Abstract
Mitochondrial and extracellular vesicles (EV) transplantation have emerged as promising therapeutic strategies targeting mitochondrial dysfunction, a central feature of numerous pathologies. This review synthesizes preclinical data on artificial mitochondrial and EV transfer, emphasizing their therapeutic potential and underlying mechanisms. A systematic analysis of 123 animal studies revealed consistent benefits across diverse models, including ischemia-reperfusion injury (IRI), neurological disorders, drug-induced toxicities, and sepsis. Mitochondrial transfer improved organ function, reduced inflammation and apoptosis, and enhanced survival. Mechanistic insights revealed restored bioenergetics, increased oxidative phosphorylation, redox balance through activation of specific pathways, and modulation of mitochondrial dynamics via fusion/fission proteins. Mitochondrial homeostasis was supported through elevated mitophagy and biogenesis, alongside the preservation of mitochondrial-associated membranes. EV demonstrated similar effects, offering a potentially more targeted therapeutic alternative. Although pre-clinical studies have demonstrated safety and feasibility, broader application is limited by variability in isolation methods, lack of mechanistic clarity, and minimal human data. Standardization and mechanistic validation are critical to advance clinical translation. This review underscores the therapeutic promise of mitochondrial and EV transfer while highlighting the need for continued research to refine these interventions and unlock their full potential in regenerative medicine.
Keywords: Mitochondrial transplantation, Artificial mitochondrial transfer, Treatment, Therapeutic, Pre-clinical data, Extracellular vesicles, Microvesicles
1. Introduction: mitochondrial and extracellular vesicles (EV) transfer mechanisms and therapeutic potential
Mitochondria play a central role in maintaining cellular homeostasis by regulating energy production, redox signaling, apoptosis, and immunomodulation. As the primary producers of adenosine tri-phosphate (ATP) through oxidative phosphorylation (OXPHOS), they are essential for cell viability and function. Beyond their role in energy metabolism, mitochondria interact closely with various cellular processes, including metabolic regulation, stress response, and inflammation modulation [1]. Their involvement in a wide range of pathologies, from neurodegenerative and cardiovascular diseases to cancer and metabolic disorders, underscores the importance of maintaining optimal mitochondrial function.
Recent studies have revealed that mitochondrial transfer between cells constitutes a natural biological mechanism that helps preserve cellular homeostasis and promote survival in response to environmental stress [2]. This transfer can occur through various mechanisms, including tunneling nanotubes (TNTs) [3]. For instance, mesenchymal stem cells (MSCs) have been shown to transfer functional mitochondria to damaged pulmonary epithelial cells, thereby enhancing their metabolic function and resistance to oxidative damage [4,5]. On the other hand, transfer of non-functional mitochondria from macrophages to cancer cells promoted their proliferation [6]. These observations suggest that inter-cellular mitochondrial transfer is an adaptive process aimed at restoring bioenergetic function and mitigating the deleterious effects of cellular injury.
Inspired by these endogenous biological mechanisms, therapeutic approaches based on artificial mitochondrial transfer have been investigated [7]. The concept of mitochondrial transplantation has emerged as an innovative strategy to restore mitochondrial function and improve cell viability in various pathological contexts. This approach is based on the hypothesis that healthy mitochondria, isolated from donor cells, can be introduced into recipient cells to enhance their bioenergetic capacity and stress resistance. Several studies suggest that transplanted mitochondria can be internalized by target cells, integrated into the endogenous mitochondrial network, and restore deficient metabolism [8–10]. Two main mitochondrial transplantation strategies are currently being explored: (i) direct administration of isolated mitochondria and (ii) the use of extracellular vesicles (EV), which represent a more physiological and potentially more targeted approach.
EV are lipid bound vesicles released by all cell types and play a crucial role in tissue crosstalk by transporting and delivering bioactive molecules such as proteins and RNA that contribute to intracellular signaling pathways and regulate functions in recipient cells [11,12]. They encompass various subtypes based on size and biogenesis pathway. In the last decade according to the different versions of the Minimal Information for Studies of Extracellular Vesicles (MISEV) [13,14] the EV can generally be divided into 2 classes based on size: small EV, described as <200 nm in diameter and large EV, with a diameter >200 nm. Moreover, there are also other classifications based on the biogenesis pathway: exosomes (30–150 nm) formed through the endosomal pathway, within multivesicular bodies (MVBs), which are then released into the extracellular space upon fusion of MVBs with the plasma membrane, microvesicles (100–1000 nm), formed by the outward budding and fission of the plasma membrane, apoptotic bodies (>1000 nm), formed during programmed cell death (apoptosis) through cell fragmentation, where the dying cell undergoes membrane blebbing and breaks into membrane-bound vesicles containing cytoplasmic contents, organelles, and nuclear fragments [14].
Recently another 2 classes of EV (not yet discussed in the MISEV 2023) were described: mitochondria derived vesicles (MDV) and the mitochondria-EV (mito-EV). MDV are small (~70–150 nm) vesicles that shuttle mitochondrial constituents to other organelles, through the sorting of mitochondrial components via two different pathways. The first pathway involves the delivery of mitochondrial material to EV through sorting nexin 9 (SNX9)-dependent MDVs. In the second pathway, oxidative stress induces the production of MDVs containing damaged mitochondrial components, targeted to lysosomes for degradation [15,16]. Mito-EV are a broader and functionally distinct category encompassing all EV subtypes—small-EV, large EV, exosomes and microvesicles —that carry mitochondrial cargo, including mitochondrial proteins, mtDNA, or even whole mitochondria [17]. Mito-EV originate from various biogenesis routes, including MDV–endolysosomal fusion, direct plasma membrane budding, and exocytosis of mito-lysosomes [17]. They are actively secreted and taken up by recipient cells, where they can modulate metabolism, promote survival, and activate signaling pathways. These EV have been isolated from many cell types and bodily fluids, reflecting great heterogeneity in size (tens of nm to μm) and composition [18]. Mito-EV can carry either intact mitochondria or selected mitochondrial components [19]. Despite these 2 new EV categories, in literature the main papers investigating the effects of EV on mitochondrial function didn’t explicitly isolate and characterize the EV as MDV or mito-EV as there is still not precise guidance. Moreover, the literature has shown that all EV subtypes can transfer intact mitochondria and other mitochondrial material between cells in response to stress conditions [20]. In particular, MSCs and macrophages can release EV containing functional mitochondria. These EV are then taken up by recipient cells, promoting their survival and metabolic function. Furthermore, electron microscopy and flow cytometry analyses have confirmed the presence of whole mitochondria or mitochondrial components (mtDNA, respiratory chain proteins) in EV [21–23]. It is likely that in the coming years we will be able to distinguish between MDVs and mito-EV among the many different types of EV. However, as the current literature still lacks the tools and criteria necessary to make this distinction reliably, in this review we refer more broadly to EV that carry mitochondrial cargo or exert mitochondrial-related effects.
These findings have paved the way for exploring EV as natural vectors for mitochondrial and mitochondrial related material transfer, with potential applications in cell therapy and regenerative medicine. The employment of EV represents a complementary and innovative approach. EV offers several advantages, including greater stability, more controlled biodistribution, and the potential for tissue-specific targeting due to their molecular signature. Furthermore, they can induce regenerative and adaptive responses in recipient cells by activating pathways involved in mitochondrial biogenesis and detoxification [17].
However, despite these advances, several challenges must be addressed before widespread clinical applications can be considered. Standardization of mitochondrial and EV isolation and characterization methods, understanding the mechanisms underlying their uptake and integration, and evaluating their immunogenicity and long-term safety remain critical areas for further investigation. Moreover, regulatory and ethical considerations must be considered to ensure the clinical translation of these emerging approaches.
The objective of this review is to report the therapeutic effects of mitochondrial and EV transfer in different disease models in preclinical studies and identify the related mechanisms (bioenergetics, redox signaling, organelle dynamics).
2. Materials & methods
Medline research was conducted via PubMed using the following search terms for mitochondria: [((Mitochondria OR mitochondrial) AND (Therapeutic OR supplementation OR transplantation OR enrichment OR treatment) AND (preclinical)) OR (mitotherapy) OR (mitoception) NOT biomarker]. For EV- the search term is [(“extracellular vesicles” OR microvesicles OR “extracellular vesicle” OR microvesicle) AND (mitochondria OR mitochondrial)) AND (treatment OR therapeutic OR transplantation OR supplementation)]. The search was conducted on October 10, 2024.
Only peer-reviewed papers in English investigating the effects in preclinical models were included. Studies examining the effects on cells, ex vivo organs, oocytes, and humans were excluded. For each study included in the analysis, the following parameters were recorded: first author, country of the first author, year of publication, animal model used, type of disease, source of mitochondria (cells or organs, and species), quality check of mitochondria after isolation (e.g., imaging, mitochondrial protein enrichment …), type of transplantation (xenogeneic, allogenic, autologous), the transfer parameters (dose of mitochondria, route of administration, site of administration), proof of mitochondrial transfer into host cells/organs (yes/no), and the study’s conclusions. For EV-mitochondria analysis, we also exclude paper where the EV isolation and characterization didn’t follow the MISEV statement [14]. A network visualization of author collaborations to identify the degree of independence of studies was also conducted. To identify the author networks, a Venn diagram was created using VOSviewer 1.6.20, and all authors appearing in at least two articles were included.
To evaluate potential bias in the studies included, we applied the SYRCLE risk of bias tool [24], specifically designed for animal research and adapted from the Cochrane Collaboration’s framework for assessing bias in randomized controlled trials. The assessment involved 10 criteria addressing six categories of bias: selection, performance, detection, attrition, reporting, and other potential sources of bias (incomplete report of the transfer parameters).
3. Results
3.1. Search results
Following a Medline search, 123 studies met the research criteria, with the first evidence of mitochondrial transplantation in animals published in 2013 and EV in 2017 (Fig. 1). The predominant experimental model used were rodents (52 %, n = 64 in mice and 41 %, n = 50 in rats), along with larger animals such as pigs (6 %, n = 7). The mitochondria were primarily sourced from organs (66 %, n = 69), whereas the EV were sourced exclusively from cells, leading predominantly to allogeneic (64 %, n = 93) or xenogeneic (29 %, n = 42) transplantations. Notably, 13 % (n = 13) of mitochondrial studies did not provide quality control data following mitochondrial isolation. Most studies used local administration (60 %, n = 68), although 13 % (n = 14) did not specify the method of delivery. Additionally, 22 % (n = 27) of studies did not report whether mitochondrial internalization into tissues or cells was confirmed (Fig. 2). Geographically, research on mitochondrial transplantation was predominantly led by teams in China (n = 50), followed by the USA (n = 23), then Iran (n = 11) and Taiwan (n = 10) (to note, 79 % of EV studies come from China, Fig. 2). This distribution was reflected in two key author clusters for mitochondria: one around Fu, Zang, and Su; and the other around McCully and Guarien (Fig. S1) with a high interconnection between the teams involved in the field; and one cluster for EV: one around Cai, Yang and Chen (Fig. S2) with poor interconnection between the teams involved in the field. Finally, the risk of bias of the studies was reported as supplementary data (Tables S1–7). It is worth noting that the methodological descriptions often lacked key details related to potential bias, including sample size calculations, random housing of animals, allocation concealment, and information on how incomplete data were handled during the study.
Fig. 1.

Prisma flow diagram.
Fig. 2.

General parameters of mitochondrial and EV transplantation-related studies
A. Animal models use for in vivo studies. B. Mitochondrial and EV sources. C. Type of transplantation. D. Administration root of mitochondria and EV. E. Proof of mitochondria and EV isolation. F. Proof of mitochondria and EV transfer. G. Geographical repartition of teams involved in mitochondrial transplantation.
3.2. Efficacy and safety of mitochondrial and EV transplantation on disease pathophysiology
3.2.1. Ischemia-reperfusion injury (IRI)
A total of 30 studies [25–54] explored the effects of mitochondrial transplantation on IRI (Table 1). Of these, 19 studies reported data on infarct size, with 84 % (16/19) demonstrating a reduction in infarct size (ranging from 12.6 % to 34.1 %) following transplantation. Among the three studies reporting negative outcomes, two used the lowest dose of mitochondria (104). The third study was not designed to assess the effect of mitochondrial transplantation alone, but rather to demonstrate the added benefit of combining mitochondria with CoQ10—a benefit that was successfully shown. Furthermore, the reduction in infarct size was correlated with improvements in organ function (e.g., cardiac, brain, muscle) and behavior. The reported clinical improvement also aligned with biological observations: decreased inflammatory markers (mainly TNFα, IL1β and IL6) and decreased apoptosis.
Table 1.
Mitochondrial effect on ischemia-reperfusion injury.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Bafadam 2024 [25] | Wistar rat IRI cardiac |
Pectoralis major Rat Allogenic |
Dose: 6*106 mito Administration: intraventricular Site: cardiac |
No effect on infarct size No effect on cardiac pressure and function |
↓ MMP (JC-1) ↓ cellular ROS (DCFHDA) |
| Boutonnet 2024 [26] | SWISS mice IRI limb |
Rectus muscle Mice Allogenic |
Dose: 106 mito Administration: IM Site: gastrocnemius |
No clinical data reported | ↑ mito quantity (VDAC) ↑ Ca2+ retention capacity ↑ anti-ox (GPX) ↓ fusion (Mfn2) |
| Salman 2024 [27] | C57BL/6J mice IRI stroke |
Liver Mice Allogenic |
Dose: 100 μg mito proteins Administration: intranasal |
↓ infarct size (≈33 % reduction vs. vehicle) ↑ motor coordination and cerebral oedema ↓ microglia and astrocyte activationα↑ inflammation (IL1β, NLRP3, NFκB, IgG) |
↑ mito biogenesis (SIRT1, PGC1α) No effect on autophagy (pAMPKα-Thr172) ↑ OXPHOS proteins (NDUF8, SDHB, UQCRC2, MITCO1) ↓ oxidative stress (NO, HNE) ↑ anti-ox (eNOS, SULT4A1) |
| Sun 2024 [28] | C57BL/6J mice IRI stroke |
Liver Mice Allogenic |
Dose: 107 mito Administration: IV Site: tail |
↓ infarct size (≈33 % reduction vs. vehicle) and mortality ↑ neurogenesis and inhibit pyroptosis ↓ anxious behavior, ↑ memory |
↑ ATP level ↓ cellular ROS (DCFHDA) ↑ fusion (Mfn2) |
| Wu 2024 [29] | Sprague Dawley rat IRI cardiac |
MSC cells Human Xenogeneic |
Dose: 5*109 mito Administration: oral |
↓ infarct size (≈5 % vs. 40 %) ↑ ejection fraction and fractional shortening ↓ apoptosis, ↓ gene neutrophil chemotaxis |
↑ ATP level ↓ mito swelling, disruption, cristae loss |
| Xu 2024 [30] | Sprague Dawley rat IRI global |
Gastrocnemius Rat Allogenic |
Dose: 5*108 mito Administration: IV Site: femoral |
↑ spatial memory ↓ cerebral oedema and S100β/NSE (cerebral injury marker) ↓ apoptosis |
↑ mito quantity (mitotracker green), ↓ oxidative stress marker (MDA) ↑ anti-ox (SOD) ↑ mitophagy (PINK1, Parkin) ↓ PTP opening |
| Liang 2023 [31] | C57B/6J mice IRI cardiac |
BM-MSC cells Human Xenogeneic |
Dose: 7.5*105 mito Administration: IM Site: cardiac |
↑ ejection fraction and fractional shortening ↓ fibrosis and apoptosis ↑ number of mature vessels |
↑ ATP level ↑ mito quantity (mitotracker green) |
| Mokhtari 2023 [32] | Wistar rat IRI cardiac |
Pectoralis major Rat Allogenic |
Dose: 2*105 mito Administration: intramyocardial Sites: 3 |
↑ ejection fraction and fractional shortening ↓ troponin I ↓ inflammation (TNFα, IL1β, IL6) |
↓LC3-II/LC3-I ratio |
| Norat 2023 [33] | C57BL/6J mice IRI stroke |
Gastrocnemius Mice Allogenic |
Dose: 200 μL mito preparation Administration: intra-arterial Site: cerebral |
↓ infarct size (≈34 % reduction vs. vehicle) ↑ cellular viability & ↓ apoptosis |
↑ ATP level |
| Sun 2023 [34] | C57BL/6J mice IRI cardiac |
Heart Mice Allogenic |
Dose: 7.5–10*104 mito Administration: IV Site: tail |
No effect on infarct size No effect on cardiac function (ejection/fractional shortening) No effect on apoptosis |
Not investigated |
| Zeng 2023 [35] | C57BL/6J mice IRI limb |
MSC cells Human Xenogeneic |
Doses: 107 mito Administration: IM Site: gastrocnemius |
↓ fibrosis ↓ apoptosis ↓ neurophil infiltrate |
↑ ATP level |
| Chen 2022 [36] | C57BL/6 mice IRI stroke |
Liver Mice Allogenic |
Dose: 2*107 mito Administration: injection Site: ischemic site |
↑ locomotion ↑ myelinization (FABP5/7) ↓ apoptosis |
↑ ATP level ↑ complex activity (I, II, III) No effect on complex activity (IV, V) |
| Doulamis 2022 [37] | Zucker diabetic fatty rat IRI cardiac |
Pectoralis major Rat (diabetes or not) Allogenic |
Dose: 106 mito Administration: intraventricular Sites: 6, cardiac |
↓ infarct size (7.0–5.3 % vs. 33.6 %) ↑ fractional shortening (more important if non-diabetes mitochondria) ↓ inflammation (IL1α/β, IL4, IL6, IL13, TNFα, IFNγ, CXCL1/2/10, CX3CL-1) |
↑ ATP level (more important if non-diabetes mitochondria) |
| Kubat 2021 [38] | Sprague Dawley rat IRI renal |
MSC cells Rat Allogenic |
Dose: 4*106 mito Administration: injection Site: renal cortex |
↓ apoptosis ↑ cellular proliferation No effect on tissue histology, creatinine and urea level |
↓ fission (DRP1) |
| Sun 2021 [39] | C56BL/6 mice IRI cardiac |
Cardiomyocyte cells/>2021Mice Allogenic |
Dose: 5*104 mito Administration: intraventricular Site: cardiac |
No effect on infarct size No effect on cardiac function (ejection/ fractional shortening) No effect on apoptosis |
↑ fusion (OPA1, Mfn1/2) ↑ mito biogenesis (TFAM) |
| Xie 2021 [40] | Sprague Dawley rat IRI stroke |
N2a cells Mice Xenogeneic |
Dose: 107 mito Administration: intra-arterial Site: carotid |
↓ infarct size (13.4 % vs. 26.0 %) ↑ neurological behavior |
↑ fusion (Mfn1, OPA1) |
| Blitzer 2020 [41] | Yorkshire pig IRI cardiac |
Pectoralis major Pig Autologous |
Dose: 109 mito Administration: intra-arterial Site: coronary |
↓ infarct size (7.4 % vs. 38.0 %) ↑ left ventricular pressure, ejection fraction |
Not investigated |
| Doulamis 2020 [42] | Yorkshire pig IRI renal |
Sternocleidomastoid Pig Autologous |
Dose: 109 mito Administration: intra-arterial Site: kidney |
↓ injury in the cortex, no effect on medulla ↓ creatinine and urea level No effect on ionogram ↓ inflammation (IL6) |
Not investigated |
| Guariento 2020 [43] | Yorkshire pig IRI cardiac |
Pectoralis major Pig Autologous |
Dose: 109 mito Administration: intra-arterial Site: coronary |
↓ infarct size (3.8–4.2 % vs. 37.9 %) ↑ ejection fraction, left anterior descending artery flow |
Not investigated |
| Jabbari 2020 [44] | Wistar rat IRI renal |
Pectoralis major Rat Allogenic |
Dose: 3*106 mito Administration: intra-arterial Site: kidney |
↓ apoptosis ↓ tubular necrosis ↓ creatinine and urea level |
Not investigated |
| Moskowitzova 2020 [45] | C57BL/6J mice IRI lung |
Gastrocnemius Mice Allogenic |
Doses: 108 mito injection in left pulmonary artery or 3*108 mito if ultrasonic nebulization | Same effect regarding doses/routes of administration ↓ histological tissue injury ↑ inspiration capacity, dynamic ↓ neutrophil infiltrate, apoptosis No effect on cytokines levels |
Not investigated |
| Nakamura 2020 [46] | C57BL/6 mice IRI stroke |
Placenta Mice Allogenic |
Dose: 100 μg mito proteins Administration: IV Site: not specified |
↓ infarct size (≈20 % reduction vs. vehicle) | Not investigated |
| Orfany 2020 [47] | C57BL/6J mice IRI limb |
Muscles Mice Allogenic |
Dose: 106–9/g of muscle Administration: IM Sites: 4 muscles in the limb |
↓ infarct size (69 % vs. 87 %, dose effect) ↑ percentage shared stance time ↓ stance factor ↓ apoptosis |
↓ ATP level (dose: 109) |
| Pourmohammadi-Bejarpasi 2020 [48] | Wistar rat IRI stroke |
UC-MSC cells Human Xenogeneic |
Dose: from 3*107 cells Administration: intraventricular Site: cerebral |
↓ infarct size (12 % vs. 31 %) Restore motor and balance neurobehavioral function Neuroprotective effect on cyto-architecture ↓ apoptosis, CPK, microglial activity, astrogliosis |
Not investigated |
| Shin 2019 [49] | Yorkshire pig IRI cardiac |
Pectoralis major Pig Autologous |
Dose: 109 mito Administration: intra-arterial Site: coronary |
↓ infarct size (7.3 % vs. 38.6 %) Restore contractile measures ↑ coronary blood flow |
↑ ATP level |
| Zhang 2019 [50] | Sprague Dawley rat IRI stroke |
Pectoralis major Rat Autologous |
Dose: 5*106 mito Administration: intraventricular Site: cerebral |
↓ infarct size (≈23 % vs. 35 %) ↓ oedema ↓ apoptosis ↑ neurogenesis |
↑ ATP level ↑ mito quantity (COX4) ↓ oxidative stress (MDA, 8-OHDG, NT) ↑ anti-ox (SOD, GSH) |
| Kaza 2017 [51] | Yorkshire pig IRI cardiac |
Pectoralis major Pig Autologous |
Dose: 9.9*106 mito Administration: sub endocardial Site: area at risk |
↓ infarct size (5.2 % vs. 13.0 %) ↓ creatine kinase and troponin level No effect on electrocardiogram parameters No effect on cytokines levels |
Not investigated |
| Huang 2016 [52] | Sprague Dawley rat IRI stroke |
BHK-21 cells Hamster Xenogeneic |
Doses: 75 μg intracerebral OR 750 μg in femoral artery expression in mito proteins | Better recovery if injection intracerebral than femoral artery ↓ infarct size (7.1 % cerebral vs. 12.6 arterial vs. 20.7 %) ↓ apoptosis |
↑ ALAMAR blue signal ↑ MTT assay signal |
| Lin 2013 [53] | Wistar rat IRI liver |
Liver Rat Allogenic |
Dose: 7.7*105 mito Administration: injection Site: spleen |
↓ histological tissue injuries ↓ apoptosis ↓ ALAT |
↓ oxidative stress (HNE) |
| Masuzawa 2013 [54] | New Zeeland White rabbit IRI cardiac |
Pectoralis major Rabbit Autologous |
Dose: 1.2*106 mito Administration: intracardiac Sites: 8 |
↓ infarct size (9.8 % vs. 36.6 %) ↓ apoptosis ↓ creatine kinase, troponin I No arrythmia or immune reaction after injection ↓ inflammation (TNFa, IL6, CRP) |
↑ cellular respiration |
8-OHDG: 8-hydroxyl-2′-deoxyguanosine, ALAT: alanine aminotransferase, anti-ox: anti-oxidant; ATP: adenosine tri-phosphate, BHK21: baby hamster kidney cells-clone 21, BM-MSC: bone-marrow MSC, COX: cytochrome C oxidase, CPK: creatine phosphokinase, CRP: C reactive protein, CXCL: Chemokine (CXC motif) ligand, DCFHDA: 2′,7′-dichlorodihydrofluorescein diacetate, DRP1: dynamin-related protein 1, eNOS: endogenous endothelial nitric oxide synthase, FABP: fatty acid-binding protein, GPX: glutathione peroxidase, GSH: reduced glutathione, HNE: 4-hydroxy-2-nonenal, IFN: inter-feron, IgG: immunoglobulin G, IL: interleukin, IM: intramuscular, IRI: ischemia-reperfusion injury, IV: intra-veinous, JC1: 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, LC3: microtubule-associated protein light chain 3, MDA: malondialdehyde, Mfn: mitofusin, MITCO1: protein complex IV, mito: mitochondria, MMP: Mitochondrial membrane potential, MSC: mesenchymal stromal cell, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromure, N2A: Neuro-2A, NDUF8: protein complex I, NFκB: nuclear factor-kappa B, NLRP3: NOD-, LRR- and pyrin domain-containing protein 3, NO: nitric oxide, NSE: neuron specific enolase, NT: nitrotyrosine, OPA1: opticatrophy 1, OXPHOS: oxidative phosphorylation, PAMPK-α-Thr172: Phospho-AMPK-alpha-Threonine 172, PGC1α: Pparg coactivator 1 alpha, PINK1: PTEN induced kinase 1, PTP: permeability transition pore, ROS: relative oxygen species, S1000β: S100-beta protein, SDHB: protein complex II, SIRT1: sirtuin 1, SULT4A1: sulfotransferase 4A1, SOD: superoxide dismutase, TFAM: mitochondrial transcription factor A, TNFα: tumor necrosis factor α, UC-MSC: Umbilical-cord MSC, UQCRC2: protein complex III, VDAC: voltage-dependent anion channel, ↑: increase/improve, ↓: decrease, ≈: data have been estimated from the graphs.
A total of six studies [55–60] explored the effects of EV on IRI (Table 7). They all reported improvement of organ function (brain, heart, liver) but only one reported significant reduction of infarct size (54 %).
Table 7.
EV treatment paper.
| Author/Year | Model/Disease | EV source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Gai 2024 [55] | C57BL/6J Mice IRI Stroke |
MSC cells Mice Allogenic |
Dose: 100 μg of EV proteins Administration: intra-nasal |
↓ hypoxia-ischemia-induced injury ↓ neuronal damage ↓ infarct size (≈40 % reduction HI-EV vs. HI, ≈20 % reduction H2S-EV vs HI-EV) |
↑ anti-ox (Nrf2, PARK7, PRDX1, PRDX2)
↑ mito quantity (mtDNA) ↑ fusion (mfn1 and mfn2 mRNA) |
| Wu 2023 [56] | Balb/c Mice IRI stroke |
Hypoxic neurons cells Mice Allogenic |
Dose: 108 EV Administration: stereotactic microinjection Sites: cortex or nasal drops |
↓ cerebral infarction volume (≈45 % vs. vehicle) ↑ neuronal neurite survival ↑ neuronal integrity ↑ neuroprotection |
↓ mitochondria-associated apoptosis |
| Ikeda 2021 [57] | C57BL/6 Mice IRI cardiac | Induced cardiomyocyte cells Human Xenogeneic | Dose: 108 EV Administration: Injection Site: peri-infarct region | ↑ ejection fraction (≈20 % vs. vehicle) ↑ restoration of intracellular bioenergetics and contractile property ↑ post-MI cardiac function improvement |
↑ mito biogenesis (PGC-1α) |
| Lu 2021 [58] | C57BL/6 Mice IRI liver |
UC-MSC cells Human Xenogeneic |
Dose: 109 EV Administration: IV Site: tail |
↓ liver IRI ↑ hepato-protection & ↓ liver damage ↓ NETs formation |
↑ fusion (Mfn1) ↓ oxidative stress (mtROS) |
| Zhao 2021 [59] | C57BL/6 Mouse IRI renal |
MSC cells Human and mouse Allogenic/Xenogeneic |
Dose: 2*107 EV Administration: IV Site: tail |
↓ kidney inflammation ↓ renal lesion formation |
↑ OXPHOS ↓ mtDNA and mitochondrial damage ↑ mito biogenesis (restoring TFAM protein and stabilizing the TFAM-mtDNA complex) |
| Yao 2019 [60] | Sprague-Dawley rats IRI liver |
UC-MSC cells Human Xenogeneic |
Dose: 10 mg/kg EV Administration: IV Site: tail |
↑ hepatoprotection ↓ liver damage ↓ mRNA levels of IL-1β, IL-6, TNF-α, C-C motif ligand 12, IFN-κ, and TLR4 |
↑anti-ox (MnSOD) ↓ respiratory burst ↓ ROS (CellRox Deep Red, mitosox) |
| Bao 2022 [108] | C57BL/6J Mice Sepsis associated coagulopathy |
Primary neutrophils cells Mice Allogenic |
Dose: 2*105 EV Administration: IV Site: tail |
↓ mortality 40 % with EV treatment vs ↓ mortality 20 % with vehicle treatment at 70 h ↑ neutrophil-mediated prevention of DIC ↑ antithrombotic function of neutrophils ↓ endothelial dysfunction ↓ DIC severity |
↑anti-ox (SOD2) mediated endothelial protection ↓ endothelial ROS accumulation |
| Zheng 2021 [109] | Sprague-Dawley Rat Sepsis |
MSC cells Rat Allogeneic |
Dose: 2*107 EV Administration: IV Site: tail |
↓ mortality (from 56 % to 25 %) ↓ intestinal barrier dysfunction |
↑ fusion (mfn2) ↑ mito biogenesis (PGC-1α) |
| Yao 2024 [117] | C57BL/6J Mice Wound healing |
ADSCs cells Human Xenogeneic |
Dose: 3*108 EV Administration: hydrogel MNP Site: skin |
↓ wound healing time (≈5 day less vs. vehicle) ↑ macrophage polarization (M2 subtype) |
↑ MMP ↑ ATP levels ↓ ROS (Cell Rox probe) |
| Zhuang 2024 [118] | C57BL/6 Mice Aging |
Metformin treated MSC cells Human Xenogeneic |
Dose: 1010 EV Administration: hydrogel wound beds Site: skin |
↑ aged skin repair ↓ cellular senescence |
↑ ATP production ↑ OXPHOS ↑ mitophagy (LC3 II) |
| Chen 2024 [119] | C57BL/6 Mice Aging |
Plasma Mice Allogenic |
Dose 36 μg of EV proteins Administration: IV Site: not specified |
↓ age-associated functional decline
↓ senescence ↑ tissue regeneration ↑ lifespan |
↑ mito biogenesis (PGC-1α) |
| Liang 2024 [140] | Sprague-Dawley Mice Erectile disfunction |
PC-12 cells Rat Xenogeneic |
Dose: 108 MVs Administration: IC Site: Corpus cavernosum |
↑ erectile function
↓ ferroptosis ↓ apoptosis |
↓ oxidative stress marker (MDA) |
| Shen 2024 [141] | C57BL/6 Mice Autoimmune Hepatitis |
MSC cells Human Xenogeneic |
Dose: 3*1010 MVs Administration: IP |
↓ liver injury & ↑ liver protection ↑ glycolysis inhibition ↓ CD4+ T-cell activation ↓ mRNA IFN-γ, TNF-α, and IL-2 |
↓ OXPHOS |
| Tolomeo 2024 [142] | Pig Graft quality transplant for IRI |
MSC cells Pig Allogenic |
Dose: 1011 MVs Administration: into the solution of heart perfusion machine |
↑ myocardial viability ↓ apoptosis ↓ IL-1ra, IL-2 and IL-6 |
↓ anti-ox (SOD, CAT, GPX) ↓ oxidative stress (carbonylated proteins) ↓ mitochondrial cristae loss ↓ mitochondrial swelling |
| Cao 2022 [143] | BALB/c Mice Solid tumors |
Dendritic cells Mice Allogenic |
Dose: 80 μg EV proteins Administration: IV Site: tail |
↓ tumor size (≈80 % reduction vs. vehicle) ↑ T cell activation ↑ immunogenic cell death in tumor cells ↑ immune responses against primary, distant tumors and metastases tumors ↑ efficient eradication of primary, distant and metastases tumors ↓ cancer stem cells |
Not investigated |
| Lu 2022 [144] | C57BL/6 Mice Cardiac hypertrophy |
Hypoxic MSC cells Human Xenogeneic |
Dose: 109 EV Administration: IV Site: tail |
↑ hepatoprotective effects ↓ NETs formation (suppressed by hUC-MSC-EV) ↓ liver IRI severity |
↓ mitochondrial ROS (mitosox) ↑ mitochondrial fusion in neutrophils (Mfn2) ↓ cytokine release from neutrophils (IFN-γ, IL-6 and TNF-α) |
| Li 2022 [145] | C57BL/6 Mice Liver Fibrosis |
Plasma Mice Allogenic |
Dose: 4*108 EV Administration: IV Site: tail |
↓ liver fibrosis progression ↑ collagen synthesis and degradation regulation ↓ HSC activation |
↑ anti-ox (GSH levels) ↓ ROS (CMXROS) |
| Dutra Silva 2021 [146] | C57BL/6 Mice ARDS |
BM-MSC cells Human Xenogeneic |
Dose: 98.3 μg of EV proteins Administration: IV Site: tail |
↑ barrier integrity ↑ barrier integrity with ARDS plasma ↓ LPS-induced inflammation |
↑ in vivo mitochondrial respiration (OCR) |
| Morrison 2017 [147] | C57BL/6 Mouse ARDS |
MDMs cells Human Xenogeneic |
Dose: not specified Administration: intra-nasal |
↑ expression of the M2 phenotype marker CD206 ↑ phagocytic capacity of macrophages ↑ protection in lung injury ↓ production of cytokines (TNF-α and IL-8) |
↓ OXPHOS |
ADSCS: adipose derived stem cells, anti-ox: anti-oxidant, ARDS: acute respiratory distress syndrome, BM-MSC: bone marrow MSC, CAT: catalase, DIC: disseminated intravascular coagulation, EV: extracellular vesicles, GPX: glutathione peroxidase, GSH: reduced glutathione, HI: hypoxia-ischemia, HypEV: EV release by cells under hypoxia, IC: intracavernous, IFN: interferon, IL: interleukin, IP: intraperitoneal, IRI: ischemia-reperfusion injury, IV: intravenous, LC3: microtubule-associated protein light chain 3, MDA: malondialdehyde, MDMs: monocyte derived macrophages, Mfn: mitofusin, mito: mithcondria, M2 phenotype: alternatively activated macrophages, MMP: mitochondrial membrane potential, MNP: microneedle patch, MSC: mesenchymal stromal cells, MV: microvesicles, NRF2: nuclear factor erythroid 2-related factor 2, OCR: oxygen consumption rate, OXPHOS: oxidative phosphorylation, PGC1-α: Pparg coactivator 1-alpha, PRDX: peroxiredoxin, ROS: reactive oxygen species, SOD: superoxide dismutase, TFAM: mitochondrial transcription factor A, TNFα: tumor necrosis factor alpha, UC-MSC: umbilical cord MSC, ↑: increase/ improve, ↓: decrease, ≈: data have been estimated from the graphs.
3.2.2. Neurological disorders
Mitochondrial transplantation was examined in 29 studies [61–89] on various neurological disorders, including spinal cord injury (n = 5), Parkinson’s disease (n = 4), stress/depression (n = 4), aging (n = 3), traumatic brain injury (n = 2), and other conditions (n = 11, Table 2). Notably, most of studies (22/23) reported positive clinical outcomes (motor and cognitive) aligning with biological observation: improvement of neurogenesis, inflammation decrease, regulation of microglial and astrocyte proliferation, and apoptosis decrease. However, six studies focused exclusively on biological effects (e.g., apoptosis, cell differentiation & axonal flow) without evaluating clinical outcomes, and one study found no clinical benefit in spinal cord injury, which was potentially linked to the source of mitochondria (PC12 cells and soleus muscle). No EV studies were found in the search.
Table 2.
Mitochondrial transplantation effects on neurologic disorders.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Eo 2024 [61] | C57BL/6 mice Parkinson’s disease |
UC-MSC cells Human Xenogeneic |
Doses: 0.5–10 μg mito proteins Administration: IV Site: not specified |
↑ behavioral (dose effect) ↓ neurological damage (only 10 μg tested) ↓ neuro-inflammation (TNFα, IL6) (only 10 μg tested) |
Not investigated |
| Jain 2024 [62] | C57BL/6 mice Parkinson’s disease |
Liver Mice Allogenic |
Dose: 0.5 mg/kg Administration: IV Site: not specified |
↑ locomotor activity | ↑ OXPHOS prot expression (NDUFB8, SDHB, UQCRC2, MTCO1, vATP5α) ↑ mito biogenesis (PGC1a, TFAM) |
| Xu 2024 [63] | C57BL/6 mice Spinal cord injury |
Macrophages cells Mice Allogenic |
Dose: from 107 cells, engineered mitochondria Administration: IV Site: tail |
↑ functional recovery ↑ motor coordination ↑ phagocytosis of myelin debris ↑ tissue repair ↓ apoptosis |
↑ ATP production ↑ mito quantity (mitotracker) ↑ basal & max intensity ↓ cellular ROS (DCFHDA) |
| Bamshad 2023 [64] | Wistar rat Traumatic brain injury |
UC-MSC cells Human Xenogeneic |
Dose: 3*107 mito Administration: intraventricular Site: cerebral |
↑ sensitivity recovery ↓ brain tissue destruction ↑ neurogenesis ↓ apoptosis, CPK, microglial activation, astrogliosis |
Not investigated |
| Ene 2023 [65] | Wistar rat Schizophrenia |
Brain Rat Allogenic |
Dose: 100 μg mito proteins Administration: intracerebral Site: prefrontal cortex |
↓ schizophrenia after development in sick rats, neurological impairment in healthy rats No effect on inflammation (IL1 p) |
↑ COX activity ↓ Cellular ROS (DCFHDA) |
| Hayashida 2023 [66] | Sprague Dawley rat Cardiac arrest neurotoxicity |
Brain Rat Allogenic |
Dose: 500 μL of mito preparation (fresh or fractioned) Administration: IV Site: not specified |
Results obtained only for fresh mito ↑ survival at day 3 (90.9 % vs. 54.5 %) ↑ neurological function and microperfusion cerebral ↓ cerebral oedema and lactate |
Brain: ↓ fusion (Mfn1/2), no effect on OPA1 No effect on fission (DRP1, Fis1) Spleen: ↑ fission (DRP1), no effect on Fis1 No effect on fusion (Mfn1/2, OPA1) |
| Huang 2023 [67] | Sprague Dawley rat Neuropathic pain |
Soleus, pectoralis major, biceps, gastrocnemius, abdominal muscles Rat Allogenic |
Dose: 100 μg mito proteins Administration: intracerebral Site: ganglion dorsal root |
↓ pain hyper-sensibility (mechanical, thermal) ↓ activation of glial cells ↓ neuro-inflammation (TNFα, IL1β, IL6, NFκB) ↓ apoptosis |
Not investigated |
| Javani 2023 [68] | Wistar rat Chronic stress |
Brain Rat Allogenic |
Dose: 100 μg mito proteins Administration: intraventricular Site: cerebral |
↓ apoptosis | ↑ ATP level ↑ MMP (JC-1) |
| Javani 2023 [69] | Wistar rat Chronic stress |
Brain Rat Allogenic |
Dose: 100 μg mito proteins Administration: intraventricular Site: cerebral |
↓ stress-induced spatial learning and memory losses ↑ neuronal dendritic morphology |
↓ oxidative stress (MDA) |
| Jia 2023 [70] | C57BL/6J mice Epilepsia |
Hippocampus Mice Allogenic |
Dose: 1 mg/kg Administration: IV Site: tail |
Improve cognitive impairment, epileptic depression and anxiety state ↓ microglial and astrocytes proliferation ↑ neurodegeneration |
Not investigated |
| Yang 2023 [71] | C57BL/6 mice Alzheimer’s disease |
Brain Mice Allogenic |
Dose: 3*106 mito Administration IV Site: caudal |
↑ cognitive ability | ↑ ATP/ADP ratio ↓ oxidative stress (MDA) ↑ anti-ox (GSH, MAPK and FOXO) ↑ complex activity (I, IV) ↑ autophagy (FOXO3, BNIP3, LC3II/LC3I) ↑ mito biogenesis (ERK, SIRT1) |
| Zhu 2023 [72] | Sprague Dawley rat Spinal cord injury |
Platelet cells Rat Allogenic |
Dose: 3*105 mito Administration: intracerebral |
No clinical data reported ↑ neuron quantity and myelin ↓ apoptosis |
↑ ATP level ↑ proportion of elongated mito ↑ complex activity (I, II, III, IV, V) ↑ anti-ox (Nrf2, HO-1, NQO1, SOD) ↓ oxidative stress markers (Nox2, MDA, NT, 8-OHDG) |
| Adlimoghaddam 2022 [73] | C57BL/6 mice Aging and hippocampal function |
Liver Mice Allogenic |
Dose: 10–20 mg/kg Administration: IV Site: not specified |
No dose effect No clinical data reported |
↑ OXPHOS (CII-SDHB) |
| Hosseini 2022 [74] | C57BL/6 mice Cognitive impairment post stroke |
BM-MSC cells Mice Allogenic |
Doses: 85/170/340 μg mito proteins Administration: intranasal |
↑ social interaction (only 340 μg) ↓ cognitive impairment ↓ loss of synaptic protein |
↑ ATP level ↑ MMP (JC-1) ↓ cellular ROS (DCFHDA) |
| Javani 2022 [75] | Wistar rat Depression |
Brain Rat Allogenic |
Dose: 100 μg mito proteins Administration: intraventricular Site: cerebral |
↓ anxiety ↓ depression-like behavior |
↑ ATP level ↑ MMP (JC-1) |
| Lin 2022 [76] | Sprague Dawley rat Spinal cord injury |
Soleus Rat Allogenic |
Dose: 50 μg mito proteins Administration: injection Site: spinal cord |
↑ sensitivity and motor function ↓ demyelinization ↓ inflammation (TNFα, IL6) |
↓ fission (DRP1) ↓ oxidative stress markers ↓NO & 3NT |
| Zhang 2022 [77] | TOPGAL mice Age-related cognitive decline |
Liver Mice Allogenic |
Dose: 1.5–2*106 mito Administration: injection Site: hippocamp |
↑ cognitive function | ↑ mito quantity (TOM20) ↑OXPHOS (complexes protein activity (I, II, IV)) |
| Fang 2021 [78] | Sprague Dawley rat Spinal cord injury |
Soleus Rat Allogenic |
Dose: 100 μg mito proteins Administration: IV Site: jugular |
↑ locomotor function and recovery ↓ apoptosis ↓ neuro-inflammation (TNFα, IL6) |
Not investigated |
| Sheu 2021 [79] | Sprague Dawley rat Sciatic crush injury |
BHK-21 cells Hamster Xenogeneic |
Dose: 195 μg mito proteins Administration: IM Site: gastrocnemius |
↑ nerve regeneration ↑ speed axonal flow Restore nerve and muscle morphology |
Not investigated |
| Zhao 2021 [80] | C57BL mice Traumatic brain injury |
Liver Mice Allogenic |
Dose: 1.2–1.4*106 mito Administration: intracerebral |
↓ anxiety ↑ cognitive function ↑ spatial memory ↓ apoptosis |
Not investigated |
| Ma 2020 [81] | db/db mice DT2 cognitive impairment |
Platelet cells Rat Xenogeneic |
Dose: 105 mito Administration: intraventricular Site: cerebral |
↑ cognitive function ↓ apoptosis ↓ β amyloid deposit No effect on glucose level and weight |
↑ ATP level ↑ mito quantity (mitotracker) ↓ damage mito ↑ CI, III, IV, V activity, no effect on CII ↓ oxidative stress (H2O2, MDA, NT, 8-OHDG) ↑ anti-ox (total antioxidant capacity expressed in Trolox equivalent) |
| Nascimento-Dos-Santos 2020 [82] | Lister Hooded rat Optic nerve injury |
Liver Rat Allogenic |
Dose: 1.25 μg mito proteins (fresh or fractioned) Administration: injection Site: intravitreal |
Results obtained for both (fresh, fractioned mito) Promote neuroprotection ↑ number of axons ↑ retinal electrophysiological |
Not investigated |
| Zhao 2020 [83] | BALB/c mice Cognitive motor impairment in aging |
Liver Mice Allogenic |
Dose: 5 mg/kg Administration: IV Site: tail |
↑ age-related behaviors ↑ motor performance ↑ macrophages phagocytosis |
↑ ATP level ↓ oxidative stress (MDA) ↑ anti-ox (GSH) ↑ pyruvate DH, α-ketoglutarate DH, NADH |
| Nitzan 2019 [84] | C57BL/6 mice Alzheimer’s disease |
HeLa cells Human Xenogeneic |
Dose: 200 μg mito proteins Administration: IV Site: tail |
↑ cognitive function ↑ neuronal disarrangement |
↑ citrate synthase activity ↑ COX activity |
| Wang 2019 [85] | ICR mice Depression |
Hippocampus Mice Allogenic |
Dose: 1 mg/kg Administration: IV Site: not specified |
↑ depression-like behavior ↑ neurogenesis ↓ neuro-inflammation (IL1β, TNFα, COX-2) |
↑ ATP level J oxidative stress (DCFHDA, MDA) ↑ anti-ox (SOD) |
| Gollihue 2018 [86] | Sprague Dawley rat Spinal cord injury |
PC12Adh/soleus Rat Allogenic |
Dose: 100 μg mito proteins Administration: injection Site: spinal cord |
No comparison between cell sources No effect on locomotor and sensory threshold ↓ number of neurons |
No effect on RCR |
| Robicsek 2018 [87] | Wistar rat Schizophrenia |
EBV transform lymphocyte/ brain Human/Rat Xenogeneic/Allogenic |
Dose: 100 μg mito proteins Administration: injection Sites: 3, pre-frontal cortex |
No comparison between cell sources ↑ neuronal differentiation Restore neuronal function Restore mitochondrial membrane potential |
↑ MMP (JC-1) |
| Shi 2017 [88] | C57BL/6J mice Parkinson’s disease |
HepG2 cells Human Xenogeneic |
Dose: 0.5 mg/kg Administration: IV Site: tail |
↑ motor activity | ↑ ATP level ↑ OXPHOS (complex I activity) ↓ oxidative stress (ROS) ↑ anti-ox (GSH) |
| Chang 2016 [89] | Sprague Dawley rat Parkinson’s disease |
PC12 cells Rat Allogenic |
Dose: 1.05 μg mito proteins Administration: injection Site: medial forebrain bundle |
↑ mobility ↓ dopaminergic neurons deterioration |
↓ OXPHOS (CI, CIII and IV activity activity) ↑ fusion (Mfn2) ↑ fission (Drp1) ↑ mitophagy (Parkin) ↓ oxidative stress (8-OHDG) |
8-OHDG: 8-hydroxyl-2′-deoxyguanosine, anti-ox: anti-oxidant; ATP: adenosine tri-phosphate, BM-MSC: bone-marrow mesenchymal stromal cell, BNIP3: Bcl-2/ adenovirus E1B-interacting protein 3, CII-SDHB: complex II - succinate dehydrogenase subunit B, COX: cytochrome C oxidase, CPK: creatine phosphokinase, DCFHDA: 2′,7′-dichlorodihydrofluorescein diacetate, DRP1: dynamin-related protein 1, EBV: Epstein-Barr virus, ERK: extracellular signal-regulated kinase, Fis1: mitochondrial fission protein 1, FOXO: forkhead box O, GSH: reduced glutathione, HO-1: heme oxygenase-1, IL: interleukin, IM: intra-muscular, IV: intra-veinous, JC1: 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, LC3: microtubule-associated protein light chain 3, MAPK: mitogen-activated protein kinase, MDA: malondialdehyde, Mfn: mitofusin, mito: mitochondria, MMP: Mitochondrial membrane potential, NDUF8: protein complex I, MTCO1: protein complex IV, NFκB: nuclear factor kappa B, NO: nitric oxide, NOX2: NADPH oxidase 2, NRF2: nuclear factor erythroid 2, NQO1: NAD(P)H:quinone oxidoreductase 1, NT: nitrotyrosine, OPA1: optic atrophy 1, OXPHOS: oxidative phosphorylation, PGC1α: Pparg coactivator 1 alpha, RCR: Respiratory control ratio, ROS: relative oxygen species, SDHB: protein complex II, SIRT1: sirtuine 1, SOD: superoxide dismutase, TFAM: mitochondrial transcription factor A, TOM: translocase of the outer membrane, TNFα: tumor necrosis factor α, Trolox: synthetic form of vitamin E, UC-MSC: Umbilical-cord MSC, UQCRC2: protein complex III, vATP5a: protein complex V, ↑: increase/ improve, ↓: decrease, ≈: data have been estimated from the graphs.
3.2.3. Drug-related toxicity
Eleven studies [90–100] investigated the impact of mitochondrial transplantation on drug-related toxicity (Table 3), eight of which focused on chemotherapy agents (doxorubicin and cisplatin) known for their cardiotoxicity, neurotoxicity, and nephrotoxicity via oxidative stress. Mitochondrial transplantation showed significant potential in mitigating these drug-related toxicities by reducing inflammation and cell apoptosis. Additionally, it improved histological and biochemical markers of acetaminophen-induced toxicity. No EV studies were found in the search.
Table 3.
Mitochondrial transplantation effect to prevent drug-related toxicity.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Jin 2024 [90] | C57BL/6N mice Doxorubicin cardiotoxicity |
MSC cells Not specified |
Dose: 3 mg/kg Administration: IV Site: not specified |
Restore ejection fraction and fractional shortening ↓ fibrosis ↓ apoptosis |
↑ ATP level ↑ mito quantity (image) ↑ anti-ox (mRNA Sdhaf2/4) ↓ autophagy (mRNA LC3BII, p62) ↓ autophagosomes (inhibit AMPK/mTOR pathway) |
| Kim 2024 [91] | Sprague Dawley rat Dexamethasone related amyotrophy |
UC-MSC cells Human Xenogeneic |
Doses: 0.5–5 μg/limb Administration: IM Site: soleus |
Positive effect with 5 μg Recover muscle mass, fiber area, desmin, myogenin ↓ lactate |
Act AMPK/AKT/Foxo pathway (↑Akt, ↓ FOXO3, ↓ MuRF-1, ↓ MAFbx) ↑ OXPHOS (CI, II, IV, V activity) ↑ mito biosynthesis (PGC-1, TFAM) |
| Kubat 2024 [92] | Sprague Dawley rat Doxorubicin muscular toxicity |
Femoris Rat Allogenic |
Dose: 6.5 μg mito proteins Administration: IM Sites: 3 tibialis |
No protective effect No effect on apoptosis No effect on inflammation |
↑ anti-ox (succinate DH, SOD) |
| Maia 2023 [93] | SWISS mice Oxaliplatin neurotoxicity |
Liver Mice Allogenic |
Dose: 0.5 mg/kg Administration: IV Site: retro-orbital |
↓ cold mechanical and sensitive alteration ↓ neuro-inflammation (TNFα, TLR4) |
Not investigated |
| Maleki 2023 [94] | Wistar rat Doxorubicin cardiotoxicity |
Liver Rat Allogenic |
Dose: 8*109 mito Administration: IV Site: tail |
Restore ejection fraction and fractional shortening ↓ apoptosis ↓ inflammation (TNFα, IL1β, IL6) |
↑ ATP level ↓ oxidative stress markers (MDA) ↑ anti-ox (SOD, CAT, GPX, GSH) |
| Sun 2023 [95] | C57BL/6 mice Doxorubicin cardiotoxicity |
Heart Mice Allogenic |
Dose: 105 mito Administration: intraventricular Site: cardiac |
↑ ejection fraction and fractional shortening ↓ apoptosis |
↓ Superoxide (mitosox probe) ↑ anti-ox (SOD2, OGG1) ↑ mito function (IDH2, citrate synthase) ↑ mitogenesis (PGC1-b) ↑ fusion (Opa1) ↑ OCR/ECAR ratio Act glutamine & glutamate metabolic pathway |
| Zhang 2023 [96] | C57BL/6 mice Doxorubicin cardiotoxicity |
Heart Mice Allogenic |
Dose: 5*104 mito Administration: intraventricular Site: cardiac |
↑ ejection fraction and fractional shortening | ↑ mito quantity (hs & ms mtDNA) ↓ intracellular ROS, ↑ mito ROS ↑ MMP ↑ baseline and max OCR |
| Alexander 2021 [97] | C57BL/6J mice Cisplatin neurotoxicity |
MSC cells Human Xenogeneic |
Dose: 170 μg mito proteins Administration: intra-nasal | ↑ executive functioning Restore spatial/working memory Restore synaptic damage |
↓ atypical mito morphology ↓ synaptosomal mito damage ↑ anti-ox (Nrf2) |
| Kubat 2021 [98] | Sprague Dawley rat Doxorubicin nephrotoxicity |
MSC cells Rat Allogenic |
Dose: 4*106 mito Administration: injection Site: below kidney capsule | ↑ cellular regeneration ↓ apoptosis ↓ proteinuria |
↑ anti-ox (SOD, GPX) |
| Ulger 2021 [99] | Sprague Dawley rat Acetaminophen hepatotoxicity |
MSC cells Rat Allogenic |
Dose: 8.2*106 mito Administration: injection Site: subscapular spleen |
↓ histological alteration ↓ apoptosis and ALAT |
↓ anti-ox (GSH) |
| Shi 2018 [100] | Sprague Dawley rat Acetaminophen hepatotoxicity |
HepG2 cells Human Xenogeneic |
Dose: 10 mg/kg (fresh or fractioned) Administration: IV Site: tail |
Results obtained only for fresh mito ↓ histological alteration ↓ ALAT and ASAT |
↑ ATP level, only for intact mito ↑ anti-ox (GSH), only for intact mito ↓ ROS, only for intact mito |
Akt: protein kinase B, ALAT: alanine aminotransferase, AMPK: AMP-activated protein kinase, anti-ox: anti-oxidant; ASAT: aspartate aminotransferase, ATP: adenosine tri-phosphate, CAT: catalase, ECAR: extracellular acidification rate, FOXO: forkhead box O, GPX: glutathione peroxidase, GSH: Reduced glutathione, IDH2: isocitrate dehydrogenase 2, IL: interleukin, IM: intra-muscular, IV: intra-veinous, LC3: microtubule-associated protein light chain 3, mito: mitochondria, MAFbx: muscle atrophy F-box, MDA: malondialdehyde, MMP: Mitochondrial membrane potential, MSC: mesenchymal stromal cell, mTOR: mammalian target of rapamycin, MuRF-1: muscle RING finger protein 1, NRF2: nuclear factor erythroid 2, OCR: oxygen consumption rate, OGG1: 8-oxoguanine DNA glycosylase 1, OPA1: optic atrophy 1, OXPHOS: oxidative phosphorylation, PGC1b: Pparg coactivator 1-b, ROS: relative oxygen species, Sdhaf: Succinate dehydrogenase assembly factor 1, SOD: superoxide dismutase, TFAM: mitochondrial transcription factor A, TLR4: Toll-like receptor 4, TNFα: tumor necrosis factor α, UC-MSC: Umbilical-cord MSC, ↑: increase/improve, ↓: decrease.
3.2.4. Sepsis-related outcomes
Seven studies [101–107] investigated the role of mitochondrial transplantation in sepsis (Table 4). Five of these studies reported survival rates with an improvement ranging from 21 % to 40 %, accompanied by reduced biological markers of inflammation and enhanced bacterial clearance. Two studies [108,109] investigated the role of EV in sepsis (Table 7), one showing improvement of survival rate and the other one a decrease in intestinal barrier dysfunction, both associated with improvement in inflammatory markers.
Table 4.
Mitochondrial transplantation effect on sepsis.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Kim 2023 [101] | Sprague Dawley rat Sepsis |
L6 cells Rat Allogenic |
Dose: 200 μg mito proteins Administration: IV Site: tail |
No clinical data reported ↑ expression of RT1-m2 and Cbln2 |
Not investigated |
| Kim 2023 [102] | Sprague Dawley rat Sepsis |
L6, clone 9, UC-MSC cells Rat Allogenic |
Dose: 200 μg mito proteins Administration: IV Site: tail |
↑ survival rate at day 12 (40 % vs. 0 % only L6 mitochondria) ↓ creatinine and ALAT levels |
Not investigated in vivo In vitro: ↑ basal & max respiration ↑ ATP production ↑ proton leak ↑ non mito oxygen consumption |
| Mokhtari 2023 [103] | Wistar rat Sepsis |
Pectoralis major Rat Allogenic |
Dose: 3*106 mito Administration: IV Site: tail |
↑ survival rate at day 3 (50 % vs. 10 %) ↓ inflammation (TNFα, IL1β, IL6), LDH |
↓ cellular ROS (DCFHDA) ↑ mito biogenesis (SIRT1, PCG-1α) ↑ fusion: (Mfn2) ↓ fission (DRP1) |
| de Carvalho 2021 [104] | C57BL/6 mice Sepsis |
MSC cells Not specified |
Dose: from 3*106 cells Administration: IV Site: jugular |
↑ survival rate at day 2 (57 % vs. 36 %) ↓ bacteria load ↓ inflammation (IL1β), ↑IL10 |
Vascular endothelial cells: ↑basal & max respiration, Iuncoupled respiration Alveolar epithelial cells: ↑ max respiration, no effect on basal and uncoupled respiration |
| Hwang 2021 [105] | Sprague Dawley rat Sepsis |
UC-MSC cells Rat Allogenic |
Dose: 50 μg mito proteins Administration: IV Site: tail |
↑ survival rate at day 14 (50 % vs. 10 %) ↑ bacterial clearance and ↓ lactate |
↑ ATP level ↓ anti-ox (SOD2) ↑ ROS (DCFHDA) Restore O2 consumption spleen, muscle, no effect on liver, kidney, heart |
| Zhang 2021 [106] | C57BL/6 mice Sepsis |
Pectoralis major Mice Allogenic |
Dose: 2.5–3*107 mito Administration: IV Site: tail |
↑ survival rate at day 7 (75 % vs. 36.8 %) ↑ bacterial clearance, nitrate + nitrite ↓ organ injury ↓ inflammation (IL1β and IL6) ↓ apoptosis |
↓ OCR lung, liver, kidney, no effect on brain |
| Yan 2020 [107] | C57BL/6 mice Sepsis |
Pectoralis major Mice Allogenic |
Dose: 4*106 mito Administration: intraventricular Site: cerebral |
No effect on mortality, mortice and explorative function ↑ memory retention performance ↑ cognitive impairment ↓ inflammation (TNFα, IL6, IL1β, TGFβ, IL4), no effect on IL10 ↓ apoptosis |
↓ oxidative stress (iNOS) |
ALAT: alanine aminotransferase, ATP: adenosine tri-phosphate, Cbln-2: cerebellin 2, DCFHDA: 2′,7′-dichlorodihydrofluorescein diacetate, DRP1: dynamin-related protein 1, IL: interleukin, iNOS: inducible nitric oxide synthase, IV: intra-veinous, LDH: lactate dehydrogenase, Mfn: mitofusin, mito: mitochondria, MSC: mesenchymal stromal cell, OCR: oxygen consumption rate, PGC1-α: Pparg coactivator 1-alpha, ROS: relative oxygen species, RT1-m2: major histocompatibility complex gene, SOD: superoxide dismutase, SIRT1: sirtuine 1, TGFβ: transforming growth factor beta, TNFα: tumor necrosis factor α, UC-MSC: Umbilical-cord MSC, ↑: increase/ improve, ↓: decrease.
3.2.5. Wound healing and aging
Five studies [110–116] reported the role of mitochondrial transplantation and three [117–119] the role of EV in the aging and healing process (Tables 5 and 7). They show a consistent increase in cell proliferation associated with a decrease in apoptosis, and improvement in related clinical investigation (i.e., healing, hair growth, muscular function).
Table 5.
Mitochondrial transplantation effect on wound healing and aging.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Arroum 2024 [110] | C57BL/6J mice Aging and muscular function |
Hind limbs Mice Allogenic |
Dose: 109 mito Administration: IM Sites: 3 |
↑ maximal speed and distance | ↑ ATP level ↑ COX activity Plantaris: ↑parkin, TFAM, BNIP3, Drp1 Soleus: ↑parkin, DRP1, no effect TFAM BNIP3 Femoris: No effect |
| Arroum 2024 [111] | Rat Aging and muscular function |
Femoris Rat Allogenic |
Dose: 109 mito Administration: IM Sites: 3 |
No effect on speed and distance | ↑ ATP level ↑ COX activity Plantaris and soleus: ↑ fusion (OPA1), ↑ mitochondrial homeostasis (Parkin, TFAM) |
| Li 2024 [112] | Kunming mice Burn injury |
Liver Mice Allogenic |
Dose: 10 μg/g Administration: SC Site: not specified |
↑ healing regeneration, ↓ scarring ↓ apoptosis ↓ inflammation (TNFα, IL1β, IL6) ↑ IL10 |
Not investigated |
| Raz 2024 [113] | C57BL/6 mice Corneal wound healing |
Liver Mice Allogenic |
Dose: 25 μL mito preparation (fresh or fractioned) Administration: drop Site: eyes |
↑ re-epithelialization and better cyto-architecture ↓ cellular infiltration Only the fresh ↓ wound area |
↓ ATP level |
| Kshersagar 2023 [114] | Wistar rat Endometrium Injury |
Placenta Human Xenogeneic |
Dose: from 106 cells Administration: injection Site: intrauterine |
↑ regeneration ↑ endometrial protein junction |
↑ OXPHOS (activity CI, CII, CIII, CIV, CytB5, CytP450, NADPH-CytC red, NADH-CytC red) |
| Wu 2022 [115] | RCS/Kyo rat Retinal degeneration |
Liver Rat Allogenic |
Dose: 50–100 μg mito proteins Administration: injection Site: intra-retinal |
Restore retinal layer thickness ↑ number of cells (only 100 μg) Anti-degeneration activity |
Not investigated |
| Wu 2020 [116] | C57B/6 mice Hair aging |
Liver Mice Allogenic |
Dose: 200 μg mito proteins Administration: SC Site: not specified |
↑ hair length, number of hair follicles No effect on derma thickness ↑ collagen |
↑ ATP synthase ↑ mito quantity (mtDNA) |
ATP: adenosine tri-phosphate, BNIP3: Bcl-2/adenovirus E1B-interacting protein 3, COX: cytochrome C oxidase, DRP1: dynamin-related protein 1, IL: interleukin, IM: intra-muscular, mito: mitochondria, OPA1: optic atrophy 1, SC: subcutaneous, TFAM: mitochondrial transcription factor A, TNFα: tumor necrosis factor α, ↑: increase/ improve, ↓: decrease.
3.2.6. Other diseases and conditions
A total of 28 studies [120–147] explored other potential applications of mitochondrial transplantation (Table 6). Six studies focused on lung disorders, with beneficial effects on pulmonary hypertension [127,139], hyper-reactivity [138], endotoxin-induced injury [129] and acute respiratory distress syndrome [146,147]. Four studies [124,130,133,143] explored the impact on tumors, with mixed results, and four other studies reported benefit on osteo-muscular disorders (myo-dystrophy [120,122], osteoarthritis [128], tendinopathy [131]). Additionally, there were positive outcomes reported in cardiological conditions (cold ischemia [135], right heart failure [132], hypertrophy [144] and graft quality [142]), liver conditions (CCl4 injury [134], fatty liver [137], fibrosis [145] and hepatitis [141]), and diabetes [125,126] (improved glycemic control). Two studies also demonstrated efficacy in treating erectile dysfunction [121,140]. Lastly, a study investigating the safety of mitochondrial transplantation in a large animal model [123] (horse) found no evidence of inflammation or adverse effects. This was further supported by a study examining the immunogenicity of different mitochondrial sources (allogeneic, syngeneic), with no reported immune or inflammatory reactions [136].
Table 6.
Other mitochondrial transplantation effects.
| Author/Year | Model/Disease | Mitochondria source | Transfer parameters | Clinical & biological evidences | Mitochondrial mechanisms modulation |
|---|---|---|---|---|---|
|
| |||||
| Dubinin 2024 [120] | C57BL/10 mice Muscular dystrophy |
Skeletal muscle Mice Allogenic |
Dose: 1 μg/g body weight Administration: IM Sites: gastrocnemius/ quadriceps | ↑ muscle strength, grips strength ↑ number of muscular fibers ↓ calcification ↓ creatine kinase No effect on inflammation (TNFα, IL6) |
↓ MAM surface area ↓ RCR ↓ Ca2+ retention capacity ↓ oxidative stress (TBARs) |
| Zhai 2024 [121] | Sprague Dawley rat Erectile dysfunction |
Adipose MSC cells Rat Allogenic |
Dose: 300 μg mito proteins Administration: injection Site: intra-cavernosum |
Restore erectile function Restore smooth muscle content ↓ apoptosis |
↑ ATP level ↓ oxidative stress (mitosox) ↑ anti-ox (SOD) |
| Always 2023 [122] | C57BL/6 mice Injured skeletal muscle |
Liver Mice Allogenic |
Dose: 50 μg mito proteins Administration: IV Site: not specified |
↓ collagen (day 7 not day 14) No effect on inflammation, muscular mass, maximal plantar force |
Not investigated |
| Cassano 2023 [123] | Horse Safety |
Platelet cells Horse Autologous |
Dose: 1 mL of mito preparation Administration: injection Site: intra-synovial |
No inflammation No adverse effect |
Not investigated |
| Celik 2023 [124] | Athymic nude mice Ovarian cancer |
Cardiac fibroblast cells Human Xenogeneic |
Dose: 107 mito Administration: IV Site: not specified |
No effect on tumor size | Not investigated in vivo In vitro: ↑ mtDNA, ↑ ATP level |
| Mudgal 2023 [125] | Wistar rat Diabetes |
Pectoralis major Rat Allogenic |
Dose: 0.5 mg/kg Administration: IV Site: not specified |
↓ blood glucose ↓ inflammation (NFκB, IL6) |
↑ ATP level ↑ mito quantity (mtDNA) ↑ complex activity (I, II, III) ↑ anti-ox (SOD, CAT, GSH) ↓ oxidative stress (NO, TBARs) ↑ mito biogenesis (PGC1α) |
| Paliwal 2023 [126] | Wistar rat Diabetes |
Pectoralis major Rat Allogenic |
Dose: 100 μg mito proteins Administration: IV Site: not specified |
↓ diastolic/systolic pressure ↓ abdominal fat, cholesterol, BMI, ALAT, ASAT ↓ glycaemia |
↑ ATP level ↑ complex activity (I, II, III, IV, V) ↑ anti-ox (SOD, CAT, GSH) ↓ oxidative stress (NO, TBARs) ↓ mito biogenesis (PGC1α) |
| Hsu 2022 [127] | Sprague Dawley rat Pulmonary hypertension |
Soleus Rat Allogenic |
Dose: 100 μg mito proteins Administration: IV Site: jugular |
No effect on mortality ↑ right ventricular function, ↓ BNP Restore contractile phenotype of pulmonary artery |
↑ ATP level |
| Lee 2022 [128] | Wistar rat Osteoarthritis |
L6 cells Rat Allogenic |
Dose: 10 μg mito proteins Administration: injection Site: intra-articular |
↓ osteoarthritis progression, suppressing pain ↓ bone damage ↑ bone surface and volume ↓ inflammation (TNFα, IL1β, IL6, NFκB) |
↑ mito quantity (mitotracker) ↓ mito diameter ↑ MMP (JC-1) ↓ ROS (mitosox) ↓ autophagy (LC3–2/LC3–1 ratio, p62) |
| Pang 2022 [129] | Sprague Dawley rat Lung injury endotoxins |
Soleus Rat Allogenic |
Dose: 100 μg mito proteins Administration: IV Site: jugular |
↑ gas exchange and bicarbonate levels ↓ lung parenchyma damage |
↑ ATP level |
| Zhou 2022 [130] | BALB/c mice Hepatocellular carcinoma |
Liver Mice Allogenic |
Dose: 6*107 mito Administration: IV Site: tail |
↓ tumor volume (≈ decrease of 30 %) ↓ apoptosis and lactate ↓ tumor metabolism (hexokinase, lactic acid, ATP), ↑ pyruvate DH, ↑ succinate DH, ↑ isocitrate DH |
↓ cellular ROS (DCFHDA) ↑ anti-ox (SOD, CAR, GSH) |
| Lee 2021 [131] | Sprague Dawley rat Tendinopathy |
L6 cells Rat Allogenic |
Doses: 10–50 μg mito proteins Administration: injection Site: tendon |
↓ tendon thickness (both) ↓ apoptosis ↓ inflammation (TNFα, IL6, NFκB: both; IL1β: only 50 μg) |
↑ ATP level ↑ mito quantity (mitotracker) ↓ Superoxide and cellular ROS (mitosox, DCFHDA) ↑ OXPHOS prot level ↓ fission (Fis1, DRp1) ↑ fusion (Mfn2) |
| Weixler 2021 [132] | Yorkshire pig Right heart failure |
Gastrocnemius, soleus Rat Xenogeneic |
Dose: 107 mito Administration: intraventricular Site: cardiac |
No effect on mortality Restore fractional area change ↑ contractile function ↓ fibrosis and apoptosis |
Not investigated in vivo In vitro: ↑ ATP level |
| Yu 2021 [133] | BALB/c mice Melanoma |
Liver Mice Allogenic |
Dose: 106 mito Administration: IV Site: not specified |
↓ tumor volume (≈ decrease of 50 %) ↓ lactate |
↑ ATP level ↑ autophagy (LC3), ↑ mitophagy (Parkin) |
| Zhao 2021 [134] | Kunming mice CCl4 hepatic lesion |
Liver Mice Allogenic |
Dose: 0.2–0.4 mg/kg Administration: IV Site: tail |
Trend for a dose effect ↓ lesion area and fibrosis ↓ ALAT and ASAT |
↑ ATP level ↑ MMP (JC-1) ↑ anti-ox (GSH, SOD, NADH DH, pyruvate DH) ↓ oxidative stress (MDA) |
| Moskowitzova 2019 [135] | C57BL/6J mice Cold ischemia time and cardiac function |
Gastrocnemius Mice Allogenic |
Dose: 108 mito Administration: IV Site: coronary |
↑ ejection fraction and fractional shortening ↓ necrosis ↓ neutrophil infiltrate No effect on apoptosis |
Not investigated |
| Ramirez-Barbieri 2019 [136] | BALB/c mice Safety and immune reaction |
Gastrocnemius Mice Allogenic and syngenic |
Doses: 105–7 mito Administration: intraperitoneal |
No dose effect, no type of transplantation effect No rejection of arterial graft No immune reaction (TNFα, IFNγ, IL2, IL4, IgM) |
Not investigated |
| Fu 2017 [137] | C57BL/6J mice Fatty liver |
HepG2 cells Human Xenogeneic |
Dose: 0.5 mg/kg Administration: IV Site: tail |
↓ cholesterol and hepatic lipid infiltrate ↓ ALAT and ASAT |
↑ ATP level ↑ CCO ↓ oxidative stress (Fenton reaction, MDA) ↑ anti-ox (SOD, GSH) |
| Su 2016 [138] | Sprague Dawley rat Hyper-reactivity bronchial |
Airway epithelial cells Rat Allogenic |
Dose: 3.75*107 mito Administration: Intratracheal |
↓ airway hyper-reactivity | ↑ MMP (JC-1) |
| Zhu 2016 [139] | Sprague Dawley rat Hypoxia and pulmonary hypertension |
Pulmonary artery/lung Rat Allogenic |
Dose: 2.25*108 mito (fresh or fractioned) Administration: IV Site: tail |
Results obtained for both (fresh and fractioned mito) Inhibit hypoxia pulmonary vasoconstriction Restore pulmonary artery pressure ↓ apoptosis ↓ vascular remodeling |
↑ mitochondrial quantity (ROS increase) |
ALAT: alanine aminotransferase, anti-ox: anti-oxidant, ASAT: aspartate aminotransferase, ATP: adenosine tri-phosphate, BMI: body mass index, BNP: B-type natriuretic peptide, CAT: catalase, DCFHDA: 2′,7′-dichlorodihydrofluorescein diacetate, DRP1: dynamin-related protein 1, Fis1: mitochondrial fission protein 1, GSH: reduced glutathione, IgM: immunoglobulin M, IL: interleukin, IM: intra-muscular, IV: intra-veinous, JC1: 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazo-lylcarbocyanine iodide, LC3: microtubule-associated protein light chain 3, MAM: Mitochondria-associated endoplasmic reticulum membranes, MDA: malondialdehyde, Mfn: mitofusin, mito: mitochondria, MMP: Mitochondrial membrane potential, MSC: Mesenchymal stromal cell, NFκB: nuclear factor kappa B, NO: nitric oxide, RCR: respiration control rate, PGC1-α: Pparg coactivator 1-alpha, ROS: reactive oxygen species, SOD: superoxide dismutase, TBARS: Thiobarbituric Acid Reactive Substances, TNFα: tumor necrosis factor α ↑: increase/improve, ↓: decrease, ≈: data have been estimated from the graphs.
3.3. Safety of mitochondrial transplantation
Across all studies, the majority were conducted under significant stress conditions (IRI, sepsis, etc.), where the transplantation of EV led to a reduction in pro-inflammatory cytokines (IL-1β, TNF-α, etc.) in 87 % (27/31) of cases.
However, in several studies, the administration of mitochondria under non-stress conditions has also been investigated to assess potential reactions. First, the study by Cassano et al. [123] showed that there was no inflammatory reaction at the injection site of the mitochondria (joint), and no systemic inflammatory response was observed (no change in blood cell counts).
However, with 94 % (115/123) of the studies using a transplantation type other than autologous, immunogenic reactions would theoretically be possible. The safety results observed in the autologous model have been confirmed in various studies using rat or mouse models with allogeneic [76,94,107,138] or xenogeneic [81] mitochondria transplantation, which did not lead to changes in the number of immune cells (leukocytes, T lymphocytes), oxidative stress, or pro-inflammatory cytokines (IL-6, TNFα, IL-1β).
Finally, these results were confirmed biologically by Ramirez-Barbieri et al., [136] showing an absence of acute direct or indirect alloreactivity. This team further investigated by verifying that mitochondrial administration did not lead to chronic immunization in the mice. They sequentially injected syngeneic or allogeneic mitochondria into mice (3 injections), and after a few days, they performed an allogeneic skin graft. The graft rejection time was comparable between the groups, suggesting an absence of immunization of these mice by the previously administered mitochondria.
3.4. Unraveling mitochondrial complexity: functional and structural mechanisms underlying mitochondrial-based therapies
Although the positive therapeutic outcomes reported across various diseases are promising, the underlying biological mechanisms remain incompletely understood. As recently emphasized by Monzel, Enríquez, and Picard (2023), mitochondria are far more than cellular powerhouses. They participate in a wide array of critical functions, including membrane potential generation, calcium uptake and release, lipid metabolism, mtDNA maintenance and expression, sodium ion exchange, OXPHOS, permeability transition, respiration, redox homeostasis, and reactive oxygen species (ROS) production. Importantly, the authors highlighted not only the diverse functions of mitochondria but also their dynamic behaviors—such as cristae remodeling, mitochondrial fusion and fission, and overall mitochondrial homeostasis—underscoring a more nuanced and integrative view of mitochondrial biology [148]. In this review, we examine both well-established and underexplored mitochondrial functions modulated by mitochondrial and EV transplantation, aiming to clarify how these alterations may contribute to the observed therapeutic benefits across disease models (Fig. 3). However, it is important to acknowledge that 17 % of the selected studies did not assess mitochondrial activity or behavior, representing a limitation in fully understanding the mechanistic basis of these interventions.
Fig. 3. Mechanisms of action and clinical outcomes of mitochondrial and extracellular vesicle (EV) transplantation.
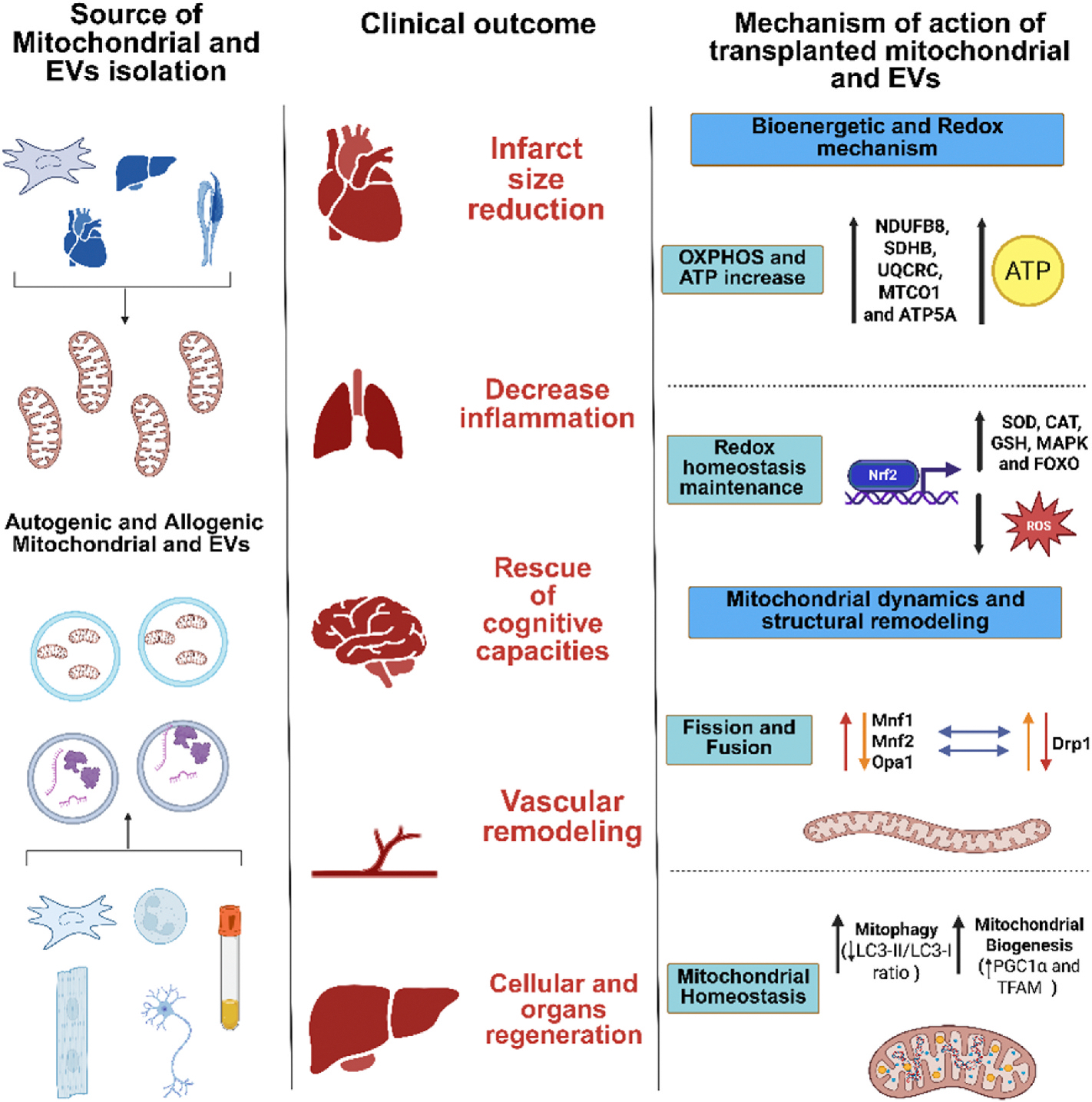
Mitochondria and EV can be isolated from various autologous or allogeneic sources, including immune cells, cardiac and neuronal tissues, organs and biological fluids. These mitochondrial therapies have been associated with multiple clinical outcomes such as infarct size reduction, decreased inflammation, rescue of cognitive functions, vascular remodeling, and regeneration of cells and organs. Mechanistically, transplanted mitochondria and EV exert their effects through: (1) enhancement of bioenergetic and redox processes by increasing oxidative phosphorylation (OXPHOS) and ATP production via upregulation of NDUFB8, SDHB, UQCRC, MTCO1, and ATP5A; (2) redox homeostasis maintenance through antioxidant pathways involving Nrf2, SOD, CAT, GSH, MAPK, and FOXO signaling; (3) modulation of mitochondrial dynamics via regulation of fission (Drp1) and fusion (Mfn1, Mfn2, Opa1) processes; and (4) preservation of mitochondrial homeostasis through enhanced mitophagy (increased LC3-II/LC3-I ratio) and mitochondrial biogenesis (upregulation of PGC1α and TFAM). These mechanisms underpin the therapeutic potential of mitochondrial and EV-based interventions across a range of diseases.
3.5. Bioenergetic and redox mechanisms underlying the therapeutic effects of mitochondrial and EV transplantation
Mitochondria are essential organelles responsible for producing ATP through OXPHOS, a process that converts energy from nutrients into a useable form for cellular functions [149,150]. This energy production is vital for maintaining cellular activities and overall organismal health. Additionally, mitochondria play a crucial role in regulating redox homeostasis, balancing the production and neutralization of ROS to prevent oxidative stress and cellular damage [151,152]. In this section, we explore the impact of mitochondrial and EV transplantation on enhancing OXPHOS efficiency, increasing ATP production, and maintaining redox balance. We also examine how these interventions contribute to restoring mitochondrial function and improving clinical outcomes across various disease models.
3.5.1. OXPHOS and ATP increase
The OXPHOS system, composed of five multi-protein complexes, includes the electron transport chain (ETC), which transfers electrons to oxygen, generating an electrochemical gradient. This gradient powers key mitochondrial functions, including ATP synthesis, ion/metabolite transport, protein import, and maintaining mitochondrial dynamics [153]. In many studies utilizing mitochondrial or EV transplantation, researchers observed increased expression of OXPHOS proteins—such as NDUFB8, SDHB, UQCRC2, MTCO1, and ATP5A—alongside enhanced activities of complexes I through V, indicating improved mitochondrial function [154]. In a majority of publications, the authors showed an increase in ATP production in IRI disease models [27,33,36,37], in neurological disease models (increase in ATP level and increase in pyruvate DH, α-ketoglutarate DH, NADH) [62,71–73,77,81,83,88,89], in the prevention of drug-related toxicity [91], in sepsis [106], in wound healing [114], in diabetes [125,126], and in osteoarthritis [128]. In all these papers, where an increase in OXPHOS protein expression/activity, and in particular ATP level increase was observed, the authors also described clinical benefits. In the IRI disease model publications, the authors showed an increase in ATP level concomitant with a reduction of infarct size [28,29,31,33,35–37,47,49,50]. However, it is important to note that not all improvements in OXPHOS protein expression and ATP levels translated into clinical benefits. In studies focused on neurological disorders, drug-related toxicity, aging/wound healing, and tumors, some papers reported increases in ATP levels and OXPHOS protein expression without corresponding clinical effects [72,73,86,110, 124].
Based on the selected literature, we can formulate three hypotheses to explain how mitochondria and EV transplantation may enhance OXPHOS system activity and increase ATP level. First, the increase in ATP levels can be explained by the integration of healthy mitochondria into the recipient cell’s network, where they actively generate ATP through OXPHOS [155]. Second, transplanted mitochondria may regulate intracellular Ca2+ levels, which subsequently increase ATP synthesis [156]. Indeed, in one of the selected papers in IRI disease [26], the authors showed an increase in Ca2+ retention capacity (CRC), although in another article in a muscular dystrophy model, the CRC decreased after mitochondrial transplantation into muscles [157]. However, it is important to consider that even though CRC is crucial for efficient ATP production, excessive Ca2+ can lead to mitochondrial dysfunction and cell death [158]. Third, mitochondrial morphology and dynamics (particularly fusion and fission) may also play a role, although this process will be discussed further in later paragraphs.
3.5.2. Redox homeostasis maintenance
Another essential mitochondrial function is redox homeostasis as maintenance of redox reactions are central to the existence of life [159]. Indeed, reactive oxygen species (ROS), nitrogen and sulfur species, mediate redox control of a wide range of essential cellular processes [160]. Moreover, excessive levels of these species are associated with many diseases. Maintaining a finely tuned balance between ROS production and scavenging is essential for cellular homeostasis [160]. Several studies have shown that mitochondrial transplantation can effectively reduce oxidative stress across various conditions. In IRI, mitochondrial transplantation has been associated with decreased ROS levels and lower oxidative stress markers, such as MDA, TBARS, lipid peroxidation, 8-OHDG, and nitrotyrosine [25,27,28,30,50,53] Similarly, in neurological diseases, reductions in both ROS content and oxidative stress markers following mitochondrial transplantation have been reported [63,65,68,71,74,81,83,85,88]. Studies on drug-related toxicity suggest that mitochondrial transplantation helps prevent oxidative stress, as evidenced by decreased ROS levels and oxidative stress markers such as MDA and 8-OHDG [94–96,99,100]. In sepsis, mitochondrial transplantation has also been linked to a reduction in ROS content and oxidative stress [103,105,107]. Furthermore, studies on erectile dysfunction, diabetes, osteoarthritis, hepatocellular carcinoma, and tendinopathy have reported a decrease in oxidative stress following mitochondrial transplantation [121,125,126,134,137]. In muscular dystrophy, diabetes, osteoarthritis, hepatic lesions, and fatty liver, the reduction in oxidative stress markers such as MDA, TBARS, lipid peroxidation, 8-OHDG, and nitro-tyrosine further supports the potential therapeutic role of mitochondrial transplantation in mitigating oxidative stress-related damage [120,125,126,134,137]. Regarding the transplantation of EV, the selected papers reported a decrease in ROS levels and respiratory burst in various conditions, including IRI [58,60], sepsis [108], wound healing [117] and liver fibrosis [145]. Only one of the selected articles which conducted EV transplantation explicitly investigated the reduction of oxidative stress markers, demonstrating that EV decrease levels of carbonylated proteins, thereby enhancing graft quality [142].
The decrease in ROS, oxidative stress markers, and the overall oxidative stress environment, essential for maintaining redox homeostasis and restoring the cellular steady state, is primarily mediated by endogenous antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GSR), and peroxiredoxins (PRxs), along with antioxidant molecules like glutathione (GSH). The expression of many antioxidant enzymes is orchestrated by the stress-responsive transcription factor Nuclear factor erythroid 2-related factor 2 (Nrf2), which also regulates stress-response proteins, such as heat shock proteins (HSPs), to maintain cellular redox homeostasis [161]. It is well known that mitochondrial respiration produces ROS essential for signaling and the mitochondria themselves possess antioxidant enzyme systems for the maintenance of Redox balance [162]. In the papers selected for this review, the majority of studies indicate that a decrease in ROS is associated with an increase in antioxidant enzymes content, particularly manganese superoxide dismutase (SOD2, the mitochondrial form), glutathione peroxidase (GPX, which utilizes glutathione (GSH) as a substrate), heme oxygenase-1 (HO-1), NAD(P)H-quinone oxidoreductase 1 (NQO1), 8-oxoguanine DNA glycosylase 1 (OGG1), catalase (CAT), and total antioxidant capacity. This increase is also associated with the translocation of transcription factors such as Nrf2, following the inhibition of its co-factor, Kelch-like ECH-associated protein 1 (Keap1). This inhibition allows Nrf2 to translocate into the nucleus, promoting the transcription of antioxidant enzymes such as CAT and SOD [163]. Additionally, there is an upregulation of Succinate Dehydrogenase Complex Assembly Factor (Sdhaf2/4) mRNA, along with the activation of other signaling pathways, including mitogen-activated protein kinase (MAPK) and Forkhead box class O (FOXO) [164]. These effects have been observed in IRI [26, 30,50], neurological disorders [71,72,81,83,85,88], drug-related toxicity prevention [90,92,94,95,97,98,100], erectile dysfunction [121], diabetes [125,126], hepatocellular carcinoma [130], hepatic lesions [134] and fatty liver disease [137]. Additionally, EV can trigger an antioxidant enzyme response in IRI [55,60], sepsis [108] and liver fibrosis [145]. However, the activation of the antioxidant response and reduction in ROS levels do not always translate into clinical benefits. In the selected papers on IRI, some studies reported no effect on clinical outcomes [25,26], as well as in studies on neurological diseases [72] and drug-related toxicity prevention [92].
Based on the review of the selected articles, it is reasonable to suggest that the observed decrease in ROS levels is due to an increase of the antioxidant system response after the mitochondria and EV transplantation. Several hypotheses can be proposed to explain this phenomenon. First, Mitochondria and EV can increase intracellular ROS levels, leading to a transient activation of the Nrf2 pathway through disruption of Keap1-mediated repression. This allows Nrf2 to translocate into the nucleus, where it binds to antioxidant response elements (AREs) and promotes the expression of key antioxidant enzymes such as SOD, CAT, GPx1, and glutamate–cysteine ligase (GCL), thereby restoring the GSH:GSSG ratio critical for redox buffering and detoxification of lipid peroxides [163,165,166]. Additionally, ROS generated by mitochondrial respiration can induce epigenetic modifications—such as histone acetylation (e.g., H3K27ac) and DNA demethylation—at antioxidant gene loci, enhancing chromatin accessibility to Nrf2 and other transcription factors, and contributing to sustained antioxidant enzyme expression [167]. Second, mitochondrial-derived signals modulate FOXO transcription factor activity through phosphorylation by AKT and JNK resulting in nuclear translocation or cytoplasmic retention of FOXO1/3a, thereby regulating expression of genes involved in oxidative stress resistance (e.g., catalase, SOD2, and GADD45) [165]. Third, the exposure to mitochondrial material activates redox-sensitive MAPK cascades (e.g., p38, ERK1/2), which in turn phosphorylate key transcription factors or co-activators, enhancing transcription of stress-responsive genes (e.g., HO-1, NQO1) [168].
3.5.3. Mitochondrial dynamics and structural remodeling in mitochondrial and EV transplantation
Mitochondria are enclosed by two membranes with distinct structures and functions: the outer membrane and the inner membrane, which are further organized into specialized regions. The outer membrane separates mitochondria from the cytosol and is the site where fission and fusion processes occur, mediated by key mitochondrial-shaping proteins such as Mitofusin 1 and 2 (Mfn1 and Mfn2) and Fission 1 protein (Fis1) [169]. The inner membrane is subdivided into the inner boundary membrane (IBM) and the cristae [169]. The IBM contains the translocase of the inner membrane, which imports proteins into the matrix. Cristae are invaginated, bag-like structures separated from the intermembrane space by narrow tubular junctions, creating specialized compartments that limit the diffusion of molecules critical for the OXPHOS system [170]. A variety of proteins, many of which remain incompletely characterized, regulate cristae biogenesis and structure. Among these, optic atrophy protein (OPA1), another essential protein involved in mitochondrial fusion and the mitochondrial contact site and cristae organizing system (MICOS complex) are the primary regulators of cristae dynamics [171].
3.5.4. Fission and fusion
Mitochondrial fission (fragmentation) and fusion (elongation) are fundamental processes that regulate mitochondrial morphology and function. Their dynamic balance is essential for maintaining cellular homeostasis and preventing disease. Together with cristae remodeling, these interconnected mechanisms continuously shape the mitochondrial network, adapting it to cellular needs and stress conditions. This structural plasticity plays a critical role in supporting mitochondrial quality control, energy metabolism, and overall cellular health [172]. Indeed, mitochondrial fusion allows the transfer of gene products between mitochondria for optimal functioning, especially under metabolic and environmental stress. On the other hand, fission is crucial for mitochondrial division and quality control [173].
In the literature, fission is described as a faster process than fusion. In most cases, fission is used for the removal of damaged mitochondria by mitophagy or the homogeneous distribution of mitochondria in dividing cells. Fusion is a slower process that allows mitochondria to share their contents (proteins, lipids, mitochondrial DNA), facilitating repair and optimizing ATP production essential for mitochondria that are unable to perform their respiratory function optimally because of a disease condition [174]. Moreover, fusion can trigger fission because fused mitochondria form elongated, interconnected networks that enhance cellular energy distribution. However, these extended structures cannot be maintained indefinitely and require fission to reshape and preserve mitochondrial dynamics.
This delicate balance between fusion and fission is tightly regulated by key proteins. DRP1, a major driver of fission, can be recruited even after a fusion event to break down mitochondrial networks when needed. Meanwhile, MFN1, MFN2, and OPA1 orchestrate fusion but also influence subsequent fission, ensuring mitochondria remain adaptable to cellular demands. Indeed, where fusion and fission were studied, a majority of publications found an increase in fusion processes, rather than fission. In 3 studies, fission was also observed to coincide with an increase in ATP levels [28,40,72]. An increase in fission was also found to coincide with a reduction in infract size in IRI [28,40], improvement of cognitive ability [71] and improve in tendon thickness in Tendinopathy condition [131].
Many of the selected papers showed that mitochondrial transplantation led to modulation of mitochondrial dynamics, affecting both fission and fusion processes. In IRI, this involved a decrease in the fission protein DRP1 [38], and an increase in fusion proteins such as OPA1 [39] and Mfn1/2 [39,40]. In neurological diseases, there was a decrease in fusion proteins Mfn1/2 [66] and a decrease in the fission protein DRP1 [76], along with an increase in both fusion (Mfn2) and fission (DRP1) [89] as well as a publication that showed an increase in the proportion of elongated mitochondria [72]. Additionally, there was a reduction in atypical mitochondrial morphology and synaptosome mitochondrial damage [97]. In drug-related toxicity, mitochondrial fusion increased (OPA1) [95], while in sepsis, fusion increased (Mfn2) and fission decreased (DRP1) [103]. In healing and aging, there was an increase in mitochondrial fission (DRP1) [110], accompanied by reduced mitochondrial swelling, disruption, and cristae loss, along with upregulation of genes related to mitochondrial structure in IRI [26,28,29].
Regarding EV transplantation several studies have reported modulation of mitochondrial dynamics, particularly fusion and fission processes, across various disease models. In IRI, increased of MFN1 and MFN2 mRNA levels was observed following EV treatment [55]. One study demonstrated that human-derived mitochondria delivered via EV successfully fused with native mitochondrial networks in mouse cardiomyocytes, enhancing mitochondrial function and decreasing the dysfunctional mitochondrial fission [57]. In an IRI liver model, EV promoted mitochondrial fusion by increasing MFN1 [58] while in sepsis this resulted in an increase of MFN2 [109]. Similarly, in cardiac hypertrophy, EV transplantation led to increased mitochondrial fusion and elongation in neutrophils [144]. Additionally, EV were shown to reduce mitochondrial cristae loss and swelling, improving mitochondrial structural integrity in graft quality for transplant after IRI disease in pig [142].
Among the 15 publications that examined mitochondrial fusion and fission processes, 11 reported an increase in fusion. One study observed no effect on either process, while two studies noted an increase in fission. The increase of fusion process with the mitochondrial transplantation can be explained with different hypotheses. First, fusion of transplanted mitochondria when they enter in the recipient cells, share protein, lipids and mitochondrial DNA and thereby improve complex mitochondrial cell function which could also explain the increase in ATP levels [175]. Second, the fusion of transplanted mitochondria could be a mechanism of metabolic adaptation to efficiently distribute respiratory chain enzymes and optimize ATP production [175]. Third, the mitochondria of the recipient cells could be damaged and in a hyper-fragmented status, and the healthy mitochondria transplant may promote fusion to dilute damage and restore mitochondrial function, reducing oxidative stress and decreasing mitochondrial dysfunction [176].
Alternative hypotheses can be formulated to explain the observed increase in fission. First, following transplantation, the transplanted mitochondria may need to be efficiently distributed and reorganized within the recipient cell. Fission would allow the transplanted mitochondria to be split into smaller pieces, facilitating their distribution and integration into the cell’s pre-existing mitochondrial networks [177]. Second, balance between fusion and fission: If mitochondrial transplantation leads to initial fusion to restore mitochondrial function, fission could subsequently be activated to maintain mitochondrial balance and turnover, preventing the formation of overly long mitochondrial networks that would be unsustainable [177]. Third, stress response: Under conditions of cellular or metabolic stress (e.g., following mitochondrial transplantation), the cell may increase fission to remove damaged mitochondria or to better adapt to new energy demands. Fission also allows the separation of malfunctioning mitochondria, reducing oxidative stress and improving energy efficiency [176].
3.5.5. Mitochondria homeostasis
Mitochondria play a critical role in maintaining cellular homeostasis, requiring regulated mitochondrial mass and function to protect mtDNA and meet the cell’s energy demands. This balance is preserved through a fine coordination between two opposing processes: mitochondrial biogenesis, which generates new mitochondria, and mitophagy/autophagy, which removes damaged ones [178,179]. Many of the selected papers showed that mitochondrial transplantation maintained mitochondrial homeostasis by increasing mitophagy in IRI, thanks to proteins that promote the clearance of damaged mitochondria (PINK1, Parkin) [30]. In IRI, one study also reported a decrease in the LC3-II/LC3-I ratio [32], which could reflect an increase in autophagic flux, where formed autophagosomes are rapidly degraded in the lysosomes. This is associated with more efficient clearance of cellular materials. Additionally, two studies in IRI demonstrated downregulation of mitochondrial apoptosis [37,53]. In addition to functional and quantitative alterations, structural changes in mitochondria can also impair homeostasis. For instance, in muscular dystrophy models, mitochondrial transplantation was associated with restoration of mitochondrial-associated membrane (MAM) surface area, which is typically reduced in the disease. In neurological diseases, there is evidence of increased mitochondrial biogenesis as showed by elevated expression of proteins such as PGC1α and TFAM [62], as well as ERK and SIRT1, accompanied by increased autophagy, indicated by the expression of FOXO3, BNIP3, and LC3-II/LC3-I [71]. In the prevention of drug related toxicity, there is a decrease in autophagy (mRNA LC3BII, p62 decrease) and autophagosomes (inhibit AMPK/mTOR pathway, decrease of p70S6K) [90] as well as an increase in mitochondrial biogenesis with an increase of PGC-1, mtTFAM [91], Ppargc1b [95], SIRT1, PGC-1α [103]. One study also observed an increase in mitochondrial content, determined by mitochondrial DNA quantification and mitochondrial-specific staining [96]. Another publication shows mitochondrial biogenesis in sepsis with an increase of PGC-1α expression followed by a decrease in mitochondria fragmentation, following EV transplantation [109]. In the context of healing and aging, one study on mitochondrial transplantation reported an increase in TFAM expression [110], while another study demonstrated an elevation in mitochondrial quantity, as indicated by increased mitochondrial DNA levels [116] while a publication with EV showed an increase in mitophagy, specifically with the increase of LC3 II [118]. In various disease models, studies have reported alterations in mitochondrial dynamics, including changes in mitochondrial biogenesis, content, autophagy, and mitophagy. For instance, in diabetes, an increase in mitochondrial biogenesis has been observed, indicated by elevated levels of markers such as PGC-1α, mtTFAM and increase in mitochondrial DNA [125]. Conversely, in a metabolic syndrome model study, authors reported a decrease in mitochondrial biogenesis, evidenced by reduced PGC-1α expression [126]. In osteoarthritis, a decrease in autophagy markers, such as the LC3-II/LC3-I ratio and p62 levels, has been noted [128]. In melanoma models, there is evidence of increased autophagy, indicated by elevated LC3 levels, and enhanced mitophagy activity, as shown by increased Parkin expression [133]. Additionally, in acute kidney injury (AKI) models induced by IRI, the transplantation of EV contributes to the restoration of TFAM levels and stabilization of the TFAM-mtDNA complex, leading to a reduction in mitochondrial DNA damage [59]. These findings highlight the complex and context-dependent nature of mitochondrial dynamics in various pathological conditions.
We can formulate several hypotheses regarding the impact of mitochondrial transplantation on mitochondria homeostasis. First, mitochondrial and EV transplantation work like a “quality control jump-start”, reactivating mitophagy via known pathways (PINK1/Parkin, LC3-II), which clears dysfunctional mitochondria, restores energy homeostasis, and reduces stress signals This is especially critical in injured or diseased tissues where these pathways are often suppressed [180]. Second, mitochondrial and EV transplantation stimulates mitochondrial biogenesis with the upregulation of transcriptional regulators such as PGC1α, TFAM, and SIRT1 in multiple disease models, indicating the activation of endogenous renewal programs that support energy recovery and mtDNA stability. This can be possible because of metabolic and signaling stimulus, pushing the cell to increase mitochondrial production to restore energy balance [181]. Third, another mechanism that can restore mitochondrial homeostasis is through the structural reorganization of the MAMs. Indeed, transplanted mitochondria may contribute directly by physically integrating into host mitochondrial networks and re-establishing Endoplasmic Reticulum contact sites [182]. This can be possible also with the delivery of essential MAM-regulatory proteins such as Mfn2 or VDAC1, or modulate signaling pathways (e.g., SIRT1 activation) that facilitate membrane tethering [183]. These structural effects improve ER-mitochondria calcium exchange and support efficient OXPHOS, as observed in muscular dystrophy models [182].
4. Discussion
4.1. Study heterogeneity and lack of standardized quality control
The field of mitochondrial and EV transplantation is beginning to expand into various applications, which makes the generalization of results complex. Importantly, the reliability of the current body of evidence is constrained by heterogeneous study quality as evidence by the presence of high risk of bias in the preclinical studies (determined by the SYRCLE guidelines). Many studies lacked critical design features such as randomization, or blinded outcome assessment, leading to an unclear or high risk of bias. This distinction is crucial for guiding translational priorities and identifying research gaps. Moreover, the lack of consistency between studies is evident, both in terms of post-isolation quality controls for mitochondria or EV, as well as their subsequent internalization. Furthermore, several instances of over-interpretation have been noted, such as attributing the observed increase in fusion proteins to the fusion between exogenous and endogenous mitochondria, although this conclusion is not definitively supported by the data. This raises a major question: are the observed effects directly related to the function of exogenous mitochondria? In other words, is it necessary to transplant functional and viable mitochondria to achieve clinical and biological effects, as well as an impact on redox homeostasis? All the studies have demonstrated (or assumed in the absence of quality control) the use of functional mitochondria, with only four comparing the efficacy of functional and non-functional mitochondria [66,82,100,139]. Two studies reported beneficial effects only with functional mitochondria, while the other two found that the effects were similar regardless of whether the mitochondria were functional or not. Moreover, studies rarely assessed respiratory competence directly (e.g., oxygen consumption rate or RCR), limiting mechanistic insight. It is still unclear whether mitochondrial structural preservation, biochemical cargo, or actual respiratory activity drives therapeutic effects. Further research should prioritize standardized functional assessments (e.g., ATP generation, membrane potential, electron transport chain activity) to clarify the precise determinants of efficacy in mitochondrial-based therapies. The question remains open for debate and has also been investigated in vitro, with diverging results. However, if the use of non-functional mitochondria proves effective, it will be possible to identify the factors responsible for the observed effects, thus developing a potentially more robust and consistent therapeutic solution. Moreover, future studies on EV transplantation should include comprehensive characterization of the different markers of EV subtypes to clarify their origin—particularly to distinguish them from already known EV subtypes (small-EV, large-EV, exosomes, microvesicles, and apoptotic bodies), as well as from the emerging classes of mitochondrial-derived extracellular vesicles (MDEV) and mito-EV.
4.2. Perspectives: from pre-clinical to clinical application
Although numerous in vitro and preclinical studies have demonstrated the benefits of mitochondrial transplantation (particularly in the context of IRI), clinical data remains limited.
The first study, published by Emani et al., in 2017 [184], described an improvement in systolic dysfunction and a decrease or disappearance of regional segmental hypokinesia in 5 children, with no acute adverse events related to the injection of 107 mitochondria into the myocardium. In 4 out of 5 patients, this led to the cessation of ECMO cannulation, which had not been possible before. Although encouraging, the lack of a control group in this study prevents any conclusions about whether the improvement was directly linked to mitochondrial administration. The same team subsequently published data in 2021 [185] on 24 patients (14 without mitochondrial administration and 10 with), confirming the absence of acute adverse events (e.g., arrhythmia, inflammation), and better successful and durable separation from ECMO following mitochondrial transplantation, fewer cardiovascular events, and enhanced ventricular strain. More recently, in 2024, another team conducted a Phase 1, prospective, parallel-group, randomized study to investigate the safety and efficacy of the transplantation of 1.2 × 108 mitochondria via coronary artery infusion in ischemic heart disease in 30 patients [186]. While no acute adverse events were reported, efficacy was limited to an improvement in exercise capacity compared to non-treated patients. No significant difference was observed in the improvement of ejection fraction between the groups. There is hope that the three ongoing clinical trials investigating the effects of mitochondria on cardiac ischemia (NCT02851758, NCT05669144) and cerebral ischemia (NCT04998357) will provide positive results to improve patient care and further investigate the potential benefits of mitochondrial transplantation.
5. Conclusion
Mitochondrial and EV transplantation represents innovative and versatile strategies to counteract mitochondrial dysfunction across a wide spectrum of preclinical disease models. By modulating mitochondrial bioenergetics, redox signaling, structural integrity, and organelle homeostasis, these therapies offer a multifactorial mechanism of action that aligns with the complex pathophysiology of many disorders.
While preclinical evidence supports the therapeutic potential of mitochondrial and EV transplantation (particularly in models of IRI, neurodegeneration, and drug-induced damage) translational readiness remains limited. Moreover, preclinical studies mostly do not adhere to the SYRCLE guidelines, which further limits the strength of the evidence they provide. The existing clinical data are preliminary, derived from small patient cohorts with limited control groups and heterogeneous methodologies. Furthermore, several critical barriers must be addressed before broader clinical application can be envisioned. These include the lack of standardized protocols for mitochondrial or EV isolation and characterization, incomplete understanding of the mechanisms of action, and unresolved questions surrounding immunogenicity and long-term safety. Regulatory challenges also remain considerable, especially regarding product classification, quality control, and clinical-grade manufacturing. As such, mitochondrial-based therapies should still be considered experimental, and ongoing efforts must prioritize mechanistic clarity, methodological harmonization, and rigorously designed clinical trials. A cautious yet optimistic outlook is warranted as the field progresses toward therapeutic maturity.
Supplementary Material
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2025.06.040.
Acknowledgments
Authors VL and SM were supported by the National Heart Lung and Blood Institute under R00 HL157690. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- 8-OHDG
8-hydroxyl-2′-deoxyguanosine
- ADSCS
adipose derived stem cells
- Akt
protein kinase B
- ALAT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- anti-ox
anti-oxidant
- ARDS
acute respiratory distress syndrome
- ASAT
aspartate aminotransferase
- ATP
adenosine tri-phosphate
- BHK21
baby hamster kidney cells-clone 21
- BM-MSC
bone marrow mesenchymal stromal cell
- CAT
catalase
- CII-SDHB
complex II – succinate dehydrogenase subunit B
- COX
cytochrome C oxidase
- CPK
creatine phosphokinase
- CRP
C-reactive protein
- CXCL
chemokine (CXC motif) ligand
- DCFHDA
2′,7′-dichlorodihydrofluorescein diacetate
- DIC
disseminated intravascular coagulation
- DRP1
dynamin-related protein 1
- ECAR
extracellular acidification rate
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- EV
extracellular vesicles
- FABP
fatty acid-binding protein
- FOXO
forkhead box O
- GPX
glutathione peroxidase
- GSH
reduced glutathione
- HI
hypoxia-ischemia
- HNE
4-hydroxy-2-nonenal
- HO-1
heme oxygenase-1
- IC
intracavernous
- IDH2
isocitrate dehydrogenase 2
- IFN
interferon
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL
interleukin
- IM
intra-muscular
- IP
intraperitoneal
- IRI
ischemia-reperfusion injury
- iNOS
inducible nitric oxide synthase
- IV
intra-veinous/intravenous
- JC1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- LC3
microtubule-associated protein light chain 3
- MAFbx
muscle atrophy F-box
- MDA
malondialdehyde
- MDMs
monocyte derived macrophages
- Mfn
mitofusin
- MITCO1
protein complex IV
- MMP
mitochondrial membrane potential
- MNP
microneedle patch
- MSC
mesenchymal stromal cell
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromure
- mTOR
mammalian target of rapamycin
- MuRF-1
muscle RING finger protein 1
- N2A
Neuro-2A
- NDUF8
protein complex I
- NFκB
nuclear factor-kappa B
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NO
nitric oxide
- NOX2
NADPH oxidase 2
- NQO1
NAD(P)H:quinone oxidoreductase 1
- NRF2
nuclear factor erythroid 2–related factor 2
- NSE
neuron specific enolase
- NT
nitrotyrosine
- OCR
oxygen consumption rate
- OGG1
8-oxoguanine DNA glycosylase 1
- OPA1
optic atrophy 1
- OXPHOS
oxidative phosphorylation
- PAMPK-α
Thr172 phospho-AMPK-alpha-Threonine 172
- Parkin
E3 ubiquitin ligase involved in mitophagy
- PGC1-α
Pparg coactivator 1-alpha
- PINK1
PTEN induced kinase 1
- PRDX
peroxiredoxin
- PTP
permeability transition pore
- RCR
respiration control ratio
- ROS
reactive oxygen species
- RT1-m2
major histocompatibility complex gene
- S100β
S100-beta protein
- SDHB
protein complex II
- Sdhaf
succinate dehydrogenase assembly factor
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- SULT4A1
sulfotransferase 4A1
- TBARS
thiobarbituric acid reactive substances
- TFAM
mitochondrial transcription factor A
- TGFβ
transforming growth factor beta
- TLR4
Toll-like receptor 4
- TNFα
tumor necrosis factor α
- TOM
translocase of the outer membrane
- Trolox
synthetic vitamin E analogue
- UC-MSC
umbilical cord mesenchymal stromal cell
- UQCRC2
protein complex III
- VDAC
voltage-dependent anion channel
- vATP5a
protein complex V
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Quentin Perrier: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Veronica Lisi: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Kelsey Fisherwellman: Writing – review & editing, Visualization, Validation, Supervision, Methodology. Sandrine Lablanche: Writing – review & editing, Validation, Resources. Amish Asthana: Writing – review & editing, Validation. Giuseppe Orlando: Writing – review & editing, Validation. Sophie Maiocchi: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology.
This article is a contribution to the special issue entitled “Redox Medicine: Translation of Basic Science to the Clinic”. Guest Edited by Prof. Giovanni E. Mann (King’s College London), Prof. Harry van Goor (University Medical Center Groningen), & Dr. Arno Bourgonje (Icahn School of Medicine at Mount Sinai).
Data sharing
The datasets generated during the current study are available from the corresponding authors on reasonable request.
References
- [1].Suomalainen A, Nunnari J, Mitochondria at the crossroads of health and disease, Cell 187 (11) (May 2024) 2601–2627, 10.1016/j.cell.2024.04.037. [DOI] [PubMed] [Google Scholar]
- [2].Borcherding N, Brestoff JR, The power and potential of mitochondria transfer, Nature 623 (7986) (Nov. 2023) 283–291, 10.1038/s41586-023-06537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu J, et al. , Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells, Oncotarget 8 (9) (Jan. 2017) 15539–15552, 10.18632/oncotarget.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang D, et al. , Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage, Cell Death Dis. 7 (11) (Nov. 2016) e2467, 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mas-Bargues C, Mitochondria pleiotropism in stem cell senescence: mechanisms and therapeutic approaches, Free Radic. Biol. Med. 208 (Nov. 2023) 657–671, 10.1016/j.freeradbiomed.2023.09.019. [DOI] [PubMed] [Google Scholar]
- [6].Kidwell CU, et al. , Transferred mitochondria accumulate reactive oxygen species, promoting proliferation, eLife 12 (Mar. 2023) e85494, 10.7554/eLife.85494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu Z, Sun Y, Qi Z, Cao L, Ding S, Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases, Cell Biosci. 12 (1) (May 2022) 66, 10.1186/s13578-022-00805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caicedo A, et al. , MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function, Sci. Rep. 5 (Mar. 2015) 9073, 10.1038/srep09073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cloer CM, et al. , Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion, J. Heart Lung Transplant. 42 (5) (May 2023) 575–584, 10.1016/j.healun.2023.01.002. [DOI] [PubMed] [Google Scholar]
- [10].Doulamis IP, et al. , Mitochondrial transplantation normalizes transcriptomic and proteomic shift associated with ischemia reperfusion injury in neonatal hearts donated after circulatory death, Sci. Rep. 14 (1) (Dec. 2024) 31236, 10.1038/s41598-024-82578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dong J, Wu B, Tian W, Human adipose tissue-derived small extracellular vesicles promote soft tissue repair through modulating M1-to-M2 polarization of macrophages, Stem Cell Res. Ther. 14 (1) (Apr. 2023) 67, 10.1186/s13287-023-03306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Raposo G, Stahl PD, Extracellular vesicles: a new communication paradigm? Nat. Rev. Mol. Cell Biol. 20 (9) (Sep. 2019) 509–510, 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- [13].Théry C, et al. , Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines, J. Extracell. Vesicles 7 (1) (Dec. 2018) 1535750, 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Welsh JA, et al. , Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches, J. Extracell. Vesicles 13 (2) (Feb. 2024) e12404, 10.1002/jev2.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matheoud D, et al. , Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation, Cell 166 (2) (Jul. 2016) 314–327, 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- [16].Soubannier V, et al. , A vesicular transport pathway shuttles cargo from Mitochondria to lysosomes, Curr. Biol. 22 (2) (Jan. 2012) 135–141, 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- [17].Zhou X, Liu S, Lu Y, Wan M, Cheng J, Liu J, MitoEV: a new player in multiple disease pathology and treatment, J. Extracell. Vesicles 12 (4) (Apr. 2023) 12320, 10.1002/jev2.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Iorio R, Petricca S, Di Emidio G, Falone S, Tatone C, Mitochondrial Extracellular Vesicles (mitoEV): emerging mediators of cell-to-cell communication in health, aging and age-related diseases, Ageing Res. Rev. 101 (Nov. 2024) 102522, 10.1016/j.arr.2024.102522. [DOI] [PubMed] [Google Scholar]
- [19].Amari L, Germain M, Mitochondrial extracellular vesicles – origins and roles, Front. Mol. Neurosci. 14 (Oct. 2021) 767219, 10.3389/fnmol.2021.767219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liang W, et al. , Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired, Nat. Commun. 14 (1) (Aug. 2023) 5031, 10.1038/s41467-023-40680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gao Y, et al. , Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis, J. Extracell. Vesicles 13 (2) (Feb. 2024) e12410, 10.1002/jev2.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zorova LD, et al. , Do extracellular vesicles derived from mesenchymal stem cells contain functional mitochondria? Int. J. Mol. Sci. 23 (13) (Jul. 2022) 7408, 10.3390/ijms23137408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tortolici F, Di Biagio C, Lettieri-Barbato D, Aquilano K, Protocol for detecting mitochondria extracellular vesicles of brown adipose tissue in mice, STAR Protoc. 5 (3) (Sep. 2024) 103161, 10.1016/j.xpro.2024.103161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW, SYRCLE’s risk of bias tool for animal studies, BMC Med. Res. Methodol. 14 (Mar. 2014) 43, 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bafadam S, Mokhtari B, Vafaee MS, Oscuyi ZZ, Nemati S, Badalzadeh R, Mitochondrial transplantation combined with coenzyme Q10 induces cardioprotection and mitochondrial improvement in aged Male rats with reperfusion injury, Exp. Physiol. (Mar. 2024), 10.1113/EP091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boutonnet L, et al. , Autologous mitochondrial transplantation in Male mice as a strategy to prevent deleterious effects of peripheral ischemia-reperfusion, Am. J. Physiol. Cell Physiol. 326 (2) (Feb. 2024) C449–C456, 10.1152/ajpcell.00639.2023. [DOI] [PubMed] [Google Scholar]
- [27].Salman M, et al. , Intranasal delivery of mitochondria attenuates brain injury by AMPK and SIRT1/PGC-1α pathways in a murine model of photothrombotic stroke, Mol. Neurobiol. 61 (5) (May 2024) 2822–2838, 10.1007/s12035-023-03739-4. [DOI] [PubMed] [Google Scholar]
- [28].Sun L, et al. , Mitochondrial transplantation confers protection against the effects of ischemic stroke by repressing microglial pyroptosis and promoting neurogenesis, Neural Regen. Res. 19 (6) (Jun. 2024) 1325–1335, 10.4103/1673-5374.385313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu Z, et al. , Oral mitochondrial transplantation using nanomotors to treat ischaemic heart disease, Nat. Nanotechnol. 19 (9) (Sep. 2024) 1375–1385, 10.1038/s41565-024-01681-7. [DOI] [PubMed] [Google Scholar]
- [30].Xu M, Zhu J, Wang Z, Yan J, Zhou X, Neuroprotective effect of autologous mitochondrial transplantation against global ischemia/reperfusion injury in a rat model of cardiac arrest, Mitochondrion 78 (Sep. 2024) 101924, 10.1016/j.mito.2024.101924. [DOI] [PubMed] [Google Scholar]
- [31].Liang X, et al. , Direct administration of mesenchymal stem cell-derived mitochondria improves cardiac function after infarction via ameliorating endothelial senescence, Bioeng. Transl. Med. 8 (1) (Jan. 2023) e10365, 10.1002/btm2.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mokhtari B, Badalzadeh R, Mitochondria-targeted combination treatment strategy counteracts myocardial reperfusion injury of aged rats by modulating autophagy and inflammatory response, Mol. Biol. Rep. 50 (5) (May 2023) 3973–3983, 10.1007/s11033-023-08318-3. [DOI] [PubMed] [Google Scholar]
- [33].Norat P, et al. , Intraarterial transplantation of mitochondria after ischemic stroke reduces cerebral infarction, Stroke Hoboken NJ 3 (3) (May 2023) e000644, 10.1161/svin.122.000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun X, et al. , Intravenous transplantation of an ischemic-specific Peptide-TPP-mitochondrial compound alleviates myocardial ischemic reperfusion injury, ACS Nano 17 (2) (Jan. 2023) 896–909, 10.1021/acsnano.2c05286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zeng J, et al. , Mitochondrial transplantation reduces lower limb ischemia-reperfusion injury by increasing skeletal muscle energy and adipocyte browning, Mol. Ther. Methods Clin. Dev. 31 (Dec. 2023) 101152, 10.1016/j.omtm.2023.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen T, et al. , Mitochondrial transplantation promotes remyelination and long-term locomotion recovery following cerebral ischemia, Mediat. Inflamm. 2022 (2022) 1346343, 10.1155/2022/1346343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doulamis IP, et al. , Transcriptomic and proteomic pathways of diabetic and non-diabetic mitochondrial transplantation, Sci. Rep. 12 (1) (Dec. 2022) 22101, 10.1038/s41598-022-25858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kubat GB, et al. , Investigation of the effect of isolated mitochondria transplantation on renal ischemia-reperfusion injury in rats, Toxicol. Appl. Pharmacol. 433 (Dec. 2021) 115780, 10.1016/j.taap.2021.115780. [DOI] [PubMed] [Google Scholar]
- [39].Sun X, et al. , Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury, Bioact. Mater. 6 (7) (Jul. 2021) 2058–2069, 10.1016/j.bioactmat.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xie Q, et al. , Mitochondrial transplantation attenuates cerebral ischemia-reperfusion injury: possible involvement of mitochondrial component separation, Oxid. Med. Cell. Longev. 2021 (2021) 1006636, 10.1155/2021/1006636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blitzer D, et al. , Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model, Ann. Thorac. Surg. 109 (3) (Mar. 2020) 711–719, 10.1016/j.athoracsur.2019.06.075. [DOI] [PubMed] [Google Scholar]
- [42].Doulamis IP, et al. , Mitochondrial transplantation by intra-arterial injection for acute kidney injury, Am. J. Physiol. Ren. Physiol. 319 (3) (Sep. 2020) F403–F413, 10.1152/ajprenal.00255.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guariento A, et al. , Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection, J. Thorac. Cardiovasc. Surg. 160 (2) (Aug. 2020) e15–e29, 10.1016/j.jtcvs.2019.06.111. [DOI] [PubMed] [Google Scholar]
- [44].Jabbari H, et al. , Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat, Biochim. Biophys. Acta Mol. Basis Dis. 1866 (8) (Aug. 2020) 165809, 10.1016/j.bbadis.2020.165809. [DOI] [PubMed] [Google Scholar]
- [45].Moskowitzova K, et al. , Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury, Am. J. Physiol. Lung Cell. Mol. Physiol. 318 (1) (Jan. 2020) L78–L88, 10.1152/ajplung.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nakamura Y, Lo EH, Hayakawa K, Placental mitochondria therapy for cerebral ischemia-reperfusion injury in mice, Stroke 51 (10) (Oct. 2020) 3142–3146, 10.1161/STROKEAHA.120.030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Orfany A, et al. , Mitochondrial transplantation ameliorates acute limb ischemia, J. Vasc. Surg. 71 (3) (Mar. 2020) 1014–1026, 10.1016/j.jvs.2019.03.079. [DOI] [PubMed] [Google Scholar]
- [48].Pourmohammadi-Bejarpasi Z, et al. , Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model, Brain Res. Bull. 165 (Dec. 2020) 70–80, 10.1016/j.brainresbull.2020.09.018. [DOI] [PubMed] [Google Scholar]
- [49].Shin B, et al. , A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy, JACC Basic Transl. Sci. 4 (8) (Dec. 2019) 871–888, 10.1016/j.jacbts.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang Z, et al. , Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury, Behav. Brain Res. 356 (Jan. 2019) 322–331, 10.1016/j.bbr.2018.09.005. [DOI] [PubMed] [Google Scholar]
- [51].Kaza AK, et al. , Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion, J. Thorac. Cardiovasc. Surg. 153 (4) (Apr. 2017) 934–943, 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- [52].Huang P-J, et al. , Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains, Cell Transplant. 25 (5) (2016) 913–927, 10.3727/096368915X689785. [DOI] [PubMed] [Google Scholar]
- [53].Lin H-C, Liu S-Y, Lai H-S, Lai I-R, Isolated mitochondria infusion mitigates ischemia-reperfusion injury of the liver in rats, Shock Augusta Ga 39 (3) (Mar. 2013) 304–310, 10.1097/SHK.0b013e318283035f. [DOI] [PubMed] [Google Scholar]
- [54].Masuzawa A, et al. , Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury, Am. J. Physiol. Heart Circ. Physiol. 304 (7) (Apr. 2013) H966–H982, 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gai C, et al. , Mesenchymal stromal cells deliver H2S-enhanced Nrf2 via extracellular vesicles to mediate mitochondrial homeostasis for repairing hypoxia-ischemia brain damage, Free Radic. Biol. Med. 225 (Nov. 2024) 528–545, 10.1016/j.freeradbiomed.2024.10.292. [DOI] [PubMed] [Google Scholar]
- [56].Wu Y, et al. , FUS-mediated HypEV: neuroprotective effects against ischemic stroke, Bioact. Mater. 29 (Nov. 2023) 196–213, 10.1016/j.bioactmat.2023.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ikeda G, et al. , Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium, J. Am. Coll. Cardiol. 77 (8) (Mar. 2021) 1073–1088, 10.1016/j.jacc.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lu T, et al. , Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia–reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps, Biomaterials 284 (May 2022) 121486, 10.1016/j.biomaterials.2022.121486. [DOI] [PubMed] [Google Scholar]
- [59].Zhao M, et al. , Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA, ACS Nano 15 (1) (Jan. 2021) 1519–1538, 10.1021/acsnano.0c08947. [DOI] [PubMed] [Google Scholar]
- [60].Yao J, et al. , Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 33 (2) (Feb. 2019) 1695–1710, 10.1096/fj.201800131RR. [DOI] [PubMed] [Google Scholar]
- [61].Eo H, et al. , Mitochondrial transplantation exhibits neuroprotective effects and improves behavioral deficits in an animal model of Parkinson’s disease, Neurother. J. Am. Soc. Exp. Neurother. 21 (4) (Jul. 2024) e00355, 10.1016/j.neurot.2024.e00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jain R, Begum N, Rajan S, Tryphena KP, Khatri DK, Role of F-actin-mediated endocytosis and exercise in mitochondrial transplantation in an experimental Parkinson’s disease mouse model, Mitochondrion 74 (Jan. 2024) 101824, 10.1016/j.mito.2023.11.007. [DOI] [PubMed] [Google Scholar]
- [63].Xu J, et al. , Targeted transplantation of engineered mitochondrial compound promotes functional recovery after spinal cord injury by enhancing macrophage phagocytosis, Bioact. Mater. 32 (Feb. 2024) 427–444, 10.1016/j.bioactmat.2023.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bamshad C, et al. , Human umbilical cord-derived mesenchymal stem cells-harvested mitochondrial transplantation improved motor function in TBI models through rescuing neuronal cells from apoptosis and alleviating astrogliosis and microglia activation, Int. Immunopharmacol. 118 (May 2023) 110106, 10.1016/j.intimp.2023.110106. [DOI] [PubMed] [Google Scholar]
- [65].Ene HM, Karry R, Farfara D, Ben-Shachar D, Mitochondria play an essential role in the trajectory of adolescent neurodevelopment and behavior in adulthood: evidence from a schizophrenia rat model, Mol. Psychiatr. 28 (3) (Mar. 2023) 1170–1181, 10.1038/s41380-022-01865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hayashida K, et al. , Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest, BMC Med. 21 (1) (Mar. 2023) 56, 10.1186/s12916-023-02759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Huang C-C, et al. , Mitochondrial transplantation attenuates traumatic neuropathic pain, neuroinflammation, and apoptosis in rats with nerve root ligation, Mol. Pain 19 (2023) 17448069231210423, 10.1177/17448069231210423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Javani G, Ghaffari-Nasab A, Farajdokht F, Mohaddes G, Chronic stress-induced apoptosis is mitigated by young mitochondria transplantation in the prefrontal cortex of aged rats, Iran. J. Basic Med. Sci. 26 (6) (2023) 725–730, 10.22038/IJBMS.2023.69551.15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Javani G, Babri S, Farajdokht F, Ghaffari-Nasab A, Mohaddes G, Mitotherapy restores hippocampal mitochondrial function and cognitive impairment in aged Male rats subjected to chronic mild stress, Biogerontology 24 (2) (Apr. 2023) 257–273, 10.1007/s10522-022-10014-x. [DOI] [PubMed] [Google Scholar]
- [70].Jia X, et al. , Mitochondrial transplantation ameliorates hippocampal damage following status epilepticus, Anim. Models Exp. Med. 6 (1) (Feb. 2023) 41–50, 10.1002/ame2.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang X, et al. , Improvement effect of mitotherapy on the cognitive ability of alzheimer’s disease through NAD+/SIRT1-Mediated autophagy, Antioxid. Basel Switz. 12 (11) (Nov. 2023) 2006, 10.3390/antiox12112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhu Z, et al. , Photobiomodulation augments the effects of mitochondrial transplantation in the treatment of spinal cord injury in rats by facilitating mitochondrial transfer to neurons via connexin 36, Bioeng. Transl. Med. 8 (3) (May 2023) e10473, 10.1002/btm2.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Adlimoghaddam A, Benson T, Albensi BC, Mitochondrial transfusion improves mitochondrial function through Up-regulation of mitochondrial complex II protein subunit SDHB in the hippocampus of aged mice, Mol. Neurobiol. 59 (10) (Oct. 2022) 6009–6017, 10.1007/s12035-022-02937-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hosseini L, et al. , Intranasal administration of mitochondria alleviated cognitive impairments and mitochondrial dysfunction in the photothrombotic model of mPFC stroke in mice, J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 31 (12) (Dec. 2022) 106801, 10.1016/j.jstrokecerebrovasdis.2022.106801. [DOI] [PubMed] [Google Scholar]
- [75].Javani G, Babri S, Farajdokht F, Ghaffari-Nasab A, Mohaddes G, Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats, Mech. Ageing Dev. 202 (Mar. 2022) 111632, 10.1016/j.mad.2022.111632. [DOI] [PubMed] [Google Scholar]
- [76].Lin M-W, et al. , Mitochondrial transplantation attenuates neural damage and improves locomotor function after traumatic spinal cord injury in rats, Front. Neurosci. 16 (2022) 800883, 10.3389/fnins.2022.800883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhang Z, Wei D, Li Z, Guo H, Wu Y, Feng J, Hippocampal mitochondrial transplantation alleviates age-associated cognitive decline via enhancing wnt signaling and neurogenesis, Comput. Intell. Neurosci. 2022 (2022) 9325302, 10.1155/2022/9325302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fang S-Y, et al. , Transplantation of viable mitochondria attenuates neurologic injury after spinal cord ischemia, J. Thorac. Cardiovasc. Surg. 161 (5) (May 2021) e337–e347, 10.1016/j.jtcvs.2019.10.151. [DOI] [PubMed] [Google Scholar]
- [79].Sheu M-L, et al. , Dual regeneration of muscle and nerve by intramuscular infusion of mitochondria in a nerve crush injury model, Neurosurgery 89 (1) (Jun. 2021) E49–E59, 10.1093/neuros/nyab105. [DOI] [PubMed] [Google Scholar]
- [80].Zhao J, et al. , Mitochondria transplantation protects traumatic brain injury via promoting neuronal survival and astrocytic BDNF, Transl. Res. J. Lab. Clin. Med. 235 (Sep. 2021) 102–114, 10.1016/j.trsl.2021.03.017. [DOI] [PubMed] [Google Scholar]
- [81].Ma H, et al. , Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice, Clin. Sci. Lond. Engl. 1979 134 (16) (Aug. 2020) 2161–2175, 10.1042/CS20200530. [DOI] [PubMed] [Google Scholar]
- [82].Nascimento-Dos-Santos G, et al. , Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina, Biochim. Biophys. Acta Mol. Basis Dis. 1866 (5) (May 2020) 165686, 10.1016/j.bbadis.2020.165686. [DOI] [PubMed] [Google Scholar]
- [83].Zhao Z, Yu Z, Hou Y, Zhang L, Fu A, Improvement of cognitive and motor performance with mitotherapy in aged mice, Int. J. Biol. Sci. 16 (5) (2020) 849–858, 10.7150/ijbs.40886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nitzan K, et al. , Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in alzheimer’s disease mice, J. Alzheimers Dis. JAD 72 (2) (2019) 587–604, 10.3233/JAD-190853. [DOI] [PubMed] [Google Scholar]
- [85].Wang Y, et al. , Mitochondrial transplantation attenuates lipopolysaccharide-induced depression-like behaviors, Prog. Neuropsychopharmacol. Biol. Psychiatry 93 (Jul. 2019) 240–249, 10.1016/j.pnpbp.2019.04.010. [DOI] [PubMed] [Google Scholar]
- [86].Gollihue JL, et al. , Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after Spinal cord injury, J. Neurotrauma 35 (15) (Aug. 2018) 1800–1818, 10.1089/neu.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Robicsek O, et al. , Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues deficits in a rat model of the disorder, Schizophr. Bull. 44 (2) (Feb. 2018) 432–442, 10.1093/schbul/sbx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shi X, Zhao M, Fu C, Fu A, Intravenous administration of mitochondria for treating experimental Parkinson’s disease, Mitochondrion 34 (May 2017) 91–100, 10.1016/j.mito.2017.02.005. [DOI] [PubMed] [Google Scholar]
- [89].Chang J-C, et al. , Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity, Transl. Res. J. Lab. Clin. Med. 170 (Apr. 2016) 40–56.e3, 10.1016/j.trsl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- [90].Jin N, et al. , Mitochondria transplantation alleviates cardiomyocytes apoptosis through inhibiting AMPKα-mTOR mediated excessive autophagy, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 38 (10) (May 2024) e23655, 10.1096/fj.202400375R. [DOI] [PubMed] [Google Scholar]
- [91].Kim MJ, Lee JM, Min K, Choi Y-S, Xenogeneic transplantation of mitochondria induces muscle regeneration in an in vivo rat model of dexamethasone-induced atrophy, J. Muscle Res. Cell Motil. 45 (2) (Jun. 2024) 53–68, 10.1007/s10974-023-09643-7. [DOI] [PubMed] [Google Scholar]
- [92].Kubat GB, et al. , The effects of exercise and mitochondrial transplantation alone or in combination against Doxorubicin-induced skeletal muscle atrophy, J. Muscle Res. Cell Motil. (Jun. 2024), 10.1007/s10974-024-09676-6. [DOI] [PubMed] [Google Scholar]
- [93].Maia JRLCB, et al. , Mitotherapy prevents peripheral neuropathy induced by oxaliplatin in mice, Neuropharmacology 245 (Mar. 2024) 109828, 10.1016/j.neuropharm.2023.109828. [DOI] [PubMed] [Google Scholar]
- [94].Maleki F, Rabbani S, Shirkoohi R, Rezaei M, Allogeneic mitochondrial transplantation ameliorates cardiac dysfunction due to doxorubicin: an in vivo study, Biomed. Pharmacother. 168 (Dec. 2023) 115651, 10.1016/j.biopha.2023.115651. Biomedecine Pharmacother. [DOI] [PubMed] [Google Scholar]
- [95].Sun X, et al. , Mitochondrial transplantation ameliorates doxorubicin-induced cardiac dysfunction via activating glutamine metabolism, iScience 26 (10) (Oct. 2023) 107790, 10.1016/j.isci.2023.107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang A, et al. , Delivery of mitochondria confers cardioprotection through mitochondria replenishment and metabolic compliance, Mol. Ther. J. Am. Soc. Gene Ther 31 (5) (May 2023) 1468–1479, 10.1016/j.ymthe.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Alexander JF, et al. , Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits, Theranostics 11 (7) (2021) 3109–3130, 10.7150/thno.53474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kubat GB, et al. , The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats, J. Biochem. Mol. Toxicol. 35 (1) (Jan. 2021) e22612, 10.1002/jbt.22612. [DOI] [PubMed] [Google Scholar]
- [99].Ulger O, et al. , The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats, Life Sci. 279 (Aug. 2021) 119669, 10.1016/j.lfs.2021.119669. [DOI] [PubMed] [Google Scholar]
- [100].Shi X, et al. , Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice, Transl. Res. J. Lab. Clin. Med. 196 (Jun. 2018) 31–41, 10.1016/j.trsl.2018.02.003. [DOI] [PubMed] [Google Scholar]
- [101].Kim S, et al. , Effects of mitochondrial transplantation on transcriptomics in a polymicrobial sepsis model, Int. J. Mol. Sci. 24 (20) (Oct. 2023) 15326, 10.3390/ijms242015326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kim Y-S, et al. , The effects of mitochondrial transplantation on sepsis depend on the type of cell from which they are isolated, Int. J. Mol. Sci. 24 (12) (Jun. 2023) 10113, 10.3390/ijms241210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mokhtari B, Hamidi M, Badalzadeh R, Mahmoodpoor A, Mitochondrial transplantation protects against sepsis-induced myocardial dysfunction by modulating mitochondrial biogenesis and fission/fusion and inflammatory response, Mol. Biol. Rep. 50 (3) (Mar. 2023) 2147–2158, 10.1007/s11033-022-08115-4. [DOI] [PubMed] [Google Scholar]
- [104].de Carvalho LRP, et al. , Mitochondria-rich fraction isolated from mesenchymal stromal cells reduces lung and distal organ injury in experimental sepsis, Crit. Care Med. 49 (9) (Sep. 2021) e880–e890, 10.1097/CCM.0000000000005056. [DOI] [PubMed] [Google Scholar]
- [105].Hwang JW, et al. , The immune modulatory effects of mitochondrial transplantation on cecal slurry model in rat, Crit. Care Lond. Engl. 25 (1) (Jan. 2021) 20, 10.1186/s13054-020-03436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang Z, Yan C, Miao J, Pu K, Ma H, Wang Q, Muscle-derived mitochondrial transplantation reduces inflammation, enhances bacterial clearance, and improves survival in sepsis, Shock Augusta Ga 56 (1) (Jul. 2021) 108–118, 10.1097/SHK.0000000000001681. [DOI] [PubMed] [Google Scholar]
- [107].Yan C, et al. , Mitochondrial transplantation attenuates brain dysfunction in sepsis by driving microglial M2 polarization, Mol. Neurobiol. 57 (9) (Sep. 2020) 3875–3890, 10.1007/s12035-020-01994-3. [DOI] [PubMed] [Google Scholar]
- [108].Bao W, et al. , Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis, Nat. Commun. 13 (1) (Aug. 2022) 4583, 10.1038/s41467-022-32325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zheng D, et al. , Mesenchymal stem cell-derived microvesicles improve intestinal barrier function by restoring mitochondrial dynamic balance in sepsis rats, Stem Cell Res. Ther. 12 (1) (May 2021) 299, 10.1186/s13287-021-02363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Arroum T, Hish GA, Burghardt KJ, McCully JD, Hüttemann M, Malek MH, Mitochondrial transplantation’s role in rodent skeletal muscle bioenergetics: recharging the engine of aging, Biomolecules 14 (4) (Apr. 2024) 493, 10.3390/biom14040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Arroum T, et al. , Mitochondria transplantation: rescuing innate muscle bioenergetic impairment in a model of aging and exercise intolerance, J. Strength Condit Res. 38 (7) (Jul. 2024) 1189–1199, 10.1519/JSC.0000000000004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li Z, Cao X, Liu Z, Wu F, Lin C, Wang C-M, Therapeutic effect of mitochondrial transplantation on burn injury, Free Radic. Biol. Med. 215 (Mar. 2024) 2–13, 10.1016/j.freeradbiomed.2024.02.019. [DOI] [PubMed] [Google Scholar]
- [113].Raz D, et al. , Mitochondria transplantation promotes corneal epithelial wound healing, Investig. Ophthalmol. Vis. Sci. 65 (6) (Jun. 2024) 14, 10.1167/iovs.65.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kshersagar J, Pulgam L, Damle MN, Tardalkar K, Sharma R, Joshi MG, Transplantation of human placenta derived mitochondria promotes cell communication in endometrium in a murine model of disturbed endometrium, Stem Cell Rev. Rep. 19 (5) (Jul. 2023) 1384–1401, 10.1007/s12015-023-10516-2. [DOI] [PubMed] [Google Scholar]
- [115].Wu S-F, et al. , Mitochondrial transplantation moderately ameliorates retinal degeneration in Royal college of surgeons rats, Biomedicines 10 (11) (Nov. 2022) 2883, 10.3390/biomedicines10112883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu H-C, et al. , Comparison of mitochondrial transplantation by using a stamp-type multineedle injector and platelet-rich plasma therapy for hair aging in naturally aging mice, Biomed. Pharmacother. 130 (Oct. 2020) 110520, 10.1016/j.biopha.2020.110520. Biomedecine Pharmacother. [DOI] [PubMed] [Google Scholar]
- [117].Yao W-D, et al. , Hydrogel microneedle patches loaded with stem cell mitochondria-enriched microvesicles boost the chronic wound healing, ACS Nano 18 (39) (Oct. 2024) 26733–26750, 10.1021/acsnano.4c06921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhuang Y, et al. , Energy metabolism as therapeutic target for aged wound repair by engineered extracellular vesicle, Sci. Adv. 10 (15) (Apr. 2024) eadl0372, 10.1126/sciadv.adl0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chen X, et al. , Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism, Nat. Aging 4 (6) (Jun. 2024) 814–838, 10.1038/s43587-024-00612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Dubinin MV, et al. , Mitochondrial transplantation therapy ameliorates muscular dystrophy in mdx Mouse model, Biomolecules 14 (3) (Mar. 2024) 316, 10.3390/biom14030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhai J, Chen Z, Chen P, Yang W, Wei H, Adipose derived mesenchymal stem cells-derived mitochondria transplantation ameliorated erectile dysfunction induced by cavernous nerve injury, World J. Mens Health 42 (1) (Jan. 2024) 188–201, 10.5534/wjmh.220233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Alway SE, et al. , Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle, J. Cachexia Sarcopenia Muscle 14 (1) (Feb. 2023) 493–507, 10.1002/jcsm.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cassano JM, et al. , Evaluating the safety of intra-articular mitotherapy in the equine model: a potential novel treatment for osteoarthritis, J. Equine Vet. Sci. 120 (Jan. 2023) 104164, 10.1016/j.jEV.2022.104164. [DOI] [PubMed] [Google Scholar]
- [124].Celik A, Orfany A, Dearling J, Del Nido PJ, McCully JD, Bakar-Ates F, Mitochondrial transplantation: effects on chemotherapy in prostate and ovarian cancer cells in vitro and in vivo, Biomed. Pharmacother. 161 (May 2023) 114524, 10.1016/j.biopha.2023.114524. Biomedecine Pharmacother. [DOI] [PubMed] [Google Scholar]
- [125].Mudgal P, Pareek J, Paliwal S, Biodistribution of intravenously transplanted Mitochondria conjugated with graphene quantum dots in diabetic rats, J. Fluoresc. (Oct. 2023), 10.1007/s10895-023-03480-0. [DOI] [PubMed] [Google Scholar]
- [126].Paliwal S, Jain S, Mudgal P, Verma K, Paliwal S, Sharma S, Mitochondrial transfer restores impaired liver functions by AMPK/mTOR/PI3K-AKT pathways in metabolic syndrome, Life Sci. 332 (Nov. 2023) 122116, 10.1016/j.lfs.2023.122116. [DOI] [PubMed] [Google Scholar]
- [127].Hsu C-H, et al. , Transplantation of viable mitochondria improves right ventricular performance and pulmonary artery remodeling in rats with pulmonary arterial hypertension, J. Thorac. Cardiovasc. Surg. 163 (5) (May 2022) e361–e373, 10.1016/j.jtcvs.2020.08.014. [DOI] [PubMed] [Google Scholar]
- [128].Lee AR, et al. , Mitochondrial transplantation ameliorates the development and progression of osteoarthritis, Immune Netw 22 (2) (Apr. 2022) e14, 10.4110/in.2022.22.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pang Y-L, et al. , Viable allogeneic mitochondria transplantation improves gas exchange and alveolar-capillary permeability in rats with endotoxin-induced acute lung injuries, Int. J. Med. Sci. 19 (6) (2022) 1036–1046, 10.7150/ijms.73151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhou W, Zhao Z, Yu Z, Hou Y, Keerthiga R, Fu A, Mitochondrial transplantation therapy inhibits the proliferation of malignant hepatocellular carcinoma and its mechanism, Mitochondrion 65 (Jul. 2022) 11–22, 10.1016/j.mito.2022.04.004. [DOI] [PubMed] [Google Scholar]
- [131].Lee JM, et al. , Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro, Antioxid. Basel Switz. 10 (5) (Apr. 2021) 696, 10.3390/antiox10050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Weixler V, et al. , Autogenous mitochondria transplantation for treatment of right heart failure, J. Thorac. Cardiovasc. Surg. 162 (1) (Jul. 2021) e111–e121, 10.1016/j.jtcvs.2020.08.011. [DOI] [PubMed] [Google Scholar]
- [133].Yu Z, Hou Y, Zhou W, Zhao Z, Liu Z, Fu A, The effect of mitochondrial transplantation therapy from different gender on inhibiting cell proliferation of malignant melanoma, Int. J. Biol. Sci. 17 (8) (2021) 2021–2033, 10.7150/ijbs.59581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zhao Z, Hou Y, Zhou W, Keerthiga R, Fu A, Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes, Bioeng. Transl. Med. 6 (2) (May 2021) e10209, 10.1002/btm2.10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Moskowitzova K, et al. , Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation, J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant 38 (1) (Jan. 2019) 92–99, 10.1016/j.healun.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ramirez-Barbieri G, et al. , Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria, Mitochondrion 46 (May 2019) 103–115, 10.1016/j.mito.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [137].Fu A, Shi X, Zhang H, Fu B, Mitotherapy for fatty liver by intravenous administration of exogenous mitochondria in Male mice, Front. Pharmacol. 8 (2017) 241, 10.3389/fphar.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Su Y, et al. , Mitochondrial transplantation attenuates airway hyperresponsiveness by inhibition of cholinergic hyperactivity, Theranostics 6 (8) (2016) 1244–1260, 10.7150/thno.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhu L, et al. , Mitochondrial transplantation attenuates hypoxic pulmonary hypertension, Oncotarget 7 (31) (Aug. 2016) 48925–48940, 10.18632/oncotarget.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Liang Z, et al. , Mitochondria-rich microvesicles alleviate CNI ED by transferring mitochondria and suppressing local ferroptosis, Int. J. Nanomed. 19 (2024) 11745–11765, 10.2147/IJN.S488163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Shen M, et al. , Metabolic reprogramming of CD4+ T cells by mesenchymal stem cell-derived extracellular vesicles attenuates autoimmune hepatitis through mitochondrial protein transfer, Int. J. Nanomed. 19 (2024) 9799–9819, 10.2147/IJN.S472086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tolomeo AM, et al. , Protective effects of mesenchymal stem cells-derived extracellular vesicles against ischemia-reperfusion injury of hearts donated after circulatory death: preliminary study in a pig model, Biomed. Pharmacother. 178 (Sep. 2024) 117256, 10.1016/j.biopha.2024.117256. [DOI] [PubMed] [Google Scholar]
- [143].Cao H, et al. , Biosynthetic dendritic cell-exocytosed aggregation-induced emission nanoparticles for synergistic photodynamic immunotherapy, ACS Nano 16 (9) (Sep. 2022) 13992–14006, 10.1021/acsnano.2c03597. [DOI] [PubMed] [Google Scholar]
- [144].Lu Y, et al. , Extracellular vesicles DJ-1 derived from hypoxia-conditioned hMSCs alleviate cardiac hypertrophy by suppressing mitochondria dysfunction and preventing ATRAP degradation, Pharmacol. Res. 187 (Jan. 2023) 106607, 10.1016/j.phrs.2022.106607. [DOI] [PubMed] [Google Scholar]
- [145].Li Y, et al. , Tetramethylpyrazine prevents liver fibrotic injury in mice by targeting hepatocyte-derived and mitochondrial DNA-enriched extracellular vesicles, Acta Pharmacol. Sin. 43 (8) (Aug. 2022) 2026–2041, 10.1038/s41401-021-00843-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dutra Silva J, et al. , Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS, Eur. Respir. J. 58 (1) (Jul. 2021) 2002978, 10.1183/13993003.02978-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Morrison TJ, et al. , Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer, Am. J. Respir. Crit. Care Med. 196 (10) (Nov. 2017) 1275–1286, 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Monzel AS, Enríquez JA, Picard M, Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction, Nat. Metab. 5 (4) (Apr. 2023) 546–562, 10.1038/s42255-023-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mitchell P, Moyle J, Chemiosmotic Hypothesis of oxidative phosphorylation, Nature 213 (5072) (Jan. 1967) 137–139, 10.1038/213137a0. [DOI] [PubMed] [Google Scholar]
- [150].Morciano G, Imamura H, Patergnani S, Pedriali G, Giorgi C, Pinton P, Measurement of ATP concentrations in mitochondria of living cells using luminescence and fluorescence approaches, Methods Cell Biol. 155 (2020) 199–219, 10.1016/bs.mcb.2019.10.007. Elsevier. [DOI] [PubMed] [Google Scholar]
- [151].Wang Y, et al. , Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit–pathogen interactions, Int. J. Mol. Sci. 20 (12) (Jun. 2019) 2994, 10.3390/ijms20122994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tirichen H, Yaigoub H, Xu W, Wu C, Li R, Li Y, Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress, Front. Physiol. 12 (Apr. 2021) 627837, 10.3389/fphys.2021.627837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Papa S, et al. , The oxidative phosphorylation system in Mammalian mitochondria, in: Scatena R, Bottoni P, Giardina B (Eds.), Advances in Mitochondrial Medicine vol. 942, Springer; Netherlands, Dordrecht, 2012, pp. 3–37, 10.1007/978-94-007-2869-1_1. Advances in Experimental Medicine and Biology, vol. 942. [DOI] [PubMed] [Google Scholar]
- [154].Adlimoghaddam A, Benson T, Albensi BC, Mitochondrial transfusion improves mitochondrial function through Up-regulation of mitochondrial complex II protein subunit SDHB in the hippocampus of aged mice, Mol. Neurobiol. 59 (10) (Oct. 2022), 10.1007/s12035-022-02937-w, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Spinelli JB, Haigis MC, The multifaceted contributions of mitochondria to cellular metabolism, Nat. Cell Biol. 20 (7) (Jul. 2018) 745–754, 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lee SH, Duron HE, Chaudhuri D, Beyond the TCA cycle: new insights into mitochondrial calcium regulation of oxidative phosphorylation, Biochem. Soc. Trans. 51 (4) (Aug. 2023) 1661–1673, 10.1042/BST20230012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Dubinin MV, et al. , Mitochondrial transplantation therapy ameliorates muscular dystrophy in mdx Mouse model, Biomolecules 14 (3) (Mar. 2024), 10.3390/biom14030316, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Mendoza AM, Karch J, Simultaneous acquisition of mitochondrial calcium retention capacity and swelling to measure permeability transition sensitivity, in: Tomar N (Ed.), Methods in Molecular Biology, vol. 2497, Springer US, New York, NY, 2022, pp. 129–140, 10.1007/978-1-0716-2309-1_9. Mitochondria, vol. 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Le Gal K, Schmidt EE, Sayin VI, Cellular redox homeostasis, Antioxidants 10 (9) (Aug. 2021) 1377, 10.3390/antiox10091377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sies H, Mailloux RJ, Jakob U, Fundamentals of redox regulation in biology, Nat. Rev. Mol. Cell Biol. 25 (9) (Sep. 2024) 701–719, 10.1038/s41580-024-00730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Hensen SMM, Heldens L, Van Enckevort CMW, Van Genesen ST, Pruijn GJM, Lubsen NH, Activation of the antioxidant response in methionine deprived human cells results in an HSF1-independent increase in HSPA1A mRNA levels, Biochimie 95 (6) (Jun. 2013) 1245–1251, 10.1016/j.biochi.2013.01.017. [DOI] [PubMed] [Google Scholar]
- [162].Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP, Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders, Antioxidants Redox Signal. 13 (11) (Dec. 2010) 1763–1811, 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yamamoto M, Kensler TW, Motohashi H, The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis, Physiol. Rev. 98 (3) (Jul. 2018) 1169–1203, 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Van Vranken JG, et al. , SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration, Cell Metab. 20 (2) (Aug. 2014) 241–252, 10.1016/j.cmet.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kawamori D, et al. , The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation, J. Biol. Chem. 281 (2) (Jan. 2006) 1091–1098, 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- [166].Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC, Mitochondrial glutathione, a key survival antioxidant, Antioxidants Redox Signal. 11 (11) (Nov. 2009) 2685–2700, 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Guo Y, Yu S, Zhang C, Kong A-NT, Epigenetic regulation of Keap1-Nrf2 signaling, Free Radic. Biol. Med. 88 (Nov. 2015) 337–349, 10.1016/j.freeradbiomed.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Canovas B, Nebreda AR, Diversity and versatility of p38 kinase signalling in health and disease, Nat. Rev. Mol. Cell Biol. 22 (5) (May 2021) 346–366, 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Giacomello M, Pyakurel A, Glytsou C, Scorrano L, The cell biology of mitochondrial membrane dynamics, Nat. Rev. Mol. Cell Biol. 21 (4) (Apr. 2020) 204–224, 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- [170].Frey TG, Mannella CA, The internal structure of mitochondria, Trends Biochem. Sci. 25 (7) (Jul. 2000) 319–324, 10.1016/S0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- [171].Darshi M, et al. , ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function, J. Biol. Chem. 286 (4) (Jan. 2011) 2918–2932, 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Chen H, et al. , Titration of mitochondrial fusion rescues Mff -deficient cardiomyopathy, J. Cell Biol. 211 (4) (Nov. 2015) 795–805, 10.1083/jcb.201507035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Adebayo M, Singh S, Singh AP, Dasgupta S, Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis, FASEB J. 35 (6) (Jun. 2021), 10.1096/fj.202100067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Van Der Bliek AM, Shen Q, Kawajiri S, Mechanisms of mitochondrial fission and fusion, Cold Spring Harbor Perspect. Biol. 5 (6) (Jun. 2013) a011072, 10.1101/cshperspect.a011072, a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yang L, et al. , Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA, Cell. Mol. Life Sci. 72 (13) (Jul. 2015) 2585–2598, 10.1007/s00018-015-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chen W, Zhao H, Li Y, Mitochondrial dynamics in health and disease: mechanisms and potential targets, Signal Transduct. Targeted Ther. 8 (1) (Sep. 2023) 333, 10.1038/s41392-023-01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mishra P, Chan DC, Mitochondrial dynamics and inheritance during cell division, development and disease, Nat. Rev. Mol. Cell Biol. 15 (10) (Oct. 2014) 634–646, 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A, Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment, CNS Neurosci. Ther. 23 (1) (Jan. 2017) 5–22, 10.1111/cns.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Ploumi C, Daskalaki I, Tavernarakis N, Mitochondrial biogenesis and clearance: a balancing act, FEBS J. 284 (2) (Jan. 2017) 183–195, 10.1111/febs.13820. [DOI] [PubMed] [Google Scholar]
- [180].Wang S, et al. , The mitophagy pathway and its implications in human diseases, Signal Transduct. Targeted Ther. 8 (1) (Aug. 2023) 304, 10.1038/s41392-023-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Abu Shelbayeh O, Arroum T, Morris S, Busch KB, PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response, Antioxidants 12 (5) (May 2023) 1075, 10.3390/antiox12051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Rowland AA, Voeltz GK, Endoplasmic reticulum–mitochondria contacts: function of the junction, Nat. Rev. Mol. Cell Biol. 13 (10) (Oct. 2012) 607–615, 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Chen Y, Xin Y, Cheng Y, Liu X, Mitochondria-endoplasmic reticulum contacts: the promising regulators in diabetic cardiomyopathy, Oxid. Med. Cell. Longev. 2022 (Apr. 2022) 1–13, 10.1155/2022/2531458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD, Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury, J. Thorac. Cardiovasc. Surg. 154 (1) (Jul. 2017) 286–289, 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- [185].Guariento A, et al. , Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury, J. Thorac. Cardiovasc. Surg. 162 (3) (Sep. 2021) 992–1001, 10.1016/j.jtcvs.2020.10.151. [DOI] [PubMed] [Google Scholar]
- [186].Baharvand F, et al. , Safety and efficacy of platelet-derived mitochondrial transplantation in ischaemic heart disease, Int. J. Cardiol. 410 (Sep. 2024) 132227, 10.1016/j.ijcard.2024.132227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors on reasonable request.


