Abstract
Improved animal models of endometriosis are needed to accurately represent the pathophysiology of human disease and identify new therapeutic targets that do not compromise fertility. There is tremendous heterogeneity among published rodent models of endometriosis, and the etiology and pathogenesis of endometriosis remain undetermined. The vast majority of endometriosis is found in menstruating women; however, no published mouse models have induced endometriosis in a menstruating mouse, further limiting our understanding of the disease. Our goal was to develop a novel, translationally relevant mouse model of endometriosis in a menstruating mouse by transplanting donor menstrual endometrium into the peritoneal cavity of menstruating, immunocompetent, intact recipients. We initially compared 4 different experimental groups to optimize implanted menstrual tissue type and method of implantation into intact, normally cycling recipient mice. To further optimize this model, a novel fifth experimental group was compared in which discrete pieces of menstrual donor endometrium were implanted via laparoscopy into menstruating recipient mice. Lesions were confirmed to be endometriosis based on histopathology. The use of laparoscopy to place discrete fragments of menstrual phase endometrium intraabdominally was the most effective method for induction of endometriosis. This method was just as effective when used to induce endometriosis in menstruating recipient mice. Menstruating mice returned to normal estrus cyclicity after induction of disease, which can allow for assessment of therapeutic interventions on fertility. This is a novel translationally relevant mouse model of endometriosis in a menstruating mouse that can be used to explore and elucidate the etiology and pathogenesis of this disease.
Abbreviations and Acronyms: B6, B6(Cg)-Tyrc-2J/J; mCherry, B6(Cg)-Tyrc-2JTg(UBC-mCherry)1Phbs/J
Introduction
Endometriosis is an estrogen-dependent gynecologic disease that affects an estimated 10% to 15% of women of reproductive age.1 It is characterized by endometrial tissue proliferating in ectopic locations and is associated with dysmenorrhea, chronic pelvic pain, dyspareunia, infertility, and increased risk of ovarian cancer. The classification, diagnosis, and treatment of endometriosis are complicated, as clinical presentation and stage/classification of endometriosis lesions do not correlate well with symptoms, response to treatment, or prognosis. In addition, the true prevalence of endometriosis is unknown, as time from initiation of disease to onset of symptoms is variable and confirmed diagnosis is often delayed, if achieved at all.1 The etiology, epidemiology, and pathogenesis of endometriosis remain largely undetermined, emphasizing the critical need for research in this field.
Although endometriosis is theorized to develop from either uterine endometrium or extrauterine tissue,2 the most widely accepted hypothesis for the etiology of this disease involves retrograde menstruation into the abdominal cavity via the fallopian tubes followed by implantation of uterine endometrial tissues to extrauterine locations.3 In fact, retrograde menstruation is found in 90% of menstruating women with patent fallopian tubes.4 However, it has yet to be determined why only a small percentage of these women go on to develop endometriosis, suggesting that the etiology and pathogenesis of this disease is likely multifactorial.
In animal species other than humans, naturally occurring endometriosis has been observed only in certain nonhuman primates, but at a very low incidence rate.5–8 Based on the hypothesis of retrograde menstruation, this is likely explained by the paucity of species that menstruate naturally, that is, humans, some higher order nonhuman primates, the elephant shrew, some species of bats, and the Egyptian spiny mouse,9–13 none of which is optimized for use as a preclinical model. Laboratory mice are well characterized, cost-effective, prolific, allow for genetic manipulations, and have been used to study various aspects of endometriosis. However, mice do not menstruate naturally and have a closed tubo-ovarian junction resulting in the need for significant manipulation to model the theory of retrograde menstruation. Mice have an estrus cycle, and the endometrium thickens with the peak at estrus. Natural endometrial decidualization in the mouse only occurs during embryo implantation.14,15 If the mouse is not pregnant, the thickened endometrium is resorbed. In menstruating species, endometrium undergoes decidualization independent of pregnancy, and in the absence of embryo implantation and subsequent decrease in progesterone, the decidualized endometrium breaks down and is shed as menstrual endometrium.16 An effective model of mouse menstruation mimicking the human menstrual cycle via induced decidualization and subsequent menstruation in a pseudopregnant mouse is well established. These menstruating intact (ovaries present and no exogenous hormone supply) mice display overt vaginal bleeding, as well as similar gene expression and histologic changes to the menstrual endometrium when compared with humans, with the uterus also displaying re-epithelialization of the luminal endometrial surface.14 Menstrual endometrium has been used to induce endometriosis in baboons and mice.5,17 In baboons, this menstrual endometrium was injected into actively menstruating baboons, but historically in mice, the recipient is either in varying stages of the estrus cycle or is ovariectomized and supplemented with exogenous hormones. The peritoneal environment changes during menstruation in women, during the estrus cycle in mice, and in rats that have been ovariectomized and supplemented with estrogen,18–22 and the peritoneum likely plays a significant role in endometriosis.23–25 Menstruation also induces a variety of physiologic changes in women with effects on immune function, microflora, and autoimmune and systemic diseases.26 Both menstruation and the peritoneal environment should be considered in an animal model of endometriosis. Development of a novel model of endometriosis using menstrual endometrium implanted into an actively menstruating recipient mouse mimics retrograde menstruation in a more complete way by approximating the peritoneal environment, the hormonal milieu, and subsequent physiologic changes in a menstruating woman. This could lead to novel, translational discoveries about etiology and pathogenesis.
Cummings and Metcalf described the first murine model of endometriosis in 1995,27 and many iterations of murine models have followed that have been reviewed in detail28–32 (see Table 1). In brief, the 3 common rodent endometriosis models are autologous (autotransplantation of tissue), syngeneic (donor and recipient are the same strain), or heterologous (human tissue into immunocompromised mice). For syngeneic models, 3 main types of tissue are used to induce endometriosis: full thickness uterine implants, naive endometrium, and menstrual endometrium. Most of these published models implant the donor endometrial tissue intraperitoneally using either surgical engraftment of a set number of discrete tissue pieces, or intraperitoneal injection or surgical injection of endometrial slurry. Surgical engraftment using suture or adhesives introduces variability due to their effects on tissues and bypasses natural attachment of tissues, one of the early factors of the disease that needs elucidation. Laparoscopic implantation of discrete tissue pieces has also been described and developed. This technique allows for adhesion of tissue pieces to peritoneal surfaces without the need for suture or artificial adhesives.33,34 Injection techniques17,35 more closely mimic true retrograde menstruation and allow for study of early disease stages, but the number of lesions generated is variable and tissue slurry composition fluctuates based on technique. Published models also vary on the use of ovariectomized and hormonally supplemented donor and recipient mice, compared with those that are intact and normally cycling. Rodent models of endometriosis that involve ovariohysterectomy and hormone replacement are impractical for studies of physiologic functions that require natural fluctuations in sex hormones, such as fertility, and heterologous models preclude the assessment of the role of immune cells in pathogenesis. Regardless of model used, ectopic endometrial implants must escape immune clearance, invade, and attach to ectopic locations, proliferate, neovascularize, and survive.2,36 Investigation of these factors requires appropriate translational animal models that recapitulate all or part of the human disease process.
Table 1.
| Method of induction | Transplant type | Tissue type | Donor status | Recipient status | Limitations | Benefits |
|---|---|---|---|---|---|---|
| Surgical (laparotomy with/without OVX) | Autologous Syngeneic |
• Unspecified • Naive endometrium • Uterine horn full thickness • Endometrium + myometrium |
• Intact • Intact + hormones • OVX • OVX + hormones |
• Intact • OVX • OVX + hormones |
|
|
| Heterologous | • Naive endometrium• Menstrual endometrium | • Human | • Immunodeficient, Intact • Immunodeficient/OVX + hormones |
|
|
|
| Injection | Syngeneic | • Unspecified • Naive endometrium • Uterine horn full thickness • Endometrium + myometrium • Menstrual endometrium |
• Intact • Intact + hormones • OVX • OVX + hormones • Induced menstruation/OVX + hormones |
• Intact • OVX • OVX + hormones |
|
|
| Laparoscopy | Syngeneic | • Uterine horn full thickness • Menstrual endometrium |
• Intact • Induced menstruation/OVX + hormones |
• Intact • Intact |
|
|
| Laparoscopya | Syngeneic | • Menstrual endometrium | • Pseudo-pregnant induced menstruation | • Pseudo-pregnant induced menstruation |
|
|
OVX, ovariectomized.
The novel model of induction of endometriosis in a menstruating mouse model described in this study.
A hypothesized ‘best fit’ model of murine endometriosis has been described, one that uses immunocompetent mice with intact ovaries to approximate spontaneous menstrual endometrial attachment in the peritoneal cavity, allowing for longitudinal assessment of lesions and ideally iterative endometrial transplantation.29 While other published models have implanted menstrual endometrium into recipient mice with various manipulations, to date, implantation of menstrual endometrium into an actively menstruating recipient mouse has not been described. In this study, our aim was to optimize a murine model of endometriosis in a menstruating recipient mouse using donor syngeneic menstrual endometrium to recapitulate the retrograde menstruation hypothesis.
Materials and Methods
Ethical review.
The facility where this research was conducted is accredited by AAALAC International and follows all U.S. Public Health Service federal statutes and regulations pertaining to animal care and use. This study was performed in accordance with the Guide for Care and Use of Laboratory Animals and was approved by Creighton University’s IACUC.
Animals, housing conditions, and diet.
All mice were maintained in environmentally controlled rooms (20 to 26 °C; humidity 30% to 55%) with diurnal lighting (12-h light/12-h dark cycle; lights on at 0700). Forty-four 8 ± 8-wk-old female B6(Cg)-Tyrc-2JTg(UBC-mCherry)1Phbs/J mice (stock no. 017614; The Jackson Laboratory, Bar Harbor, ME) were used as donor mice and 55 8 ± 8-wk-old female B6(Cg)-Tyrc-2J/J mice (stock no 000058; The Jackson Laboratory, Bar Harbor, ME) were used as recipient mice. Hereafter, these strains are referred to as mCherry for B6(Cg)-Tyrc-2JTg(UBC-mCherry)1Phbs/J mice and B6 for B6(Cg)-Tyrc-2J/J mice. Mice were maintained in autoclaved individually ventilated cages prefilled with 7099 TEK-Fresh (paper) bedding, 8 g of nesting material (Enviro-dri from Shepherd Specialty Papers, Watertown, TN and Rodent Nesting Sheets from Bio-Serv, Flemington, NJ), a wood gnawing block, mouse trapeze, and mouse tunnel (Bio-Serv, Flemington, NJ), and soy protein-free extruded autoclavable diet 2020SX (Teklad Global Rodent Diets, Madison, WI) fed ad libitum. Soy protein-free food was used to decrease exposure to any exogenous estrogens (that is, the mycotoxin zearalenone in corncob bedding and/or phytoestrogens in soy-based rodent diets)37–39 because supplemental estrogen has been shown to promote endometrial lesion growth in rats and mice.27,40 Autoclaved tap water was provided in bottles. Female mice were group housed until the day of vaginal plug, and then single housed until euthanized to avoid the Lee–Boot effect.41 Eight vasectomized male mice (B6(Cg)-Tyrc-2J/J, stock no. 000058) were obtained from The Jackson Laboratory (Bar Harbor, ME) and were single housed on corncob bedding and fed Teklad Global 18% protein 2018S ad libitum. Health surveillance using alternating serology of dirty bedding sentinels or PCR of colony animals was performed quarterly. The mice were free from Rodentibacter heylii, Mycoplasma pulmonis, Staphylococcus aureus, β-hemolytic Streptococcus (groups A, B, C, and G), Bordetella bronchiseptica, Bordetella pseudohinzii/hinzii, Corynebacterium kutscheri, Streptobacillus moniliformis, Streptococcus pneumoniae, Filobacterium rodentium, Klebsiella oxytoca, Klebsiella pneumoniae, Salmonella spp., Citrobacter rodentium, Clostridium piliforme, Pseudomonas aeruginosa, Campylobacter coli, Campylobacter jejuni, Proteus mirabilis, Corynebacterium bovis, Corynebacterium spp., Staphylococcus xylosus, Pneumocystis spp., murine norovirus, pinworms, fur mites, Spironucleus muris, Entamoeba muris, Tritrichomonas muris, Cryptosporidium spp., Giardia spp., Ornithonyssus, Demodex, Sendai virus, epizootic diarrhea of infant mice, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus (1 to 5), Theiler encephalomyelitis virus, ectromelia virus, mouse adenovirus 1 and 2, K virus, Hantaan virus, reovirus type 3, lymphocytic choriomeningitis virus, polyoma virus, and mouse kidney parvovirus. This colony is endemic for Rodentibacter pneumotropica and Helicobacter spp.
Study design.
Experimental groups 1 to 5, animal numbers (n = 10 per group), and timeline of experiment are shown in (Figure 1). For experimental groups 1 to 4 there was no control group, as mice do not develop spontaneous endometriosis, and we were comparing incidence of endometriosis and number of confirmed endometrial lesions between groups. Group 1 is considered the control for group 5 for comparison of these 2 metrics. The methods used in this study combine techniques used in different studies,27,33–35,42 all with different sample sizes varying from n = 4 to n = 26. We used this range and our previous experience with similar experiments to select n = 10. The histopathologist was blinded to group allocation at all stages of the experiment. A single donor mouse could be used for up to 4 recipients based on the amount of menstrual endometrium generated.
Figure 1.
(A) Overview of experimental design. Groups 1 to 4 compared implantation of 2 different types of menstrual endometrium and 2 different implantation techniques into cycling recipient mice. Group 5 implanted discrete biopsies of menstrual endometrium into menstruating recipient mice. (B) Timeline of procedures and data collections. Donor mice are color-coded pink and recipient mice are color-coded blue. For recipient mice, cycling means sexually intact with a natural estrus cycle. The visual presence of vaginal plug was considered day 0.5. Decidualization was performed on day 4.5. Menstrual tissue was harvested on the same day of implantation into recipient mice. For groups 1 to 4 this was between day 7.5 to 9.5 after the vaginal plug, and for group 5 this occurred on day 9.5 after the vaginal plug. Discrete biopsies are sectioned from menstrual endometrium into ∼2 mm3. Slurry was made from 10 discrete biopsies, minced and mixed with 0.2 mL of sterile saline for an average wet weight of 94.16 mg. Endometrial tissue was implanted via laparoscopy or laparotomy in discrete pieces or via injection of slurry. Recipient mice were sacrificed in proestrus, ∼30 d after resuming the estrus cycle after the procedure.
Induction of decidualization/menstruation.
Induction of menstruation was performed using a combination of methods with modifications.14,33 Naive donor mCherry (groups 1 to 5) and B6 recipient female mice (group 5) were placed (using simple randomization) with vasectomized males overnight (day 0) and checked for the presence of a vaginal plug the next morning (day 0.5). Females with a confirmed vaginal plug were individually housed. On day 4.5 pseudopregnant mice were anesthetized using isoflurane, and decidualization of endometrium was induced by intrauterine injection of 100 µL of sterile sesame oil using a blunt 27-gauge, 0.86-in. needle (ALZET LLC, Campbell, CA) followed by mechanical stimulation. Sustained release buprenorphine was administered after the procedure. Decidualized endometrium transitioned to menstrual endometrium when progesterone naturally decreased during pseudopregnancy. Menstruation was confirmed using vaginal cytology and/or the visual presence of overt vaginal bleeding. Donor mice were excluded from an experiment if they did not show evidence of menstrual endometrium in the uterus when euthanized. For group 5, recipient mice were excluded from an experiment if they did not show evidence of induced menstruation based on vaginal cytology results on the day of transplant. These criteria were set a priori.
Induction of endometriosis.
On day 7.5 to 9.5, induced menstruating donor mCherry mice for groups 1 to 4 were anesthetized with isoflurane and cervically dislocated. The uterus was removed en bloc using a sterile technique, placed in sterile cold (4 °C) PBS (0.01 M PBS composed of 13.7 mM NaCl, 0.27 mM KCl, 0.15 mM KH2PO4, and 0.8 mM Na2HPO4; pH 7.4). Each uterine horn was opened longitudinally, and menstrual endometrium was dissected from the myometrium, then sectioned into ∼2-mm3 discrete biopsies. Ten biopsies were implanted into recipient mice in groups 1, 2, and 5. For groups 3 and 4, menstrual endometrial slurry was made using the Greaves and colleagues protocol with modifications.17 Ten menstrual endometrial discrete biopsies were weighed, minced, and mixed with 0.2 mL of sterile saline (0.9% NaCl). This slurry was then drawn into a syringe through a 19-gauge hypodermic needle to ensure uniform suspension. The average wet weight of menstrual endometrium implanted was 94.16 mg per recipient mouse. Groups 1 to 4 used sexually intact cycling B6 recipient mice (n = 10) pseudorandomized based on the amount of available donor menstrual endometrium, surgery schedule, and to complete the n for each group. Group 5 used menstruating B6 recipient mice (n = 10) that were time-matched for date of vaginal plug and decidualization with donor/menstruating mCherry mice. On day 9.5 after vaginal plug, donor mCherry mice were sacrificed and menstrual endometrial tissue harvested as described and implanted into the menstruating B6 recipients.
For all 5 groups, recipient B6 mice were weighed and then anesthetized with ketamine (87.5 mg/kg) and xylazine (12.5 mg/kg) given intraperitoneally. Hair was removed from the abdomen using depilatory cream (Nair), and heat support was provided using a homeothermic monitoring system (Harvard Apparatus, Holliston, MA). Stage of estrus of recipients was determined via cytologic evaluation of vaginal smears for groups 1 to 4. For group 5, menstruation of recipient B6 mice was confirmed via cytologic evaluation of vaginal smears and/or presence of overt vaginal bleeding. If a laparoscope was used, mice were intubated using a 22-gauge, 1-in. intravenous catheter (Pivetal Veterinary Supplies) and ventilated with a MiniVent (model 845, Harvard Apparatus, Holliston, MA) with stroke volume calculated at 10 µL/kg and stroke rate of 200 strokes/min. Sustained-release buprenorphine was administered after the procedure for analgesia.
Laparotomy (groups 2 and 4).
A 5-mm cranial ventral midline incision was made caudal to the xiphoid process. The discrete endometrial biopsies were placed with forceps or endometrial slurry was injected intraabdominally through this incision. The incision was closed using 5-0 polyglactin 910 suture (Patterson Veterinary, Loveland, CO).
Laparoscopy (groups 1, 3, and 5).
Laparoscopic guided implantation of menstrual endometrial biopsies was performed using the Peterse and colleagues protocol with modifications.33,34 A 5-mm cranial ventral midline incision was made caudal to the xiphoid process. A 2-mm endoscope covered with a 3-mm insufflation shield (Stryker, Kalamazoo, MI) was placed into the peritoneal cavity. The abdomen was insufflated with warmed CO2 at a flow of 1 L/min to maintain an intraabdominal pressure of 5 mm Hg. For implantation of discrete biopsies, a 14-gauge intravenous catheter (SurFlash, Patterson Veterinary, Loveland, CO) was inserted into the right lower abdominal quadrant to serve as a port. The biopsies were placed into the tip of the catheter and advanced into the abdomen using a blunt stylet. For implantation of menstrual endometrial slurry, the suspension was injected intraperitoneally with a 19-gauge needle through the right lower abdominal quadrant. The incision and port opening were closed using 5-0 polyglactin 910 suture (Patterson Veterinary, Loveland, CO).
Lesion harvest.
Recipient mice were followed with cytologic evaluation of vaginal smears postoperatively to confirm return to estrus cyclicity. All mice were euthanized in proestrus, at least 30 d after return to normal estrus cyclicity to decrease variability of lesion size and appearance. Mice were anesthetized with isoflurane and cervically dislocated. The abdominopelvic cavity was closely examined by visual inspection for endometriosis-like lesions; number and location of each lesion were recorded, along with a detailed description of gross appearance. Lesions were photographed, excised from surrounding tissue, and immediately fixed in 10% neutral-buffered formalin.
Histologic and immunohistochemistry protocols.
Endometriosis was confirmed histologically by the presence of endometrial stroma. Lesions were processed in the Creighton University Histology Core Facility using the Excelsior ES tissue processor (Thermo Fisher Scientific, Waltham, MA) and embedded in paraffin. Tissue sections (5 μm) were cut on a Leica RM 2135 microtome (Leica Biosystems, Deer Park, IL) and placed on Fisherbrand Superfrost Plus microscope slides (Thermo Fisher Scientific, Waltham, MA). Gemini automated cell stainer was used for hematoxylin and eosin staining. Lesion sections were evaluated by a histopathologist blinded to the experimental group. Immunohistochemical staining was performed on a subset of confirmed endometriotic lesions to qualitatively investigate the presence of angiogenesis (CD31), proliferation (Ki67), mast cells (toluidine blue), and macrophages (CD68). Immunohistochemical staining was performed on a Discovery Ultra advanced staining system (Ventana Medical Systems; Roche Diagnostics, Indianapollis, IN). Sections were deparaffinized using a mild detergent solution and vortex mixing at 69 °C for 24 min (catalog no. 950-102; Roche Diagnostics, Indianapolis, IN). Tris-based reaction buffer (pH 7.6) was used throughout the protocol to maintain aqueous conditions and rinse slides (catalog no. 950-300; Roche Diagnostics, Indianapolis, IN). Antigen retrieval was accomplished using cell conditioning (CC1; pH 8.2) Tris-borate-EDTA buffer at 95 °C for 24 min (catalog no. 950-124; Roche Diagnostics, Indianapolis, IN); treatment with a Discovery ChromoMap DAB kit (RUO) inhibitor for 8 min (catalog no. 760-159; Roche Diagnostics, Indianapolis, IN); primary antibody for CD31, rabbit polyclonal, 1:100, with incubation at 37 °C for 44 min (catalog no. ab28364; Abcam, Fremont, CA), primary antibody for Ki67, rabbit polyclonal, 1:200, with incubation at 37 °C for 32 min (catalog no. ab16667; Abcam, Fremont, CA), or primary antibody for CD68, rabbit polyclonal, 1:200, with incubation at 37 °C for 32 min (catalog no. ab125212; Abcam, Fremont, CA); secondary antibody HRP detection, Discovery anti-rabbit HQ RTU, incubation at 37 °C for 16 min (catalog no. 760-4815; Roche Diagnostics, Indianapolis, IN); enzyme conjugate biotin-free Discovery anti-HQ HRP RTU, incubation at 37 °C for 16 min (catalog no. 760-4820; Roche Diagnostics, Indianapolis, IN); and chromogen staining with a Discovery Purple kit (RUO) for 32 min and counterstained with hematoxylin (catalog nos. 760-229, 790-2208, and 760-2037; Roche Diagnostics, Indianapolis, IN). Sections were stained with toluidine blue, resulting in red-purple (metachromatic) staining of mast cells on a blue background.
Statistical analysis.
Endometriosis incidence.
Endometriosis incidence was defined as the percentage of mice in a group with at least one confirmed endometrial lesion. To test for differences in the incidence of endometriosis among groups, we first conducted a 2 (yes/no) × 5 (induction method) omnibus Fisher exact test, followed by individual 2 × 2 Fisher exact tests.
Confirmed endometrial lesions.
To test for differences in mean number of confirmed lesions among different endometriosis induction methods, we used a generalized linear model with a Poisson distribution and a log link function using the GLM (generalized linear model) function in R (R Core Team, 2022). Significant differences are defined as P < 0.05, and marginal differences are defined as P < 0.10. To test for differences in the incidence of endometriosis among groups, we first conducted a 2 (yes/no) × 5 (induction method) omnibus Fisher exact test, followed by individual 2 × 2 Fisher exact tests.
Results
Induction of menstruation.
We achieved successful menstruation in 34 of 44 (77.27% ±12.38%, 95% CI) mCherry donor mice. We considered menstruation successful if red blood cells were present in vaginal cytology on day 9.5 and enough endometrial tissue could be harvested from the donor uterus to implant into 1 recipient mouse. In general, 1 donor mouse provided enough tissue to implant into 2 recipient mice. Decidualization and subsequent decidualized endometrial breakdown and shedding (menstrual endometrium) occurred unilaterally, bilaterally, and segmentally throughout the uterine horns (Figure 2). In the 10 of 44 (22% ±12.38%, 95% CI) mice that failed to menstruate, the uterus was either normal in appearance, or the breakdown was incomplete, and the endometrium could not be adequately separated from the myometrium.
Figure 2.
Images of menstruating mouse with bloody vaginal discharge (A), blood-tinged vaginal cytology from menstruating mouse (B), bicornuate menstrual phase uterus (C), and histomorphology of cross-section of menstrual endometrium with myometrium removed showing expansion of endometrial stroma (arrows) and loss of epithelium and lumen (circle) (D).
Estrus cyclicity after surgery.
All recipient mice in all groups (n = 50) returned to normal estrus cyclicity after surgery as determined by vaginal cytology. B6 menstruating recipient mice in group 5 (n = 10) returned to estrus cyclicity between 3 and 14 d (mean = 6.8 d, SD = 3.4, 95% CI(4.70, 8.90)) postoperatively.
Endometriosis incidence.
For all 5 groups, the average days of disease from day of induction to lesion harvest was 34.85 d (SD = 2.05, 95% CI(34.45, 35.25)). The endometriosis incidence differed across induction methods and tissue type (P < 0.001, Table 2). Post hoc Fisher exact tests showed that group 1 produced higher rates of endometriosis than groups 3 or 4 (P < 0.001) but did not differ from groups 2 or 5 (P > 0.47). Using laparoscopy with discrete biopsies, groups 1 and 5 resulted in 100% incidence of endometriosis, while group 2 (laparotomy with discrete biopsies) had an 80% incidence. For groups 3 and 4, 2 and 3 recipient mice, respectively, were excluded from analysis due to death after the procedure.
Table 2.
Individual mouse data per group
| Group | Recipient estrus stage | Donor euthanasia: no. of days after vaginal plug | Days of disease | Individual take ratea (%) | Mean counts of confirmed lesionsb | Endometriosis incidencec (%) |
|---|---|---|---|---|---|---|
| 1: Laparoscopy with discrete endometrial biopsies | Estrus | 8.5 | 41 | 20 (2/10) | 2.7 | 100 (95% CI: 65, 100) |
| Estrus | 8.5 | 42 | 40 (4/10) | |||
| Estrus | 9.5 | 36 | 20 (2/10) | |||
| Estrus | 9.5 | 34 | 30 (3/10) | |||
| Estrus | 10.5 | 36 | 25 (2/8) | |||
| Estrus | 9.5 | 34 | 20 (2/10) | |||
| Estrus | 9.5 | 34 | 20 (2/10) | |||
| Proestrus | 8.5 | 35 | 10 (1/10) | |||
| Diestrus | 9.5 | 33 | 60 (6/10) | |||
| Metestrus | 9.5 | 34 | 30 (3/10) | |||
| 2: Laparotomy with discrete endometrial biopsies | Proestrus | 9.5 | 35 | 10 (1/10) | 1.1 | 80 (95% CI: 44, 96) |
| Estrus | 9.5 | 35 | 10 (1/10) | |||
| Proestrus | 9.5 | 36 | 10 (1/10) | |||
| Proestrus | 9.5 | 35 | 0 (0/10) | |||
| Proestrus | 9.5 | 34 | 20 (2/10) | |||
| Proestrus | 9.5 | 35 | 0 (0/10) | |||
| Estrus | 9.5 | 34 | 2 (2/10) | |||
| Estrus | 9.5 | 35 | 10 (1/10) | |||
| Estrus | 9.5 | 37 | 10 (1/10) | |||
| Estrus | 9.5 | 37 | 20 (2/10) | |||
| 5: Laparoscopy with discrete endometrial biopsies in menstruating recipients | Menstruating | 9.5 | 35 | 20 (2/10) | 2.6 | 100 (95% CI: 65, 100) |
| 35 | 40 (4/10) | |||||
| 35 | 20 (2/10) | |||||
| 44 | 30 (3/10) | |||||
| 36 | 30 (3/10) | |||||
| 20 (2/10) | ||||||
| 40 (4/10) | ||||||
| 20 (2/10) | ||||||
| 40 (4/10) | ||||||
| 10 (1/10) | ||||||
| Individual lesion countd | ||||||
| 3: Laparoscopy with endometrial slurry | Estrus | 8.5 | 36 | 1 | 0.2 | 20 (95% CI: 3.5, 56) |
| Estrus | 8.5 | 34 | 1 | |||
| Estrus | 8.5 | 33 | 0 | |||
| Estrus | 8.5 | 33 | 0 | |||
| Proestrus | 8.5 | 36 | 0 | |||
| Diestrus | 8.5 | 32 | 0 | |||
| Diestrus | 9.5 | 32 | 0 | |||
| Diestrus | 9.5 | 35 | 0 | |||
| Diestrus | 9.5 | 34 | 0 | |||
| Diestrus | 8.5 | 34 | 0 | |||
| 4: Laparotomy with endometrial slurry | Estrus | 8.5 | 34 | 1 | 0.1 | 10 (95% CI: 0.5, 46) |
| Estrus | 8.5 | 35 | 0 | |||
| Estrus | 8.5 | 38 | 0 | |||
| Estrus | 8.5 | 35 | 0 | |||
| Estrus | 9.5 | 34 | 0 | |||
| Estrus | 9.5 | 35 | 0 | |||
| Estrus | 9.5 | 33 | 0 | |||
| Proestrus | 8.5 | 33 | 0 | |||
| Diestrus | 8.5 | 33 | 0 | |||
| Diestrus | 8.5 | 33 | 0 | |||
| Average days of disease | 34.85 (SD = 0.05) |
Individual take rate = number confirmed lesions/number of implanted biopsies × 100, expressed as a percentage.
Mean counts of confirmed lesions per mouse: group 1 SD = 1.4, group 2 SD = 0.74, group 3 SD = 0.42, group 4 SD = 0.32, group 5 SD = 1.17.
Endometriosis incidence per group, expressed as percentage of animals (n = 10) per paradigm that had confirmed endometriosis lesions histologically.
Individual confirmed endometrial lesion counts for slurry implanted animals.
Histologic confirmation.
In addition to endometrial stroma, some lesions contained hemosiderin, glandular epithelium, and calcifications (Figure 3). Lesions with glandular epithelium were only found in groups 1 and 5. Most endometrial lesions were found on skeletal muscle (body wall), adipose tissue, mesentery, on/near the bladder, and on broad ligaments of uterine horns (Figure 4). The method of endometriosis induction and type of endometrial tissue implanted significantly impacted the presence of confirmed (χ2 = 55.16, df = 4, P < 0.001; Figure 5) lesions. Groups 1 (mean = 2.7 lesions, SD = 1.4, 95% CI(1.81, 3.580)) and 5 (mean = 2.6 lesions, SD = 1.17, 95% CI(1.87, 3.32)) produced significantly more confirmed lesions than groups 3 (mean = 0.2 lesions, SD = 0.42, 95% CI(−0.05, 0.45)) and 4 (mean = 0.1 lesion, SD = 0.32, 95% CI(−0.09, 0.29)). Group 1 also produced marginally more confirmed lesions than did group 2 (mean = 1.1 lesions, SD = 0.74, 95% CI(0.64, 1.55)) (P = 0.088).
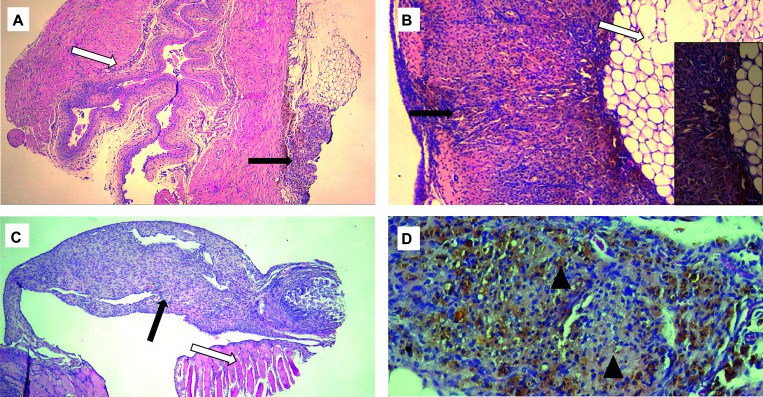
Figure 3.
Representative histopathology of confirmed endometriotic lesions (solid arrows) showing hemosiderin and stroma on abdominal organs and tissues identified with open arrows. (A) Bladder wall. (B) Abdominal adipose tissue with inset showing endometrial stroma and hemosiderin (golden brown) at 20× original magnification. (C) Peritoneal body wall. (D) Higher original magnification (20×) of endometriotic lesion with hemosiderin (golden brown) and endometrial stroma shown by arrowheads.
Figure 4.
Gross endometriotic lesions in situ with brown pigmentation. Arrows point to lesions on (A) bifurcation of uterus, (B) urinary bladder and body wall, and (C) inguinal fat.
Figure 5.
Mean (±95% CI) counts of confirmed endometriotic lesions across the 5 groups. The letters a, b, and c are used to denote which groups have significant differences between the mean number of confirmed lesions. The numbers at the top of the bars are the mean counts of confirmed lesions per group. Bars with no letters in common are significantly (P < 0.05) different based on post hoc tests from the Poisson generalized linear model of the log difference between groups. The numbers alongside each group description on the x-axis correlate to experimental group number. Figures were created in ggplot2 using R version 4.2.2.100
Immunohistochemistry.
Immunohistochemical staining performed on a subset of confirmed endometriotic lesions indicated the presence of angiogenesis (CD31), proliferation (Ki67) within the endometrial stroma, and mast cells (toluidine blue) and macrophages (CD68) in close proximity or within the endometrial stroma (Figure 6). The presence of angiogenesis and proliferation confirmed neovascularization and proliferation of confirmed endometriotic lesions, while the macrophages and mast cells confirmed the presence of potentially important immune cells in and around confirmed endometriotic lesions.
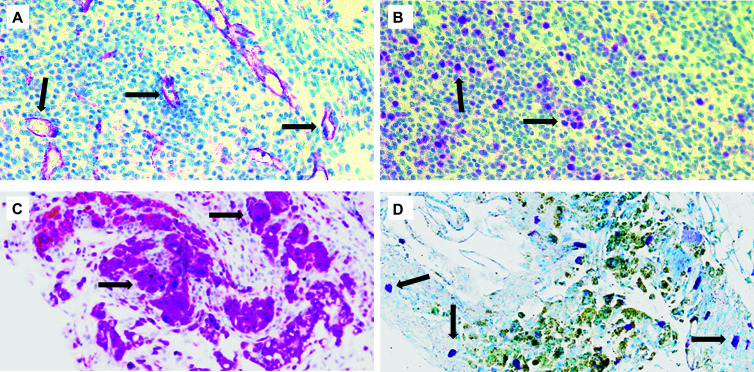
Figure 6.
Endometriotic lesions with immunohistochemical staining. Sections were stained using primary antibodies for (A) the endothelial cell marker CD31 and (B) the cell proliferation marker Ki67, demonstrating angiogenesis and cell replication, respectively. The presence of macrophages and mast cells was also confirmed using (C) the macrophage marker CD68 and (D) toluidine blue. Arrows indicate antigen-positive cells. Original magnification 20×.
Discussion
In this study, we developed and optimized a translationally relevant preclinical murine model of endometriosis using implantation of donor mouse menstrual endometrium into the peritoneal cavity of a menstruating recipient mice to mimic retrograde menstruation. We initially combined and tested methodologies from published murine models of endometriosis to optimize menstrual tissue type and implantation method.14,17,33–35 The most successful of these methods was used to induce endometriosis using transplantation of donor menstrual endometrium into menstruating recipient mice. The endometriosis incidence of 100% and average lesion count of 2.6 in group 5 are similar to published models of rodent endometriosis using different induction techniques and tissue types.17,27,33–35,43–46 Our model closely mimics the methods of successful induction of endometriosis using menstrual endometrium implantation during menses in baboons.42
In the original study design, mCherry mice were selected as donors due to ubiquitous expression of monomeric red mCherry fluorescent protein, with the goal of using longitudinal noninvasive fluorescent imaging (IVIS Lumina XR) to monitor progression of implanted endometriotic lesions. These transgenic mice with the albino tyrosinase point mutation avoid imaging issues associated with absorption of light due to skin pigment, improve deep in vivo imaging due to wavelength of light, and are reported to have normal development, physiology, and reproduction.47–49 However, results of noninvasive fluorescent imaging of live mice were suboptimal (inadequate signal and inadequate resolution of discrete lesions; data not shown), and this aspect of the study was not pursued. Our experience differed from the success reported using noninvasive fluorescent monitoring of endometrial tissue labeled with mCherry adeno-associated viral vectors.50,51 However, in Ferrero and colleagues, the strongest, most reliable signal was generated from endometrial tissue implanted subcutaneously on the ventral abdomen compared with intraperitoneal implantation, the more translationally relevant location.51 The use of the adeno-associated viral vector would increase mCherry signal compared with the transgenic mouse alone, potentially allowing noninvasive longitudinal assessment of lesion progression, but this was beyond the scope of this project. If perfected, this would allow for evaluation of lesions during different phases of the estrus cycle, progression/regression of lesions over time, and real-time evaluation of potential therapies.
Laparoscopic implantation of discrete biopsies of menstrual endometrium into immunocompetent, intact menstruating mice resulted in a 100% incidence of endometriosis and mean confirmed lesion counts of >2 per mouse (20% take rate). Implantation of discrete biopsies via laparoscopy was successful in a previous study by Peterse and colleagues with the same incidence of endometriosis (100%) and higher reported take rate (60%); however, uterine biopsies included myometrium and nonmenstrual endometrium compared with only menstrual phase endometrium in our study.33,34 Stage of estrus has been shown to affect endometriotic lesion development in mice,35 highlighting the importance of controlling for this variable in recipient mice. Importantly, note that we induced menstruation using intact pseudopregnant mice compared with ovariectomized mice treated with exogenous hormones. The goal of this was 2-fold: 1) to maintain normal physiologic levels of ovarian hormones and other factors produced by the ovaries (growth factors, transcription factors, cytokines, prostaglandins, proteoglycans, and proteolytic enzymes) involved in signaling pathways that regulate the menstrual cycle, and 2) develop a model of endometriosis that would return to normal estrus cyclicity, thereby enabling studies concerning fertility. Utilizing menstruating recipient mice that were time matched to menstruating donor mice ensured that both mice transitioned from the proliferative to the secretory phase simultaneously and were in the menstrual phase of the cycle at the time of transplant surgery. Consequently, both mice were subject to the same hormonal milieu, and the recipient mice were in the same hormonal phase physiologically as women experiencing retrograde menstruation (Figure 7). Our use of pseudopregnant mice resulted in the modulation of the endometrium and peritoneal environments in donor and recipient mice by physiologic hormone levels and could allow for further exploration of prolactin and other hormones.52–54
Figure 7.
Timing of menstrual cycle phases in women compared with pseudopregnant decidualized menstruating mice. Red mice are mCherry donor mice (D), and gray mice are B6 recipient mice (R). Menstrual endometrium was harvested from donor mice and implanted into menstruating recipient mice to mimic retrograde menstruation. Menstruating mice mimic the hormonal milieu and peritoneal microenvironment of menstruating women.101 Proliferation of ectopic lesions extended for at least 30 d past confirmed return to normal estrus cyclicity in the recipient mouse. Estrus cycles in mice are ∼4 to 5 d. The ectopic lesions would be exposed to the cyclical nature of estrogen and progesterone ∼6 to 7 times during their implantation and growth.
Endometriosis is an estrogen-dependent disease, and local estrogen production plays a role in pathogenesis, while endometriotic tissue displays progesterone resistance.55,56 These hormones play an important role in lesion development, survival, and progression, which still need further exploration. In women, hormone levels are cyclical, but rodent models using ovariectomy and exogenous hormones prevent this natural cyclicity and may affect local steroid hormone production and receptors, which could certainly affect study outcomes. Menstruation and hormonal cyclicity affect immune function and response in women,26 and immune response and dysregulation affect implantation, proliferation, and angiogenesis of ectopic endometrium.24,57 In intact mice, the stage of estrus (proliferative compared with secretory) impacts proliferation and apoptosis of uterine epithelium and stromal cells, extracellular matrix, and inflammatory cell populations,15 while ovariectomized mice can have changes in estrogen receptor α expression.58–60 Experimental manipulations to recipient and donor mouse hormones and naive endometrium can affect lesion pathology and immune response.43,61 In other mouse models, the use of naive endometrium harvested at various stages of estrus, or endometrium from ovariectomized donors primed by exogenous estrogen administration, may yield different lesion phenotypes and experimental outcomes compared with implantation of time-matched menstrual endometrium.
In group 5, we optimized the date of euthanasia of the donor and implantation into recipient mice to day 9.5 of pseudopregnancy (with induced decidualization on day 4.5). In menstruating mice, day 9.5 correlates with the highest vaginal bleeding scores, the largest decrease in systemic progesterone levels, and maximum mRNA expression levels of prostaglandin-endoperoxide synthase 2 (Ptgs2), vascular endothelial growth factor (Vegfa), and platelet endothelial cell adhesion molecule-1 (PECAM-1),14 which correspond to the elevated mRNA profiles of these genes observed in ectopic pelvic endometriotic lesions in women suffering from endometriosis.62–64 Utilization of a menstruating recipient mouse for induction of endometriosis is physiologically relevant; the peritoneal microenvironment during menses likely plays an important role in the survival of refluxed menstrual endometrium and subsequent development of endometriosis lesions. The refluxed menstrual endometrium is proinflammatory, with potential mutations of epithelial cells and epigenetic alterations to stromal cells. Survival and proliferation of ectopic endometrium is facilitated by upregulation of local estrogen production, immune cell dysfunction, reactive oxygen species/oxidative stress, and hypoxia in the peritoneal cavity.24 Our model replicates expression of potentially important factors and conditions in both the donor and the recipient mouse. Experimental manipulations to donor and recipient mice in the menstrual state could help elucidate innate and/or acquired factors contributing to the etiology and pathogenesis of this disease.
Injection of menstrual endometrial slurry (groups 3 and 4) was largely unsuccessful when compared with placement of discrete menstrual endometrium biopsies (groups 1, 2, and 5) and compared with results reported by others using injection of menstrual endometrium.17 In Greaves and colleagues, both donor and recipient mice were ovariectomized and administered supraphysiologic doses of hormones, with the recipient mice under constant exposure to estradiol-17β.17 High levels of estrogen, as found in proestrus in C57BL/6 mice or with administration of estradiol-17 β, have been shown to increase the total number of endometriotic lesions,27,35 which could explain the higher proportion of mice that developed lesions and higher mean count of lesions per mouse in their study compared with our results. However, in our groups 1 to 4, stage of estrus was recorded at time of endometrial implantation, and the lack of lesions produced with slurry injection cannot be explained by hormonal state alone. To have similar conditions between our groups, we made an incision in mice injected with slurry, comparable to the incision made for placement of the laparoscope. This differs from other reported models that perform an intraperitoneal injection without laparotomy. The physiologic effects of anesthesia and laparotomy cannot be ignored, and although the laparotomy incision was small and should decrease stress responses compared with larger incisions,65 this could have negatively affected the development of endometriotic lesions with the slurry injection model. All 3 groups using discrete biopsies had higher group incidences of endometriosis and higher mean confirmed lesions counts compared with the slurry injection groups. It has been previously reported in a chicken chorioallantoic membrane model of implantation that larger biopsies (>1 mm3) resulted in lesion formation compared with no lesion development when endometrial cells were transplanted.66 This suggests that intact tissue architecture is crucial to implantation, survival, and proliferation, and could explain the lack of lesions when endometrial slurry was used. However, further exploration of injected menstrual phase endometrial slurry into menstruating mice is warranted, as this could be a noninvasive model allowing for iterative transplantation.
When comparing groups 1 and 2, the discrete biopsies adhered to abdominal tissues without the use of suture or glue in both groups, with a higher mean count of lesions in group 1, using laparoscopy, compared with laparotomy in group 2. The spontaneous attachment of menstrual phase endometrium allows for early study of disease progression, including angiogenesis, apoptosis, proliferation, inflammation, and chemotactic homing response. There are reported differences in the morphology of mouse peritoneum and postoperative adhesion formation after laparoscopy or laparotomy,67,68 with bulging of mesothelial cells with the presence of intercellular clefts occurring 1 to 2 h after CO2 insufflation.69 This phenomenon has been hypothesized to play a role in peritoneal metastasis and infiltration of tumor cells into the submesothelial connective tissue matrix.70 The biologic response of the peritoneum to laparoscopy aids in spontaneous endometrial biopsy attachment and proliferation, as a compromised mesothelial barrier may play a role in the pathogenesis of the disease.71 Surgical treatment has also been reported as a potential risk factor for recurrence of ovarian endometriomas,72 and surgery has been shown to affect endometriosis lesion weight and angiogenesis in mice.73 It is hypothesized that laparoscopy alone potentially contributed to spontaneous development of endometriosis in a control group of baboons.74 In addition, laparoscopy has been shown to affect inflammatory cells and cytokines in rodents.75,76 Further investigation into the roles that diagnostic and therapeutic laparoscopy potentially play in recurrence and progression of endometriosis is warranted and would be possible using this model.
Infertility is a common complaint of women suffering from endometriosis, and 30% to 50% of women with endometriosis seek care due to infertility alone.77 In fact, a recent study in which neither the donor nor recipient mice were treated with exogenous hormones showed that chronic exposure to endometriosis affected perinatal outcomes.45 The use of intact mice and the confirmed return of estrus cyclicity in intact menstruating recipient mice with endometriosis will enable future studies to examine the impact of disease on fertility as well as testing new therapeutic strategies to treat disease symptoms, while simultaneously preserving and/or improving fertility in recipient mice.
In addition to infertility, pelvic pain is a common reason women seek diagnosis and treatment of endometriosis. Ovarian cycle, estradiol, neuroimmune/neuroendocrine factors, and a nervous system responsive to hormones all play a role in the pain a woman with endometriosis experiences.78 One recent study comparing 4 model variations of endometriosis (induced via injected slurry) in mice noted that endometriosis models using intact cycling mice could be preferable for assessment of pain-related behaviors.43 There is additional evidence that prior manipulations with exogenous hormones or surgery can affect future pain response,79,80 although laparoscopy has less of an effect on postoperative pain–related behaviors compared with laparotomy.81 Pain-related behaviors were not assessed in the development of this model, and thus further testing of our model to assess pain and to compare pain indices between methods of induction should be examined.
Pathologists have classically defined endometriosis in women as the presence of endometroid glands and stroma in biopsied lesions; however, there is tremendous heterogeneity in human endometriotic lesions, and evidence of ordered progression of lesions is lacking.82–84 In fact, some publications call into question the stringent definition of the presence of both glandular epithelium and stroma to diagnose endometriosis, which could lead to false negatives, as well as missed contributions of additional lesion phenotypes and adhesions to the clinical signs observed and response to treatments.56,82–84 Fibrosis and the presence of collagen and myofibroblasts in and around lesions are also features of endometriosis and likely contribute to infertility and pain.85–88 Most endometriotic lesions contain primarily stromal cells,56 and the stromal cells are hypothesized to play a supportive role in the lesions as well as being the primary cell type that interacts with the peritoneal mesothelial cells, impacting implantation.86 The periglandular stroma in lesions also contains inflammatory cells and blood vessels, important factors in pathogenesis.89 Although most confirmed endometrial lesions in this study did not contain glandular epithelium, endometrial stroma was present in all confirmed lesions, and some lesions also contained calcification and hemosiderin. The primary stromal component and heterogeneity of confirmed lesions in our study can certainly be reflective of the dynamic nature of endometriotic lesions in humans, primates, and mice.43,56,74,86 The findings of Dorning and colleagues43 align with our results, with confirmed lesions containing stroma and variable evidence of epithelial cells (20% to 40% of lesions based on cytokeratin-positive glandular structures). In our study, confirmed endometriotic lesions with glandular epithelium were only found in groups 1 and 5, although there was no significant difference detected between these groups for lesions with glands.
The lesions in our model are most similar to superficial peritoneal endometriosis based on their location and variable gross appearance and histology. Immunohistochemistry demonstrated positive staining for proliferation (Ki67) and angiogenesis (CD31) in our endometriotic lesions, indicating survivability and a potential progressive nature. In fact, we have also successfully induced endometriosis using group 5 methodology and menstruating B6(Cg)-Tyrc-2J/J females and menstruating C57BL/6 females as both donors and recipients and found confirmed endometriotic lesions at 60 and 90 d timepoints, demonstrating prolonged survival of lesions (unpublished data). Ki67 expression correlates with cellular atypia and malignant transformation in human lesions89 and is only expressed in the nuclei of proliferating cells.90 CD31, also known as PECAM-1, is involved in initiation of inflammation and wound healing, and is present in immature blood vessels,91 which would be present during early lesion angiogenesis and proliferation. As mentioned previously, it is highly expressed in pelvic endometriotic lesions in women, and its expression is upregulated in menstrual endometrium in mice. Immune cell dysfunction in endometriosis is associated with impaired phagocytosis/clearance of menstrual debris, increased survival/proliferation of ectopic tissue, neuroangiogenesis, and pain. Immunohistochemical staining revealed the presence of both macrophages (CD68) and mast cells (toluidine blue) within or near ectopic endometriotic lesions. Importantly, the density of these cells was increased in human endometriotic lesions with hyperplastic and malignant foci, and they are markers of a proinflammatory environment.89 Macrophages are the most abundant immune cells present in endometriotic lesions, and, in response to local estrogen, are recruited to the peritoneum and adopt a “wound-healing" (anti-inflammatory) M2 phenotype compatible with survival and growth of ectopic tissue.92 The M1 macrophage phenotype detects and responds to refluxed endometrial tissue and damage caused by hypoxia and oxidative stress and contributes to a proinflammatory state.24,93 The proinflammatory state activates other proinflammatory cell types, including mast cells, that contribute to the promotion of angiogenesis, fibrotic adhesions, and pain. The mast cell stabilizer ketotifen effectively reduced hyperalgesia in a rat model of endometriosis, indicating that mast cells may play a role in endometriosis pain.94 As both of these critically important immune cells were found in or near our confirmed lesions, our model could be used for future studies of these immune cells and potentially for evaluation of pain and pain modulation with the stipulations outlined previously. It is critical that our model is evaluated in other widely used strains of mice, as differences in endometrial lesion phenotype have been demonstrated between C57BL/6 and BALB/c mice,35 and inbred mouse lines can differ in their immunologic and inflammatory pathways.95
We found confirmed lesions in translationally relevant locations such as the bladder, uterine ligaments, and serosal surface of the uterus. Lesions in women are generally found in gravity-dependent areas such as the posterior cul de sac,2 and consistent with the quadruped nature of the mouse, most lesions were located on the ventral aspect of serosal surfaces. Although the ovaries are a common site of endometriosis in women, no lesions were found on the ovaries in recipient mice. This is not surprising as mice have an ovarian bursa and closed tubo-ovarial junction, and there is no bursa surrounding the ovaries in women.96 The closed tubo-ovarial junction also precludes the study of spontaneous endometriosis development due to retrograde menstruation in a menstruating mouse, thus requiring the use of both a donor and recipient mouse and a surgical or injectable method of implantation. Many lesions were attached to adipose tissue. The significance of this needs further exploration, as there is a strong relationship between estrogen and adipose tissue in women,97 and endometriosis is associated with lean body mass index and a low waist-to-hip ratio.1 In addition, higher levels of gene expression characteristic of M2 phenotype macrophages are associated with adipose tissue in lean mice,98 and endometriosis alters adipose stem cell population and metabolic gene expression.99
As a scientific community we are continually called upon to improve animal models of disease so they can reliably inform human studies. There is an urgent, unmet need for endometriosis research that leads to discoveries in etiology, pathogenesis, accurate diagnosis, and treatments. Currently, there is no standardized preclinical animal model for endometriosis, and a murine model is ideal due to size, fecundity, and amenability to genetic manipulation. Due to the use of intact, immunocompetent, menstruating recipient mice and the potential for long-term lesion survival, this model could be used to study fertility, immune factors, lesion progression, and etiology and pathogenesis of this disease. However, this model is challenging logistically, as it requires time-matched experimental manipulations of both donor and recipient mice that rely on the natural estrus cycle, making high-throughput studies difficult. The laparoscopic induction model requires technical expertise/specialized equipment and may not be best suited for studies examining pain but could provide insight into surgical manipulations and their effects on endometriosis. This model is a novel addition to the toolbox of animal models of endometriosis, and further explorations could elucidate the relationship between menstruation and ectopic endometriosis lesions.
Acknowledgments
We thank Alex Hall, Research Statistician II and Jack Taylor, PhD Biostatistician for consultations on statistics, the Department of Pharmacology and Neuroscience and the Department of Obstetrics and Gynecology (Creighton University School of Medicine) for support, the Stryker Corporation for donation of equipment, as well as Melissa Holzapfel, University of Nebraska Medical Center Tissue Sciences Facility, Toni Howard, Creighton University Histology Core Facility, and the Creighton Animal Resource Facility and animal husbandry staff for assistance.
Conflict of Interest
C.A.H., C.T.S, J.J.H., and M.R.B. have no conflicts of interest to declare. J.J.C. was a Principal Investigator on endometriosis industry-sponsored trials for AbbVie and Myovant and received compensation as a member of the endometriosis speaker’s bureau for AbbVie and Pfizer. J.B.G.v.W. received donation of laparoscopic devices from Stryker Corporation. Partial financial support was received from Robin Farias-Eisner, MD, PhD, MBA, FACOG and the OB/GYN Department, and Peter Abel, PhD, and Department of Pharmacology and Neuroscience (Creighton University School of Medicine).
Funding
This work was supported by the George F. Haddix President’s Faculty Research Fund (April 1, 2019 to March 31, 2020) and the Health Sciences Strategic Investment Fund (July 1, 2021 to June 30, 2023). Partial financial support was received from Robin Farias-Eisner, MD, PhD, MBA, FACOG and the Department of Obstetrics and Gynecology, and from Peter Abel, PhD and Department of Pharmacology and Neuroscience (Creighton University School of Medicine). The funding sources had no role in the design, practice, or analysis of this study.
References
- 1.Zondervan KT, Becker CM, Missmer SA. Endometriosis N Engl J Med. 2020382131244–1256 [DOI] [PubMed] [Google Scholar]
- 2.Saunders PT, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects Cell. 2021184112807–2824 [DOI] [PubMed] [Google Scholar]
- 3.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity Am. J. Obstet. Gynecol. 1927144422–469 [Google Scholar]
- 4.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis Obstet Gynecol. 1984642151–154 [PubMed] [Google Scholar]
- 5.D’Hooghe TM, Nugent NP, Cuneo S. et al. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study Biol Reprod. 2006741131–136 [DOI] [PubMed] [Google Scholar]
- 6.Dick EJ, Hubbard GB, Martin LJ, Leland MM. Record review of baboons with histologically confirmed endometriosis in a large established colony J Med Primatol. 200332139–47 [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie WF, Casey HW. Animal model of human disease. Endometriosis. Animal model: endometriosis in rhesus monkeys Am J Pathol. 1975802341–344 [PMC free article] [PubMed] [Google Scholar]
- 8.Yamanaka A, Kimura F, Takebayashi A, Kita N, Takahashi K, Murakami T. Primate model research for endometriosis Tohoku J Exp Med. 2012226295–99 [DOI] [PubMed] [Google Scholar]
- 9.Bellofiore N, Evans J. Monkeys, mice and menses: the bloody anomaly of the spiny mouse J Assist Reprod Genet. 2019365811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamlett GD. Uterine bleeding in a bat, Glossophaga soricina Anat Rec. 19346019–17 [Google Scholar]
- 11.Rasweiler JJ. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater Am J Anat. 199119111–22 [DOI] [PubMed] [Google Scholar]
- 12.Rasweiler JJ, de Bonilla H. Menstruation in short-tailed fruit bats (Carollia spp.) J Reprod Fertil. 1992951231–248 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zhu C, Lin H. et al. Wild fulvous fruit bats (Rousettus leschenaulti) exhibit human-like menstrual cycle Biol Reprod. 2007772358–364 [DOI] [PubMed] [Google Scholar]
- 14.Rudolph M, Döcke WD, Müller A et al. Induction of overt menstruation in intact mice. PLoS One. 2012;7(3):e32922. doi: 10.1371/journal.pone.0032922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus Reproduction. 200713351035–1044 [DOI] [PubMed] [Google Scholar]
- 16.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives Semin Reprod Med. 2007256445–453 [DOI] [PubMed] [Google Scholar]
- 17.Greaves E, Cousins FL, Murray A. et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium Am J Pathol. 201418471930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koninckx PR, Heyns W, Verhoeven G. et al. Biochemical characterization of peritoneal fluid in women during the menstrual cycle J Clin Endocrinol Metab. 19805161239–1244 [DOI] [PubMed] [Google Scholar]
- 19.Koninckx PR, Renaer M, Brosens IA. Origin of peritoneal fluid in women: an ovarian exudation product Br J Obstet Gynaecol. 1980873177–183 [DOI] [PubMed] [Google Scholar]
- 20.Hartveit F, Thunold S. Peritoneal fluid volume and the oestrus cycle in mice Nature. 196621050411123–1125 [DOI] [PubMed] [Google Scholar]
- 21.McGowan L, Davis RH. Effect of the mouse estrous cycle on peritoneal serous fluid cytology Endocrinology. 1969841175–177 [DOI] [PubMed] [Google Scholar]
- 22.Modat G, Benmbarek A, Lalaurie M. Variations quantitatives du nombre et de la taille des mastocytes péritonéaux chez la ratte au cours du cycle sexuel normal, après ovariectomie et administration d'oestrogènes [Quantitative variations in the number and size of peritoneal mastocytes in the female rat during the normal sexual cycle, following ovariectomy and administration of estrogens] C R Seances Soc Biol Fil. 19821765675–680 [PubMed] [Google Scholar]
- 23.Huang Q, Yu Y, Xu W, Li S, Zhou Y, Shu J. The role of peritoneal immunity in peritoneal endometriosis and related infertility. Front Biosci (Landmark Ed)). 2023;28(8):166. doi: 10.31083/j.fbl2808166. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Imanaka S, Yoshimoto C, Matsubara S, Shigetomi H. Rethinking the pathogenesis of endometriosis: complex interactions of genomic, epigenetic, and environmental factors J Obstet Gynaecol Res. 202450101771–1784 [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Yuan M, Jiao X. et al. Metabolite profiles in the peritoneal cavity of endometriosis patients and mouse models Reprod Biomed Online. 2021435810–819 [DOI] [PubMed] [Google Scholar]
- 26.Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle: a review Obstet Gynecol Surv. 200964158–72 [DOI] [PubMed] [Google Scholar]
- 27.Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen Reprod Toxicol. 199593233–238 [DOI] [PubMed] [Google Scholar]
- 28.Bruner-Tran K, Mokshagundam S, Herington JL, Ding T, Osteen KG. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets Curr Womens Health Rev. 2018142173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns KA, Pearson AM, Slack JL et al. Endometriosis in the mouse: challenges and progress toward a ‘best fit’ murine model. Front Physiol. 2021;12:806574. doi: 10.3389/fphys.2021.806574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greaves E, Critchley HOD, Horne AW, Saunders PTK. Relevant human tissue resources and laboratory models for use in endometriosis research Acta Obstet Gynecol Scand. 2017966644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaves E, Rosser M, Saunders PTK. Endometriosis-associated pain—Do preclinical rodent models provide a good platform for translation? Adv Anat Embryol Cell Biol. 202023225–55 [DOI] [PubMed] [Google Scholar]
- 32.Simitsidellis I, Gibson DA, Saunders PTK. Animal models of endometriosis: replicating the aetiology and symptoms of the human disorder Best Pract Res Clin Endocrinol Metab. 2018323257–269 [DOI] [PubMed] [Google Scholar]
- 33.Peterse D, Binda MM, O DF. et al. Of mice and women: a laparoscopic mouse model for endometriosis J Minim Invasive Gynecol. 2018254578–579 [DOI] [PubMed] [Google Scholar]
- 34.Peterse DP, Fassbender A, O DF. et al. Laparoscopic surgery: a new technique to induce endometriosis in a mouse model Reprod Sci. 201623101332–1339 [DOI] [PubMed] [Google Scholar]
- 35.Dodds KN, Beckett EAH, Evans SF, Hutchinson MR. Lesion development is modulated by the natural estrous cycle and mouse strain in a minimally invasive model of endometriosis Biol Reprod. 2017976810–821 [DOI] [PubMed] [Google Scholar]
- 36.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis Fertil Steril. 20017511–10 [DOI] [PubMed] [Google Scholar]
- 37.Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets Toxicol Lett. 20021281–3145–157 [DOI] [PubMed] [Google Scholar]
- 38.Jones SL, Antonie RA, Pfaus JG. The inhibitory effects of corncob bedding on sexual behavior in the ovariectomized Long-Evans rat treated with estradiol benzoate are overcome by male cues Horm Behav. 20157239–48 [DOI] [PubMed] [Google Scholar]
- 39.Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain Endocrinol. 20121532949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings AM, Metcalf JL. Effects of estrogen, progesterone, and methoxychlor on surgically induced endometriosis in rats Fundam Appl Toxicol. 1995272287–290 [DOI] [PubMed] [Google Scholar]
- 41.Van Der Lee S, Boot LM. Spontaneous pseudopregnancy in mice Acta Physiol Pharmacol Neerl. 195543442–444 [PubMed] [Google Scholar]
- 42.Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4(Suppl 1) doi: 10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorning A, Dhami P, Panir K et al. Bioluminescent imaging in induced mouse models of endometriosis reveals differences in four model variations. Dis Model Mech. 2021;14(8):dmm049070. doi: 10.1242/dmm.049070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model Endocrinology. 201215383960–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsherbini M, Koga K, Maki E et al. Impact of chronic exposure to endometriosis on perinatal outcomes: establishment of a mouse model. Biomedicines. 2022;10(10):2627. doi: 10.3390/biomedicines10102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Adur MK, Kannan A et al. Progesterone alleviates endometriosis via inhibition of uterine cell proliferation, inflammation and angiogenesis in an immunocompetent mouse model. PLoS One. 2016;11(10):e0165347. doi: 10.1371/journal.pone.0165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deliolanis NC, Kasmieh R, Wurdinger T, Tannous BA, Shah K, Ntziachristos V. Performance of the red-shifted fluorescent proteins in deep-tissue molecular imaging applications. J Biomed Opt. 2008;13(4):044008. doi: 10.1117/1.2967184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink D, Wohrer S, Pfeffer M, Tombe T, Ong CJ, Sorensen PH. Ubiquitous expression of the monomeric red fluorescent protein mCherry in transgenic mice Genesis. 20104812723–729 [DOI] [PubMed] [Google Scholar]
- 49.Frangioni JV. In vivo near-infrared fluorescence imaging Curr Opin Chem Biol. 200375626–634 [DOI] [PubMed] [Google Scholar]
- 50.Martinez J, Bisbal V, Marin N, Cano A, Gómez R. Noninvasive monitoring of lesion size in a heterologous mouse model of endometriosis J Vis Exp. 2019144e58358 [DOI] [PubMed] [Google Scholar]
- 51.Ferrero H, Buigues A, Martínez J, Simón C, Pellicer A, Gómez R. A novel homologous model for noninvasive monitoring of endometriosis progression Biol Reprod. 2017962302–312 [DOI] [PubMed] [Google Scholar]
- 52.Yang JJ, Larsen CM, Grattan DR, Erskine MS. Mating-induced neuroendocrine responses during pseudopregnancy in the female mouse J Neuroendocrinol. 200921130–39 [DOI] [PubMed] [Google Scholar]
- 53.Franchimont P, Dourcy C, Legros JJ. et al. Prolactin levels during the menstrual cycle Clin Endocrinol (Oxf). 197656643–650 [DOI] [PubMed] [Google Scholar]
- 54.Mirabi P, Alamolhoda SH, Golsorkhtabaramiri M, Namdari M, Esmaeilzadeh S. Prolactin concentration in various stages of endometriosis in infertile women JBRA Assist Reprod. 2019233225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laganà AS, Garzon S, Götte M et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci. 2019;20(22):5615. doi: 10.3390/ijms20225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bulun SE, Yilmaz BD, Sison C. et al. Endometriosis Endocr Rev. 20194041048–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. 2015;2015:795976. doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham JD, Clarke CL. Physiological action of progesterone in target tissues Endocr Rev. 1997184502–519 [DOI] [PubMed] [Google Scholar]
- 59.Kurita T, Lee K, Saunders PT. et al. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse Biol Reprod. 2001641272–283 [DOI] [PubMed] [Google Scholar]
- 60.Carley ME, Rickard DJ, Gebhart JB, Webb MJ, Podratz KC, Spelsberg TC. Distribution of estrogen receptors alpha and beta mRNA in mouse urogenital tissues and their expression after oophorectomy and estrogen replacement Int Urogynecol J Pelvic Floor Dysfunct. 2003142141–145 [DOI] [PubMed] [Google Scholar]
- 61.He Y, Liang B, Hung SW et al. Re-evaluation of mouse models of endometriosis for pathological and immunological research. Front Immunol. 2022;13:986202. doi: 10.3389/fimmu.2022.986202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santulli P, Borghese B, Noël JC. et al. Hormonal therapy deregulates prostaglandin-endoperoxidase synthase 2 (PTGS2) expression in endometriotic tissues J Clin Endocrinol Metab. 2014993881–890 [DOI] [PubMed] [Google Scholar]
- 63.Van Langendonckt A, Eggermont J, Casanas-Roux F, Scholtes HE, Donnez J. Expression of platelet endothelial cell adhesion molecule-1 in red and black endometriotic lesions Fertil Steril. 2004824984–985 [DOI] [PubMed] [Google Scholar]
- 64.Li G, Lin Y, Zhang Y et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022;8(1):29. doi: 10.1038/s41420-022-00821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishibashi S, Takeuchi H, Fujii K, Shiraishi N, Adachi Y, Kitano S. Length of laparotomy incision and surgical stress assessed by serum IL-6 level Injury. 2006373247–251 [DOI] [PubMed] [Google Scholar]
- 66.Nap AW, Groothuis PG, Demir AY. et al. Tissue integrity is essential for ectopic implantation of human endometrium in the chicken chorioallantoic membrane Hum Reprod. 200318130–34 [DOI] [PubMed] [Google Scholar]
- 67.Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model Fertil Steril. 2001763560–567 [DOI] [PubMed] [Google Scholar]
- 68.Suematsu T, Hirabayashi Y, Shiraishi N, Adachi Y, Kitamura H, Kitano S. Morphology of the murine peritoneum after pneumoperitoneum vs laparotomy Surg Endosc. 2001159954–958 [DOI] [PubMed] [Google Scholar]
- 69.Volz J, Köster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum Surg Endosc. 1999136611–614 [DOI] [PubMed] [Google Scholar]
- 70.Brokelman WJ, Lensvelt M, Borel Rinkes IH, Klinkenbijl JH, Reijnen MM. Peritoneal changes due to laparoscopic surgery Surg Endosc. 20112511–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albertsen HM, Ward K. Genes linked to endometriosis by GWAS are integral to cytoskeleton regulation and suggests that mesothelial barrier homeostasis is a factor in the pathogenesis of endometriosis Reprod Sci. 2017246803–811 [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo S. Patterns of and risk factors for recurrence in women with ovarian endometriomas Obstet Gynecol. 200710961411–1420 [DOI] [PubMed] [Google Scholar]
- 73.Long Q, Liu X, Guo SW. Surgery accelerates the development of endometriosis in mice Am J Obstet Gynecol. 20162153320.e1–320.e15 [DOI] [PubMed] [Google Scholar]
- 74.Harirchian P, Gashaw I, Lipskind ST. et al. Lesion kinetics in a non-human primate model of endometriosis Hum Reprod. 20122782341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filho IA, Sobrinho AAH, Meneses do Rego AC. et al. Influence of laparoscopy and laparotomy on gasometry, leukocytes and cytokines in a rat abdominal sepsis model Acta Cirurgica Brasil. 20062174–79 [DOI] [PubMed] [Google Scholar]
- 76.Huang SG, Li YP, Zhang Q, Redmond HP, Wang JH, Wang J. Laparotomy and laparoscopy diversely affect macrophage-associated antimicrobial activity in a murine model BMC Immunol. 20131427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners Fertil Steril. 200992168–74 [DOI] [PubMed] [Google Scholar]
- 78.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications Hum Reprod Update. 2011173327–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hains LE, Loram LC, Weiseler JL. et al. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming J Pain. 201011101004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loram LC, Taylor FR, Strand KA. et al. Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats J Pain. 201213121162–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Préfontaine L, Hélie P, Vachon P. Postoperative pain in Sprague Dawley rats after liver biopsy by laparotomy versus laparoscopy Lab Anim (NY). 2015445174–178 [DOI] [PubMed] [Google Scholar]
- 82.Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects Adv Anat Pathol. 2007144241–260 [DOI] [PubMed] [Google Scholar]
- 83.Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature Hum Reprod. 2018333347–352 [DOI] [PubMed] [Google Scholar]
- 84.Boyle DP, McCluggage WG. Peritoneal stromal endometriosis: a detailed morphological analysis of a large series of cases of a common and under-recognised form of endometriosis J Clin Pathol. 2009626530–533 [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation Mol Cell Endocrinol. 20164281–16 [DOI] [PubMed] [Google Scholar]
- 86.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis Fertil Steril. 2012983511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia Garcia JM, Vannuzzi V, Donati C, Bernacchioni C, Bruni P, Petraglia F. Endometriosis: cellular and molecular mechanisms leading to fibrosis Reprod Sci. 20233051453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lac V, Huntsman DG. Distinct developmental trajectories of endometriotic epithelium and stroma: implications for the origins of endometriosis J Pathol. 20182463257–260 [DOI] [PubMed] [Google Scholar]
- 89.Istrate-Ofiţeru AM, Mogoantă CA, Zorilă GL et al. Clinical characteristics and local histopathological modulators of endometriosis and its progression. Int J Mol Sci. 2024;25(3):1789. doi: 10.3390/ijms25031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer Acta Histochem. 20161185544–552 [DOI] [PubMed] [Google Scholar]
- 91.da Silva BB, Lopes-Costa PV, dos Santos AR. et al. Comparison of three vascular endothelial markers in the evaluation of microvessel density in breast cancer Eur J Gynaecol Oncol. 2009303285–288 [PubMed] [Google Scholar]
- 92.Heublein S, Vrekoussis T, Kuhn C. et al. Inducers of G-protein coupled estrogen receptor (GPER) in endometriosis: potential implications for macrophages and follicle maturation J Reprod Immunol. 201397195–103 [DOI] [PubMed] [Google Scholar]
- 93.Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, Marín-Sánchez P, Machado-Linde F, García-Peñarrubia P. The role of peritoneal macrophages in endometriosis. Int J Mol Sci. 2021;22(19):10792. doi: 10.3390/ijms221910792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu T-H, Zou G, Ding S-J. et al. Mast cell stabilizer ketotifen reduces hyperalgesia in a rodent model of surgically induced endometriosis J Pain Res. 2019121359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice Vet Pathol. 201249132–43 [DOI] [PubMed] [Google Scholar]
- 96.Boyd KL, Muehlenbachs A, Rendi MH, Garcia RL, Gibson-Corley KN. Female reproductive system Treuting PM, Dintzis SM, Montine KS. eds Comparative Anatomy and Histology. 2nd ed Academic Press; 2018303–334 [Google Scholar]
- 97.Hetemäki N, Mikkola TS, Tikkanen MJ et al. Adipose tissue estrogen production and metabolism in premenopausal women. J Steroid Biochem Mol Biol. 2021;209:105849. doi: 10.1016/j.jsbmb.2021.105849. [DOI] [PubMed] [Google Scholar]
- 98.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization J Clin Invest. 20071171175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zolbin MM, Mamillapalli R, Nematian SE, Goetz TG, Taylor HS. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. 2019;17(1):36. doi: 10.1186/s12958-019-0480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wickham H. ggplot2: Elegant Graphics for Data Analysis New York, NY: Springer-Verlag; 2016. 10.1007/978-3-319-24277-4_9 [DOI]
- 101.Thiyagarajan DK, Basit H, Jeanmonod R. Physiology, menstrual cycle [Updated 2024 Sep 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Jan. https://www.ncbi.nlm.nih.gov/books/NBK500020/ [PubMed]