Abstract
Background
The risk of post-contrast acute kidney injury (PC-AKI) following subclinical acute kidney injury (sAKI) remains unclear. This study was performed to determine whether sAKI increases the risk of PC-AKI and to assess the utility of multiparametric MRI, including diffusion kurtosis imaging (DKI) and arterial spin labeling (ASL), for detecting pathological changes with high sensitivity.
Methods
A rat model of subclinical kidney injury was established through subcutaneous injection of carbon tetrachloride for 6 weeks. Subclinical kidney injury and control groups (n = 30 each) received intravascular iopamidol (4 g iodine/kg). Kidney MRI, including DKI and ASL, was conducted at 24 h before and at 1, 24, 48, and 72 h after iopamidol administration (n = 6 per time point). Key parameters measured included renal mean kurtosis (MK), mean diffusion (MD), renal blood flow (RBF), blood urea nitrogen (BUN), serum creatinine (SCr), histopathological changes assessed by hematoxylin-eosin (HE) score, hypoxia-inducible factor-1α (HIF-1α) expression, and renal tissue neutrophil gelatinase-associated lipocalin (NGAL) expression.
Results
At baseline, the subclinical kidney injury group showed higher mRNA expression of renal NGAL, increased HE scores, and a greater HIF-1α-positive area in all renal compartments compared with controls. Additionally, MD in the outer stripe of the outer medulla (OSOM) and RBF were lower in the subclinical kidney injury group than in the control group. At 1 h after iopamidol injection, HE scores, NGAL expression, and HIF-1α expression further increased, whereas MD and RBF further decreased in both groups compared with their respective baselines. At 24 h in the control group, all indicators returned to baseline levels, but changes in the subclinical kidney injury group persisted. By 48 and 72 h, all indicators in both groups had returned to baseline levels. SCr and BUN levels remained unchanged in the control group at all time points but increased at 1 and 24 h in the subclinical kidney injury group. MRI parameters showed strong correlations with histopathological findings and NGAL expression.
Conclusions
Rats with subclinical kidney injury exhibited more severe PC-AKI and slower resolution of microstructural and oxygenation changes compared with controls.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-04413-8.
Keywords: Subclinical kidney injury, Post-contrast acute kidney injury, Arterial spin labeling, Diffusion kurtosis imaging, Iodinated contrast medium
Introduction
Post-contrast acute kidney injury (PC-AKI), a leading cause of hospital-acquired acute kidney injury (AKI), is defined as decreased renal function after intravascular injection of contrast medium (CM) [1]. Patients with PC-AKI have higher mortality rates, a greater risk of kidney failure, extended hospital stays, and increased healthcare costs [2]. The latest diagnostic criteria for PC-AKI includes elevated serum creatinine (SCr) [1]. However, SCr is a suboptimal biomarker for kidney injury because clinically significant renal damage (> 50%) is typically required to produce a clear increase in SCr due to the substantial functional reserve of the kidneys [3, 4]. The term “subclinical kidney injury” describes a condition in which kidney injury biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL) or kidney injury molecule-1 (KIM-1), are elevated without concurrent changes in conventional markers of AKI (i.e., SCr and urine output) [5]. This condition is associated with tubular injury and subsequent tubulointerstitial damage [6]. A “second hit” to the kidneys in the context of subclinical kidney injury can result in overt kidney damage [7].
In our previous study, rats exhibited subclinical kidney injury after 6 weeks of subcutaneous injection with carbon tetrachloride (CCl4) [8]. This injury was characterized by histologically confirmed substantial tubular damage, despite the absence of increased blood urea nitrogen (BUN) or SCr levels. Based on these findings, we developed a rat model of subclinical kidney injury via subcutaneous injection of CCl4 for 6 weeks. Iodine-based contrast media directly damage renal tubular epithelial and endothelial cells, leading to cell swelling, vacuolation, apoptosis, and necrosis. Necrotic cells detach from the basement membrane and migrate into the tubular lumen [9], contributing to increased heterogeneity within the kidney microenvironment. Indirect effects of iodine contrast media include alterations in renal hemodynamics. After the administration of radiocontrast, a sustained reduction in renal blood supply of 10–25% below baseline is often observed [10].
Multiparametric magnetic resonance imaging (MRI) is a valuable technique widely used to monitor the progression of renal diseases [11–14]. Diffusion kurtosis imaging (DKI) quantifies the non-Gaussian behavior of water diffusion [15], offering a sensitive measure of changes in the renal microenvironment [13, 14]. The progression of PC-AKI is characterized by increased complexity in the renal microenvironment. Elevated mean kurtosis (MK) reflects greater heterogeneity within the renal microenvironment, as well as functional impairments and structural abnormalities [16]. Arterial spin labeling (ASL), a non-invasive imaging method for quantifying tissue perfusion, uses arterial blood as an endogenous tracer; thus eliminates the need for exogenous contrast agents [17, 18]. Preclinical studies have demonstrated that ASL reliably indicates renal hypoxia and tracks the progression of PC-AKI [19, 20]. Together, DKI and ASL imaging provide comprehensive insights into renal microstructural changes and oxygenation after PC-AKI.
This study was performed to investigate the progression of PC-AKI in rats with subclinical kidney injury relative to controls and to assess the utility of DKI and ASL imaging as noninvasive tools for detecting kidney injury. Using DKI and ASL MRI data, we aimed to characterize renal hypoxia and microstructural damage during PC-AKI in rats with subclinical kidney injury. These findings were validated through renal pathology assessments and the expression levels of hypoxia-inducible factor-1α (HIF-1α) and NGAL.
Materials and methods
Animal preparation and study groups
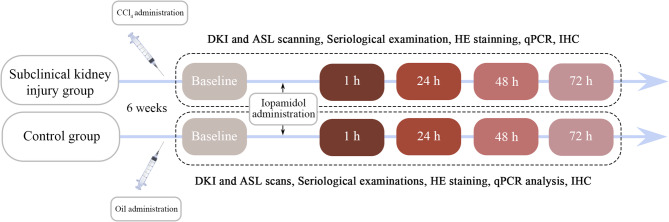
Male Sprague–Dawley rats, weighing 260–280 g each, were obtained from Beijing HFK Bioscience Co., Ltd. (Beijing, China) and housed under a 12-hour light/dark cycle with ad libitum access to food and water. In total, 60 rats were randomly divided into two groups: subclinical kidney injury and control. Rats in the subclinical kidney injury group received subcutaneous injections of CCl4 (Yi En Chemical Technology Co., Ltd., Shanghai, China) twice weekly for 6 weeks. The initial dose was 5 mL/kg and subsequent doses were 3 mL/kg; all were diluted in olive oil at a ratio of 2:3. Rats in the control group received injections of olive oil alone. After the modeling period, baseline MRI scans were performed. Twenty-four hours after baseline scans, all rats received a tail vein injection of iopamidol (370 mg/mL; Shanghai Starry Pharmaceutical Co., Ltd., Shanghai, China) at a dose of 4 g iodine/kg body weight, which is through the conversion of body surface area between human and rats [21]. MRI scans of rats in both groups were conducted at the following time points: 24 h before CM administration (baseline) and at 1 h, 24 h, 48 h, and 72 h after CM administration. Six rats were examined at each time point. The experimental animals were euthanized under the use of inhalational anesthetics. Figure 1 presents the study flowchart.
Fig. 1.
Flowchart of experimental design. qPCR: quantitative polymerase chain reaction; IHC: immunohistochemistry
MRI acquisition
After an 8-hour fast, rats were placed in the supine position on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthineers, Forchheim, Germany) equipped with a 16-channel animal coil (Chenguang Medical Technology Co., Ltd., Shanghai, China). Inhalational anesthesia was maintained with 2% isoflurane (Yuyan Scientific Instrument Co., Ltd., Shanghai, China) throughout the scanning process.
T2-weighted images were initially acquired to visualize the anatomical structures of the kidneys. DKI was performed using a single-shot echo planar imaging sequence with six b-values (0, 500, 800, 1000, 1500, and 2000 s/mm²) applied in three diffusion directions. DKI-derived parameters, including MD and MK, were extracted using research post-processing software (MR Body Diffusion Toolbox, Siemens Healthineers). ASL data were acquired using a three-dimensional turbo-gradient spin-echo pulsed ASL research sequence with flow-sensitive alternating inversion recovery preparation, incorporating seven inversion times (TI): 300, 500, 700, 900, 1100, 1300, and 1500 ms. Quantitative renal blood flow (RBF) maps were generated after data acquisition. Axial images of the kidneys were captured during all MRI sessions. Detailed parameters used in this study are listed in Supplementary Table 1.
Image analysis
A commercial post-processing workstation, syngo.via (Siemens Healthineers, Forchheim, Germany), was used to analyze all imaging data. The DKI data were processed using a research post-processing software (MR Body Diffusion Toolbox, Siemens Healthineers, Forchheim, Germany) to derive the MK and MD parameters. RBF was calculated using the following equation:
 |
where f is the perfusion rate (in mL/100 g/min), λ is the blood-tissue partition coefficient (80 mL/100 g), TI is the inversion time, M0 is the equilibrium magnetization intensity, and T1 is the longitudinal relaxation time. Regions of interest (ROIs) were carefully delineated in the cortex (CO), outer stripe of the outer medulla (OSOM), and inner stripe of the outer medulla (ISOM) by two radiologists blinded to the animal group assignments (Supplementary Fig. 1). On each axial slice, three ROIs were strategically placed to ensure precise quantitative evaluation of the MRI parameters. ROIs were placed independently on different parametric maps, but consistency is maintained as much as possible. Intraclass correlation coefficients (ICCs) were used to assess interobserver reliability.
Serum analysis and histopathological assessment
Tissue and blood samples, including liver, kidney, and serum, were collected immediately after the MRI scans. Blood samples from the inferior vena cava were centrifuged at 3000 rpm for 10 min, and the resulting serum was stored at − 80 °C. Serum levels of alanine aminotransferase, aspartate aminotransferase, SCr, and BUN were measured using a URIT-8210 fully automated biochemical analyzer (URIT Medical Electronic Co. Ltd., Guangxi, Guilin, China). Kidney sections were stained with hematoxylin-eosin (HE) using standard protocols. Renal damage was assessed based on criteria that included swelling, vacuolization, desquamation, proteinaceous casts, tubular dilation, and tubular necrosis. Each kidney compartment was evaluated in ten randomly selected fields at ×400 magnification. Each microscopic field was divided into a 10 × 10 grid (100 squares in total). Squares exhibiting the above pathological features were labeled positive, and the percentage of injury for that field was calculated as the number of positive squares divided by 100. Damage was scored on a scale from 0 to 5 (0: normal; 1: ≤ 5%; 2: 5–25%; 3: 25–50%; 4: 50–75%; 5: 75–100%) [22].
For immunohistochemistry, primary antibodies against HIF-1α (1:50 dilution, sc-53546, Santa Cruz Biotechnology) were used in combination with a commercially available reagent kit (PV-6002 & ZLI-9017, ZSGB-BIO Technology). HIF-1α expression was quantified using ImageJ software and reported as percentage of cells displaying HIF-1a positivity.
RNA extraction and quantitative polymerase chain reaction
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. One microgram of RNA was reverse-transcribed using the StarScript Pro All-in-One RT Mix with gDNA Remover (Cat#A240-10, GenStar Technology). Quantitative polymerase chain reaction (qPCR) was performed with SYBR qPCR Green Master Mix (GK10002, GlpBio Technology) on a real-time thermal cycler (Bio-Rad, Hercules, CA, USA). The following primers were used: NGAL forward, 5′-GATGAACTGAAGGAGCGATTC-3′; NGAL reverse, 5′-TCG GTGGGAACAGAGAAAAC-3′; β-actin forward, 5′-CCTAGACTTCGAGCAAGAGA-3′; β-actin reverse, 5′-GGAAGGAAGGCTGGAAGA-3′. Data were analyzed using the 2–ΔΔCt method, normalized to β-actin, and compared with control samples.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software, version 9.5.0 (GraphPad Inc., La Jolla, CA, USA). Data are presented as mean ± standard deviation (SD). MD, MK, and RBF values were derived from the right kidney. For normally distributed data, one-way analysis of variance with post hoc multiple comparisons was used for intragroup analyses; independent samples t-tests were utilized for intergroup comparisons. Pearson correlation analyses were performed to evaluate relationships between MRI parameters and histological changes. A p-value < 0.05 was considered statistically significant.
Results
Iopamidol worsened renal function in rats with subclinical kidney injury
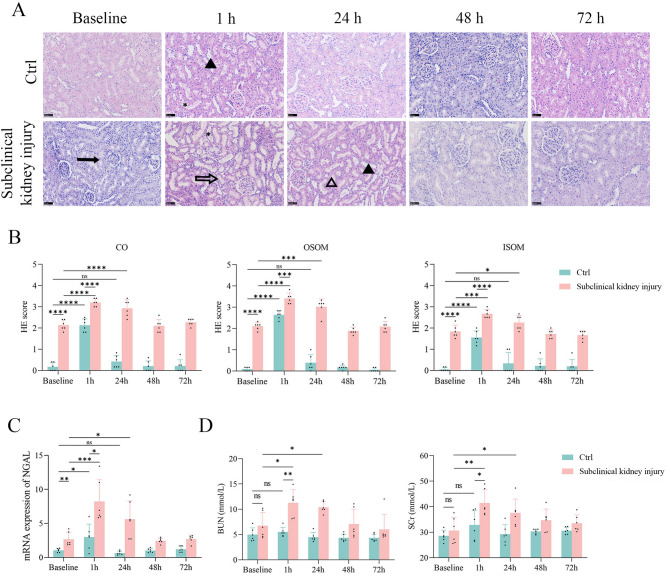
HE staining and qPCR confirmed the presence of mild renal injury in rats with subclinical kidney injury at baseline. HE scores (Fig. 2A, B; Supplementary Table 2) and renal NGAL expression levels (Fig. 2C) were significantly higher in the subclinical kidney injury group than in the control group (all p < 0.05). However, serum BUN and SCr levels did not show significant increases (Fig. 2D), indicating that the kidney injury in the subclinical kidney injury group was not detectable via routine serum analyses.
Fig. 2.
Contrast administration worsened renal function in rats with subclinical kidney injury. (A-B) HE scores increased at 1 h in both groups. The control group recovered at 24 h, while the subclinical kidney injury group recovered at 48 h. The asterisk indicates tubular dilation. The solid arrow marks swelling and vacuolization. The open arrow highlights proteinaceous casts. The solid triangle denotes desquamation. The open triangle signifies tubular necrosis. Scale bar, 50 μm. (C) Quantitative polymerase chain reaction assay results for NGAL mRNA expression shows that renal injury was most severe at 1 h, and the control group recovered at 24 h, while the subclinical kidney injury group recovered at 48 h. (D) Rats in the subclinical kidney injury group exhibited increased levels of blood urea nitrogen and serum creatinine levels at 1 h and 24 h compared to baseline
Following iopamidol administration, HE scores for all renal compartments significantly increased in both groups at 1 h (all p < 0.05). NGAL mRNA expression was 2.67-fold higher in the subclinical kidney injury group than in the control group (p = 0.004). At 24, 48, and 72 h, HE scores and renal NGAL expression in the control group did not differ from baseline levels (all p > 0.05). In contrast, HE scores and NGAL expression in the subclinical kidney injury group remained elevated at 24 h (all p < 0.05) but showed no significant difference from baseline at 48 and 72 h. Serum BUN and SCr levels fluctuated within a narrow range. In the subclinical kidney injury group, both parameters increased at 1 and 24 h (both p < 0.05); in the control group, they remained unaffected by iopamidol at all time points (all p > 0.05).
DKI measurements and correlations with renal injury
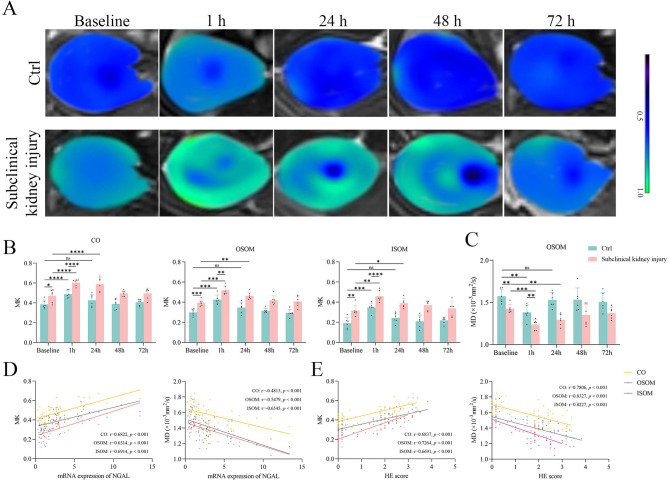
DKI parameters revealed distinct differences in PC-AKI progression between rats with subclinical kidney injury and control rats. At baseline, MK values in all renal compartments were significantly higher in the subclinical kidney injury group than in the control group (all p < 0.05). After iopamidol injection, MK values in the subclinical kidney injury group showed increasing trends in the CO, OSOM, and ISOM at 1 and 24 h post-injection (all p < 0.05); these values did not differ from baseline at 48 and 72 h (all p > 0.05). At 48 and 72 h, no inter-group differences in MK values were observed (all p > 0.05). The control group also exhibited elevated MK values in the CO, OSOM, and ISOM at 1 h post-contrast (all p < 0.05); these values returned to baseline by 24 h and remained stable thereafter (all p > 0.05 for all compartments). No inter-group differences in MK values were detected at 24, 48, and 72 h (all p > 0.05) (Fig. 3A, B).
Fig. 3.
DKI measurements and correlations with renal injury. (A-B) MK values increased at 1 h, and the control group recovered at 24 h, while the subclinical kidney injury group recovered at 48 h. (C) MD values decreased at 1 h, and the control group recovered at 24 h, while the subclinical kidney injury group recovered at 48 h. (D-E) DKI parameters showed great correlations with NGAL levels and HE scores in all renal compartments
MD values in all compartments were significantly lower at baseline in the subclinical kidney injury group compared with the control group (all p < 0.05). After contrast administration, MD values in the OSOM decreased at 1 h in both the control group (p = 0.0002) and the subclinical kidney injury group (p = 0.003). At 24 h, MD values in the OSOM remained lower in the subclinical kidney injury group (p = 0.0042); in the control group, MD values did not significantly differ from baseline (p > 0.05). At 48 and 72 h, MD values returned to baseline levels in both groups (both p > 0.05) (Fig. 3D). In the subclinical kidney injury group, there were no significant differences between 48 and 72 h (p > 0.05). Similarly, in the control group, no significant differences were detected among 24, 48, and 72 h (all p > 0.05). MD values in the CO and ISOM remained unchanged in both groups at all post-iopamidol time points (all p > 0.05) (Fig. 3C; Supplementary Fig. 2). DKI data are summarized in Supplementary Table 3. The results of inter-observer agreement analysis are presented in Supplementary Table 4.
Figure 3D, E shows strong correlations of MK and MD values with NGAL expression and histological injury scores. MK values in the CO, OSOM, and ISOM, as well as MD values in the OSOM, showed moderate correlations with NGAL expression (r = 0.6822, p < 0.001; r = 0.6514, p < 0.001; r = 0.6914, p < 0.001; and r = -0.6545, p < 0.001, respectively) and with HE scores (r = 0.6691–8327, p < 0.001).
ASL measurements and correlations with HIF-1α expression
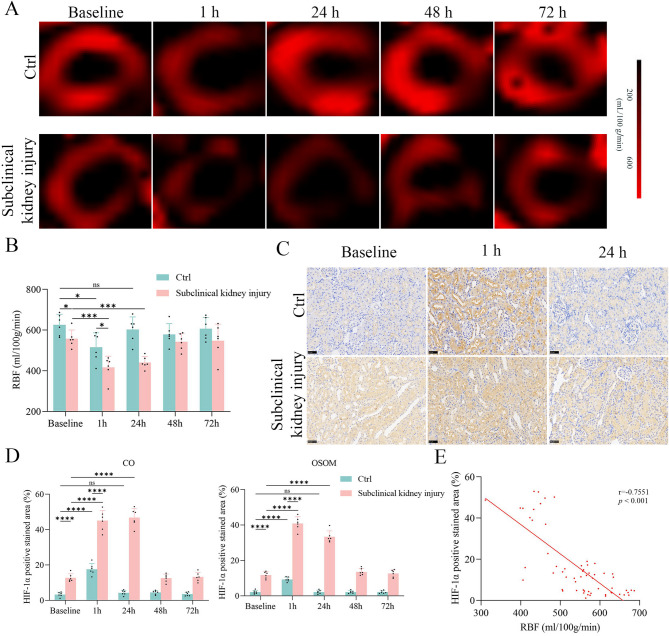
ASL-derived RBF exhibited distinct changes after iopamidol administration in rats with subclinical kidney injury relative to controls (Fig. 4A, B). At baseline, RBF values were 10.9% lower in rats with subclinical kidney injury than in controls (p = 0.0309). One hour after CM injection, both groups showed a decreasing trend in RBF (both p < 0.05). At 24 h, RBF in the control group did not significantly differ from baseline (p > 0.05); rats with subclinical kidney injury continued to display reduced RBF (p = 0.0002). At 48 and 72 h, RBF values in both groups nearly returned to their respective baselines (all p > 0.05). No significant differences were observed between 48 and 72 h in the subclinical kidney injury group (p > 0.05) or among 24, 48, and 72 h in the control group (all p > 0.05). ASL data are presented in Supplementary Table 3.
Fig. 4.
ASL measurements and correlations with HIF-1α expression. (A-B) RBF decreased at 1 h, and the control group recovered at 24 h, while the subclinical kidney injury group recovered at 48 h. (C) Immunohistochemical staining of HIF-1α in kidney tissue (400×). Scale bar, 50 μm. (D) Relative quantitation shown as percentage of cells displaying HIF-1a positivity. (E) RBF showed great correlations with HIF-1α expression
HIF-1α expression during PC-AKI was assessed by immunohistochemical staining (Fig. 4C, D; Supplementary Table 5). Baseline HIF-1α expression in the subclinical kidney injury group was upregulated in all renal compartments (all p < 0.05) and increased further at 1 and 24 h after CM administration (all p < 0.05); no significant differences were observed at 48 and 72 h compared with baseline (all p > 0.05). However, HIF-1α was upregulated at 1 h in the control group (all p < 0.05); it returned to baseline levels by 24 h and persisted at those levels during subsequent time points (all p > 0.05). Additionally, a negative correlation was identified between RBF and HIF-1α expression (r = -0.7551, p < 0.0001) (Fig. 4E).
Discussion
Our study demonstrated that rats with subclinical kidney injury exhibited greater susceptibility to kidney damage and delayed histological resolution after PC-AKI. DKI and ASL effectively delineated pathological changes and showed strong correlations with histological findings, highlighting its diagnostic utility. These results suggest that subclinical kidney injury significantly increases susceptibility to PC-AKI, emphasizing the need for greater clinical attention to this condition.
Previous clinical studies have mainly identified subclinical kidney injury by elevated novel biomarkers such as KIM-1 and NGAL [5]. However, the acquisition of kidney biopsy results remains a key clinical challenge. In our study, we confirmed subclinical kidney injury in rats through pathological investigations, including HE staining to assess structural damage and HIF-1α analysis to evaluate oxygenation status. Rats with subclinical kidney injury exhibited a distinct response to CM compared with rats who had normal kidney function. In the control group, iopamidol caused only mild pathological kidney injury at 1 h; indicators of kidney injury resolved by 24 h. In contrast, rats with subclinical kidney injury experienced more severe damage at 1 h post-contrast, including higher renal injury scores and increased NGAL expression. This damage persisted at 24 h but resolved by 48 h. Renal oxygenation, represented by HIF-1α expression, followed a similar pattern. These findings suggest that rats with subclinical kidney injury experience more severe CM-induced renal damage and hypoxia, along with a 1-day delay in resolution, relative to controls. Studies comparing PC-AKI between normal and diseased rats remain limited. Wang et al. [23] reported delayed recovery of baseline HIF-1α and VEGF expression in diabetic rats compared with controls, accompanied by higher HE scores, which supports our findings. However, in their study, renal damage and hypoxia, as measured by HE scores and HIF-1α expression, had not resolved by 72 h. This slower resolution may be attributable to the higher baseline SCr levels in diabetic nephropathy rats, as well as their more severe underlying renal injury (evidenced by higher HE scores and HIF-1α expression), compared with the rats in our study.
We observed that iodine had minimal impact on BUN and SCr levels in the control group. In contrast, rats with subclinical kidney injury exhibited elevated levels of these markers after contrast injection. Collectively, these findings suggest that subclinical kidney injury predisposes rats to increased susceptibility to CM, potentially enhancing the risk of PC-AKI. This increased susceptibility to exogenous nephrotoxicants aligns with findings from another preclinical study [7], where a low dose of cisplatin did not induce functional or structural damage in control rats but triggered AKI and elevated SCr levels in rats with subclinical kidney injury. These results underscore the importance of recognizing subclinical kidney injury as a latent pathological state that, although clinically elusive, is highly sensitive to even mild injurious agents and warrants further attention.
DKI indirectly measures microstructural changes, reflecting the complexity and cellular heterogeneity within the kidneys [24, 25]. At baseline, MK values were significantly higher in the subclinical kidney injury group; they showed correlations with pathologically confirmed renal damage. After CM administration, MK values increased while MD values decreased in both groups, consistent with a previous study regarding PC-AKI [21]. These changes likely result from the direct toxic effects of iodine, including renal tubular necrosis, increased microstructural complexity, and restricted water molecule diffusion [21]. In the control group, DKI indices returned to baseline by 24 h post-CM administration, whereas rats with subclinical kidney injury required 48 h for recovery, indicating delayed resolution of PC-AKI. MK and MD values, particularly in the OSOM, were strongly correlated with pathological and histological findings. These results demonstrate that DKI is a reliable tool for assessing histological renal injury.
ASL, a technique for imaging-based quantification of tissue perfusion, uses arterial blood as an endogenous tracer; this approach eliminates the need for exogenous contrast agents [17]. The present findings indicate that renal RBF in rats with subclinical kidney injury was reduced after modeling, consistent with our previous study [8] and another report [26]. This reduction was correlated with increased HIF-1α expression at baseline. Iopamidol administration caused a further decrease in RBF in both groups at 1 h relative to baseline. This decrease may be due to the vasoconstrictive effects of iodine-induced angiotensin II, which reduces blood flow and leads to oxygen deprivation. Additionally, contrast-induced renal dysfunction increases tubular resistance and dilation, compressing adjacent blood vessels and impairing renal oxygen delivery [11, 12]. By 24 h, the reduction in RBF had resolved in the control group, whereas resolution in the subclinical kidney injury group was delayed until 48 h. These findings suggest that rats with subclinical kidney injury experience more severe renal hypoxia during PC-AKI and require a longer recovery period. Compared with the control group, the subclinical kidney injury group exhibited consistently lower ASL-derived RBF values and higher HIF-1α expression from 1 to 72 h, indicating a prolonged and more severe hypoxic state during PC-AKI progression. Exogenous iodine may exacerbate pre-existing tubular swelling, vacuolization, and dilation in subclinical kidney injury, further impairing renal oxygen supply and perfusion [11, 12]. RBF values showed a strong correlation with the HIF-1α-positive area; the pattern of RBF variation during PC-AKI closely mirrored that of HIF-1α expression. These results highlight the potential of ASL as a noninvasive indicator for assessing renal oxygenation during PC-AKI in rats with subclinical kidney injury.
Li et al. [27] reported that both intravoxel incoherent motion (IVIM) and blood oxygenation level-dependent (BOLD) measurements exhibited immediate changes at 1 h post-contrast in control rats and rats with diabetic nephropathy. Whereas MRI indices in control rats returned to baseline at 48 h, rats with diabetic nephropathy required up to 72 h for recovery. This delayed recovery aligns with our findings. However, the model group in our study recovered by 48 h; the difference might be due to the more severe kidney damage in rats with diabetic nephropathy, which prolonged their recovery period. Our results suggest that the renal injury induced by CM in this study was less severe compared with other studies of PC-AKI. Multiple studies have shown that the peak of renal injury and MRI parameter variations typically occurs between 48 and 72 h, with a longer recovery time than observed in our study [20, 23, 27]. Additionally, iopamidol had minimal effects on BUN and SCr levels in our control group, which contradicts some previous findings [20, 21]. Wang et al. [23] also reported no post-contrast renal function impairment in control rabbits. These discrepancies may be explained by the observation that contrast administration alone rarely causes significant renal cell damage in animal models unless combined with additional insults [28].
Our study has several limitations. First, the small sample size may reduce the reliability of the results; a larger sample size could provide more robust conclusions. Second, the study did not include human patients, which precluded validation of the clinical applicability of DKI and ASL for PC-AKI. Third, the route of CM administration, along with the dose and osmolality, significantly influences the incidence of AKI. Further studies are needed to address these factors.
Conclusion
This study confirmed a higher risk of PC-AKI in rats with subclinical kidney injury. Multiparametric MRI, including DKI and ASL, offers a sensitive method for detecting pathological changes. DKI and ASL may serve as valuable tools for monitoring the progression of PC-AKI over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely appreciate the support provided by Siemens Healthineers in the application of MRI sequences in our experiments.
Abbreviations
- AKI
Acute Kidney Injury
- ASL
Arterial Spin Labeling
- BUN
Blood Urea Nitrogen
- CCl4
Carbon Tetrachloride
- CM
Contrast Medium
- CO
Cortex
- DKI
Diffusion Kurtosis Imaging
- HE
Hematoxylin-Eosin
- HIF-1α
Hypoxia-Inducible Factor-1α
- ICC
Intraclass Correlation Coefficient
- IHC
Immunohistochemistry
- ISOM
Inner Stripe of the Outer Medulla
- KIM-1
Kidney Injury Molecule-1
- MD
Mean Diffusion
- MK
Mean Kurtosis
- MRI
Magnetic Resonance Imaging
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
- OSOM
Outer Stripe of the Outer Medulla
- PC-AKI
Post-Contrast Acute Kidney Injury
- qPCR
Quantitative Polymerase Chain Reaction
- RBF
Renal Blood Flow
- SCr
Serum Creatinine
- sAKI
Subclinical Acute Kidney Injury
Author contributions
Hongtao Yuan: Formal analysis; Methodology; Writing – original draft. Jiaming Qin: Visualization. Xinzhe Du: Formal analysis. Jiaqi Yang: Data curation. Jinxia Zhu: Software. Bernd Kuehn: Software. Lihua Chen: Data curation. Shuangshuang Xie: Conceptualization; Methodology; Writing – review & editing. Wen Shen: Funding acquisition; Supervision.
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 82271971), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-041A) and Science and Technology Project in Health of Tianjin (TJWJ2025MS012).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by Ethics Committee of Nankai University, approval number 2022-SYDWLL-000236 in accordance with the 3R principles. Consent to participate is not applicable here.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuangshuang Xie and Wen Shen contributed equally to this work and are co-corresponding authors.
Contributor Information
Shuangshuang Xie, Email: xiess1989_happy@163.com.
Wen Shen, Email: shenwen66happy@126.com.
References
- 1.van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin M-F, Bertolotto M, et al. Post-contrast acute kidney injury – Part 1: definition, clinical features, incidence, role of contrast medium and risk factors. Eur Radiol. 2018;28(7):2845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cashion W, Weisbord SD. Radiographic contrast media and the kidney. Clin J Am Soc Nephrol. 2022;17(8):1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dépret F, Hollinger A, Cariou A, Deye N, Vieillard-Baron A, Fournier MC, et al. Incidence and outcome of subclinical acute kidney injury using penkid in critically ill patients. Am J Respir Crit Care Med. 2020;202(6):822–29. [DOI] [PubMed] [Google Scholar]
- 4.Koyner JL. Subclinical acute kidney injury is acute kidney injury and should not be ignored. Am J Respir Crit Care Med. 2020;202(6):786–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase M, Kellum JA, Ronco C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8(12):735–39. [DOI] [PubMed] [Google Scholar]
- 6.Silva-Aguiar RP, Teixeira DE, Peres RAS, Peruchetti DB, Gomes CP, Schmaier AH et al. Subclinical acute kidney injury in COVID-19: Possible mechanisms and future perspectives. Int J Mol Sci. 2022;23(22). [DOI] [PMC free article] [PubMed]
- 7.Succar L, Pianta TJ, Davidson T, Pickering JW, Endre ZH. Subclinical chronic kidney disease modifies the diagnosis of experimental acute kidney injury. Kidney Int. 2017;92(3):680–92. [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Xie S, Yu Y, Yang J, Zhao Y, Qiu C et al. Evaluation of kidney injury using arterial spin labeling and blood oxygen level-dependent MRI: an experimental study in rats with carbon tetrachloride‐induced liver cirrhosis. J Magn Reson Imaging. 2024. [DOI] [PubMed]
- 9.Li Y, Wang J. Contrast-induced acute kidney injury: a review of definition, pathogenesis, risk factors, prevention and treatment. BMC Nephrol. 2024;25(1). [DOI] [PMC free article] [PubMed]
- 10.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of Radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3(1):288–96. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YD, Wang J, Zhang J, Wang X, Jiang X. Effect of iodinated contrast media on renal function evaluated with dynamic three-dimensional MR renography. Radiology. 2014;270(2):409–15. [DOI] [PubMed] [Google Scholar]
- 12.Wu C-J, Bao M-L, Wang Q, Wang X-N, Liu X-S, Shi H-B, et al. Acute kidney damage induced by low- and iso-osmolar contrast media in rats: comparison study with physiologic MRI and histologic-gene examination. J Magn Reson Imaging. 2017;45(1):291–302. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wang B, Qin J, Yan H, Chen H, Guo J, et al. Use of multiparametric MRI to noninvasively assess iodinated contrast-induced acute kidney injury. Magn Reson Imaging. 2024;114:110248. [DOI] [PubMed] [Google Scholar]
- 14.Qin J, Xie S, Yu Y, Zhang C, Zhao Y, Tong D et al. The value of diffusion kurtosis imaging to assess liver and kidney recovery after mesenchymal stem cell intervention in CCl4-Induced cirrhotic rats. Am J Nephrol 2025 Feb 11:1–15. [DOI] [PubMed]
- 15.Lodhia N, Kader M, Mayes T, Mantry P, Maliakkal B. Risk of contrast-induced nephropathy in hospitalized patients with cirrhosis. World J Gastroenterol. 2009;15(12). [DOI] [PMC free article] [PubMed]
- 16.Li A, Liang L, Liang P, Hu Y, Xu C, Hu X, et al. Assessment of renal fibrosis in a rat model of unilateral ureteral obstruction with diffusion kurtosis imaging: comparison with α-SMA expression and 18F-FDG PET. Magn Reson Imaging. 2020;66:176–84. [DOI] [PubMed] [Google Scholar]
- 17.Nery F, Buchanan CE, Harteveld AA, Odudu A, Bane O, Cox EF, et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. Magn Reson Mater Phys Biol Med. 2019;33(1):141–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Q, Zhang Q, An Z, Chen Z, Feng Y. Multi-Parametric MRI for evaluating variations in renal structure, function, and endogenous metabolites in an animal model with acute kidney injury induced by ischemia reperfusion. J Magn Reson Imaging: JMRI. 2024;60(1):245–55. [DOI] [PubMed] [Google Scholar]
- 19.Liang L, Chen W-b, Chan KWY, Li Y-g, Zhang B, Liang C-h, et al. Using intravoxel incoherent motion MR imaging to study the renal pathophysiological process of contrast-induced acute kidney injury in rats: comparison with conventional DWI and arterial spin labelling. Eur Radiol. 2015;26(6):1597–605. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Wang Y, Li L, Guo J, Wu P-Y, Zhang H, et al. Diffusion kurtosis imaging and arterial spin labeling for the noninvasive evaluation of persistent post-contrast acute kidney injury. Magn Reson Imaging. 2022;87:47–55. [DOI] [PubMed] [Google Scholar]
- 21.Dai H, Zhao C, Xiong Y, He Q, Su W, Li J et al. Evaluation of contrast-induced acute kidney injury using IVIM and DKI MRI in a rat model of diabetic nephropathy. Insights into Imaging. 2022;13(1). [DOI] [PMC free article] [PubMed]
- 22.Ulusoy S, Ozkan G, Mungan S, Orem A, Yulug E, Alkanat M, et al. GSPE is superior to NAC in the prevention of contrast-induced nephropathy: might this superiority be related to caspase 1 and Calpain 1? Life Sci. 2014;103(2):101–10. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang X, Wang B, Xie Y, Wang Y, Jiang X et al. Evaluation of renal pathophysiological processes induced by an iodinated contrast agent in a diabetic rabbit model using intravoxel incoherent motion and blood oxygenation level-dependent magnetic resonance imaging. Korean J Radiol. 2019;20(5). [DOI] [PMC free article] [PubMed]
- 24.Mao W, Ding Y, Ding X, Wang Y, Fu C, Zeng M, et al. Pathological assessment of chronic kidney disease with DWI: is there an added value for diffusion kurtosis imaging? J Magn Reson Imaging. 2021;54(2):508–17. [DOI] [PubMed] [Google Scholar]
- 25.Fokkinga E, Hernandez-Tamames JA, Ianus A, Nilsson M, Tax CMW, Perez‐Lopez R, et al. Advanced diffusion‐weighted MRI for cancer microstructure assessment in body imaging, and its relationship with histology. J Magn Reson Imaging. 2023;60(4):1278–304. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang X, Hua Z, Xie L, Jiang X, Wang R, et al. Blood oxygen Level-Dependent imaging and intravoxel incoherent motion MRI of early Contrast-Induced acute kidney injury in a rabbit model. Kidney Blood Press Res. 2019;44(4):496–512. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Shi D, Zhang H, Yao X, Wang S, Wang R et al. The application of functional magnetic resonance imaging in type 2 diabetes rats with Contrast-Induced acute kidney injury and the associated innate immune response. Front Physiol. 2021;12. [DOI] [PMC free article] [PubMed]
- 28.Sůva M, Kala P, Poloczek M, Kaňovský J, Štípal R, Radvan M et al. Contrast-induced acute kidney injury and its contemporary prevention. Front Cardiovasc Med. 2022;9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.