Abstract
Background
Migraine is a neurological disorder affecting approximately 12% of the population, more frequent in women, causing disability. Preventive treatment is recommended to prevent chronification and analgesics’ abuse and to improve quality of life, but not all candidates receive it. Common preventive drugs include amitriptyline, flunarizine, propranolol, and topiramate. Their effectiveness and safety have not yet been assessed in our setting. The objective of this study is to evaluate the effectiveness of these drugs in reducing the monthly migraine days (MMD) and to evaluate their safety.
Methods
Phase IV, pragmatic randomized clinical trial of four parallel groups, open and multicentric, in Primary Care in Catalonia, Spain.
We will include adults with migraine eligible for preventive treatment. They will be randomized to the following: (1) propranolol, (2) amitriptyline, (3) flunarizine, or (4) topiramate.
The main efficacy measure will be the change in the mean MMD at week 12 in comparison to the mean MMD at baseline. Other secondary efficacy and safety measures will be assessed.
The main non-inferiority analysis will be conducted in the per protocol population at week 12, comparing topiramate versus propranolol as the main analysis, and amitriptyline and flunarizine versus propranolol as exploratory analyses.
Discussion
There are epidemiological, clinical and pharmacological sex-differences in migraine which may result in differences in the use of migraine preventive drugs. However, the effectiveness of the most frequent preventive treatment and the differences in effectiveness and safety between women and men have not yet been studied. Moreover, as new expensive drugs have been authorized for preventive treatment, indicated when the usual drugs have failed, assessing the effectiveness and safety of the usual oral preventive drugs through a clinical trial is especially important. We also plan to evaluate their cost-effectiveness, and the clinical trial will count on an active contribution from patients through an app designed for this purpose, which may help in the management of the disease in the future.
Trial registration
ClinicalTrials.gov ID: NCT06499116 (https://clinicaltrials.gov/study/NCT06499116).
Trial registration date: July 12, 2024.
EU CT Number: 2024–513597-22.
Protocol ID: IJG-PREMI-2024.
Sponsor: Fundació d’Investigació en Atenció Primària Jordi Gol i Gurina.
Gran Via de les Corts Catalanes 587, àtic. 08007, Barcelona, Spain.
Keywords: Migraine, Monthly Migraine Days (MMD), Randomized Clinical Trial, Preventive Treatment, Primary Care, Adherence, Patient Reported Outcomes Measures (PROM)
Introduction
Background and rationale
Migraine is a disabling neurological disease that affects approximately 12% of the adult population [1, 2]. Its overall age-standardized prevalence in 2016 was 14.4%, higher in women (18.9%) than in men (9.8%) [3]. Migraine is the seventh most prevalent disease worldwide [2–4], the second cause of longer years with disability, the 14th according to disability-adjusted life years [3], and it is a public health problem, especially in the most productive ages of life, as it has a great impact on quality of life and in the family, social, academic and occupational settings [5].
Migraine is a primary headache characterized by episodes lasting between 4 and 72 h. Its typical characteristics are unilateral localization, pulsating characteristics, worsening with movement and with moderate to severe intensity, as well as the associated symptoms of photophobia, nausea and vomiting [6]. In approximately one-third of patients, the headache may be preceded or accompanied by transient neurological disturbances known as aura, which most often manifests visually, but may also present with other symptoms (sensory, language, unsteadiness, etc.) [5, 7]. The frequency, duration and disability caused by migraine attacks are higher in women than in men [8].
Migraine is usually classified as episodic migraine, when there are 14 or fewer monthly headache days (MHD), or as chronic migraine, when there are at least 15 MHD, of which at least 8 days the pain is of the migraine type (8 monthly migraine days [MMD]) [6, 9]. Approximately 2% of the general population is affected by chronic migraine and 12% is affected by episodic migraine [5, 10].
Adequate management of migraine requires the detection of precipitating factors, the symptomatic treatment of migraine attacks, the use of preventive treatment and the control of factors related to chronification [5, 11]. The aim of symptomatic treatment is to relieve pain and associated symptoms. The most frequently used treatments are non-steroidal anti-inflammatory drugs (NSAID), analgesics, triptans and ergotics [5, 7]. The treatment basis is to administer NSAID for mild-moderate attacks and triptans for moderate-severe attacks [5]. In 2022, rimegepant and lasmiditan were authorized by the European Medicines Agency for acute treatment of migraine. Rimegepant is an antagonist of the human calcitonin gene-related peptide receptor (CGRP) [12]. Lasmiditan is a high-affinity agonist of the serotonin 1 F receptor (5-HT1F), which is located in the central nervous system [13]. In Spain, only lasmiditan is reimbursed for this indication since 2024.
The aim of preventive pharmacological treatment is to prevent chronification and the abuse of analgesics and to improve the quality of life, reduce the frequency of migraine attacks, the MMD and their intensity, and improve the response to acute treatment. Candidates of preventive treatment in episodic migraine are those patients with at least three MMD and those experiencing more than one migraine attack per week for several days, intense and with insufficient response or intolerance to acute treatment [5, 14]. Approximately 25% of migraine patients require preventive treatment, but only 2% to 14% of them actually receive it [5, 7, 15]. Currently, the use of a single drug is recommended [16, 17], and the drugs with proven efficacy and approved indications include amitriptyline, flunarizine, topiramate, some beta-blockers such as propranolol or metoprolol, or botulinum toxin A. There are also some drugs with less evidence or with a safety profile that limits their use, such as valproate, candesartan, lisinopril or venlafaxine [5]. In recent years, rimegepant and the monoclonal antibodies eptinezumab, erenumab, fremanezumab and galcanezumab, which act on the CGRP, have been authorized [12, 18, 19]. Atogepant, another CGRP antagonist, has recently been authorized by the European Medicines Agency [20].
Despite being one of the most prevalent diseases worldwide, with a high impact on quality of life [22], the usual preventive drugs have never been directly compared between them to assess their efficacy and safety in episodic migraine. There is a high rate of treatment discontinuation, but the reasons have not been studied.
The PERSEC study revealed that only 30.6% of patients receiving preventive treatment for migraine continued with the treatment after 6 months, with the highest discontinuation rate during the first 3 months [21], considering that the minimum recommended duration of preventive treatment is, precisely, 3 months [22]. The authors also highlighted the greater associated cost with non-persistence in comparison to treatment persistence. Thus, it is necessary to understand the reasons for treatment discontinuation: lack of efficacy, adverse events (AE), adequate dosage, etc. This study found that approximately 76% of these treatments were initiated in Primary Care, followed by Neurology [21].
In an observational study with data from 2012 to 2019 in the UK, 42.4% of migraine patients had previously used at least one preventive treatment, 7.7% of them had started and discontinued at least three different pharmacological groups and 21.7% of them had been referred to Neurology to weigh up alternative treatments [23].
Differences in effectiveness and safety between women and men have not been ascertained either, notwithstanding that migraine prevalence is higher in women [1, 3, 5]; there are also differences in the clinical presentation of symptoms, as most studies point to a longer duration of migraine attacks in women, as well as more concomitant symptoms such as nausea, vomiting, phonophobia or photophobia [8]; or differences exist in the response to the drugs caused by pharmacokinetic and pharmacodynamic sex-particularities, including preventive drugs [24]. Differences were also observed in the Global Burden of Diseases, Injuries, and Risk Factors’ studies, where migraine is the fifth disease in disability-adjusted life years in women and the 20th in men [3].
According to the 2018 migraine Atlas, people with migraine reported disease-related fears of daily life limitations, pain and loss of quality of life. With respect to economic expenses, the Atlas reported a total annual cost per patient with episodic migraine of 5041.38€, split into direct healthcare costs (19.1%), direct costs sustained by the patients (17.4%) and indirect costs attributable to work productivity losses (63.5%) [25].
New drugs have been authorized for the preventive treatment, indicated when the usual drugs have failed [12, 13, 18–20, 26–28], with high economic costs. In this context, it is necessary to study the effectiveness and safety of the usual preventive drugs to update the clinical guidelines, prescription preferences and possible sex differences among treatments.
Objectives
We hypothesize that the drugs usually prescribed for preventive treatment in Primary Care, propranolol, amitriptyline, flunarizine and topiramate, will have similar effectiveness. Thus, we aim to evaluate the effectiveness of these four drugs for migraine preventive treatment in reducing the MMD and to evaluate their tolerance and safety as well as their differences between women and men. We also plan to assess the cost-effectiveness of the four study drugs.
Trial design
Phase IV, pragmatic randomized clinical trial of parallel groups, open, and multicentric will be conducted in Primary Care centres in Catalonia, Spain. The allocation ratio will be 1:1:1:1.
The clinical trial will be conducted under a non-inferiority hypothesis with respect to the propranolol group with the primary endpoint of the number of MMD (difference between week 12 and baseline). The main non-inferiority analysis will be performed on the per protocol (PP) population. If we confirm the study hypothesis, a sensitivity analysis will be performed on the intention-to-treat (ITT) population. The ITT population will be defined as those randomized participants who received at least one dose of the study drug. The PP population will be defined as those ITT participants who complete all the study information in the app, attend all the study visits, and have all effectiveness measures.
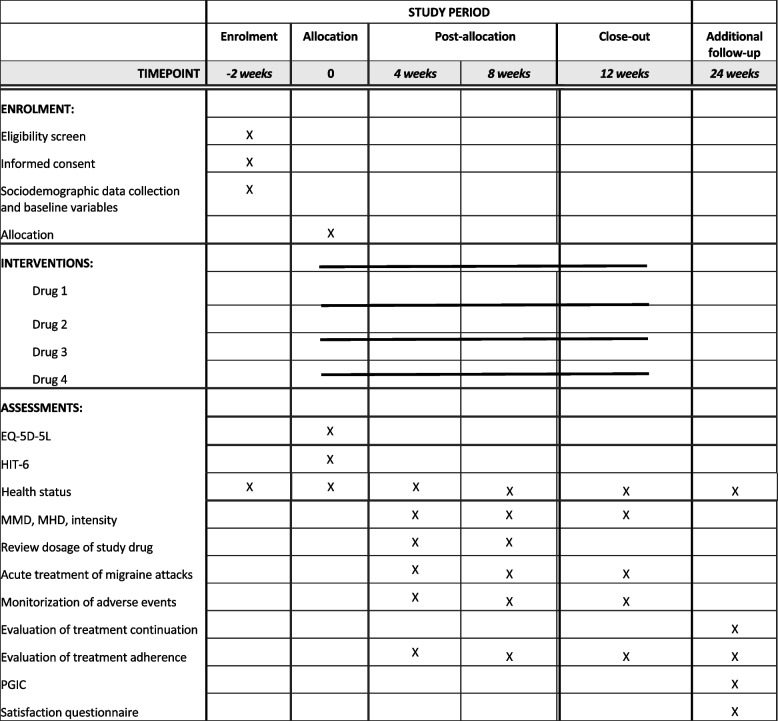
The study calendar will consist of: pre-selection visit, baseline visit, two follow-up phone calls at weeks 4 and 8, and a final visit at week 12. There will be an additional follow-up phone call at week 24 (Fig. 1). The planned enrolment, interventions and assessments to be conducted during the clinical trial are described in Fig. 2.
Fig. 1.
Participant timeline
Fig. 2.
SPIRIT figure: enrolment, interventions and assessments
Methods: participants, intervention, and outcomes
Study setting
Migraine patients attended in Primary Care centres in Catalonia, Spain, will be invited to participate in the clinical trial. In Annex 1, there is a list of the centres who have currently accepted to participate. More centres can be added in the future.
Eligibility criteria
Inclusion criteria are as follows: adults (≥ 18 years) candidates for migraine preventive treatment; those with ≥ 4 MMD who accept to participate and sign the informed consent form. Patients meeting the inclusion criteria will be invited to participate.
Exclusion criteria are as follows: people with migraine who are not candidates for preventive treatment, people with a chronic migraine diagnosis, those not having a smartphone, simultaneous participation in another clinical trial, being pregnant or the possibility of pregnancy in the upcoming 3 months, breastfeeding, migraine patients who are already treated with a preventive drug, people with chronic treatment with opioids, analgesics or NSAID not used for migraine acute treatment, people with an absolute contraindication to at least one of the study drugs.
Who will take informed consent?
During the pre-selection visit, the investigators (general practitioner [GP] and/or family nurse) will explain the clinical trial, delivering the corresponding participant information sheet and the informed consent form.
Additional consent provisions for collection and use of participant data and biological specimens
Not applicable.
Interventions
Explanation for the choice of comparators
Propranolol will be considered the reference treatment, against which the other three drugs will be compared. This treatment was chosen because it has the most experience of use in Primary Care [10, 29] and has shown evidence in the treatment of episodic migraine [30]. The four study arms represent four drug families with different mechanisms of action, which are the most prescribed preventive drugs in our Primary Care setting (unpublished internal data from Primary Care in Catalonia).
Intervention description
Once the participants are included in the trial, they will be randomized to one of the four study arms, to be prescribed through the usual circuit of drug prescription in Primary Care at the usual dosage for migraine prevention according to the following ranges and administered for 12 weeks: (1) propranolol, 20–120 mg/12 h; (2) amitriptyline, 10–75 mg/24 h; (3) flunarizine, 2.5–10 mg/24 h; or (4) topiramate, 25–100 mg/12 h.
Criteria for discontinuing or modifying allocated interventions
There are no criteria for treatment modification during this trial. In case of treatment failure, worsening of the migraine, or occurrence of adverse effects requiring treatment discontinuation, the patient will be discontinued from the trial.
Strategies to improve adherence to interventions
The participants will download a mobile app, specifically designed for this clinical trial, where they can register the migraine management during the study duration. The app will send reminders to improve adherence to the intervention and the collection of variables.
Relevant concomitant care permitted or prohibited during the trial
The prohibited care during the trial is specified in the exclusion criteria; thus, patients will be excluded if they meet at least one of these conditions.
Provisions for post-trial care
After the end of the trial, patients will be attended as per standard care. At week 24, the investigator will contact the participant by telephone for an additional follow-up aimed at evaluating the preventive treatment continuation and its effectiveness after the end of the clinical trial.
Outcomes
The main efficacy measure is the clinical effectiveness, measured by the modification in the mean MMD at week 12 in comparison to the MMD at baseline.
The secondary measures will include the following: proportion of responding patients at week 12 (non-responsive, < 25% of change in MMD; partial responders, 25–49%; responders, 50–75%; excellent responders, > 75%), change in mean MMD at weeks 4 and 8, reduction in the mean moderate-severe MMD, proportion of patients with associated symptoms at week 12, proportion of adherent and non-adherent patients at week 12, change in the mean number of drugs used for symptomatic treatment at weeks 4, 8 and 12, reconsultations, patients’ global impression of change (PGIC) scale at week 12, changes in HIT-6 and EQ-5D-5L questionnaires at week 12, change in the mean number of days of sick leave, absenteeism, and presentism at week 12. As complementary measures, adherence to preventive treatment and switch or discontinuation of the preventive treatment will be assessed at 6 months after the clinical trial initiation.
The safety measures at week 12 will include the following: number of AE, number of AE causing a dose reduction or a discontinuation of the study drug, proportion of patients with AA in each treatment arm, number of severe AE.
In case of treatment failure, patients will be discontinued from the trial and followed up by usual care. Concomitant treatments will be collected if required.
Variables’ collection will be conducted through the mobile app mentioned above, which has been designed specifically for this trial.
Participant timeline
The participant timeline is described in Fig. 1.
Sample size
The clinical trial will be conducted under a non-inferiority hypothesis with respect to the propranolol group with the primary endpoint of the number of MMD (difference between week 12 and baseline). We expect a mean reduction of 2.5 MMD with an SD of 3.1 days (according to the results of recent clinical trials in the same indication with the new monoclonal antibodies [19, 26–28]), a non-inferiority margin of 1 day and assuming a 15% loss rate. A total of 115 patients per treatment arm (sample size, 460 patients) is required to obtain a statistical power of 80% at a significance level of 5%.
Recruitment
During the pre-selection visit, the investigators will explain the clinical trial, delivering the corresponding participant information sheet and the informed consent form. The investigator will complete the baseline characteristics and migraine history and symptoms reported by the patient in the electronic case report form (eCRF). All procedures and variables collected in the eCRF are described in Table 1.
Table 1.
Variables to be collected in the eCRF
| Visits | Pre-selection visit (1) | Baseline visit (2) | Follow-up phone call (3) | Follow-up phone call (4) | Final visit (5) | Additional phone call (6) |
|---|---|---|---|---|---|---|
| Week | −2 weeks | 0 (randomization) | 4 weeks post-randomization | 8 weeks post-randomization | 12 weeks post-randomization | 24 weeks post-randomization |
| Face-to-face visit | X | X | X | |||
| Informed consent | X | |||||
| Eligibility (inclusion/exclusion criteria) | X | |||||
| Sociodemographic data collection | X | |||||
| Citation for the baseline visit | X | |||||
| Randomization | X | |||||
| Prescription of the study drug and instructions of use/administration | X | |||||
| Phone call | X | X | X | |||
| Review of the dosage of the study drug | X | X | ||||
| Review of the health status: migraine-related visits to Primary Care, Emergency Department, Hospital, other centres, migraine related test (CT, MRI, others) | X | X | X | |||
| Acute treatment of migraine: prescription of analgesics/NSAID and specific drugs for migraine (registered in the electronic health records) | X | X | X | |||
| Monitorization of adverse events to study medication | X | X | X | |||
| Citation for the final visit | X | |||||
| Evaluation of treatment continuation: switch or discontinuation of preventive treatment | X | |||||
| Evaluation of adherence to preventive treatment | X |
CT computerized tomography, MRI magnetic resonance image, NSAID non-steroidal anti-inflammatory drugs
The participants will download a mobile app, specifically designed for this clinical trial, where they can register the migraine management during the following two weeks and during the study duration. Variables collected through the app are described in Table 2.
Table 2.
Variables to be collected by the participant in the mobile app
| Visits | Pre-selection visit (1) | Baseline visit (2) | During the study | Final visit (5) | |
|---|---|---|---|---|---|
| Week | −2 weeks | 0 (randomization) | Weekly notifications in the app | In case of migraine attack | 12 weeks post-randomization |
| App download | X | ||||
| Introduction of data of the 2 previous weeks up to the baseline visit: migraine episodes, intensity, daily life limitations, acute treatment, visits, etc | X | X | |||
| EQ-5D-5L, HIT-6 questionnaires | X | X | |||
| Review of the data introduced in the app | X | X | |||
| Reminder of data registration during the trial | X | ||||
| Monthly migraine days (MMD) | X | X | X | ||
| Monthly headache days (MHD) | X | X | |||
| Intensity of migraine attacks | X | ||||
| Migraine acute treatment: number of tablets/capsules of the specific treatment, dose of analgesics/NSAID | X | X | |||
| Symptoms associated to the migraine attack and pharmacological treatment | X | ||||
| Work/school absenteeism | X | ||||
| Sick leave | X | ||||
| Work presenteeism | X | ||||
| Migraine-related visits: Primary Care, Emergency Department, Hospital, etc | X | X | X | ||
| Extra-cost related with migraine attacks | X | ||||
| PGIC questionnaire | X | ||||
| Evaluation of adherence to preventive treatment | X | X | |||
| Satisfaction questionnaire (treatment, app, clinical trial) | X | ||||
EQ-5D-5L EuroQol-5 Dimensions-5 Levels, HIT-6 Headache Impact Test, NSAID non-steroidal anti-inflammatory drugs, PGIC patients’ global impression of change
After 14 days, the participant will attend the baseline visit to review the data introduced in the app, and the investigator will review again that all inclusion criteria are met. Then, he/she will be randomized to one of the four treatment arms through the eCRF. The GP will prescribe the preventive drug through the usual electronic prescription system.
At weeks 4 and 8 after study initiation, the research team will contact the participant by telephone for study follow-up: health status, information registered in the app, study treatment compliance, AE and tolerance.
At week 12, the participants will attend the final visit at the Primary Care centre to assess their health status, information registered in the app, treatment compliance, AE, etc. The GP will also assess the need to continue with the preventive treatment after the finalization of the clinical trial.
At week 24, the investigator will contact the participant by telephone for an additional follow-up aimed at evaluating the preventive treatment continuation and its effectiveness after the end of the clinical trial.
Assignment interventions: allocation
Sequence generation
Randomization will be done by registering the patient in the eCRF during the baseline visit. Once the inclusion and exclusion criteria have been reviewed, the investigator will randomize the patient to one of the four treatment groups and prescribe the assigned treatment using the usual electronic prescription system. Randomization to one of the four treatment groups will be done in a 1:1:1:1 ratio.
Concealment mechanism
Patients will be assigned sequentially as they are recruited during the baseline visit, two weeks later after inclusion, through eCRF. This automatic allocation will maintain study group ratios.
Implementation
An independent statistician will generate the randomization list. Site investigators will enrol participants and prescribe the allocated medication.
Assignment interventions: Blinding
Not applicable.
Data collection and management
Plans for assessment and collection of outcomes
The main efficacy measure is the clinical effectiveness, measured by the modification in the mean MMD at week 12 in comparison to the MMD at baseline. This variable will be collected by the participant in the mobile app (Table 2).
The secondary measures and the safety measures will be collected by the investigator in the eCRF (Table 1) or by the participant in the app (Table 2).
Plans to promote participant retention and complete follow-up
Monthly calls will be performed to collect follow-up information, as well as through the app; both sources of information will engage patients during the study period.
Data management
A data management plan will be established, and procedures will be implemented to ensure homogenisation, traceability and quality of data. Study data will be collected both in the eCRF (Table 1) and in the app (Table 2). For the eCRF, we will use REDCap. Data are hosted on a server of the sponsor that complies with all legal requirements regarding data protection. Access to the eCRF needs a personal username and password for each investigator. When participants agree to take part in the study, they will be assigned a study code to collect all data without identification. For the app, participants will collect the data on migraine evolution and trial conduct. The app has been developed by a provider with extensive experience in the development of mobile applications for patients in clinical practice and research. A username and password are necessary, and data will be identified by patient code.
The sponsor is responsible for the data treatment. There will be no international transfer of data. The FAIR (findable, accessible, interoperable and reusable) principles will be followed. The datasets created will be shared on the Zenodo platform, if possible, first ensuring compliance with the General Data Protection Regulation. All publications will be made open access. Publications and datasets will be identified using the Digital Object Identifier (DOI), unique identification and the datasets that can be shared will be saved using standard formats (.txt,.csv, pdf…). They will always be accompanied by metadata created following the guidelines for this type of research.
Confidentiality
Data processing will be carried out in compliance with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons regarding the processing of personal data and on the free movement of such data, and with Law 3/2018 of 5th December on the protection of personal data and guarantee of digital rights. Patients will be identified with a study code on all data collection documents.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use
Not applicable.
Statistical methods
Statistical methods for primary and secondary outcomes and methods for additional analyses
The main non-inferiority analysis will be performed on the PP population. If we confirm the study hypothesis, a sensitivity analysis will be performed on the ITT population. Safety will be assessed by analysing the AA observed, by severity, in each treatment arm.
The primary endpoint will be assessed after 12 weeks of treatment, comparing topiramate versus propranolol as a reference group, as it is the most widely prescribed in Primary Care [10] and has demonstrated evidence in episodic migraine [30]. Amitriptyline and flunarizine will be compared with propranolol as exploratory analyses.
To determine the effect of possible confounders, a comparison of the clinical and demographic variables of interest will be performed between treatment arms. If any of the variables are not balanced between the study groups, the main analysis will adjust for those variables with ANCOVA or similar models. In addition, we plan to perform a stratified analysis by sex/gender and other relevant factors to assess the consistency of the observed effects on the main outcomes. For the other arms, amitriptyline and flunarizine, information on their efficacy will be obtained via exploratory analyses, which will be complementary to the main analysis.
Continuous variables will be described as mean and standard deviation (SD) or median and interquartile range (IQR) if the variable is not normally distributed. Qualitative variables will be described by calculating absolute and relative frequencies. For bivariate analysis, the Student’s t-test or nonparametric tests such as the Mann–Whitney U test will be used. If the variable to be contrasted is categorical, Fisher’s exact test or chi-square will be used.
To evaluate the non-inferiority hypothesis, the t-Student test will be performed, calculating the p-value according to the established non-inferiority margin. The main variable will be evaluated after 12 weeks of treatment, and the four groups will be compared, taking propranolol as the reference. To determine the effect of possible confounding factors, we will perform a stratified analysis by sex and other relevant factors to assess the consistency of the effect observed in the main results.
The secondary variables will be assessed at weeks 4, 8, 12 and/or 24.
All analyses will be performed with R statistical software (v4.1 or higher) and will be presented in a clear and detailed manner in the final study report.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data
If there is evidence of non-random missing data, the characteristics of patients with and without these missing values will be described separately. If missing data occur in a non-random manner, the characteristics of the patients with and without missing data should be described separately.
Plans to give access to the full protocol, participant level data and statistical code
The full protocol and participant level data will be provided upon request once the trial has ended. An anonymized dataset will be generated to protect participants’ identities.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee
IDIAPJGol will be responsible for coordinating the trial. Project management, monitoring and pharmacovigilance activities will be carried out by qualified personnel.
Composition of the data monitoring committee, its role and reporting structure
Not applicable.
Adverse event reporting and harms
It is the investigator’s responsibility to detect and document any event that meets the criteria and definitions of AE and to report all severe AE. AE will be collected during all the study duration from clinical observation of the patient, laboratory analyses, spontaneous communication from the patient, open questioning by the investigator, and review of the patient’s records and data entered into the app, including start and end date, description of the event, severity, course, outcome, relationship with the study drug and actions taken. Participants with AE will be followed up during the remaining visits until the end of the study follow-up.
This is a low-intervention clinical trial using medication already authorized by the Spanish Medicines Agency (AEMPS), in the authorized indication and with a well-documented safety profile. For this reason, in this trial, AEs not related to the study medication will be recorded in the patient’s own health records. The sponsor is responsible for pharmacovigilance of this trial.
Frequency and plans for auditing trial conduct
No audits planned, apart from the usual trial monitoring.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees)
Substantial modifications will be notified according to Good Clinical Practices. All investigators involved in the trial will be notified via email of any modification to the initial authorisation procedures that have a substantial impact on the safety or rights of the subjects or on the reliability and robustness of the data generated in the clinical trial.
Dissemination plans
All members of the research team will have the responsibility to communicate and disseminate the study in their respective areas, with special emphasis in the dissemination activities to the public, as migraine is a highly prevalent condition, being necessary to raise awareness and disseminate at a general level. We propose to conduct scientific communication and dissemination of all study results in conferences and in scientific articles, communication and dissemination to the stakeholders involved in decision-making and communication and dissemination to patients and citizens. We have planned at least one communication to a congress and at least five scientific manuscripts.
Discussion
Migraine is a disease that causes significant disability. Its occupational and emotional impact is important, and not always recognized, but it has consequences for quality of life. When migraine attacks are frequent and disabling, preventive treatment is also required, and this approach is not used as often as it should be.
The epidemiological, clinical, and pharmacological sex differences in migraine may result in different prescription patterns of migraine preventive drugs. However, differences in effectiveness and safety between women and men have not yet been studied.
In the current context, new drugs with high economic costs have been authorized for preventive treatment, indicated when the usual drugs have failed. Thus, it is of special importance to assess the effectiveness and safety of the usual oral preventive drugs through a clinical trial.
The results of the clinical trial that we propose will be published and will allow to update the clinical guidelines for migraine prevention recommendations, considering the sex and gender differences described. In addition, the continuous increase in pharmaceutical expenses raises the need to implement economic principles in order to reduce costs, ensuring the health outcomes for patients. Additionally, we plan to evaluate the efficiency of the four study drugs by conducting a cost-effectiveness analysis from a societal perspective. The clinical trial will count on an active contribution from patients by their own evaluation through an app designed for this purpose, which may help in the management of the disease in the future. This patient and public involvement (PPI) will improve the quality and implementation of health services, addressing patients’ needs and expectations and promoting shared decision-making models between patients and professionals.
Trial status
Protocol version number 2.0, 02/September/2024.
The recruitment started in April 2025. The first patient was included on 29th April.
Acknowledgements
The authors want to thank in advance all GPs and nurses who will be participating in the recruitment of participants of the trial.
Abbreviations
- AE
Adverse events
- AEMPS
Spanish Medicines Agency
- CGRP
Calcitonin gene-related peptide receptor
- CEIm
Ethics Committee
- eCRF
Electronic case report form
- EQ-5D-5L
EuroQol-5 Dimensions-5 Levels
- GP
General practitioner
- HIT-6
Headache Impact Test
- IQR
Interquartile range
- ITT
Intention-to-treat
- MHD
Monthly headache days
- MMD
Monthly migraine days
- NSAID
Non-steroidal anti-inflammatory drugs
- PGIC
Patients’ global impression of change
- PP
Per protocol
- PPI
Patient and public involvement
- SD
Standard deviation
- 5-HT1F
Serotonin 1F receptor
Appendix
List of centres and principal investigators in each centre
| Centros | Investigador Principal |
|---|---|
| CAP Florida | Yolanda Rando Matos |
| CAP Rambla | Sebastian Vignoli Carradori |
| CAP Dr. Martí i Julià | Esther Gómez Martin |
| CAP Cornellà | Bàrbara Pina Sánchez-Arjona |
| CAP Sant Ildefons | Jose Antonio Escamilla Fresnadillo |
| CAP Can Mòritz (Jaume Soler) | Laura Illamola Martin |
| CAP Amadeu Torner | Marta Bandrés Mingueza |
| CAP Sant Martí de Provençals | Carme Gisbert Revilla |
| CAP Canet de Mar | Eva Maria Calvo |
| CAP Temple | Rosa Ripollès Vicente |
| CAP Balàfia | Antoni Plana Blanco |
| CAP Santa Coloma de Queralt | Angelina Marcela Aumala Aguilera |
| CAP Santa Clara | Dan Marian Oltean Oltean |
| CAP El Castell | Lluïsa Gardeñes Moron |
| CAP La Sagrera | Maria del Mar Gili Riu |
| CAP La Granja | Lluïsa Gardeñes Moron |
Authors’ contributions
Conceptualization and design of the study: RMP, MGS, AGS. Clinical expertise: SFG, CV, SBM, SCF, ECM, RBN, NMSM. Methodological and statistical expertise: MGS, RMP, DO, SFG, RME, AGS. CEIm and AEMPS approval: MGS, RME, AGS. Funding acquisition: All authors. Original draft of the manuscript: MGS. Review and editing of the manuscript: All authors.
Funding
This study has been funded by the Instituto Carlos III in the 2023 call of Independent Clinical Research through the project ICI23/00047.
Instituto de Salud Carlos III,ICI23/00047,Maria Giner-Soriano
Availability of data and materials
The datasets created will be shared on the Zenodo platform, if possible, first ensuring compliance with the General Data Protection Regulation. If not, the datasets generated during the current study will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The clinical trial will be conducted in accordance with the protocol, the principles of the Declaration of Helsinki, in accordance with the Tripartite Harmonized Guide for Good Clinical Practice and following the current legal regulations (Royal Decree 1090/2015, December 4th).
The study protocol has been evaluated and approved by the Ethics Committee (CEIm) of the Hospital de la Santa Creu i Sant Pau and authorized by the Spanish Medicines Agency (AEMPS).
The informed consent will be requested from all the participants in the trial, respecting their willingness and autonomy in the decision. The participant information sheet will be provided beforehand, and any doubts they may have will be resolved before the trial initiation. Consent will be documented by the signature and date of the patient on the corresponding form, together with the signature and date of the person in charge of the trial explanation and request for consent. The patient or patient’s legal representative must be informed in a timely manner of any information that may have a relevant influence on the patient’s willingness to participate in the study. Two copies of the informed consent will be signed. One copy will be kept by the participant and the other by the investigator, who will keep it in the investigator’s file along with the rest of the study documents. Likewise, it will be documented in the clinical records of the patient that he/she agrees to participate in the clinical study and who signs the consent form.
Consent for publication
Consent will be obtained directly from the participants, as explained above.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matías-Guiu J, Porta-Etessam J, Mateos V, Díaz-Insa S, Lopez-Gil A, Fernández C. One-year prevalence of migraine in Spain: a nationwide population-based survey. Cephalalgia [Internet]. 2011 ;21;31(4):463–70. Available from: https://journals.sagepub.com/doi/https://doi.org/10.1177/0333102410382794?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed. [DOI] [PubMed]
- 2.Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton RB, Scher AI, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia [Internet]. 2007;27(3):193–210. Available from: https://journals.sagepub.com/doi/https://doi.org/10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed]
- 3.Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol [Internet]. 2018;17(11):954–76. Available from: http://www.thelancet.com/article/S1474442218303223/fulltext. [DOI] [PMC free article] [PubMed]
- 4.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet [Internet]. 2012 Dec 15 [cited 2023 Feb 21];380(9859):2163–96. Available from: http://www.thelancet.com/article/S0140673612617292/fulltext. [DOI] [PMC free article] [PubMed]
- 5.Grup d’estudi de cefalees de la Societat Catalana de Neurologia (SCN), Grup d’estudi de la cefalea (GRECAP) de la Societat Catalana de Medicina Familiar i Comunitària (CAMFiC). Consens català sobre l’atenció al pacient amb migranya. 2015.
- 6.Olesen J., Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition Copyright. [cited 2023 Feb 21]; Available from: www.uk.sagepub.com.
- 7.Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, et al. Diagnosis and management of migraine in ten steps. Nature Reviews Neurology 2021 17:8 [Internet]. 2021;17(8):501–14. Available from: https://www.nature.com/articles/s41582-021-00509-5 [DOI] [PMC free article] [PubMed]
- 8.Allais G, Chiarle G, Sinigaglia S, Airola G, Schiapparelli P, Benedetto C. Gender-related differences in migraine. Neurol Sci. 2020;41(S2):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riesco N, García-Cabo C, Pascual J. Migraine. Medicina Clínica (English Edition) [Internet]. 2016;146(1):35–9. Available from: https://www.elsevier.es/en-revista-medicina-clinica-english-edition--462-articulo-migraine-S2387020616300894 [DOI] [PubMed]
- 10.Adell V, Belvís R, Gómez JB, Cuadrado E, Fabregat N, García J, et al. Diagnòstic i tractament de la cefalea Guies mèdiques de la Societat Catalana de Neurologia Diagnòstic i tractament de la cefalea 2019 COORDINADORS [Internet]. 2019. Available from: https://www.scneurologia.cat/wp-content/uploads/2019/07/Guia-CEFALEES_Societat-Catalana-de-Neurologia_Actualitzaci%C3%B3-2019_DEF.pdf
- 11.Cuadrado-Godia E, Garrido-Alejos G, Marrero P. Tractament de la migranya en adults: de la crisi a la profilaxi. Butlletí d’informació terapéutica [Internet]. 2019;30(5):26–32. Available from: http://scientiasalut.gencat.cat/handle/11351/4348
- 12.EMA - European Medicines Agency. Vydura | European Medicines Agency [Internet]. 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vydura
- 13.EMA - European Medicines Agency. Rayvow | European Medicines Agency [Internet]. 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rayvow
- 14.Scotish Intercollegiate Guidelines Network (SIGN). Pharmacological management of migraine. [Internet]. Edinburgh; 2022. Available from: http://www.sign.ac.uk
- 15.Santos Lasaosa S, Rosich PP, Nieto RB, Latorre González G, González Oria C. Manual de práctica clínica en cefaleas. Recomendaciones diagnóstico-terapéuticas de la Sociedad Española de Neurología. 2020.
- 16.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ [Internet]. 2009;339(7728):1015. Available from: https://www.bmj.com/content/339/bmj.b3914 [DOI] [PMC free article] [PubMed]
- 17.Kurth T, Kase CS, Schürks M, Tzourio C, Buring JE. Migraine and risk of haemorrhagic stroke in women: prospective cohort study. BMJ [Internet]. 2010;341(7771):492. Available from: https://www.bmj.com/content/341/bmj.c3659 [DOI] [PMC free article] [PubMed]
- 18.Programa d’harmonització farmacoterapèutica. Erenumab, fremanezumab i galcanezumab per al tractament profilàctic de la migranya. [Internet]. Barcelona: Servei Català de la Salut. Departament de Salut. Generalitat de Catalaunya; 2019. Available from: http://catsalut.gencat.cat/ca/proveidors-professionals/farmacia-medicaments/programa-
- 19.Ashina M, Lanteri-Minet M, Ettrup A, Christoffersen CL, Josiassen MK, Phul R, et al. Efficacy and safety of eptinezumab for migraine prevention in patients with prior preventive treatment failures: subgroup analysis of the randomized, placebo-controlled DELIVER study. https://doi.org/101177/03331024231170807 [Internet]. 2023;43(5):033310242311708. Available from: https://journals.sagepub.com/doi/https://doi.org/10.1177/03331024231170807 [DOI] [PubMed]
- 20.EMA - European Medicines Agency. Aquipta | European Medicines Agency [Internet]. 2024. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/aquipta
- 21.Irimia P, García-Azorín D, Núñez M, Díaz-Cerezo S, de Polavieja PG, Panni T, et al. Persistence, use of resources and costs in patients under migraine preventive treatment: the PERSEC study. J Headache Pain. 2022D 7;23(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilar-Shea AL, Diaz-de-Teran J. Migraine review for general practice. Aten Primaria. 2022;54(2). [DOI] [PMC free article] [PubMed]
- 23.Kernick D, Kondori N, Pain A, Mount J, Appel C, Ranopa M, et al. Preventive treatment patterns in the adult migraine population: an observational UK study over 7 years. BMC Primary Care [Internet]. 2024;25(1):1–9. Available from: https://bmcprimcare.biomedcentral.com/articles/https://doi.org/10.1186/s12875-023-02242-y [DOI] [PMC free article] [PubMed]
- 24.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asociación Española de Migraña y Cefalea. Impacto y situación de la migraña en España: Atlas 2018. Universidad de Sevilla, editor. 2018.
- 26.Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine. JAMA Neurol. 2018S 1;75(9):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine. JAMA. 2018M 15;319(19):1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. New England Journal of Medicine [Internet]. 2017;377(22):2123–32. Available from: https://www.nejm.org/doi/https://doi.org/10.1056/NEJMoa1705848 [DOI] [PubMed]
- 29.Domingues RB, da Silva ALP, Domingues SA, Aquino CCH, Kuster GW. A double-blind randomized controlled trial of low doses of propranolol, nortriptyline, and the combination of propranolol and nortriptyline for the preventive treatment of migraine. Arq Neuropsiquiatr. 2009;67(4):973–7. [DOI] [PubMed] [Google Scholar]
- 30.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults. Neurology [Internet]. 2012;78(17):1337–45. Available from: https://www.neurology.org/doi/https://doi.org/10.1212/WNL.0b013e3182535d20?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets created will be shared on the Zenodo platform, if possible, first ensuring compliance with the General Data Protection Regulation. If not, the datasets generated during the current study will be available from the corresponding author upon reasonable request.