Abstract
Patient: Male, 68-year-old
Final Diagnosis: Abdominal aortic aneurysm (AAA) • duodeno-aortic fistula
Symptoms: Bloating • mass in abdomen • urinary tract infection
Clinical Procedure: —
Specialty: Surgery
Objective: Unusual clinical course
Background
Aorto-duodenal fistula (ADF) is a communication between the aorta and the duodenum and requires urgent aortic repair. However, it often leads to life-threatening complications. Thus, an early and appropriate diagnostic method is necessary to deliver adequate treatment. This report describes the case of a 68-year-old man with anaorto-duodenal fistula following emergency endovascular repair of a 14-cm abdominal aortic aneurysm (AAA) identified using oral contrast computed tomography (CT), and discusses the consequences of delayed treatment and ADF management.
Case Report
We present a case of a 68-year-old White man with abdominal bloating, who upon further examination was diagnosed with an AAA, which was successfully treated by endovascular aneurysm repair (EVAR). However, 1 year later, an endoleak was discovered and, despite the physicians’ efforts, the overall treatment was complicated due to the patient’s continuous postponement of treatment over several years. The aneurysm continued to expand until it reached 14 cm. Despite the successful endovascular repair of the endoleak, a duodeno-aortic fistula (DAF) was diagnosed using CT with oral contrast. The patient was managed conservatively with aneurysmal sac drainage and antibiotic therapy, which decreased the size of the aneurysmal sac. Despite early diagnostic efforts and interventions, the patient’s condition deteriorated due to further complications, ultimately resulting in death.
Conclusions
This case report demonstrates a potential useful diagnostic role of CT with oral contrast in detecting DAF, in situations when other methods fail to do so, and emphasizes the importance of prompt intervention to prevent life-threatening complications. However, further investigations to explore the underlying mechanism and its replicability are required.
Keywords: Aortic Aneurysm, Abdominal; Diagnosis; Endovascular Aneurysm Repair; Fistula; Case Reports
Introduction
Abdominal aortic aneurysm (AAA) is a potentially life-threatening condition characterized by permanent, localized dilation of the abdominal aorta [1]. The global incidence of AAA varies geographically but affects approximately 4–8% of men and 1–2% of women over the age of 65 [2]. The incidence of AAA peaks in the seventh and eighth decades of life. Risk factors include atherosclerosis, smoking, advanced age, male sex, White race, family history of AAA, hypertension, hypercholesterolemia, and history of aortic dissection. Other predisposed groups are those with cystic medial necrosis, syphilis, human immunodeficiency virus, and connective tissue diseases (Ehlers-Danlos syndrome, Marfan syndrome, and Loeys-Dietz syndrome) [3]. Most AAAs are asymptomatic and are often discovered incidentally on imaging performed for unrelated reasons [4]. When symptoms occur, they may include abdominal, flank, and back pain, abdominal bloating, and a pulsatile abdominal mass [4,5]. Gastrointestinal and renal manifestations can be caused by compression of adjacent viscera [3]. A ruptured AAA typically presents dramatically with sudden abdominal pain, hypotension, abdominal distention, and a pulsatile abdominal mass [6]. Diagnosis is typically confirmed via imaging, with abdominal ultrasound being the initial screening modality [7]. Computed tomography (CT) is considered the criterion standard for preoperative planning and post-EVAR surveillance [8]. Endovascular aneurysm repair (EVAR) has become the treatment of choice for many patients with large AAAs, particularly those exceeding 55 mm in men and 50 mm in women [9]. EVAR is a minimally invasive procedure that involves placement of a stent graft within the aorta to exclude the aneurysm from circulation and prevent rupture by accessing the aorta through the femoral artery [10]. Compared with open surgical repair (OSR), EVAR offers a less invasive option with lower perioperative morbidity, decreased intraoperative mortality, and improved early survival [11,12], but is associated with a 20% reintervention rate within 5 years of the procedure. EVAR carries risks of both device- and procedure-related complications such as acute limb ischemia from graft limb thrombosis or kinking, embolic events causing colonic, spinal cord, or renal ischemia, graft infections, and stent-graft migration or occlusion morbidity or mortality [13]. Long-term outcomes of EVAR did not show superiority compared to OSR, as demonstrated in 3 large randomized controlled trials: EVAR-1, DREAM, and OVER [14–16]. Lifelong surveillance after EVAR is required due to complications such as endoleaks, which occur in up to 20% of patients within 5 years and can lead to aneurysm rupture if untreated [17]. Endoleak, defined as continued expanding of an aneurysmal sac following placement of an endograft, is the most frequent complication following EVAR [18]. Among the most severe but rare complications following EVAR is the development of an aorto-enteric fistula (AEF), particularly a aorto-duodenal fistula (ADF), involving a pathological communication between the aorta and the duodenum [19]. Primary causes of AEF formation involve the native aorta, while the main secondary cause is synthetic graft material that erodes into adjacent enteric viscera [20]. AEFs are often linked to infection or persistent endoleaks and are associated with very high mortality rates [21]. In ADF, we observe leakage of aortic sac content causing gastrointestinal bleeding into the duodenum [22], as seen in previous cases [23–26]. However, in this case study, a distinction between an ADF and a duodeno-aortic fistula (DAF) needs to be made, as this was a unique case in which duodenal contents had leaked into the aortic sac, unlike ADFs. Diagnosis remains challenging; while CT with contrast is typically the first-line imaging modality, it is not always sufficient, as presented in this case, necessitating additional diagnostic approaches [27,28]. This report describes a 68-year-old man with a DAF but no ADF, following EVAR of a 14-cm AAA identified using oral contrast CT.
Case Report
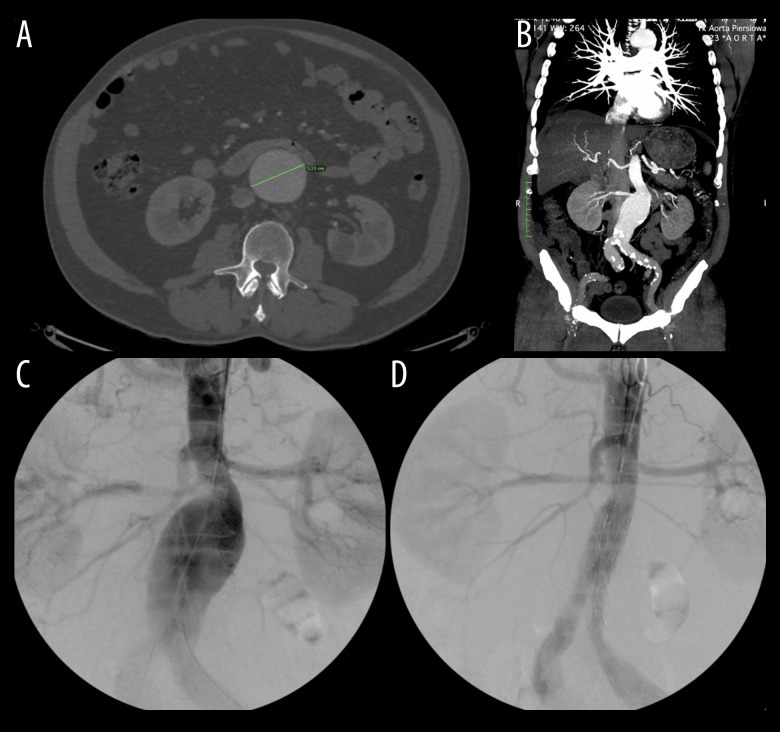
In September 2014, a 68-year-old White man presented to the emergency room with abdominal bloating. While on holiday, where he was admitted to a hospital due to a urinary tract infection, during which he had a CT scan that revealed an incidental AAA. Subsequently, upon physical examination in our hospital, an abdominal palpable mass was found with no other unusual findings or symptoms. CT showed a 55-mm-diameter AAA (Figure 1). He did not smoke and drank alcohol occasionally. He had no significant medical history and denied connective tissue disorder and chronic diseases in the family, including family history of AAA. Additionally, he reported that both of his parents died of stroke at the age of 70. He was admitted to the hospital for further investigation, and the following year, a Cook Fenestrated 28/24-mm stent was placed successfully and without complications (Figure 1).
Figure 1.
Non-contrast-enhanced CT (A) and contrast-enhanced CT (B) of the abdominal aortic aneurysm (AAA) measuring 55 mm. The same AAA in 2015, before the EVAR procedure (C) and after it (D). CT – computed tomography.
A year later, he returned for a follow-up CT of the stent graft, which suggested an endoleak type 2 at the L4 level and an increase of the aneurysmal sac to 58 mm in diameter. However, he declined endovascular treatment. In 2017, a follow-up revealed the aneurysm had grown to 77 mm. Additionally, he had significant stenosis of the right popliteal artery and an occlusion of the tibial arteries. However, he again declined any form of treatment or intervention.
Throughout the years the patient kept delaying treatment and the aneurysm continued to grow. In 2020 the aneurysm reached a size of 104 mm (Figure 2) and treatment was offered again. However, the patient left the hospital on his own request and appeared a year later in 2021, when he decided to undergo the procedure to repair the endoleak via EVAR. The same year, the patient was admitted to the hospital due to a COVID-19 infection and thus he did not appear for the follow-up CT that was supposed to occur following the procedure.
Figure 2.
CT (contrast-enhanced) scan of the abdominal aortic aneurysm (AAA) in 2020, measuring 104 mm. (A) and CT (non-contrast-enhanced) of AAA in 2022, measuring 145 mm (B). CT – computed tomography.
In early May 2022, he came to the hospital with melena and fatigue. On CT, a massive endoleak was detected, with the aneurysmal sac measuring 145 mm (Figure 2). There was fluid accumulation outside the sac, indicating a suspended aneurysmal rupture. Additionally, the duodenum had stretched across the sac, enveloping the aneurysm. Considering these findings, an emergency stent graft extension was done. During the surgery, dissection of the right common iliac artery was found, but it could not be successfully repaired, leading to a femoral-femoral bypass surgery.
Postoperative CT showed air and fat stranding on the segment of the duodenum overlying the aorta, which suggested a duodeno-aortic fistula with inflammation of fat tissues surrounding the aneurysm (Figure 3). Additionally, after the procedure, it was noted that the right common iliac artery and the right external iliac artery were obstructed; however, the superior mesenteric artery, renal arteries, and the bypass graft were still patent. Despite the typical CT picture indicating fistula, the CT with intravenous contrast did not confirm this diagnosis. However, considering the fact that melena was no longer present, a CT with oral contrast was done instead, which successfully identified a fistula between the duodenum and the aortic sac (Figure 3). After the diagnosis of the fistula and the patient’s decision not to undergo open surgery, a drain was placed with daily flushing with saline, and antibiotic therapy was administered according to the antibiogram (Figure 4).
Figure 3.
CT (non-contrast-enhanced) with visible characteristic features of duodeno-aortic fistula (DAF) such as: fat stranding on the segment of the duodenum overlying the aorta (arrow 1), inflammation of fat tissues surrounding the aneurysm with gas bubbles in the aortic sac (arrow 2). CT with oral contrast present in the duodenum (arrow 3) and aneurysmal sac (arrow 4). CT – computed tomography.
Figure 4.
CT (non-contrast-enhanced) of abdominal aortic aneurysm (AAA) after applying conservative treatment using the drain (arrows). CT – computed tomography.
A CT done in August 2022 showed that the aneurysmal sac had decreased in size to 92 mm, with aneurysmal wall thickening. The duodeno-aortic fistula was no longer detected with oral or intravenous contrast CT. Despite the successful but still ongoing conservative treatment with drainage, the patient requested to be discharged and the drain was subsequently removed.
Two months later, the patient was readmitted due to a loss of sensation and paresis in the distal extremities bilaterally after a 10-hour aircraft flight. On CT, the aneurysmal sac was larger with more gas, but no leakage was detected. The patient underwent a percutaneous transluminal angioplasty for revascularization, which was successful, but his circulation deteriorated and as a consequence his right leg was amputated. There were no post-amputation complications, but within the next few weeks, his condition progressively worsened and he died.
Discussion
This article presents a case of endovascular repair of a 14-cm AAA, which was later complicated with DAF, and an alternative approach of detecting it using CT with oral contrast. In radiological images, formation of the fistula can be seen on CT presented as periaortic fluid collection, aortic fat stranding, and visualization of the fistula itself [29]. To confirm such a finding, endoscopy, CT, angiography, or surgical exploration can be used [28]. In similar case reports, describing post-EVAR ADF formation, diagnosis was made with CT and surgical exploration [23–25]. However, this case presents a diagnosis of DAF using oral contrast, which was not previously described by any other article. We believe that the reason for such an uncommon finding is that the pressure inside the aneurysmal sac was lower than in the duodenum, and the duodenal content was leaking into the aneurysmal sac, which complicated detection of the fistula by CT angiography (CTA). CTA is primarily used to detect ADF, where blood with contrast is leaking to the duodenum. The previously mentioned case reports described the ADF in AAA measuring 5.8 cm, 5.7 cm, and 5.5 cm, respectively, in contrast to the 14-cm AAA and DAF we describe in this case. Thus, further studies should be conducted to determine the relationship between pressures in the aortic sac and the duodenum with respect to the AAA size and occurrence of DAF and ADF. Nevertheless, clinicians should be aware that in the presence of typical features of AEF on CT, an oral contrast agent can also serve as a diagnostic approach in situations when CT angiography fails to confirm the diagnosis. Treatment of AEF involves either total graft resection together with arterial reconstruction, bowel repair, and antibiotic therapy, or a conservative approach [29]. However, studies show 30–40% mortality rates among patients with such fistulas treated surgically [30,31] and almost 100% on conservative treatment within 1 year [29]. It was reported that the overall postoperative morbidity rate is higher in patients with ADF treated by OSR in comparison to patients treated with EVAR [26]. In the literature, 14-cm aneurysms are uncommon and patients with large AAAs (5.5 cm or larger) have significantly lower survival rates compared to patients with small AAAs (less than 5.5 cm). Moreover, outcomes after EVAR are also poorer in patients with large aneurysms [32]. Thus, considering high surgical risk and patient refusal to OSR, another approach needed to be considered. Spontaneous closure of AEF is highly uncommon. However, a case report by Shindo et al noted a non-surgical closure of ADF after administering CT-guided drainage. In addition to spontaneous closure of the fistula, they reported full recovery of the patient without further complications [33]. Similarly, in our case, after applying treatment consisting of stent graft preservation, aneurysmal sac drainage using saline solution, and antibiotic therapy, closure of DAF and a decrease in aneurysmal sac size to 92 mm were observed. Despite initial improvement of our patient’s condition, he requested to be discharged from the hospital and the treatment had to be discontinued. ADF is a rare but serious complication of AAA, which can be preceded by persistent endoleaks, which were also present in our case. However, management of endoleaks is not always simple. Studies show that more than 50% of type 2endoleaks resolve on their own with time [34]. Therefore, it is difficult to decide when to start treatment for this type of endoleak. Nevertheless, the decision to start the treatment is based on an increase in the aneurysmal sac measuring at least 5 mm over 6 months [35]. In our case, the treatment was delivered with a delay due to consistent non-adherence to treatment recommendations over the course of management. This demonstrates that it is crucial for treatment to be initiated immediately and for possible complications to be acknowledged by doctors and patients alike, but it is up to the patient to adhere to the treatment.
Conclusions
This case presents an alternative approach to diagnosing DAF through the use of oral contrast, highlighting its potential use in identifying such an uncommon finding, especially in situations where conventional imaging in ADF diagnosis may be inconclusive. Further studies should be conducted to evaluate the relationship between the aneurysmal sac and the duodenum with respect to the pressure inside them and the implementation of appropriate diagnostic methods. This case also highlights the importance of prompt intervention in managing persistent leakage, as well as the crucial role of patient cooperation, as both are essential to prevent life-threatening complications.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Patient Consent: Patient’s consent was obtained.
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452–58. doi: 10.1067/mva.1991.26737. [DOI] [PubMed] [Google Scholar]
- 2.Svensjö S, Björck M, Gürtelschmid M, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124(10):1118–23. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PM, Loree J, Oropallo A. StatPearls [Internet[ Treasure Island (FL): StatPearls Publishing; 2025. 2025. Abdominal aortic aneurysm. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470237/ [Google Scholar]
- 4.Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099–105. doi: 10.1016/s0741-5214(99)70049-2. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson KJ, Webster DJ. Abdominal aortic aneurysm causing duodenal and ureteric obstruction. J Vasc Surg. 1986;3(2):364–68. doi: 10.1067/mva.1986.avs0030364. [DOI] [PubMed] [Google Scholar]
- 6.Bown MJ, Sutton AJ, Bell PR, et al. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89(6):741–30. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 7.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: Validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160(10):1425–30. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 8.Hu DK, Pisimisis GT, Sheth RA. Repair of abdominal aortic aneurysms: Preoperative imaging and evaluation. Cardiovasc Diagn Ther. 2018;8(Suppl 1):S157–S67. doi: 10.21037/cdt.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaikof EL, Brewster DC, Dalman RL, et al. Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4 Suppl):S2–49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Lederle FA, Freischlag JA, Kyriakides TC, et al. Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: A randomized trial. JAMA. 2009;302(14):1535–42. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 12.Prinssen M, Verhoeven EL, Buth J, et al. Dutch Randomized Endovascular Aneurysm Management (DREAM)Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 13.Maleux G, Koolen M, Heye S. Complications after endovascular aneurysm repair. Semin Intervent Radiol. 2009;26(1):3–9. doi: 10.1055/s-0029-1208377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenhalgh RM, Brown LC, Kwong GP, et al. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet. 2004;364(9437):843–48. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 15.Van Schaik TG, Yeung KK, Verhagen HJ, et al. DREAM trial participants. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66(5):1379–89. doi: 10.1016/j.jvs.2017.05.122. [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, Kyriakides TC, Stroupe KT, et al. OVER Veterans Affairs Cooperative Study Group. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380(22):2126–35. doi: 10.1056/NEJMoa1715955. [DOI] [PubMed] [Google Scholar]
- 17.Jones JE, Atkins MD, Brewster DC, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46(1):1–8. doi: 10.1016/j.jvs.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Urquijo M, Lozano-Balderas G, Fabiani MA. Type II endoleaks after EVAR: A literature review of current concepts. Vasc Endovascular Surg. 2020;54(8):718–24. doi: 10.1177/1538574420945448. [DOI] [PubMed] [Google Scholar]
- 19.Kakkos SK, Bicknell CD, Tsolakis IA, et al. Hellenic Co-operative Group on Aortic Surgery. Editor’s choice – Management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: A review and pooled data analysis. Eur J Vasc Endovasc Surg. 2016;52(6):770–86. doi: 10.1016/j.ejvs.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Dorosh J, Lin JC. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2025. Aortoenteric fistula. [Updated 2022 Sep 26] Available from: https://www.ncbi.nlm.nih.gov/books/NBK567729/ [PubMed] [Google Scholar]
- 21.Batt M, Jean-Baptiste E, O’Connor S, et al. Early and late results of contemporary management of 37 secondary aortoenteric fistulae. Eur J Vasc Endovasc Surg. 2011;41(6):748–57. doi: 10.1016/j.ejvs.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Busuttil SJ, Goldstone J. Diagnosis and management of aortoenteric fistulas. Semin Vasc Surg. 2001;14(4):302–11. doi: 10.1053/svas.2001.27888. [DOI] [PubMed] [Google Scholar]
- 23.Zaki M, Tawfick W, Alawy M, et al. Secondary aortoduodenal fistula following endovascular repair of inflammatory abdominal aortic aneurysm due to Streptococcus anginosus infection: A case report and literature review. Int J Surg Case Rep. 2014;5(10):710–13. doi: 10.1016/j.ijscr.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg. 2007;45(4):834–36. doi: 10.1016/j.jvs.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Koda Y, Murakami H, Yoshida M, et al. EJVES Short Rep. Secondary aorto-enteric fistula and type II endoleak five years after endovascular abdominal aortic aneurysm repair. 2019;43:12–17. doi: 10.1016/j.ejvssr.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omran S, Raude B, Bürger M, et al. Aortoduodenal fistulas after endovascular abdominal aortic aneurysm repair and open aortic repair. J Vasc Surg. 2021;74(3):711–19e1. doi: 10.1016/j.jvs.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Šumskienė J, Šveikauskaitė E, Kondrackienė J, et al. A case report. Aorto-duodenal fistula: A rare but serious complication of gastrointestinal hemorrhage. Acta Med Litu. 2016;23(3):165–68. doi: 10.6001/actamedica.v23i3.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Liu Q, Shen H, et al. Diagnosis and management of primary aortoenteric fistulas--experience learned from eighteen patients. Surgery. 2008;143(1):43–50. doi: 10.1016/j.surg.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Gueifão I, Quintas A, Alves G, et al. Secondary aortoenteric fistula after endovascular aortic aneurysm repair – a narrative review. Angiol Cir Vasc. 2023;19(3):186–90. [Google Scholar]
- 30.Genovés-Gascó B, Torres-Blanco Á, Plaza-Martínez Á, et al. Primary aortoduodenal fistula in a patient with pararenal abdominal aortic aneurysm. Ann Vasc Surg. 2012;26(5):730e1–5. doi: 10.1016/j.avsg.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Beuran M, Negoi I, Negoi RI, et al. Primary aortoduodenal fistula: First you should suspect it. Braz J Cardiovasc Surg. 2016;31(3):261–63. doi: 10.5935/1678-9741.20160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouriel K, Srivastava SD, Sarac TP, et al. Disparate outcome after endovascular treatment of small versus large abdominal aortic aneurysm. J Vasc Surg. 2003;37(6):1206–12. doi: 10.1016/s0741-5214(02)75449-9. [DOI] [PubMed] [Google Scholar]
- 33.Shindo S, Inoue H, Motohashi S, Uchiyama H, Akasaka J. Spontaneous evacuation of a vascular metallic stent through a graft-duodenal fistula with concomitant non-surgical fistula closure. Ann Vasc Dis. 2016;9(4):338–41. doi: 10.3400/avd.cr.16-00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JX, Stavropoulos SW. Type 2 endoleak management. Semin Intervent Radiol. 2020;37(4):365–70. doi: 10.1055/s-0040-1715873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Stavropoulos SW. Management of endoleaks. Semin Intervent Radiol. 2015;32(3):259–64. doi: 10.1055/s-0035-1556825. [DOI] [PMC free article] [PubMed] [Google Scholar]