Abstract
Purpose
Brain metastases (BrMs) frequently manifest in patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC), and the optimal treatment approach for these individuals remains controversial. This study aimed to evaluate the cost-effectiveness of adding pemetrexed-platinum chemotherapy to first-line gefitinib in EGFR-mutant NSCLC patients with BrMs, considering the perspective of the Chinese healthcare system.
Patients and methods
Constructing a Markov model, we simulated disease progression over a 10-year horizon in 4-week intervals. The model included three main health states: progression-free survival (PFS), progressive disease (PD), and death, along with a temporary PFS health state. Transition probabilities were estimated based on the data from the GAP BRAIN trial. Costs and utilities were collected from local public database and literature. One-way and probabilistic sensitivity analyses were employed to assess the robustness of the model.
Results
In the base case analysis, adding pemetrexed-platinum chemotherapy to first-line gefitinib resulted in an incremental effectiveness of 0.59 quality-adjusted life years (QALYs) and an increase in healthcare costs by $12,298.51. Consequently, the incremental cost-effectiveness ratio (ICER) amounted to $24,887.67/QALY, which fell below the prespecified willingness-to-pay (WTP) threshold of $37,654.50 per QALY. Deterministic sensitivity analysis indicated that, apart from the utility value of the PFS health state of the gefitinib plus chemotherapy (GPP) group, other parameters exerted minimal influence on the results. The probabilistic sensitivity analysis demonstrated that, at the predefined WTP threshold, the combination of gefitinib and chemotherapy was a cost-effective treatment strategy.
Conclusion
Adding pemetrexed-platinum chemotherapy to first-line gefitinib for EGFR-mutant NSCLC patients with BrMs in China represented a cost-effective treatment strategy from the perspective of the Chinese healthcare system.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-025-13322-3.
Keywords: Markov, Gefitinib, Chemotherapy, NSCLC, China, Cost-effectiveness
Background
Brain metastases (BrMs) represent a prevalent occurrence in lung cancer with a grim prognosis [1]. Around 20–25% of advanced non-small cell lung cancer (NSCLC) cases manifest BrMs at the time of initial diagnosis, while 30–40% of NSCLC cases develop BrMs as the disease progresses [2]. Clinical evidence indicates a significant correlation between epidermal growth factor receptor (EGFR) mutations and the prevalence of BrMs in NSCLC [3, 4]. This correlation is particularly evident in Asian countries, where data shows that approximately 44–63% of Epidermal Growth Factor Receptor (EGFR)-mutated NSCLC patients may develop BrMs during anticancer treatment [5–7].
Currently, the standard-of-care for first-line treatment of metastatic NSCLC patients with EGFR mutations involves the use of EGFR tyrosine kinase inhibitors (TKIs) [8, 9]. Notably, results from the BRAIN trial demonstrated that the first-generation EGFR-TKI drug icotinib significantly prolonged intracranial progression-free survival (PFS) (10.0 months vs. 4.8 months) in NSCLC patients with brain metastases compared to whole-brain radiation therapy (WBR), while also reducing the incidence of adverse reactions (3% vs. 28%)[10]. However, the clinical application of first-generation EGFR-TKIs is hindered by the emergence of widespread resistance. Encouragingly, findings from a large-scale, randomized, double-blind, intent-to-treat clinical trial known as GAP BRAIN (NCT01951469) revealed that first-line gefitinib combined with pemetrexed-platinum (GPP) significantly extended intracranial PFS (median, 15.6 months vs. 9.1 months) in EGFR-mutated NSCLC patients with BrMs compared to gefitinib monotherapy (GM) [11]. Furthermore, the combination therapy exhibited higher objective response rates in the brain (85.0% vs. 63.0%) as well as overall objective response rates (80.0% vs. 64.2%) compared to gefitinib alone.
Beyond assessing efficacy, it is essential to gather evidence regarding the cost-effectiveness of GPP to inform clinical and healthcare decision-making. Consequently, the primary objective of this study is to conduct a comprehensive cost-effectiveness analysis comparing GPP versus GM in the treatment of EGFR-mutated NSCLC patients with BrMs, considering the perspective of the Chinese healthcare system.
Materials and methods
Analytical overview
Drawing upon data from the GAP BRAIN (NCT01951469) trial, we undertook a meticulous cost-effectiveness analysis to compare first-line GPP and GM in EGFR-mutated NSCLC patients with BrMs [11]. Through Markov cohort analyses, we projected the long-term economic and clinical outcomes associated with each treatment strategy. The primary outputs of our model encompassed total costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). To evaluate cost-effectiveness, we juxtaposed the ICER generated by our model against a willingness-to-pay (WTP) threshold of $38,223.34 per QALY gained. This WTP threshold was defined based on three times China’s per capita gross domestic product (GDP) in 2022, aligning with published guidelines [12, 13]. Our Markov model was constructed and analyzed using TreeAge Pro software 2022 R1 (https://www.treeage.com/) and R version 4.2.0 (http://www.r-project.org).
This economic evaluation relied on existing data, including clinical efficacy and safety data sourced from the GAP BRAIN trial, health utility data, as well as cost data extracted from published literature and local public databases. As a result, our study did not involve human subjects and secured an exemption from approval by the Clinical Ethics Committee of Henan Provincial Chest Hospital. Moreover, our study adhered to the guidelines for pharmacoeconomic evaluation in China, ensuring that our methodology and findings adhere to the highest standards and best practices within this realm of research [12].
Model structure and clinical data
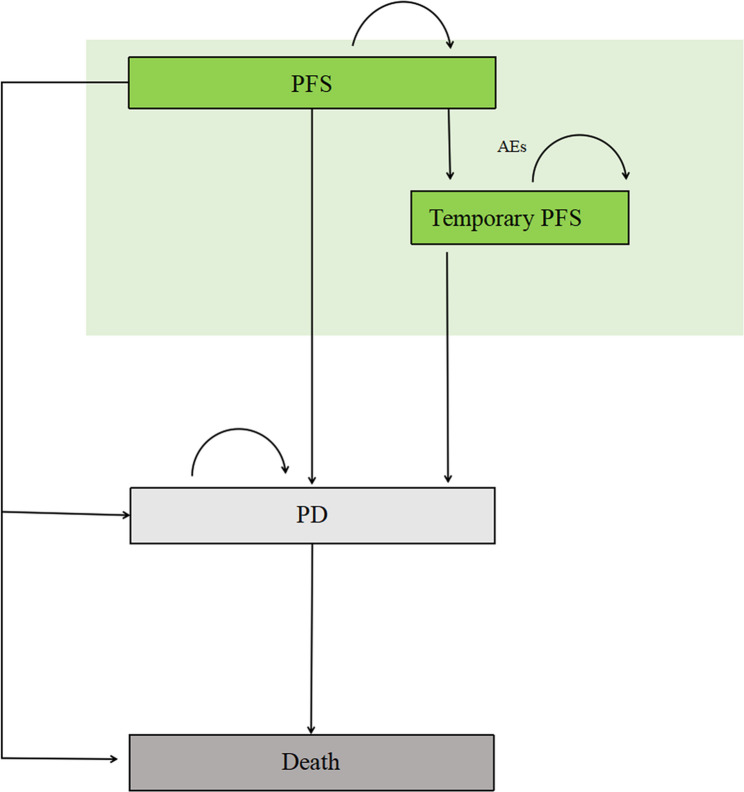
In our Markov model, we incorporated three main health states including PFS, progressive disease (PD), and death. Additionally, we introduced a temporary PFS health state to account for first-line treatment interruptions resulting from adverse events (AEs) that occur in real-world settings. Figure 1 provides an overview of the model structure. All patients initially started in the PFS health state and were randomized to receive either first-line GPP or GM therapy. Patients who experienced treatment failure progressed to the PD state and received second-line therapy. Subsequent treatments, such as third-generation TKI osimertinib or WBR, was modeled based on follow-up anticancer treatment data from the GAP BRAIN trial [11]. In cases where patients experienced intolerable toxicity but did not exhibit disease progression, they were transferred to the temporary PFS health state, where the triggering drug was discontinued while other drugs were maintained. To align with the trial administration interval, we utilized a 28-day Markov cycle length, and a 10-year time horizon was select to ensure that over 99% of model patients reach the death health state. The treatment dosages and administration schedule mentioned above followed those outlined in the clinical trial (see Table s1). To avoid overestimating expected survival, the half-cycle correction was used in the model.
Fig. 1.
Markov Model Diagram. The diagram shows the Markov model used in the analysis, which includes three main health states: intracranial progression-free survival (iPFS), progressive disease (PD), and death. Additionally, a temporary health state related to adverse events (AEs) was incorporated into the model. All patients started in the PFS state and were randomly assigned to receive either first-line gefitinib plus pemetrexed-platinum based chemotherapy (GPP) or gefitinib monotherapy (GM). During each four-week cycle, patients had a probability of transitioning to another health state based on the calculated transition probabilities
Transition probabilities for intracranial PFS (iPFS) and OS were estimated based on the Kaplan-Meier (KM) curves from the GAP BRAIN study. The individual patient data was extracted from the curves using GetData Graph Digitizer software package (version 2.26; http://www.getdatagraphdigitizer.com/index.php) and then fitted and extrapolated using four commonly used survival distributions (exponential, log-normal, Weibull, and log-logistic). After statistical measureing of goodness-of-fit, including the Akaike information criterion (AIC) and Bayesian information criterion (BIC), we selected the log-logistic and Weibull distributions for OS and iPFS data, respectively (see Table s2) [15]. The R software (version 4.2.0, http://www.r-project.org) was employed to estimate the scale and shape parameters of the Weibull distribution and the lambda and gamma parameters of the log-logistic distribution. According to survival analysis, the transition probability at the tth Markov cycle can be derived from the survival function of the fitted parametric distribution.
For the Weibull distribution, the transition probability is:
 |
For the log-logistic distribution, the corresponding formula is:
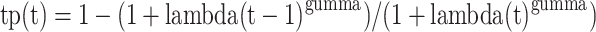 |
Where tp(t) is the probability of transitioning out of the current state during cycle t. The transition probability from PD state to death was calculated as the difference between the transition probabilities derived from the OS and iPFS curves at each time point. The details of distribution parameters can be found in Table 1. Finally, we calculated the probabilities of temporary health states resulting from treatment interruptions caused by AEs utilizing the probability calculation method proposed by Rachael L. et al., based on the data from the GAP BRAIN study (refer to Table s3) [16]. After recovery, these patients resume the initially assigned treatment regimen—excluding the drug that triggered the AEs—and continue treatment until disease progression. Therefore, the transition probability from the temporary iPFS state to the PD state is considered to be identical to that of patients in the standard PFS state, as both groups follow the same clinical course once the adverse event has resolved. Table s4 presents the transition probabilities between health states during the first 20 cycles of the Markov model.
Table 1.
Model inputs
| Variable | Baseline value | Range | Distribution | Source |
|---|---|---|---|---|
| Survival | ||||
| iPFS for first-line GPP | Lambda=6.091e-05; Gamma=3.452 | Fixed in DSA | Fixed in PSA | Estimateda |
| iPFS for first-line GM | Scale=0.006607; Shape=2.039062 | Fixed in DSA | Fixed in PSA | Estimateda |
| OS for first-line GPP | Scale=0.001477; Shape=1.708060 | Fixed in DSA | Fixed in PSA | Estimateda |
| OS for first-line GM | Scale=0.0009051; Shape=1.9709346 | Fixed in DSA | Fixed in PSA | Estimateda |
| 1-cycle probability of GPP treatment discontinuation due to AEs | 0.003554505 | 0.001777253–0.005331756 | Normal | Estimatedb |
| 1-cycle probability of GM treatment discontinuation due to AEs | 0.002914722 | 0.001457361–0.004372083 | Normal | Estimatedb |
| Cost (US$) | ||||
| Gefitinib per cycle | 155.83 | 77.92-233.745 | Lognormal | Local charge |
| Pemetrexed per cycle | 305.32 | 152.66-457.98 | Lognormal | Local charge |
| Cisplatin per cycle | 26.46 | 13.23–39.69 | Lognormal | Local charge |
| Premedication for the use of pemetrexede | 1.5 | 0.75–2.25 | Lognormal | Estimatedc |
| Osimertinib | 693.29 | 346.65−1,039.94 | Lognormal | Local charge |
| WBI | 3419.52 | 1709.76−5,129.28 | Lognormal | Local charge |
| BSC | 347.68 | 173.84−521.52 | Lognormal | [22, 23] |
| Cost of end-of-life care | 2,706.63 | 1,353.32−4,059.95 | Lognormal | [22, 23] |
| AEs cost for GPP group | 295.83 | 147.92−443.75 | Lognormal | Estimatedd |
| AEs cost for GM group | 96.72 | 48.36−145.08 | Lognormal | Estimatedd |
| Routine follow-ups | 148.06 | 74.03−222.09 | Lognormal | [22, 23] |
| MRI | 162.02 | 81.01−243.03 | Lognormal | Local charge |
| Health state utilities | ||||
| PFS state for GPP group | 0.65 | 0.0.26–0.78 | Beta | [17, 18] |
| PFS state for GM group | 0.67 | 0.27–0.80 | Beta | [17, 18] |
| PD state | 0.321 | 0.1605–0.4815 | Beta | [17, 18] |
| AEs disutility for GPP group | 0.0439 | 0.02195–0.06585 | Beta | Estimatedd |
| AEs disutility for GM group | 0.0012 | 0.0006–0.0018 | Beta | Estimatedd |
| Other parameters | ||||
| Discount-rate | 0.03 | 0−0.08 | Fixed in DSA | [14] |
| Proportion of first-line cisplatin in GPP group | 0.5 | 0.25–0.75 | Normal | Estimated |
| Proportion of first-line pemetrexed discontinuation in GPP group | 0.5 | 0.25–0.75 | Normal | Estimated |
| Proportion of first-line gefitinib discontinuation in GPP group | 0.7 | 0.375-1 | Normal | Estimated |
| Proportion of subsequent osimertinib therapy in GPP group | 0.675 | 0.3375-1 | Normal | Estimated |
| Proportion of subsequent WBI therapy in GPP group | 0.25 | 0.125–0.275 | Normal | Estimated |
| Proportion of subsequent osimertinib therapy in GM group | 0.6296 | 0.3148–0.9444 | Normal | Estimated |
| Proportion of subsequent WBI therapy in GM group | 0.2963 | 0.1481–0.4444 | Normal | Estimated |
| PFS of subsequent osimertinib therapy in GPP group(month) | 7.7 | 3.85–11.55 | Normal | Estimated |
| PFS of subsequent osimertinib therapy in GM group(month) | 8.5 | 4.25–12.75 | Normal | Estimated |
GPP Gefitinib + pemetrexed-platinum, GM Gefitinib monotherapy, OS Overall survival, PFS Progression-free survival, PD Progressive disease, AEs Adverse events, BSC Best support care, WBI Whole-brain irradiation, MRI Magnetic resonance imaging
aEstimated by the survival fifitting implemented in R software
bEstimated in Supplementary Table S3
cEstimated in Supplementary Table S1
dEstimated in Supplementary Table S4
eThe cost of premedication for the use of pemetrexed was based on the sum of the costs of folic acid, vitamin B12, and dexamethasone as listed in Table S1
Utilities and cost estimates
In the model, we derived the QALY by weighting the survival time with the health state utility values. To account for the improved quality of life associated with oral gefitinib compared to intravenous treatment, we assigned a health state utility value of 0.65 to the GPP group and 0.67 to the GM group for the iPFS health state [17, 18]. For the PD health state, we assumed an equivalent utility value of 0.321 for both treatment groups [18]. – [19] Additionally, our model factored in the negative impact of AEs by incorporating disutilities obtained from previously reported economic studies. These disutility scores were added to the first cycle using a frequency-weighted sum method.
From the perspective of the Chinese healthcare system, we collected direct costs encompassing drug costs, general cancer treatment costs and AE treatment costs. In this study, the first-line treatment regimen includes gefitinib, pemetrexed and platinum-based (assuming an equal distribution of cisplatin and nedaplatin, each comprising 50%) chemotherapy, while the second-line treatment consists of osimertinib and WBI. According to local oncologists’ opinions and clinical practices, patients receiving pemetrexed treatment also receive folic acid, vitamin B12, and corticosteroids as premedication to mitigate the risk of neurotoxicity (see Table s1). All information for drugs is obtained from the China Health Industry Data Platform, while the cost for WBR is sourced from a local comprehensive tertiary hospital [20]. The calculation of costs per cycle for each drug were involved multiplying the actual consumption of drug vials by the unit cost per vial. To determine drug dosages in this mode, we utilized an average body surface area of 1.72 m [2] [21]. Adhering to local guideline, the general cancer treatment include follow-up (comprising routine follow-up and a cranial magnetic resonance imaging (MRI) scan every 8 weeks), best supportive care (BSC) required by patients during cancer treatment, as well as the palliative care provided at the end of life [14]. Except for the MRI, which was obtained from a local comprehensive tertiary hospital, all other costs are sourced from the literature [22, 23].
In our model, we accounted for the costs associated with various AEs: leukopenia, anemia, neutropenia, vomiting, liver damage (including increased aspartate aminotransferase, increased alanine aminotransferase, and increased blood creatinine), diarrhea, nausea, and thrombocytopenia. The costs of treating these AEs were sourced from previously economic studies and added to the first cycle using the same algorithm as the disutilities(see Table s4) [22, 23]. All costs were converted to 2022 US dollars using an annual rate of 3% and the exchange rate of the RMB against the US dollar in 2022 (1 USD = 6.7261 RMB) [13].
Sensitivity analyses
To address the uncertainty surrounding model parameters, both deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were performed. In the DSA, all parameters were systematically tested within a plausible range, independently varied, and their influence on ICER was assessed. The ranges for costs and proportions of subsequent therapy were set at ± 50% of the base-case value, while other parameters were tested based on ranges reported in relevant literature. To visually represent the result of DSA, a tornado diagram was utilized.
For the PSA, appropriate statistical distributions were assigned to key parameters based on recommendations from the ISPOR-SMDM Modeling Good Research Practices Task Force [24]. Utilities followed a beta distribution, costs followed a log-normal distribution, discount rates and proportions followed normal distributions. By simultaneously varying all parameters through 1,000 iterations, the model was recalculated to estimate the impact of uncertainty on the outcomes. The results of the PSA were presented as a cost-effectiveness acceptability curve (CEAC) and a Incremental Cost-Effectiveness Plane (ICEP). Table 1 provides an overview of the baseline values, ranges, and distributions of the model parameters.
Result
Base case results
Figure s1 exemplifies the comparison between the curves derived from the best-fit models, as well as the original K-M curves, showcasing that the robustness of the Markov model employed in this study. Within the specified 10-year time horizon, it is projected that all patients in the GPP group (100.00%) and an overwhelming majority in the GM group (99.98%) were died.
Table 2 presents comprehensive details regarding the base-case analysis. In the iPFS state, using first-line GPP gave patients more health benefits — with an increase of 0.59 QALYs (1.09 vs. 0.50) and 0.94 life years (1.68 vs. 0.74) — but also led to higher costs, adding $16,069.57 ($23,162.54 vs. $7,092.97) compared to first-line GM. In contrast, in the PS state, first-line GPP cost less ($12,149.00 vs. $15,863.48), but provided slightly fewer QALYs (0.40 vs. 0.49) and life years (1.25 vs. 1.54) than first-line GM. Notably, the administration cost of first-line treatment showed the largest cost difference between the two strategies ($23,162.53 vs. $7,092.97). Overall, first-line GPP provided 0.65 more QALYs than GM, at an extra cost of $12,298.51. This led to an ICER of $24,887.67 per QALY, which is well below the WTP threshold of $38,223.34 per QALY.
Table 2.
Summary of cost (US dollars) and outcome results in the base-case analysis
| Items | GPP | GM | Incremental |
|---|---|---|---|
| Mean LYs | |||
| iPFS state | 1.68 | 0.74 | 0.94 |
| PD state | 1.25 | 1.54 | −0.29a |
| Total | 2.93 | 2.28 | 0.65 |
| Mean QALYs | |||
| iPFS state | 1.09 | 0.50 | 0.59 |
| PD state | 0.40 | 0.49 | −0.09a |
| Total | 1.49 | 0.99 | 0.50 |
| Cost(US$) | |||
| iPFS state | 23,162.53 | 7,092.97 | 16,069.57 |
| PD state | 12,149.00 | 15,863.48 | −4,118.05a |
| Drug administration cost of 1 st line treatment | 10,432.64 | 1,512.57 | 8,920.07 |
| Drug administration cost of 2nd line treatment | 2,742.94 | 4,344.47 | −1,901.53a |
| AEs management | 307.25 | 100.89 | 206.36 |
| Follow-up visit | 8,609.38 | 6,685.91 | 1,923.47 |
| BSC | 13,235.93 | 10,317.16 | 2,918.77 |
| End-of-life care | 2,470.29 | 2,526.87 | −56.58a |
| Total | 37,781.82 | 25,483.32 | 12,298.51 |
| ICER((US$) | |||
| Per LY | 18,920.78 | ||
| Per QALY | 24,887.67 | ||
GPP Gefitinib + pemetrexed-platinum, GM Gefitinib monotherapy, QALY Quality-adjusted life-year, LY Life-year, ICER Incremental cost-effectiveness ratio, PFS Progression-free survival, PD Progressive disease, OS Overall survival, BSC Best support care, AEs Adverse events
aindicate that the values of the GPP group are lower than those of the GM group
Sensitivity analyses
The results of the DSA are presented in Figure s2. The range of ICERs varied from -$77,479.93/QALY to $208,808.96/QALY. The most influential parameters were identified as the utility for iPFS state in the GPP group, the utility for iPFS state in the GM group, and the cost of pemetrexed per cycle identified. Other parameters, such as the cost of BSC, osimertinib, and gefitinib, had a minor impact on the ICERs. Notably, changing any parameter individually, except for the utility value of the iPFS state in the GPP group, did not alter the cost-effectiveness of switching from first-line GPP to first-line GM treatment.
The CEAC is shown in figure S3 and demonstrates that the probability of first-line GPP being cost-effective increases as the WTP threshold rises. In 1,000 Monte Carlo simulations, first-line GPP has a 50% chance of being cost-effective at a WTP threshold of $24,887.67/QALY. At a WTP threshold of three times the per capita GDP of China in 2022 ($38,223.34), GPP achieves cost-effectiveness in 79.9% of Monte Carlo iterations. As shown in Figure s4, the ICEP indicates that the majority of simulated ICERs fall below the willingness-to-pay (WTP) threshold. This indicates that, in most scenarios, the GPP strategy achieves better health outcomes at an acceptable additional cost.
Discussion
In this study, we developed a Markov model to estimate the cost-effectiveness of first-line GPP over a 10-year time horizon for EGFR-mutated NSCLC patients with BrMs. The model was populated with clinical efficacy and safety data from a large, randomized, phase III clinical trial, as well as cost information from local public databases and published literature. Our findings indicate that first-line GPP is a cost-effective option compared to GM, with an ICER of $24,887.67/QALY, falling below the WTP threshold of $38,223.34/QALY. This analysis also revealed that the most significant difference in QALYs between the two strategies was observed in the iPFS health state, where first-line GPP provided an additional 0.59 QALY at an incremental cost of $16,069.57 relative to GM. The higher total medical cost associated with first-line GPP primarily stemmed from the inclusion of pemetrexed, resulting in an increase of $305.32 in drug acquisition cost per cycle, as well as a relatively longer treatment duration. It is worth noting that since 2018, the efforts of the National Healthcare Security Administration (NHSA) has contributed significantly to establishing a basic medical insurance system and reducing of drug prices [25]. The Chinese government’s policy initiatives have led to significant price reductions—often exceeding 90%—for cancer drugs such as gefitinib, pemetrexed, and platinum-based agents, all of which are now included in the National Drug Reimbursement List (NDRL). Nevertheless, substantial regional disparities in economic development persist, particularly between eastern and western provinces. For instance, in less developed regions such as Gansu, the per capita GDP remains as low as $6,688—far below the national average [13]. In such areas, the financial burden of healthcare remains a major concern, and access to affordable cancer treatment is still limited. Under these economic constraints, the GPP regimen may no longer be considered cost-effective. To enhance its affordability and restore its cost-effectiveness, further reductions in drug prices—particularly for pemetrexed, which has the most significant impact on ICER—are needed. Therefore, strategic drug price negotiations and flexible reimbursement policies are essential to improving equitable access to cancer care across China’s economically diverse regions.
Globally, lung cancer accounts for 11.4% of all cancer cases and 18.0% of all cancer deaths. In China alone, there were over 810,000 new cases of lung cancer and 710,000 deaths in 2020, making it the leading cause of cancer mortality in the country and representing 23.8% of all cancer-related deaths [26, 27]. Additionally, NSCLC patients with BrMs have an average survival time of only 1–2 months, further highlighting the urgency to address this issue [28]. BrMs significantly contribute to lung cancer mortality and incidence, as well as impacting patients’ quality of life. Therefore, this study holds important implications for the Chinese government in balancing its population and medical resources. To our knowledge, this is the first cost-effectiveness analysis examing the combination of TKI treatment and traditional chemotherapy for EGFR-Mutated NSCLC patients with BrMs. The results demonstrated that GPP treatment increased effectiveness by 0.59 QALYs. A similar cost-effectiveness analysis comparing erlotinib and WBI/WBI with or without chemotherapy also showed that erlotinib was more cost-effective [18]. These findings further highlight the importance of combining gefitinib with traditional chemotherapy in treating NSCLC patients with EGFR mutations and brain metastases, offering valuable insights for developing more effective treatment strategies.
Strengths and limitations
This study has several strengths that contribute to its significance. Firstly, it addressed a crucial research gap by conducting the first cost-effectiveness analysis of the combination of TKI and traditional pemetrexed-platinum chemotherapy for EGFR-mutated NSCLC patients with BrMs in China. This fills an important void in the existing literature and offers valuable insights into the cost-effectiveness of this treatment option within the Chinese healthcare system. Secondly, the model used in this study is comprehensive and taking into account a wide range of factors that can influence the cost-effectiveness of the treatment. It incorporated considerations such as first-line and second-line treatments, adverse events, and end-of-life care, ensuring that the findings are applicable to real-world clinical practice in China.Thirdly, this study provides important information for the Chinese government, which is the main payer for healthcare in the country. Our results show that using first-line GPP treatment for patients with EGFR-mutated NSCLC and BrMs could be a cost-effective choice in China’s healthcare system. These findings can help the government make better decisions about offering affordable cancer treatments while using healthcare resources wisely. Lastly, the Markov model used in this study is particularly well-suited to our research context. It enabled us to integrate clinical data from multiple sources and to perform survival curve fitting for several health states—including PFS, PS, death, and a temporary PFS state. Multiple parametric distributions (exponential, log-normal, Weibull, and log-logistic) were tested, and the model with the best fit (i.e., the lowest error) was selected. This approach provided robust evidence for accurately estimating transition probabilities and enhanced the credibility of the model outputs.
However, it is essential to acknowledge some limitations of this study. Firstly, due to the limited follow-up duration in the included clinical trials, long-term survival had to be extrapolated from published K–M curves. Although such extrapolation techniques are widely accepted and commonly applied in health economic evaluations, they inevitably introduce uncertainty, particularly when predictions extend beyond the observed trial period. The validity of the extrapolated outcomes largely depends on the selected survival models and how well they fit the observed data. To address this uncertainty, we performed goodness-of-fit assessments and selected the most appropriate parametric distributions for modeling survival. Nonetheless, we acknowledge that as more mature survival data become available from ongoing studies, the projected long-term outcomes may change. Future research should focus on incorporating updated real-world evidence or extended trial follow-up data to validate and improve the accuracy of model predictions. Additionally, scenario analyses using alternative survival distributions can offer a broader perspective on the potential range of outcomes and support more robust decision-making under uncertainty. Secondly, for simplification purposes, the subsequent second-line treatments in the two groups were modeled as WBR and osimertinib chemotherapy. This approach may resulted in an underestimation of PS costs. Nonethless, sensitivity analysis demonstrated that changing the cost of WBR and osimertinib did not affect the conclusion that GPP was cost-effective in this study. Finally, the lack of quality-of-life data for Chinese EGFR-mutated NSCLC patients with BrMs necessitated reliance on health utilities from published literature. Sensitivity analysis indicated that the utility value of the iPFS state in the GPP group had the most substantial impact on the study outcomes. Therefore, future research should aim to develop suitable quality-of-life assessment tools for Chinese patients in order to enhance the accuracy and relevance of findings in this population.
Conclusions
The findings of this study suggest that adding pemetrexed-platinum chemotherapy to first-line gefitinib for EGFR-mutated NSCLC patients with BrMs in China represents a cost-effective treatment strategy. This combination not only improves clinical outcomes by enhancing PFS and OS, but also provides good economic value within the context of China’s healthcare system, where resource allocation and treatment affordability are critical concerns. These results may support evidence-based decision-making for clinicians and policymakers, helping to optimize treatment protocols and promote more equitable access to effective cancer therapies across different regions in China.
Supplementary Information
Acknowledgements
I certify that no individuals other than the listed co-authors contributed to this publication.
Abbreviations
- BrMs
Brain metastases
- EGFR
Epidermal growth factor receptor
- NSCLC
Non-small cell lung cancer
- iPFS
Progression-free survival
- PD
Progressive disease
- QALYs
Quality-adjusted life years
- ICER
Incremental cost-effectiveness ratio
- WTP
Willingness-to-pay
- GPP
Gefitinib plus chemotherapy
- GM
Gefitinib monotherapy
- TKIs
Tyrosine kinase inhibitors
- LYs
Life-years
- WBR
Whole-brain radiation therapy ()
- AEs
Adverse events
- iPFS
Intracranial PFS
- KM
Kaplan-Meier
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
- MRI
Magnetic resonance imaging
- BSC
Best supportive care
- DSA
Deterministic sensitivity analyses
- PSA
Probabilistic sensitivity analyses
- CEAC
Cost-effectiveness acceptability curve
- NHSA
National Healthcare Security Administration
Authors’ contributions
Z Z and Y Z were primarily responsible for writing the main manuscript. J D prepared the figures and table. All authors, including Y Z, L Z, L F, M C and Q L, reviewed the manuscript collectively.
Funding
This research was supported by the Henan Provincial Medical Science and Technology Research Program (Grant No. 202401006).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable, because our economic evaluation is based on a mathematical model analysis, and does not contain any studies with human participants or animals performed.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Q, Abdo R, Iosef C, Kaneko T, Cecchini M, Han VK, Li SS. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat Commun. 2022;13(1): 5983. 10.1038/s41467-022-33365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Kelly WJ, Shah NJ, Subramaniam DS. Management of brain metastases in epidermal growth factor receptor mutant non-small-cell lung cancer. Front Oncol. 2018;8: 208. 10.3389/fonc.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge M, Zhuang Y, Zhou X, Huang R, Liang X, Zhan Q. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol. 2017;135(2):413–8. 10.1007/s11060-017-2590-x. [DOI] [PubMed] [Google Scholar]
- 5.Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE, Huberman MS, Costa DB. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–11. Epub 2015 Feb 4. PMID: 25682925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195–9. 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 7.Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–7. 10.1016/j.lungcan.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 10.Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, Huang C, Wu G, Zhang L, Cheng Y, Hu C, Chen G, Zhang L, Liu X, Yan HH, Tan FL, Zhong W, Wu YL. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5(9):707–16. 10.1016/S2213-2600(17)30262-X. [DOI] [PubMed] [Google Scholar]
- 11.Hou X, Li M, Wu G, Feng W, Su J, Jiang H, Jiang G, Chen J, Zhang B, You Z, Liu Q, Chen L. Gefitinib plus chemotherapy vs gefitinib alone in untreated EGFR-mutant non-small cell lung cancer in patients with brain metastases: the GAP BRAIN open-label, randomized, multicenter, phase 3 study. JAMA Netw Open. 2023;6(2): e2255050. 10.1001/jamanetworkopen.2022.55050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese Pharmaceutical Association. Chinese Guidelines for Pharmacoeconomic Evaluations. (2020) Available at: https://www.zixin.com.cn/doc/4150052.pdf.
- 13.National Bureau Of Statistics Of China. China statistical yearbook 2022. Accessed November 14. 2022. http://www.stats.gov.cn
- 14.Guidelines Working Committee of Chinese society of Clinical Oncology. Guidelines of Chinese society of clinical oncology (CSCO) for non-small cell lung cancer [M] 2021 edition. Beijing: People’s Medical Publishing House; 2021. p. 201. [Google Scholar]
- 15.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. 10.1186/1471-2288-12-9. PMID: 22297116; PMCID: PMC3313891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6. 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Carlson JJ, Garrison LP, Ramsey SD, Veenstra DL. The potential clinical and economic outcomes of pharmacogenomic approaches to EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. Value Health. 2009;12(1):20–7. 10.1111/j.1524-4733.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Bai R, Qian L, Chen N, Zhao Y, Han F, Bai L, Li J, Yu Y, Cui J. Cost-effectiveness of icotinib versus whole-brain irradiation with or without chemotherapy in EGFR-mutant NSCLC patients with brain metastases. Asia Pac J Clin Oncol. 2021;17(2):e40-7. 10.1111/ajco.13291. [DOI] [PubMed] [Google Scholar]
- 19.Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195-203. 10.1111/ajco.12477. [DOI] [PubMed] [Google Scholar]
- 20.China’s health industry data platform. Bid winning information of drugs. Accessed September 14. 2021. https://www.yaozh.com/
- 21.Liu Q, Zhou Z, Luo X, Yi L, Peng L, Wan X, Tan C, Zeng X. First-line ICI monotherapies for advanced non-small-cell lung cancer patients with PD-L1 of at least 50%: a cost-effectiveness analysis. Front Pharmacol. 2021;12: 788569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Liu Q, Zhou Z, Yi L, Peng L, Wan X, Zeng X, Tan C, Li S. Cost-effectiveness of bevacizumab biosimilar LY01008 combined with chemotherapy as first-line treatment for Chinese patients with advanced or recurrent nonsquamous non-small cell lung cancer. Front Pharmacol. 2022;13: 832215. 10.3389/fphar.2022.832215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Zhou Z, Zeng X, Liu Q. The cost-effectiveness of Tislelizumab plus chemotherapy for locally advanced or metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. 2022;13:935581. 10.3389/fphar.2022.935581. PMID: 35935852; PMCID: PMC9354466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32(5):722–32. 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 25.National Healthcare Security Administration. Dynamic deepening of medical insurance reform of drugs and high value medical consumables centralized procurement progress of the state Council policy routine briefing nhsa.gov.cn (Accessed 23 sep 2022).
- 26.International Agency for Research on Cancer. Cancer Fact Sheets [Internet]. 2020 Dec 18 [cited 2023 May 5]. Available from: https://gco.iarc.fr/today/en/dataviz/tables?mode=population.
- 27.Pan F. Comprehensive management is needed to reduce the incidence and mortality of lung Cancer - An interview with professor Li danqing, director of thoracic surgery, Peking union medical college hospital. Chin J Med Gui. 2021;18(20):1-3. 10.20047/j.issn1673-7210.2021.20.001.
- 28.Shi YK, Sun Y, Yu JM, Ding CM, Ma ZY, Wang ZP, Wang D, Wang Z, Wang MZ, Wang Y, Lu Y, Ai B, Feng JF, Liu YP, Liu XQ, Liu JW, Wu G, Qu BL, Li XJ, Li EX, Li W, Song Y, Chen GY, Chen ZT, Chen J, Yu P, Wu N, Wu ML, Xiao WH, Xiao JP, Zhang L, Zhang Y, Zhang YP, Zhang SC, Song X, Luo RC, Zhou CC, Zhou ZM, Zhao Q, Hu CP, Hu Y, Nie LG, Guo QS, Chang JH, Huang C, Han BH, Li G, Huang YW, Shi YW. Chinese expert consensus on the diagnosis and treatment of brain metastases in lung cancer (2017 Version). Chin J Lung Cancer. 2017;20(1):1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.