemental calcium) |
5–20 (0.5-2.0) |
Intravenous slowly, over 10–15 min to avoid bradycardia; diluted in 0.9% sodium chloride or 5% dextrose |
|
Asymptomatic hypocalcemia or normocalcemia |
Carbonate (40% of elemental calcium) Citrate (21% of elemental calcium) |
30–75 5 years old: 500 mg/day 10 years old: 1000 mg/day |
Oral route, divided into 2–3 doses daily |
Treatment of refractory rickets
When a child with suspected nutritional rickets exhibits no improvement with appropriate vitamin D therapy, a diagnosis of refractory rickets must be made [20]. Vitamin D refractory rickets are usually vitamin D dependent rickets including type 1 A,1B,2 A, 2B and type 3, FGF-23 dependent hypophosphatemic rickets including X linked, autosomal dominant, autosomal recessive (type 1,2, and 3) and FGF-23 independent hypophosphatemic rickets including tubulopathy like Fanconi syndrome, Dent disease, drug induced proximal tubulopathy [21]. Drug induced vitamin D deficiency may need 400–4000 IU of vitamin D supplementation [22].
Vitamin D-dependent rickets (VDDR)
VDDR has been broadly classified into three categories– VDDR type 1 (VDDR1), VDDR type 2 (VDDR2) and VDDR type 3 (VDDR3). VDDR1 again subdivided into VDDR type 1 A (VDDR1A) and VDDR type 1B (VDDR1B) caused by renal 1ɑ-hydroxylase and hepatic 25-hydroxylase deficiency, respectively [4, 23]. VDDR2, currently termed as hereditary vitamin D resistant rickets [24] is caused by either mutation of vitamin D receptor gene (VDDR2A) or impaired interaction between vitamin D receptor and DNA (VDDR2B). VDDR3 results from genetic mutation leading to excessive inactivation of vitamin D metabolites [23].
Treatment of vitamin D-dependent rickets (VDDR)
In case of VDDR, treatment is based on the combination of vitamin D analogs and calcium (Table 3). The commonly used metabolite is calcitriol, which regulates the active transport of calcium from the intestine and suppresses the secretion of parathyroid hormone, and alfacalcidiol, which bypasses the need for renal activation. Calcitriol has a half-life of approximately 5–8 h; requiring at least 2–3 daily doses. In contrast, alfacalcidiol may be administered once a day as it has a longer half-life (approximately 24 h). Patients must be treated for a lifelong period in case of genetic vitamin D-dependent rickets with patient-tailored doses. The best treatment of VDDR1B is oral calcifediol as it bypasses the defect of hepatic 25-hydroxylation. Wide variability is noted regarding response to vitamin D therapy in VDDR2. Patients with VDDR2 without alopecia show a better response to treatment than patients having alopecia. About half of them with alopecia are refractory to high doses of active form of vitamin D [4, 25].
Table 3.
| Drug | VDDR1A | VDDR1B | VDDR2 | VDDR3 |
|---|---|---|---|---|
| Vitamin D | NI | Heterozygous 5000–10,000 IU/day Homozygous:600,000 every 3 months | LI | 50,000/day |
| Calcifediol | NI |
15–50 ug/day (preferred choice) |
20–200 ug/day | 50 to? |
| Alfacalcidol |
10–100 ng/kg/day 0.5-3ug/day |
0.5-3 ug/day (less effective) |
10–400 ng/kg/day 5–60 ug/day |
2 to? |
| Calcitriol |
10–100 ng/kg/day 0.3-2 ug/day |
0.3-2 ug/day (alternative choice) |
10-400ng/kg/day 5-60ug/day |
1 to? |
|
Calcium salts (by oral route) |
0.5–3 g/day | 0.5–2 g/day |
3–5 g/day 400–1400 mg/m2/day (IV route) |
? |
NI, Not indicated; LI, little indicated
Some patients may need intravenous overnight calcium infusion for 12 h to maintain normocalcemia [23, 25]. The role of cinacalcet has also been found effective in these cases, encouraging its short-term use [26, 27].
Phosphopenic rickets
Classification of phosphopenic rickets
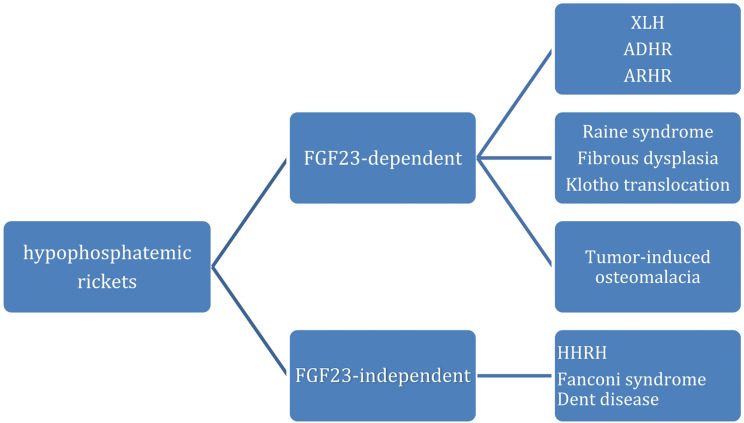
Phosphopenic or hypophosphatemic rickets include disorders that may be FGF23-dependent and FGF-23 independent forms (Fig. 1).
Fig. 1.
classification of different forms of hypophosphatemic rickets [4]
Hypophosphatemic rickets may be attributed to secondary hyperparathyroidism. This condition results from failure of organ involved in vitamin D metabolism, i.e. liver, kidney or intestine and inadequate supply of phosphate as occurs in malnutrition, prematurity, total parenteral nutrition, use of phosphate chelators [6].
There are some other rare form of FGF23 dependant rickets including genetic mutations of DMP1 (Dentin matrix protein 1) and ENPP1, which constitute less than 20% of hypophosphatemic rickets [28]. The latter is most important as current recommendations argue against treatment with anti-FGF23 antibody therapy [29].
Treatment of phosphopenic rickets
Conventional treatment of patients with FGF23-dependent hypophosphatemic rickets consists of inorganic oral phosphate salts combined with vitamin D active metabolites, such as calcitriol or alfacalcidol. Most of the data regarding the conventional treatment of hypophosphatemic rickets comes from the management of patients with X-linked hypophosphatemic rickets (XLH). The recommended starting and maintenance doses of inorganic phosphate and vitamin D active metabolites in patients with hypophosphatemic rickets are summarized in Table 4.
Table 4.
Starting and maintenance doses of inorganic phosphate and active vitamin D analogs in hypophosphatemic rickets [30–35]
| Newborn or before the development of clinical or radiological signs of rickets |
Evidence of clinical or radiological signs of rickets | |
|---|---|---|
|
Starting Doses |
Alfacalcidol,25-40ng/kg/day (0.8-1ug/day), once/day. Inorganic phosphate salts, 20–40 mg/kg/day, 4 to 6 intakes/daily |
Alfacalcidol, 40–80 ng/kg/day (1-1.5 ug/kg) once/day. Calcitriol, 20–40 ng/kg/day, 2–3 times/day Inorganic phosphate salts, 40–60 mg/kg/day, 4 to 6 intakes/daily |
|
Maintenance doses |
Alfacalcidol,25–40 ng/kg/day (1–2 ug/day), once/day; calcitriol,20–30 ng/kg/day. 2–3 times/day. inorganic phosphate salts, 20–60 mg/kg/day. 4 to 6 intakes/ day |
|
N.B. A progressive increase in the dose of phosphate and active vitamin D analogs is recommended. The treatment should be individualized and tailored according to the severity of the patient’s condition and tolerability. The two medications must be administered combinedly at a balanced dosage with careful monitoring.
Patients who respond poorly to conventional treatment or have significant side effects are candidates for therapy with Burosumab, a humanized IgG1 monoclonal antibody directed against the FGF23 hormone. Some patients with XLH have also been treated with growth hormone (GH) in combination with conventional treatment to improve growth although results are inconclusive [36].
In patients with autosomal dominant hypophosphatemic rickets (ADHR) and autosomal recessive hypophosphatemic rickets (ARHR), the dosages of phosphate supplements and vitamin D active metabolites may vary according to the severity of the disease and the response to treatment [4].
Monitoring and follow-up
Vitamin D deficiency
Therapeutic monitoring of treatment with vitamin D and calcium varies with the severity of rickets and response to therapy. Normalization of serum calcium and phosphate level.
usually occurs within 3 weeks of initiation of treatment [37], but it may be earlier (after 6–10 days of treatment) [38]. Serum parathyroid hormone (PTH) concentration falls within the normal range by normal feedback loop after restoration of serum Ca level. Serum 25(OH)D concentration rises rapidly and reaches its normal value after 4–6 weeks. Serum 1,25(OH)2D concentration increases rapidly after treatment that may remain elevated for 10 weeks [36]. Alkaline phosphatase activity declines progressively but may remain elevated for several months (3–6 months) depending on the severity of the vitamin D deficiency. Serum alkaline phosphatase level serves as a reliable and economic biochemical marker of activity of rickets and monitoring tool of therapeutic response [39].
Radiological examinations are useful to demonstrate the effectiveness of treatment with vitamin D in patients with nutritional rickets. The zone of provisional calcification at the end of the metaphyses is usually appeared within 3–4 weeks of treatment [38].
Clinical response pattern of nutritional rickets include improvements of symptoms like aches and pains disappear by 2 weeks, the disappearance of metaphyseal swelling by 6 months. Total correction of bowed legs & knock knees may need 2 years, adolescents are usually left with some residual deformities which require surgical correction [40].
Calcium deficiency
Radiological healing of the growth plate usually demonstrates after 3–6 months of calcium administration. It requires a longer time to improve the clinical signs than the biochemical alterations and radiological healing [41].
Vitamin D-dependent rickets
Response to therapy with active vitamin D metabolites (calcitriol or alfacalcidol) is usually faster. Radiological healing of rickets and normalization of the biochemical parameters occur within 7–9 weeks of therapy [42]. Strict adherence to long term treatment is of paramount importance. Serum concentrations of calcium, phosphate, PTH and urinary calcium excretion should be monitored routinely to see the response during the maintenance phase of therapy (at least every 4–6 months). Time interval may be shortened if hypocalcemia presents. Target should be made to keep serum calcium concentration in the low-normal range and PTH level below the upper limit of normal [43]. Urinary calcium excretion should be maintained below the normal limit for age and weight. Renal ultrasound should be performed every 1–2 years to detect nephrocalcinosis earlier and more frequently if there is evidence of hypercalciuria [23].
Hypophosphatemic rickets
Some recommendations for follow up of XLH patients who are receiving conventional treatment have been suggested (Table 5).
Table 5.
Recommendations for the follow-up of patients with XLH rickets receiving conventional treatment [4, 25]
| Clinical assessment | Timing |
|---|---|
| Clinical and auxological examination |
< 5 years:1–3 months > 5 years: 3–6 months |
| Dental Examination | Every 6–12 months or based on clinical symptoms |
| Orthopedic examination | Every 12 months or based on clinical symptoms |
| Hearing test | >8 years or based on clinical symptoms |
| Biochemical parameters | |
| aSerum calcium, inorganic phosphate, creatinine, alkaline phosphatase, PTH | Every 3–6 months |
|
Urinary calcium creatinine ratio, bTmP/GFR |
Every 3–6 months in patients on active vitamin D or Burosumab treatment |
| Imaging examinations | |
|
Radiographs of wrists, knees, standing lower limbs |
Every 1–2 years or based on clinical signs |
| Renal ultrasonography | Every year |
| Fundoscopy and brain MRI |
In the presence of craniosynostosis or skull shape malformation, headache, neurological symptoms or visual disturbances. |
| Quality of life | Every year |
aBiochemical parameters including serum phosphate, ALP and TmP/GFR must be interpreted with age-specific normative value
bTmP/GFR is the maximum rate of tubular reabsorption of phosphate per glomerular filtration rate; calculated by entering the fasting urine and plasma concentrations (same unit) into following equation: TmP/GFR =  [25]
[25]
Age specific upper normal range of TmP/GFR: 2.2 (< 1 year), 1.4 (1–3 years), 1.1 (3–5 years), 0.8 (5–7 years), 0.7 (> 7 years)
Treatment of hereditary hypophosphatemic rickets with hypercalciuria (HHRH)
Treatment includes supplementation of phosphate in a monitored way to avoid nephrocalcinosis. 1,25(OH)2 D is not recommended as it is high in HHRH. High fluid intake, avoidance of high salt and protein along with thiazide diuretics are useful in preventing nephrocalcinosis [44].
Management of vitamin D deficiency in CKD-MBD
The therapeutic goal of pediatric CKD-MBD is to prevent or minimize complications in the growing skeleton, achieve adequate growth, and prevent vascular calcification by managing hyperphosphatemia, vitamin D deficiency, and secondary hyperparathyroidism [45, 46].
Vitamin D deficiency is common and often severe in children and adults with CKD. Treatment of vitamin D deficiency is important because vitamin D deficiency contributes to secondary hyperparathyroidism [47].
The European Society for Paediatric Nephrology (ESPN) CKD-MBD clinical practice guidelines suggest using native vitamin D (e.g., ergocalciferol and cholecalciferol) supplements to treat vitamin D insufficiency or deficiency in children with CKD G2-G5D, and to prevent or treat secondary hyperparathyroidism in children with CKD G2-G3 [48]. Active vitamin D analogs (e.g., alfacalcidol, calcitriol, paricalcitol, and doxercalciferol) should not be used to treat vitamin D deficiency [49]. Mega-dose vitamin D therapy is not recommended [50] (Tables 6 and 7).
Table 6.
Classification of vitamin D status in children with CKD [51]
| Sufficiency | > 75 nmol/L (> 30 ng/mL) |
|---|---|
| Insufficiency | 50–75 nmol/L (20–30 ng/mL) |
| Deficiency | 12–50 nmol/L (5–20 ng/mL) |
| Severe deficiency | < 12 nmol/L (< 5 ng/mL) |
Table 7.
Vitamin D supplementation in children with end stage renal disease (ESRD) [48]
| Age | 25(OH)D level (nmoI/L) | Daily dose | Monitoring |
|---|---|---|---|
| Intensive replacement therapy | |||
| < 1 year | < 12 | 600 IU/day |
Serum Ca and urinary Ca levels at 1–3 months. 25(OH)D after 3 months |
| > 1 year | 12–50 | 8000 IU/day |
Serum Ca and urinary Ca levels at 1–3 months. 25(OH)D after 3 months |
| 50–75 | 4000 IU/day |
Serum Ca and urinary Ca levels at 1–3 months. 25(OH)D after 3 months |
|
| Maintenance therapy | |||
| < 1 year | > 75 | 400 IU/day | 25(OH)D level at 6–12 months |
| > 1 year | > 75 |
1000/2000 IU/day (more in advanced CKD) |
25(OH)D level at 6–12 months |
Treatment of other forms of rickets
Fibrous dysplasia can cause hypophosphatemic rickets via secretion of excess of FGF23 in dysplastic tissue, resulting in some degree of phosphate wasting [52]. Management of fibrous dysplasia primarily focuses on non-rachitic complications as hypophosphatemia is less severe. Treatment with phosphate salt and active vitamin D therapy is considered when hypophosphatemic rickets develop. Failure to respond needs burosumab treatment. Bisphosphonates has limited role [25, 53].
Tumor-induced osteomalacia (TI0) may be solved by complete resection of the tumor. When tumor removal is not possible, conventional treatment of XLH should consider such as phosphate supplementation at a dose of 15–30 mg/kg/day in 4–6 divided doses and calcitriol or alphacalcidiol at 15–60 ng/kg/day. Doses should be adjusted as per clinical and biochemical state & presence of side effects. Alternatively, burosumab can be used [54].
Phosphate supplements along with active metabolites of Vitamin D are suggested for the treatment of patients with hypophosphatemic rickets secondary to tubulopathies, Dent disease, Fanconi syndrome or other systemic diseases in association with the disease- specific treatments for each of them [55].
Vitamin D toxicity (VDT)
VDT is a rare, but serious clinical condition characterized by severe hypercalcemia, usually develops after excessive intake of vitamin-D megadose or its metabolite [56]. According to pediatric endocrine society, serum 25 (OH)D levels > 100 ng/ml (250 nmol/L) have been defined as hypervitaminosis D and > 150 ng/ml (375nmol/L) have been proposed to define vitamin-D intoxication [57]. According to a updated guideline in Poland (2023), VDT is defined as hypercalcemia, hypercalciuria and serum 25(OH)D concentration > 100ng/ml [58]. So, disparity is noted on VDT threshold amongst different guidelines. Priority must be given on laboratory reference value. There is delay in diagnosis of refractory rickets due to limited access to advance diagnostic facilities (i.e. genetic testing) in resource poor settings. Repeated treatment of mega dose of vitamin D leads to the risk of VDT. The long term effects of high dose vitamin D therapies require further investigations.
The clinical manifestations of VDT are primarily related to hypercalcemia, ranging from asymptomatic to severe life-threatening features [59]. Laboratory investigations reveal hypercalcemia and hypercalciuria. Nephrocalcinosis may be observed on renal ultrasonography. Treatment of toxicity includes discontinuing vitamin D, adequate hydration and management of hypercalcemia by calcitonin, steroid and hemodialysis with or without diuretics [60].
Prevention of rickets
Exclusively breast-fed infants consume an average of 750 ml of breast milk and ingest 10–40 IU (0.25 to 1 µg/day) of vitamin D daily. The vitamin D content is usually lower in mothers with dark skinned and on vitamin-D deficient diets. Breast-fed infants need to be exposed to sunlight 30 min/day while wearing a diaper to maintain 25(OH)D concentration at ˃ 20 ng/ml. Infant formula and orange juice in the United States is fortified to contain 40–100 IU (1-2.5 µgm/L) and 400 IU (10 µg) of vitamin D per liter respectively [61]. This rule should be made universal to all nations of the world. In case of light-skinned people, sufficient cutaneous vitamin D synthesis needs 10–15 min of sun exposure to the arm, legs, hands and face between 10.00 am to 3.00 pm during spring, summer and fall. Prolonged sun exposure results in a minimum serum 25(OH) D level of ˂80 ng/ml (200 nmol/L) [62]. Among Indian children, role of sunlight was found to be effective in improving vitamin D status who had a sunlight exposure of 17–30 min in infants and 30–45 min in older children over 15–40% of body surface area [63].
American Academy of Pediatrics (AAP) recommends universal supplementation of vitamin D 400IU per day throughout infancy regardless of mode of feeding (breast feeding or formula feeding). Infant can receive supplementation indirectly through breast milk from women who fortify their own milk by ingesting high doses of vitamin D (4000 to 6400 IU/day) [64]. AAP also recommends 1 L of vitamin-D fortified milk to all children beyond infancy. Unfortunately, only one-third of American children used to consume the recommended amount as per the 2022 report because only 30% o clinicians believe that recommendation [65]. 1 cup of milk contains 276 mg calcium as well as enough phosphate. The habit of drinking milk is very essential [22].
The food safety Standards Authority of India recommends milk fortification by using a fat soluble premix. Vitamin D supplementation with 400–600 IU/day is set as an estimated average requirement for Indian children. So, food fortification is a cost effective strategy of prevention of vitamin D deficiency in low resource settings [66].
Daily 4000 to 6000 IU (100–160 µg) of vitamin-D supplementation to lactating mothers is another strategy to improve vitamin D status in infant. Vitamin-D enriched foods are very little in number. Consumption of spinach, green vegetables, bread (wheat, brown), almonds rich in calcium can mitigate vitamin D deficiency [67].
Universal supplementation of vitamin-D during national immunization program to all infants and children can be an alternative option. More than 1100 foods are vitamin-D fortified in USA. Milk fortification in India has been found to be successful in school children. Education to all level of health workers, mass campaign in social media can be useful [67, 68]. All nation of the globe can fortify their staple food with the recommended daily amount. Parliamentary legal approval of universal fortification of vitamin D may be helpful as per author’s view.
CKD-MBD prevention can be ensured by optimum CKD nutrition with concomitant daily or monthly oral D2 or D3 supplementation, keeping normal 25(OH)D and calcium. Higher doses of ergocalciferol and repeat course of intensive replacement treatment may be needed [48].
Conclusion
Early diagnosis and institution of proper treatment are the main targets to avoid severe complications in later life. The differentiation among the varieties of rickets is an important step for definitive management. Although nutritional rickets is the commonest form, refractory patterns must be considered based on specific biochemical and radiographic findings along with special clinical features, so that appropriate treatment and monitoring can be instituted promptly in a timely- manner.
Acknowledgements
Not applicable.
Abbreviations
- IU
International unit
- ESPGHAN
European society of pediatric gastroenterology, nutrition and hepatology
- FGF23
Fibroblast growth factor-23
- VDDR
Vitamin D-dependent rickets
- RXR
Retinoid X receptor
- ADHR
Autosomal dominant hypophosphatemic rickets
- ARHR
Autosomal recessive hypophosphatemic rickets
- CKD-MBD
Chronic kidney disease– mineral bone disease
- NKF-KDOQI
National Kidney Foundation- Kidney Disease Outcomes Quality Initiative
- TI0
Tumor-induced: osteomalacia
- VDT
Vitamin D toxicity
- ESRD
End stage renal disease
- AAP
American Academy of Pediatrics
Author contributions
Rummana Tazia Tonny was a major contributor in writing the manuscript.Ranjit Ranjan Roy made a substantial, direct and intellectual contribution to the work and approved it for publication.Tahmina Jesmin was actively involved in overall scrutiny of this review based on updated literature.Abdullah Al Mamun reviewed the manuscript.Nadira Sultana was actively involved in writing the manuscript and searching literature.
Funding
The research received no external funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haffner D, Leifheit-Nestler M, Grund A, Schnabel D. Rickets guidance: part I—diagnostic workup. Pediatric Nephrology. 2021 37:9 [Internet]. 2021 Dec 15 [cited 2024 May 15];37(9):2013–36. Available from: https://link.springer.com/article/10.1007/s00467-021-05328-w [DOI] [PMC free article] [PubMed]
- 2.Uday S, Högler W. Nutritional rickets & osteomalacia: A practical approach to management. Indian Journal of Medical Research [Internet]. 2020 Oct 1 [cited 2025 Feb 26];152(4):356–67. Available from: https://journals.lww.com/ijmr/fulltext/2020/52040/nutritional_rickets___osteomalacia__a_practical.6.aspx [DOI] [PMC free article] [PubMed]
- 3.Selvamanojkumar S, Gupta N. Rickets is Not Always Nutritional! Indian J Pediatr [Internet]. 2023 Dec 1 [cited 2024 May 15];90(12):1169–70. Available from: https://link.springer.com/article/10.1007/s12098-023-04808-1 [DOI] [PubMed]
- 4.Baroncelli GI, Comberiati P, Aversa T, Baronio F, Cassio A, Chiarito M, et al. Diagnosis, treatment, and management of rickets: a position statement from the bone and mineral metabolism group of the Italian society of pediatric endocrinology and diabetology. Front Endocrinol (Lausanne). 2024;15:1383681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allgrove J, Shaw NJ. A Practical Approach to Vitamin D Deficiency and Rickets. Endocr Dev [Internet]. 2015 [cited 2024 May 30];28:119–33. Available from: https://karger.com/books/book/171/chapter/5106050/A-Practical-Approach-to-Vitamin-D-Deficiency-and [DOI] [PubMed]
- 6.Biasucci G, Donini V, Cannalire G. Rickets Types and Treatment with Vitamin D and Analogues. Nutrients 2024, Vol 16, Page 416 [Internet]. 2024 Jan 31 [cited 2024 May 18];16(3):416. Available from: https://www.mdpi.com/2072-6643/16/3/416/htm [DOI] [PMC free article] [PubMed]
- 7.Chinoy A, Padidela R, Refractory Rickets. Indian J Pediatr [Internet]. 2023 Jun 1 [cited 2024 May 18];90(6):574–81. Available from: https://link.springer.com/article/10.1007/s12098-023-04538-4 [DOI] [PMC free article] [PubMed]
- 8.Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr [Internet]. 2020 Nov 1 [cited 2024 May 18];74(11):1498. Available from: http://www.pmc/articles/PMC7091696/ [DOI] [PMC free article] [PubMed]
- 9.Chowdhury A, Roy RR, Jesmin T, Tonny RT. The evolution of rickets through the ages. Int J Contemp Pediatrics [Internet]. 2025 Dec 24 [cited 2025 Mar 18];12(1):142–6. Available from: https://www.ijpediatrics.com/index.php/ijcp/article/view/6406
- 10.Cannalire G, Pilloni S, Esposito S, Biasucci G, Di Franco A, Street ME. Alkaline phosphatase in clinical practice in childhood: focus on rickets. Front Endocrinol (Lausanne). 2023;14:1111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert AS, Linglart A. Hypocalcaemic and hypophosphatemic rickets. Best Pract Res Clin Endocrinol Metab. 2018;32(4):455–76. [DOI] [PubMed] [Google Scholar]
- 12.Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proceedings of the Nutrition Society [Internet]. 2017 Aug 1 [cited 2024 May 18];76(3):392–9. Available from: https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/vitamin-d-deficiency-as-a-public-health-issue-using-vitamin-d2-or-vitamin-d3-in-future-fortification-strategies/49816B8345AFC98DB16320F12608E2A2 [DOI] [PubMed]
- 13.Rusinska A, Płudowski P, Walczak M, Borszewska-Kornacka MK, Bossowski A, Chlebna-Sokół D et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front Endocrinol (Lausanne) [Internet]. 2018 May 31 [cited 2025 Feb 26];9(MAY):246. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5990871/ [DOI] [PMC free article] [PubMed]
- 14.Corsello A, Spolidoro GCI, Milani GP, Agostoni C. Vitamin D in pediatric age: Current evidence, recommendations, and misunderstandings. Front Med (Lausanne) [Internet]. 2023 [cited 2025 Feb 26];10:1107855. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10060648/ [DOI] [PMC free article] [PubMed]
- 15.Aguiar M, Atapattu N, Bhatia V, Braegger C, Butler G, Cassinelli H et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab [Internet]. 2016 Feb 1 [cited 2025 Feb 26];101(2):394–415. Available from: https://pubmed.ncbi.nlm.nih.gov/26745253/ [DOI] [PMC free article] [PubMed]
- 16.Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr [Internet]. 2013 Jun [cited 2025 Feb 26];56(6):692–701. Available from: https://pubmed.ncbi.nlm.nih.gov/23708639/ [DOI] [PubMed]
- 17.Zakharova I, Klimov L, Kuryaninova V, Nikitina I, Malyavskaya S, Dolbnya S et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front Endocrinol (Lausanne) [Internet]. 2019 [cited 2025 Feb 26];10(MAR):103. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6406072/ [DOI] [PMC free article] [PubMed]
- 18.Sahay M, Sahay R. Rickets–vitamin D deficiency and dependency. Indian J Endocrinol Metab [Internet]. 2012 [cited 2025 Feb 26];16(2):164. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3313732/ [DOI] [PMC free article] [PubMed]
- 19.Vitamin D. insufficiency and deficiency in children and adolescents - UpToDate [Internet]. [cited 2025 Feb 26]. Available from: https://www.uptodate.com/contents/vitamin-d-insufficiency-and-deficiency-in-children-and-adolescents
- 20.Roy A, Chowdhury AS, Ray A, Baidya A, Roychowdhury B, Sarkar D et al. Diagnostic approach to rickets: an Endocrine Society of Bengal (ESB) consensus statement. Ann Pediatr Endocrinol Metab [Internet]. 2024 Oct 1 [cited 2025 Feb 26];29(5):284. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC11541088/ [DOI] [PMC free article] [PubMed]
- 21.Shore RM, Chesney RW, Rickets. Part II. Pediatr Radiol [Internet]. 2013 Feb [cited 2025 Feb 26];43(2):152–72. Available from: https://pubmed.ncbi.nlm.nih.gov/23179485/ [DOI] [PubMed]
- 22.Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG et al. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics [Internet]. 2015 Oct 1 [cited 2025 Feb 26];136(4):625–34. Available from: https://pubmed.ncbi.nlm.nih.gov/26416936/ [DOI] [PMC free article] [PubMed]
- 23.Levine MA. Diagnosis and Management of Vitamin D Dependent Rickets. Front Pediatr [Internet]. 2020 Jun 12 [cited 2025 Feb 26];8:315. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7303887/ [DOI] [PMC free article] [PubMed]
- 24.Ghazi AA, Zadeh-Vakili A, Yeganeh MZ, Alamdari S, Amouzegar A, Khorsandi AA et al. Hereditary Vitamin D Resistant Rickets: Clinical, Laboratory, and Genetic Characteristics of 2 Iranian Siblings. Int J Endocrinol Metab [Internet]. 2017 [cited 2025 Apr 8];15(3):e12384. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5702004/ [DOI] [PMC free article] [PubMed]
- 25.Haffner D, Leifheit-Nestler M, Grund A, Schnabel D. Rickets guidance: part II-management. Pediatr Nephrol [Internet]. 2022 Oct 1 [cited 2025 Feb 26];37(10):2289–302. Available from: https://pubmed.ncbi.nlm.nih.gov/35352187/ [DOI] [PMC free article] [PubMed]
- 26.Nicolescu RC, Lombet J, Cavalier E. Vitamin D-resistant rickets and cinacalcet-one more favorable experience. Front Pediatr [Internet]. 2018;6:415871. Available from: www.frontiersin.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas J, Badia JL, Lucas E, Remon A. Cinacalcet treatment experience in hereditary vitamin D resistant rickets. Journal of Pediatric Endocrinology and Metabolism [Internet]. 2020 Feb 1 [cited 2025 Feb 26];33(2):313–8. Available from: https://www.researchgate.net/publication/338535515_Cinacalcet_treatment_experience_in_hereditary_vitamin_D_resistant_rickets [DOI] [PubMed]
- 28.Pavone V, Testa G, Gioitta Iachino S, Evola FR, Avondo S, Sessa G. Hypophosphatemic rickets: etiology, clinical features and treatment. Eur J Orthop Surg Traumatol [Internet]. 2015 Feb 1 [cited 2025 Feb 26];25(2):221–6. Available from: https://pubmed.ncbi.nlm.nih.gov/24957364/ [DOI] [PubMed]
- 29.Höppner J, Kornak U, Sinningen K, Rutsch F, Oheim R, Grasemann C. Autosomal recessive hypophosphatemic rickets type 2 (ARHR2) due to ENPP1-deficiency. Bone [Internet]. 2021 Dec 1 [cited 2025 Feb 26];153. Available from: https://pubmed.ncbi.nlm.nih.gov/34252603/ [DOI] [PubMed]
- 30.Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM, Rickets. Nature Reviews Disease Primers 2017 3:1 [Internet]. 2017 Dec 21 [cited 2024 May 19];3(1):1–20. Available from: https://www.nature.com/articles/nrdp2017101 [DOI] [PubMed]
- 31.Baroncelli GI, Mora S, X-Linked Hypophosphatemic, Rickets. Multisystemic Disorder in Children Requiring Multidisciplinary Management. Front Endocrinol (Lausanne) [Internet]. 2021 Aug 6 [cited 2025 Feb 26];12. Available from: https://pubmed.ncbi.nlm.nih.gov/34421819/ [DOI] [PMC free article] [PubMed]
- 32.Linglart A, Biosse-Duplan M, Briot K, Chaussain C, Esterle L, Guillaume-Czitrom S et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect [Internet]. 2014 Feb 19 [cited 2025 Feb 26];3(1):R13–30. Available from: https://pubmed.ncbi.nlm.nih.gov/24550322/ [DOI] [PMC free article] [PubMed]
- 33.Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol [Internet]. 2019 Jul 1 [cited 2025 Feb 26];15(7):435–55. Available from: https://pubmed.ncbi.nlm.nih.gov/31068690/ [DOI] [PMC free article] [PubMed]
- 34.Rothenbuhler A, Schnabel D, Högler W, Linglart A. Diagnosis, treatment-monitoring and follow-up of children and adolescents with X-linked hypophosphatemia (XLH). Metabolism [Internet]. 2020 Feb 1 [cited 2025 Feb 26];103S. Available from: https://pubmed.ncbi.nlm.nih.gov/30928313/ [DOI] [PubMed]
- 35.Trombetti A, Al-Daghri N, Brandi ML, Cannata-Andía JB, Cavalier E, Chandran M et al. Interdisciplinary management of FGF23-related phosphate wasting syndromes: a Consensus Statement on the evaluation, diagnosis and care of patients with X-linked hypophosphataemia. Nat Rev Endocrinol [Internet]. 2022 Jun 1 [cited 2025 Feb 26];18(6):366–84. Available from: https://pubmed.ncbi.nlm.nih.gov/35484227/ [DOI] [PubMed]
- 36.Lafage-Proust MH. What are the benefits of the anti-FGF23 antibody burosumab on the manifestations of X-linked hypophosphatemia in adults in comparison with conventional therapy? A review. Therapeutic advances in rare disease [Internet]. 2022 Jan 1 [cited 2025 Feb 26];3. Available from: https://pubmed.ncbi.nlm.nih.gov/37180412/ [DOI] [PMC free article] [PubMed]
- 37.Shaw NJ, Vitamin D. Deficiency Rickets. Endocr Dev [Internet]. 2003 [cited 2024 May 31];6:93–104. Available from: https://karger.com/books/book/2450/chapter/5735644/Vitamin-D-Deficiency-Rickets [DOI] [PubMed]
- 38.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics [Internet]. 2008 Aug [cited 2025 Feb 26];122(2):398–417. Available from: https://pubmed.ncbi.nlm.nih.gov/18676559/ [DOI] [PubMed]
- 39.Baroncelli GI, Bertelloni S, Ceccarelli C, Amato V, Saggese G. Bone turnover in children with vitamin D deficiency rickets before and during treatment. Acta Paediatr [Internet]. 2000 May 1 [cited 2024 May 31];89(5):513–8. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1651-2227.2000.tb00329.x [DOI] [PubMed]
- 40.Mughal MZ, Rickets. Curr Osteoporos Rep [Internet]. 2011 Dec 4 [cited 2025 Feb 26];9(4):291–9. Available from: https://link.springer.com/article/10.1007/s11914-011-0081-0 [DOI] [PubMed]
- 41.Thacher TD. Calcium-deficiency rickets. Endocr Dev [Internet]. 2003 [cited 2024 May 31];6:105–25. Available from: https://www.researchgate.net/publication/10576348_Calcium-Deficiency_Rickets [DOI] [PubMed]
- 42.Delvin EE, Glorieux FH, Marie PJ, Pettifor JM. Vitamin D dependency: Replacement therapy with calcitriol. J Pediatr [Internet]. 1981 Jul 1 [cited 2024 May 31];99(1):26–34. Available from: http://www.jpeds.com/article/S0022347681809523/fulltext [DOI] [PubMed]
- 43.Miller WL, Portale AA, Vitamin D, Biosynthesis, Vitamin D. 1α-Hydroxylase Deficiency. Endocr Dev [Internet]. 2003 [cited 2024 May 31];6:156–74. Available from: https://karger.com/books/book/2450/chapter/5735654/Vitamin-D-Biosynthesis-and-Vitamin-D-1-Hydroxylase [DOI] [PubMed]
- 44.Bergwitz C, Miyamoto KI. Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Arch [Internet]. 2019 Jan 31 [cited 2025 Feb 26];471(1):149–63. Available from: https://pubmed.ncbi.nlm.nih.gov/30109410/ [DOI] [PubMed]
- 45.Hanudel MR, Salusky IB. Treatment of Pediatric Chronic Kidney Disease-Mineral and Bone Disorder. Curr Osteoporos Rep [Internet]. 2017 Jun 1 [cited 2025 Feb 26];15(3):198. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5607746/ [DOI] [PMC free article] [PubMed]
- 46.McAlister L, Pugh P, Greenbaum L, Haffner D, Rees L, Anderson C et al. The dietary management of calcium and phosphate in children with CKD stages 2–5 and on dialysis-clinical practice recommendation from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet]. 2020 Mar 1 [cited 2025 Feb 26];35(3):501–18. Available from: https://pubmed.ncbi.nlm.nih.gov/31667620/ [DOI] [PMC free article] [PubMed]
- 47.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K et al. Disordered FGF23 and mineral metabolism in children with CKD. Clinical Journal of the American Society of Nephrology [Internet]. 2014 [cited 2025 Feb 26];9(2):344–53. Available from: https://pubmed.ncbi.nlm.nih.gov/24311704/ [DOI] [PMC free article] [PubMed]
- 48.Shroff R, Wesseling-Perry K, Bacchetta J. Chronic Kidney Disease– Mineral and Bone Disorder (CKD-MBD). Pediatric Nephrology: Eighth Edition [Internet]. 2022 Jan 1 [cited 2025 Feb 27];1751–78. Available from: https://link.springer.com/referenceworkentry/10.1007/978-3-030-52719-8_129
- 49.Zand L, Kumar R. The Use of Vitamin D Metabolites and Analogs in the Treatment of Chronic Kidney Disease. Endocrinol Metab Clin North Am [Internet]. 2017 Dec 1 [cited 2025 Feb 26];46(4):983. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5977979/ [DOI] [PMC free article] [PubMed]
- 50.Bacchetta J, Schmitt CP, Bakkaloglu SA, Cleghorn S, Leifheit-Nestler M, Prytula A et al. Diagnosis and management of mineral and bone disorders in infants with CKD: clinical practice points from the ESPN CKD-MBD and Dialysis working groups and the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet]. 2023 Sep 1 [cited 2025 Feb 27];38(9):3163–81. Available from: https://pubmed.ncbi.nlm.nih.gov/36786859/ [DOI] [PMC free article] [PubMed]
- 51.Shroff R, Wan M, Nagler EV, Bakkaloglu S, Fischer DC, Bishop N et al. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol Dial Transplant [Internet]. 2017 Jul 1 [cited 2025 Feb 26];32(7):1098–113. Available from: https://pubmed.ncbi.nlm.nih.gov/28873969/ [DOI] [PMC free article] [PubMed]
- 52.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res [Internet]. 2012 May [cited 2025 Feb 27];27(5):1132–41. Available from: https://pubmed.ncbi.nlm.nih.gov/22247037/ [DOI] [PMC free article] [PubMed]
- 53.De Castro LF, Ovejero D, Boyce AM, DIAGNOSIS OF ENDOCRINE DISEASE.: Mosaic disorders of FGF23 excess: Fibrous dysplasia/McCune-Albright syndrome and cutaneous skeletal hypophosphatemia syndrome. Eur J Endocrinol [Internet]. 2020 May 1 [cited 2025 Feb 26];182(5):R83–99. Available from: https://pubmed.ncbi.nlm.nih.gov/32069220/ [DOI] [PMC free article] [PubMed]
- 54.Florenzano P, Gafni RI, Collins MT. Tumor-induced osteomalacia. Bone Rep [Internet]. 2017 Dec 1 [cited 2025 Feb 26];7:90. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5633085/ [DOI] [PMC free article] [PubMed]
- 55.Flores-Aldana M, Rivera-Pasquel M, García-Guerra A, Pérez-Cortés JG, Bárcena-Echegollén JE. Effect of Vitamin D Supplementation on (25(OH)D) Status in Children 12–30 Months of Age: A Randomized Clinical Trial. Nutrients [Internet]. 2023 Jun 1 [cited 2025 Feb 26];15(12). Available from: https://pubmed.ncbi.nlm.nih.gov/37375660/ [DOI] [PMC free article] [PubMed]
- 56.Marcinowska-Suchowierska E, Kupisz-Urbanska M, Lukaszkiewicz J, Pludowski P, Jones G, Vitamin D, Toxicity.–A Clinical Perspective. Front Endocrinol (Lausanne) [Internet]. 2018 Sep 20 [cited 2025 Feb 26];9(SEP):550. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6158375/ [DOI] [PMC free article] [PubMed]
- 57.Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab [Internet]. 2014 [cited 2025 Feb 26];99(4):1132–41. Available from: https://pubmed.ncbi.nlm.nih.gov/24456284/ [DOI] [PubMed]
- 58.Płudowski P, Kos-Kudła B, Walczak M, Fal A, Zozulińska-Ziółkiewicz D, Sieroszewski P et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients [Internet]. 2023 Feb 1 [cited 2025 Mar 18];15(3). Available from: https://pubmed.ncbi.nlm.nih.gov/36771403/ [DOI] [PMC free article] [PubMed]
- 59.Vitamin DT. A Case Study| New Zealand Journal of Medical Laboratory Science [Internet]. [cited 2025 Feb 26]. Available from: https://search.informit.org/doi/abs/10.3316/INFORMIT.247484105865433
- 60.Chanchlani R, Nemer P, Sinha R, Nemer L, Krishnappa V, Sochett E et al. An Overview of Rickets in Children. Kidney Int Rep [Internet]. 2020 Jul 1 [cited 2025 Feb 26];5(7):980. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7335963/ [DOI] [PMC free article] [PubMed]
- 61.Overview of vitamin D. - UpToDate [Internet]. [cited 2025 Feb 26]. Available from: https://www.uptodate.com/contents/overview-of-vitamin-d
- 62.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D Deficiency in Children and Its Management: Review of Current Knowledge and Recommendations. Pediatrics [Internet]. 2008 Aug 1 [cited 2024 May 31];122(2):398–417. Available from: http://www.pediatrics/article/122/2/398/73042/Vitamin-D-Deficiency-in-Children-and-Its [DOI] [PubMed]
- 63.Khadilkar A, Khadilkar V, Chinnappa J, Rathi N, Khadgawat R, Balasubramanian S, et al. Prevention and treatment of vitamin D and calcium deficiency in children and adolescents: Indian academy of pediatrics (IAP) guidelines. Indian Pediatr. 2017;54(7):567–73. [DOI] [PubMed] [Google Scholar]
- 64.Aul AJ, Fischer PR, Benson MR, Oberhelman-Eaton SS, Mara KC, Thacher TD, Infant, Maternal Vitamin D, Supplementation. Clinician Perspectives and Practices. The Journal of the American Board of Family Medicine [Internet]. 2023 Feb 8 [cited 2025 Feb 26];36(1):95–104. Available from: https://www.jabfm.org/content/36/1/95 [DOI] [PubMed]
- 65.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) et al. 2011. 3, Overview of Vitamin D. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56061/ [PubMed]
- 66.Dabas A, Padidela R, Dabas A, Padidela R. Nutritional Vitamin D deficiency rickets in children– Challenges in diagnosis, management, and prevention. Wadia Journal of Women and Child Health [Internet]. 2024 Jun 4 [cited 2025 Feb 26];3(1):15–24. Available from: https://wjwch.com/nutritional-vitamin-d-deficiency-rickets-in-children-challenges-in-diagnosis-management-and-prevention/
- 67.Ritu G, Gupta A, Gupta A. Fortification of Foods with Vitamin D in India. Nutrients [Internet]. 2014 Sep 12 [cited 2025 Feb 26];6(9):3601. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4179178/ [DOI] [PMC free article] [PubMed]
- 68.Bromage S, Ahmed T, Fawzi WW. Calcium Deficiency in Bangladesh: Burden and Proposed Solutions for the First 1000 Days. Food Nutr Bull [Internet]. 2016 Dec 1 [cited 2025 Feb 26];37(4):475. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5135641/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.