Abstract
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive disease caused by the deposition of insoluble amyloid fibrils derived from misfolded transthyretin (TTR). The treatment landscape is rapidly evolving, with disease-modifying therapies now targeting distinct steps in disease progression. Management requires both disease-modifying treatment and symptom-guided treatment of heart failure and arrhythmias, along with device therapy and consideration of advanced heart failure interventions (i.e., heart transplantation) in select patients. Therapeutic advances have significantly increased treatment possibilities, selection of appropriate therapy, switching between therapies, combination strategies, and how to monitor treatment response over time. This review summarizes available and investigational therapies for ATTR-CM and considers practical questions that guide clinical decision-making, with the goal of helping clinicians navigate the evolving therapeutic landscape.
Keywords: Transthyretin amyloid cardiomyopathy (ATTR-CM), Treatment, Disease-modifying therapy, Heart failure, Tafamidis, Acoramidis, Vutrisiran
Key Summary Points
| Transthyretin amyloid cardiomyopathy is a progressive disease that requires both disease-modifying treatment and symptom-directed management. |
| In the past 6 months, the number of therapies approved by the United States Food and Drug Administration (FDA) for transthyretin amyloid cardiomyopathy has tripled. Tafamidis was approved in 2019, followed by acoramidis in November 2024 and vutrisiran in March 2025. Additional therapies targeting transthyretin silencing, amyloid clearance, and gene editing are in development. |
| This review focuses on current and emerging therapies for transthyretin amyloid cardiomyopathy and the practical challenges clinicians face in initiating, monitoring, and adjusting treatment as options continue to expand. |
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive disease characterized by the accumulation of insoluble amyloid fibrils derived from misfolded transthyretin (TTR). TTR is a tetrameric protein produced predominantly in the liver that plays a role in transporting retinol and thyroxine. In ATTR-CM, amyloid arises either from non-variant TTR (wild-type ATTR, or ATTRwt), where factors such as aging and oxidative stress drive later-onset amyloid formation, or from variant TTR (variant ATTR, or ATTRv), inherited in an autosomal-dominant pattern with incomplete penetrance [1–5]. In both forms of disease, unstable tetramers dissociate into monomers that are prone to misfolding and aggregation into amyloid fibrils. Amyloid is deposited in the extracellular space of different target organs, disrupting their structure and function. In the myocardium, amyloid infiltration leads to wall thickening which consequently causes impaired relaxation and elevated filling pressures, resulting in progressive cardiomyopathy and possible death. Median survival ranges between 2 and 5 years without treatment [1–9]. For years, treatment of ATTR-CM was limited to managing symptoms, with few options available to alter the disease course. The recent introduction of numerous disease-modifying therapies has shifted this paradigm, and ongoing research continues to expand treatment possibilities. This review outlines current and emerging treatments for ATTR-CM and examines how recent therapeutic advances are reshaping management strategies in clinical practice. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Disease-Modifying Therapies for ATTR-CM
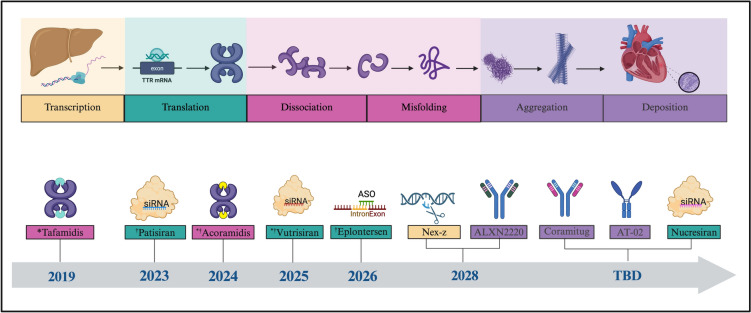
Significant progress has been achieved over the past decade in the development of therapies for ATTR-CM that target different steps of disease pathogenesis (Fig. 1). Stabilizers bind to the TTR tetramer at the thyroxine binding site to prevent dissociation into amyloidogenic monomers. Silencers, such as small interfering RNA (siRNA) and antisense oligonucleotides (ASOs), work at the level of the hepatocyte, targeting TTR messenger RNA (mRNA) to reduce hepatic TTR production, thereby limiting the supply of amyloidogenic monomers. Additionally, new treatments are being developed to bind amyloid and promote immune-mediated clearance of amyloid fibrils [2, 8, 10, 11].
Fig. 1.
The evolving treatment landscape of transthyretin amyloid cardiomyopathy. Created in BioRender. Margolin, E. (2025) https://BioRender.com/ mz5wyr4. Dates reflect projected FDA approval for treatment of transthyretin amyloid cardiomyopathy, including therapies that were not approved. *FDA-approved for the treatment of transthyretin amyloid cardiomyopathy. †FDA-approved for the treatment of transthyretin amyloid polyneuropathy. *†FDA-approved for the treatment of transthyretin amyloid cardiomyopathy and transthyretin amyloid polyneuropathy. Pink: transthyretin stabilized. Green: transthyretin silencer. Yellow: gene-editing therapy. Purple: degrader/depleter. siRNA small interfering ribonucleic acid, ASO antisense oligonucleotide, TBD to be determined
Current Therapies
Stabilizers
Tafamidis was the first FDA-approved treatment of ATTR-CM in May 2019. Tafamidis is a once-a-day oral therapy that binds to TTR’s thyroxine binding site, stabilizing its structure. The ATTR-ACT trial demonstrated that tafamidis significantly reduced mortality and cardiovascular-related hospitalizations over 30 months, with patients on tafamidis showing a 29.5% mortality rate compared with 42.9% in the placebo group (hazard ratio [HR]: 0.67, 95% CI: 0.51–0.96). Rates of cardiovascular-related hospitalizations were also 32% lower in the tafamidis group (relative risk reduction [RRR]: 0.68, 95% CI: 0.56–0.81), with additional benefits seen in attenuated decline in the 6-min walk test (6MWT) distance and Kansas City Cardiomyopathy Questionnaire Overall Summary (KCCQ-OS) score [3, 11–13].
Acoramidis, a next-generation stabilizer, was developed to replicate the stabilizing effect of the naturally occurring T119M mutation, which enhances TTR tetramer stability. In addition to binding thyroxine, it forms hydrogen bonds with serine residues at position 117 as in the T119M variant, contributing to its ability to achieve over 90% TTR stabilization [14]. Acoramidis is a twice-a-day oral therapy treatment, which became the second FDA-approved therapy for ATTR-CM (including both ATTRwt- and ATTRv-CM) in November 2024 after publication of the ATTRibute-CM trial. This trial demonstrated that acoramidis was superior to placebo, achieving a statistically significant primary endpoint as assessed by a four-step hierarchical win ratio analysis of all-cause mortality, cardiovascular hospitalizations, change from baseline in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, and change from baseline in 6MWT distance (win ratio: 1.8, 95% CI: 1.4–2.2; P < 0.001). Serial cardiac magnetic resonance imaging (MRI) scans were also used in a subset of acoramidis-treated patients to assess changes in amyloid burden. Patients on acoramidis showed favorable trends in cardiac magnetic resonance (CMR) parameters compared with placebo. The observation of potential amyloid regression warrants further investigation. The ATTRibute-CM protocol allowed enrolled patients to initiate tafamidis starting at 12 months, with 14.9% of those in the acoramidis group and 22.8% in the placebo group ultimately receiving it. The median time to initiation of tafamidis and duration of exposure were similar between groups [11, 14].
Silencers
Patisiran is an siRNA-based therapy that was FDA-approved to treat hereditary ATTR-polyneuropathy (ATTRv-PN) in 2018. It is administered as an intravenous infusion with pre-medications every 3 weeks. Patisiran’s expanded use in ATTR-CM was evaluated in the APOLLO-B phase III trial [15]. The trial met its co-primary endpoints of reduced decline in 6MWT distance and KCCQ-OS score, though the absolute differences were modest despite statistical significance. Secondary analyses evaluated composite clinical outcomes including all-cause mortality, cardiovascular events, hospitalizations, and urgent heart failure visits but did not show significant differences; of note, the trial was not powered for these outcomes. Patients on tafamidis at baseline were allowed to continue treatment (25% in both groups), and a small percentage initiated tafamidis during the 12-month double-blind period (3% in the patisiran group, 2% in the placebo group). In October 2023, the FDA declined a label extension for ATTR-CM, citing limited clinical meaningfulness, with statistically significant benefits observed only in surrogate endpoints and modest absolute differences [2, 3, 5, 8, 11, 15]. As with the 12-month results of the ATTRibute-CM trial, this may reflect insufficient duration or event rates to detect differences in hard outcomes [14].
Vutrisiran, a second-generation silencer, employs a similar siRNA mechanism to patisiran but it is administered subcutaneously every 3 months. It was FDA-approved for ATTRv-PN in June 2022 and for ATTR-CM in March 2025. The HELIOS-B phase III trial evaluated its effect in patients with ATTRv- and ATTRwt-CM and found that vutrisiran reduced all-cause mortality and recurrent cardiovascular events (HR: 0.72, 95% CI: 0.56–0.93; P = 0.01). In addition, vutrisiran led to decreased decline in functional endpoints, with patients experiencing a 26.5-m lower decline in 6MWT (P < 0.001) and a 5.8-point lower decline in KCCQ scores (P < 0.001) [2, 3, 5, 8, 11, 16]. At baseline, 60% of patients in both the vutrisiran and placebo groups were not receiving tafamidis, but 22% and 21%, respectively, initiated tafamidis after randomization. Patients not on tafamidis at baseline experienced greater benefit from vutrisiran, with reduced all-cause mortality and recurrent cardiovascular events (HR: 0.67, 95% CI: 0.49–0.93; P = 0.02).
Emerging Therapies
Silencers
Eplontersen is an antisense oligonucleotide (ASO) therapy that reduces hepatic TTR production by targeting TTR mRNA. It is administered subcutaneously every month; however, it can be administered by the patient at home, whereas vutrisiran must be administered in a healthcare setting. Eplontersen was FDA-approved in December 2023 for ATTRv-PN based on its ability to lower serum TTR levels and improve neuropathy symptoms in the NEURO-TTRansform trial. Post hoc analyses of this trial suggested potential cardiac benefits including possible structural remodeling in response to treatment, with improvements in echocardiographic parameters such as left ventricular end-diastolic volume, stroke volume, and left atrial volume [11]. While not yet approved for ATTR-CM, its effects on cardiomyopathy are being investigated in the CARDIO-TTRansform trial, which when completed will be the largest trial in ATTR-CM, enrolling over 1400 patients, and will assess whether eplontersen reduces cardiovascular mortality and recurrent hospitalizations. Concomitant tafamidis use is allowed, and this trial may provide insight into the role of combined stabilizer-silencer therapy [5, 11].
Nucresiran (ALN-TTRsc04) is a next-generation siRNA therapy that inhibits hepatic synthesis of both wild-type and variant TTR mRNA through subcutaneous administration every 6 to 12 months. In phase 1 studies, it achieved deep and sustained TTR knockdown, with reductions exceeding 96% at higher doses and suppression maintained for at least 180 days [17]. Based on these findings, the phase 3 TRITON-CM trial was recently announced to evaluate nucresiran against placebo in patients with ATTR-CM receiving standard-of-care therapy. The study plans to enroll approximately 1200 patients with either wild-type or variant ATTR-CM, including those already on stabilizer therapy [18], and will also provide insight into the possible benefit of combination therapy.
Nexiguran ziclumeran (Nex-z, also known as NTLA-2001) is a CRISPR-Cas9-based gene-editing therapy designed to silence the TTR gene with a single intravenous infusion. In a phase I study, Nex-z reduced serum TTR levels by 89% at 1 month that was sustained at 12 months (95% CI −93 to −87), with no serious adverse events reported. Functional measures and cardiac biomarkers remained stable, with unchanged NT-proBNP levels, a median increase in 6MWT of 5 m, and improvement in KCCQ-OS scores by eight points [19]. The phase III MAGNITUDE trial is investigating Nex-z’s impact on cardiovascular mortality, recurrent events, and amyloid burden. This trial was initially designed for a more advanced population, requiring NT-proBNP greater than 1000 pg/mL for entry. However, the threshold was recently lowered to 600 pg/mL, resulting in a study population that will more closely resemble those in prior silencer trials [20]. Structural changes will be evaluated using serial cardiac MRI scans to track changes in extracellular volume (ECV) and treatment response [11].
Depleters
While stabilizers and silencers disrupt the amyloidogenic cascade by decreasing the precursor to amyloid fibril formation, depleter therapies have potential effects downstream, targeting circulating amyloid fibrils and clearing existing amyloid deposits. ALXN2220 (formerly NI006) is a recombinant human monoclonal Immunoglobulin G1 (IgG1) antibody designed to recognize and bind misfolded TTR within extracellular amyloid deposits. ALXN2220 binds misfolded TTR and activates the immune system, leading to macrophage-mediated removal through Fc receptor-mediated phagocytosis. [2, 4, 5, 21, 22]. In a phase I study of 40 patients with ATTRwt- or ATTRv-CM, ALXN2220 administration was associated with an improvement in the cardiac imaging and NT proBNP level, both considered to be a surrogate for amyloid burden. The degree of circulating native TTR was unaffected in this trial [25]. The ongoing phase III DepleTTR-CM trial, launched in January 2024, is evaluating ALXN2220’s long-term efficacy and safety in 1000 patients with more advanced ATTR-CM than in other trials, enrolling patients with New York Heart Association (NYHA) class IV symptoms with a requisite NT proBNP of greater than 2000 pg/mL, with weight-based intravenous infusions administered every 4 weeks over 48 months [1].
Coramitug (formerly PRX004), another investigational TTR depleter, is a monoclonal IgG1 antibody that binds an epitope specific to TTR monomers and misfolded aggregates [22]. Like ALXN2220, binding facilitates amyloid clearance through antibody-mediated phagocytosis. In addition, by binding soluble amyloidogenic monomers, coramitug may interfere with fibril elongation, thereby preventing further amyloid formation [23, 24]. In a phase I study, coramitug demonstrated safety and tolerability in 21 patients with ATTRv-PN and/or ATTRv-CM, with some patients showing improved global longitudinal strain (GLS) [11, 23, 24]. Coramitug is currently in phase 2 trials for the treatment of ATTR-CM, with 99 patients (ATTRv or ATTRwt) receiving monthly intravenous infusions to assess changes in 6MWT and NT-proBNP over 12 months. Results are expected in late 2025 [11, 23].
AT-02 is a humanized IgG1-peptide fusion antibody designed for pan-amyloid clearance. Unlike ALXN2220 and coramitug, which specifically bind ATTR, AT-02 is a pan-amyloid antibody with an engineered p5R peptide component. The p5R peptide uses electrostatic interactions to bind all forms of amyloid deposits with high affinity, allowing AT-02 to function as a broad-spectrum amyloid depleter, potentially treating various types of amyloidosis, including ATTR-CM [11, 22, 23]. Preclinical studies showed effective amyloid clearance across multiple organs, including the heart, liver, and spleen, with sustained phagocytic activity facilitating long-term removal of deposits [22]. AT-02 is currently being evaluated in phase I and II trials for its safety and efficacy in both healthy volunteers and patients with systemic amyloidosis, including ATTR-CM [11, 23].
Supportive and Adjunctive Treatments
In addition to disease-modifying therapies, treatment of ATTR-CM includes symptomatic management of heart failure and arrhythmias, with consideration of advanced therapies in advanced disease states without significant extracardiac involvement.
Non-Disease-Specific Medical Therapies
Heart Failure
Heart failure in ATTR-CM is characterized by a restrictive cardiomyopathy, often with preserved or mildly reduced ejection fraction, with progression to reduced ejection fraction in later-stage disease. Medical therapy is often poorly tolerated, and its role in patients with heart failure with reduced ejection fraction (HFrEF) remains unclear, with expert opinion cautioning against the routine use of guideline-directed medical therapy (GDMT).
In a retrospective study of patients with ATTR-CM, the use of low-dose beta-blockers was independently associated with improved survival in those with left ventricular ejection fraction (LVEF) ≤ 40% (HR 0.61 [95% CI 0.45–0.83], P = 0.002) [26]. However, in another study, beta-blocker withdrawal was associated with improved quality of life [27]. Patients with advanced ATTR-CM may not tolerate traditional heart rate goals of less than 70 beats per minute, as they have a fixed stroke volume from restrictive physiology contributing to dependence on heart rate for cardiac output augmentation. Therefore, beta-blockers should be used with caution, and tolerance should be routinely assessed in patients receiving them [2, 5, 11, 28].
Similarly, vasodilatory therapies such as angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), and angiotensin receptor-neprilysin inhibitors (ARNIs) can exacerbate hypotension in patients who are normotensive or have baseline hypotension. This is particularly a concern in patients with ATTRv-CM, who often have debilitating autonomic dysfunction and orthostasis. While these therapies typically increase stroke volume and cardiac output in patients with HFrEF by reducing afterload, their effectiveness may be limited in patients with ATTR due to a fixed stroke volume seen in advanced restrictive heart disease. Therefore, their use should be approached with caution [5, 12, 28, 29].
In contrast, mineralocorticoid receptor antagonists (MRAs) are generally well tolerated by patients with ATTR-CM and have been associated with improved outcomes in retrospective propensity-matched analyses. Additionally, sodium-glucose cotransporter-2 inhibitors (SGLT2i) have shown benefit across various heart failure phenotypes, including ATTR-CM, though prospective trials are lacking [5, 11, 28, 30, 31]. Both MRAs and SGLT2is are therefore increasingly considered in ATTR-CM management.
Diuretics remain the cornerstone of symptomatic treatment for heart failure in ATTR-CM, with the goal of maintaining euvolemia. Loop diuretics are commonly used but require careful titration, given the narrow euvolemic window in restrictive cardiomyopathies. Over-diuresis may lead to hypotension and acute kidney injury, particularly when using sequential nephron blockade with thiazide diuretics [2, 5, 28]. Importantly, progressive diuretic escalation and decreased glomerular filtration rate (GFR) are markers of disease progression in ATTR-CM and have been associated with worse survival [5, 32].
Arrhythmias
Atrial fibrillation (AF) is common in ATTR-CM and can worsen hemodynamics, often leading to symptom progression. Loss of atrial contraction may be particularly poorly tolerated in this population, and rhythm control is often preferred in early-stage disease when more effective, though it may not be feasible in those with advanced cardiomyopathy [33]. Amiodarone is generally well tolerated as a first-line agent, and catheter ablation appears more effective when pursued earlier in the disease course. The benefit of rhythm control over rate control remains uncertain, with no demonstrated mortality difference in available studies [2, 28].
Rate control is typically pursued when rhythm control is not achievable, but standard agents such as beta-blockers and calcium channel blockers may be poorly tolerated due to their negative inotropic effects as described above [2, 5]. Digoxin has traditionally been avoided due to concerns about amyloid binding and toxicity, but recent studies suggest it may be used cautiously for rate control in select patients with close monitoring for toxicity [5, 28].
Given the high thromboembolic risk in ATTR-CM, anticoagulation is recommended in all patients with AF, regardless of CHA2DS2-VASc score. Direct oral anticoagulants (DOACs) are preferred over warfarin due to their favorable safety profile and ease of use. Additionally, transesophageal echocardiography (TEE) may be considered prior to cardioversion, regardless of prior anticoagulation status, due to the increased risk of intracardiac thrombi even in normal sinus rhythm [2, 28, 29].
Devices and Advanced Therapies
Devices:
Conduction disease is common in ATTR-CM, and pacemaker implantation is indicated based on standard guidelines. Cardiac resynchronization therapy (CRT) may be considered in patients with significant ventricular dyssynchrony or those requiring a high degree of ventricular pacing, though data on ATTR-CM remain limited [2, 28]. Implantable cardioverter-defibrillators (ICDs) are generally reserved for secondary prevention, as primary prevention remains controversial. ICD placement should be individualized based on patient risk factors and clinical context [2, 28, 29].
Advanced Therapies:
For ATTRv-CM, orthotopic liver transplantation or combined heart-liver transplantation was historically considered to be the only available disease-modifying treatment [2]. In patients with ATTRwt-CM, liver transplantation is not a curative option, and heart transplantation is an important consideration for patients with advanced (American College of Cardiology [ACC]/American Heart Association [AHA] stage D) heart failure and minimal extracardiac involvement [2, 28]. In the contemporary era, appropriately selected patients with ATTR-CM achieve favorable outcomes following heart transplantation. For instance, a cohort from Cedars-Sinai Medical Center demonstrated a 3-year survival rate of 86% with no amyloid recurrence [34], while data from Stanford University Medical Center and Kaiser Permanente Santa Clara Medical Center reported a 3-year survival of 88% without graft failure or amyloid-related mortality [35]. Similarly, the Mayo Clinic reported a 3-year post-transplant survival of 90.8% in patients with ATTR-CM [36]. Additionally, in the modern era, adjunct silencer treatment post-heart transplant is often employed for patients with ATTRv-CM with concurrent peripheral neuropathy.
Monitoring Disease Progression and Treatment Response
Based on expert consensus, monitoring disease progression in patients with ATTR-CM requires consideration of three domains: (1) clinical and functional endpoints, (2) biomarkers and laboratory markers, and (3) imaging parameters and electrocardiography (ECG). Disease progression is defined by worsening in at least one marker in each domain [37].
Markers of Prognosis and Disease Progression
Clinical and Functional Endpoints
Clinical and functional endpoints include cardiovascular-related hospitalizations, increases in NYHA class, declines in KCCQ scores, and changes in the 6MWT or peak oxygen uptake (pVO2). An absolute reduction of > 35 m or a relative reduction of > 5% in 6MWT is associated with disease progression and is independently linked to a worse prognosis [31]. Both daily diuretic dose and NYHA functional class are independent predictors of mortality in ATTR-CM [11, 32]. In a cohort of patients with ATTR-CM, higher daily diuretic dose was significantly associated with all-cause mortality, with a 1 mg/kg increase in furosemide equivalents nearly doubling the unadjusted mortality risk [32]. More recently, outpatient diuretic escalation (ODI) has been identified as a longitudinal marker of disease worsening in ATTR-CM. Defined as a sustained increase in loop diuretic dose for more than a month, ODI was independently associated with higher mortality in cohorts of patients receiving tafamidis [38], patisiran [39], and vutrisiran [40].
Biomarkers and Staging:
Elevated NT-proBNP (> 3000 pg/mL) is associated with worse survival and serves as a key component of multiple staging systems for ATTR-CM, which are outlined below. Changes in NT-proBNP levels also provide important prognostic information. Relative increases > 30% or absolute increases > 700 ng/L indicate disease progression and are independently associated with increased mortality in ATTR-CM [2, 4, 5, 7, 9, 11, 41, 42].
For ATTRwt-CM, the Mayo staging system stratifies patients into three stages based on NT-proBNP > 3000 pg/mL and troponin T (TnT) > 0.05 ng/mL, with increasing stage correlating with worse median survival [28, 41]. This system has not been validated for ATTRv-CM. In contrast, the United Kingdom (UK) National Amyloidosis Centre (NAC) system applies to both ATTRwt-CM and ATTRv-CM, using NT-proBNP > 3000 ng/L and estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 to classify patients into three stages, with corresponding median survival rates of approximately 2, 4, and 6 years, respectively. A more recent adaptation includes a fourth stage (NT-proBNP > 10,000 ng/L) to identify patients at the highest risk of early mortality [11]. To further refine prognostication, the Columbia staging system was developed by adding diuretic dose and NYHA functional class to the Mayo and UK NAC risk scores. Since these variables are independent predictors of mortality in ATTR-CM, they provide incremental value to existing risk scores [32].
High-sensitivity troponin is also considered in the assessment of ATTR-CM, with a 30% relative increase indicating disease progression [37]. While troponin’s prognostic utility is well established in ATTRwt-CM, its significance in ATTRv-CM remains unproven [37, 43, 44]. Similarly, serum TTR has been proposed as a potential marker of disease progression in ATTR-CM, though current evidence is specific to ATTRwt-CM [37]. In ATTRwt, lower baseline serum TTR concentrations (< 18 mg/dL) have been independently associated with shorter survival, with longitudinal assessments showing that declining levels correspond to worsening cardiac function [4, 45]. While serum troponin and TTR may offer future insights into disease severity, further research is needed to clarify their applications in monitoring ATTRv-CM progression.
The Role of Imaging and Amyloid Burden
Echocardiographic markers of disease progression include increased left ventricular (LV) wall thickness, worsening diastolic dysfunction, reduced systolic function, and deterioration in global longitudinal strain (GLS). Clinically meaningful thresholds from trial data include ≥ 2 mm increases in LV wall thickness, ≥ 5% reductions in ejection fraction (EF), or ≥ 1% worsening in GLS [2, 5, 6, 8, 10, 12, 46]. New-onset conduction abnormalities on ECG, including supraventricular arrhythmias—particularly atrial fibrillation—as well as atrioventricular and intraventricular conduction disturbances, also indicate disease progression [37, 47].
Amyloid burden, defined as the extent of amyloid deposition in the heart, can be assessed using cardiac MRI (CMR) and nuclear scintigraphy. CMR evaluation includes native T1 mapping, late gadolinium enhancement (LGE), and extracellular volume (ECV) measurement, with ECV serving as a surrogate for amyloid burden. Increases in ECV occur early in the disease process, preceding LGE and structural changes [2, 5, 6, 8, 10, 11]. While CMR has significant prognostic value and potential for monitoring treatment response, its broader use is limited by cost and challenges with serial assessments. Similarly, the role of cardiac scintigraphy with bone-avid tracers in tracking ATTR-CM progression remains unclear and requires further study [6, 11, 48].
Practical Considerations in Therapy Selection
Therapy selection for ATTR-CM requires consideration of patient-centered factors such as disease stage, medication tolerability, cost, and access to treatment. Practical considerations also include treatment logistics, such as mode of administration and dosing schedules.
Disease Stage
As the only disease-modifying therapies currently FDA-approved for ATTR-CM, tafamidis, acoramidis, and vutrisiran are recommended as first-line therapies for patients with NYHA class I–III symptoms [3, 10, 22, 23]. However, the trials supporting these approvals enrolled different patient populations, particularly with respect to disease severity. ATTR-ACT included a greater proportion of patients with NYHA class III symptoms, while ATTRibute-CM and HELIOS-B enrolled more patients in class II [12, 14, 16]. These differences complicate direct comparisons and should be considered when applying trial data to clinical care. Early findings indicate a more consistent benefit in NYHA III, but additional studies are needed [10, 23]. For patients with advanced disease (NYHA class IV), disease-modifying therapies are unproven and identify a gap in data for this high-risk population. Table 1 summarizes key baseline characteristics across the three trials to help clarify differences in patient populations.
Table 1.
Baseline characteristics of trial populations for FDA-approved ATTR-CM therapies
| ATTR-ACT | ATTRibute-CM | HELIOS-B | ||
|---|---|---|---|---|
| Trial design | Therapy studied | Tafamidis | Acoramidis | Vutrisiran |
| Study duration (months) | 30 | 30 | 36 | |
| Population size (n) | 441 | 632 | 654 | |
| Age (years) | Treatment (mean ± SD) | 74.5 ± 7.2 | 77.4 ± 6.5 | *77 (45–85) |
| Placebo (mean ± SD) | 74.1 ± 6.7 | 77.1 ± 6.8 | *76 (46–85) | |
| Gender | Male (n, %) | 398 (90.2) | 570 (90.2) | 605 (92.5) |
| (n, %) | 43 (9.8) | 62 (9.8) | 49 (7.5) | |
| Race | White (n, %) | 357 (81) | 555 (87.8) | 552 (84.4) |
| Black (n, %) | 63 (14.3) | 30 (4.7) | 47 (7.2) | |
| Asian (n, %) | 18 (4.1) | 13 (2.1) | 37 (5.7) | |
| Other (n, %) | 3 (0.7) | 34 (5.4) | 18 (2.8) | |
| Genotype | ATTRv (n, %) | 106 (24) | 61 (9.7) | 76 (11.6) |
| ATTRwt (n, %) | 335 (76) | 571 (90.3) | 578 (88.4) | |
| NYHA Class | I (n, %) | 37 (8.4) | 68 (10.8) | 84 (12.8) |
| II (n, %) | 263 (59.6) | 455 (72) | 508 (77.7) | |
| III (n, %) | 141 (32) | 103 (17.2) | 62 (9.5) | |
| NT-proBNP (pg/mL) | Treatment (median, IQR) | 2995.9 (1751.5–4861.5) | 2326 (1332–4019) | 2021 (1138–3312) |
| Placebo (median, IQR) | 3161 (1864.4–4825) | 2306 (1128–3754) | 1801 (1042–3082) | |
| Tafamidis use | Treatment (n, %) | Not applicable | †61 (14.9) | 130 (40%) |
| Placebo (n, %) | †46 (22.8) | 129 (39%) | ||
NYHA New York Heart Association, NT-proBNP N-terminal pro-B-type natriuretic peptide
pg/mL picograms per milliliter, SD standard deviation, ATTRv hereditary transthyretin amyloidosis
ATTRwt wild-type transthyretin amyloidosis, IQR interquartile range
*Median (IQR) shown; mean ± SD not reported in HELIOS-B
†Tafamidis use was permitted after 12 months into the trial and at physician discretion
Cost and Access
The cost of ATTR-CM disease-modifying therapies is a significant barrier to patient access. Tafamidis and acoramidis, for example, cost over $240,000 per year. Silencer therapies are even more expensive, with annual costs surpassing $500,000 for vutrisiran and eplontersen [3, 5]. Cost-effectiveness analyses estimate that an 80–92% price reduction would be necessary to align tafamidis with established value benchmarks, as its current incremental cost-effectiveness ratio (ICER) exceeds $180,000 per quality-adjusted life-year (QALY) gained [3, 5, 10, 28, 29]. Financial assistance programs may be required to improve patient access to any disease-modifying therapy, but navigating these programs presents additional challenges [28].
Safety, Tolerability, and Administration
Stabilizer therapies are generally well tolerated and offer a convenient approach to TTR stabilization through oral administration. Tafamidis, taken once daily, has a favorable safety profile, with adverse events occurring less frequently than in placebo groups [12, 28]. Similarly, acoramidis, taken twice daily, has shown a comparable rate of adverse events to placebo and fewer serious adverse events overall. A transient increase in serum creatinine (mean 0.2 mg/dL) is observed within the first month of acoramidis therapy and returns to baseline after discontinuation [5, 10, 14, 49].
Silencer therapies require periodic injections and vary in dosing frequency. Unlike intravenous formulations, vutrisiran (25 mg subcutaneously every 3 months) and eplontersen (45 mg subcutaneously once monthly) do not require premedication due to their subcutaneous administration. However, because TTR transports vitamin A, all siRNA and ASO agents reduce serum retinol levels, leading to vitamin A deficiency. Supplementation with 3000 international units of vitamin A daily is recommended for all patients on silencer therapies, including patisiran, vutrisiran, and eplontersen. In addition to vitamin A deficiency, vutrisiran has been associated with extremity pain, peripheral edema, dizziness, and gout. Although the CARDIO-TTRansform is still underway, adverse effects observed in NEURO-TTRansform for hereditary ATTRv-PN were mild, with headache, diarrhea, and nausea reported less frequently with eplontersen than with placebo [5, 16, 50].
Investigational therapies offer alternative administration strategies. Nex-z, a CRISPR-based gene-editing therapy, is administered as a single intravenous infusion, potentially eliminating the need for ongoing dosing. Reported adverse effects from the phase 1 trials include self-limited elevated liver enzymes and infusion-related reactions, highlighting the need for close monitoring during initial use [5, 19]. ALXN2220 is given as an intravenous infusion every 4 weeks. No serious drug-related adverse events have been reported, but the most common side effects included cytokine release syndrome, dose-related arthralgia and arthritis, and transient thrombocytopenia [21, 25].
Therapy Optimization
Optimizing therapy in patients with ATTR-CM is complicated by limited data on when and how to adjust treatment. As disease-modifying therapies continue to evolve, questions remain about whether switching between agents or combining treatments can improve outcomes.
Switching Agents
Currently, there are no established guidelines on when or how to switch disease-modifying therapy in ATTR-CM. While criteria for disease progression in ATTR-CM have been proposed as outlined above, these have not been validated for assessing response to or failure of therapy [23, 37]. A key challenge is distinguishing between treatment failure and the natural course of ATTR-CM progression. While biomarkers and imaging have been proposed to track disease trajectory, their role in differentiating treatment failure from expected progression remains unclear. A recent retrospective study found that 50% of tafamidis-treated patients experienced worsening heart failure events—including HF hospitalization or sustained diuretic escalation—after a mean of 3.3 years from diagnosis. Those with worsening heart failure had higher subsequent mortality rates than those without [38]. Although disease progression can occur despite treatment, determining whether this reflects treatment failure or the expected course of ATTR-CM is challenging. Until more is known, progression should not be interpreted as a reason to discontinue treatment altogether, as long-term data on treated patients with ATTR-CM are incomplete at this time [3, 37].
Stabilizer to Stabilizer
Tafamidis and acoramidis are TTR stabilizers that have both been approved for use in ATTR-CM. No head-to-head trials directly compare these therapies, and differences in trial populations and study designs complicate cross-trial comparisons, as ATTRibute-CM included fewer NYHA III or ATTRv patients than ATTR-ACT [23]. In ATTRibute-CM, acoramidis-treated patients exhibited a 39% increase in serum TTR levels at 12 months, compared to a 30% increase observed in ATTR-ACT with tafamidis [3, 12, 14]. A greater increase in serum TTR was associated with improved cardiovascular outcomes in ATTRibute-CM [51], but whether this relationship holds across different stabilizers remains unknown. Whereas acoramidis was associated with a larger reduction in cardiovascular hospitalizations, tafamidis led to a greater relative reduction in mortality [12, 14]. While switching between stabilizers may be considered on an individualized basis, no definitive evidence favors one over the other at this time in the absence of head-to-head trials. Post hoc data from ATTRibute-CM showed a ~ 9 mg/dL rise in TTR with acoramidis, versus ~ 6 mg/dL in those on placebo or placebo-to-tafamidis. In the open-label phase, switching from tafamidis to acoramidis led to an additional ~ 3 mg/dL increase [52]. The clinical significance of these differences remains unclear.
Silencer to Silencer
TTR silencers have progressed from first-generation (patisiran) to second-generation agents (vutrisiran, eplontersen). While second-generation silencers theoretically achieved greater TTR knockdown in their respective phase III trials (~ 85–90% vs. ~ 80% with patisiran), head-to-head comparisons are lacking, and it remains unclear whether this translates to improved clinical outcomes. [23]. Given that patisiran was denied FDA approval for use beyond ATTRv-polyneuropathy, future direct comparisons between first- and second-generation silencers in cardiomyopathy will be limited.
Stabilizer to Silencer
There is currently no direct evidence comparing stabilizers to silencers, making it difficult to determine whether switching between these medication classes is beneficial. Retrospective analysis from the ATTR-ACT trial suggests that patients with worsening NT-proBNP levels or cardiac symptoms despite tafamidis therapy may show clinical improvement when switched to patisiran [4]. However, these findings do not provide definitive guidance on when or how to transition from a stabilizer to a silencer and do not necessarily support routine transitions between stabilizers and silencers [3, 23]. Switching agents should be guided by disease progression, tolerability, and individualized patient considerations.
Combination Therapy
Combination therapy with both silencers and stabilizers has been proposed as a strategy to target complementary aspects of disease pathophysiology [3, 10, 53]. Although second-generation silencers achieve substantial TTR knockdown (~ 80–90%), residual circulating TTR may still misfold and contribute to fibril formation. This raises the possibility of a synergistic effect when combining therapies, but clinical benefits have not yet been established by current evidence [23].
Recent phase III clinical trials have permitted the concomitant use of tafamidis but were not designed or powered to detect significant differences between monotherapy and combination therapy [5, 10, 14, 16, 23]. Subgroup analyses from APOLLO-B provide the most robust data on combination therapy to date; however, patisiran was not superior to placebo in improving 6MWT or KCCQ among patients receiving concurrent tafamidis [15]. Similarly, in ATTRibute-CM, 14.9% of patients in the acoramidis group initiated tafamidis after 12 months, but no benefit from combining stabilizers was observed [14]. In HELIOS-B, 40% of participants were taking tafamidis at baseline, and 22% of those receiving vutrisiran monotherapy started tafamidis during follow-up. However, the trial design did not allow for valid comparisons between combination therapy and monotherapy due to the lack of randomization to tafamidis use [3, 16, 23].
Ongoing trials like CARDIO-TTRansform may answer this question, as they are designed to include subgroup analyses powered to assess combination therapy. Until more robust evidence emerges, expert panels emphasize that monotherapy remains the standard of care due to the lack of supporting evidence for combination therapy and concerns about cost and accessibility [3, 23].
Discussion
The treatment landscape of ATTR-CM is evolving rapidly, with recent advances fundamentally changing disease management. Given the current pace of development, treatment strategies will likely look very different in the next 3 to 5 years. Current therapies focus on stabilizing TTR, reducing its synthesis, or promoting amyloid clearance, but it remains unclear how to best select, sequence, or combine them for individual patients. Optimizing therapy requires assessing treatment response, defining progression, and deciding when to adjust or escalate treatment.
Current markers of treatment response remain imperfect, raising the question of whether we are even measuring the right signals. Serum TTR levels have been proposed as a surrogate for stabilizer efficacy, with larger increases correlating with better outcomes. In a cross-study comparison of ATTRibute-CM and ATTR-ACT, acoramidis led to a 39% increase in serum TTR at 12 months versus 30% with tafamidis [3]. TTR stabilization alone may not fully reflect treatment response, however, and whether these changes actually translate into meaningful clinical benefit remains unclear. Beyond total TTR levels, circulating transthyretin amyloid aggregates have emerged as a possible marker of disease burden, correlating with amyloid load and declining with treatment [54]. If validated, this could provide a noninvasive way to estimate residual amyloid and determine whether escalation or a change in management approach is needed.
Defining progression in ATTR-CM is challenging. Trials rely on functional decline, biomarkers, and survival endpoints to demonstrate treatment efficacy, but these do not always reflect the more subtle signs of worsening disease seen in clinical practice. Whether real-world markers like diuretic dose escalation should be incorporated into broader staging systems is unclear, but in practice, it often signals an important change in a patient’s clinical status.
With more treatment options available for ATTR-CM, knowing when to switch or escalate therapy is becoming increasingly relevant. Currently, there are no standardized criteria to guide these decisions. While ongoing and upcoming trials will provide useful data, they may not answer many of the practical questions clinicians face, especially when managing patients with advanced disease or those already on disease-modifying therapy. A large-scale multicenter amyloid registry could help fill this gap by providing long-term data on treatment response, patterns of treatment change, and whether combination therapy improves outcomes compared to monotherapy. It could also capture the factors driving therapy changes, including symptoms, biomarkers, imaging, and patient preferences. Without clear real-world data to guide these decisions, clinicians risk both premature switching, if effective therapies are discontinued too soon, or missing the window for a necessary switch between therapeutic classes when a different approach could still impact the disease trajectory.
Looking ahead, ATTR-CM management may eventually shift from slowing disease progression to actively reversing it. Antibody-based depleters, such as ALXN2220, coramitug, and AT-02, offer the potential to remove existing amyloid rather than stabilizing TTR or reducing its production. What remains unknown is whether amyloid fibril removal translates to improved cardiac function and whether the immune response triggered by these antibody therapies contributes to fibrosis, potentially limiting their overall benefit. If these therapies prove effective, they could provide meaningful benefits even in those with more advanced disease. This approach may attenuate the consequences of a late or delayed diagnosis, as these therapies may reverse the clinical course.
As the treatment landscape expands, clinical decision-making will need to evolve. A more individualized framework that combines biomarkers, pragmatic clinical markers like ODI, and real-world data will be essential. ATTR-CM management is no longer a one-size-fits-all approach. The next few years will define not just which therapies are available, but how they are used.
Acknowledgments
Medical Writing/Editorial Assistance
During editing of this work, the first author used ChatGPT to assist with language refinement. All content was reviewed and approved by the authors, who take full responsibility for the final publication.
Author Contribution
Emily Margolin organized the review framework, conducted the literature review, and drafted the manuscript. Kevin Alexander and Marcus Urey contributed to content development, structure, and interpretation, and provided critical revisions. Lily Stern, Alessia Argiro, and Julie Rosenthal provided guidance on content, structure, and manuscript revisions. All authors reviewed and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
Emily Margolin, MD, and Alessia Argiro, MD, have nothing to disclose. Lily Stern, MD, has received research grants from Intellia Therapeutics and Pfizer. Julie Rosenthal, MD, has received consulting fees from BridgeBio and Novo Nordisk, and research funding for clinical studies from Novo Nordisk, Ionis, and Alnylam. Marcus Urey, MD, has received consulting fees from Alnylam, AstraZeneca, BridgeBio, Pfizer, and research funding for clinical studies from Alexion, Intellia, and Ionis. Kevin Alexander, MD, has received consulting fees from Alexion, Alnylam, Arbor Biotechnologies, Bridgebio, Novo Nordisk, and Pfizer. Kevin Alexander, MD, is an Editorial Board member of Cardiology and Therapy. Kevin Alexander was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Simões MV, Fernandes F, Dabarian A, Valicelli FH, Mesquita CT. Evolving therapies for transthyretin cardiac amyloidosis: new clinical trials with amyloid fibrils depleter. Int J Cardiovasc Sci. 2024;37: e20240112. 10.36660/ijcs.20240112. [Google Scholar]
- 2.Tomasoni D, Bonfioli GB, Aimo A, Adamo M, Canepa M, Inciardi RM, Lombardi CM, Nardi M, Pagnesi M, Riccardi M, Vergaro G, Vizzardi E, Emdin M, Metra M. Treating amyloid transthyretin cardiomyopathy: lessons learned from clinical trials. Front Cardiovasc Med. 2023;10:1154594. 10.3389/fcvm.2023.1154594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasfy JH, Winn AN, Touchette DR, Nikitin D, Shah KK, Richardson M, Lee W, Kim S, Rind DM. Disease modifying therapies for the treatment of transthyretin amyloid cardiomyopathy; draft evidence report. Institute for Clinical and Economic Review, July 17, 2024.
- 4.Griffin J, Rosenthal J, Grodin J, et al. ATTR amyloidosis: current and emerging management strategies: JACC: cardiooncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2021;3(4):488–505. 10.1016/j.jaccao.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellenbart EL, Ipema HJ, Rodriguez-Ziccardi MC, Krishna H, DiDomenico RJ. Disease-modifying therapies for amyloid transthyretin cardiomyopathy: current and emerging medications. Pharmacotherapy. 2024. 10.1002/phar.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorbala S, et al. ASNC/AHA/ase/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—evidence base and standardized methods of imaging. Circulation Cardiovasc Imaging. 2021. 10.1161/hci.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Falk RH. ‘A new staging system for cardiac transthyretin amyloidosis’: is it already on the verge of obsolescence? Eur Heart J. 2018;39(30):2807–9. 10.1093/eurheartj/ehx740. [DOI] [PubMed] [Google Scholar]
- 8.Kittleson MM, et al. Cardiac amyloidosis: evolving diagnosis and management: A scientific statement from the American Heart Association. Circulation. 2020. 10.1161/cir.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 9.Kuyama N, Takashio S, Oguni T, Yamamoto M, Hirakawa K, Ishii M, Hanatani S, Oda S, Matsuzawa Y, Usuku H, Yamamoto E, Hirai T, Ueda M, Tsujita K. Cardiac biomarker change at 1 year after tafamidis treatment and clinical outcomes in patients with transthyretin amyloid cardiomyopathy. J Am Heart Assoc. 2024;13(10): e034518. 10.1161/JAHA.124.034518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer MS, Soman P, Hernandez A, et al. Advancing transthyretin amyloidosis drug development in an evolving treatment landscape: amyloidosis forum meeting proceedings. Adv Ther. 2024;41:2723–42. 10.1007/s12325-024-02891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana M, et al. “The last decade in cardiac amyloidosis.” JACC: Cardiovasc Imaging. 2025, 10.1016/j.jcmg.2024.10.011. [DOI] [PMC free article] [PubMed]
- 12.Maurer MS, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. New Engl J Med. 2018;379(11):1007–16. 10.1056/nejmoa1805689. [DOI] [PubMed] [Google Scholar]
- 13.Rapezzi C, Kristen V, Gundapaneni B, Sultan MB, Hanna M. Benefits of tafamidis in patients with advanced transthyretin amyloid cardiomyopathy. Eur Heart J. 2020;41(Suppl. 2): ehaa946.2115. 10.1093/ehjci/ehaa946.2115. [Google Scholar]
- 14.Gillmore JD, et al. Efficacy and safety of ACORAMIDIS in transthyretin amyloid cardiomyopathy. New England J Med. 2024;390(2):132–42. 10.1056/nejmoa2305434. [DOI] [PubMed] [Google Scholar]
- 15.Maurer MS, Kale P, et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. New England J Med. 2023;389(17):1553–65. 10.1056/nejmoa2300757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana M, Berk JL, et al. Vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy. New England J Med. 2025;392(1):33–44. 10.1056/nejmoa2409134. [DOI] [PubMed] [Google Scholar]
- 17.Murad A. A Phase 1, single ascending dose study to evaluate ALN-TTRsc04, a next-generation RNA interference therapeutic, in healthy participants for potential treatment of transthyretin amyloidosis. Am Heart Assoc Sci Sess. 2024;2024:16–8. [Google Scholar]
- 18.“Alnylam Highlights Significant Pipeline Progress and Platform Innovation at R&D Day.” BioSpace, Alnylam Pharmaceuticals, 25 Feb. 2025, www.biospace.com/press-releases/alnylam-highlights-significant-pipeline-progress-and-platform-innovation-at-r-d-day.
- 19.Fontana M, Solomon SD, et al. CRISPR-Cas9 gene editing with Nexiguran Ziclumeran for ATTR cardiomyopathy. New Engl J Med. 2024;391(23):2231–41. 10.1056/nejmoa2412309. [DOI] [PubMed] [Google Scholar]
- 20.“MAGNITUDE: A Phase 3 Study of NTLA-2001 in Participants with Transthyretin Amyloidosis with Cardiomyopathy (ATTR-CM).” Clinicaltrials.Gov, National Cancer Institute, clinicaltrials.gov/study/NCT06128629?intr=NCT06128629&rank=1.
- 21.Michalon A, Hagenbuch A, Huy C, et al. A human antibody selective for transthyretin amyloid removes cardiac amyloid through phagocytic immune cells. Nat Commun. 2021;12:3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel AGM, Li P, Badrish N, et al. Transthyretin cardiac amyloidosis: current and emerging therapies. Curr Cardiol Rep. 2025;27:33. 10.1007/s11886-024-02172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Lopez E, et al. Transthyretin amyloid cardiomyopathy: a paradigm for advancing precision medicine. Eur Heart J. 2025. 10.1093/eurheartj/ehae811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suhr OB, Grogan M, da Silva AM, Karam C, Garcia-Pavia P, Drachman B, Kinney GG. PRX004 in variant amyloid transthyretin (ATTRv) amyloidosis: results of a phase 1, open-label, dose-escalation study. Amyloid. 2024. 10.1080/13506129.2024.2420809. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Pavia P, et al. Phase 1 trial of antibody NI006 for depletion of cardiac transthyretin amyloid. New Engl J Med. 2023;389(3):239–50. 10.1056/nejmoa2303765. [DOI] [PubMed] [Google Scholar]
- 26.Ioannou, Massa P, Patel RK, Razvi Y, Porcari A, Rauf MU, Jiang A, Cabras G, Filisetti S, Bolhuis RE, Bandera F, Venneri L, Martinez-Naharro Ana, Law S, Kotecha T, Virsinskaite R, Knight DS, Emdin M, Petrie A, Lachmann H, Wechelakar A, Petrie M, Hughes A, Freemantle N, Hawkins PN, Whelan C, McMurray JJV, Gillmore JD, Fontana M. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur Heart J. 2023;44(31):2893–907. 10.1093/eurheartj/ehad347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera-Romero E, Serrao-Faria LA, Mora-Ayestarán N, Peiró-Aventín B, Espinoza A, de Castro D, Cobo-Marcos M, Domínguez F, González-López E, Garcia-Pavia P. Clinical impact of beta-blocker withdrawal in transthyretin amyloid cardiomyopathy. Amyloid. 2025;32(1):87–9. 10.1080/13506129.2024.2431082. (Epub 2024 Nov 20 PMID: 39565050). [DOI] [PubMed] [Google Scholar]
- 28.Kittleson MM, Ruberg FL, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis. J Am Coll Cardiol. 2023;81(11):1076–126. 10.1016/j.jacc.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Heidenreich PA, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines”. Circulation. 2022. 10.1161/cir.0000000000001063.36378760 [Google Scholar]
- 30.Porcari A, et al. SGLT2 inhibitor therapy in patients with transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2024;83(24):2411–22. 10.1016/j.jacc.2024.03.429. [DOI] [PubMed] [Google Scholar]
- 31.Ioannou A, Fumagalli C, Razvi Y, et al. Prognostic value of a 6-minute walk test in patients with transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2024;84:43–58. 10.1016/j.jacc.2024.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng RK, et al. “Diuretic dose and NYHA functional class are independent predictors of mortality in patients with transthyretin cardiac amyloidosis. JACC: CardioOncology. 2020;2(3):414–24. 10.1016/j.jaccao.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnellan Eoin, et al. Atrial fibrillation in transthyretin cardiac amyloidosis. JACC: Clin Electrophysiol. 2020;6(9):1118–27. 10.1016/j.jacep.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Vaidya GN, Patel JK, Kittleson M, Chang DH, Kransdorf E, Geft D, Czer L, Vescio R, Esmailian F, Kobashigawa JA. Intermediate-term outcomes of heart transplantation for cardiac amyloidosis in the current era. Clin Transplant. 2021;35(6): e14308. 10.1111/ctr.14308. (Epub 2021 Apr 19 PMID: 33825224). [DOI] [PubMed] [Google Scholar]
- 35.Barrett CD, Alexander KM, Zhao H, Haddad F, Cheng P, Liao R, Wheeler MT, Liedtke M, Schrier S, Arai S, Weisshaar D, Witteles RM. Outcomes in patients with cardiac amyloidosis undergoing heart transplantation. JACC Heart Fail. 2020;8(6):461–8. 10.1016/j.jchf.2019.12.013. (Epub 2020 May 6 PMID: 32387068). [DOI] [PubMed] [Google Scholar]
- 36.Lyle MA, et al. Amyloidosis and heart transplantation in a new era. Clin Transpl. 2025. 10.1111/ctr.70070. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Pavia P, et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021;23(6):895–905. 10.1002/ejhf.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeldin L, Eichler JBS, Teruya SL, Weinsaft AY, Mirabal A, Cuomo MO, Mateo K, Helmke S, Maurer MS. Outpatient worsening of heart failure and mortality in transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2024. 10.1002/ejhf.3540. (Epub ahead of print. PMID: 39638976). [DOI] [PubMed] [Google Scholar]
- 39.Fontana M, et al. Worsening of heart failure in outpatients with transthyretin amyloidosis and cardiomyopathy in the Apollo-B trial. J Am Coll Cardiol. 2025;85(7):744–52. 10.1016/j.jacc.2024.10.097. [DOI] [PubMed] [Google Scholar]
- 40.Fontana M, Maurer MS, Gillmore JD, Bender S, Aldinc E, Eraly SA, Jay PY, Solomon SD. Outpatient worsening heart failure in patients with transthyretin amyloidosis with cardiomyopathy in the HELIOS-B Trial. J Am Coll Cardiol. 2025;85(7):753–61. 10.1016/j.jacc.2024.11.015. (Epub 2024 Nov 18 PMID: 39566871). [DOI] [PubMed] [Google Scholar]
- 41.Grogan M, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–20. 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Law S, et al. Change in N-terminal pro-B-type natriuretic peptide at 1 year predicts mortality in wild-type transthyretin amyloid cardiomyopathy. Heart. 2021;108(6):474–8. 10.1136/heartjnl-2021-319063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takashio S, et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2018;5:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oghina S, et al. Prognostic Value of N-terminal pro-brain natriuretic peptide and high-sensitivity troponin T levels in the natural history of transthyretin amyloid cardiomyopathy and their evolution after tafamidis treatment. J Clin Med. 2021;10(21):4868. 10.3390/jcm10214868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson JLS, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circulation Heart Fail. 2018. 10.1161/circheartfailure.117.004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aimo A, Tomasoni D, Porcari A, Vergaro G, Castiglione V, Passino C, et al. Left ventricular wall thickness and severity of cardiac disease in women and men with transthyretin amyloidosis. Eur J Heart Fail. 2023;25(4):510–3. 10.1002/ejhf.2824. [DOI] [PubMed] [Google Scholar]
- 47.Takigawa M, Hashimura K, Ishibashi-Ueda H, Yamada N, Kiso K, Nanasato M, Yoshida Y, Hirayama H. Annual electrocardiograms consistent with silent progression of cardiac involvement in sporadic familial amyloid polyneuropathy: a case report. Intern Med. 2010;49:139–44. 10.2169/internalmedicine.49.2703. [DOI] [PubMed] [Google Scholar]
- 48.Ruberg F, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. JACC. 2019;73(22):2872–91. 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ATTRUBY (acoramidis) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/216540s000lbl.pdf. November 2024.
- 50.Coelho T, Marques W Jr, Dasgupta NR, Chao CC, Parman Y, NEURO-TTRansform Investigators, et al. Eplontersen for hereditary transthyretin amyloidosis with polyneuropathy. JAMA. 2023;330(15):1448–58. 10.1001/jama.2023.18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maurer M, Cappelli F, Fontana M, Garcia-Pavia P, Grogan M, Hanna M, Judge D, Masri A, Du J, Siddhanti S, Tamby J, Ji A, Sinha U, Fox J, Gilmore J, ATTRibute – CM Study Group and Open-Label Extension Study Group. Increase in serum TTR levels observed with acoramidis treatment in patients with transthyretin amyloid cardiomyopathy (ATTR-CM): insights from ATTRibute-CM and its open-label extension. Eur Heart J. 2024;45(Supplement_1): ehae666.2088. 10.1093/eurheartj/ehae666.2088. [Google Scholar]
- 52.“Additional Data Showing Acoramidis Increases Serum Transthyretin Which Is Associated with Improved Cardiovascular Outcomes Presented at ISA from Bridgebio Pharma’s Phase 3 Attribute-Cm Study in Transthyretin Amyloid Cardiomyopathy (ATTR-Cm).” GlobeNewswire News Room, BridgeBio Pharma, Inc., 29 May 2024, www.globenewswire.com/news-release/2024/05/29/2890251/0/en/Additional-Data-Showing-Acoramidis-Increases-Serum-Transthyretin-Which-is-Associated-with-Improved-Cardiovascular-Outcomes-Presented-at-ISA-from-BridgeBio-Pharma-s-Phase-3-ATTRibut.html.
- 53.Qarni TN, Jones FJS, Drachman B, et al. Treatment characteristics of patients with hereditary transthyretin amyloidosis: a cohort study. Orphanet J Rare Dis. 2024;19:191. 10.1186/s13023-024-03198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedretti R, Wang L, Hanna M, Benson MD, Grodin JL, Tang WHW, Masri A, Saelices L. Detection of circulating transthyretin amyloid aggregates in plasma: a novel biomarker for transthyretin amyloidosis. Circulation. 2024;149(21):1696–9. 10.1161/CIRCULATIONAHA.123.067225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.