Abstract
Background
This study aimed to investigate the potential toxic effects of Molybdenum disulfide nano-flowers (MoS2 NF), which have been suggested as a chemotherapeutic agent, but lack previous toxicity studies.
Methods
Acute, sub-acute and developmental toxicity studies were conducted following OECD guidelines 425, 407 and 414, respectively.
Results
In the acute toxicity study, female Wistar rats received logarithmic doses (1.75–550 mg/kg) of MoS2NF over 14 days. Results indicated a decrease in oxidative stress markers (CAT, SOD and GSH) and increased MDA levels, along with significant decrease in organ weight compared to normal control. Alterations in liver enzymes, CBC profile and lipid profile and histopathological analysis were observed in MoS2 NF groups. Sub-acute toxicity (28-day at 3 and 10 mg/kg in both male and female rats) resulted in increased levels of ALT and AST, decreased levels of CAT, SOD and GSH and increased MDA and urea levels. Sperm analysis in male group showed increased motility and concentration, with more defective morphology. In developmental toxicity studies, a 10 mg/kg dose for 21 days decreased all oxidative markers except MDA, which increased. Fetal crown-to-rump length increased, while uterine SOD, CAT and GSH levels decreased. Histopathology revealed organ damage in both sub-acute and developmental studies. Maternal weight remained unaffected, whereas fetal weight showed an increased.
Conclusion
MoS2 NF exhibited mild-to-moderate toxicity, however, long-term and studies are recommended to assess the safety and therapeutic potential of MoS2NF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40360-025-00881-8.
Keywords: Acute toxicity, Sub-acute toxicity, Developmental toxicity, In vivo, Nano-flowers
Introduction
The process of transforming matter to an extent near to the atomic level to produce innovative structures, materials, and devices is known as nanotechnology [1]. Nanoparticles can readily penetrate the skin because of their tiny size and accessibility to sensitive organs. The size, morphology, charged surface, and surface modifications can all have an impact on how pathogenic they are. Their adverse effects are mostly caused by the generation of reactive oxygen species, which results in cell death, damage to cells, and reduced function [2].
Molybdenum belongs to second row transitional metals having molecular weight 95.94 and is represented symbolically by Mo [3]. Pure metallic molybdenum does not occur naturally. In solution, molybdenum readily becomes polymerized to form an array of intricate polymolybdate compounds [4].
Molybdenum disulfide, also known as molybdenite, is the primary source of molybdenum. MoS2 levels in mineral deposits are typically quite low, although the flotation process can concentrate them. Nowadays, molybdenum disulfide is mainly generated as a byproduct of copper extraction [5]. Nanoparticles of molybdenum disulfide are utilized for the identification and examination of biomolecules and hazardous chemicals [6]. Recent research has indicated that MoS2 may pose a risk under certain biological conditions, particularly when used as imaging agents or drug carriers are bio-distributed to the liver. MoS2 causes dose-dependent cytotoxicity in liver kupffer cells [7]. We have synthesized and characterized the MoS2NF and have studied its therapeutic potential in breast cancer [8]. However, its toxicity has not yet been evaluated. Therefore, this study aimed to provide a comprehensive understanding of the toxicological profile of MoS2 NF through acute, sub-acute and developmental toxicity studies in Wistar rats and inform safe usage guidelines for their potential applications in medicine and industry.
Material and methods
Ethical committee
All the procedures and techniques used in this study were approved by the Ethical committee (REC/RIPS/LHR/2023/068) of Riphah International University, Raiwind Campus, Lahore. Pakistan.
Study design
Albino wistar rats of weight range 100–160 g (6–7 weeks old) were chosen for conducting the toxicity studies according to the OECD guidelines. The animals were purchased from the University of Veterinary and Animal Science’s animal house. For acute toxicity study conducted on female rats, main test was the choice using a onetime dose and observing for 14 days. Sub-acute toxicity study was conducted by dividing the animals into three groups separate from both sex rats (n = 5 for each group) group-I; the control group (CMC), group-II the treatment group at dose 3 mg/kg MoS2 NF and group-III being dosed at 10 mg/kg MoS2 NF. As there are no in-vivo study standards available for MoS2 NF so we use Cisplatin (the only anti-cancer registered metal compound) as standard to choose the dose for the current study. This study was conducted on both the male and female rats for 28 days orally with a once daily dose. Developmental toxicity study was designed by dividing the animals into 2 groups (n = 5 for each group); the control group and the treatment group (10 mg/kg MoS2NF dose). Animals in this study group were treated for 19–21 days based on their pregnancy cycle.
Drugs and chemicals
Molybdenum disulfide, carboxymethylcellulose (CMC), monobasic potassium phosphate, dibasic potassium phosphate, Pyrogallol, hydrogen peroxide (H2O2), trichloroacetic acid, sodium hydrogen carbonate, monobasic sodium phosphate, DTNB (5,5-dithio-bis-2-nitrobenzoic acid), hydrochloric acid, dibasic sodium phosphate, thiobarbituric acid (TBA), Sodium hydroxide, sodium potassium tartarate, copper sulfate, folin phenol. All the chemicals were of analytical grade and purchased from local vendors dealing with the companies.
Acute toxicity study
OECD guidelines 425 were followed to conduct this study. As the toxicity profile of the drug was not available, so directly main test was conducted. Female rats were given a dose of 1.75 mg as per kg of the body weight. The rats were keenly noticed for first 30 minutes and kept under observation for next 14 days. All the animals survived so new animals were given the next dose of 5.5 mg/kg and the procedure continued till 550 mg/kg (following the log doses of 17.5, 55, 175 and 550 mg/kg) as no animal showed sign of mortality. The animals were sacrificed after 14-days to analyze different parameters [9].
Sub-acute toxicity study
This study was conducted under OECD guidelines 407. Rats of both sex (male and female) were used. Animals were divided into six groups (n = 5 for each group). Group I -The female control group was given 0.5% CMC. Group II (female) received molybdenum disulfide (MoS2) NF 3 mg/kg and Group III (female) was being treated with 10 mg/kg MoS2 NF. Group IV- male control group treated with 0.5% CMC and Group V (male) was given a dose of 3 mg/kg MoS2 NF. Group VI (male) treated with 10 mg/kg MoS2NF. The dosing was continued orally for 28 days daily. After completion of the dosing period, blood samples were collected from each group for CBC, LFTs, RFTs and lipid profiling. Animals were dissected and different organs were removed for analysis of anti-oxidant activity and Histopathology study [10–12].
Developmental toxicity
Developmental toxicity study was designed according to OECD guidelines 414. Male and female rats were kept for mating until a vaginal plug appeared. The pregnant rats were then divided into two groups (control and treatment group), with n = 5 for each group. Treatment group received 10 mg/kg MoS2 NF daily for 19–21. Following that time period C- section was performed a day before the completion of gestational period carefully collecting the fetus to check different vitals including weight of fetus, no. of live and dead fetus, crown to rump length, total no. of fetus, etc.
Soft tissue examination
Fetuses were dipped in the saturated solution of picric acid in alcohol (Bouin’s solution) for 10–15 days. Following that time period, the fetus got hardened, its skin was removed carefully and all vital organs were removed and viewed individually for any visual abnormality. Each tissue’s length and width were measured as well [10].
Sperm analysis
All the rats were killed by a dislocation of the cervical spine. Castration method was used to remove the testis. Testicles were collected by making an excision on the prescrotal line. Cauda epididymis thus removed was transferred to petri plate having 1 ml of 7.4 pH sodium phosphate buffer. Sperm cells were diffused into the buffer at 37℃. The sperm suspension thus made was used to measure different parameters, including sperm count, its morphology, DNA integrity (using Acridine Orange test) and sperm motility [13, 14].
Separation of plasma
After the completion of the experimental protocol, blood samples of experimental rats were collected in the EDTA tube by cardiac puncture. Blood samples were centrifuged for 15 minutes at 4000 rpm to separate the blood plasma for biochemical analysis [15].
Biochemical analysis
The blood plasma that was separated by centrifugation was used for analysis of ALT, AST, ALP levels, Bilirubin, total proteins, globulins, albumins levels in the liver. Renal function tests (RFTs) including creatinine and urea and analysis of lipid profile that included cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), very-low-density lipoproteins (VLDL) and triglycerides using particular kits for each parameter [16].
Tissue homogenate preparation
After dissecting all the test animals, desired organs (liver, heart, kidney, lungs, brain, ovaries and testis respectively from female and male animals) were removed and weighed. Sodium phosphate buffer was used in ratio 1:10 to prepare homogenates which were centrifuged at 6000 rpm, 4℃ temperature for 10 minutes. A clear supernatant was collected and used for the analysis of different parameters [17].
Oxidative stress markers analysis
Tissue homogenates were used for the analysis of oxidative parameters that are listed below:
Assessment of superoxide dismutase (SOD) level
To measure the anti-oxidant SOD levels, 0.1 ml of pyrogallol solution, 0.1 mL of tissue homogenate and 2.8 ml of 0.1 M Potassium phosphate buffer (pH 7.4) were mixed and the optical density of the mixture were recorded at a wavelength of 325 nm. The formula given below was used to calculate the results [18].
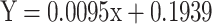 |
Catalase activity (CAT)
All the three constituents, including 0.05 ml of tissue homogenate, 1.95 mL of 50 mM Phosphate buffer,and 1 mL of H2O2 solution were mixed together to measure the absorbance of reaction mixture at a wavelength of 240 nm. Using the following equation, CAT was calculated [19]
 |
Where; S.O.D = absorbance measured, E = 0.071 [20].
Malondialdehyde (MDA) estimation
1 ml of the tissue supernatant, 1 ml of TBA, 1 ml of 15% TCA and 1 ml of HCL were all mixed. The whole reaction mixture was placed for 15 minutes in a hot water bath. It was then chilled in an ice bath and centrifuged later on at 3500 rpm for 10 minutes. Supernatant was taken to measure absorbance at 532 nm wavelength. MDA was then calculated by the formula.
 |
Where; Vu = Aliquot volume, Vt = total mixture volume, Wt = weight of dissected organ [17, 20].
Estimation of protein levels
To perform this test, reagent A, B, C were prepared to make reagent 1, where Reagent A: 2% sodium carbonate (Na2CO3) in sodium hydroxide (0.1 N), Reagent B: Sodium potassium tartrate 1 % in 100 ml H2O and Reagent C: 0.5% CuSO4.5H2O in 100 ml water.
Reagent-I: 48 ml of reagent A, 1 ml of B and 1 ml of C were combined to make reagent-I.
Reagent-II was prepared by mixing 1 part folin phenol (2 N) in part of water. 0.2 ml of the tissue homogenate taken and distilled water was added to make it 1 ml. In this diluted homogenate, 4.5 ml of reagent-I was added to incubate for 10 minutes. Reagent-II (0.5 ml) was added to the mixture after incubation and was incubated again for 30 minutes. Absorbance of the sample was observed at a wavelength of 660 nm. Calculate results using the formula:
 |
Estimation of GSH (glutathione) levels
1 ml of supernatant of the tissue homogenate, 1 ml of TCA (10%) was added in 4 ml of phosphate buffer and 0.5 ml of DTNB and mixed well. Precipitates were formed; the supernatant was removed to estimate the absorbance of the sample at 412 nm. Results were then calculated using the equation:
 |
Where; Y = absorbance of sample, DF = dilution factor, Bt = organ weight calculated, Vv = volume of homogenate used [20].
Histopathological analysis
Histopathology of some selected organs, including kidney, lungs, liver, brain, heart, ovaries, testis, were analyzed by fixing with neutral buffered formalin (10%), processed and embedded in the paraffin. The tissues were then sliced into 3–4 µm sections in longitudinal direction. Eosin and hematoxylin dyes were then used to stain the organ slices. Histology of the slides formed was then studied using YJ-2005DN digital articulated free binocular microscope having 1.30 MP camera [21].
Statistical analysis
Mean ± SEM was used to express all the calculated values. T-test and One-way analysis of variance (ANOVA) were used to examine all the treatment outcomes before a post-hoc Dunnett’s test was run to investigate the entire test procedures used.
Results
Effect of MoS2 NF on body and organ weights in acute, sub-acute and developmental toxicity studies
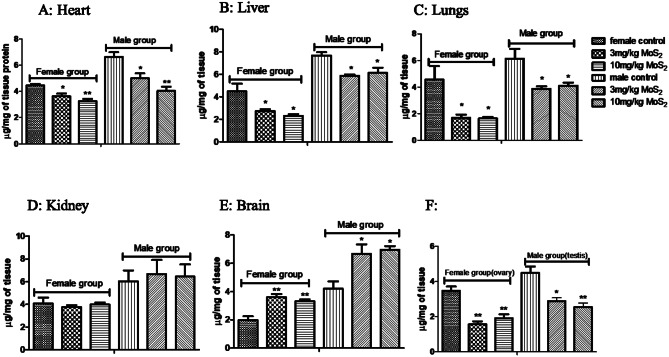
Body weights were found to be parallel to normal control in each study group for acute, sub-acute and developmental toxicity (Supplementary data; Table 1–3). In acute toxicity study, organ weights of brain, lungs, liver and ovary at a high dose of 175 mg/kg showed a significant increase as compared to control, as shown in Table 1. In sub-acute toxicity study, weight of all organs in the male treatment group at both dosage levels of 3 and 10 mg/kg were significantly increased while in females, weight of only liver and lungs were increased as compared to control group (Table 2).
Table 1.
Effect of treatment on organ weight of animals calculated as per 100 g of animal weight in acute toxicity studies at different doses of MoS2 Nano flowers
| Organs | Control group | MoS2 NF | |||||
|---|---|---|---|---|---|---|---|
| 1.75 mg/kg | 5.5 mg/kg | 17.5 mg/kg | 55 mg/ kg | 175 mg/kg | 550 mg/kg | ||
| Brain | 1.08 ± 0.062 | 1.75 ± 0.068*** | 1.60 ± 0.046*** | 1.70 ± 0.08*** | 1.907 ± 0.049 *** | 1.457 ± 0.083 *** | 1.38 ± 0.09* |
| Heart | 0.437 ± 0.04 | 0.63 ± 0.038** | 0.457 ± 0.03 | 0.57 ± 0.029 | 0.44 ± 0.029 | 0.57 ± 0.02 | 0.53 ± 0.029 |
| Liver | 3.78 ± 0.14 | 6.58 ± 0.15*** | 2.63 ± 0.22*** | 7.15 ± 0.518*** | 7.35 ± 0.28*** | 7.51 ± 0.27*** | 5.85 ± 0.19*** |
| Lung | 0.82 ± 0.07 | 1.43 ± 0.06** | 1.07 ± 0.049 | 2.33 ± 0.10*** | 2.57 ± 0.16*** | 2.45 ± 0.11*** | 2.06 ± 0.07*** |
| Ovary | 0.084 ± 0.009 | 0.09 ± 0.01 | 0.09 ± 0.012 | 0.134 ± 0.02 | 0.086 ± 0.009 | 0.219 ± 0.021*** | 0.125 ± 0.018 |
| Kidney | 0.74 ± 0.05 | 1.13 ± 0.03* | 0.77 ± 0.06 | 1.423 ± 0.13*** | 1.07 ± 0.07* | 0.99 ± 0.06 | 1.077 ± 0.072* |
Data are showed as mean ± SEM, **P < 0.01, ***P < 0.001 as compared with normal control. *indicates increase in weight
Table 2.
Effect of treatment on organ weights of Male and Female rats calculated per 100 g of animal weight at different doses of MoS2 NF in sub-acute toxicity studies
| Organs | Female | Male | ||||
|---|---|---|---|---|---|---|
| Control group | MoS2NF | Control group | MoS2NF | |||
| 3 mg/kg | 10 mg/kg | 3 mg/kg | 10 mg/kg | |||
| Brain | 2.567 ± 0.127 | 2.933 ± 0.156 | 2.94 ± 0.107 | 2.770 ± 0.15 | 3.343 ± 0.143 | |
| Heart | 1.00 ± 0.064 | 1.103 ± 0.067 | 1.047 ± 0.081 | 0.767 ± 0.07 | 0.917 ± 0.105 | |
| Liver | 9.92 ± 0.18 | 11.81 ± 0.13*** | 12.61 ± 0.23*** | 6.82 ± 0.23 | 11.47 ± 0.26*** | |
| Lungs | 2.137 ± 0.27 | 3.99 ± 0.24** | 4.82 ± 0.326*** | 2.117 ± 0.102 | 3.13 ± 0.13** | |
| Ovary | 0.289 ± 0.107 | 0.250 ± 0.093 | 0.147 ± 0.029 | 2.96 ± 0.061 | 4.44 ± 0.121*** | |
| Kidney | 1.743 ± 0.142 | 1.110 ± 0.097* | 1.237 ± 0.137 | 1.087 ± 0.068 | 2.650 ± 0.144*** | |
Data are expressed as mean ± SEM, n = 5, *p < 0.05, **p < 0.01, ***p < 0.001 as compared with control. *indicates an increase in animals weight
Effect of MoS2 NF on CBC profile in acute, sub-acute and developmental toxicity studies
CBC profile of the acute toxicity study after 28 days of dosing showed a significant increase (p < 0.001) in Hb at higher doses, while WBCs and leukocytes were increased at low doses. Lymphocytes were significantly (p < 0.001) decreased (Table 3). Sub-acute and developmental toxicity studies showed a significant increase (p < 0.001) in platelets and lymphocytes count while the neutrophils were decreased (p < 0.01) in both studies as compared to normal control (Table 4).
Table 3.
Effect of MoS2 Nano flowers on complete blood count (CBC) in acute toxicity studies at different logarithmic doses
| Parameters | Unit | Normal control | MoS2 NF | |||||
|---|---|---|---|---|---|---|---|---|
| 1.75 mg/kg | 5.5 mg/kg | 17.5 mg/kg | 55 mg/ kg | 175 mg/kg | 550 mg/kg | |||
| Hemoglobin | g/dL | 10.3 ± 0.1 | 8.60 ± 0.1^^^ | 8.30 ± 0.1^^^ | 7.80 ± 0.1^^^ | 14.0 ± 0.1*** | 12.0 ± 0.1*** | 13.5 ± 0.1*** |
| RBCs | ×1012/L | 5.650 ± 0.33 | 4.90 ± 0.11 | 4.61 ± 0.190 | 4.07 ± 0.03^^ | 7.31 ± 0.31** | 6.75 ± 0.25* | 7.85 ± 0.25** |
| Neutrophils | % | 34.650 ± 2.15 | 26.50 ± 1.70 | 54.05 ± 1.55** | 42.20 ± 1.4 | 23.0 ± 3.0^ | 84.0 ± 1.0*** | 61.5 ± 4.50*** |
| Lymphocyte | % | 66.60 ± 1.60 | 63.10 ± 2.20 | 39.0 ± 2.0^^^ | 55.25 ± 0.95^ | 74.50 ± 1.5 | 13.0 ± 3.0^^^ | 40.0 ± 2.0^^^ |
| WBCs | ×109/L | 3.60 ± 0.10 | 3.30 ± 0.20 | 6.65 ± 0.150*** | 10.40 ± 0.20*** | 5.30 ± 0.1*** | 4.60 ± 0.20* | 3.35 ± 0.15 |
| Platelet count | ×109/L | 632.5 ± 17.5 | 470.0 ± 10.0 | 509 ± 11.0 | 565.0 ± 15.0 | 626.0 ± 7.50 | 737.0 ± 8.0 | 846.5 ± 5.50 |
Data are expressed as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 as compared with control. *indicates increase levels while ^indicate decreased levels
Table 4.
Effect of MoS2 NF on CBC profile of rats group in sub-acute and pregnant rats in developmental toxicity studies
| Parameters | Units | Sub-acute toxicity | Developmental toxicity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
| Control | MoS2NF | Control | MoS2NF | Control | MoS2 NF | ||||
| 3 mg/kg | 10 mg/kg | 3 mg/kg | 10 mg/kg | 10 mg/kg | |||||
| Hemoglobin | g/dL | 15.37 ± 0.51 | 14.16 ± 0.92 | 13.60 ± 0.81 | 10.96 ± 1.4 | 12.23 ± 0.77 | 14.56 ± 1.10 | 14.56 ± 1.10 | 13.40 ± 1.03 |
| RBCs | ×1012/L | 6.77 ± 0.26 | 7.3 ± 0.49 | 7.36 ± 0.42 | 4.67 ± 0.38 | 6.26 ± 0.31 | 7.5 ± 0.5** | 5.00 ± 0.89 | 6.43 ± 1.10 |
| WBCs | ×109/L | 9.07 ± 1.3 | 8.40 ± 1.09 | 10.6 ± 1.27 | 4.20 ± 1.3 | 6.5 ± 1.26 | 3.92 ± 0.97 | 807.3 ± 63.04 | 1109 ± 68.45* |
| Platelet count | ×109/L | 402.6 ± 9.1 | 850.6 ± 42.67 *** | 533.33 ± 28.6 * | 752.33 ± 7.2 | 656.3 ± 29.9 | 836.6 ± 37.8 | 4.66 ± 0.63 | 6.83 ± 1.04 |
| Neutrophils | % | 66.66 ± 4.4 | 7.33 ± 0.88^^^ | 10.33 ± 1.45^^^ | 29.27 ± 0.65 | 10.66 ± 1.2^^^ | 25.0 ± 1.15^ | 48.33 ± 3.38 | 20.33 ± 2.02^^ |
| Lymphocytes | % | 39.66 ± 2.6 | 90.0 ± 2.3*** | 85.33 ± 2.02*** | 64.0 ± 2.6 | 82.3 ± 4.8* | 70.0 ± 3.46 | 40.00 ± 2.64 | 74.33 ± 2.64 |
Data are represented as mean ± SEM, n = 5, *P < 0.05, **P < 0.01, ***P < 0.001 as compared with normal control. *indicates increase levels while ^indicate decreased levels
Effect of MoS2 NF on oxidative stress markes in acute and sub-acute and developmental toxicity studies
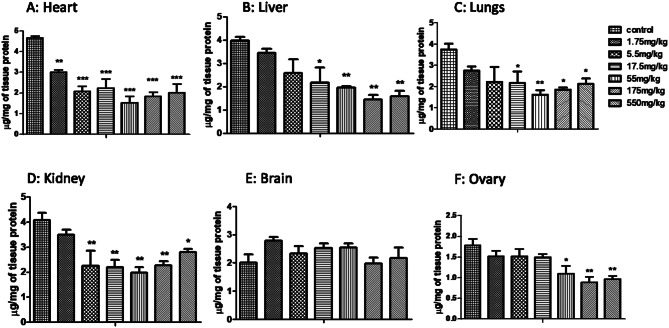
In acute toxicity study, CAT showed decrease levels in all the selected organs except ovary, which showed increased levels at low doses and decrease at high doses (Fig. 1). Glutathione (GSH) (Fig. 2) and superoxide dismutase (SOD) levels were also decreased (Fig. 3) at all doses. MDA levels showed increased activity in all organs except brain that showed significant MDA levels when compared to control (Fig. 4).
Fig. 1.
Effect of MoS2-NF (1.75 to 550 mg/kg) on Catalase levels in various tissues at the end of acute toxicity study. Data represented as Mean ± SEM (n = 5). ***p<0.001 and *p<0.05 in comparison to control groups group
Fig. 2.
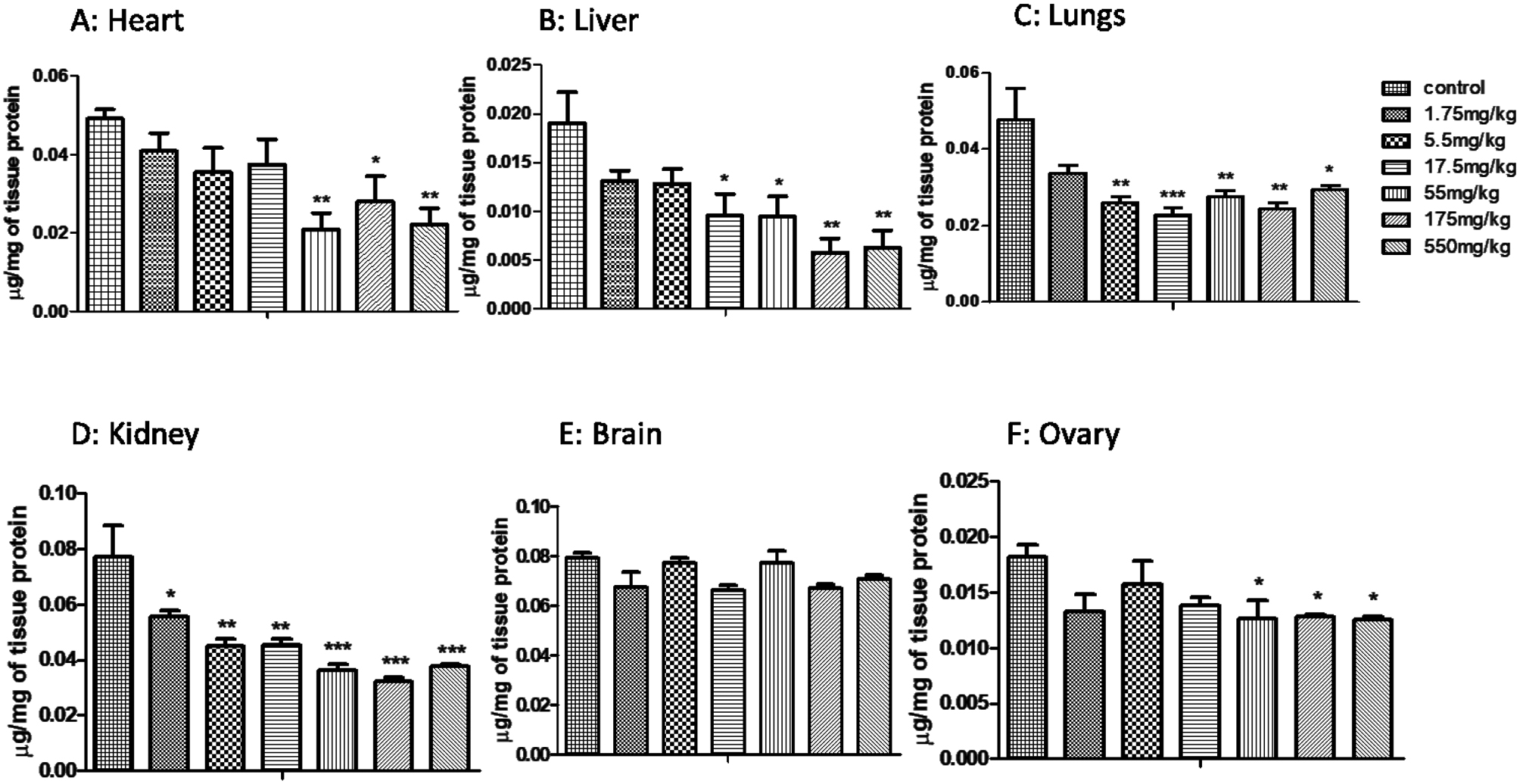
Effect of MoS2 NF treatment on GSH levels in acute toxicity studies of different organs at different logarithmic doses. Data represented as Mean ± SEM (n = 5). ***p<0.001 and *p<0.05 in comparison to control groups group
Fig. 3.
Effect of MoS2 NF treatment on SOD levels in acute toxicity studies of different organs at different logarithmic doses. Data represented as Mean ± SEM (n = 5). ***p<0.001, **P< 0.01 and *p<0.05 in comparison to control groups group
Fig. 4.
Effect of MoS2 NF treatment on MDA levels in acute toxicity studies of different organs at different logarithmic doses. Data represented as Mean ± SEM (n = 5). ***p<0.001, **p< 0.01 and *p<0.05 in comparison to control groups group
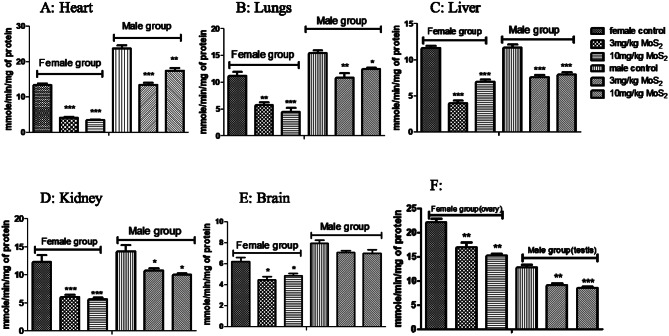
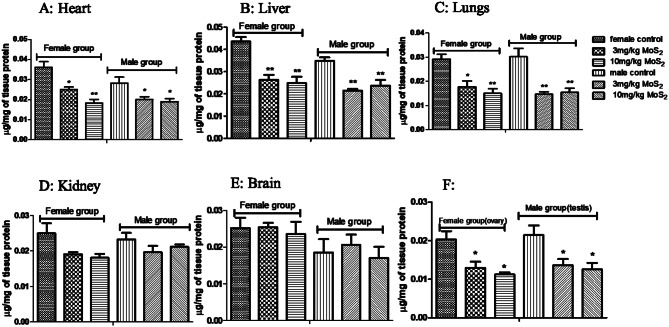
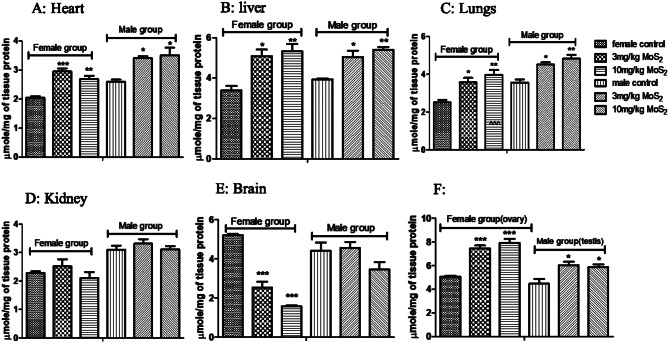
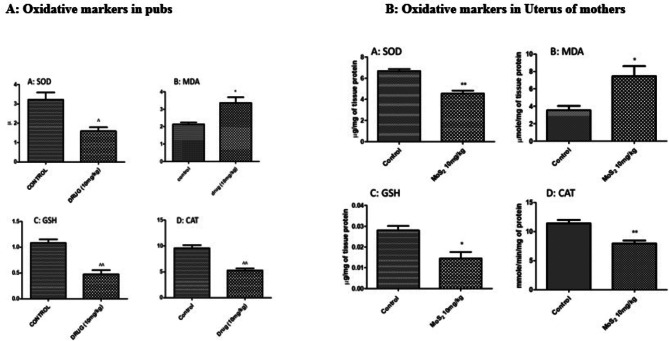
CAT levels were significantly decreased at both dose levels in both male and female treatment groups (Fig. 5). GSH levels were significantly decreased in both male and female treatment groups at both dose levels (Fig. 6), while MDA levels were increased in both treatment groups in all organs except brain (Fig. 7) SOD was also decreased at both doses in male and female rats groups as compared to normal control (Fig. 8). In the teratogenicity study, all the oxidative markers CAT, SOD and GSH were decreased while MDA levels were increased in the fetus when compared to the control (Fig. 9).
Fig. 5.
Effect of MoS2-NF (3 mg/kg and 10 mg/kg) on Catalase levels in various tissues at the end of sub-acute toxicity study. Data represented as Mean ± SEM (n = 5). ***p<0.001, **p< 0.01 and *p<0.05 in comparison to control groups group
Fig. 6.
Effect of MoS2-NF (3 mg/kg and 10 mg/kg) on GSH levels in various tissues at the end of sub-acute toxicity study. Data represented as Mean ± SEM (n = 5). **p< 0.01 and *p<0.05 in comparison to control groups group
Fig. 7.
Effect of MoS2-NF (3 mg/kg and 10 mg/kg) on MDA levels in various tissues at the end of sub-acute toxicity study. Data represented as Mean ± SEM (n = 5). ***p<0.001, **p< 0.01 and *p<0.05 in comparison to control groups group
Fig. 8.
Effect of MoS2-NF (3 mg/kg and 10 mg/kg) on SOD levels in various tissues at the end of sub-acute toxicity study. Data represented as Mean ± SEM (n = 5). ***p<0.001, **p< 0.01 and *p<0.05 in comparison to control groups group
Fig. 9.
Effect of MoS2 NF 10 mg/kg on oxidative stress markers in pubs (A) and in uterus of mother (B) in developmental toxicity studies
Effect of MoS2 NF on biochemical markers in acute, sub-acute and developmental toxicity study
All the toxicity studies conducted in the present research showed a significant increase (p < 0.001) in ALT, AST levels in the liver (see Table 5 for acute, Table 7 for subacute and Table 8 for developmental toxicity study). RFTs showed a significant increase (p < 0.001) in Urea levels in acute toxicity study (Table 5) and female group of sub-acute toxicity study (Table 7) while the creatinine levels were significantly decreased (p < 0.001) in the same groups when compared to normal control. RFTs and lipid profile were comparable to normal control in developmental toxicity study (Table 9). In acute toxicity study, levels of cholesterol, LDL and Triglycerides were decreased while the HDL was increased (p < 0.001) (Table 6). Cholesterol level was increased (p < 0.01) in male treatment group while decreased (p < 0.001) in females. LDL was decreased (p < 0.001) at low dose of 3 mg/kg while increased at high dose of 10 mg/kg MoS2 NF. Triglycerides were significantly increased at in both sex groups at both dose levels (Table 7).
Table 5.
Effect of treatment on Liver and Renal functions in acute toxicity studies of MoS2 NF at different logarithmic doses
| Parameters | Unit | Normal control | MoS2 NF | |||||
|---|---|---|---|---|---|---|---|---|
| LFTs | 1.75 mg/kg | 5.5 mg/kg | 17.5 mg/kg | 55 mg/ kg | 175 mg/kg | 550 mg/kg | ||
| ALT | U/L | 32.66 ± 1.20 | 87.0 ± 1.15*** | 41.33 ± 1.76* | 50.33 ± 1.76*** | 77.33 ± 1.76*** | 73.66 ± 1.85*** | 108.0 ± 3.51*** |
| AST | U/L | 38.33 ± 2.02 | 135.66 ± 2.02*** | 106.66 ± 2.96*** | 107.66 ± 4.48*** | 261.0 ± 2.30*** | 215.0 ± 2.08*** | 208.66 ± 3.38*** |
| Albumins | g/dl | 3.53 ± 0.20 | 4.23 ± 0.176 | 3.50 ± 0.173 | 3.36 ± 0.20 | 3.76 ± 0.14 | 4.86 ± 0.14*** | 4.76 ± 0.145*** |
| Globulins | g/dl | 3.0 ± 0.11 | 3.16 ± 0.14 | 4.66 ± 0.14*** | 4.76 ± 0.14*** | 4.56 ± 0.145*** | 3.63 ± 0.176 | 3.76 ± 0.20* |
| RFTs | ||||||||
| Urea | mg/dL | 37.66 ± 1.45 | 59.0 ± 1.15*** | 62.33 ± 1.76*** | 56.00 ± 2.64*** | 53.0 ± 2.08*** | 67.0 ± 1.52*** | 65.0 ± 2.08*** |
| Creatinine | mg/dL | 0.70 ± 0.012 | 0.61 ± 0.01 | 0.59 ± 0.02 | 0.48 ± 0.02 | 0.63 ± 0.01 | 0.55 ± 0.03 | 0.45 ± 0.03 |
Data are expressed as mean ± SEM, n = 5, *p < 0.05, p < 0.01, ***p < 0.001 as compared with normal control. *indicates increase levels while ^indicate decreased levels
Table 7.
Effect of MoS2 on liver, renal function tests and lipid profile in sub-acute toxicity studies at 3 mg/kg and 10 mg/kg dose levels
| Male | Control group | Female | |||||
|---|---|---|---|---|---|---|---|
| LFTs | Units | Control group | MoS2 NF | MoS2 NF | |||
| 3 mg/kg | 10 mg/kg | 3 mg/kg | 10 mg/kg | ||||
| ALT | U/L | 39.33 ± 2.60 | 86.0 ± 3.4*** | 56.0 ± 3.2* | 35.3 ± 1.76 | 88.0 ± 3.4*** | 58.33 ± 3.48** |
| ALP | U/L | 113.6 ± 8.9 | 183.3 ± 5.54** | 216.3 ± 10.4*** | 107.33 ± 2.9 | 203.0 ± 10.4** | 414.0 ± 16.0*** |
| AST | U/L | 38.3 ± 2.3 | 147.3 ± 7.8*** | 106.3 ± 4.6*** | 31.33 ± 1.76 | 135.33 ± 4.91*** | 136.0 ± 5.68*** |
| RFTs | |||||||
| Creatinine | mg/dL | 0.53 ± 0.06 | 0.60 ± 0.05 | 0.68 ± 0.04 | 0.76 ± 0.03 | 0.61 ± 0.04 | 0.53 ± 0.03^^ |
| Urea | mg/dL | 48.0 ± 1.52 | 43.3 ± 2.60 | 53.33 ± 1.76 | 42.66 ± 1.45 | 56.0 ± 2.08** | 51.0 ± 2.30* |
| Lipid Profile | |||||||
| Cholesterol | mg/dL | 98.3 ± 2.3 | 116.0 ± 3.05** | 90.6 ± 2.9 | 105.3 ± 1.7 | 93.6 ± 3.5 ^ | 72.3 ± 1.76^^^ |
| Triglycerides | mg/dL | 80.66 ± 2.96 | 94.6 ± 2.9* | 97.0 ± 3.05 * | 88.6 ± 2.02 | 114.3 ± 3.2 ** | 104.0 ± 3.60* |
| HDL | mg/dL | 48.3 ± 1.45 | 50.0 ± 2.08 | 49.0 ± 1.73 | 45.6 ± 1.4 | 5.1.3 ± 1.76 | 53.3 ± 2.3* |
| LDL | mg/dL | 48.6 ± 2.02 | 46.0 ± 2.8 | 49.6 ± 2.02 | 63.6 ± 2.02 | 28.6 ± 2.02^^^ | 81.0 ± 2.6** |
| VLDL | mg/dL | 19.0 ± 1.15 | 17.3 ± 0.8 | 20.0 ± 1.15 | 24.0 ± 1.15 | 20.6 ± 0.8 | 23.6 ± 1.45 |
Data are expressed as mean ± SEM, n = 5, *p < 0.05, **p < 0.01, ***p < 0.001 as compared with normal control. *indicates increase levels while ^indicate decreased levels
Table 8.
Effect of MoS2 NF on sperm morphology in sub-acute toxicity studies
| Parameters | Sperm defects | |||||
|---|---|---|---|---|---|---|
| Headless (%) | Hookless (%) | Curved tail (%) | Bend (%) | Coil tail (%) | Normal (%) | |
| CMC | 9 | 1.0 | 8 | 6.2 | 5.3 | 70.5 |
| MoS2 NF (3 mg/kg) | 13* | 1.3 | 12.2 | 8.3* | 8.0 | 57.2^^^ |
| MoS2 NF (10 mg/kg) | 20*** | 1.5* | 14.5** | 10.56*** | 12.0** | 41.44^^^ |
Data are expressed as mean ± SEM, n = 5, *p < 0.05, **p < 0.01, ***p < 0.001 as compared to control. *indicates increase levels while ^indicate decreased levels
Table 9.
Effect of MoS2 NF on liver, renal functions, lipid profile, crown to rump length, placental and fetal weight of animals in developmental toxicity studies
| LFTs | Units | Control group (CMC) | MoS2NF (10 mg/kg) |
|---|---|---|---|
| Bilirubin | mg/dL | 0.750 ± 0.076 | 0.533 ± 0.145 |
| ALT | U/L | 41.67 ± 2.02 | 106.7 ± 7.83** |
| AST | U/L | 35.33 ± 4.33 | 189.3 ± 8.25*** |
| ALP | U/L | 142.3 ± 3.38 | 115.0 ± 11.55 |
| Globulins | g/dL | 3.55 ± 0.24 | 2.93 ± 0.24 |
| RFTs | |||
| Urea | mg/dL | 43.67 ± 2.96 | 45.33 ± 5.23 |
| Creatinine | mg/dL | 0.873 ± 0.063 | 0.626 ± 0.093 |
| Lipid Profile | |||
| Cholesterol | mg/dL | 111.0 ± 3.0 | 118.0 ± 3.0 |
| Triglycerides | mg/dL | 93.0 ± 3.00 | 106.0 ± 4.00 |
| HDL | mg/dL | 47.00 ± 2.00 | 48.50 ± 2.50 |
| LDL | mg/dL | 61.00 ± 2.00 | 53.50 ± 2.50 |
| VLDL | mg/dL | 22.00 ± 2.00 | 24.50 ± 1.50 |
| Crown rump length | cm | 4.760 ± 0.242 | 6.060 ± 0.06782*** |
| Placental weight variation | g | 0.7240 ± 0.1256 | 0.6480 ± 0.04510 |
| Fetal weight variation | g | 2.296 ± 0.136 | 5.286 ± 0.2685*** |
Data are expressed as mean ± SEM, n = 5, **P < 0.01, ***P < 0.001 as compared to normal control. *indicates increase levels while ^indicate decreased levels
Table 6.
Effect of MoS2 NF on lipid profile of different organs at logarithmic doses in acute toxicity studies
| Parameters | Unit | Normal control | MoS2 NF | |||||
|---|---|---|---|---|---|---|---|---|
| group | 1.75 mg/kg | 5.5 mg/kg | 17.5 mg/kg | 55 mg/kg | 175 mg/kg | 550 mg/kg | ||
| Cholesterol | mg/dL | 144.66 ± 1.45 | 90.33 ± 2.72^^^ | 67.66 ± 3.38^^^ | 72.00 ± 3.60^^^ | 74.00 ± 3.05^^^ | 77.33 ± 3.28^^^ | 60.66 ± 2.96^^^ |
| Triglycerides | mg/dL | 135.0 ± 1.73 | 108.66 ± 1.45 | 90.0 ± 2.64^^^ | 103.66 ± 2.84^^^ | 107.0 ± 4.58^^^ | 98.0 ± 3.21^^^ | 88.0 ± 3.0^^^ |
| LDL | mg/dL | 75.33 ± 1.45 | 25.33 ± 1.76^^^ | 34.0 ± 2.64^^^ | 33.33 ± 1.76^^^ | 44.33 ± 1.76^^^ | 25.33 ± 2.33^^^ | 24.66 ± 1.45^^^ |
| HDL | mg/dL | 35.33 ± 2.02 | 50.0 ± 2.0*** | 48.66 ± 1.45*** | 50.0 ± 2.00*** | 48.0 ± 2.00** | 46.5 ± 1.50** | 52.0 ± 1.15*** |
| VLDL | mg/dL | 18.33 ± 0.88 | 20.33 ± 0.88 | 18.0 ± 1.15 | 18.66 ± 0.88 | 21.0 ± 1.15 | 20.0 ± 1.15 | 17.33 ± 0.88 |
Data are expressed as mean ± SEM, n = 5, *P < 0.05, **P < 0.01, ***P < 0.001 as compared with normal control. *indicates increase levels while ^indicate decreased levels
Effect of sub-acute toxicity study on sperm count, motility, morphology and DNA integrity of sperms
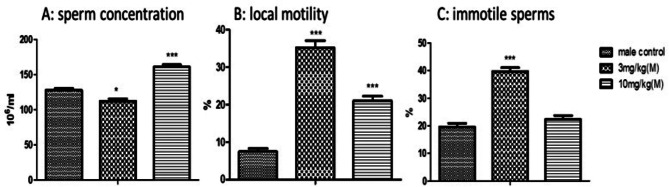
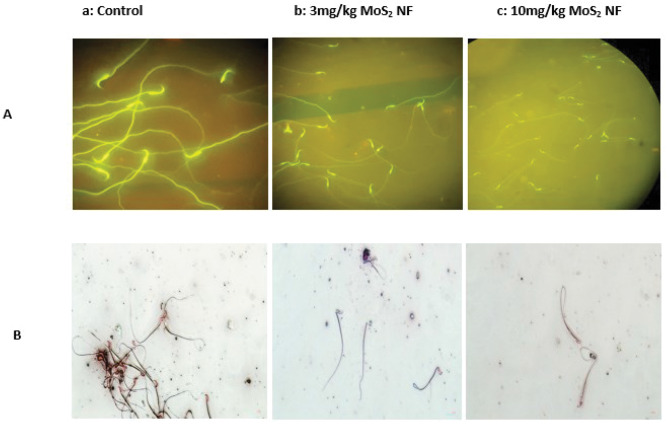
Sperm concentration expressed an unexpected increase (p < 0.001) at a dose of 10 mg/kg. Local motility of sperms appeared to rise (p < 0.001) significantly at both doses but more at 3 mg/kg. Immotile sperms were notably increased (p < 0.001) at 3 mg/kg dose of MoS2 NF while 10 mg/kg dose group of MoS2 NF was parallel to that of control group (Fig. 10). DNA integrity was not affected by the treatment (Fig. 11) while the sperms morphology was greatly affected (Fig. 11 and Table 8).
Fig. 10.
Effect of treatment on sperm Motility and Concentration in Sub-acute toxicity. ***p < 0.001 and *p < 0.05 in comparison to control group
Fig. 11.
Effect of treatment on sperm’s DNA integrity (A) and morphology (B) in Sub-Acute toxicity study
Measurement of teratogenic parameters after MoS2 NF treatment
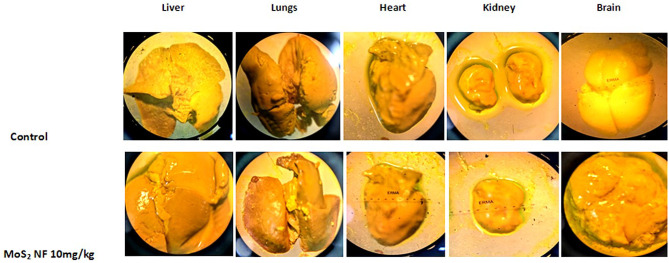
soft tissue examination showed normal growth of organs as compared to the control group (Fig. 12). The crown to rump length was found to be significantly increased (p < 0.001) when compared to normal control (Fig. 1 in supplementary data and Table 9). Weight of fetus was significantly increased while that of placental weight remained parallel to control (Table 9)
Fig. 12.
Effect of MoS2 NF on pubs’ organs in developmental toxicity study
Effect of MoS2 NF on histopathology of different organs in acute, sub-acute and developmental toxicity studies
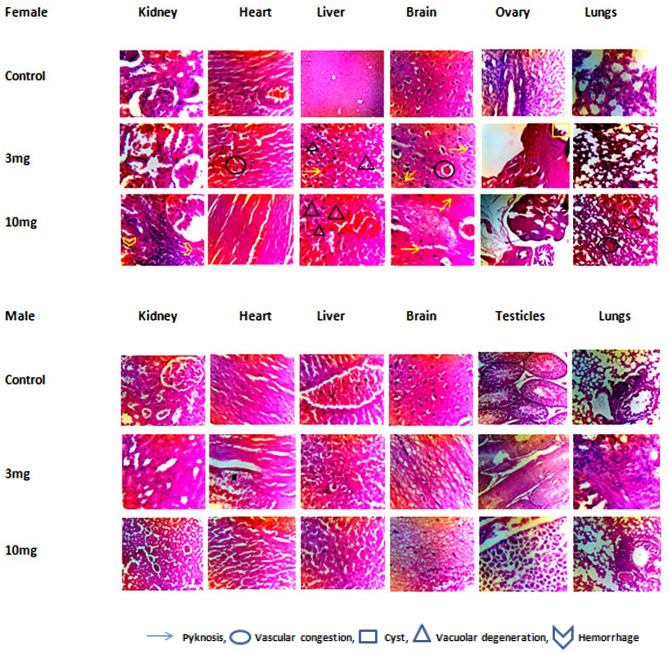
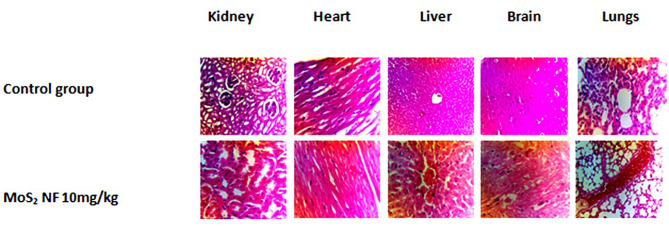
Histopathology showed vacuolar degeneration in the brain in acute, developmental and sub acute study except in female at 3 mg/kg dose of subacute toxicity. Degeneration of cardiomyocyte in heart in all toxicity studies conducted. Degeneration in different regions of kidney and dilation of Bowman’s capsule as well at all doses given in acute and sub-acute study (male group). Pyknosis and vascular congestion were also reported in the kidney at 10 mg/kg dose in the female treatment group in the subacute toxicity study. In lungs, Vascular congestion was reported at the highest dose of 550 mg/kg and hemorrhage at 175 mg/kg dose. Histopathology of the liver showed hyaline degeneration in parenchyma cells in acute toxicity at a dose of 1.75 mg/kg and degenerative changes in the sinusoidal space at 5.5 mg/kg dose. Hepatocyte degeneration at dose 17.5 mg/kg, vascular congestion at 55 mg/kg dose level was seen in the histopathological analysis of the liver. Swelling of hepatocytes was observed at 175 mg/kg in acute toxicity and at high dose (10 mg/kg) of sub-acute in female treatment group. Degenerative changes, pyknosis and necrosis were caused by the highest dose of 550 mg/kg in acute toxicity. Degenerative changes were also reported in sub-acute toxicity study (Fig. 13). Swelling of hepatocytes was reported in developmental toxicity (Fig. 14) and sub-acute female 10 mg/kg group. Low doses of 1.75 and 5.5 mg/kg showed degeneration in ovarian parenchyma. Cysts and numerous immature follicle structures were formed in the Ovaries in acute at doses of 17.5 and 55 mg/kg (Fig. 15) and sub-acute toxicity study (Fig. 13).
Fig. 13.
Histopathological analysis of male and female rats’ organs in sub-acute toxicity
Fig. 14.
Represents histopathological changes of the maternal organs in developmental toxicity
Fig. 15.
Histopathology of different organs in acute toxicity study
Discussion
Prior to clinical trials, toxicity assessment is crucial in the development and screening of newly developed drugs. The primary goal of the toxicity study of any compound is to make sure that the exposed population is secure from the toxic reactions of that compound [22]. International regulatory authorities for toxicity research (GSH, EPA, OECD, EEC etc.) can discover any possible hazards and risks of the substances on people by assessing the dose-dependent toxic outcomes that manifest in animal models [23].
In acute oral toxicity studies, a single drug dosage is used to determine lethal dose (LD50). In this study, main test was conducted using logarithmic doses of 1.75 mg/kg to 550 mg/kg of MoS2 Nano flowers following the OECD guidelines. This was done because mostly toxicities studies are conducted at high doses, but humans have a less probability of getting large doses so this study was aimed at checking the toxicity at low doses of metal NF [24]. Both the acute and sub-acute toxicity investigation showed no evidence of mortality at any dose.
Body weights were significantly decreased during the 14-day study duration of acute toxicity studies and were comparable to control in sub-acute toxicity studies, while in developmental toxicity studies fetal weights were increased and maternal weights were comparable to normal control. Organ weights were increased in acute toxicity studies and sub-acute male treatment group. This increase and decrease in body weights might be an indication of adverse drug reaction or it might be due to improved or healthy diet [11, 25].
Changes in liver and renal function parameters might be associated with their toxicity, as they are the major sites for metabolism and elimination of substances. Increase in ALT and AST levels may be due to liver injury that makes the membrane more permeable and causes the release of aminotransferases in the blood [26–28]. In the current research, ALT, AST, globulins and albumin levels were increased in all the three types acute, sub-acute and developmental toxicity studies. Previous studies revealed that increase in ALT, AST levels are indicative of hepatocellular damage, so MoS2 NF might be hepatotoxic [25, 29, 30]
Renal function remains comparable to normal control in all study groups except in the female treatment groups of sub-acute toxicity studies where blood urea level was increased at both dose levels which in accordance to the previous studies are due to renal damage because most of the drugs are excreted by kidney [31]
Lipid profile of the acute oral toxicity study exhibited a remarkable decrease that indicates MoS2 might be hypolipidemic [31]. Lipid profile assessment of sub-acute toxicity studies showed increased levels of triglycerides in both sex groups at all dose levels, which in accordance with previous studies might be a cause of cardiovascular disease [32]. Cholesterol level was increased in 3 mg/kg MoS2 NF group while decrease levels were observed in all female treatment groups. Variation in cholesterol levels are related to hypo and hyperlipidemia. In the male treatment group, LDL was decreased at low dose while increase at high dose of 10 mg/kg MoS2 NF with a notable increase in HDL level at the same dosage [31]. In developmental toxicity studies, lipid profile remains intact.
Hematological assay of the acute toxicity study showed increased levels of hemoglobin, RBCs WBCs and neutrophils at high logarithmic doses while the lymphocyte count was decreased at the high dose level. This rise might be an immune-protective effect in response to the toxic outcomes of the medicine [33]. Hematological analysis of sub-acute toxicity assessment showed an increase in RBC in female 10 mg/kg MoS2 NF treatment group which might be due to low oxygen level in the blood circulation which in turn stimulate erythropoietin secretion in the kidney [32]. Neutrophils were decreased in the sub-acute and developmental toxicity investigation while lymphocytes were increased in sub-acute female 3 mg/kg MoS2 NF treatment group and developmental toxicity and decreased in all male treatment groups. Platelet count was increased in male treatment group of sub-acute toxicity studies and developmental toxicity studies. According to previous toxicities studies, this increase or decrease might be due to immune response [11].
During toxicity, ROS are responsible for creating oxidative stress pathways. They are usually responsible for cell signaling and homeostasis [34]. Disease state, toxins and radiation exposure are the factors that lead to an abnormal elevation in ROS [16]. Prolonged exposure or production of reactive oxygen species in organisms may result in altered oxidative balance, which can harm lipids, proteins and nucleic acid [35]. It can cause apoptosis, necrosis and irreparable cell damage to the affected cell. A number of defense mechanism pathways are then activated to protect against these reactive oxygen species [11]. Antioxidant enzymes, including SOD, CAT, and GSH, are involved in this defense mechanism.
In the acute toxicity studies CAT, SOD and GSH showed decrease levels in all the selected organs except brain at almost all doses that might indicate antioxidant property of MoS2 NF.
MDA levels showed increased activity except Brain. This increase might indicate potential oxidative stress and harm to tissues [11]
In sub-acute toxicity research SOD, CAT, GSH and MDA were measured in different tissues to check for any change or cell damage caused by MoS2 NF. CAT, SOD and GSH levels were significantly decreased at all dose levels in both male and female treatment groups while MDA levels were increased. In teratogenicity study all the oxidative markers were decreased in fetus except MDA which was increased which might show the toxic potential of the drug.
DNA integrity was analyzed in male treatment groups of sub-acute toxicity as it is the major indicator of fertility and is strongly linked to mobility of sperm; a parameter for sperm functioning. In the ongoing research, DNA integrity of the sperms was normal, indicating that Blood-testis barrier is not affected by the drug [13]. Sperm count was increased at a high dose of 10 mg/kg and was decreased at a low dose. Sperm motility was increased at both dose levels. Immotile sperms increased at low dose while comparable to control at high dose. Histopathology of different organs in all the three toxicity studies performed was altered by drug treatment.
Conclusion
This study provide a comprehensive toxilogical assessment of the (MoS₂ NFs) through acute, sub-acute, and developmental toxicity studies in Wistar rats, following OECD guidelines. The finding demonstrates that MoS2 NF exhibit mild-to-moderate level toxicity, characterized by hepatic and renal dysfunction, oxidative stress induction, hematological alterations, and histopathological changes in vital organs. The observed toxicities are likely mediated by altered liver enzymes, hematological disturbances, oxidative stress and histopathological changes, including cellular degeneration and inflammatory responses. Although MoS₂ NFs did not cause mortality at the tested doses, the histopathological changes, alterations in biochemical markers, and oxidative stress induction suggest potential risks associated with prolonged exposure.
However, further studies are essential to ensure the safe and effective utilization of MoS₂ NFs in therapeutic applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not Applicable
Abbreviations
- ALT
Alkaline transaminase
- ALP
Alkaline phosphatase
- AST
Aspartate Transaminase
- ANOVA
Analysis of Variance
- CAT
Catalase
- CBC
Complete blood count
- CMC
Carboxymethyl cellulose
- DNA
Deoxyribonucleic Acid
- EDTA
Ethylene diamine tetra acetic acid
- GSH
Glutathione
- HDL
High density lipoprotein
- LD
Lethal dose
- LDL
Low density lipoprotein
- VLDL
Very low density lipoprotein
- LFTs
Liver function test
- MoS₂ NF
Molybdenum disulfide Nanoflowers
- MDA
Malonaldehyde
- OECD
Organization for Economic Co-operation and Development
- RFTs
Renal function test
- RBCs
Red blood cells
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- SEM
Standard error of Mean
- WBCs
White blood cells
Author contributions
FH carried out experimental work and prepared the first draft of the manuscript. AK designed and supervised the project, prepared and revised the manuscript, and conducted data analysis. FA Designed and supervised the research project; helped in experimental work and data analysis. HU Synthesized, characterized and provided the MoS2NF. MFA and MIK helped in the revision of the manuscript, data analysis, and critical revision of the drafted manuscript. All authors approved the final version of the manuscript.
Funding
No funding is provided for this study.
Data availability
Data is provided within the manuscript or supplementary information files.
Declaration
Ethical approval
All the procedures and techniques used in this study were approved by the Ethical committee (REC/RIPS/LHR/2023/068) of Riphah International University, Raiwind Campus, Lahore. Pakistan.
Consent to participate and consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hulla J, Sahu S, Hayes A. Nanotechnology: history and future. Hum Exp Toxicol. 2015;34(12):1318–21. [DOI] [PubMed] [Google Scholar]
- 2.Nikzamir M, Akbarzadeh A, Panahi Y. Technology. An overview on nanoparticles used in biomedicine and their cytotoxicity. 2021;61:102316.
- 3.Odularu AT, Ajibade PA, Mbese JZ. Impact of molybdenum compounds as anticancer agents. Bioinorg Chem Appl. 2019;2019. [DOI] [PMC free article] [PubMed]
- 4.Barceloux DG, Barceloux D. Molybdenum. J Toxicol Clin Toxicol. 1999;37(2):231–37. [DOI] [PubMed] [Google Scholar]
- 5.Benavente E, Santa Ana M, Mendizábal F, González G. Intercalation chemistry of molybdenum disulfide. Coord Chem Rev. 2002;224(1–2):87–109. [Google Scholar]
- 6.Vilian A, Dinesh B, Kang S-M, Krishnan UM, Huh YS, Han Y-K. Recent advances in molybdenum disulfide-based electrode materials for electroanalytical applications. Mikrochim Acta. 2019;186(3):1–29. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Guiney LM, Downing JR, Wang X, Chang CH, Jiang J, et al. Dissolution of 2D Molybdenum disulfide generates differential toxicity among liver cell types compared to Non-Toxic 2D Boron Nitride effects. Small 2021;17:2101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roheel A, Khan A, Anwar F, Ullah H, Rehman AU, Ullah N, et al. Evaluation of anti-tumor activity of molybdenum disulfide nanoflowers per se and in combination with berberine against mammary gland cancer in rats. J Nanopart Res 2024;26(10):1–24. 10.1007/s11051-024-06153-6. https://link.springer.com/article/10.1007/s11051-024-06153-6 [Google Scholar]
- 9.Guideline OO. 425: acute oral toxicity—up-and-down procedure. OECD Guidelines for the Test Chem. 2001;2:12–16. [Google Scholar]
- 10.Anwar F, Saleem U, Ahmad B, Ismail T, Mirza MU, Ahmad S. Acute oral, subacute, and developmental toxicity profiling of naphthalene 2-yl, 2-chloro, 5-nitrobenzoate: assessment based on stress response, toxicity, and adverse outcome pathways. Front Pharmacol. 2022;12:3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anwar F, Saleem U, Rehman A-U, Ahmad B, Froeyen M, Mirza MU, et al. Toxicity evaluation of the naphthalen-2-yl 3, 5-dinitrobenzoate: a drug candidate for alzheimer disease. Front Pharmacol. 2021;12:607026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunimatsu T, Yamada T, Miyata K, Yabushita S, Seki T, Okuno Y, et al. Evaluation for reliability and feasibility of the draft protocol for the enhanced rat 28-day subacute study (OECD Guideline 407) using androgen antagonist flutamide. Toxicology 2004;200:77–89. [DOI] [PubMed] [Google Scholar]
- 13.Saleem U, Zubair S, Riaz A, Anwar F, Ahmad B. Effect of venlafaxine, pramipexole, and valsartan on spermatogenesis in male rats. ACS Omega. 2020;5(32):20481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anwar F, Saleem U, Rehman AU, Ahmad B, Ismail T, Mirza MU, et al. Toxicological screening of 4-Phenyl-3, 4-dihydrobenzo [h] Quinolin-2 (1 H)-one: a new potential candidate for Alzheimer’s treatment. ACS Omega 2021;6:10897–909. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol. 2015;29:104–10. [DOI] [PubMed] [Google Scholar]
- 16.Anwar F, Saleem U, Ahmad B, Ismail T, Mirza MU, Ahmad S. Acute oral, subacute, and developmental toxicity profiling of naphthalene 2-yl, 2-chloro, 5-nitrobenzoate: assessment based on stress response, toxicity, and adverse outcome pathways. Front Pharmacol. 2022;12:810704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleem U, Ahmad B, Ahmad M, Hussain K, Bukhari NI. Investigation of in vivo antioxidant activity of Euphorbia helioscopia latex and leaves methanol extract: a target against oxidative stress induced toxicity. Asian Pac J Trop Med. 2014;7:S369–S75. [DOI] [PubMed] [Google Scholar]
- 18.Parambi DGT, Saleem U, Shah MA, Anwar F, Ahmad B, Manzar A, et al. Exploring the therapeutic potentials of highly selective oxygenated chalcone based MAO-B inhibitors in a haloperidol-induced murine model of Parkinson’s disease. Neurochem Res. 2020;45:2786–99. [DOI] [PubMed] [Google Scholar]
- 19.Mir NT, Saleem U, Anwar F, Ahmad B, Ullah I, Hira S, et al. Lawsonia Inermis markedly improves cognitive functions in animal models and modulate oxidative stress markers in the brain. Medicina 2019;55:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhangale JO, Acharya SR. Anti-Parkinson activity of petroleum ether extract of Ficus religiosa (L.) leaves. Adv Pharmacol Pharma Sci. 2016;2016. [DOI] [PMC free article] [PubMed]
- 21.Saleem S, Anwar F, Khan A, Saleem U, Akhtar MF, Shahzadi I, et al. Toxicity profiling of Burgmansia aurea Lagerh. Leaves using acute and sub-acute toxicity studies in rats. J Ethnopharmacol. 2023;311:116447. [DOI] [PubMed] [Google Scholar]
- 22.Morton DM. Importance of species selection in drug toxicity testing. Toxicol Lett. 1998;102:545–50. [DOI] [PubMed] [Google Scholar]
- 23.Lewis RW, Billington R, Debryune E, Gamer A, Lang B, Carpanini F. Recognition of adverse and nonadverse effects in toxicity studies. Toxicol Pathol. 2002;30(1):66–74. [DOI] [PubMed] [Google Scholar]
- 24.Kaur J, Khatri M, Puri S. Toxicological evaluation of metal oxide nanoparticles and mixed exposures at low doses using zebra fish and THP1 cell line. Environ Toxicol. 2019;34(4):375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mounnissamy VM, Kavimani S, Sankari G, Quine SD, Subramani K. Evaluation of acute and sub-acute toxicity of ethanol extracts of Cansjera rheedii J. Gmelin (Opiliaceae). J Brew Distilling. 2010;1(1):011–4. [Google Scholar]
- 26.Ain QU, Saleem U, Ahmad B, Khalid I. Pharmacological screening of silibinin for antischizophrenic activity along with its acute toxicity evaluation in experimental animals. Front Pharmacol. 2023;14:1111915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogunlana OO, Ogunlana OE, Adeneye AA, Udo-Chijioke O, Dare-Olipede T, Olagunju JA, et al. Evaluation of the toxicological profile of the leaves and young twigs of Caesalpinia bonduc (Linn) roxb. Afr J Traditional, Complementary Altern Med 2013;10:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasarala G, Tillmann HL. Standard liver tests. Clin Liver Dis. 2016;8(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olaniyan JM, Muhammad HL, Makun HA, Busari MB, Abdullah AS. Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J Acute Dis. 2016;5(1):62–70. [Google Scholar]
- 30.Khan SM, Kour G. Subacute oral toxicity of chlorpyriphos and protective effect of green tea extract. Pesticide Biochem Physiol. 2007;89(2):118–23. [Google Scholar]
- 31.Hosseinzadeh H, Shakib SS, Sameni AK, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12(1):93. [PMC free article] [PubMed] [Google Scholar]
- 32.Magaye RR, Yue X, Zou B, Shi H, Yu H, Liu K, et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomed. 2014;1393–402. [DOI] [PMC free article] [PubMed]
- 33.Mythilypriya R, Shanthi P, Sachdanandam P. Oral acute and subacute toxicity studies with Kalpaamruthaa, a modified indigenous preparation, on rats. J Health Sci. 2007;53(4):351–58. [Google Scholar]
- 34.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.