Abstract
Purpose:
The contributions from the central auditory and cognitive systems play a major role in communication. Understanding the relationship between auditory and cognitive abilities has implications for auditory rehabilitation for clinical patients. The purpose of this systematic review is to address the question, “In adults, what is the relationship between central auditory processing abilities and cognitive abilities?”
Method:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed to identify, screen, and determine eligibility for articles that addressed the research question of interest. Medical librarians and subject matter experts assisted in search strategy, keyword review, and structuring the systematic review process. To be included, articles needed to have an auditory measure (either behavioral or electrophysiologic), a cognitive measure that assessed individual ability, and the measures needed to be compared to one another.
Results:
Following two rounds of identification and screening, 126 articles were included for full analysis. Central auditory processing (CAP) measures were grouped into categories (behavioral: speech in noise, altered speech, temporal processing, binaural processing; electrophysiologic: mismatch negativity, P50, N200, P200, and P300). The most common CAP measures were sentence recognition in speech-shaped noise and the P300. Cognitive abilities were grouped into constructs, and the most common construct was working memory. The findings were mixed, encompassing both significant and nonsignificant relationships; therefore, the results do not conclusively establish a direct link between CAP and cognitive abilities. Nonetheless, several consistent relationships emerged across different domains. Distorted or noisy speech was related to working memory or processing speed. Auditory temporal order tasks showed significant relationships with working memory, fluid intelligence, or multidomain cognitive measures. For electrophysiology, relationships were observed between some cortical evoked potentials and working memory or executive/inhibitory processes. Significant results were consistent with the hypothesis that assessments of CAP and cognitive processing would be positively correlated.
Conclusions:
Results from this systematic review summarize relationships between CAP and cognitive processing, but also underscore the complexity of these constructs, the importance of study design, and the need to select an appropriate measure. The relationship between auditory and cognitive abilities is complex but can provide informative context when creating clinical management plans. This review supports a need to develop guidelines and training for audiologists who wish to consider individual central auditory and cognitive abilities in patient care.
Supplemental Material:
Communication under adverse listening conditions—such as with unclear talkers, in background noise, or in reverberant acoustic environments—is a frequent complaint expressed to clinical audiologists. In some cases, the patient's listening difficulty may be due to poor signal audibility from peripheral hearing loss or energetic masking of the target by background noise. However, clinicians and researchers also recognize the role of higher level abilities, specifically contributions from the central auditory and cognitive systems. Central auditory processing (CAP) refers to the perceptual processing of information by the neural centers and transmission pathways between the cochlea and the primary auditory cortex (American Speech-Language-Hearing Association [ASHA], n.d.). Cognitive processing refers to processing of information at the level of the cortex such as memory, speed, and language. Together with peripheral processing, these functions allow a listener to hear and attend to a signal, extract relevant information, and use that information to determine meaning, reflect, and respond.
CAP skills relevant to communication include discriminating and recognizing patterns, integrating and discriminating temporal information, dichotic processing of competing or concurrent stimuli, and localizing or lateralizing sound. Figure 1 provides a schematic of CAP categories (information from Chermak & Musiek, 1997) that identifies six primary categories (temporal, dichotic, low redundancy, binaural interaction, discrimination, and electrophysiology) for evaluation with examples of tests for each category. The categories are all connected in that they represent CAP abilities, but they do not necessarily depend on or relate to one another. Common tests include pitch pattern sequencing, temporal ordering, gaps in noise, simple dichotic speech tests using single words or spoken digits, and low-redundancy speech tests. Chermak and Musiek recommend testing between three and five tests in a CAP battery, with no more than one test per category.
Figure 1.
Central auditory processing schematic defining the six main categories used to evaluate auditory processing abilities.
Electrophysiologic measures at the level of the auditory brainstem and/or auditory cortex may also be used. The electrophysiologic measures proposed to directly assess central auditory function include the auditory brainstem response (ABR), middle latency response (MLR), late cortical responses, and mismatch negativity (MMN). Electrophysiologic measures have been recommended to assess for neurological disorder, or when the patient cannot reliably complete behavioral testing. While electrophysiology measures are more common in research studies than clinical assessment (Chermak & Musiek, 2011; Emanuel et al., 2011; Tremblay & Kraus, 2002), their lack of bias and sensitivity to individual differences justifies their inclusion in this systematic review.
Although auditory processing is not analogous with general speech recognition, individual differences in auditory processing have consequences for communication especially in environments with distorted, complex, or rapidly varying signals. Listeners with poor CAP may have difficulty localizing sounds, distinguishing between target and background signals, or comprehending rapid speech. Central auditory processing disorder (CAPD) refers to performance that is lower than normal in one or more auditory processing domains, as defined by test scores. Much of the literature on CAPD is focused on children and motivated by educational achievement or learning differences. CAPD can also occur in adults, either persisting from early life or associated with a specific etiology such as traumatic brain injury (Fausti et al., 2009; Gallun et al., 2012; Hoover et al., 2017), stroke (Bamiou et al., 2012), or exposure to neurotoxins (Fuente & McPherson, 2007; Guthrie et al., 2014). In general, listeners with poor auditory processing may not be able to extract as much information from the auditory signal as listeners with better auditory processing. CAPD in adults is associated with reduced hearing aid benefit and/or satisfaction (Davidson et al., 2021; Givens et al., 1998; Humes, 2004; Lopez-Poveda et al., 2017) and with increased risk of dementia (Gates et al., 2011). Therefore, understanding how auditory processing contributes to listeners' communication abilities in different scenarios may assist clinicians in making differential diagnoses and recommending appropriate auditory rehabilitation.
A large body of work also recognizes the role of cognitive abilities in communication. Several well-established frameworks have described cognitive abilities as representing distinct but related domains (e.g., Baddeley, 2012; Cowan, 2017; Diamond, 2013; Salthouse, 2000). Although comprehensive descriptions of cognitive models and their theoretical frameworks are beyond the scope of this review, the following section summarizes cognitive abilities that have been shown to impact spoken communication and therefore may be relevant to clinical assessment and treatment.
Working memory refers to the simultaneous processing and short-term storage of incoming information (Baddeley, 1992, 2012). Listeners with higher working memory capacity have better recognition for speech in noise (Akeroyd, 2008; Rönnberg et al., 2010), speech in reverberant listening environments (Kjellberg, 2004; McCreery et al., 2019; Reinhart & Souza, 2016), and accented speech (Ingvalson et al., 2017; McLaughlin et al., 2018). Working memory also contributes to variance in the perception of some types of hearing aid–processed speech, particularly if the processing substantially modifies the acoustic cues (Arehart et al., 2013; Kates et al., 2013; Lunner & Sundewall-Thorén, 2007; Rallapalli et al., 2021). Some cognitive models (Pichora-Fuller et al., 2016; Rönnberg et al., 2013) theorize this occurs because working memory capacity is taxed when the listener cannot easily reconcile an altered or degraded acoustic signal with stored lexical representations. Other models (e.g., Cowan, 2008, 2010, 2017; Oberauer, 2002; Unsworth & Spillers, 2010) explicitly define the role of attention and theorize that working memory is essentially a limited-capacity, short-term storage system where attention (or other executive processes) direct and utilize stored information. For example, in Cowan's (2010) description, attention could either fill working memory with important, relevant items, or fill it with distracting, irrelevant items. Empirical data show that working memory declines with age, albeit with considerable variability (Wingfield et al., 1988). In general, the strongest empirical evidence linking working memory to speech perception is for older adults, particularly those who also have hearing loss (Füllgrabe & Rosen, 2016).
Because the ability to rapidly process information in the acoustic signal is expected to improve speech recognition, processing speed is often measured in cognitive hearing studies. Lexical processing speed (e.g., Burleson & Souza, 2022) may be more strongly related to speech recognition than processing speed measured via generic or visual tasks (e.g., Nagaraj, 2021; Nuesse et al., 2018). Executive or inhibitory functions are of interest to researchers who study speech in complex listening environments where the listener must focus on the target and inhibit distractors. Listeners with better executive or inhibitory functions may be able to communicate more effectively in noise than those with poorer executive functions (Moberly et al., 2018; Neher et al., 2011; Stenbäck et al., 2016; Tamati et al., 2013).
When a listener has good peripheral auditory sensitivity but poor measured or perceived listening ability, how should we view the relative contributions of CAP and cognitive abilities? Some guidelines describe CAPD and cognitive processing as distinct and independent domains (American Academy of Audiology, 2010; ASHA, n.d.). Other frameworks (Alles et al., 2011; Bellis & Bellis, 2015; Millett et al., 2012) suggest that one domain influences the other. A systematic review and meta-analysis aimed to address the association between cognitive ability and speech-in-noise performance and whether that association depended on the type of outcome measure (Dryden et al., 2017). Dryden and colleagues categorized speech in noise according to foreground and background signals and lexical complexity, and they categorized cognitive measures according to primary and subdomain measures. CAP measures were not reviewed. Overall, the results from the work of Dryden et al. found an association (r = ~.3) between speech in noise and cognitive performance, although the strength of the association depended on the specific category.
From a practical standpoint, understanding whether central auditory and cognitive processing abilities are related has implications for auditory rehabilitation. Audiologists have indicated interest in—but lack of knowledge to interpret—both CAP (Emanuel, 2002; Erickson, 2008) and cognitive processing (Raymond et al., 2020). Audiologists who see patients with speech-in-noise complaints that are not accounted for by pure-tone threshold elevation may refer those patients for auditory processing evaluation, cognitive follow-up, or neither, without knowledge of how the two systems interact (Koerner et al., 2020). While there are reviews of subsets of this information, particularly the relationships between cognitive abilities and speech in noise (e.g., Akeroyd, 2008; Dryden et al., 2017), a systematic review encompassing recent data for both central auditory and cognitive processing could inform clinical decisions, counseling, and future research. Accordingly, the main objective of this systematic review is to answer the question, “In adults, what is the relationship—if any—between central auditory processing abilities and cognitive abilities?”
Method
The CAP constructs described by Chermak and Musiek (1997; i.e., temporal, dichotic, low redundancy, binaural interaction, discrimination, and electrophysiology) were used in this systematic review. Some of these constructs were further divided, and additional categories of auditory processing were added (e.g., spatial and other) to more comprehensively represent the data reviewed.
Cognitive measures were separated into their construct domain or grouped by multidomain or global cognition. There were six construct domains used in this systematic review, grouped based on cognitive theories (Baddeley, 2012; Cowan, 2017; Diamond, 2013; Salthouse, 2000): working memory (e.g., digit span), processing speed (e.g., Trail-Making Test-A [TMT-A]), executive/inhibitory processes (e.g., Stroop), fluid intelligence (e.g., reasoning), visual perception (e.g., visuospatial processing), and multidomain or global cognitive measures (e.g., Montreal Cognitive Assessment [MoCA]).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed (Moher et al., 2009). Included in these guidelines is a 27-item checklist that was used to ensure a robust systematic review protocol. Following the PRISMA guidelines, four phases of data collection were included: (a) identification, (b) screening, (c) eligibility, and (d) inclusion.
Identification
Phase 1 of the PRISMA guidelines is to identify potentially relevant articles in the existing research literature. Four bibliographic databases were systematically searched to identify articles for this review: Scopus (Elsevier platform: 1970 to present), PubMed (MEDLINE platform: 1973 to present), Web of Science (Clarivate platform: 1995 to present), and PsycINFO (ProQuest platform: 1990 to present). In addition to subject matter experts, medical librarians at Northwestern assisted with the search strategy development and keyword review (e.g., MeSH terms). Two main ideas were represented in the search strategy—terminology related to “central auditory processing” and terminology related to “cognitive abilities.” The desired population for this review, adults aged 18 years or older, was also specified in the search strategy. The primary search strategy was conducted in PubMed:
(“Cognitive Dysfunction“[Mesh] OR “Cognition Disorders”[Mesh] OR “Mental Status and Dementia Tests”[Mesh] OR “Neuropsychological Tests”[Mesh]) OR (“mild cognitive impairment” OR “mci” OR “cognitive processing*” OR “mild cognitive dysfunction” OR “cognitive decline” OR “MoCA” OR “Mini mental state” OR “montreal cognitive assessment” OR “SLUMS” OR “MMSE” OR “GPCOG” OR “Alzheimer's*”) AND ((“Auditory Perception” [Mesh] OR “Auditory Perceptual Disorders”[Mesh] OR “Spatial Processing”[Mesh] OR “Hearing Loss, Central”[Mesh] OR “Dichotic Listening Tests”[Mesh] OR “Evoked Potentials, Auditory, Brain Stem”[Mesh] OR “Evoked Potentials, Auditory”[Mesh]) OR (“auditory processing*” OR “central auditory processing*” OR “binaural processing” OR “temporal processing” OR “listening effort” OR “dichotic listening*” OR “spatial release*” OR “masking level difference” OR “duration pattern test” OR “frequency pattern test” OR “speech in noise*” OR “frequency following response” OR “auditory electrophysiology” OR “middle latency response“)) AND ((“Aged”[Mesh] OR “Middle Aged”[Mesh] OR “Aged, 80 and over”[Mesh]) OR (“elderly” OR “adult“ OR “older”))
Other databases were searched with the same search terms converted to the search strategy syntax specific to that platform. Gathered articles were managed using EndNote. The identification phase took place in December 2021. Duplicates were removed prior to Phase 2. A follow-up literature search was conducted in March 2023 to identify any additional articles published after the initial search and review.
Screening
Phase 2 of the PRISMA guidelines involves title and abstract screening of records identified from Phase 1. Identification and screening of articles also occurred by searching relevant papers' reference lists and citation titles using the SnowGlobe program (McWeeny et al., 2021) and from suggested references by subject matter experts. Snowglobe is a platform that reviews relevant articles on the topics of interest and searches the reference lists of these articles, as well as the studies that have cited them, to help identify more records. Records were eliminated at this phase if the title or abstract was not relevant to the topics of interest. The authors of this systematic review (A.D. and P.S.) blindly screened all records for relevance using the online platform, Rayyan (Ouzzani et al., 2016). Rayyan is a web-based tool that creates a repository of all uploaded records to be evaluated and rated blindly. Although not needed here, a third-party subject matter expert was available to resolve any disagreements for screening inclusion. That is, all conflicts of whether to include or exclude based on the title and abstract were resolved between the two authors. Once a consensus was made for all records, full-text articles were assessed for eligibility (Phase 3).
Eligibility
Phase 3 of the PRISMA guidelines is to determine eligibility. This was accomplished using the following criteria: (a) population: The study included human participants 18+ years old; (b) main ideas assessed: The study reported on a measure of CAP or auditory evoked potential and assessed behavioral cognitive abilities; and (c) study design/publication type: The study was experimental and published in a peer-reviewed journal. Other review studies, gray literature (e.g., theses, conference proceedings), and studies not reported in English were excluded.
Inclusion and Data Collection
Phase 4 of the PRISMA guidelines is inclusion and data collection based on the full text of the articles. The authors of the systematic review (A.D. and P.S.) blindly evaluated the screened records for inclusion and recorded eligibility information on a data extraction document. The data extraction document included the following: study characteristics (e.g., author, title, journal, year published, design), sample (e.g., number of participants, participant characteristics and demographics), auditory processing assessment (e.g., what measure was used, how it was scored, electrophysiology or behavioral), cognitive processing ability assessed (e.g., what measure was used, how it was scored), the relationship between the measures, the relevant statistics (e.g., p values, effect sizes), and study quality assessment (described below).
The hypothesis for this systematic review was that assessments of CAP and cognitive processing would be positively correlated with one another. That is, if CAP was impaired, cognitive processing would be poor as well. As such, if a significant correlation was determined in an article, it was notated with a plus sign (+) on the data extraction document. If no significant relationship was determined, a tilde (~) was used to indicate the null finding. Results from the data extraction document can be found in Appendix Table A1 and A2.
Study quality assessment was conducted to critically appraise the included studies and offer an explanation to some of the possible limitations. ASHA offers a level of evidence (LOE) system for appraising the quality of studies (Mullen, 2007). In the field of cognitive hearing science, it is crucial for an article to provide enough detail to be reproducible (individual-level replication) and have appropriately powered analyses for group-level reliability (Parsons et al., 2019). The eight quality indicators included in the LOE system were modified based on the idea that reporting basic psychometrics improves the reliability of the information (Parsons et al., 2019): (a) study design, (b) assessor blinding procedures, (c) sampling procedures, (d) subject information, (e) validity and reliability of outcomes, (f) reporting of significance, (g) reporting precision, and (h) intention to treat. Intention to treat was not included in the quality ratings because it pertains to clinical trial treatments only; instead, a category was added for statistical power. A “yes” or “no” system was implemented if an article met the quality indicator standards for each of the eight categories. The standards for a “yes” were as follows: (a) controlled trials and cohort studies, (b) assessors were blinded, (c) random sampling implemented, (d) enough information was provided about the participants to be reproducible. Because auditory abilities were a primary outcome for this review, hearing status was required to be reported to meet this quality criterion: (e) CAP and cognitive measures were valid and reliable. The outcome measures were valid and reliable if they were a standardized test with published normative ranges or a published validation study was provided for unique lab measures, (f) p value was reported, (g) effect size was reported, and (h) a power analysis was provided or the sample size was large enough to produce valid findings given the specific analysis performed. The number of “yes” responses for each article was summed, and the sum was used to classify study quality. A “yes” score of 0–2 was classified as weak, 3–5 as moderate, 6–7 as strong (modified from de Wit et al., 2016), and 8 as very strong. Quality rating was independently determined by each author (A.D. and P.S.) then compared for discrepancies. Again, a third party could be consulted as needed to settle any unresolved conflicts, but was not needed.
Results
Identification of Included Studies
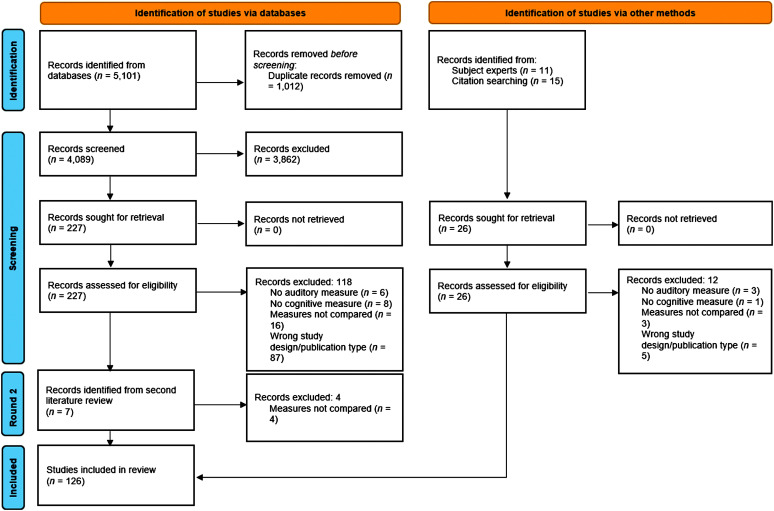
The PRISMA 2020 flow diagram for systematic reviews is shown in Figure 2. This diagram includes the identification, screening, and included stages from studies identified via databases as well as through other sources. A total of 5,101 records were identified from databases, and an additional 26 were added from subject experts and bibliography review. From the database review, 1,012 duplicates were removed, resulting in 4,089 articles screened by title and abstract. From the title and abstract review, 3,862 articles were excluded based on relevance to the topic of this systematic review. Then, 227 articles were blindly assessed by both authors (A.D. and P.S.) for eligibility through a full-text review. Strict inclusion and exclusion criteria were applied for the 227 from databases as well as for the 26 articles from other methods. During this process, 118 articles were removed from the database articles and 12 were removed from the other methods articles. Across identification methods, articles were removed based on the following exclusion criteria: no auditory measure (n = 9), no behavioral cognitive measure (n = 9), auditory and cognitive measures not compared (n = 19), and wrong study design or publication type (n = 92), resulting in 123 studies. The secondary literature review conducted resulted in seven additional studies for review. The same process was followed for inclusion, and four were excluded based on the auditory and cognitive measures not being compared. This resulted in a total of 126 articles included for analysis.
Figure 2.
Flow diagram following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for identifying, screening, and including articles.
Characteristics of Included Studies
The 126 articles spanned the time period 1988–2022 and included a total of 520,975 participants from 18 to 98 years old. The two study designs represented in this systematic review were cohort (n = 52) and cross-sectional (n = 74). The number of articles increased with each decade. Specifically, there were three articles between 1980 and 1990, six articles between 1991 and 2000, 28 articles between 2001 and 2010, 74 articles between 2011 and 2020, and 15 articles in just 2021 and 2022. Participants within the studies were evaluated across 24 countries: the United States (46), Sweden (13), Italy (seven), Brazil (seven), Japan (five), Germany (five), Denmark (five), China (five), the United Kingdom (four), Poland (four), the Netherlands (four), India (four), Australia (three), Spain (two), France (two), Finland (two), Turkey (one), Switzerland (one), Malaysia (one), Korea (one), Israel (one), Greece (one), Egypt (one), and Chile (one). The included 126 articles represented 62 unique peer-reviewed journals with the top five being: Ear and Hearing (15), International Journal of Audiology (nine), Journal of the American Academy of Audiology (seven), Journal of Speech, Language, and Hearing Research (six), and PLOS ONE (five).
The most common behavioral auditory processing measure utilized was sentence recognition in speech-shaped noise (26 times used for comparison). The most common electrophysiologic auditory measure was the latency of the P300 (50 times used for comparison). There were a large number of cognitive assessments included, so for the purpose of this review, we report the relationship to the six cognitive constructs (i.e., working memory, processing speed, executive/inhibitory processes, fluid intelligence, visual perception, and multidomain global cognitive measures) rather than to the assessment itself. Working memory included tasks or composite scores that evaluated verbal, auditory, short-term memory, and long-term memory; processing speed included tasks that evaluated information processing, lexical access speed, phonological processing, and verbal information processing; executive/inhibitory processes included executive functioning and inhibition tasks; fluid intelligence included tasks that evaluated inference making, abstract reasoning, nonverbal reasoning, intellectual ability, intellectual resources, and psychomotor function; visual perception included tasks that evaluated visual organization, visuospatial processing, and visual memory; and multidomain/global cognition included multidomain screeners and composites of multiple domains.
Tables 1 and 2 provide a breakdown of the number of relationships tested between central auditory and cognitive measures, with behavioral and electrophysiologic categories represented separately. The most common cognitive construct was working memory (223 times used for comparison for both behavioral and electrophysiologic studies). The total numbers of significant and nonsignificant relationships were tallied based on these groupings. Hearing status was reported inconsistently across the studies and, as such, was not considered in the analyses.
Table 1.
Count of significant and nonsignificant relationships between auditory behavioral constructs and cognitive measures, grouped by the cognitive construct.
| Construct | Unique studies | Behavioral auditory measure | No. of sig correlations | No. of nonsig correlations |
|---|---|---|---|---|
| Working memory | 58 | Aided-Sentences/Modulateda | 2 | 2 |
| Aided-Sentences/SS | — | 1 | ||
| Aided-Sentences/Steadya | 2 | 1 | ||
| ATTR | 2 | 2 | ||
| Beat Alignment Test | 2 | 1 | ||
| Composite AP | 1 | — | ||
| Composite SINa | 4 | — | ||
| Composite TP | — | 1 | ||
| Consonants/VCV-shaped | 1 | 2 | ||
| CRM | — | 1 | ||
| DDTa | — | 2 | ||
| DDT-LFa | 1 | 2 | ||
| DDT-RFa | 1 | 1 | ||
| Dichotic Sentences Ident-LF | — | 1 | ||
| Dichotic Speech-FRa | 2 | — | ||
| FPTa | 2 | — | ||
| Freq./Pitch Pattern Sequence | — | 2 | ||
| Gap Detection | 4 | — | ||
| Harmonic Mistuning | 1 | — | ||
| HINTa | 5 | 1 | ||
| LPFS | — | 2 | ||
| MBEA | 1 | 2 | ||
| MLDa | 1 | 1 | ||
| Modulation Detectiona | 4 | 1 | ||
| Phase Audiometry | 2 | — | ||
| QSINa | 7 | 7 | ||
| Seashore Rhythm Test | 1 | 2 | ||
| Sentences/1-talker | — | 1 | ||
| Sentences/2-talkera | 7 | 7 | ||
| Sentences/3-talkera | 2 | — | ||
| Sentences/4-talker | 1 | — | ||
| Sentences/Babblea | 4 | 1 | ||
| Sentences/Cafeteriaa | 2 | — | ||
| Sentences/Fluctuating | — | 1 | ||
| Sentences/Modulateda | 3 | 3 | ||
| Sentences/SSa | 4 | 6 | ||
| Sentences/Steady | 1 | — | ||
| Sentences/Unmodulateda | 2 | — | ||
| Sentences/Whitea | — | 2 | ||
| SPINa | — | 4 | ||
| SSI-CCM | — | 1 | ||
| SSI-ICM | — | 1 | ||
| Stream Segregation | — | 1 | ||
| TC Speecha | 8 | 5 | ||
| Temporal Envelope | — | 4 | ||
| Temporal Fine Structurea | 2 | — | ||
| Temporal Order Identificationa | 5 | 2 | ||
| Temporal Masking | 1 | — | ||
| Time Discrimination | 1 | — | ||
| Vocoded Speech | — | 1 | ||
| WINa | 2 | 4 | ||
| Words/Babble | — | 2 | ||
| Words/SSa | — | 2 | ||
| Words/White | — | 1 | ||
| Processing speed | 19 | Aided-Sentences/SS | 2 | 1 |
| ATTR | — | 1 | ||
| Auditory Processing Speed | 1 | — | ||
| Composite SIN | 2 | — | ||
| DDT-LFa | 1 | 4 | ||
| DDT-RF | — | 1 | ||
| Dichotic Sentences Ident-LF | 1 | — | ||
| Dichotic Sentences Ident-RF | — | 1 | ||
| Dichotic Speech-FRa | 5 | — | ||
| Gap Detection | 2 | — | ||
| ILD JND | 1 | — | ||
| IPD Freq. Limits | 1 | — | ||
| IPD JND | — | 1 | ||
| Phase Audiometry | 3 | — | ||
| Sentences/2-talker | 1 | — | ||
| Sentences/4 kHz | — | 1 | ||
| Sentences/16 kHz | — | 1 | ||
| Sentences/Babblea | 2 | 2 | ||
| Sentences/Cafeteria | 1 | — | ||
| Sentences/SSa | 3 | 3 | ||
| TC Speecha | 4 | 2 | ||
| Temporal Envelope | 1 | — | ||
| Temporal Order Identification | 2 | — | ||
| Time Discrimination | 1 | — | ||
| WIN | — | 1 | ||
| Words/Babble | 2 | — | ||
| Words/White | — | 1 | ||
| Executive/inhibitory processes | 19 | ATTR | 1 | 1 |
| Composite SINa | 1 | 1 | ||
| Consonants/VCV-shaped | 1 | — | ||
| DDTa | — | 3 | ||
| DDT-LF | 1 | — | ||
| DDT-RF | 2 | 1 | ||
| Dichotic Sentences Ident-RF | 1 | — | ||
| Dichotic Speech-FR | 2 | — | ||
| Dichotic Speech-RF | 1 | — | ||
| ILD JND | — | 2 | ||
| IPD Freq. Limits | 2 | — | ||
| IPD JND | 2 | — | ||
| LISN Low Cue | 1 | — | ||
| LISN Spatial Advantage | 1 | — | ||
| QSIN | 1 | — | ||
| Sentences/1-talker | 1 | 1 | ||
| Sentences/2-talkera | 2 | 2 | ||
| Sentences/Babble | — | 1 | ||
| Sentences/SSa | 2 | 1 | ||
| SPINa | 1 | 3 | ||
| SSI-CCM | — | 2 | ||
| SSI-ICM | — | 2 | ||
| TC Speech | 1 | 1 | ||
| Temporal Envelopea | 1 | 2 | ||
| Temporal Fine Structure | 1 | — | ||
| Time Discrimination | 1 | — | ||
| Vocoded Speech | 1 | — | ||
| WIN | — | 1 | ||
| Words/1-talker | 1 | — | ||
| Words/White | 1 | 1 | ||
| Fluid intelligence | 8 | Auditory Processing Speed | 1 | — |
| Composite SIN | — | 1 | ||
| DDT | — | 1 | ||
| Gap Detection | 1 | — | ||
| Phase Audiometry | 1 | — | ||
| TC Speech | 1 | — | ||
| Temporal Order Identificationa | 3 | 1 | ||
| Vocoded Speech | 1 | — | ||
| Visual perception | 8 | Auditory Processing Speed | 1 | — |
| DDTa | — | 2 | ||
| Gap Detection | 1 | — | ||
| LISN Spatial Advantage | 1 | — | ||
| QSIN | 1 | — | ||
| Sentences/SS | — | 1 | ||
| SPINa | — | 2 | ||
| SSI-CCM | — | 1 | ||
| SSI-ICM | — | 1 | ||
| WIN | 1 | — | ||
| Words/White | — | 1 | ||
| Multidomain/global cognition | 22 | ATTR | 1 | 1 |
| DDTa | 1 | 1 | ||
| Dichotic CV Syllables | 1 | — | ||
| Digits/Stationarya | 3 | — | ||
| Duration Discrimination | 1 | — | ||
| Gap Detection | 1 | — | ||
| GIN | — | 1 | ||
| HINTa | 3 | — | ||
| ILD JND | — | 1 | ||
| IPD Freq. Limits | — | 1 | ||
| IPD JND | — | 1 | ||
| LISN | — | 1 | ||
| QSINa | 1 | 1 | ||
| Sentences/Babble | 2 | — | ||
| Sentences/4-talker | 1 | — | ||
| Sentences/SSa | 3 | — | ||
| SSI-ICMa | 3 | 1 | ||
| TC Speech | 1 | — | ||
| Temporal Fine Structure | 1 | — | ||
| Temporal Masking | — | 1 | ||
| Temporal Order Identificationa | 2 | — | ||
| WIN | 1 | — | ||
| Words/White | — | 1 |
Note. Em dashes indicate no data. sig = significant; nonsig = nonsignificant; SS = Speech Shaped; ATTR = Adaptive Tests of Temporal Resolution; AP = Auditory Processing; SIN = Speech in Noise; TP = Temporal Processing; VCV = Vowel–Consonant–Vowel; CRM = Coordinate Response Measure; DDT = Dichotic Digits Test; LF = Left Ear Focused; RF = Right Ear Focused; FR = Free Recall; FPT = Frequency Pattern Test; Freq. = frequency; HINT = Hearing in Noise Test; LPFS = Low Pass Filtered Speech; MBEA = Montreal Battery for Evaluation of Amusia; MLD = Masking Level Difference; QSIN = Quick Speech in Noise Test; SPIN = Speech Perception in Noise Test; SSI = Synthetic Sentence Identification Test; CCM = Contralateral Competing Message; ICM = Ipsilateral Competing Message; TC = Time Compressed; WIN = Words in Noise Test; ILD = Interaural Level Difference; JND = Just Noticeable Difference; IPD = Interaural Phase Difference; LISN = Listening in Spatialized Noise Sentence Test; GIN = Gaps in Noise.
Indicates relationships found in more than one study.
Table 2.
Count of significant and nonsignificant relationships between auditory electrophysiologic constructs and cognitive measures, grouped by the cognitive construct.
| Construct | No. of unique studies | Electrophysiologic auditory measure | No. of sig correlations | No. of nonsig correlations |
|---|---|---|---|---|
| Working memory | 18 | Auditory Brainstem Response | 2 | — |
| ABR Wave 1 Amplitude | — | 1 | ||
| ABR Speech | 1 | 1 | ||
| N100 Suppression | 2 | 2 | ||
| N200 Mean | 1 | — | ||
| N200 Latency* | 2 | — | ||
| Mismatch Negativity Amp* | 1 | 4 | ||
| Mismatch Negativity Latency | 2 | 1 | ||
| P200 Amplitude | — | 1 | ||
| P300 Amplitude* | 3 | 3 | ||
| P300 Latency* | 7 | 6 | ||
| P300 Mean | 1 | — | ||
| P50 Ratio | — | 2 | ||
| P50 Suppression* | 1 | 4 | ||
| P50 Difference | — | 2 | ||
| Processing speed | 8 | Auditory Brainstem Response | 3 | — |
| ABR Wave 1 Amplitude | 2 | — | ||
| Mismatch Negativity Amp* | — | 2 | ||
| Mismatch Negativity Latency | — | 1 | ||
| N200 Amplitude | 2 | 1 | ||
| N200 Latency | 3 | — | ||
| P200 Amplitude | 1 | 2 | ||
| P200 Latency | 1 | 2 | ||
| P300 Amplitude* | 5 | 1 | ||
| P300 Latency* | 3 | 2 | ||
| P50 Ratio | — | 2 | ||
| P50 Difference | — | 2 | ||
| Executive/inhibitory processes | 14 | ABR Wave 1 Amplitude | — | 2 |
| Mismatch Negativity Amp* | — | 2 | ||
| Mismatch Negativity Latency | — | 1 | ||
| N200 Amplitude | 1 | — | ||
| N200 Latency* | 2 | — | ||
| P200 Amplitude* | 2 | 1 | ||
| P200 Latency | — | 1 | ||
| P300 Amplitude* | 3 | 6 | ||
| P300 Latency* | 4 | 6 | ||
| P50 Difference | — | 3 | ||
| P50 Ratio | — | 3 | ||
| P50 Suppression | 2 | 1 | ||
| Fluid intelligence | 5 | Auditory Brainstem Response | 1 | — |
| N200 Mean | — | 3 | ||
| P300 Amplitude* | 1 | 1 | ||
| P300 Latency* | 1 | 1 | ||
| P300 Mean | — | 3 | ||
| Visual perception | 2 | N200 Mean | 1 | 2 |
| P300 Amplitude | 1 | — | ||
| P300 Mean | 2 | 1 | ||
| P50 Ratio | — | 1 | ||
| P50 Difference | — | 1 | ||
| Multidomain/global cognition | 23 | Mismatch Negativity Amp* | — | 2 |
| Mismatch Negativity Latency | — | 1 | ||
| N100 Amplitude | 1 | — | ||
| N100 Latency | 5 | — | ||
| N200 Mean | 1 | — | ||
| N200 Amplitude* | 2 | 2 | ||
| N200 Latency* | 1 | 1 | ||
| P200 Amplitude* | 2 | — | ||
| P200 Latency | 1 | — | ||
| P300 Amplitude* | 7 | 7 | ||
| P300 Latency* | 9 | 11 | ||
| P300 Mean | 1 | — | ||
| P50 Ratio* | 1 | 3 | ||
| P50 Suppression | 1 | — | ||
| P50 Difference | — | 2 |
Note. Em dashes indicate no data. sig = significant; nonsig = nonsignificant; ABR = auditory brainstem response.
Indicates relationships found in more than one study.
Quality of Studies
As described above, the potential range of study quality was 0–8 based on ASHA's LOE (Mullen, 2007). For the 126 articles included in this review, the range was between 1 and 6. The average quality rating was 4.3 (SD = 1.0), suggesting moderate quality across all studies. As suggested by the average, the majority of the studies (88.6%) were between 3 and 5. For study design, 51 studies were a “yes,” suggesting they met the quality standard, while 75 studies were “no.” There were 7 “yes” (119 “no”) scores for blinding; 3 “yes” (123 “no”) for sampling; 97 “yes” (29 “no”) for subjects; 80 “yes” (46 “no”) for outcomes; 123 “yes” (3 “no”) for significance; 114 “yes” (12 “no”) for precision; and 63 “yes” (63 “no”) for power. See Table 3 for a further breakdown.
Table 3.
Distribution of study quality ratings.
| Rating | Value | Cohort (N = 126) |
|---|---|---|
| Classification | ||
| 0 | 0 (0%) | Weak |
| 1 | 1 (0.8%) | Weak |
| 2 | 3 (2.4%) | Weak |
| 3 | 22 (17.4%) | Moderate |
| 4 | 51 (40.5%) | Moderate |
| 5 | 33 (26.2%) | Moderate |
| 6 | 16 (12.7%) | Strong |
| 7 | 0 (0%) | Strong |
| 8 | 0 (0%) | Very strong |
Relationship Between Auditory Processing and Cognitive Abilities
This systematic review set out to answer the question, “In adults, what is the relationship between central auditory processing abilities and cognitive abilities?” Note that there were studies that evaluated more than one measure and found both significant and nonsignificant relationships within the same study. In fact, there were two studies (Bergemalm & Lyxell, 2005; Kamerer et al., 2019) that evaluated both behavioral and electrophysiologic measures in comparison to cognitive abilities. As a result of the overlap, many of the articles will be reported multiple times to include the measures they found significant correlations with and the measures that were not found to be significant.
In total for both categories of central auditory measures (behavioral and electrophysiologic), there were 292 significant relationships and 261 nonsignificant relationships evaluated, regardless of quality rating. Appendix Table A1 lists the relationships between the specific cognitive construct and behavioral central auditory measure. Results are provided in the following sections for the relationships found in more than one study (indicated by a in Tables 1 and 2). No conclusions were drawn if the relationship was only represented one time, but these relationships can be seen in Appendix Table A3.1
The average quality rating was calculated for each central auditory measure category, grouped by cognitive construct. These relationships are visually represented in Figure 3. Quality is plotted as a function of the CAP measure, with behavioral CAP grouped in the left panel and electrophysiology CAP grouped in the right panel. The specific cognitive constructs are shown as different symbols. Symbol color indicates whether the relationship was significant. For example, in the left panel, temporal CAP tests were more often related to cognitive processing, although study quality varied; whereas binaural CAP tests were rarely related to cognitive processing. In the right panel, late potentials such as the P300 were more likely to be related to cognitive processing than earlier potentials such as the P50. The following sections describe these relationships in more detail.
Figure 3.
Average quality ratings of studies with relationships between auditory behavioral (left) and electrophysiologic (right) and cognitive constructs measured in more than one study. SIN = Speech in Noise; MMN = mismatch negativity; Ex/Inhib = Executive/Inhibitory Processes; Fluid = Fluid Intelligence; Multi = Multiple-Domains; ProcS = Processing Speed; VisP = Visual Perception; WM = Working Memory.
The percentage of significant relationships by auditory construct is aggregated in Figure 4. Across all studies reviewed, there were more significant than nonsignificant relationships for temporal processing (19/25), altered speech (12/19), and speech in noise (70/126; ordered by highest percentage). For electrophysiologic measures of auditory processing, there were more significant than nonsignificant relationships for P200 (4/5) and N200 (7/10) only. Figure 4 is further divided to show the comparison of significant relationships for studies with a total quality rating of 4 or more. Overall, altered speech declined in percentage from 63% to 50%, P50 increased from 22% to 50%, and the other measures remained relatively the same.
Figure 4.
Clustered column chart of the percentage of significant relationships between auditory and cognitive constructs. Columns are separated by all quality ratings and ratings of 4 or more. MMN = mismatch negativity; QR = quality ratings.
Behavioral Auditory Measures
When evaluating the reliability of data across multiple studies, it is important to consider whether enough detail was provided to reproduce the sample and the number of participants was large enough to find a generalizable result. As such, a more granular analysis was conducted to evaluate the studies by LOE quality rating categories subjects and power (see Table 4). For the studies that found significant relationships between working memory and speech in noise, 28/30 (93%) had subject descriptions that were reproducible and 17/30 (57%) analyses had sufficient power to support the finding. Conversely, 23/24 (96%) of the studies that found nonsignificant findings had adequate subject descriptions and 10/24 (42%) had adequate power. In other words, there were consistently higher quality ratings for subject compared to power, regardless of the cognitive construct or auditory processing ability. There were no striking differences in subject or power quality ratings between the significant and nonsignificant relationships.
Table 4.
Percentage of “yes” ratings for subjects and power level of evidence (LOE): behavioral.
| Variable | Significant | Nonsignificant | ||
|---|---|---|---|---|
| Working memory | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| 28/30 (93%) | 17/30 (57%) | 23/24 (96%) | 10/24 (42%) | |
| Altered speech | Subjects | Power | Subjects | Power |
| 5/5 (100%) | 3/5 (60%) | 3/3 (100%) | 3/3 (100%) | |
| Temporal processing | Subjects | Power | Subjects | Power |
| 8/8 (100%) | 6/8 (75%) | 1/1 (100%) | 1/1 (100%) | |
| Binaural processing | Subjects | Power | Subjects | Power |
| 4/4 (100%) | 2/4 (50%) | 6/7 (86%) | 3/7 (43%) | |
| Processing speed | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| 2/4 (50%) | 1/4 (25%) | 3/3 (100%) | 0/3 (0%) | |
| Altered speech | Subjects | Power | Subjects | Power |
| 2/2 (100%) | 1/2 (50%) | 2/2 (100%) | 2/2 (100%) | |
| Temporal processing | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Binaural processing | Subjects | Power | Subjects | Power |
| 3/3 (100%) | 1/3 (33%) | 1/1 (100%) | 0/1 (0%) | |
| Executive/inhibitory processes | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| 5/6 (83%) | 3/6 (50%) | 5/6 (83%) | 2/6 (33%) | |
| Altered speech | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Temporal processing | Subjects | Power | Subjects | Power |
| 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | 0/1 (0%) | |
| Binaural processing | Subjects | Power | Subjects | Power |
| N/A | N/A | 1/2 (50%) | 0/2 (0%) | |
| Fluid intelligence | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Altered speech | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Temporal processing | Subjects | Power | Subjects | Power |
| 3/3 (100%) | 2/3 (67%) | 1/1 (100%) | 0/1 (0%) | |
| Binaural processing | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Visual perception | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| N/A | N/A | 2/3 (67%) | 0/3 (0%) | |
| Altered speech | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Temporal processing | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Binaural processing | Subjects | Power | Subjects | Power |
| N/A | N/A | 1/2 (50%) | 0/2 (0%) | |
| Multidomain/global cognition | ||||
| Speech in noise | Subjects | Power | Subjects | Power |
| 10/12 (83%) | 9/12 (75%) | 1/1 (100%) | 1/1 (100%) | |
| Altered speech | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| Temporal processing | Subjects | Power | Subjects | Power |
| 2/2 (100%) | 2/2 (100%) | N/A | N/A | |
| Binaural processing | Subjects | Power | Subjects | Power |
| 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | 0/1 (0%) | |
Note. N/A = not applicable.
The number of comparisons for each behavioral CAP category was tallied. For the speech in noise category, the number of comparisons in decreasing size (number of comparisons) were sentences in speech-shaped noise (22), sentences in two-talker maskers (18), Quick Speech-in-Noise Test (QuickSIN; 16; Killion et al., 2004), and Speech Perception in Noise Test (10; Kalikow et al., 1977). Other measures had less than 10 comparisons. For the altered speech category, time-compressed speech was the most commonly used measure with 19 comparisons. The temporal processing category had 13 comparisons for temporal order tasks, and the remaining measures had five or fewer comparisons. For the binaural processing category, Dichotic Digits Test (DDT; Musiek, 1983), DDT-Left Focused, Dichotic Speech with free recall, DDT-Right Focused, and Masking Level Difference and had nine, eight, seven, two, and two comparisons, respectively. The measure with the highest number of comparisons in each category was chosen for further analysis.
Speech in Noise
Twenty-two total relationships were tested for sentences in speech-shaped noise. Working memory: Four significant relationships were found: reading span (Lunner, 2003; Schoof & Rosen, 2014) and nonword repetition (Millman & Mattys, 2017; Moberly et al., 2017). Six nonsignificant relationships were found: reading span (Miller et al., 2017; Ng et al., 2014), n-back (Giroud et al., 2021; Rosemann & Thiel, 2020), Word Auditory Recognition and Recall Measure (Miller et al., 2017), and digit span (Van Rooij & Plomp, 1992). Processing speed: Three significant relationships were found: TMT-A (Ellis et al., 2016), rhyme judgment test (Lunner, 2003), and letter digit substitution test (Schoof & Rosen, 2014). Three nonsignificant relationships were found: physical matching, rhyme judgment test, and lexical decision making (Ng et al., 2014). Executive/inhibitory processes: Two significant relationships were found: TMT (Rosemann & Thiel, 2020) and TMT-B (Ellis et al., 2016). One nonsignificant relationship was found: the Stroop test (Rosemann & Thiel, 2020). Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: Three significant relationships were found: Mini-Mental State Examination (MMSE; Folstein et al., 1975), Alzheimer's Disease Assessment Scale (ADAS; Carvalho et al., 2017), and MoCA (Castiglione et al., 2019). No nonsignificant relationships were found.
Speech in noise, specifically sentences in speech-shaped noise, appears to be most strongly related to executive/inhibitory processes and global measures of cognition, although the findings are mixed. For example, both significant and nonsignificant relationships were found for working memory, processing speed, and executive/inhibitory processes. The significant relationships were in the expected direction. That is, better performance on the executive/inhibitory processes and better performance on multidomain cognitive assessments were associated with better performance on sentences in speech-shaped noise.
Altered Speech
Nineteen total relationships were tested for time-compressed speech. Working memory: Eight significant relationships were found: digit span tests (Bergemalm & Lyxell, 2005; Cervera et al., 2009; Kim et al., 2020), reading span test (Bergemalm & Lyxell, 2005), serial recall (Cervera et al., 2009), list sorting (Kamerer et al., 2019), listening span (Kim et al., 2020), and a composite of three sequential working memory tasks (Vaughan et al., 2008). Five nonsignificant relationships were found: digit span (Fostick et al., 2013; O'Brien et al., 2021), spatial span (O'Brien et al., 2021), n-back, and nonsequential working memory (Vaughan et al., 2008). Processing speed: Five significant relationships were found: lexical decision making, semantic decision making, the rhyme judgment test (Bergemalm & Lyxell, 2005), and auditory reaction time (Vaughan et al., 2008). Two nonsignificant relationships were found: visual reaction times (Vaughan et al., 2008) and the TMT-A task (O'Brien et al., 2021). Executive/inhibitory processes: no relationships tested. Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: no relationships tested.
Altered speech, specifically time-compressed speech, appears to be most strongly related to working memory and processing speed, although both significant and nonsignificant relationships were found for each cognitive construct. The significant relationships were in the expected direction. That is, better working memory scores and faster processing speed resulted in better performance on time-compressed speech.
Temporal Processing
Thirteen total relationships were tested for temporal order tasks. Working memory: Five significant relationships were found: the Wechsler Adult Intelligence Scale–Third Edition (WAIS-3) Digit Span (two and four items), WAIS-3 Letter Number (two and four items), WAIS-3 Episodic long-term memory pairing (two items only), WAIS-3 Episodic free recall (two items only; Danielsson et al., 2019), and digit span (Ulbrich et al., 2009). Two nonsignificant relationships were found: the WAIS-3 Episodic long-term memory pairing (four items only) and the WAIS-3 Episodic free recall (four items only; Danielsson et al., 2019). Processing speed: no relationships tested. Executive/inhibitory processes: no relationships tested. Fluid intelligence: Three significant relationships were found: WAIS-3 Arithmetic (Danielsson et al., 2019; Humes, 2021b) and the Mosaik Test assessed monaurally (Szymaszek et al., 2009). One nonsignificant relationship was found: the Mosaik Test assessed binaurally (Szymaszek et al., 2009). Visual perception: no relationships tested. Multidomain/global: Two significant relationships were found: WAIS-R/3 (Humes, 2005; Humes et al., 2010). No nonsignificant relationships were found.
Temporal processing, specifically temporal order tasks, appears to be most strongly related to working memory, fluid intelligence, and global measures of cognition, although findings show both significant and nonsignificant relationships for working memory and fluid intelligence. The significant relationships were in the expected direction. For example, better working memory scores, better scores on fluid intelligence tasks, and better scores for multidomain assessments were related to better performance on temporal order tasks.
Binaural Processing
Nine total relationships were tested for the DDT. Working memory: No significant relationships were found. Two nonsignificant relationships were found: Paired Associates Learning Test and one-back test (Ghannoum et al., 2018). Processing speed: no relationships tested. Executive/inhibitory processes. No significant relationships were found. Three nonsignificant relationships were found: the TMT, Controlled Oral Word Association Test (Ghannoum et al., 2018), and the Groton Maze Learning Test (Nixon et al., 2019). Fluid intelligence: no relationships tested. Visual perception: No significant relationships were found. Two nonsignificant relationships were found: clock drawing (Ghannoum et al., 2018) and the one card learning test (Nixon et al., 2019). Multidomain/global: One significant relationship was found: the ADAS (Pinheiro et al., 2012). One nonsignificant relationship was found: the MoCA (Mukari et al., 2020). Binaural processing, as measured by the DDT, does not appear to be strongly related to any of the cognitive constructs, although there are mixed findings for global measures of cognition.
Electrophysiologic Auditory Measures
A full breakdown of the relationships between the cognitive constructs and specific electrophysiologic measures is provided in Appendix Table A2. Results evaluating the quality of the significant and nonsignificant relationships between the electrophysiologic measures and cognitive constructs can be found in Table 5. As an example, 2/4 (50%) of the significant relationships between P300 and working memory had adequate subject descriptions and 1/4 (25%) of the significant relationships had adequate power. For nonsignificant relationships between P300 and working memory, 2/4 (50%) had adequate subject descriptions and power. The subject and power quality ratings between the significant and nonsignificant relationships were not notably different, although it is important to acknowledge that most of these comparisons were between one and two studies.
Table 5.
Percentage of “yes” ratings for subjects and power level of evidence (LOE): electrophysiologic.
| Variable | Significant | Nonsignificant | ||
|---|---|---|---|---|
| Working memory | ||||
| MMN | Subjects | Power | Subjects | Power |
| 1/1 (100%) | 1/1 (100%) | 2/3 (67%) | 1/3 (33%) | |
| P50 | Subjects | Power | Subjects | Power |
| 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | |
| N200 | Subjects | Power | Subjects | Power |
| 1/2 (50%) | 1/2 (50%) | N/A | N/A | |
| P200 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P300 | Subjects | Power | Subjects | Power |
| 2/4 (50%) | 1/4 (25%) | 2/4 (50%) | 2/4 (50%) | |
| Processing speed | ||||
| MMN | Subjects | Power | Subjects | Power |
| N/A | N/A | 1/2 (50%) | 0/2 (0%) | |
| P50 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| N200 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P200 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P300 | Subjects | Power | Subjects | Power |
| 1/3 (33%) | 0/3 (0%) | 0/2 (0%) | 0/2 (0%) | |
| Executive/inhibitory processes | ||||
| MMN | Subjects | Power | Subjects | Power |
| N/A | N/A | 1/2 (50%) | 0/2 (0%) | |
| P50 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| N200 | Subjects | Power | Subjects | Power |
| 0/2 (0%) | 0/2 (0%) | N/A | N/A | |
| P200 | Subjects | Power | Subjects | Power |
| 1/2 (50%) | 0/2 (0%) | 0/1 (0%) | 0/1 (0%) | |
| P300 | Subjects | Power | Subjects | Power |
| 2/5 (40%) | 1/5 (20%) | 0/4 (0%) | 0/4 (0%) | |
| Fluid intelligence | ||||
| MMN | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P50 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| N200 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P200 | Subjects | Power | Subjects | Power |
| N/A | N/A | N/A | N/A | |
| P300 | Subjects | Power | Subjects | Power |
| 0/2 (0%) | 0/2 (0%) | 0/1 (0%) | 0/1 (0%) | |
| Multidomain/global cognition | ||||
| MMN | Subjects | Power | Subjects | Power |
| N/A | N/A | 1/2 (50%) | 1/2 (50%) | |
| P50 | Subjects | Power | Subjects | Power |
| 1/1 (100%) | 1/1 (100%) | 0/2 (0%) | 1/2 (50%) | |
| N200 | Subjects | Power | Subjects | Power |
| 1/2 (50%) | 1/2 (50%) | 1/2 (50%) | 1/2 (50%) | |
| P200 | Subjects | Power | Subjects | Power |
| 1/2 (50%) | 0/2 (0%) | N/A | N/A | |
| P300 | Subjects | Power | Subjects | Power |
| 0/9 (0%) | 2/9 (22%) | 2/9 (22%) | 2/9 (22%) | |
Note. MMN = mismatch negativity; N/A = not applicable.
The number of comparisons for each electrophysiologic auditory category were tallied, and the specific measures were identified. The MMN amplitude had 11 comparisons, the P50 had nine comparisons (five for suppression, four for ratio), the N200 had 10 comparisons (six for latency, four for amplitude), the P200 amplitude had five comparisons, and the P300 had 87 comparisons (37 for amplitude and 50 for latency). Again, the measure with the highest number of comparisons in each category was chosen for further analysis.
MMN
Eleven total relationships were tested for amplitude. Working memory: One significant relationship was found: spatial span (Bonetti et al., 2018). Four nonsignificant relationships were found: letter number sequencing (Bonetti et al., 2018), immediate and delayed retrieval of logical memory, digit symbol test (Kärgel et al., 2014), and digit span (Naismith et al., 2012). Processing speed: No significant relationships were found. Two nonsignificant relationships were found: TMT-A (Kärgel et al., 2014; Naismith et al., 2012). Executive/inhibitory processes. No significant relationships were found. Two nonsignificant relationships were found: TMT-B (Kärgel et al., 2014; Naismith et al., 2012). Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: No significant relationships were found. Two nonsignificant relationships were found: MoCA (Brückmann et al., 2021) and Rey Auditory Verbal Learning Test (Naismith et al., 2012).
The MMN amplitude does not appear to be strongly related to any of the cognitive constructs, although there are mixed findings for working memory. For this relationship, the expected direction was found: The MMN amplitude was higher for those with better performance on the spatial span.
P50. Five total relationships were tested for P50 suppression. Working memory: One significant relationship was found: digit span (Thomas et al., 2010). Four nonsignificant relationships were found to Wechsler memory scale tasks: letter string, color pattern, visual reproduction, and verbal pair associate (Hsieh et al., 2004). Processing speed: no relationships tested. Executive/inhibitory processes: no relationships tested. Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: no relationships tested. P50 suppression does not appear to be strongly related to working memory, with one exception, where the relationship was as expected, better sensory gating was suggestive of better performance on the digit span.
N200. Six total relationships were tested for the N200 latency. Working memory: Two significant relationships were found: the Wechsler memory scale (Bodis-Wollner et al., 1995) and digit span (Pokryszko-Dragan et al., 2009). No nonsignificant relationships were found. Processing speed: no relationships tested. Executive/inhibitory processes. Two significant relationships were found: TMT (Pokryszko-Dragan et al., 2009; Stuckenschneider et al., 2020). No nonsignificant relationships were found. Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: One significant relationship was found: MoCA (Stuckenschneider et al., 2020). One nonsignificant relationship was found: Mattis Dementia Rating Scale (MDRS; Bodis-Wollner et al., 1995).
The N200 latency appears to be most strongly related to working memory and executive/inhibitory processes. Both significant and nonsignificant relationships were found for global measures of cognition. The significant relationships were in the expected direction. That is, earlier N200 latencies were associated with better working memory and executive/inhibitory performance.
P200
Five total relationships were tested for P200 amplitude. Working memory: no relationships tested. Processing speed: no relationships tested. Executive/inhibitory processes: Two significant relationships were found: TMT-B (Nagasawa et al., 1999) P200 standard tone and TMT-B (Stuckenschneider et al., 2020). One nonsignificant relationship was found: P200 target tone and TMT-B (Stuckenschneider et al., 2020). Fluid intelligence: no relationships tested. Visual perception: no relationships tested. Multidomain/global: Two significant relationships were found: MoCA (Oliveira et al., 2021; Stuckenschneider et al., 2020). No nonsignificant relationships were found.
P200 amplitude appears to be most strongly related to executive/inhibitory processes and global measures of cognition, although the findings were mixed. That is, both significant and nonsignificant relationships were found for executive/inhibitory processes. The significant relationships were in the expected direction. Higher P200 amplitudes were associated with better performance on the TMT-B and the MoCA.
P300
The P300 latency was evaluated 50 times, 24 were significant relationships and 26 were nonsignificant. Working memory: Seven significant relationships were found: digit symbol (Gurrera et al., 2005; Portin et al., 2000), digit span (Gurrera et al., 2005), digit span forward and backwards (Volpato et al., 2010), prose memory immediate and delayed recall (Volpato et al., 2010), and dot location recall (Dichter et al., 2006). Six nonsignificant relationship was found: prose memory immediate and delayed recall (Portin et al., 2000), digit span (Dichter et al., 2006; Katsarou et al., 2004; Portin et al., 2000), digit symbol (Katsarou et al., 2004), and the Wechsler memory scale (Bodis-Wollner et al., 1995). Processing speed: Three significant relationships were found: TMT-A (Gurrera et al., 2005; Stuckenschneider et al., 2020) and verbal fluency-letter (Stuckenschneider et al., 2020). Two nonsignificant relationships were found: verbal fluency-category (Stuckenschneider et al., 2020) and TMT-A (Dichter et al., 2006). Executive/inhibitory processes: Four significant relationships were found: TMT-B (Dichter et al., 2006; Gurrera et al., 2005), overall score on the Wisconsin card sorting test (Katsarou et al., 2004), and the Wisconsin card sorting test categories completed subscale (Dichter et al., 2006). Six nonsignificant relationships were found: TMT-B (Podemski et al., 2008; Stuckenschneider et al., 2020), Wisconsin card sorting test perseverative errors subscale, Tower of London, Continuous Performance task (Dichter et al., 2006), and TMT (Pokryszko-Dragon et al., 2009). Fluid intelligence: One significant relationship was found: Raven Coloured Progressive Matrices (Katsarou et al., 2004). One nonsignificant relationship was found: WAIS-Information (Dichter et al., 2006). Visual perception: no relationships tested. Multidomain/global: Nine significant relationships were found: MMSE (Marsh et al., 1990; Pokryszko-Dragan et al., 2003; Tanaka et al., 2000), ADAS (Katada et al., 2003), WAIS (Neshige et al., 1988), MoCA (Stuckenschneider et al., 2020; Zeng et al., 2017), and two composites of multiple cognitive assessments (Cooray et al., 2008). Eleven nonsignificant relationships were found: MMSE (Hayashi et al., 1993; Katada et al., 2003; Krishnamurthy et al., 2019; de Miranda et al., 2012; Podemski et al., 2008; Zeng et al., 2017), ADAS (de Miranda et al., 2012), WAIS (Pokryszko-Dragan et al., 2009), Rey Auditory Verbal Learning Test (Podemski et al., 2008; Pokryszko-Dragan et al., 2009), and MDRS (Bodis-Wollner et al., 1995).
The P300 latency appears to be most strongly related to working memory and processing speed, although the findings are mixed. For example, both significant and nonsignificant relationships were found for working memory, processing speed, executive/inhibitory processes, fluid intelligence, and global measures of cognition. For the significant relationships, the expected direction was determined. That is, earlier P300 latency was associated with better scores on the cognitive constructs.
Discussion
This systematic review sought to answer, “In adults, what is the relationship between central auditory processing and cognitive abilities?” In order to address this question comprehensively, both behavioral and electrophysiologic CAP measures were considered for evaluation. There is no single answer from the literature as to whether CAP and cognitive abilities are related. Indeed, this review highlights that lack of consensus, such that each of the behavioral and electrophysiologic constructs demonstrated both significant and nonsignificant correlations with cognitive abilities. Findings must also be qualified by noting that some of the studies were of relatively low quality particularly with regard to number of subjects and adequate statistical power to support results. However, we can draw conclusions from those relationships that were more often found to be significant.
The individual CAP constructs that were more often than not related to cognitive abilities across the reviewed studies were temporal processing (76%), altered speech (63%), and speech in noise (56%). Auditory temporal processing is crucial to speech recognition and is processed via the neural networks and circuitry of the auditory cortex (Mauk & Buonomano, 2004). It is a reasonable conclusion that temporal processing would be related to cognition (i.e., working memory, fluid intelligence, and global measures of cognition, as shown in the studies reviewed).
Altered speech was significantly related to working memory and processing speed. When speech is altered, such as with time compression, the listener must actively fill in missed information or reconcile aberrant acoustic cues in order to interpret information correctly. To be efficient and accurate at such a task, individuals also need to inhibit the irrelevant “noise” in the stimuli or background noise to make sense of the target. This, logically, will require more time to process than nonaltered speech and will require tapping into one's phonological understanding of language (Wagner & Torgesen, 1987) and activating working memory in order to reconstruct degraded input or resolve perceptual conflicts (e.g., Rönnberg et al., 2010, 2013).
Speech in some types of background noise was shown to have significant relationships to cognitive constructs, specifically the constructs of working memory, processing speed, and multidomain composites. During speech-in-noise tests such as QuickSIN (Killion et al., 2004), individuals must engage working memory, at least to some degree, in order to accurately recall the target after it ends. In previous work, the strongest relationships with working memory were for sentences in modulated noise (e.g., Lunner & Sundewall-Thoren, 2007; Ohlenforst et al., 2016). This may be because the process of extracting and assembling spectral and/or temporal glimpses of the target requires working memory to a greater extent than speech in constant-amplitude noise, which has a more constant spectro-temporal structure. Individuals with poorer processing speed would also presumably have poorer speech recognition. Indeed, listeners with hearing loss are cognizant that part of listening in noise includes “keeping up” with a rapid stream of information and that doing so is fatiguing (Preminger & Laplante-Levesque, 2014).
Although sentences in speech-shaped noise had more significant than nonsignificant relationships with executive/inhibitory processes, the speech-in-noise category as a whole did not (13 significant compared to 15 nonsignificant). The finding that speech in noise was not significantly related to executive/inhibitory processes is surprising because listening to speech in noise requires inhibiting the background noise to some degree. However, the speech tests reviewed in this review article may have underrepresented the executive/inhibitory skills used to listen in realistic noisy situations. That is, in the speech-in-noise tests typically used in clinical or experimental settings, the listener's attention is directed to a single talker who produces speech (sentences) at predictable intervals, usually with similar syntactic structure and with the talker's voice at an audible level over a relatively constant noise level. We can speculate that in a realistic scenario with more distractions that occur at unpredictable times and perhaps with the need to direct attention to different signals at different times, executive/inhibitory processes could play a larger role.
With regard to electrophysiologic measures of CAP, the constructs that were more often than not related to cognitive abilities were P200 (80%) and N200 (70%). The N200 significant relationships were for working memory, executive/inhibitory processes, and multidomain constructs, while the significant relationships for the P200 were for global measures of cognition. Although there were more significant than nonsignificant relationships for the P200 amplitude and executive/inhibitory processes, the P200 category including amplitude and latency had an even number of significant and nonsignificant relationships (2 and 2). The P200 is a mid-latency evoked potential and a component of the MLR, which has an activation site distributed around the secondary auditory cortex (Golob & Starr, 2000). One possible explanation for this relationship is that the N200/P200 reflects activity in the thalamus, a brain region that serves as a relay between sensory inputs and higher cortical areas involved in executive functioning. The thalamus has been shown to play a critical role in attentional control and working memory, and dysfunction in this region has been associated with executive dysfunction in various neurological and psychiatric disorders (Gomot & Giard, 2007; Mitchell & Chakraborty, 2013). Therefore, the N200 and/or P200 may provide a useful biomarker for investigating the neural mechanisms underlying executive functioning and their relationship to thalamic activity.
While a detailed discussion of the mechanisms underlying the relationships between CAP and cognition is beyond the scope of this review, it sparks curiosity regarding how underlying changes in neural responses might influence both types of higher level processes. Some authors have proposed that neural changes could explain individual declines in processing (e.g., Pichora-Fuller et al., 2007; Salthouse et al., 1998; Uchida et al., 2019). It is plausible that such changes could exert significant effects on temporal processing, as measured in CAP tests, as well as on measures of neural physiology, such as the P200/N200. These changes may be especially relevant for older listeners, who were included in some, but not all, of the reviewed studies. Indeed, Salthouse et al. (1998) suggests that the neural mechanisms associated with timing (such as temporal processing) play a fundamental role in the speed at which an individual can execute and process a task, an aspect influenced by age-related differences.
Finally, quality ratings for each study were assessed separately for their significant and nonsignificant relationships. No distinct patterns were observed, and most of the studies regardless of whether they showed significant or nonsignificant findings were rated as mid-quality. This is consistent with previous observations (e.g., Cox, 2005; Hahs-Vaughn & Nye, 2008) that many studies in this literature rely on lower quality designs.
Clinical Relevance
Because adults are less likely to have been evaluated or diagnosed with CAPD compared to children (Agrawal et al., 2021; Hind et al., 2011), an audiologist may be unaware that CAPD is a potential source of communication difficulties. In addition, although there is professional interest in assessing the cognitive abilities that contribute to overall communication, such assessments are not common in audiology practice (Anderson et al., 2018). Recent calls to action to revise the auditory battery and assess individual abilities beyond sound sensitivity (e.g., Fitzgerald et al., 2023; Herbert et al., 2023; Sanchez-Lopez et al., 2021) may lead to a more complete understanding of individual abilities and their aggregate effect on communication. At minimum, cognitive and central auditory measures should be considered when appropriate remediation (e.g., well-fit hearing aids and/or hearing assistive technology) does not improve communication for listeners whose audiometric data indicate that audibility concerns have been addressed. An individualized assessment that includes CAP and cognitive tests could also help direct aural habilitation. Currently, the most-used training programs focus on either central auditory (Cameron & Dillon, 2011; Sweetow & Sabes, 2006; Weihing et al., 2015) or cognitive abilities (George & Whitehouse, 2011). Some remediation programs propose to recruit cognitive processes as a means of compensating for CAP deficits (Chermak, 1998), an approach that may fail if the patient has (unrecognized) relatively poor cognitive abilities. Even when the remediation plan is more general and targeted to improving the communication environment rather than training specific abilities, counseling patients about their own CAP and cognitive processing abilities may allow more individualized support. Both patients and audiologists react positively to inclusion of such topics as part of the evaluation and treatment process (Broome et al., 2023; Cavitt & Hill, 2022).
The relationship between auditory and cognitive abilities is a critical piece of information to add to the literature on brain health. Understanding this relationship can inform future research endeavors on the topic of cognitive decline and dementia, as well as at the individual level for clinical management plans. Audiologists and researchers alike agree that the audiogram (i.e., determining pure-tone thresholds), a measure used across all hearing clinics in determining candidacy for management of auditory dysfunction, is not cognitively demanding. Herein lies the problem—our lives in the real world, outside of the soundproofed booths, are cognitively demanding. However, auditory abilities beyond the pure-tone audiogram, such as those described in this review, are not routinely being measured. It is clear that there are aspects of our auditory and cognitive networks that intertwine and depend, at least in part, on one another.
As such, this review brings light to what many already know and accept. Creating a management plan for a patient without understanding the role of specific abilities (auditory or cognitive) does not represent the individual well and is not fully utilizing patient-centered care.
Conclusions
In this systematic review, our objective was to address the question, “In adults, what is the relationship between central auditory processing abilities and cognitive abilities?” The CAP categories considered by this review included speech in noise, altered speech, temporal processing, binaural processing, and electrophysiology processing. Cognitive measures included working memory, processing speed, executive/inhibitory processes, fluid intelligence, visual perception, and multidomain measures. Across a comprehensive set of reviewed papers employing different approaches, research designs, and findings, the most robust relationships emerged between speech perception in noisy conditions and either executive/inhibitory or multidomain cognitive abilities. Similarly, altered (especially time-compressed) speech exhibited strong relationships with working memory or processing speed. Auditory temporal order tasks showed significant relationships with working memory, fluid intelligence, or multidomain cognitive measures. In the context of electrophysiology, specifically N200 and P300 latency, the most consistent relationships were observed between cortical evoked potentials and working memory or executive/inhibitory processes. The variety of experimental approaches and the diversity of findings emphasize the intricate interplay between auditory and cognitive abilities, particularly in communication scenarios characterized by noise or distortion, especially in older adults or those who have hearing loss. The results also highlight the pivotal role of higher level processes in speech recognition and underscore the need to assess individual listener abilities beyond the constraints of the pure-tone audiogram.
Acknowledgments
This work was partially supported by funding from the National Institutes of Health (R01 DC012289 to P. Souza). The authors thank Maya Reid and Kendra Marks for their assistance in preparing the review article.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Defense (DoD) of the U.S. Government. The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the authors, DoD, or any component agency.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within this article and Supplemental Material S1.
Supplementary Material
Appendix
Table A1.
Correlations between behavioral auditory measures and cognitive constructs.
| Behavioral auditory construct | Study | N | Auditory measure | Cognitive construct | Correlation | p value | Effect size (r unless specified) |
|---|---|---|---|---|---|---|---|
| Speech in noise | Anderson et al., 2013 | 120 | QSIN | WM | ~ | > .05 | −.42 |
| WIN | ^^ | ~ | > .05 | −.16 | |||
| Anderson et al., 2013 | 120 | HINT | WM | + | < .01 | .27 | |
| ^^ | ^^ | + | < .05 | −.22 | |||
| QSIN | ^^ | + | < .01 | −.29 | |||
| WIN | ^^ | + | < .01 | −.27 | |||
| Anzivino et al., 2019 | 44 | SentBab | Multi | + | .04 | NR | |
| ^^ | ^^ | + | .02 | NR | |||
| Arehart et al., 2015 | 31 | Sent4 | WM | + | .04 | t = 2.15 | |
| Cahana-Amitay et al., 2016 | 173 | SPIN | ExP | ~ | > .05 | NR | |
| ^^ | WM | ~ | > .05 | NR | |||
| ^^ | ^^ | ~ | > .05 | NR | |||
| Cahana-Amitay et al., 2016 | 173 | SPIN | ExP | + | .002 | t = −3.1 | |
| Carvalho et al., 2017 | 25 | SentSS | Multi | + | .003 | −.57 | |
| ^^ | ^^ | + | .002 | .58 | |||
| Castiglione et al., 2019 | 166 | SentSS | Multi | + | < .001 | −.54 | |
| Cox et al., 2008 | 45 | QSIN | WM | ~ | > .05 | .39 | |
| ^^ | ^^ | ~ | > .05 | .44 | |||
| Ellis et al., 2016 | 1,509 | SentSS | ProcS | + | < .001 | .37 | |
| ^^ | ExP | + | < .001 | .33 | |||
| Foo et al., 2007 | 32 | SentUnMod | WM | + | < .01 | −.67 | |
| SentMod | ^^ | + | < .01 | −.65 | |||
| HINT | ^^ | + | < .01 | −.53 | |||
| Fostick et al., 2013 | 89 | WordsSS | WM | ~ | > .05 | .1 | |
| WordsW | ^^ | ~ | > .05 | .16 | |||
| Füllgrabe et al., 2015 | 30 | VCV | WM | ~ | > .05 | .28 | |
| ^^ | ^^ | ~ | > .05 | .13 | |||
| Sent2 | ^^ | ~ | > .05 | .45 | |||
| ^^ | ^^ | ~ | > .05 | .27 | |||
| Füllgrabeet al., 2015 | 30 | VCV | WM | + | ≤ .05 | .52 | |
| ^^ | ExP | + | ≤ .05 | .40 | |||
| Sent2 | ^^ | + | ≤ .05 | .61 | |||
| ^^ | WM | + | ≤ .05 | .65 | |||
| Ghannoum et al., 2018 | 71 | SPIN | ExP | ~ | .07 | .15 | |
| SSI-ICM | ^^ | ~ | .09 | .15 | |||
| SSI-CCM | ^^ | ~ | .48 | .17 | |||
| SPIN | ^^ | ~ | .34 | .08 | |||
| SSI-ICM | ^^ | ~ | 2.49 | .03 | |||
| SSI-CCM | ^^ | ~ | 2.65 | .17 | |||
| SPIN | WM | ~ | .69 | .11 | |||
| SSI-ICM | ^^ | ~ | 1.3 | .07 | |||
| SSI-CCM | ^^ | ~ | 1.69 | .02 | |||
| SPIN | VisP | ~ | 1.84 | .04 | |||
| SSI-ICM | ^^ | ~ | 1.0 | .09 | |||
| SSI-CCM | ^^ | ~ | .88 | .04 | |||
| Giroud et al., 2021 | 38 | SentSS | WM | ~ | .65 | .32 | |
| Gordon-Salant & Cole, 2016 | 28 | WordsBab | WM | + | < .01 | −.63 | |
| ^^ | ^^ | + | < .01 | −.48 | |||
| ^^ | ProcS | + | < .01 | −.59 | |||
| ^^ | ^^ | + | < .01 | −.52 | |||
| SentBab | WM | + | < .01 | −.63 | |||
| ^^ | ^^ | + | < .01 | −.55 | |||
| ^^ | ProcS | + | < .05 | −.36 | |||
| ^^ | ^^ | + | < .05 | −.33 | |||
| Helfer & Freyman, 2014 | 45 | Sent2 | ExP | ~ | > .05 | −.17 | |
| Sent1 | ^^ | ~ | > .05 | −.25 | |||
| Helfer & Freyman, 2014 | 45 | Sent2 | WM | + | ≤ .05 | .42 | |
| ^^ | ExP | + | ≤ .05 | .42 | |||
| Sent1 | ^^ | + | ≤ .05 | .45 | |||
| Humes, 2021 | 137 | QSIN | WM | + | < .01 | −.16 | |
| WIN | ^^ | + | < .01 | −.27 | |||
| Humes et al., 2013 | 125 | CompSIN | WM | + | < .01 | .54 | |
| Jain & Dwarakanath, 2019 | 92 | QSIN | WM | ~ | .75 | .04 | |
| Jain & Dwarakanath, 2019 | 92 | QSIN | WM | + | .01 | .48 | |
| Kamerer et al., 2019 | 32 | WordsSS | WM | ~ | > .05 | NR | |
| Kim et al., 2020 | 96 | SentBab | WM | + | .001 | .38 | |
| ^^ | ^^ | + | .001 | .38 | |||
| Koelewijn et al., 2014 | 32 | SentFluc | WM | ~ | > .05 | −.13 | |
| Sent1 | ^^ | ~ | > .05 | −.19 | |||
| Krause et al., 2014 | 15 | Sent16Hz | ProcS | ~ | > .05 | −.02 | |
| Sent4kHz | ^^ | ~ | > .05 | .24 | |||
| Krause et al., 2014 | 15 | Sent2 | ProcS | + | < .05 | .60 | |
| Lee et al., 2018 | 46 | Sent3 | WM | + | < .05 | .35 | |
| Lunner, 2003 | 72 | SentSS | WM | + | < .05 | −.53 | |
| ^^ | ProcS | + | < .05 | .39 | |||
| Lunner & Sundewall-Thoren, 2007 | 23 | SentUnMod | WM | + | < .05 | −.44 | |
| Mamo & Helfer, 2021 | 39 | SentMod | WM | ~ | .46 | .10 | |
| Mamo & Helfer, 2021 | 39 | Sent2 | WM | + | .05 | .30 | |
| Mamo et al., 2019 | 250 | QSIN | WM | + | .01 | .82 | |
| ^^ | ExP | + | .02 | .81 | |||
| Merten et al., 2020 | 1,274 | Words1 | ExP | + | < .05 | .14 | |
| Miller et al., 2017 | 76 | SentSS | WM | ~ | .15 | F = 2.2 | |
| ^^ | ^^ | ~ | .15 | F = 2.1 | |||
| Millman & Mattys, 2017 | 30 | SentMod | WM | ~ | .40 | .16 | |
| ^^ | ^^ | ~ | .82 | .05 | |||
| Millman & Mattys, 2017 | 30 | SentSS | WM | + | .05 | .40 | |
| Moberly et al., 2017 | 60 | SentSS | VisP | ~ | > .05 | NR | |
| Moberly et al., 2017 | 60 | SentSS | WM | + | .007 | .50 | |
| Moore et al., 2014 | 502,642 | DigitStat | Multi | + | < .001 | NR | |
| Mosnier et al., 2015 | 94 | WordsW | Multi | ~ | > .05 | NR | |
| ^^ | VisP | ~ | > .05 | NR | |||
| ^^ | ProcS | ~ | > .05 | NR | |||
| ^^ | ExP | ~ | > .05 | NR | |||
| Mosnier et al., 2015 | 94 | WordsW | ExP | + | < .05 | NR | |
| Mukari et al., 2020 | 72 | HINT | Multi | + | < .001 | −.44 | |
| Murphy et al., 2016 | 177 | SentW | WM | ~ | .70 | −.03 | |
| Murphy et al., 2018 | 77 | SentW | WM | ~ | > .05 | .03 | |
| Mussoi, 2021 | 31 | QSIN | WM | ~ | .04 | .38 | |
| HINT | ^^ | ~ | .86 | .03 | |||
| SPIN | ^^ | ~ | .16 | .27 | |||
| Neher, 2014 | 60 | SentCafe | WM | + | < .01 | .35 | |
| Neher et al., 2009 | 20 | Sent3 | WM | + | < .01 | −.58 | |
| Neher et al., 2012 | 17 | Sent2 | WM | + | < .05 | −.48 | |
| Neher et al., 2013 | 40 | SentCafe | WM | + | < .05 | NR | |
| Neher et al., 2014 | 40 | SentCafe | ProcS | + | < .001 | −.28 | |
| Ng et al., 2014 | 27 | SentSS | WM | ~ | > .05 | −.31 | |
| ^^ | ProcS | ~ | > .05 | .26 | |||
| ^^ | ^^ | ~ | > .05 | .32 | |||
| ^^ | ^^ | ~ | > .05 | −.23 | |||
| A-SentSS | ^^ | ~ | > .05 | −.42 | |||
| Ng et al., 2014 | 27 | A-SentSS | WM | + | < .01 | −.53 | |
| ^^ | ProcS | + | .47 | ≤ .05 | |||
| ^^ | ^^ | + | ≤ .05 | .39 | |||
| O'Brien et al., 2021 | 213 | WIN | ProcS | ~ | > .05 | .05 | |
| ^^ | ExP | ~ | > .05 | .11 | |||
| ^^ | WM | ~ | > .05 | .12 | |||
| ^^ | ^^ | ~ | > .05 | .13 | |||
| O'Brien et al., 2021 | 213 | WIN | Multi | + | < .01 | −.31 | |
| Parbery-Clark et al., 2011 | 37 | WIN | WM | ~ | .32 | −.17 | |
| Parbery-Clark et al., 2011 | 37 | QSIN | WM | + | .01 | −.40 | |
| HINT | ^^ | + | .03 | −.35 | |||
| Patro et al., 2021 | 61 | SentBab | ProcS | ~ | > .05 | NR | |
| ^^ | ExP | ~ | > .05 | NR | |||
| Pronk et al., 2013 | 1,298 | DigitStat | Multi | + | < .001 | −.10 | |
| Pronk et al., 2019 | 1,029 | DigitStat | Multi | + | NR | NR | |
| Quaranta et al., 2014 | 488 | SSI-ICM | Multi | + | .03 | OR = 2.4 | |
| Rönnberg et al., 2016 | 200 | Sent4 | Multi | + | < .001 | .20 | |
| Rosemann & Thiel, 2020 | 38 | SentSS | WM | ~ | > .05 | NR | |
| ^^ | ExP | ~ | > .05 | NR | |||
| Rosemann & Thiel, 2020 | 38 | SentSS | ExP | + | .02 | .58 | |
| Rudner et al., 2007 | 32 | A-SentMod | WM | ~ | > .05 | NR | |
| HINT | ^^ | ~ | > .05 | NR | |||
| Rudner et al., 2007 | 32 | SentMod | WM | + | < .05 | −.65 | |
| HINT | ^^ | + | < .05 | −.53 | |||
| Rudner et al., 2009 | 31 | A-SentSteady | WM | ~ | > .05 | −.01 | |
| A-SentMod | ^^ | ~ | > .05 | −.43 | |||
| Rudner et al., 2009 | 31 | A-SentSteady | WM | + | < .05 | −.58 | |
| A-SentMod | ^^ | + | < .05 | −.64 | |||
| HINT | ^^ | + | < .05 | −.58 | |||
| Rudner et al., 2011 | 30 | SentSteady | WM | + | .01 | −.46 | |
| SentMod | ^^ | + | .01 | −.48 | |||
| A-SentSteady | ^^ | + | < .05 | −.37 | |||
| A-SentMod | ^^ | + | < .01 | −.53 | |||
| HINT | ^^ | + | < .01 | −.60 | |||
| Sardone et al., 2020 | 1,647 | SSI-ICM | Multi | + | < .01 | .04 | |
| Sardone et al., 2021 | 1,929 | SSI-ICM | Multi | + | NR | OR = 1.9 | |
| Saunders et al., 2018 | 61 | QSIN | Multi | + | < .001 | −.55 | |
| Schoof & Rosen, 2014 | 38 | SentBab | WM | ~ | > .05 | NR | |
| ^^ | ProcS | ~ | > .05 | NR | |||
| Schoof & Rosen, 2014 | 38 | SentSS | ProcS | + | .01 | .44 | |
| Sheft et al., 2015 | 124 | QSIN | Multi | ~ | .70 | −.04 | |
| Slater & Kraus, 2016 | 54 | QSIN | WM | ~ | .15 | −.21 | |
| Smith & Pichora-Fuller, 2015 | 72 | SPIN | VisP | ~ | > .003 | NR | |
| Smith & Pichora-Fuller, 2015 | 72 | WIN | VisP | + | < .003 | −.77 | |
| QSIN | ^^ | + | < .003 | −.71 | |||
| Souza & Arehart, 2015 | 124 | QSIN | WM | + | .02 | −.18 | |
| Stroi & Souza, 2022 | 19 | QSIN | WM | ~ | > .05 | NR | |
| Strori & Souza, 2022 | 19 | QSIN | WM | + | .02 | .54 | |
| Van Rooij & Plomp, 1992 | 80 | SentSS | WM | ~ | .33 | .31 | |
| Wayne et al., 2016 | 26 | Sent2 | WM | ~ | > .05 | −.34 | |
| ^^ | ^^ | ~ | > .05 | −.23 | |||
| ^^ | ^^ | ~ | > .05 | .26 | |||
| ^^ | ^^ | ~ | > .05 | .31 | |||
| ^^ | ^^ | ~ | > .05 | −.17 | |||
| ^^ | ExP | ~ | > .05 | −.06 | |||
| Wayne et al., 2016 | 26 | Sent2 | WM | + | < .05 | .45 | |
| ^^ | ^^ | + | < .05 | −.42 | |||
| ^^ | ^^ | + | < .01 | .58 | |||
| Wong et al., 2014 | 34 | HINT | Multi | + | < .005 | .33 | |
| Yeend et al., 2019 | 122 | CompSIN | WM | + | < .001 | .02 | |
| Yumba, 2019 | 195 | CompSIN | ExP | ~ | .33 | t = −0.97 | |
| ^^ | Fluid | ~ | > .05 | NR | |||
| Yumba, 2019 | 195 | CompSIN | WM | + | .02 | t = −2.4 | |
| ^^ | ^^ | + | .03 | t = −2.2 | |||
| ^^ | ProcS | + | .05 | t = −2.5 | |||
| ^^ | ^^ | + | .02 | t = −2.5 | |||
| ^^ | ExP | + | .05 | t = −2.0 | |||
| Zhan et al., 2018 | 166 | HINT | Multi | + | .02 | .17 | |
| Altered speech | Bergemalm & Lyxell, 2005 | 22 | TCSpch | WM | + | NR | NR |
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ProcS | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | Fluid | + | NR | NR | |||
| Cervera et al., 2009 | 55 | TCSpch | WM | + | < .05 | .37 | |
| ^^ | ^^ | + | < .05 | .36 | |||
| Cox, 2008 | 45 | LPFS | WM | ~ | > .05 | .46 | |
| ^^ | ^^ | ~ | > .05 | .55 | |||
| Fostick et al., 2013 | 89 | TCSpch | WM | ~ | > .05 | −.02 | |
| Kamerer et al., 2019 | 32 | TCSpch | WM | + | < .01 | .40 | |
| ^^ | ExP | + | < .01 | .63 | |||
| Kim et al., 2020 | 96 | TCSpch | WM | + | .002 | .36 | |
| Moberly et al., 2019 | 83 | VocodSpch | ExP | + | .02 | −.35 | |
| ^^ | Fluid | + | .03 | .34 | |||
| O'Brien et al., 2021 | 213 | TCSpch | ProcS | ~ | > .05 | −.03 | |
| ^^ | ExP | ~ | > .05 | −.12 | |||
| ^^ | WM | ~ | > .05 | −.08 | |||
| ^^ | ^^ | ~ | > .05 | .13 | |||
| O'Brien et al., 2021 | 213 | TCSpch | Multi | + | < .01 | .36 | |
| Shader et al., 2020 | 30 | VocodSpch | WM | ~ | > .05 | NR | |
| Vaughan et al., 2008 | 225 | TCSpch | WM | ~ | > .05 | NR | |
| ^^ | ^^ | ~ | > .05 | .14 | |||
| ^^ | ProcS | ~ | > .05 | NR | |||
| Vaughan et al., 2008 | 225 | TCSpch | WM | + | < .05 | .28 | |
| ^^ | ProcS | + | < .05 | .12 | |||
| Temporal processing | Cox, 2008 | 45 | PPS | WM | ~ | > .05 | .25 |
| ^^ | ^^ | ~ | > .05 | .36 | |||
| Danielsson et al., 2019 | 245 | TempOr | WM | ~ | > .05 | −.10 | |
| ^^ | ^^ | ~ | > .05 | −.13 | |||
| Danielsson et al., 2019 | 245 | Gap | ProcS | + | < .05 | −.16 | |
| ^^ | ^^ | + | < .01 | −.23 | |||
| ^^ | Fluid | + | < .01 | −.19 | |||
| ^^ | WM | + | < .01 | −.29 | |||
| ^^ | ^^ | + | < .01 | −.24 | |||
| ^^ | ^^ | + | < .01 | −.19 | |||
| ^^ | ^^ | + | < .05 | −.16 | |||
| TempOr | ProcS | + | < .01 | −.44 | |||
| ^^ | ^^ | + | < .01 | −.42 | |||
| ^^ | Fluid | + | < .01 | −.27 | |||
| ^^ | WM | + | < .01 | −.4 | |||
| ^^ | ^^ | + | < .01 | −.49 | |||
| ^^ | ^^ | + | < .01 | −.31 | |||
| ^^ | ^^ | + | < .05 | −.18 | |||
| Füllgrabe et al., 2015 | 30 | Envelope | WM | ~ | > .05 | −.15 | |
| ^^ | ^^ | ~ | > .05 | −.15 | |||
| ^^ | ^^ | ~ | > .05 | .09 | |||
| ^^ | ExP | ~ | > .05 | −.26 | |||
| ^^ | ^^ | ~ | > .05 | −.11 | |||
| Füllgrabe et al., 2015 | 30 | TempFS | WM | + | ≤ .05 | .49 | |
| ^^ | ExP | + | ≤ .05 | .54 | |||
| Humes, 2005 | 213 | Duration | Multi | + | < .01 | .32 | |
| TempOr | ^^ | + | < .01 | .26 | |||
| Humes, 2021b | 98 | Gap | VisP | + | < .05 | .22 | |
| TempOr | Fluid | + | < .01 | −.32 | |||
| Humes et al., 2010 | 339 | TempMas | Multi | ~ | > .05 | NR | |
| Humes et al., 2010 | 339 | Gap | Multi | + | < .01 | .87 | |
| TempOr | ^^ | + | < .01 | .54 | |||
| Humes et al., 2013 | 125 | ModD | Multi | ~ | > .01 | −.25 | |
| CompTP | ^^ | ~ | > .01 | −.25 | |||
| Kamerer et al., 2019 | 32 | ModD | WM | + | < .01 | .17 | |
| ^^ | ^^ | + | < .01 | .24 | |||
| Lentz et al., 2022 | 155 | StrSeg | WM | ~ | .35 | −.08 | |
| Lentz et al., 2022 | 155 | ModD | WM | + | < .001 | .09 | |
| ^^ | ^^ | + | < .001 | −.34 | |||
| TempMas | ^^ | + | .002 | −.25 | |||
| Mioni et al., 2021 | 247 | TimeDis | WM | + | < .01 | .009 | |
| ^^ | ExP | + | < .05 | .01 | |||
| ^^ | ProcS | + | < .05 | .02 | |||
| Mukari et al., 2020 | 72 | GIN | Multi | ~ | > .05 | −.21 | |
| Murphy et al., 2016 | 177 | FPT | WM | + | .004 | .21 | |
| Murphy et al., 2018 | 77 | FPT | WM | + | < .001 | .43 | |
| Neher et al., 2012 | 17 | TempFS | WM | + | .06 | .48 | |
| O'Brien et al., 2021 | 213 | ATTR | WM | ~ | > .05 | −.06 | |
| ^^ | ^^ | ~ | > .05 | −.17 | |||
| ^^ | Multi | ~ | > .05 | −.07 | |||
| ^^ | ExP | ~ | > .05 | .12 | |||
| ^^ | ProcS | ~ | > .05 | .009 | |||
| O'Brien et al., 2021 | 213 | ATTR | ExP | + | < .01 | .40 | |
| ^^ | Multi | + | < .05 | −.21 | |||
| ^^ | WM | + | < .05 | −.22 | |||
| ^^ | ^^ | + | < .05 | −.27 | |||
| Patro et al., 2021 | 61 | Envelope | ProcS | + | < .05 | NR | |
| ^^ | ExP | + | < .05 | NR | |||
| Rönnberg et al., 2016 | 200 | TempFS | Multi | + | < .001 | .30 | |
| Strelcyk et al., 2019 | 20 | ILD JND | ExP | ~ | > .05 | .60 | |
| ^^ | ^^ | ~ | > .05 | .34 | |||
| ^^ | Multi | ~ | > .05 | −.48 | |||
| IPD JND | ^^ | ~ | > .05 | −.38 | |||
| ^^ | ProcS | ~ | > .05 | .61 | |||
| IPD Freq | Multi | ~ | > .05 | .42 | |||
| Strelcyk et al., 2019 | 20 | IPD Freq | ProcS | + | < .05 | −.69 | |
| ^^ | ExP | + | < .001 | −.83 | |||
| ^^ | ExP | + | < .05 | −.68 | |||
| IPD JND | ^^ | + | < .05 | .67 | |||
| ^^ | ExP | + | < .01 | .79 | |||
| ILD JND | ProcS | + | < .05 | .72 | |||
| Szymaszek et al., 2009 | 86 | TempOr | Fluid | ~ | > .05 | NR | |
| Szymaszek et al., 2009 | 86 | TempOr | Fluid | + | < .07 | .20 | |
| Ulbrich et al., 2009 | 100 | TempOr | WM | + | ≤ .001 | −.33 | |
| Binaural processing | Ghannoum et al., 2018 | 71 | DDT | ExP | ~ | 1.29 | .02 |
| ^^ | ^^ | ~ | 1.23 | .02 | |||
| ^^ | WM | ~ | .11 | .07 | |||
| ^^ | VisP | ~ | 1.21 | .05 | |||
| Hällgren et al., 2001 | 30 | DDT-LF | WM | ~ | > .05 | NR | |
| ^^ | ProcS | ~ | > .05 | NR | |||
| ^^ | ^^ | ~ | > .05 | NR | |||
| ^^ | ^^ | ~ | > .05 | NR | |||
| ^^ | ^^ | ~ | > .05 | NR | |||
| Hällgren et al., 2001 | 30 | DiSpch-FR | WM | + | < .05 | .57 | |
| ^^ | ProcS | + | < .05 | −.38 | |||
| ^^ | ^^ | + | < .05 | −.46 | |||
| ^^ | ^^ | + | < .05 | .46 | |||
| ^^ | ^^ | + | < .05 | −.44 | |||
| Hommet et al., 2010 | 53 | DiSpch-FR | ProcS | + | < .05 | −.52 | |
| ^^ | ExP | + | < .05 | −.54 | |||
| ^^ | WM | + | < .05 | .48 | |||
| DiSpch-RF | ExP | + | < .05 | .66 | |||
| Humes, 2005 | 213 | DiCV | Multi | + | < .05 | .48 | |
| Humes et al., 2013 | 125 | MLD | WM | ~ | > .01 | NR | |
| Lentz et al., 2022 | 155 | MLD | WM | + | .005 | −.37 | |
| Mukari et al., 2020 | 72 | DDT | Multi | ~ | > .05 | .07 | |
| Murphy et al., 2016 | 177 | DDT-RF | WM | ~ | .07 | .15 | |
| Murphy et al., 2016 | 177 | DDT-LF | WM | + | .04 | .14 | |
| Murphy et al., 2018 | 77 | DDT-LF | WM | ~ | .09 | .20 | |
| Murphy et al., 2018 | 77 | DDT-RF | WM | + | < .01 | .30 | |
| Nixon et al., 2019 | 85 | DDT | ExP | ~ | > .05 | NR | |
| ^^ | Fluid | ~ | > .05 | NR | |||
| ^^ | VisP | ~ | > .05 | NR | |||
| ^^ | WM | ~ | > .05 | NR | |||
| O'Brien et al., 2021 | 213 | DSI-RF | ProcS | ~ | > .05 | −.19 | |
| DDT-RF | ^^ | ~ | > .05 | −.15 | |||
| ^^ | ExP | ~ | > .05 | −.19 | |||
| DSI-LF | WM | ~ | > .05 | .06 | |||
| DDT-LF | ^^ | ~ | > .05 | .14 | |||
| O'Brien et al., 2021 | 213 | DSI-LF | ProcS | + | < .01 | −.34 | |
| DDT-LF | ^^ | + | < .05 | −.25 | |||
| ^^ | ExP | + | < .01 | −.31 | |||
| DSI-RF | ^^ | + | < .05 | .20 | |||
| DDT-RF | ^^ | + | < .05 | .27 | |||
| ^^ | ^^ | + | < .01 | .31 | |||
| Pinheiro et al., 2012 | 60 | DDT | Multi | + | .01 | NR | |
| Spatial processing | Bergemalm & Lyxell, 2005 | 22 | PhasAud | WM | + | NR | NR |
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ProcS | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | Fluid | + | NR | NR | |||
| Glyde et al., 2013 | 80 | LISN | Multi | ~ | |||
| Humes et al., 2013 | 125 | CRM | WM | ~ | > .01 | NR | |
| Nixon et al., 2019 | 85 | LISN-LC | ExP | + | .01 | .28 | |
| LISN-SA | ^^ | + | .03 | −.22 | |||
| ^^ | VisP | + | .03 | −.16 | |||
| Other | Biagianti et al., 2016 | 131 | APS | VisP | + | < .05 | −.25 |
| ^^ | ^^ | + | < .01 | −.29 | |||
| ^^ | ^^ | + | < .001 | −.36 | |||
| ^^ | ProcS | + | < .001 | −.34 | |||
| ^^ | Fluid | + | < .05 | −.34 | |||
| Biswas et al., 2016 | 21 | SRhT | WM | ~ | > .05 | .25 | |
| ^^ | ^^ | ~ | > .05 | .4 | |||
| MBEA | ^^ | ~ | > .05 | .22 | |||
| ^^ | ^^ | ~ | > .05 | .24 | |||
| BAT | ^^ | ~ | > .05 | .37 | |||
| Biswas et al., 2016 | 21 | SRhT | WM | + | < .01 | .55 | |
| MBEA | ^^ | + | < .05 | .44 | |||
| BAT | ^^ | + | < .05 | −.48 | |||
| ^^ | ^^ | + | < .05 | .47 | |||
| Jain et al., 2022 | 60 | CompAP | WM | + | < .001 | .58 | |
| Lentz et al., 2022 | 155 | HarMis | WM | + | < .001 | −.35 |
Note. Correlations indicated using “positive” (+), “negative” (−), or “null” (~) symbols. ^^ signifies “same as above.” A- = Aided; APS = Auditory Processing Speech; Bab = Babble; BAT = Beat Alignment Test; CafÕ = Cafeteria Noise; Comp = Composite; Digit = Digits; Dis = Discrimination; DiSpch = Dichotic Speech; DSI = Dichotic Sentence Identification; ExP = Executive Processes; Fluc = Fluctuating Noise; Fluid = fluid intelligence; FS = FineStructure; Gap = Gap Detection; HarMis = Harmonic Mistuning; LC = Low Cue; Mas = Masking; Mod = Modulated Noise; ModD = Modulation Detection; Multi = multiple-domains; Sent = Sentences; Or = Order; PhasAud = Phase Audiometry; ProcS = processing speed; SA = Spatial Advantage; Sent# = Sentences in #-talker masker; SIN = Speech in Noise; Spch = Speech; Stat = Stationary Noise; SRhT= Seashore Rhythm Test; Steady = Steady State Noise; StrSeg = Stream Segregation; Temp = Temporal; UnMod = Unmodulated Noise; VisP = visual perception; Vocod = Vocoded; WM = working memory.
Table A2.
Correlations between electrophysiologic auditory measures and cognitive constructs.
| Electrophysiologic construct | Study | N | Measure | Cognitive construct | Correlation | p value | Effect size |
|---|---|---|---|---|---|---|---|
| Mismatch negativity | Bonetti et al., 2018 | 86 | Amplitude | WM | ~ | .97 | .14 |
| Bonetti et al., 2018^^ | 86 | Amplitude | WM | + | < .05 | .37 | |
| Brückmann et al., 2021 | 54 | Latency | Multi | ~ | .53 | .09 | |
| Amplitude | ^^ | ~ | .75 | −.05 | |||
| Kärgel et al., 2014 | 56 | Amplitude | WM | ~ | .65 | −.07 | |
| ^^ | ^^ | ~ | .88 | −.03 | |||
| ^^ | ProcS | ~ | .97 | -.01 | |||
| ^^ | ExP | ~ | .81 | -.04 | |||
| Latency | ^^ | ~ | .94 | .01 | |||
| ^^ | ProcS | ~ | .23 | .20 | |||
| ^^ | WM | ~ | .64 | .08 | |||
| Kärgel et al., 2014^^ | 56 | Latency | WM | + | .01 | .40 | |
| ^^ | ^^ | + | .02 | .38 | |||
| Naismith et al., 2012 | 34 | Amplitude | Multi | ~ | .97 | .01 | |
| ^^ | ProcS | ~ | .96 | −.01 | |||
| ^^ | ExP | ~ | .78 | .07 | |||
| ^^ | WM | ~ | .58 | .01 | |||
| Auditory brainstem response | Bergemalm & Lyxell, 2005 | 22 | ABR | WM | + | NR | NR |
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ProcS | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | ^^ | + | NR | NR | |||
| ^^ | Fluid | + | NR | NR | |||
| Delano et al., 2020 | 101 | Wave 1 | ExP | ~ | .21 | .14 | |
| ^^ | ^^ | ~ | .82 | −.02 | |||
| ^^ | WM | ~ | .33 | −.10 | |||
| Delano et al., 2020^^ | 101 | Wave 1 | ProcS | + | .05 | .20 | |
| ^^ | ^^ | + | .01 | −.27 | |||
| Kamerer et al., 2019 | 32 | Speech | WM | ~ | NR | NR | |
| Speech | WM | + | NR | .57 | |||
| P50 | Hamilton et al., 2018 | 93 | Ratio | Multi | ~ | .74 | −.06 |
| Hamilton et al., 2018^^ | 93 | Ratio | Multi | + | .01 | −.41 | |
| Hsieh et al., 2004 | 20 | Suppression | WM | ~ | > .05 | .06 | |
| ^^ | ^^ | ~ | > .05 | .04 | |||
| ^^ | ^^ | ~ | > .05 | .17 | |||
| ^^ | ^^ | ~ | > .05 | .43 | |||
| Sánchez-Morla et al., 2013 | 225 | Ratio | Multi | ~ | .28 | .02 | |
| ^^ | ProcS | ~ | .35 | .09 | |||
| ^^ | ^^ | ~ | .77 | −.03 | |||
| ^^ | WM | ~ | .06 | .02 | |||
| ^^ | ^^ | ~ | .60 | −.13 | |||
| ^^ | Multi | ~ | .56 | −.19 | |||
| ^^ | VisP | ~ | .08 | −.18 | |||
| ^^ | ExP | ~ | .90 | .06 | |||
| ^^ | ^^ | ~ | .99 | .06 | |||
| ^^ | ^^ | ~ | .50 | .09 | |||
| Difference | ^^ | ~ | .50 | −.07 | |||
| ^^ | ^^ | ~ | .90 | .01 | |||
| ^^ | ^^ | ~ | .99 | −.10 | |||
| ^^ | VisP | ~ | .08 | .04 | |||
| ^^ | Multi | ~ | .56 | .15 | |||
| ^^ | WM | ~ | .06 | .03 | |||
| ^^ | ^^ | ~ | .60 | .12 | |||
| ^^ | ProcS | ~ | .35 | −.08 | |||
| ^^ | ^^ | ~ | .77 | .07 | |||
| ^^ | Multi | ~ | .28 | .10 | |||
| Thomas et al., 2010 | 53 | Suppression | WM | + | .01 | −.46 | |
| ^^ | Multi | + | .003 | −.49 | |||
| Xia et al., 2020 | 70 | Ratio | Multi | ~ | > .05 | NR | |
| Yadon et al., 2009 | 25 | Suppression | ExP | ~ | > .05 | −.01 | |
| Yadon et al., 2009^^ | 25 | Suppression | ExP | + | .01 | −.52 | |
| ^^ | ^^ | + | .02 | .45 | |||
| N100 | Cooray et al., 2008 | 180 | Amplitude | Multi | + | < .04 | .25 |
| Latency | ^^ | + | < .003 | −.35 | |||
| ^^ | ^^ | + | < .001 | −.29 | |||
| ^^ | ^^ | + | < .01 | −.25 | |||
| ^^ | ^^ | + | < .01 | −.22 | |||
| ^^ | ^^ | + | < .03 | −.20 | |||
| ^^ | ^^ | + | < .01 | −.22 | |||
| ^^ | ^^ | + | < .03 | −.20 | |||
| Hsieh et al., 2004 | 20 | Suppression | WM | ~ | > .05 | .35 | |
| ^^ | ^^ | ~ | > .05 | .52 | |||
| Hsieh et al., 2004^^ | 20 | Suppression | WM | + | < .01 | .79 | |
| ^^ | ^^ | + | < .01 | .76 | |||
| N200 | Bodis-Wollner et al., 1995 | 50 | Latency | WM | + | < .025 | −.45 |
| ^^ | Multi | ~ | > .10 | −.06 | |||
| Folmer et al., 2021 | 70 | Amplitude | Multi | + | < .02 | .39 | |
| Pang et al., 1990 | 43 | Mean | Fluid | ~ | < .10 | −.33 | |
| ^^ | VisP | ~ | > .10 | −.32 | |||
| ^^ | ^^ | ~ | < .10 | −.34 | |||
| ^^ | Fluid | ~ | > .10 | −.30 | |||
| ^^ | ^^ | ~ | > .10 | −.35 | |||
| Pang et al., 1990^^ | 43 | Mean | Multi | + | < .05 | −.42 | |
| ^^ | WM | + | < .001 | −.70 | |||
| ^^ | VisP | + | < .01 | −.54 | |||
| Pokryszko-Dragan et al., 2009 | 21 | Amplitude | Multi | ~ | NR | NR | |
| ^^ | ^^ | ~ | NR | NR | |||
| Pokryszko-Dragan et al., 2009^^ | 21 | Latency | ExP | + | < .05 | .54 | |
| ^^ | WM | + | < .05 | .49 | |||
| Stuckenschneider et al., 2020 | 26 | Amplitude | ProcS | ~ | .06 | .37 | |
| Stuckenschneider et al., 2020 | 26 | Amplitude | Multi | + | < .001 | −.75 | |
| ^^ | ProcS | + | < .001 | −.65 | |||
| ^^ | ExP | + | .001 | −.60 | |||
| ^^ | ProcS | + | .04 | .41 | |||
| Latency | Multi | + | < .001 | −.71 | |||
| ^^ | ExP | + | .01 | .48 | |||
| ^^ | ProcS | + | < .001 | .72 | |||
| ^^ | ^^ | + | .03 | −.43 | |||
| ^^ | ^^ | + | < .001 | −.79 | |||
| P200 | Nagasawa et al., 1999 | 20 | Amplitude | ExP | + | < .05 | .50 |
| Nagasawa et al., 1999 | 20 | ^^ | WM | ~ | > .05 | −.11 | |
| Oliveira et al., 2021 | 30 | Amplitude | Multi | + | < .001 | .45 | |
| Stuckenschneider et al., 2020 | 26 | Latency | ExP | ~ | .21 | .26 | |
| ^^ | ProcS | ~ | .49 | −.14 | |||
| ^^ | ^^ | ~ | .14 | −.30 | |||
| Amplitude | ExP | ~ | .06 | .37 | |||
| ^^ | ProcS | ~ | .11 | −.32 | |||
| ^^ | ^^ | ~ | .24 | −.24 | |||
| Stuckenschneider et al., 2020^^ | 26 | Amplitude | Multi | + | < .001 | −.72 | |
| ^^ | ProcS | + | .01 | .49 | |||
| Latency | Multi | + | .03 | −.43 | |||
| ^^ | ProcS | + | .02 | .45 | |||
| P300 | Bodis-Wollner et al., 1995 | 50 | Latency | Multi | ~ | > .10 | −.03 |
| ^^ | WM | ~ | > .10 | −.28 | |||
| Cooray et al., 2008 | 180 | Amplitude | Multi | + | < .003 | .35 | |
| ^^ | ^^ | + | < .003 | .27 | |||
| ^^ | ^^ | + | < .02 | .21 | |||
| Latency | ^^ | + | < .01 | −.24 | |||
| ^^ | ^^ | + | < .05 | −.18 | |||
| Dichter et al., 2006 | 25 | Amplitude | Fluid | ~ | > .05 | .39 | |
| ^^ | WM | ~ | > .05 | −.06 | |||
| ^^ | ExP | ~ | > .05 | −.34 | |||
| ^^ | ^^ | ~ | > .05 | .32 | |||
| ^^ | ^^ | ~ | > .05 | .16 | |||
| ^^ | ProcS | ~ | > .05 | −.29 | |||
| Latency | Fluid | ~ | > .05 | −.15 | |||
| ^^ | WM | ~ | > .05 | .33 | |||
| ^^ | ProcS | ~ | > .05 | .22 | |||
| ^^ | ExP | ~ | > .05 | .43 | |||
| ^^ | ^^ | ~ | > .05 | .001 | |||
| ^^ | ^^ | ~ | > .05 | −.27 | |||
| Dichter et al., 2006^^ | 25 | Amplitude | ProcS | + | < .05 | −.69 | |
| ^^ | ExP | + | < .05 | −72 | |||
| Latency | ^^ | + | < .05 | .69 | |||
| ^^ | WM | + | < .05 | .71 | |||
| ^^ | ExP | + | < .05 | −.70 | |||
| Gurrera et al., 2005 | 43 | Amplitude | WM | + | < .01 | .54 | |
| ^^ | ProcS | + | < .01 | .43 | |||
| Latency | WM | + | < .05 | −.34 | |||
| ^^ | ^^ | + | < .05 | −.33 | |||
| ^^ | ProcS | + | < .05 | −.32 | |||
| ^^ | ExP | + | < .05 | −.33 | |||
| Hayashi et al., 1993 | 53 | Latency | Multi | ~ | < .05 | NR | |
| Katada et al., 2003 | 13 | Amplitude | Multi | ~ | > .05 | NR | |
| Latency | ^^ | ~ | > .05 | NR | |||
| Katada et al., 2003^^ | 13 | Latency | Multi | + | < .01 | .68 | |
| Katsarou et al., 2004 | 85 | Latency | WM | ~ | > .05 | −.03 | |
| ^^ | ^^ | ~ | > .05 | .26 | |||
| Amplitude | ^^ | ~ | > .05 | NR | |||
| ^^ | ^^ | ~ | > .05 | NR | |||
| Katsarou et al., 2004^^ | 85 | Latency | Fluid | + | < .02 | −.35 | |
| ^^ | ExP | + | < .001 | .49 | |||
| Krishnamurthy et al., 2019 | 84 | Latency | Multi | ~ | .08 | .19 | |
| Amplitude | ^^ | ~ | .21 | .14 | |||
| Marsh et al., 1990 | 35 | Latency | Multi | + | < .001 | −.58 | |
| De Miranda et al., 2012 | 60 | Latency | Multi | ~ | > .05 | NR | |
| ^^ | ^^ | ~ | > .05 | NR | |||
| Nagasawa et al., 1999 | 20 | Amplitude | VisP | + | < .01 | .65 | |
| Neshige et al., 1988 | 27 | Amplitude | Multi | ~ | > .05 | NR | |
| Neshige et al., 1988^^ | 27 | Latency | Multi | + | < .001 | −.71 | |
| Ozcan et al., 2016 | 72 | Amplitude | ExP | + | ≤.05 | −.39 | |
| Pang et al., 1990 | 43 | Mean | Fluid | ~ | < .10 | −.45 | |
| ^^ | ^^ | ~ | > .10 | −.24 | |||
| ^^ | VisP | ~ | > .10 | −.22 | |||
| ^^ | Fluid | ~ | > .10 | −.19 | |||
| Pang et al., 1990^^ | 43 | Mean | Multi | + | < .05 | −.37 | |
| ^^ | WM | + | < .01 | −.62 | |||
| ^^ | VisP | + | < .05 | −.44 | |||
| ^^ | ^^ | + | < .05 | −.44 | |||
| Podemski et al., 2008 | 61 | Latency | Multi | ~ | > .05 | NR | |
| ^^ | ^^ | ~ | > .05 | NR | |||
| ^^ | ExP | ~ | > .05 | NR | |||
| Amplitude | Multi | ~ | > .05 | NR | |||
| ^^ | ExP | ~ | > .05 | NR | |||
| Podemski et al., 2008^^ | 61 | Amplitude | Multi | + | .03 | −.45 | |
| Pokryszko-Dragan et al., 2003 | 26 | Latency | Multi | + | < .05 | .66 | |
| Pokryszko-Dragan et al., 2009 | 21 | Amplitude | Multi | ~ | NR | NR | |
| ^^ | ^^ | ~ | NR | NR | |||
| ^^ | ExP | ~ | NR | NR | |||
| Latency | ^^ | ~ | NR | NR | |||
| ^^ | Multi | ~ | NR | NR | |||
| ^^ | ^^ | ~ | NR | NR | |||
| Portin et al., 2000 | 200 | Latency | WM | ~ | > .05 | −.04 | |
| ^^ | ^^ | ~ | > .05 | −.02 | |||
| Portin et al., 2000^^ | 200 | Latency | ^^ | + | < .05 | −.19 | |
| Amplitude | ^^ | + | < .01 | .24 | |||
| Stuckenschneider et al., 2020 | 26 | Latency | ExP | ~ | .06 | .37 | |
| ^^ | ProcS | ~ | .16 | −.28 | |||
| Amplitude | ExP | ~ | .08 | .36 | |||
| Stuckenschneider et al., 2020^^ | 26 | Amplitude | Multi | + | < .001 | −.73 | |
| ^^ | ProcS | + | .002 | .57 | |||
| ^^ | ^^ | + | .05 | −.39 | |||
| ^^ | ^^ | + | .02 | −.45 | |||
| Latency | Multi | + | < .001 | .74 | |||
| ^^ | ProcS | + | .01 | .53 | |||
| ^^ | ^^ | + | .02 | −.45 | |||
| Tanaka et al., 2000 | 69 | Amplitude | Multi | + | .03 | .40 | |
| Latency | ^^ | + | .02 | −.43 | |||
| Volpato et al., 2010 | 41 | Amplitude | Fluid | + | .02 | .43 | |
| ^^ | WM | + | .02 | .47 | |||
| ^^ | ExP | + | .05 | −.42 | |||
| Latency | WM | + | .04 | −.39 | |||
| ^^ | ^^ | + | .01 | −.41 | |||
| ^^ | ^^ | + | .004 | −.56 | |||
| Zeng et al., 2017 | 96 | Latency | Multi | ~ | .40 | −.10 | |
| Amplitude | ^^ | ~ | .78 | .03 | |||
| Zeng et al., 2017^^ | 96 | Latency | Multi | + | < .01 | −.59 | |
| Amplitude | ^^ | + | < .01 | .77 |
Note. ABR = auditory brainstem response; ExP = Executive Processes; Fluid = Fluid Intelligence; Multi = Multiple-Domains; ProcS = Processing Speed; VisP = Visual Perception; WM = Working Memory.
Table A3.
Relationships examined in only one study.
| Significant | Nonsignificant | Mixed findings | ||||
|---|---|---|---|---|---|---|
| Working memory | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| CompAP | ABR | A-Sent/SS | ABR1 | ATTR | ABRSp | |
| Gap | N200M | CompTP | P200A | BAT | N100S | |
| HarMis | P300M | CRM | P50D | MBEA | MMNL | |
| PhasAud | DSI-LF | P50R | SRhT | |||
| Sent4 | Envelope | VCV | ||||
| SentSteady | LPFS | |||||
| TempMask | PPS | |||||
| TimeDis | Sent1 | |||||
| SentFluc | ||||||
| SSI-CCM | ||||||
| SSI-ICM | ||||||
| StrSeg | ||||||
| VocodSpch | ||||||
| WordsBab | ||||||
| Words/W | ||||||
| Processing speed | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| APS | ABR | ATTR | MMNL | A-Sent/SS | N200A | |
| CompSIN | ABR1 | DDT-RF | P50R | N200L | ||
| DSI-LF | DSI-RF | P50D | P200A | |||
| Gap | IPD JND | P200L | ||||
| ILD JND | Sent4k | |||||
| IPD Freq | Sent16k | |||||
| PhasAud | WIN | |||||
| Sent2 | Words/W | |||||
| SentCafe | ||||||
| Envelope | ||||||
| TempOr | ||||||
| TimeDis | ||||||
| WordsBab | ||||||
| Executive/inhibitory processes | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| DDT-LF | N200A | ILD JND | ABR1 | ATTR | P50S | |
| DiSpch-FR | SentBab | MMNL | DDT-RF | |||
| DiSpch-RF | SSI-CCM | P200L | Sent1 | |||
| DSI-RF | SSI-ICM | P50R | WordsW | |||
| IPD Freq | WIN | |||||
| IPD JND | ||||||
| LISN-LC | ||||||
| LISN-SA | ||||||
| QSIN | ||||||
| TempFS | ||||||
| TimeDis | ||||||
| VCV | ||||||
| VocodSpch | ||||||
| Words1 | ||||||
| Fluid intelligence | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| APS | ABR | CompSIN | N200M | P300M | ||
| Gap | DDT | |||||
| PhasAud | VocodSpch | |||||
| TCSpch | ||||||
| Visual perception | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| APS | P300A | SentSS | P50R | N200M | ||
| Gap | SSI-CCM | P50D | P300M | |||
| LISN-SA | SSI-ICM | |||||
| QSIN | Words/W | |||||
| WIN | ||||||
| Multidomain/global cognition | ||||||
| Behavioral | Electro | Behavioral | Electro | Behavioral | Electro | |
| DiCV | N100A | GIN | MMNL | ATTR | ||
| Duration | N100L | ILD JND | P50D | |||
| Gap | N200M | IPD Freq | ||||
| Sent4 | P200L | IPD JND | ||||
| SentBab | P300M | LISN | ||||
| TCSpch | P50S | TempMask | ||||
| TempFS | Words/W | |||||
| WIN | ||||||
Note. Correlations indicated using “positive” (+), “negative” (−), or “null” (~) symbols. ^^ signifies “same as above.” A- = Aided; A = Amplitude; ABR1 = Wave1; APS = Auditory Processing Speech; Bab = Babble; BAT = Beat Alignment Test; CafÕ = Cafeteria Noise; Comp = Composite; D = Difference; Dis = Discrimination; DiSpch = Dichotic Speech; DSI = Dichotic Sentence Identification; Fluc = Fluctuating Noise; FS = FineStructure; Gap = Gap Detection; HarMis = Harmonic Mistuning; L = Latency; LC = Low Cue; M = Mean; Mas = Masking; Sent = Sentences; Or = Order; PhasAud = Phase Audiometry; R = Ratio; S = Suppression; SA = Spatial Advantage; Sent# = Sentences in #-talker masker; SIN = Speech in Noise; Sp = Speech; Spch = Speech; SRhT= Seashore Rhythm Test; Steady = Steady State Noise; StrSeg = Stream Segregation; Temp = Temporal; Vocod = Vocoded; W = White Noise.
Footnote
There were no relationships measured in more than one study for behavioral auditory measures in the spatial processing or other categories, or ABR and N100 categories for electrophysiologic auditory measures. As such, these categories were not included in the analyses.
References
- Agrawal, D., Dritsakis, G., Mahon, M., Mountjoy, A., & Bamiou, D. E. (2021). Experiences of patients with auditory processing disorder in getting support in health, education, and work settings: Findings from an online survey. Frontiers in Neurology, 12. 10.3389/fneur.2021.607907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeroyd, M. A. (2008). Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. International Journal of Audiology, 47(Suppl. 2), S53–S71. 10.1080/14992020802301142 [DOI] [PubMed] [Google Scholar]
- Alles, R., Bamiou, D., Batchelor, L., Campbell, N.G., Canning, D., Grant, P., Luxon, L., Moore, D., Murray, P., Nairn, S., Rosen, S., Sirimanna, T., Treharne, D., & Wakeham, K. (2011). BSA position statement: Auditory processing disorder (APD).
- American Academy of Audiology. (2010). Diagnosis, treatment and management of children and adults with central auditory processing disorder. https://www.audiology.org/wp-content/uploads/2021/05/CAPD-Guidelines-8-2010-1.pdf_539952af956c79.73897613-1.pdf
- American Speech-Language-Hearing Association. (n.d.). Central auditory processing disorder [Practice Portal]. Retrieved December 4, 2022 from www.asha.org/Practice-Portal/Clinical-Topics/Central-Auditory-Processing-Disorder/
- Anderson, M., Arehart, K. H., & Souza, P. (2018). Survey of current practice in the fitting and fine-tuning of common signal-processing features in hearing aids for adults. Journal of the American Academy of Audiology, 29(2), 118–124. 10.3766/jaaa.16107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, S., White-Schwoch, T., Parbery-Clark, A., & Kraus, N. (2013). A dynamic auditory-cognitive system supports speech-in-noise perception in older adults. Hearing Research, 300, 18–32. 10.1016/j.heares.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzivino, R., Conti, G., Di Nardo, W., Fetoni, A. R., Picciotti, P. M., Marra, C., Guglielmi, V., Fortunato, S., Forli, F., Paludetti, G., & Berrettini, S. (2019). Prospective evaluation of cognitive functions after rehabilitation with cochlear implant or hearing aids: Preliminary results of a multicentric study on elderly patients. American Journal of Audiology, 28(3S), 762–774. 10.1044/2019_AJA-HEAL18-18-0176 [DOI] [PubMed] [Google Scholar]
- Arehart, K. H., Souza, P., Baca, R., & Kates, J. M. (2013). Working memory, age, and hearing loss: Susceptibility to hearing aid distortion. Ear and Hearing, 34(3), 251–260. 10.1097/AUD.0b013e318271aa5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arehart, K. H., Souza, P., Kates, J. M., Lunner, T., & Pedersen, M. S. (2015). Relationship among signal fidelity, hearing loss, and working memory for digital noise suppression. Ear and Hearing, 36, 505–516. 10.1097/AUD.0000000000000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley, A. (1992). Working memory. Science, 255(5044), 556–559. 10.1126/science.1736359 [DOI] [PubMed] [Google Scholar]
- Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63(1), 1–29. 10.1146/annurev-psych-120710-100422 [DOI] [PubMed] [Google Scholar]
- Bamiou, D.-E., Werring, D., Cox, K., Stevens, J., Musiek, F. E., Brown, M. M., & Luxon, L. M. (2012). Patient-reported auditory functions after stroke of the central auditory pathway. Stroke, 43(5), 1285–1289. 10.1161/STROKEAHA.111.644039 [DOI] [PubMed] [Google Scholar]
- Bellis, T. J., & Bellis, J. D. (2015). Chapter 30—Central auditory processing disorders in children and adults. In Aminoff M. J., Boller F., & Swaab D. F. (Eds.), Handbook of clinical neurology (Vol. 129, pp. 537–556). Elsevier. [DOI] [PubMed] [Google Scholar]
- Bergemalm, P. O., & Lyxell, B. (2005). Appearances are deceptive? Long-term cognitive and central auditory sequelae from closed head injury. ¿Las apariencias engañan? Secuelas cognitivas y auditivas centrales a largo plazo después de un traumatismo cráneo-encefálico cerrado. International Journal of Audiology, 44(1), 39–49. 10.1080/14992020400022546 [DOI] [PubMed] [Google Scholar]
- Biagianti, B., Fisher, M., Neilands, T. B., Loewy, R., & Vinogradov, S. (2016). Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology, 30(8), 998–1008. 10.1037/neu0000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, A., Hegde, S., Jhunjhunwala, K., & Pal, P. K. (2016). Two sides of the same coin: Impairment in perception of temporal components of rhythm and cognitive functions in Parkinson's disease. Basal Ganglia, 6(1), 63–70. 10.1016/j.baga.2015.12.001 [DOI] [Google Scholar]
- Bodis-Wollner, I., Borod, J. C., Cicero, B., Haywood, C. S., Raskin, S., Mylin, L., Sliwinski, M., Falk, A., & Yahr, M. D. (1995). Modality dependent changes in event-related potentials correlate with specific cognitive functions in nondemented patients with Parkinson's disease. Journal of Neural Transmission - Parkinson's Disease and Dementia Section, 9(2), 197–209. 10.1007/BF02259661 [DOI] [PubMed] [Google Scholar]
- Bonetti, L., Haumann, N. T., Brattico, E., Kliuchko, M., Vuust, P., Särkämö, T., & Näätänen, R. (2018). Auditory sensory memory and working memory skills: Association between frontal MMN and performance scores. Brain Research, 1700, 86–98. 10.1016/j.brainres.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Broome, E. E., Tannirandorn, P., Straus, J., Beale, P., Heffernan, E., Dening, T., & Henshaw, H. (2023). Patient perceptions of cognitive screening in adult audiology services: A qualitative exploration. Frontiers in Neurology, 14, Article 567. 10.3389/fneur.2023.1143128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückmann, M., Pagliarin, K. C., & Garcia, M. V. (2021). Mismatch negativity in older adults and its relationship with the cognitive and behavioral aspects of central auditory processing. Clinics, 76, Article e1830. 10.6061/clinics/2021/e1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson, A. M., & Souza, P. E. (2022). Cognitive and linguistic abilities and perceptual restoration of missing speech: Evidence from online assessment. Frontiers in Psychology, 13. 10.3389/fpsyg.2022.1059192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahana-Amitay, D., Spiro, A., III, Sayers, J. T., Oveis, A. C., Higby, E., Ojo, E. A., Duncan, S., Goral, M., Hyun, J., Albert, M. L., & Obler, L. K. (2016). How older adults use cognition in sentence-final word recognition. Aging, Neuropsychology, and Cognition, 23(4), 418–444. 10.1080/13825585.2015.1111291 [DOI] [PubMed] [Google Scholar]
- Cameron, S., & Dillon, H. (2011). Development and evaluation of the LiSN & learn auditory training software for deficit-specific remediation of binaural processing deficits in children: Preliminary findings. Journal of the American Academy of Audiology, 22(10), 678–696. 10.3766/jaaa.22.10.6 [DOI] [PubMed] [Google Scholar]
- Carvalho, L. M. A. D., Gonsalez, E. C. D. M., & Iorio, M. C. M. (2017). Speech perception in noise in the elderly: Interactions between cognitive performance, depressive symptoms, and education. Brazilian Journal of Otorhinolaryngology, 83(2), 195–200. 10.1016/j.bjorl.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione, A., Casa, M., Gallo, S., Sorrentino, F., Dhima, S., Cilia, D., Lovo, E., Gambin, M., Previato, M., Colombo, S., Caserta, E., Gheller, F., Giacomelli, C., Montino, S., Limongi, F., Brotto, D., Gabelli, C., Trevisi, P., Bovo, R., & Martini, A. (2019). Correspondence between cognitive and audiological evaluations among the elderly: A preliminary report of an audiological screening model of subjects at risk of cognitive decline with slight to moderate hearing loss. Frontiers in Neuroscience, 13, Article 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavitt, K., & Hill, H. (2022). Ear-to-brain: The role of cognition in evidence-based audiology. Audiology Online. Retrieved from April 15, 2023, https://www.audiologyonline.com/audiology-ceus/course/ear-to-brain-role-cognition-36136 [Google Scholar]
- Cervera, T. C., Soler, M. J., Dasi, C., & Ruiz, J. C. (2009). Speech recognition and working memory capacity in young-elderly listeners: Effects of hearing sensitivity. Canadian Journal of Experimental Psychology, 63(3), 216–226. 10.1037/a0014321 [DOI] [PubMed] [Google Scholar]
- Chermak, G. D. (1998). Managing central auditory processing disorders: Metalinguistic and metacognitive approaches [Paper presentation]. Seminars in Hearing. [Google Scholar]
- Chermak, G. D., Musiek, F. E. (1997). Central auditory processing disorders: New perspectives. Singular. [Google Scholar]
- Chermak, G. D., & Musiek, F. E. (2011). Neurological substrate of central auditory processing deficits in children. Current Pediatric Reviews, 7(3), 241–251. 10.2174/157339611796548393 [DOI] [Google Scholar]
- Cooray, G. K., Maurex, L., & Brismar, T. (2008). Cognitive impairment correlates to low auditory event-related potential amplitudes in type 1 diabetes. Psychoneuroendocrinology, 33(7), 942–950. 10.1016/j.psyneuen.2008.04.013 [DOI] [PubMed] [Google Scholar]
- Cowan, N. (2008). What are the differences between long-term, short-term, and working memory? Progress in Brain Research, 169, 323–338. 10.1016/S0079-6123(07)00020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, N. (2010). The magical mystery four: How is working memory capacity limited, and why? Current Directions in Psychological Science, 19(1), 51–57. 10.1177/0963721409359277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, N. (2017). The many faces of working memory and short-term storage. Psychonomic Bulletin & Review, 24(4), 1158–1170. 10.3758/s13423-016-1191-6 [DOI] [PubMed] [Google Scholar]
- Cox, C. L., McCoy, S. L., Tun, P. A., & Wingfield, A. (2008). Monotic auditory processing disorder tests in the older adult population. Journal of the American Academy of Audiology, 19(04), 293–308. 10.3766/jaaa.19.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. M. (2005). Evidence-based practice in audiology. Journal of the American Academy of Audiology, 16(7), 408–409. 10.1055/s-0040-1715694 [DOI] [PubMed] [Google Scholar]
- Danielsson, H., Humes, L. E., & Rönnberg, J. (2019). Different associations between auditory function and cognition depending on type of auditory function and type of cognition. Ear and Hearing, 40(5), 1210–1219. 10.1097/AUD.0000000000000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, A., Musiek, F., Fisher, J. M., & Marrone, N. (2021). Investigating the role of auditory processing abilities in long-term self-reported hearing aid outcomes among adults age 60+ years. Journal of the American Academy of Audiology, 32(7), 405–419. 10.1055/s-0041-1728771 [DOI] [PubMed] [Google Scholar]
- de Miranda, E. C., Pinheiro, M. M. C., Pereira, L. D., & Iorio, M. C. M. (2012). Correlation of the P300 evoked potential in depressive and cognitive aspects of aging. Brazilian Journal of Otorhinolaryngology, 78(5), 83–89. 10.5935/1808-8694.20120013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, E., Visser-Bochane, M. I., Steenbergen, B., van Dijk, P., van der Schans, C. P., & Luinge, M. R. (2016). Characteristics of auditory processing disorders: A systematic review. Journal of Speech, Language, and Hearing Research, 59(2), 384–413. 10.1044/2015_JSLHR-H-15-0118 [DOI] [PubMed] [Google Scholar]
- Delano, P. H., Belkhiria, C., Vergara, R. C., Martínez, M., Leiva, A., Andrade, M., Marcenaro, B., Torrente, M., Maass, J. C., & Delgado, C. (2020). Reduced suprathreshold auditory nerve responses are associated with slower processing speed and thinner temporal and parietal cortex in presbycusis. PLOS ONE, 15(5), Article e0233224. 10.1371/journal.pone.0233224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter, G. S., van der Stelt, O., Boch, J. L., & Belger, A. (2006). Relations among intelligence, executive function, and P300 event related potentials in schizophrenia. The Journal of Nervous and Mental Disease, 194(3), 179–187. 10.1097/01.nmd.0000202490.97425.de [DOI] [PubMed] [Google Scholar]
- Dryden, A., Allen, H. A., Henshaw, H., & Heinrich, A. (2017). The association between cognitive performance and speech-in-noise perception for adult listeners: A systematic literature review and meta-analysis. Trends in Hearing, 21. 10.1177/2331216517744675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, R. J., Molander, P., Rönnberg, J., Lyxell, B., Andersson, G., & Lunner, T. (2016). Predicting speech-in-noise recognition from performance on the trail making test: Results from a large-scale internet study. Ear and Hearing, 37(1), 73–79. 10.1097/aud.0000000000000218 [DOI] [PubMed] [Google Scholar]
- Emanuel, D. C. (2002). The auditory processing battery: Survey of common practices. Journal of the American Academy of Audiology, 13(2), 093–117. 10.1055/s-0040-1715952 [DOI] [PubMed] [Google Scholar]
- Emanuel, D. C., Ficca, K. N., & Korczak, P. (2011). Survey of the diagnosis and management of auditory processing disorder. American Journal of Audiology, 20(1), 48–60. 10.1044/1059-0889(2011/10-0019) [DOI] [PubMed] [Google Scholar]
- Erickson, K. (2008). CAP testing and interpreting 101: Recommendations for audiologists. Audiology Online. Retrieved April 15, 2023, from https://www.audiologyonline.com/articles/c-apd-testing-and-interpreting-896 [Google Scholar]
- Fausti, S. A., Wilmington, D. J., Gallun, F. J., Myers, P. J., & Henry, J. A. (2009). Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. Journal of Rehabilitation Research and Development, 46(6), 797–810. 10.1682/JRRD.2008.09.0118 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M. B., Gianakas, S. P., Qian, Z. J., Losorelli, S., & Swanson, A. C. (2023). Preliminary guidelines for replacing word-recognition in quiet with speech in noise assessment in the routine audiologic test battery. Ear and Hearing, 44(6), 1548–1561. 10.1097/AUD.0000000000001409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer, R. L., Vachhani, J. J., & Riggins, A. (2021). Electrophysiological evidence of auditory and cognitive processing deficits in Parkinson disease. BioMed Research International, 2021, Article 6610908. 10.1155/2021/6610908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Foo, C., Rudner, M., Rönnberg, J., & Lunner, T. (2007). Recognition of speech in noise with new hearing instrument compression release settings requires explicit cognitive storage and processing capacity. Journal of the American Academy of Audiology, 18(07), 618–631. 10.3766/jaaa.18.7.8 [DOI] [PubMed] [Google Scholar]
- Fostick, L., Ben-Artzi, E., & Babkoff, H. (2013). Aging and speech perception among the elderly: beyond hearing threshold and cognitive ability. Journal of Basic and Clinical Physiology and Pharmacology, 24, 175–183. 10.1515/jbcpp-2013-0048 [DOI] [PubMed] [Google Scholar]
- Fuente, A., & McPherson, B. (2007). Central auditory processing effects induced by solvent exposure. International Journal of Occupational Medicine and Environmental Health, 20(3), 271–279. 10.2478/v10001-007-0030-4 [DOI] [PubMed] [Google Scholar]
- Füllgrabe, C., Moore, B. C., & Stone, M. A. (2015). Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Frontiers in Aging Neuroscience, 6, Article 347. 10.3389/fnagi.2014.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe, C., & Rosen, S. (2016). On the (un)importance of working memory in speech-in-noise processing for listeners with normal hearing thresholds. Frontiers in Psychology, 7, Article 1268. 10.3389/fpsyg.2016.01268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun, F. J., Lewis, M. S., Folmer, R. L., Diedesch, A. C., Kubli, L. R., McDermott, D. J., Walden, T. C., Fausti, S. A., Lew, H. L., & Leek, M. R. (2012). Implications of blast exposure for central auditory function: A review. Journal of Rehabilitation Research and Development, 49(7), 1059–1074. 10.1682/JRRD.2010.09.0166 [DOI] [PubMed] [Google Scholar]
- Gates, G. A., Anderson, M. L., McCurry, S. M., Feeney, M. P., & Larson, E. B. (2011). Central auditory dysfunction as a harbinger of Alzheimer dementia. Archives of Otolaryngology–Head & Neck Surgery, 137(4), 390–395. 10.1001/archoto.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, D. R., & Whitehouse, P. J. (2011). Marketplace of memory: What the brain fitness technology industry says about us and how we can do better. The Gerontologist, 51(5), 590–596. 10.1093/geront/gnr042 [DOI] [PubMed] [Google Scholar]
- Ghannoum, M. T., Shalaby, A. A., Farghaly, M., Hamdy, M., & Hamdy, H. S. (2018). Central auditory processing findings in a group of cognitively impaired individuals. Hearing, Balance and Communication, 16(3), 145–154. 10.1080/21695717.2018.1490117 [DOI] [Google Scholar]
- Giroud, N., Keller, M., & Meyer, M. (2021). Interacting effects of frontal lobe neuroanatomy and working memory capacity to older listeners' speech recognition in noise. Neuropsychologia, 158, Article 107892. 10.1016/j.neuropsychologia.2021.107892 [DOI] [PubMed] [Google Scholar]
- Givens, G. D., Arnold, T., & Hume, W. G. (1998). Auditory processing skills and hearing aid satisfaction in a sample of older adults. Perceptual and Motor Skills, 86(3), 795–801. 10.2466/pms.1998.86.3.795 [DOI] [PubMed] [Google Scholar]
- Glyde, H., Cameron, S., Dillon, H., Hickson, L., & Seeto, M. (2013). The effects of hearing impairment and aging on spatial processing. Ear and Hearing, 34(1), 15–28. 10.1097/aud.0b013e3182617f94 [DOI] [PubMed] [Google Scholar]
- Golob, E. J., & Starr, A. (2000). Age-related qualitative differences in auditory cortical responses during short-term memory. Clinical Neurophysiology, 111(12), 2234–2244. 10.1016/S1388-2457(00)00468-5 [DOI] [PubMed] [Google Scholar]
- Gomot, M., & Giard, M. H. (2007). N200, P300 and MMN in autistic adults: Evidence for a neural dysfunction in error monitoring. NeuroReport, 18(5), 463–467. [Google Scholar]
- Gordon-Salant, S., & Cole, S. S. (2016). Effects of age and working memory capacity on speech recognition performance in noise among listeners with normal hearing. Ear and Hearing, 37(5), 593–602. 10.1097/aud.0000000000000316 [DOI] [PubMed] [Google Scholar]
- Gurrera, R. J., Salisbury, D. F., O'Donnell, B. F., Nestor, P. G., & McCarley, R. W. (2005). Auditory P3 indexes personality traits and cognitive function in healthy men and women. Psychiatry Research, 133(2–3), 215–228. 10.1016/j.psychres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Guthrie, O. W., Xu, H., Wong, B. A., McInturf, S. M., Reboulet, J. E., Ortiz, P. A., & Mattie, D. R. (2014). Exposure to low levels of jet-propulsion fuel impairs brainstem encoding of stimulus intensity. Journal of Toxicology and Environmental Health, Part A, 77(5), 261–280. 10.1080/15287394.2013.862892 [DOI] [PubMed] [Google Scholar]
- Hahs-Vaughn, D. L., & Nye, C. (2008). Understanding high quality research designs for speech language pathology. Evidence-Based Communication Assessment and Intervention, 2(4), 218–224. 10.1080/17489530802646323 [DOI] [Google Scholar]
- Hällgren, M., Larsby, B., Lyxell, B., & Arlinger, S. (2001). Cognitive effects in dichotic speech testing in elderly persons. Ear and Hearing, 22(2), 120–129. 10.1097/00003446-200104000-00005 [DOI] [PubMed] [Google Scholar]
- Hamilton, H. K., Williams, T. J., Ventura, J., Jasperse, L. J., Owens, E. M., Miller, G. A., Subotnik, K. L., Nuechterlein, K. H., & Yee, C. M. (2018). Clinical and cognitive significance of auditory sensory processing deficits in schizophrenia. American Journal of Psychiatry, 175(3), 275–283. 10.1176/appi.ajp.2017.16111203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, R., Hanyu, N., Shindo, M., Tamaru, F., & Yanagisawa, N. (1993). Event-related potentials, reaction time, and cognitive state in patients with Parkinson's disease. Advances in Neurology, 60, 429–433. [PubMed] [Google Scholar]
- Helfer, K. S., & Freyman, R. L. (2014). Stimulus and listener factors affecting age-related changes in competing speech perception. The Journal of the Acoustical Society of America, 136(2), 748–759. 10.1121/1.4887463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert, C. J., Kronenberger, W. G., Wolfert, K., Nelson, R. F., Yates, C. W., & Pisoni, D. B. (2023). Extraordinary speech and language outcomes after auditory brainstem implantation: Guidance from a case study. American Journal of Audiology, 32(4), 761–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind, S. E., Haines-Bazrafshan, R., Benton, C. L., Brassington, W., Towle, B., & Moore, D. R. (2011). Prevalence of clinical referrals having hearing thresholds within normal limits. International Journal of Audiology, 50(10), 708–716. 10.3109/14992027.2011.582049 [DOI] [PubMed] [Google Scholar]
- Hommet, C., Mondon, K., Berrut, G., Gouyer, Y., Isingrini, M., Constans, T., & Belzung, C. (2010). Central auditory processing in aging: The dichotic listening paradigm. The Journal of Nutrition, Health & Aging, 14(9), 751–756. 10.1007/s12603-010-0097-7 [DOI] [PubMed] [Google Scholar]
- Hoover, E. C., Souza, P. E., & Gallun, F. J. (2017). Auditory and cognitive factors associated with speech-in-noise complaints following mild traumatic brain injury. Journal of the American Academy of Audiology, 28(4), 325–339. 10.3766/jaaa.16051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, M. H., Liu, K., Liu, S. K., Chiu, M. J., Hwu, H. G., & Chen, A. C. (2004). Memory impairment and auditory evoked potential gating deficit in schizophrenia. Psychiatry Research: Neuroimaging, 130(2), 161–169. 10.1016/j.pscychresns.2002.12.001 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. (2004). Factors affecting long-term hearing aid success [Paper presentation]. Seminars in Hearing. [Google Scholar]
- Humes, L. E. (2021). Factors underlying individual differences in speech-recognition threshold (SRT) in noise among older adults. Frontiers in Aging Neuroscience, 13, Article 702739. 10.3389/fnagi.2021.702739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. (2021b). Longitudinal changes in auditory and cognitive function in middle-aged and older adults. Journal of Speech, Language, and Hearing Research, 64(1), 230–249. 10.1044/2020_JSLHR-20-00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. (2005). Do ‘auditory processing’ tests measure auditory processing in the elderly? Ear and Hearing, 26(2), 109–119. 10.1097/00003446-200504000-00001 [DOI] [PubMed] [Google Scholar]
- Humes, L. E., Kewley-Port, D., Fogerty, D., & Kinney, D. (2010). Measures of hearing threshold and temporal processing across the adult lifespan. Hearing Research, 264(1–2), 30–40. 10.1016/j.heares.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E., Kidd, G. R., & Lentz, J. J. (2013). Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Frontiers in Systems Neuroscience, 7, Article 55. 10.3389/fnsys.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvalson, E. M., Lansford, K. L., Fedorova, V., & Fernandez, G. (2017). Cognitive factors as predictors of accented speech perception for younger and older adults. The Journal of the Acoustical Society of America, 141(6), 4652–4659. 10.1121/1.4986930 [DOI] [PubMed] [Google Scholar]
- Jain, C., & Dwarakanath, V. M. (2019). Influence of subcortical auditory processing and cognitive measures on cocktail party listening in younger and older adults. International Journal of Audiology, 58(2), 87–96. 10.1080/14992027.2018.1543962 [DOI] [PubMed] [Google Scholar]
- Jain, S., Rathnamala, M., & Narne, V. K. (2022). Evaluation of auditory processing and working memory abilities in individuals with chronic renal disease undergoing hemodialysis. Auditory Perception & Cognition, 5(1–2), 129–150. 10.1080/25742442.2022.2036538 [DOI] [Google Scholar]
- Kalikow, D. N., Stevens, K. N., & Elliott, L. L. (1977). Development of a test of speech intelligibility in noise using sentence materials with controlled word predictability. The Journal of the Acoustical Society of America, 61(5), 1337–1351. [DOI] [PubMed] [Google Scholar]
- Kamerer, A. M., AuBuchon, A., Fultz, S. E., Kopun, J. G., Neely, S. T., & Rasetshwane, D. M. (2019). The role of cognition in common measures of peripheral synaptopathy and hidden hearing loss. American Journal of Audiology, 28(4), 843–856. 10.1044/2019_AJA-19-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärgel, C., Sartory, G., Kariofillis, D., Wiltfang, J., & Müller, B. W. (2014). Mismatch negativity latency and cognitive function in schizophrenia. PLOS ONE, 9(4), Article e84536. 10.1371/journal.pone.0084536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada, E., Sato, K., Sawaki, A., Dohi, Y., Ueda, R., & Ojika, K. (2003). Long-term effects of donepezil on P300 auditory event-related potentials in patients with Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology, 16(1), 39–43. 10.1177/0891988702250561 [DOI] [PubMed] [Google Scholar]
- Kates, J. M., Arehart, K. H., & Souza, P. E. (2013). Integrating cognitive and peripheral factors in predicting hearing-aid processing effectiveness. The Journal of the Acoustical Society of America, 134(6), 4458–4469. 10.1121/1.4824700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsarou, Z., Bostantjopoulou, S., Kimiskidis, V., Rossopoulos, E., & Kazis, A. (2004). Auditory event-related potentials in Parkinson's disease in relation to cognitive ability. Perceptual and Motor Skills, 98(Suppl. 3), 1441–1448. 10.2466/pms.98.3c.1441-1448 [DOI] [PubMed] [Google Scholar]
- Killion, M. C., Niquette, P. A., Gudmundsen, G. I., Revit, L. J., & Banerjee, S. (2004). Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 116(4), 2395–2405. [DOI] [PubMed] [Google Scholar]
- Kim, S., Choi, I., Schwalje, A. T., Kim, K., & Lee, J. H. (2020). Auditory working memory explains variance in speech recognition in older listeners under adverse listening conditions. Clinical Interventions in Aging, 15, 395–406. 10.2147/CIA.S241976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellberg, A. (2004). Effects of reverberation time on the cognitive load in speech communication: Theoretical considerations. Noise and Health, 7(25), 11–21. [PubMed] [Google Scholar]
- Koelewijn, T., Zekveld, A. A., Festen, J. M., & Kramer, S. E. (2014). The influence of informational masking on speech perception and pupil response in adults with hearing impairment. The Journal of the Acoustical Society of America, 135(3), 1596–1606. 10.1121/1.4863198 [DOI] [PubMed] [Google Scholar]
- Koerner, T. K., Papesh, A. M., & Gallun, F. J. (2020). A questionnaire survey of current rehabilitation practices for adults with normal hearing sensitivity who experience auditory difficulties. American Journal of Audiology, 29(4), 738–761. 10.1044/2020_AJA-20-00027 [DOI] [PubMed] [Google Scholar]
- Krause, M. O., Kennedy, M. R., & Nelson, P. B. (2014). Masking release, processing speed and listening effort in adults with traumatic brain injury. Brain Injury, 28(11), 1473–1484. 10.3109/02699052.2014.920520 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, S., Sivagnaname, Y., & Gumallapu, G. C. (2019). Identification of subclinical cognitive impairment in chronic obstructive pulmonary disease using auditory P300 event related potential. Monaldi Archives for Chest Disease, 89(2). 10.4081/monaldi.2019.1039 [DOI] [PubMed] [Google Scholar]
- Lee, S. J., Park, K. W., Kim, L.-S., & Kim, H. (2018). Association between frontal-executive dysfunction and speech-in-noise perception deficits in mild cognitive impairment. Journal of Clinical Neurology, 14(4), 513–522. 10.3988/jcn.2018.14.4.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz, J. J., Humes, L. E., & Kidd, G. R. (2022). Differences in auditory perception between young and older adults when controlling for differences in hearing loss and cognition. Trends in Hearing, 26. 10.1177/23312165211066180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda, E. A., Johannesen, P. T., Pérez-González, P., Blanco, J. L., Kalluri, S., & Edwards, B. (2017). Predictors of hearing-aid outcomes. Trends in Hearing, 21. 10.1177/2331216517730526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunner, T. (2003). Cognitive function in relation to hearing aid use. International Journal of Audiology, 42(Supp. 1), 49–58. 10.3109/14992020309074624 [DOI] [PubMed] [Google Scholar]
- Lunner, T., & Sundewall-Thorén, E. (2007). Interactions between cognition, compression, and listening conditions: Effects on speech-in-noise performance in a two-channel hearing aid. Journal of the American Academy of Audiology, 18(07), 604–617. 10.3766/jaaa.18.7.7 [DOI] [PubMed] [Google Scholar]
- Mamo, S. K., & Helfer, K. S. (2021). Speech understanding in modulated noise and speech maskers as a function of cognitive status in older adults. American Journal of Audiology, 30(3), 642–654. 10.1044/2021_AJA-20-00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo, S. K., Reed, N. S., Sharrett, A. R., Albert, M. S., Coresh, J., Mosley, T. H., Knopman, D., Lin, F. R., & Deal, J. A. (2019). Relationship between domain-specific cognitive function and speech-in-noise performance in older adults: The Atherosclerosis Risk in Communities Hearing Pilot Study. American Journal of Audiology, 28(4), 1006–1014. 10.1044/2019_AJA-19-00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, J. T., Schubarth, G., Brown, W. S., Riege, W., Strandburg, R., Dorsey, D., Maltese, A., & Kuhl, D. (1990). PET and P300 relationships in early Alzheimer's disease. Neurobiology of Aging, 11(4), 471–476. 10.1016/0197-4580(90)90015-R [DOI] [PubMed] [Google Scholar]
- Mauk, M. D., & Buonomano, D. V. (2004). The neural basis of temporal processing. Annual Review of Neuroscience, 27(1), 307–340. 10.1146/annurev.neuro.27.070203.144247 [DOI] [PubMed] [Google Scholar]
- McCreery, R. W., Walker, E. A., Spratford, M., Lewis, D., & Brennan, M. (2019). Auditory, cognitive, and linguistic factors predict speech recognition in adverse listening conditions for children with hearing loss. Frontiers in Neuroscience, 13, Article 1093. 10.3389/fnins.2019.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, D. J., Baese-Berk, M. M., Bent, T., Borrie, S. A., & Van Engen, K. J. (2018). Coping with adversity: Individual differences in the perception of noisy and accented speech. Attention, Perception, & Psychophysics, 80(6), 1559–1570. 10.3758/s13414-018-1537-4 [DOI] [PubMed] [Google Scholar]
- McWeeny, S., Choe, J., & Norton, E. (2021). SnowGlobe: An iterative search tool for systematic reviews and meta-analyses. OSF. 10.17605/OSF.IO/U25RN [DOI]
- Merten, N., Fischer, M. E., Tweed, T. S., Breteler, M. M., & Cruickshanks, K. J. (2020). Associations of hearing sensitivity, higher-order auditory processing, and cognition over time in middle-aged adults. The Journals of Gerontology: Series A, 75(3), 545–551. 10.1093/gerona/glz189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. W., Stewart, E. K., Wu, Y.-H., Bishop, C., Bentler, R. A., & Tremblay, K. (2017). Working memory and speech recognition in noise under ecologically relevant listening conditions: Effects of visual cues and noise type among adults with hearing loss. Journal of Speech, Language, and Hearing Research, 60(8), 2310–2320. 10.1044/2017_JSLHR-H-16-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett, P., Jutras, B., Noel, G., Pichora-Fuller, K., Watson, C., & Nelson, A. (2012). Canadian guideline on auditory processing disorder in children and adults: Assessment and intervention. Canadian Interorganizational Steering Group for Audiology Speech-Language Pathology. https://www.ooaq.qc.ca/media/qf4ar4jk/canadian_guidelines_en.pdf [Google Scholar]
- Millman, R. E., & Mattys, S. L. (2017). Auditory verbal working memory as a predictor of speech perception in modulated maskers in listeners with normal hearing. Journal of Speech, Language, and Hearing Research, 60(5), 1236–1245. 10.1044/2017_JSLHR-S-16-0105 [DOI] [PubMed] [Google Scholar]
- Mioni, G., Cardullo, S., Ciavarelli, A., & Stablum, F. (2021). Age-related changes in time discrimination: The involvement of inhibition, working memory and speed of processing. Current Psychology, 40(5), 2462–2471. 10.1007/s12144-019-00170-8 [DOI] [Google Scholar]
- Mitchell, A. S., & Chakraborty, S. (2013). What does the mediodorsal thalamus do? Frontiers in Systems Neuroscience, 7, Article 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly, A. C., Harris, M. S., Boyce, L., & Nittrouer, S. (2017). Speech recognition in adults with cochlear implants: The effects of working memory, phonological sensitivity, and aging. Journal of Speech, Language, and Hearing Research, 60(4), 1046–1061. 10.1044/2016_jslhr-h-16-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly, A. C., Mattingly, J. K., & Castellanos, I. (2019). How does nonverbal reasoning affect sentence recognition in adults with cochlear implants and normal-hearing peers? Audiology and Neurotology, 24(3), 127–138. 10.1159/000500699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly, A. C., Patel, T. R., & Castellanos, I. (2018). Relations between self-reported executive functioning and speech perception skills in adult cochlear implant users. Otology and Neurotology, 39(2), 250–257. 10.1097/MAO.0000000000001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group*. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Moore, D. R., Edmondson-Jones, M., Dawes, P., Fortnum, H., McCormack, A., Pierzycki, R. H., & Munro, K. J. (2014). Relation between speech-in-noise threshold, hearing loss and cognition from 40–69 years of age. PLOS ONE, 9(9), Article e107720. 10.1371/journal.pone.0107720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier, I., Bebear, J.-P., Marx, M., Fraysse, B., Truy, E., Lina-Granade, G., Mondain, M., Sterkers-Artières, F., Bordure, P., Robier, A., Godey, B., Meyer, B., Frachet, B., Poncet-Wallet, C., Bouccara, D., & Sterkers, O. (2015). Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngology–Head & Neck Surgery, 141(5), 442–450. 10.1001/jamaoto.2015.129 [DOI] [PubMed] [Google Scholar]
- Mukari, S. Z. M. S., Yusof, Y., Ishak, W. S., Maamor, N., Chellapan, K., & Dzulkifli, M. A. (2020). Relative contributions of auditory and cognitive functions on speech recognition in quiet and in noise among older adults. Brazilian Journal of Otorhinolaryngology, 86(2), 149–156. 10.1016/j.bjorl.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, R. (2007). The state of the evidence: ASHA develops levels of evidence for communication sciences and disorders. The ASHA Leader, 12(3), 8–25. 10.1044/leader.FTR4.12032007.8 [DOI] [Google Scholar]
- Murphy, C. F., Rabelo, C. M., Silagi, M. L., Mansur, L. L., & Schochat, E. (2016). Impact of educational level on performance on auditory processing tests. Frontiers in Neuroscience, 10, Article 97. 10.3389/fnins.2016.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. F., Rabelo, C. M., Silagi, M. L., Mansur, L. L., Bamiou, D. E., & Schochat, E. (2018). Auditory processing performance of the middle-aged and elderly: Auditory or cognitive decline? Journal of the American Academy of Audiology, 29(01), 005–014. 10.3766/jaaa.15098 [DOI] [PubMed] [Google Scholar]
- Musiek, F. E. (1983). Assessment of central auditory dysfunction: The dichotic digit test revisited. Ear and Hearing, 4(2), 79–83. [DOI] [PubMed] [Google Scholar]
- Mussoi, B. S. (2021). The impact of music training and working memory on speech recognition in older age. Journal of Speech, Language, and Hearing Research, 64(11), 4524–4534. 10.1044/2021_jslhr-20-00426 [DOI] [PubMed] [Google Scholar]
- Nagaraj, N. K. (2021). Effect of auditory distraction on working memory, attention switching, and listening comprehension. Audiology Research, 11(2), 227–243. 10.3390/audiolres11020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa, T., Kamiya, T., Kawasaki, Y., Higashima, M., Urata, K., Sakai, N., & Koshino, Y. (1999). The relationship between auditory ERP and neuropsychological assessments in schizophrenia. International Journal of Psychophysiology, 34(3), 267–274. 10.1016/s0167-8760(99)00083-5 [DOI] [PubMed] [Google Scholar]
- Naismith, S. L., Mowszowski, L., Ward, P. B., Diamond, K., Paradise, M., Kaur, M., Lewis, S. J., Hickie, I. B., & Hermens, D. F., & Hermens, D. F. (2012). Reduced temporal mismatch negativity in late-life depression: an event-related potential index of cognitive deficit and functional disability? Journal of Affective Disorders, 138(1–2), 71–78. 10.1016/j.jad.2011.12.028 [DOI] [PubMed] [Google Scholar]
- Neher, T. (2014). Relating hearing loss and executive functions to hearing aid users' preference for, and speech recognition with, different combinations of binaural noise reduction and microphone directionality. Frontiers in Neuroscience, 8, Article 391. 10.3389/fnins.2014.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, T., Behrens, T., Carlile, S., Jin, C., Kragelund, L., Petersen, A. S., & Schaik, A. (2009). Benefit from spatial separation of multiple talkers in bilateral hearing-aid users: Effects of hearing loss, age, and cognition. International Journal of Audiology, 48, 758–774. 10.3109/14992020903079332 [DOI] [PubMed] [Google Scholar]
- Neher, T., Grimm, G., & Hohmann, V. (2014). Perceptual consequences of different signal changes due to binaural noise reduction: Do hearing loss and working memory capacity play a role? Ear and Hearing, 35, e213–227. 10.1097/AUD.0000000000000054 [DOI] [PubMed] [Google Scholar]
- Neher, T., Grimm, G., Hohmann, V., & Kollmeier, B. (2013). Do hearing loss and cognitive function modulate benefit from different binaural noise-reduction settings? Ear and Hearing, 35, e52–62. 10.1097/AUD.0000000000000003 [DOI] [PubMed] [Google Scholar]
- Neher, T., Laugesen, S., Søgaard Jensen, N., & Kragelund, L. (2011). Can basic auditory and cognitive measures predict hearing-impaired listeners' localization and spatial speech recognition abilities? The Journal of the Acoustical Society of America, 130(3), 1542–1558. 10.1121/1.3608122 [DOI] [PubMed] [Google Scholar]
- Neher, T., Lunner, T., Hopkins, K., & Moore, B. C. (2012). Binaural temporal fine structure sensitivity, cognitive function, and spatial speech recognition of hearing-impaired listeners. Journal of the Acoustical Society of America, 131, 2561–2564. 10.1121/1.3689850 [DOI] [PubMed] [Google Scholar]
- Neshige, R., Barrett, G., & Shibasaki, H. (1988). Auditory long latency event-related potentials in Alzheimer's disease and multi-infarct dementia. Journal of Neurology, Neurosurgery & Psychiatry, 51(9), 1120–1125. 10.1136/jnnp.51.9.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, E. H., Classon, E., Larsby, B., Arlinger, S., Lunner, T., Rudner, M., & Rönnberg, J. (2014). Dynamic relation between working memory capacity and speech recognition in noise during the first 6 months of hearing aid use. Trends in Hearing, 18. 10.1177/2331216514558688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon, G., Sarant, J. Z., Tomlin, D., & Dowell, R. (2019). The relationship between peripheral hearing loss and higher order listening function on cognition in older Australians. International Journal of Audiology, 58(12), 933–944. 10.1080/14992027.2019.1641752 [DOI] [PubMed] [Google Scholar]
- Nuesse, T., Steenken, R., Neher, T., & Holube, I. (2018). Exploring the link between cognitive abilities and speech recognition in the elderly under different listening conditions. Frontiers in Psychology, 9. 10.3389/fpsyg.2018.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, J. L., Lister, J. J., Fausto, B. A., Morgan, D. G., Maeda, H., Andel, R., & Edwards, J. D. (2021). Are auditory processing and cognitive performance assessments overlapping or distinct? Parsing the auditory behaviour of older adults. International Journal of Audiology, 60(2), 123–132. 10.1080/14992027.2020.1791366 [DOI] [PubMed] [Google Scholar]
- Oberauer, K. (2002). Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition, 28(3), 411–421. [PubMed] [Google Scholar]
- Ohlenforst, B., Souza, P. E., & MacDonald, E. (2016). Exploring the relationship between working memory, compressor speed and background noise characteristics. Ear and Hearing, 37(2), 137–143. 10.1097/AUD.0000000000000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, M. D. F. F. D., Menezes, P. D. L., Carnaúba, A. T. L., Pereira, L. D., Andrade, K. C. L. D., Frizzo, A. C. F., & Soares, I. D. A. (2021). Cognitive performance and long-latency auditory evoked potentials: a study on aging. Clinics, 76. 10.6061/clinics/2021/e1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan—A web and mobile app for systematic reviews. Systematic Reviews, 5(1), 1–10. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan, H., Ozer, S., & Yagcioglu, S. (2016). Neuropsychological, electrophysiological and neurological impairments in patients with obsessive compulsive disorder, their healthy siblings and healthy controls: identifying potential endophenotype(s). Psychiatry Research, 240, 110–117. 10.1016/j.psychres.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Pang, S., Borod, J. C., Hernandez, A., Bodis-Wollner, I., Raskin, S., Mylin, L., Coscia, L., & Yahr, M. D. (1990). The auditory P300 correlates with specific cognitive deficits in Parkinson's disease. Journal of Neural Transmission-Parkinson's Disease and Dementia Section, 2(4), 249–264. 10.1007/BF02252920 [DOI] [PubMed] [Google Scholar]
- Parbery-Clark, A., Strait, D. L., Anderson, S., Hittner, E., & Kraus, N. (2011). Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLOS ONE, 6(5), Article e18082. 10.1371/journal.pone.0018082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, S., Kruijt, A.-W., & Fox, E. (2019). Psychological science needs a standard practice of reporting the reliability of cognitive-behavioral measurements. Advances in Methods and Practices in Psychological Science, 2(4), 378–395. 10.1177/2515245919879695 [DOI] [Google Scholar]
- Patro, C., Kreft, H. A., & Wojtczak, M. (2021). The search for correlates of age-related cochlear synaptopathy: Measures of temporal envelope processing and spatial release from speech-on-speech masking. Hearing Research, 409, Article 108333. 10.1016/j.heares.2021.108333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K., Kramer, S. E., Eckert, M. A., Edwards, B., Hornsby, B. W., Humes, L. E., Lemke, U., Lunner, T., Matthen, M., Mackersie, C. L., Naylor, G., Phillips, N. A., Richter, M., Rudner, M., Sommers, M. S., Tremblay, K. L., & Wingfield, A. (2016). Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear and Hearing, 37(1), 5S–27S. 10.1097/AUD.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K., Schneider, B. A., Macdonald, E., Pass, H. E., & Brown, S. (2007). Temporal jitter disrupts speech intelligibility: A simulation of auditory aging. Hearing Research, 223(1–2), 114–121. 10.1016/j.heares.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Pinheiro, M. M. C., Iório, M. C. M., Miranda, E. C., Dias, K. Z., & Pereira, L. D. (2012). The influence of cognitive aspects and auditory processes on the hearing aid acclimatization in the elderly. Jornal Da Sociedade Brasileira De Fonoaudiologia, 24, 309–315. 10.1590/s2179-64912012000400004 [DOI] [PubMed] [Google Scholar]
- Podemski, R., Pokryszko-Dragan, A., Zagrajek, M., Słotwiński, K., Bilińska, M., Sąsiadek, M., Filarski, J., & Mazur, K. (2008). Mild cognitive impairment and event-related potentials in patients with cerebral atrophy and leukoaraiosis. Neurological Sciences, 29(6), 411–416. 10.1007/s10072-008-1057-4 [DOI] [PubMed] [Google Scholar]
- Pokryszko-Dragan, A., Słotwiński, K., & Podemski, R. (2003). Modality-specific changes in P300 parameters in patients with dementia of the Alzheimer type. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 9(4), CR130–CR134. [PubMed] [Google Scholar]
- Pokryszko-Dragan, A., Zagrajek, M., Slotwinski, K., Gruszka, E., Bilinska, M., & Podemski, R. (2009). Neuropsychological testing and event-related potentials in the assessment of cognitive performance in the patients with multiple sclerosis—a pilot study. Clinical Neurology and Neurosurgery, 111(6), 503–506. 10.1016/j.clineuro.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Portin, R., Kovala, T., Polo‐Kantola, P., Revonsuo, A., Müller, K., & Matikainen, E. (2000). Does P3 reflect attentional or memory performances, or cognition more generally? Scandinavian Journal of Psychology, 41(1), 31–40. 10.1111/1467-9450.00168 [DOI] [PubMed] [Google Scholar]
- Preminger, J. E., & Laplante-Lévesque, A. (2014). Perceptions of age and brain in relation to hearing help-seeking and rehabilitation. Ear and Hearing, 35(1), 19–29. 10.1097/AUD.0b013e31829c065c [DOI] [PubMed] [Google Scholar]
- Pronk, M., Deeg, D. J., Festen, J. M., Twisk, J. W., Smits, C., Comijs, H. C., & Kramer, S. E. (2013). Decline in older persons' ability to recognize speech in noise: The influence of demographic, health-related, environmental, and cognitive factors. Ear and Hearing, 34(6), 722–732. 10.1097/AUD.0b013e3182994eee [DOI] [PubMed] [Google Scholar]
- Pronk, M., Lissenberg-Witte, B. I., van der Aa, H. P., Comijs, H. C., Smits, C., Lemke, U., Zekveld, A. A., & Kramer, S. E. (2019). Longitudinal relationships between decline in speech-in-noise recognition ability and cognitive functioning: The longitudinal aging study Amsterdam. Journal of Speech, Language, and Hearing Research, 62(4S), 1167–1187. 10.1044/2018_JSLHR-H-ASCC7-18-0120 [DOI] [PubMed] [Google Scholar]
- Quaranta, N., Coppola, F., Casulli, M., Barulli, O., Lanza, F., Tortelli, R., Capozzo, R., Leo, A., Tursi, M., Grasso, A., Solfrizzi, V., Sobbà, C., & Logroscino, G. (2014). The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiology and Neurotology, 19(Suppl. 1), 10–14. 10.1159/000371597 [DOI] [PubMed] [Google Scholar]
- Rallapalli, V., Ellis, G., & Souza, P. (2021). Effects of directionality, compression, and working memory on speech recognition. Ear and Hearing, 42(3), 492–505. 10.1097/AUD.0000000000000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, M. J., Lee, A. C., Schader, L. M., Moore, R. H., Raol, N. R., & Vivas, E. X. (2020). Practices and perceptions of cognitive assessment for adults with age-related hearing loss. Laryngoscope Investigative Otolaryngology, 5(1), 137–144. 10.1002/lio2.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart, P. N., & Souza, P. E. (2016). Intelligibility and clarity of reverberant speech: Effects of wide dynamic range compression release time and working memory. Journal of Speech, Language, and Hearing Research, 59(6), 1543–1554. 10.1044/2016_JSLHR-H-15-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg, J., Lunner, T., Ng, E. H. N., Lidestam, B., Zekveld, A. A., Sörqvist, P., Lyxell, B., Träff, U., Yumba, W., Classon, E., Hällgren, M., Larsby, B., Signoret, C., Pichora-Fuller, M. K., Rudner, M., Danielsson, H., & Stenfelt, S. (2016). Hearing impairment, cognition and speech understanding: Exploratory factor analyses of a comprehensive test battery for a group of hearing aid users, the n200 study. International Journal of Audiology, 55(11), 623–642. 10.1080/14992027.2016.1219775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg, J., Lunner, T., Zekveld, A., Sörqvist, P., Danielsson, H., Lyxell, B., Dahlström, O., Signoret, C., Stenfelt, S., Pichora-Fuller, M. K., & Rudner, M. (2013). The ease of language understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7, Article 31. 10.3389/fnsys.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg, J., Rudner, M., Lunner, T., & Zekveld, A. A. (2010). When cognition kicks in: Working memory and speech understanding in noise. Noise and Health, 12(49), 263–269. 10.4103/1463-1741.70505 [DOI] [PubMed] [Google Scholar]
- Rosemann, S., & Thiel, C. M. (2020). Neural signatures of working memory in age-related hearing loss. Neuroscience, 429, 134–142. 10.1016/j.neuroscience.2019.12.046 [DOI] [PubMed] [Google Scholar]
- Rudner, M., Foo, C., Ronnberg, J., & Lunner, T. (2007). Phonological mismatch makes aided speech recognition in noise cognitively taxing. Ear and Hearing, 28(6), 879. 10.1097/AUD.0b013e3181576c9c [DOI] [PubMed] [Google Scholar]
- Rudner, M., Foo, C., Rönnberg, J., & Lunner, T. (2009). Cognition and aided speech recognition in noise: Specific role for cognitive factors following nine‐week experience with adjusted compression settings in hearing aids. Scandinavian Journal of Psychology, 50(5), 405–418. 10.1111/j.1467-9450.2009.00745.x [DOI] [PubMed] [Google Scholar]
- Rudner, M., Rönnberg, J., & Lunner, T. (2011). Working memory supports listening in noise for persons with hearing impairment. Journal of the American Academy of Audiology, 22, 156–167. 10.3766/jaaa.22.3.4 [DOI] [PubMed] [Google Scholar]
- Sánchez-Morla, E. M., Santos, J. L., Aparicio, A., García-Jiménez, M. Á., Soria, C., & Arango, C. (2013). Neuropsychological correlates of P50 sensory gating in patients with schizophrenia. Schizophrenia Research, 143(1), 102–106. 10.1016/j.schres.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2000). Aging and measures of processing speed. Biological Psychology, 54(1–3), 35–54. 10.1016/S0301-0511(00)00052-1 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A., Hambrick, D. Z., & McGuthry, K. E. (1998). Shared age-related influences on cognitive and noncognitive variables. Psychology and Aging, 13(3), 486–500. 10.1037/0882-7974.13.3.486 [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez, R., Fereczkowski, M., Santurette, S., Dau, T., & Neher, T. (2021). Towards auditory profile-based hearing-aid fitting: Fitting rationale and pilot evaluation. Audiology Research, 11(1), 10–21. 10.3390/audiolres11010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardone, R., Battista, P., Donghia, R., Lozupone, M., Tortelli, R., Guerra, V., Grasso, A., Griseta, C., Castellana, F., Zupo, R., Lampignano, L., Sborgia, G., Capozzo, R., Bortone, I., Stallone, R., Fiorella, M. L., Passantino, A., Giannelli, G., Seripa, D., Panza, F., … Quaranta, N. (2020). Age-related central auditory processing disorder, MCI, and dementia in an older population of Southern Italy. Otolaryngology–Head and Neck Surgery, 163(2), 348–355. 10.1177/0194599820913635 [DOI] [PubMed] [Google Scholar]
- Sardone, R., Castellana, F., Bortone, I., Lampignano, L., Zupo, R., Lozupone, M., Griseta, C., Dibello, V., Seripa, D., Guerra, V., Donghia, R., Logroscino, G., Solfrizzi, V., Quaranta, N., Ferrucci, L., Giannelli, G., & Panza, F. (2021). Association between central and peripheral age-related hearing loss and different frailty phenotypes in an older population in Southern Italy. JAMA Otolaryngology–Head & Neck Surgery, 147(6), 561–571. 10.1001/jamaoto.2020.5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, G. H., Odgear, I., Cosgrove, A., & Frederick, M. T. (2018). Impact of hearing loss and amplification on performance on a cognitive screening test. Journal of the American Academy of Audiology, 29(07), 648–655. 10.3766/jaaa.17044 [DOI] [PubMed] [Google Scholar]
- Schoof, T., & Rosen, S. (2014). The role of auditory and cognitive factors in understanding speech in noise by normal-hearing older listeners. Frontiers in Aging Neuroscience, 6, 307. 10.3389/fnagi.2014.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader, M. J., Yancey, C. M., Gordon-Salant, S., & Goupell, M. J. (2020). Spectral-temporal trade-off in vocoded sentence recognition: Effects of age, hearing thresholds, and working memory. Ear and Hearing, 41(5), 1226. 10.1097/AUD.0000000000000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheft, S., Shafiro, V., Wang, E., Barnes, L. L., & Shah, R. C. (2015). Relationship between auditory and cognitive abilities in older adults. PLOS ONE, 10(8), Article e0134330. 10.1371/journal.pone.0134330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, J., & Kraus, N. (2016). The role of rhythm in perceiving speech in noise: A comparison of percussionists, vocalists and non-musicians. Cognitive Processing, 17, 79–87. 10.1007/s10339-015-0740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. L., & Pichora-Fuller, M. K. (2015). Associations between speech understanding and auditory and visual tests of verbal working memory: Effects of linguistic complexity, task, age, and hearing loss. Frontiers in Psychology, 6, Article 1394. 10.3389/fpsyg.2015.01394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, P., & Arehart, K. (2015). Robust relationship between reading span and speech recognition in noise. International Journal of Audiology, 54(10), 705–713. 10.3109/14992027.2015.1043062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbäck, V., Hällgren, M., & Larsby, B. (2016). Executive functions and working memory capacity in speech communication under adverse conditions. Speech, Language and Hearing, 19(4), 218–226. 10.1080/2050571X.2016.1196034 [DOI] [Google Scholar]
- Strelcyk, O., Zahorik, P., Shehorn, J., Patro, C., & Derleth, R. P. (2019). Sensitivity to interaural phase in older hearing-impaired listeners correlates with nonauditory trail making scores and with a spatial auditory task of unrelated peripheral origin. Trends in Hearing, 23. 10.1177/2331216519864499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strori, D., & Souza, P. E. (2022). The role of working memory in speech recognition by hearing-impaired older listeners: Does the task matter? International Journal of Audiology, 62(11), 1067–1075. 10.1080/14992027.2022.2128445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckenschneider, T., Askew, C. D., Weber, J., Abeln, V., Rüdiger, S., Summers, M. J., Schneider, S., & NeuroExercise Study Group. (2020). Auditory event-related potentials in individuals with subjective and mild cognitive impairment. Behavioural Brain Research, 391, Article 112700. 10.1016/j.bbr.2020.112700 [DOI] [PubMed] [Google Scholar]
- Sweetow, R. W., & Sabes, J. H. (2006). The need for and development of an adaptive listening and communication enhancement (LACE™) program. Journal of the American Academy of Audiology, 17(8), 538–558. 10.3766/jaaa.17.8.2 [DOI] [PubMed] [Google Scholar]
- Szymaszek, A., Sereda, M., Pöppel, E., & Szelag, E. (2009). Individual differences in the perception of temporal order: the effect of age and cognition. Cognitive Neuropsychology, 26(2), 135–147. 10.1080/02643290802504742 [DOI] [PubMed] [Google Scholar]
- Tamati, T. N., Gilbert, J. L., & Pisoni, D. B. (2013). Some factors underlying individual differences in speech recognition on PRESTO: A first report. Journal of the American Academy of Audiology, 24(7), 616–634. 10.3766/jaaa.24.7.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H., Koenig, T., Pascual-Marqui, R. D., Hirata, K., Kochi, K., & Lehmann, D. (2000). Event-related potential and EEG measures in Parkinson's disease without and with dementia. Dementia and Geriatric Cognitive Disorders, 11(1), 39–45. 10.1159/000017212 [DOI] [PubMed] [Google Scholar]
- Thomas, C., vom Berg, I., Rupp, A., Seidl, U., Schröder, J., Roesch-Ely, D., Kreisel, S. H., Mundt, C., & Weisbrod, M. (2010). P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiology of Aging, 31(3), 416–424. 10.1016/j.neurobiolaging.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Tremblay, K., & Kraus, N. (2002). Beyond the ear: Central auditory plasticity. Otorinolaringologia, 52, 93–100. [Google Scholar]
- Uchida, Y., Sugiura, S., Nishita, Y., Saji, N., Sone, M., & Ueda, H. (2019). Age-related hearing loss and cognitive decline—The potential mechanisms linking the two. Auris Nasus Larynx, 46(1), 1–9. 10.1016/j.anl.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Ulbrich, P., Churan, J., Fink, M., & Wittmann, M. (2009). Perception of temporal order: The effects of age, sex, and cognitive factors. Aging, Neuropsychology, and Cognition, 16(2), 183–202. 10.1080/13825580802411758 [DOI] [PubMed] [Google Scholar]
- Unsworth, N., & Spillers, G. J. (2010). Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. Journal of Memory and Language, 62(4), 392–406. 10.1016/j.jml.2010.02.001 [DOI] [Google Scholar]
- van Rooij, J. C., & Plomp, R. (1992). Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion. The Journal of the Acoustical Society of America, 91(2), 1028–1033. 10.1121/1.402628 [DOI] [PubMed] [Google Scholar]
- Vaughan, N., Storzbach, D., & Furukawa, I. (2008). Investigation of potential cognitive tests for use with older adults in audiology clinics. Journal of the American Academy of Audiology, 19(07), 533–541. 10.3766/jaaa.19.7.2 [DOI] [PubMed] [Google Scholar]
- Volpato, C., Piccione, F., Silvoni, S., Cavinato, M., Palmieri, A., Meneghello, F., & Birbaumer, N. (2010). Working memory in amyotrophic lateral sclerosis: Auditory event-related potentials and neuropsychological evidence. Journal of Clinical Neurophysiology, 27(3), 198–206. 10.1097/WNP.0b013e3181e0aa14 [DOI] [PubMed] [Google Scholar]
- Wagner, R. K., & Torgesen, J. K. (1987). The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin, 101(2), 192–212. 10.1037/0033-2909.101.2.192 [DOI] [Google Scholar]
- Wayne, R. V., Hamilton, C., Jones Huyck, J., & Johnsrude, I. S. (2016). Working memory training and speech in noise comprehension in older adults. Frontiers in Aging Neuroscience, 8, Article 49. 10.3389/fnagi.2016.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing, J., Chermak, G. D., & Musiek, F. E. (2015). Auditory training for central auditory processing disorder [Paper presentation]. Seminars in Hearing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield, A., Stine, E. A., Lahar, C. J., & Aberdeen, J. S. (1988). Does the capacity of working memory change with age? Experimental Aging Research, 14(2), 103–107. 10.1080/03610738808259731 [DOI] [PubMed] [Google Scholar]
- Wong, L. L. N., Yu, J. K. Y., Chan, S. S., & Tong, M. C. F. (2014). Screening of cognitive function and hearing impairment in older adults: A preliminary study. BioMed Research International, 2014, Article 867852. 10.1155/2014/867852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. C., Jin, J. X., Gunasekera, G. M., Abel, R., Lee, E. R., & Dhar, S. (2009). Aging and cortical mechanisms of speech perception in noise. Neuropsychologia, 47(3), 693–703. 10.1016/j.neuropsychologia.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, L., Wang, D., Wang, J., Xu, H., Huo, L., Tian, Y., Dai, Q., Wei, S., Wang, W., Zhang, G., Du, X., Jia, Q., Zhu, X., Wang, L., Tang, W., & Zhang, X. Y. (2020). Association of cognitive and P50 suppression deficits in chronic patients with schizophrenia. Clinical Neurophysiology, 131(3), 725–733. 10.1016/j.clinph.2019.12.405 [DOI] [PubMed] [Google Scholar]
- Yadon, C. A., Bugg, J. M., Kisley, M. A., & Davalos, D. B. (2009). P50 sensory gating is related to performance on select tasks of cognitive inhibition. Cognitive, Affective, & Behavioral Neuroscience, 9(4), 448–458. 10.3758/CABN.9.4.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend, I., Beach, E. F., & Sharma, M. (2019). Working memory and extended high-frequency hearing in adults: Diagnostic predictors of speech-in-noise perception. Ear and Hearing, 40(3), 458–467. 10.1097/AUD.0000000000000640 [DOI] [PubMed] [Google Scholar]
- Yumba, W. K. (2019). Selected cognitive factors associated with individual variability in clinical measures of speech recognition in noise amplified by fast-acting compression among hearing aid users. Noise & Health, 21(98), 7. 10.4103/nah.NAH_59_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q., Dong, X., Ruan, C., Hu, B., Zhou, B., Xue, Y., Liu Y, & Yang, H. (2017). Cognitive impairment in Chinese IIDDs revealed by MoCA and P300. Multiple Sclerosis and Related Disorders, 16, 1–7. 10.1016/j.msard.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Zhan, Y., Fellows, A. M., Qi, T., Clavier, O. H., Soli, S. D., Shi, X., Gui, J., Shi, Y., & Buckey, J. C. (2018). Speech in noise perception as a marker of cognitive impairment in HIV infection. Ear and Hearing, 39(3), 548–554. 10.1097/AUD.0000000000000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within this article and Supplemental Material S1.



 This work is licensed under a
This work is licensed under a