Abstract
Background
The two well-known quality-assessment metrics of head and neck oncosurgery are the status of surgical margin (SM) and lymph node yield (LNY). While the clinical importance of LNY has been well-established, several unresolved controversies around the SM have deterred its practical application.
Methods
This article reviews some of the issues with the SM and ongoing efforts to improve its clinical application and reliability.
Results
Several variations exist around SM, regarding its definition, designation, procurement, handling, and pathological processing, which could hinder its reliability. Until newer instruments that could improve the safety of surgical resection are validated robustly and are accessible widely, the surgeons need to adhere to the standardized approach of using the SM in clinical practice.
Conclusions
Unless not available, the SM based on the surgical specimen should be given priority for all practical purposes over the tissue taken from the surgical bed; with the latter serving only as an intraoperative guide, to facilitate an appropriate margin revision whenever needed and feasible.
Keywords: Surgical margins, Oral cancer, Oropharynx, Head and neck, Frozen section, Close margin, Survival outcomes, Transoral robotic surgery
Introduction
Surgery is integral to the therapeutic armamentarium in head and neck squamous cell carcinoma (HNSCC), and is particularly pertinent to oral squamous cell carcinoma(OSCC) [1–3]. The surgical excision of the primary tumor with an appropriate margin and a concurrent neck dissection, apart from eliminating the disease burden, will also provide crucial information about the pathological prognostic factors, such as the depth of invasion (DOI), worst pattern of invasion, lymphovascular invasion, perineural invasion, cervical lymph nodes and extranodal extension (ENE), that also dictate the adjuvant treatment [4–8]. Among these factors, the status of cervical lymph nodes, ENE, and DOI, owing to their stronger predictive ability, are relied upon heavily for the decision-making on the need and the type of adjuvant therapy [9, 10]. While the status of cervical lymph node in HNSCC has been a part of the American Joint Committee on Cancer (AJCC) staging system for a couple of decades, the status of ENE and DOI (for oral cancer) have also been incorporated into the latest (VIII) edition of the AJCC staging manual, thanks to the mounting evidence in the recent years on their profound impact on the overall survival (OS) [11]. There is another critical pathological prognostic factor in the surgically treated cases of HNSCC, in the form of the status of surgical margin (SM), which, like the ENE, has a direct implication on the decision about the need for adjuvant chemoradiotherapy [1, 12–15] However, despite its proven prognostic relevance, the SM is yet to find its way into the staging manual, owing to the several technical and practical hurdles that are undermining its reliability in clinical practice [16–19]. This article reviews some of the issues with the SM that are probably responsible for hindering its clinical application in the management of HNSCC.
To simplify the otherwise complex narratives around the SM, this review is organised peculiarly into four sections, with each section discussing a particular controversy, practical hurdle, or technical issue related to the SM, followed by the suggested remedial measures to overcome the respective challenge. A special emphasis is given to the emerging techniques in surgical practice that could offer potentially durable solutions for this clinical conundrum. Also, considering the pathological and prognostic heterogeneity between the HNSCC that arise from the different sites of the upper aerodigestive tract, this review focuses its discussion about the SM around the surgically treated OSCC, the principles of which may or may not be applicable to the other sites of the head and neck. However, whenever essential or feasible, we have drawn parallels to and from the surgical procedures/nuances related to the other HNSCC. Nevertheless, this review focuses on OSCC, as it is the commonest HNSCC to be treated by surgery, and the prognosis of this cancer, which is also predicted by the SM, has not yet improved to the acceptable levels. Understanding the intricacies around the SM in OSCC might lead to a better disease clearance and thus improve prognosis.
Predicament 1: controversies related to the philosophy of SM
1.1. Dilemma around ‘what constitutes the SM’
A critical issue that undermines studies on the prognostic value of SM in HNSCC, including in OSCC, is the lack of a consensus on its description and standardized classification. Generally, a curative intent excision of a particular tumor would include removal of an adequate cuff of normal tissue all around it, and the adequacy of tumor clearance can only be ascertained by a histopathological examination of this normal tissue cuff around the tumor. However, there exists a lack of consensus on what constitutes an adequate SM, with two methods in use, interchangeably, to assess or designate the status of SM: the tissue sampled from the free edge of the excised specimen, called the surgical specimen margin (SSM), or the tissue sampled from the bed of the operated site, called the surgical bed margin (SBM) [16, 20–24]. One of the plausible reasons for the use of both these methods in practice is the notion that both SSM and SBM possess identical prognostic impact. However, contrary to this belief, there is a considerable body of evidence that suggests substantial differences between the two. From the practical standpoint, SBM offers a binary outcome: ‘positive (or involved)’ vs ‘negative (or clear),’ based on the presence or absence of an invasive tumor on histological assessment, and the SSM offers additional information, including the microscopic distance between the tumor and free edge of the excised surgical specimen [12, 25–28], allowing descriptors such as ‘close margin.’ The SBM, which is taken after the surgical excision of the primary tumor, as a small strip of tissue from the ‘surgeon-felt’ boundary of the tumor bed, may not necessarily be an accurate representation of the three-dimensional extent of the tumor [19]. Also, since the SBM usually involves excising an extra strip of ‘anticipated normal tissue’ from the surgical bed after the tumor removal, expectantly, the chances of finding an involved SSM in the final histopathology tend to be higher even if the SBM is negative [23, 29, 30]. By virtue of these differences between SBM and SSM, multiple studies have found SSM to have a significantly superior prognostic value than the SBM in HNSCC, including in OSCC [21, 22, 29, 31–33].
Remedy: Reliance on SSM over SBM, for all practical purposes
Recognizing the substantial prognostic value of SM in oncology practice, the Union for International Cancer Control adopted a classification in 1987 to denote the presence or absence of residual (R) disease after the treatment [34, 35]. In this ‘R classification,’ a complete disease clearance is represented by 'R0,' the presence of microscopic residual tumor is designated as 'R1,' and the presence of macroscopic residual tumor is implied by 'R2' [34, 35]. Accordingly, a curative intent surgical excision can be categorized as R0 resection only when SMs from all around the surgical specimen are negative for invasive disease; and even if one of the resected SM is positive, such resection should be considered as R1. However, as mentioned above, different institutes tend to consider either one of SSM or SBM for designating the R status. This practice needs amendment, since these two SMs do not carry the same clinical and oncological implications in operated cases of HNSCC. Considering the overwhelming evidence favouring the prognostic superiority of SSM, the surgeons, as well as the multidisciplinary tumor boards, should classify the R status based on the SSM as a standard practice in HNSCC, including in OSCC, and accordingly, should prioritize their decisions based on the status of SSM, both for prognostication and for therapeutic planning. This approach is also supported by the latest practice guidelines [11, 36]. The clinical relevance of SBM in the surgical treatment of HNSCC should be limited to ascertain the completeness of disease clearance (particularly along the third dimension of a large lesion, or from a soft tissue compartment such as infratemporal fossa or skull base), and to undertake further excisions of surgical bed at doubtful or positive residual disease. However, in some of the oncosurgical procedures, which encompass piecemeal excision of the primary tumor (as in transoral surgeries for medium-to-large sized pharyngeal or laryngeal cancers or in transnasal endoscopic excision in sinonasal carcinoma), the SBM may be the only SM available for designating the R status and for the clinical decision making. In all other cases of surgically treated HNSCC, the SSM should be considered over the SBM for all practical purposes.
1.2. Confusion about the prognostic value of SM that is revised after being positive
Theoretically, the presence of an invasive tumor along an SM, irrespective of whether it is SSM or SBM, is bad for the prognosis as it tends to increase the chances of subsequent recurrences [37, 38]. However, in clinical practice, the prognostic relevance of the SSM varies from that of SBM due to the differences in the way surgeons handle these positive margins. While there is no doubt that a positive SM in a curative setting warrants a re-resection of the corresponding surgical bed, the feasibility and reliability of such re-resection is not always given, particularly in cases of positive SSM. The reported re-resection rates of positive SSM in oral cancer are as low as around 25–10%, and even if feasible, it is challenging to accurately re-resect the appropriate site of interest [37, 39]. Although the use of intraoperative frozen section histopathology (IFSH) can enable an immediate on-table revision of the doubtful or positive margin, due to certain reliability issues with IFSH (discussed in the subsequent section), it is not uncommon in clinical practice to end up in a situation of having a positive SM on the final histology with an inability to revise it. Irrespective of the situation , an unrevised positive SM, either SSM or SBM, is among the pathological prognostic factors with highest-risk and thus warrants an aggressive adjuvant therapy in the form of chemoradiation [21, 31].
On the other hand, the relevance of ‘the positive SM that has been revised until negative’ has been generally ignored, with some even considering it to be as safe as the SM that was negative upfront. However, the best available evidence indicates that the prognostic value of ‘a positive SM even after the re-resection until negative’ is not the same as that of an upfront negative SM [16, 40]. Several studies have reported ‘a need for revising the SBM’ as one of the strongest predictors of local recurrence (LR) in HNSCC after adjusting for other known predictors [29, 33, 41–47]. This was also reflected in a meta-analysis of eight eligible studies on OSCC, which showed that the ‘re-resection of an initially positive SM until it becomes negative’ was not the same as an upfront negative SM, as the re-resection did not improve local control rates significantly [48].
Remedy: ‘Intermediate risk factor’ with reduced threshold for adjuvant treatment
By analysing the available literature in this regard, ‘a positive SM that has been revised until negative’ seems to fit appropriately as an intermediate pathological risk factor between the positive SSM at one end of the spectrum (as a high-risk factor), and the negative SSM at the other end (as a low-risk factor) [22, 32, 43, 48]. In such cases, although the decision about the adjuvant therapy should be guided by the presence or absence of other clinic-pathological risk factors, it could probably serve well to lower the threshold for adjuvant therapy in these cases, particularly in patients with aggressive disease variants and tolerable general profiles [33, 41, 45–47]. However, further studies are needed to validate this hypothesis and to understand the actual benefit of adjuvant treatment in such situations.
1.3. Inconsistencies in ‘how to designate the status of SSM’
The microscopic distance between the tumor front and the free edge of the specimen for designating a particular status to the SSM varies from institute to institute, largely due to the disparities in the literature on the ‘optimal cut-off value of this distance’ that can reliably predict the risk of the LR and local recurrence-free survival (LRFS) [49–55]. While some studies have found a margin closer than 5 millimeters (mm) to affect the LR rates [31, 39, 56], several others have failed to demonstrate a similar association [25, 27, 28, 37, 57–61]. A few studies have also reported lower cut-off values to be predictive of prognosis in OSCC: a study of 381 patients with oral tongue cancer identified SM > 2.2 mm as a better predictor of LRFS than 5 mm [51]; another study of 192 patients with oral and oropharyngeal cancers demonstrated SM of > 1.6 mm as sufficient to impart better disease-specific survival [53]; further, a larger study with a cohort of 432 patients of OSCC concluded 1 mm as sufficient margin [25]; but on the contrary, a study with 602 patients of bucco-alveolar complex reported a need of minimum margin of 5.5 mm to reduce the LRFS [49]. Table 1 summarizes some of the commonly prescribed cut-off values to designate a SM as involved, negative, or close across different research papers.
Table 1.
Various cut-off values (microscopic distance from the tumor front to the free edge of the surgical specimen) used to designate the surgical margin in oral cancer
Remedy: Standardisation; development of prognostic models
Despite all these inconsistencies, there is a broad consensus among the head and neck surgeons that a margin of ≥ 5 mm is oncologically safe in OSCC [27, 28, 36, 37, 41, 63, 65, 66]. Accordingly, the current guidelines classify SM in oral cancer surgery to be positive, when the distance between the tumor and the free edge of the surgical specimen is < 1 mm, as close, when this distance is 1–5 mm, and as negative, when it is > 5 mm [16, 25, 33]. It is noteworthy that the cut-off values for defining the negative, close, and positive margins are different for the HNSCC arising from oropharynx, larynx, paranasal sinuses, and ear canal; and further discussion on the SM in these sites is not included in this review [52, 67]. Nevertheless, unless the objective of the study is to evaluate the impact of this distance of SM (from tumor) on the oncological outcomes in OSCC, and has sufficient statistical power and methodological considerations to study these outcomes, authors should exercise caution while publishing underpowered analyses on the significance of lower cut-off values in these tumors.
Alternatively, considering the variability in prognostic value of SM at different cut-off values, it is also worthwhile to explore the feasibility of multivariable prognostic models and nomograms in OSCC that view the SM as a continuum rather than as a categorical variable. The recently expanding applications of the machine learning/deep learning methods in medicine can aid the development and validation of such models; however, this exercise might demand comprehensive clinical data of a large number of patients, with a detailed pathological report and a long-term follow-up, with information on disease status.
1.4. Lack of consensus on the value of ‘close SM’ and other findings at SM
Apart from the confusion about the safe limit of minimum distance required at the surgical margin , there is also a lack of clarity in the literature on the prognostic impact of dysplasia or carcinoma-in-situ (CIS) found at the SM, and on the subsequent management of these changes [26]. Owing to the dearth of high-quality data in the literature on the clinical implications of these findings, there is a considerable variation between the institutes on how they interpret and handle such changes at the SM while making decisions on adjuvant treatment in their patients [26].
Remedy: Assigning ‘intermediate-risk’ status to the SM with these changes
In a recently published systematic review and meta-analysis, which included 26 studies and 8435 surgically managed patients of OSCC, patients with a close SM had a lower incidence of 5-year LR rates than those with a positive SM, but a 5-year OS did not differ between the patients with close and positive margins [26]. Moreover, in this review, both the 5-year LR-free status and the OS were inferior in those with the close margins as compared to those with the negative margins, suggesting an 'intermediate status' of the close margin between that of the positive and negative SM. In other words, a close SM, like a revised SM, can be placed between the clear and involved SMs in terms of its ability to predict the LR. However, there is a need for the development and validation of multivariable prognostic models and nomograms to predict survival at various distances of SM, as these tools could provide a more realistic and clinically meaningful answer in this regard. Interestingly, a few of the published reports have also suggested a similar 'intermediate prognostic relevance' to the SMs that demonstrated either dysplasia or Cis microscopically [53, 66, 68]. Although the available evidence in this regard is not strong, there is a trend in the literature to suggest a designation of a close SM and SM with either dysplasia or Cis as an intermediate pathological risk factor, particularly in OSCC. Nevertheless, further studies are essential to validate the suggested relevancies of these SMs; until then, the final decision about the need and type of adjuvant treatment in patients with such SMs must be based on the other pathological risk factors, with a low threshold to lean towards additional therapy [25, 28, 57, 58, 65, 68]. Universal adherence to the standard practices along these lines could enable robust multicentre research outputs on these SMs and might eventually facilitate their incorporation into the staging manual.
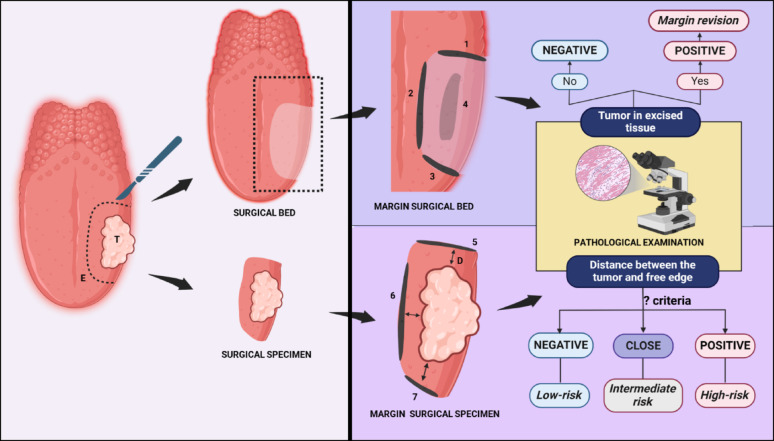
The dilemma around the description and designation of the SM and their practical implications has been summarised in Fig. 1.
Fig. 1.
Illustration depicting the differences between two types of surgical margins and their clinical implications
Key: D-Distance between the cut end of specimen and tumor; E-surgically marked excision margin; T- Tumor; 1, 2 and 3 represent surgical bed margins, respectively from the three sides of the surgical bed (along the length and breadth of the defect), and 4 is from the third dimension (depth); 5, 6 and 7 represent the surgical specimen margins corresponding respectively to 1, 2 and 3 of surgical bed margins. Note: While frozen section analysis of any of the 1 to 7 margins could be useful for intraoperative assessment and appropriate on-table revision of the microscopic residual disease, the margins 5, 6, and 7 would serve better than 1 to 4 for prognostication and decision on adjuvant therapy. [Created in BioRender. KMC, R. (2025) https://BioRender.com/l75t855].
Predicament 2: Disease characteristics offsetting the reliability of assessment methods
Precise pre-operative assessment of the tumor extent by the clinical and radiological means and achieving an optimal intra-operative exposure (open surgery or minimally invasive approaches) of the tumor all around are the most commonly practiced surgical strategies to achieve a negative SM in HNSCC, which are subsequently expected to translate into better clinical outcomes [17, 69–71]. However, some of the disease-related factors inherent to HNSCC, particularly to OSCC, could conspire to negate these measures, in turn affecting the prognostic reliability of the SM [69, 71].
2.1. Field cancerization
OSCC is known to originate from several subclinical genetic alterations cumulated over a period of time, secondary to repetitive or persistent exposure to risk factors such as tobacco and alcohol [72–76]. In a patient with OSCC who is exposed to such risk factors, studies have shown that, the subclinical pro-carcinogenic molecular aberrations can be seen in surrounding mucosal field up to several centimeters away from the invasive carcinoma underneath a clinically and histologically apparently normal mucosa [72–77]. Thus, by this concept of ‘field cancerisation,’ a pathological negative SM of > 5 mm may not necessarily be oncologically safe, as the tissue at this distance could still carry an increased risk of subsequent carcinogenesis [72–76]. Clinical studies have reported a strong correlation between the presence of such subclinical molecular changes in histologically normal SM and an increased risk of local failure in OSCC, and have blamed ‘field cancerisation’ for the increase in the LR [78–83]. However, the current standard practice of histopathological assessment of the SM does not account for (or evaluate) the pro-carcinogenic molecular alterations [84–87].
Remedy: Molecular SM (MSM)
Identification of these molecular alterations at the margin tissue can not only enhance the prognostic value of the SM in clinical practice but could also potentially permit an appropriate re-resection or revision of ‘at-risk mucosa’ whenever feasible. Studies have shown this to be an oncologically sound approach to identify the disease-free margin, with correspondingly reduced LR rates in HNSCC [78, 82, 84, 85, 88]. The evaluation of the margin tissue for the pro-carcinogenic genetic, epigenetic, or proteomic changes that are not captured by the standard histological techniques constitutes the foundation of 'MSM' [89]. It includes the application of immunostaining or DNA-based techniques, such as in situ hybridization or polymerase chain reaction, to identify the well-known molecular signatures that could improve the prognostic ability of SM [84, 86, 88]. The most common molecular signatures in histologically normal SM that have shown a strong association with an increased LR in OSCC are the loss of heterozygosity (LOH) of chromosome 9p21 (the gene which codes for p16), LOH of 17p13 (codes for TP53) and combination of LOH at 9p or overamplification of TP53 (reflected as overexpression of p53 on immunostaining) [78–80]. The presence of mutated TP53 in the SM has also shown its independent prognostic ability in operated cases of HNSCC [90]. In fact, of the several other markers and panels of molecular alterations being evaluated, alterations related to p53 protein and the TP53 genome seem to be the most appreciated for their potential utility as MSM [89]. However, in another report, the overexpression of a proto-oncogene, eukaryotic translation initiation factor 4E (eIF4E) in the SM, has demonstrated to have a better predictive value than the p53 immunopositivity [91]. A panel of methylated gene combinations identified at the SM has also shown to reliably predict the LR in HNSCC, independent of other known histological risk factors [82]. Overall, the MSM, with a known panel of markers, can complement the current clinic-pathological risk factors in operated cases of HNSCC [82, 84, 88]. Although the low coverage of the technique, additional time consumption, and financial constraints have been the major limiting factors to hinder the clinical application of MSM so far, with the recent advances in the field of biotechnology, MSM seems not too far from becoming a practical reality [85, 89].
2.2. Extension along the third dimension: difficulty in accessing the tumor depth
In an audit of 6.5 million patients with various solid cancers, the cancers arising from the oral cavity exhibited the highest rate of margin positivity across both sexes [92]. The SM in oral cancer surgery is more likely to fail along the deeper aspect (in the third dimension) than at the mucosal front, particularly in large lesions involving complex anatomical sites, such as buccal mucosa, retromolar trigone, tongue, and floor of mouth [71]. This is because, the OSCC involving these subsites can extend along the third dimension into the deeper muscles, soft tissues of the cheek/infratemporal fossa/neck, or into adjacent bony compartments, making it challenging at times to attain a clear margin all along the deeper extent [71]. The notion of having a margin of > 5 mm on the naked eye may not necessarily reflect an oncologically adequate or safe surgical margin along the third dimension, as the microscopic tumor extension in this dimension (along the nerves, muscles, and lymphatics) may go much beyond the visualized extent of the tumor. In fact, delineating the surgical margin along the deeper extent of the tumor spread, in its third dimension, is an entirely different facet than that of the mucosal margin [71]. Apart from the obvious constraints along the deeper planes such as ‘concealed microscopic spread,’ ‘difficultly in exposure of the deeper surgical site,’ and ‘potential proximity to the critical structures,’ the surgical resection in this plane, particularly while cutting through the elastic tissue, could also result in a ‘significant retraction of the cut tissue.’ These issues along the third dimension could negatively affect the orientation of the cut margins during grossing and their pathological assessment, eventually undermining the status of SM.
Remedy: Alternate surgical approaches to accomplish optimal disease clearance in OSCC
To ensure optimal clearance of a large-volume tumor, particularly at its deeper front, currently, surgeons adopt one of the two alternative approaches. In the first approach, which aligns with the traditional onco-surgical practice, surgeons tend to remove one additional layer of the uninvolved structure around the tumor as a safe surgical margin. This approach is commonly followed in buccal cancer that nears or abuts the mandible but without involving it, in which case, stripping of periosteum from the adjacent mandible can ensure an adequate disease clearance. Similar approach is also followed in other areas, such as sinonasal tumors (where lamina papyracea, periorbita, cribriform plate, or dura can be removed to account for disease that respectively abuts and breaches the lamina papyracea, or the skull base) and small pharyngeal cancers (superior constrictor resection for tonsillar cancers and middle or inferior constrictor for hypopharyngeal cancers) [93, 94]. The second approach, which has gained popularity over the last few decades, is the concept of compartmental resection, wherein the entire anatomical compartment of the involved site or the whole organ is removed in toto with the tumor. The oncological relevance of this approach is rooted in the concept of ‘natural anatomical barriers’ that contain the spread of tumors outside the confines of a particular compartment, zone, or organ itself [95–97]. This concept forms the basis for ‘compartment tongue surgery’ or ‘compartment clearance of infratemporal fossa’ for the advanced OSCC involving oral tongue and gingiva-buccal complex, respectively [96–99]. Apart from accounting for the deeper microscopic spread of the tumor, these compartment surgeries, by ensuring a complete in toto clearance of the relevant compartments, can also reduce the risk of residual disease that could have otherwise resulted from a potential retraction of the tongue muscles or masticatory muscles during the conventional surgical excision. The philosophy of compartment resection can also be witnessed in several other onco-surgical procedures, including those for laryngeal cancers (entire spectrum of cordectomy and other forms of partial laryngectomy), sinonasal cancers (various maxillectomies and orbital exenteration), and ear cancers (temporal bone resections) [98–101]. Nevertheless, though intuitively acceptable, with favorable results in studies from a few centers, the oncological safety of some of these approaches in OSCC, as opposed to the classical approach of ‘aiming for a clear SM of ≥ 5 mm,’ continues to be a matter of debate. One of the other counteractive measures to account for the deeper microscopic spread of the tumor is the use of real-time intraoperative assessment of SM, which is discussed in the next section of this review.
Predicament 3: Inadequacies of current intraoperative methods for assessing SM
Some of the image-based techniques such as confocal microscopy, tissue staining methods (e.g., Lugol’s iodine), narrow band imaging and other spectral imaging tools, have been explored for assessing the SM intraoperatively, as these tools could enable better demarcation of the interface between the tumor and normal tissue, allowing surgeon to achieve a safe SM away from the tumor [102]. However, most of these imaging instruments are of value only along the mucosal surfaces (two-dimensional assessment of SM), and their availability and modest diagnostic accuracy continue to be the critical barriers for their clinical application. On the other hand, IFSH, which is among the most practiced intraoperative methods for assessing the disease clearance in HNSCC, could be of more relevant to clinical practice [69, 103, 104]. Unlike the other methods described above, which facilitate identification of a safe SM away from the tumor in vivo to cut through, the IFSH, performed ex vivo on the excised tissue, is expected to help in achieving a better disease clearance by enabling an on-table (same-setting) re-resection of the SM, if found positive [19, 105–107]. The IFSH of SBM is particularly valuable in large tumors with potential microscopic extension into the third dimension (along the muscle, nerve, or other soft tissue channels) and in cases that could have satellite nodules or skip lesions away from the main tumor. In a retrospective study on oral and pharyngeal cancers, the frozen controlled re-resection of the positive SBM until negative resulted in a similar 5-year survival rate as in patients with immediate negative margins [108]. In this study, the use of IFSH led to an increase in OS rate by 2–3% among the operated oral and pharyngeal cancers. However, not many studies have been able to reproduce similar outcomes with IFSH. In fact, most of the other studies in the literature have reported poor reliability of IFSH in improving survival rates among the HNSCC, which could be attributable to a couple of practical and technical issues discussed below [29, 33, 41–47].
Since the IFSH involves examination of tissue taken from either SBM or SSM ex vivo, when reported positive, precise localization of the involved site on the surgical bed is prone to errors [19, 109, 110]. While some of the practices, such as the paired tagging, application of clips to the surgical field, and further re-orientation of the excised tissue fragment with sutures and colors, have all been used to counter this 'relocation errors,' they are not without practical difficulties and reliability issues [19, 24, 109–112]. Secondly, studies have reported considerable disparities between the status of ‘IFSH-designated SM’ and of the ‘final pathological classification’ (after the formalin fixation) of the same margin specimen [23, 24, 33, 44, 113, 114]. In one of the studies, the false-negative rates of IFSH in OSCC were reported to be as high as 46% [33]. Furthermore, the reliability of IFSH could also be affected by the inherent drawbacks of the SBM discussed in the earlier section; and on the other hand, the IFSH on SSM comes with the risk of mis-handling of the surgical specimen, owing to the need for a time-sensitive intraoperative reporting, which in turn, could hamper the final histopathological assessment of the SSM and its reliability [115]. Lastly, studies have also reported IFSH to be cost-ineffective as a routine practice [69, 107]. Considering these drawbacks of the IFSH approach, the guidelines that support the IFSH in HNSCC have limited its utility only to rule out the possibility of an incomplete clearance in doubtful cases, to allow an appropriate on-table re-resection [116]. In other words, the current consensus calls for attaching ‘no further clinical relevance’ to the status of SM based on IFSH, and particularly downplays the significance of SMB based IFSH in planning the adjuvant therapy.
Remedy: Newer methods for real-time intraoperative assessment of SM
With the recent advent in the field of biotechnology, several novel technology-based instruments, with different working principles, have been introduced into surgical armamentaria for the ‘real-time intraoperative margin assessment,’ and a few of these instruments have already exhibited a promising outlook on preclinical and early clinical studies [69, 70, 102]. Table 2 provides a brief summary of some of the prominent tools that are being explored for improving the intraoperative margin assessment in HNSCC. While many of these are still useful only for two-dimensional assessment of SM, a few tools that are based on spectroscopy-based molecular imaging technology and mass spectrometry-based molecular profiling can be used for delineating the SM even along the third dimension.
Table 2.
Prominent technology-based devices that are being explored for IMA in HNSCC
| Sl no | Name | Technology | Use in HNSCC |
|---|---|---|---|
| 1 | Narrow band imaging | uses a high-resolution endoscope and a filter to narrow white light into distinct wavelengths of light (415 nm blue and 540 nm green) that limits penetrance to the mucosal surface for improved visualization of vessels within | [117–120] |
| 2 | Elastic scattering spectroscopy | point spectroscopic measurement of cellular and subcellular morphological features (scattering spectral signatures) with a depth of penetration of 0.5 millimeters | [121] |
| 3 | Autofluorescence | non-invasive handpiece-based illumination with bright blue light (400–460 nm), enabling direct visualization of the mucosa in real time | [122, 123] |
| 4 | Portable 3D ultrasound system | uses 3D probe, electromagnetic or optical tracker systems, or a sensorless 3D ex vivo ultrasound system | [124, 125] |
| 5 | High-resolution microendoscopy | fluorescence microscope coupled to a fiber optic imaging probe, allowing visualization of epithelial architecture and cellular morphology | [126, 127] |
| 6 | Optical coherence tomography | non-invasive modality that uses high-resolution microstructural imaging based on architectural changes with or without epithelial thickness. | [128–130] |
| 7 | Confocal reflectance microscopy | based on a signal provided by the backscattering of light from cellular and tissue structures, and image contrast is generated by differences in the refractive indexes of cellular components. | [131–133] |
| 8 | Touch Imprint Cytology | rapid histopathological method that is simpler and cheaper than frozen section | [134–137] |
| 9 | Raman spectroscopy | based on Raman Scattering - a photon in the light will interact with molecules and scatter to produce a highly detailed and specific biochemical (molecular and optical) ‘fingerprint’ of the matter | [138–142] |
| 10 | Matrix-assisted laser desorption ionization | perform tissue microsampling and real-time molecular profiling by mass spectrometry when coupled with a special laser microprobe | [143, 144] |
| 11 | intelligent Knife | aerosol generated by the diathermy is aspirated into a dedicated mass spectrometer for analysis to classify the dissected tissue as healthy or not. | [145–147] |
Spectrometry can identify metabolites and variations in metabolic processes inside a tissue, and since the aberrant metabolic process is one of the characteristic features of cancer, spectrometry-based techniques can readily differentiate the tumor tissue from other tissues purely based on the profiles of its metabolites [148]. Intelligent knife (iKnife) (Waters Corp, Milford, Massachusetts, United States), which works on the principle of rapid evaporative ionization mass spectrometry (REIMS), is one of the most promising emerging techniques of real-time margin assessment [70, 144]. In this technology, the aerosol generated by the monopolar or bipolar diathermy during surgical excision is aspirated into a dedicated mass spectrometer for analysis, and the derived lipid profiles will be matched against a database of histologically annotated reference spectra by appropriate statistical analysis to classify the dissected tissue as healthy or not [70]. The iKnife has demonstrated a commanding ability to discriminate the normal tissue from a premalignant lesion and invasive cancer with high accuracy (with 100% sensitivity and specificity) in different types of cancers, including OSCC [145, 147, 149–157]. REIMS collects the mass spectral signal of the tissue under investigation at a given location by analyzing the lipids and small molecules present within surgical smoke without any need for sample preparation, and thus, delivers instant feedback in a few seconds, making it an ideal intraoperative tool for almost real-time assessment of SM [149]. Since its introduction a decade back, iKnife has been optimized over the years for better intraoperative usability, and with the further incorporation of complex machine learning strategies, it is expected to achieve higher accuracy and precision in the days to come [147, 149, 152, 158].
Predicament 4: Issues around the procurement, processing, and examination of SM
Apart from the lack of consensus in terms of operational designations and the inherent disease characteristics, a few of the technical issues related to the extraction and processing of the surgical specimen could also affect the reliable assessment of the SM.
Firstly, the three-dimensional orientation of the excised surgical specimens of the head and neck region, which is an essential step for appropriate pathological processing, could be a challenging task by itself, particularly in composite specimens that contain muscles, fascia, nerves, and bones [17, 18]. In addition to the anatomical complexity of large composite resections, the multipart resection of the tumor and an unintended tumor fragmentation during excision could all hinder an appropriate orientation of the surgical specimen. Although each element of these soft tissue components can be tagged, inked, mounted, or sutured separately at the time of dispatch from operating rooms, the final orientation of the formalin-fixed specimen might not guarantee an accurate assessment of SM. Secondly, the procedural artifacts and the post-resection changes in the surgical specimen, such as cautery burns and specimen shrinkage, could also affect the accuracy of its assessment. Similarly, changes during specimen processing, such as crush artifacts, inadvertent sectioning of an uninked specimen, and physical loss of margin tissue, are some of the additional concerns that could affect the pathological assessment of SM and its clinical reliability [17, 18]. Third, the ability to accurately define the margin status can also be impeded by a lack of proficient interdisciplinary communication between the surgical team and pathologists [17, 18, 159]. As per a report, a discrepancy between the ‘surgeon-felt SM’ and the ‘pathologically reported SM’ was found in 93% of the patients who had undergone transoral robotic surgery for oropharyngeal cancers, which impacted their therapeutic planning [94]. Lastly, the status of SM, despite the best efforts, could remain elusive in some of the clinical scenarios, owing to any of the above-mentioned procedural or processing errors. If a particular pathological report, for any reason, is not able to indicate whether the margin in a resected surgical specimen is positive, negative, or close, the status of such SM is considered indeterminate [160]. As per a review, up to 20% of the curative resections of head and neck cancer can have an indeterminate SM [160].
Remedy: Interdisciplinary coordination
Apart from the attempts to minimise the surgical and tissue handling artifacts, another critical factor for enhancing the reliability of SM in complex tumors like HNSCC (including OSCC) is to have an efficient scheme of communication between the surgical and pathology teams. Both ‘interdisciplinary communication’ and ‘tissue handling’ can be optimised by having a frozen section facility within- or immediately adjacent to the operating theatre complex. Such a facility not only allows the surgeon to ‘hand carry’ the surgical specimen to the pathologist (minimising the orientation and tissue handling errors) but could also facilitate an effective communication of the ‘suspicious site(s)/front(s)’ of the surgical specimen to rule out a potential close or positive margin. The ‘bread-loafing of the surgical specimen’ in the presence of both surgeon and pathologist provides the maximum opportunity to have a reliable intraoperative assessment of SM, and in addition, tends to shorten the turnaround time required for pathological processing and reporting. Considering all these advantages, institutes dealing with HNSCC must embrace such close communication facilities and workflow between the surgeons and pathologists.
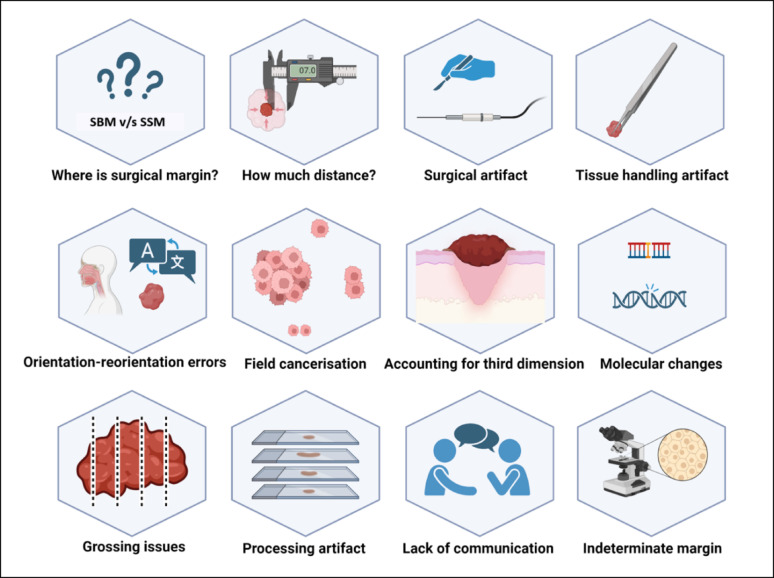
To summarise, several controversies, procedural variations, and technical errors illustrated in Fig. 2 could affect the prognostic value of the status of SM, and overcoming some of these shortcomings could work to improve the practical application of SM in oncology practice.
Fig. 2.
Various practical issues that could affect the reliability of the surgical margin in practice. Key: SBM- surgical bed margin and SSM- surgical specimen margin. [Created in BioRender. KMC, R. (2025) https://BioRender.com/h35f661]
Conclusion
Achieving a clear SM is the prime objective of oncologic resections and is among the most important prognostic factors for disease control and cure in surgically treated OSCC. Assessment of margins at the surgical bed allows for better feasibility of accurate re-resection if required, but does not allow for discriminating between clear and close margins. Assessment of margins at the surgical specimen allows differentiation between close and clear margins and an appropriately calibrated choice of adjuvant treatment. Frozen section evaluation- despite being operator and technique-dependent and not entirely specific- remains the current standard for intraoperative margin assessment. Although newer technologies incorporating molecular imaging and metabolite-based identification, such as the iKnife, show immense potential, they are not yet a clinical reality. The standardization of the clinical practice, along with the reliance on the SSM for prognostication, whenever applicable, could enhance the reliability of SM as a pathological prognostic factor, and thus, eventually may aid in streamlining its introduction into the staging manual. However, further studies are needed to validate the relevance of re-resected SM, dysplastic SM, and MSM. It might also be worthwhile to invest in multivariable prognostic models and nomograms that account for the SM as a continuum.
Author contributions
Dr Devaraja was involved in initial planning, manuscript layout, literature review, manuscript preparation, manuscript editing, and submission. Dr Thakar was involved in literature review, formulating manuscript layout, manuscript preparation, and manuscript editing before submission. Dr Paleri was involved in literature review, manuscript preparation, reframing the manuscript layout, and manuscript editing and proofreading before submission.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors did not receive support from any organization for the work.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, et al. NCCN Guidelines® insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20:224–34. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Prabhash K, Babu G, Kuriakose M, Birur P, Anand AK, et al. Indian clinical practice consensus guidelines for the management of oral cavity cancer. Indian J Cancer. 2020;57:S6–8. [DOI] [PubMed] [Google Scholar]
- 3.Joo Y-H, Cho J-K, Koo BS, Kwon M, Kwon SK, Kwon SY, et al. Guidelines for the surgical management of oral cancer: Korean society of Thyroid-Head and neck surgery. Clin Exp Otorhinolaryngol. 2019;12:107–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto F, Mori T, Matsumura S, Matsumoto Y, Fukasawa M, Teshima M, et al. Prognostic significance of surgical extranodal extension in head and neck squamous cell carcinoma patients. Jpn J Clin Oncol. 2017;47:699–704. [DOI] [PubMed] [Google Scholar]
- 5.International Consortium for Outcome Research (ICOR) in Head and Neck Cancer, Ebrahimi A, Gil Z, Amit M, Yen T-C, Liao C-T, et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140:1138–48. [DOI] [PubMed] [Google Scholar]
- 6.Liao L-J, Lo W-C, Hsu W-L, Wang C-T, Lai M-S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer. 2012;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P, Sultania M, Muduly D, Chaudhary I, Ghalige H, Patro S, et al. Triple positive oral squamous cell carcinoma patients predict poor survival outcomes: multiple factor positivity warrants the need for modified treatment approaches. J Maxillofac Oral Surg. 2024;23:923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Zhu Y, Cai H, Zhang Y, Hou J. Impact of lymphovascular invasion in oral squamous cell carcinoma: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:319–e3281. [DOI] [PubMed] [Google Scholar]
- 9.Sunkara PR, Graff JT, Cramer JD. Association of surgical margin distance with survival in patients with resected head and neck squamous cell carcinoma: A secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2023;149:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2016;62:60–71. [DOI] [PubMed] [Google Scholar]
- 11.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Springer; 2017.
- 12.Machiels J-P, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1462–75. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski LP. Eugene Nicholas myers’ lecture on head and neck cancer, 2020: the surgeon as a prognostic factor in head and neck cancer patients undergoing surgery. Int Arch Otorhinolaryngol. 2023;27:e536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843–50. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an international American head and neck society member survey. Head Neck. 2005;27:952–8. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock YE, Alava I, Dierks EJ. Pitfalls in determining head and neck surgical margins. Oral Maxillofac Surg Clin North Am. 2014;26:151–62. [DOI] [PubMed] [Google Scholar]
- 18.Magliocca KR. Surgical margins: the perspective of pathology. Oral Maxillofac Surg Clin North Am. 2017;29:367–75. [DOI] [PubMed] [Google Scholar]
- 19.Kubik MW, Sridharan S, Varvares MA, Zandberg DP, Skinner HD, Seethala RR, et al. Intraoperative margin assessment in head and neck cancer: A case of misuse and abuse?? Head Neck Pathol. 2020;14:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchakjian MR, Ginader T, Tasche KK, Pagedar NA, Smith BJ, Sperry SM. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg. 2018;159:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tassone P, Savard C, Topf MC, Keane W, Luginbuhl A, Curry J, et al. Association of positive initial margins with survival among patients with squamous cell carcinoma treated with total laryngectomy. JAMA Otolaryngol Head Neck Surg. 2018;144:1030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125:2298–307. [DOI] [PubMed] [Google Scholar]
- 23.Wu SS, Woody N, Hesse J, Cook S, Cracolici V, Ku JA, et al. Margin assessment methods in oral cavity squamous cell carcinoma and recurrence: tumor bed vs resection specimen sampling. JAMA Otolaryngol Head Neck Surg. 2023;149:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhu AV, Sturgis CD, Lai C, Maxwell JH, Merzianu M, Hernandez-Prera JC, et al. Improving margin revision: characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017;75:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasche KK, Buchakjian MR, Pagedar NA, Sperry SM. Definition of close margin in oral cancer surgery and association of margin distance with local recurrence rate. JAMA Otolaryngol Head Neck Surg. 2017;143:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamman J, Howe CL, Borgstrom M, Baker A, Wang SJ, Bearelly S. Impact of close margins in head and neck mucosal squamous cell carcinoma: A systematic review. Laryngoscope. 2022;132:307–21. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell DA, Kanatas A, Murphy C, Chengot P, Smith AB, Ong TK. Margins and survival in oral cancer. Br J Oral Maxillofac Surg. 2018;56:820–9. [DOI] [PubMed] [Google Scholar]
- 28.Lee D-H, Kim G-J, Kim H-B, Shin H-I, Kim C-S, Nam I-C et al. Close Surgical Margins in Oral and Oropharyngeal Cancer: Do They Impact Prognosis? Cancers (Basel). 2022;14:2990. [DOI] [PMC free article] [PubMed]
- 29.Maharaj DD, Thaduri A, Jat B, Poonia DR, Durgapal P, Rajkumar KS. Performance and survival outcomes of defect-driven versus specimen-driven method of frozen section intraoperative margin assessment in oral cancers. Int J Oral Maxillofac Surg. 2022;51:1131–7. [DOI] [PubMed] [Google Scholar]
- 30.Amit M, Na’ara S, Leider-Trejo L, Akrish S, Cohen JT, Billan S, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: A prospective randomized controlled study. Head Neck. 2016;38(Suppl 1):E1803–1809. [DOI] [PubMed] [Google Scholar]
- 31.Sridharan S, Thompson LDR, Purgina B, Sturgis CD, Shah AA, Burkey B, et al. Early squamous cell carcinoma of the oral tongue with histologically benign lymph nodes: A model predicting local control and vetting of the eighth edition of the American joint committee on cancer pathologic T stage. Cancer. 2019;125:3198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell JH, Thompson LDR, Brandwein-Gensler MS, Weiss BG, Canis M, Purgina B, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142:1191–8. [DOI] [PubMed] [Google Scholar]
- 34.Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract. 1994;190:115–23. [DOI] [PubMed] [Google Scholar]
- 35.Hakim SG, von Bialy R, Falougy M, Steller D, Tharun L, Rades D, et al. Impact of stratified resection margin classification on local tumor control and survival in patients with oral squamous cell carcinoma. J Surg Oncol. 2021;124:1284–95. [DOI] [PubMed] [Google Scholar]
- 36.Miller MC, Goldenberg D, Education Committee of the American Head and Neck Society (AHNS). AHNS series: do you know your guidelines? Principles of surgery for head and neck cancer: A review of the National comprehensive cancer network guidelines. Head Neck. 2017;39:791–6. [DOI] [PubMed] [Google Scholar]
- 37.P DK. Evaluation of resection margins in oral squamous cell carcinoma. Acta Chir Plast. 2023;64:110–5. [DOI] [PubMed] [Google Scholar]
- 38.Hendriksma M, Montagne MW, Langeveld TPM, Veselic M, van Benthem PPG, Sjögren EV. Evaluation of surgical margin status in patients with early glottic cancer (Tis-T2) treated with transoral CO2 laser microsurgery, on local control. Eur Arch Otorhinolaryngol. 2018;275:2333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzopardi S, Lowe D, Rogers S. Audit of the rates of re-excision for close or involved margins in the management of oral cancer. Br J Oral Maxillofac Surg. 2019;57:678–81. [DOI] [PubMed] [Google Scholar]
- 40.Shivhare V, Rath S, Rathod H, Dash NK, Parikh A, Suryanarayan Kunikullaya U. Analyzing the impact of close margins and extra-resection margins on failure rates in postoperative oral cavity cancers. Klin Onkol. 2023;37:467–72. [DOI] [PubMed] [Google Scholar]
- 41.Ettl T, El-Gindi A, Hautmann M, Gosau M, Weber F, Rohrmeier C, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17–23. [DOI] [PubMed] [Google Scholar]
- 42.Tapase TP, Patra RK, Kisku DK, Badhai SK, Panigrahi SK, Bhoopathy G, et al. Intraoperative assessment of margin accuracy in early oral squamous cell carcinoma (cT1, T2, N0): clinical versus frozen section analysis. Cureus. 2023;15:e36699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ooms M, Ponke L, Puladi B, Winnand P, Heitzer M, Katz MS, et al. Impact of secondary tumor-free resection margins in soft tissue on local, regional, and distant recurrence in R0-resected oral squamous cell carcinoma. Head Neck. 2022;44:1172–81. [DOI] [PubMed] [Google Scholar]
- 44.Long SM, Mclean T, Valero Mayor C, Fitzgerald CWR, Feit NZ, Katabi N, et al. Use of intraoperative frozen section to assess final tumor margin status in patients undergoing surgery for oral cavity squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel RS, Goldstein DP, Guillemaud J, Bruch GA, Brown D, Gilbert RW, et al. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32:1444–51. [DOI] [PubMed] [Google Scholar]
- 46.Nentwig K, Unterhuber T, Wolff K-D, Ritschl LM, Nieberler M. The impact of intraoperative frozen section analysis on final resection margin status, recurrence, and patient outcome with oral squamous cell carcinoma. Clin Oral Investig. 2021;25:6769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szewczyk M, Golusinski W, Pazdrowski J, Masternak M, Sharma N, Golusinski P. Positive fresh frozen section margins as an adverse independent prognostic factor for local recurrence in oral cancer patients. Laryngoscope. 2018;128:1093–8. [DOI] [PubMed] [Google Scholar]
- 48.Bulbul MG, Tarabichi O, Sethi RK, Parikh AS, Varvares MA. Does clearance of positive margins improve local control in oral cavity cancer?? A Meta-analysis. Otolaryngol Head Neck Surg. 2019;161:235–44. [DOI] [PubMed] [Google Scholar]
- 49.Mishra A, Malik A, Datta S, Mair M, Bal M, Nair D, et al. Defining optimum surgical margins in buccoalveolar squamous cell carcinoma. Eur J Surg Oncol. 2019;45:1033–8. [DOI] [PubMed] [Google Scholar]
- 50.Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sándor GKB. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:625–9. [DOI] [PubMed] [Google Scholar]
- 51.Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, Ganly I, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 2017;143:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alicandri-Ciufelli M, Bonali M, Piccinini A, Marra L, Ghidini A, Cunsolo EM, et al. Surgical margins in head and neck squamous cell carcinoma: what is close? Eur Arch Otorhinolaryngol. 2013;270:2603–9. [DOI] [PubMed] [Google Scholar]
- 53.Wong LS, McMahon J, Devine J, McLellan D, Thompson E, Farrow A, et al. Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg. 2012;50:102–8. [DOI] [PubMed] [Google Scholar]
- 54.Spence RN, Efthymiou V, Goss D, Varvares MA. Margin distance in oral tongue cancer surgery: A systematic review of survival and recurrence outcomes. Oral Oncol. 2023;147:106609. [DOI] [PubMed] [Google Scholar]
- 55.Szewczyk M, Pazdrowski J, Pieńkowski P, Wojtera B, Więckowska B, Golusiński P, et al. A matter of margins in oral Cancer-How close is enough?? Cancers (Basel). 2024;16:1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillon JK, Brown CB, McDonald TM, Ludwig DC, Clark PJ, Leroux BG, et al. How does the close surgical margin impact recurrence and survival when treating oral squamous cell carcinoma? J Oral Maxillofac Surg. 2015;73:1182–8. [DOI] [PubMed] [Google Scholar]
- 57.Stathopoulos P, Smith WP. Close resection margins do not influence local recurrence in patients with oral squamous cell carcinoma: A prospective cohort study. J Oral Maxillofac Surg. 2018;76:873–6. [DOI] [PubMed] [Google Scholar]
- 58.Barry CP, Ahmed F, Rogers SN, Lowe D, Bekiroglu F, Brown JS, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck. 2015;37:1176–80. [DOI] [PubMed] [Google Scholar]
- 59.Solomon J, Hinther A, Matthews TW, Nakoneshny SC, Hart R, Dort JC, et al. The impact of close surgical margins on recurrence in oral squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2021;50:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain PV, Sharan R, Manikantan K, Clark GM, Chatterjee S, Mallick I, et al. Redefining adequate margins in oral squamous cell carcinoma: outcomes from close and positive margins. Eur Arch Otorhinolaryngol. 2020;277:1155–65. [DOI] [PubMed] [Google Scholar]
- 61.Cariati P, Cabello Serrano A, Mosalve Iglesias F, Torné Poyatos P, Fernandez Solis J, Ferrari S, et al. What is the real prognostic value of close margins in oral oncology? Curr Probl Cancer. 2019;43:100500. [DOI] [PubMed] [Google Scholar]
- 62.Adriaansens CMEM, Noorlag R, Visscher WP, de Bree R, Breimer GE, van Es RJJ. A closer look at the resection margins of buccal mucosa cancer: their influence on local recurrence free survival. Head Neck. 2023;45:983–92. [DOI] [PubMed] [Google Scholar]
- 63.Lang K, Held T, Freudlsperger C, Plath K, Hoffmann J, Plinkert PK, et al. Oral cavity squamous cell carcinoma: impact of clear margin distance on locoregional control in patients undergoing postoperative radiotherapy. Technol Cancer Res Treat. 2024;23:15330338241258596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brinkman D, Callanan D, O’Shea R, Jawad H, Feeley L, Sheahan P. Impact of 3 mm margin on risk of recurrence and survival in oral cancer. Oral Oncol. 2020;110:104883. [DOI] [PubMed] [Google Scholar]
- 65.Young K, Bulosan H, Kida CC, Bewley AF, Abouyared M, Birkeland AC. Stratification of surgical margin distances by the millimeter on local recurrence in oral cavity cancer: A systematic review and meta-analysis. Head Neck. 2023;45:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada S, Kurita H, Shimane T, Kamata T, Uehara S, Tanaka H, et al. Estimation of the width of free margin with a significant impact on local recurrence in surgical resection of oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2016;45:147–52. [DOI] [PubMed] [Google Scholar]
- 67.Williamson A, Moen CM, Slim MAM, Warner L, O’Leary B, Paleri V. Transoral robotic surgery without adjuvant therapy: A systematic review and meta-analysis of the association between surgical margins and local recurrence. Oral Oncol. 2023;147:106610. [DOI] [PubMed] [Google Scholar]
- 68.Kurita H, Nakanishi Y, Nishizawa R, Xiao T, Kamata T, Koike T, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010;46:814–7. [DOI] [PubMed] [Google Scholar]
- 69.Higginson JA, Breik O, Thompson AH, Ashrafian H, Hardman JC, Takats Z, et al. Diagnostic accuracy of intraoperative margin assessment techniques in surgery for head and neck squamous cell carcinoma: A meta-analysis. Oral Oncol. 2023;142:106419. [DOI] [PubMed] [Google Scholar]
- 70.Hänel L, Kwiatkowski M, Heikaus L, Schlüter H. Mass spectrometry-based intraoperative tumor diagnostics. Future Sci OA. 2019;5:FSO373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005;41:1034–43. [DOI] [PubMed] [Google Scholar]
- 72.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. [DOI] [PubMed] [Google Scholar]
- 73.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–92. [PubMed] [Google Scholar]
- 74.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Van Der Wal JE, Snow GB, Leemans CR, et al. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol. 2002;161:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ha PK, Benoit NE, Yochem R, Sciubba J, Zahurak M, Sidransky D, et al. A transcriptional progression model for head and neck cancer. Clin Cancer Res. 2003;9:3058–64. [PubMed] [Google Scholar]
- 76.Braakhuis BJM, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–30. [PubMed] [Google Scholar]
- 77.Lavieille JP, Gazzeri S, Riva C, Reyt E, Brambilla C, Brambilla E. p53 mutations and p53, Waf-1, Bax and Bcl-2 expression in field cancerization of the head and neck. Anticancer Res. 1998;18:4741–9. [PubMed] [Google Scholar]
- 78.Wang X, Chen S, Chen X, Zhang C, Liang X. Tumor-related markers in histologically normal margins correlate with locally recurrent oral squamous cell carcinoma: a retrospective study. J Oral Pathol Med. 2016;45:83–8. [DOI] [PubMed] [Google Scholar]
- 79.Graveland AP, Golusinski PJ, Buijze M, Douma R, Sons N, Kuik DJ, et al. Loss of heterozygosity at 9p and p53 Immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int J Cancer. 2011;128:1852–9. [DOI] [PubMed] [Google Scholar]
- 80.Kamat MS, Puranik RS, Das Rai AB, Patil BR, Patil S. Assessing immuno-expression of p53 protein and TP 53 gene amplification in histologically negative surgical margins of oral squamous cell carcinoma patients and normal oral mucosa. Clin Oral Investig. 2022;26:6235–43. [DOI] [PubMed] [Google Scholar]
- 81.Sorroche BP, Talukdar FR, Lima SCS, Melendez ME, de Carvalho AC, de Almeida GC, et al. DNA methylation markers from negative surgical margins can predict recurrence of oral squamous cell carcinoma. Cancers (Basel). 2021;13:2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayashi M, Wu G, Roh J-L, Chang X, Li X, Ahn J, et al. Correlation of gene methylation in surgical margin imprints with locoregional recurrence in head and neck squamous cell carcinoma. Cancer. 2015;121:1957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devaraja K. Second primary tumor versus recurrent oral squamous cell carcinoma: effect of inconsistent definitions on evidence synthesis. Cancer Res Stat Treat. 2022;5:719–21. [Google Scholar]
- 84.Fox SA, Vacher M, Farah CS. Transcriptomic biomarker signatures for discrimination of oral cancer surgical margins. Biomolecules. 2022;12:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark DJ, Mao L. Understanding the surgical margin: A molecular assessment. Oral Maxillofac Surg Clin North Am. 2017;29:245–58. [DOI] [PubMed] [Google Scholar]
- 86.Braakhuis BJM, Bloemena E, Leemans CR, Brakenhoff RH. Molecular analysis of surgical margins in head and neck cancer: more than a marginal issue. Oral Oncol. 2010;46:485–91. [DOI] [PubMed] [Google Scholar]
- 87.Pierik AS, Leemans CR, Brakenhoff RH. Resection margins in head and neck cancer surgery: an update of residual disease and field cancerization. Cancers (Basel). 2021;13:2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baldan F, Gnan C, Lazarevic M, Nikolic N, Mio C, Tepavcevic Z, et al. Somatic genomic imbalances in tumour-free surgical margins of oral cancer. Int J Oral Maxillofac Surg. 2023;52:831–8. [DOI] [PubMed] [Google Scholar]
- 89.Stepan KO, Li MM, Kang SY, Puram SV. Molecular margins in head and neck cancer: current techniques and future directions. Oral Oncol. 2020;110:104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Houten VMM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, van den Brekel MWM, et al. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: a prospective study. Clin Cancer Res. 2004;10:3614–20. [DOI] [PubMed] [Google Scholar]
- 91.Nathan C-AO, Amirghahri N, Rice C, Abreo FW, Shi R, Stucker FJ. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope. 2002;112:2129–40. [DOI] [PubMed] [Google Scholar]
- 92.Orosco RK, Tapia VJ, Califano JA, Clary B, Cohen EEW, Kane C, et al. Positive surgical margins in the 10 most common solid cancers. Sci Rep. 2018;8:5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chatelet F, Simon F, Bedarida V, Le Clerc N, Adle-Biassette H, Manivet P, et al. Surgical management of sinonasal cancers: A comprehensive review. Cancers (Basel). 2021;13:3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parvathaneni A, Patel SA, Houlton JJ, Pang J, Futran ND. Clarification of the margin status by the multidisciplinary tumor board following transoral robotic surgery for p16 positive oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2023;8:1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jimenez L, Jayakar SK, Ow TJ, Segall JE. Mechanisms of invasion in head and neck cancer. Arch Pathol Lab Med. 2015;139:1334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oj U, Jb FS, Dm NGH, Wc F et al. F,. Invasion Patterns of External Auditory Canal Squamous Cell Carcinoma: A Histopathology Study. The Laryngoscope [Internet]. 2021 [cited 2024 Sep 8];131. Available from: https://pubmed.ncbi.nlm.nih.gov/32311775/ [DOI] [PMC free article] [PubMed]
- 97.Mor N, Blitzer A. Functional anatomy and oncologic barriers of the larynx. Otolaryngol Clin North Am. 2015;48:533–45. [DOI] [PubMed] [Google Scholar]
- 98.Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, et al. Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncol. 2011;47:174–9. [DOI] [PubMed] [Google Scholar]
- 99.Trivedi NP, Kekatpure V, Kuriakose MA. Radical (compartment) resection for advanced buccal cancer involving masticator space (T4b): our experience in Thirty patients. Clin Otolaryngol. 2012;37:477–83. [DOI] [PubMed] [Google Scholar]
- 100.Oancea ALA, Popescu CR, Bordei P. Anatomo-surgical correlations in larynx cancer. J Med Life. 2012;5:198–202. [PMC free article] [PubMed] [Google Scholar]
- 101.Peretti G, Piazza C, Mora F, Garofolo S, Guastini L. Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2016;24:135–9. [DOI] [PubMed] [Google Scholar]
- 102.Heidkamp J, Scholte M, Rosman C, Manohar S, Fütterer JJ, Rovers MM. Novel imaging techniques for intraoperative margin assessment in surgical oncology: A systematic review. Int J Cancer. 2021;149:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Kleijn BJ, Heldens GTN, Herruer JM, Sier CFM, Piazza C, de Bree R, et al. Intraoperative imaging techniques to improve surgical resection margins of oropharyngeal squamous cell cancer: A comprehensive review of current literature. Cancers (Basel). 2023;15:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Koning KJ, Adriaansens CMEM, Noorlag R, de Bree R, van Es RJJ. Intraoperative techniques that define the mucosal margins of oral cancer In-Vivo: A systematic review. Cancers (Basel). 2024;16:1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tirelli G, Hinni ML, Fernández-Fernández MM, Bussani R, Gatto A, Bonini P, et al. Frozen sections and complete resection in oral cancer surgery. Oral Dis. 2019;25:1309–17. [DOI] [PubMed] [Google Scholar]
- 106.Gauthier P, Audet N, Guertin L, Arteau-Gauthier I, Comeau L, Pilon L, et al. Complete frozen section margins (with measurable 1 or 5 mm Thick free margin) for cancer of the tongue: part 2: clinical experience. J Otolaryngol Head Neck Surg. 2010;39:20–7. [PubMed] [Google Scholar]
- 107.Datta S, Mishra A, Chaturvedi P, Bal M, Nair D, More Y, et al. Frozen section is not cost beneficial for the assessment of margins in oral cancer. Indian J Cancer. 2019;56:19–23. [DOI] [PubMed] [Google Scholar]
- 108.Kwok P, Gleich O, Hübner G, Strutz J. Prognostic importance of clear versus revised margins in oral and pharyngeal cancer. Head Neck. 2010;32:1479–84. [DOI] [PubMed] [Google Scholar]
- 109.van Lanschot CGF, Mast H, Hardillo JA, Monserez D, Ten Hove I, Barroso EM, et al. Relocation of inadequate resection margins in the wound bed during oral cavity oncological surgery: A feasibility study. Head Neck. 2019;41:2159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kerawala CJ, Ong TK. Relocating the site of frozen sections–is there room for improvement? Head Neck. 2001;23:230–2. [DOI] [PubMed] [Google Scholar]
- 111.Kirova YM, Castro Pena P, Hijal T, Fournier-Bidoz N, Laki F, Sigal-Zafrani B, et al. Improving the definition of tumor bed boost with the use of surgical clips and image registration in breast cancer patients. Int J Radiat Oncol Biol Phys. 2010;78:1352–5. [DOI] [PubMed] [Google Scholar]
- 112.Aaboubout Y, Barroso EM, Algoe M, Ewing-Graham PC, Ten Hove I, Mast H et al. Intraoperative assessment of resection margins in oral cavity cancer: this is the way. J Vis Exp. 2021. [DOI] [PubMed]
- 113.Serinelli S, Bryant SM, Williams MPA, Marzouk M, Zaccarini DJ. Frozen-Permanent section discrepancy rate in oral cavity and oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2022;16:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110:1773–6. [DOI] [PubMed] [Google Scholar]
- 115.Moratin J, Horn D, Oehme M, Semmelmayer K, Flechtenmacher C, Ristow O, et al. Variation of resection margins in oral cancer in dependence of tumor stage and subsite - a retrospective cohort study. Clin Oral Investig. 2024;28:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Helliwell TR, Giles TE. Pathological aspects of the assessment of head and neck cancers: united Kingdom National multidisciplinary guidelines. J Laryngol Otol. 2016;130:S59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plaat BEC, Zwakenberg MA, van Zwol JG, Wedman J, van der Laan BFAM, Halmos GB, et al. Narrow-band imaging in transoral laser surgery for early glottic cancer in relation to clinical outcome. Head Neck. 2017;39:1343–8. [DOI] [PubMed] [Google Scholar]
- 118.Azam MA, Sampieri C, Ioppi A, Benzi P, Giordano GG, De Vecchi M, et al. Videomics of the upper aerodigestive tract cancer: deep learning applied to white light and narrow band imaging for automatic segmentation of endoscopic images. Front Oncol. 2022;12:900451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tirelli G, Piovesana M, Marcuzzo AV, Gatto A, Biasotto M, Bussani R, et al. Tailored resections in oral and oropharyngeal cancer using narrow band imaging. Am J Otolaryngol. 2018;39:197–203. [DOI] [PubMed] [Google Scholar]
- 120.Vicini C, Montevecchi F, D’Agostino G, DE Vito A, Meccariello G. A novel approach emphasising intra-operative superficial margin enhancement of head-neck tumours with narrow-band imaging in transoral robotic surgery. Acta Otorhinolaryngol Ital. 2015;35:157–61. [PMC free article] [PubMed] [Google Scholar]
- 121.Grillone GA, Wang Z, Krisciunas GP, Tsai AC, Kannabiran VR, Pistey RW, et al. The color of cancer: margin guidance for oral cancer resection using elastic scattering spectroscopy. Laryngoscope. 2017;127(Suppl 4):S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pošta P, Kolk A, Pivovarčíková K, Liška J, Genčur J, Moztarzadeh O, et al. Clinical experience with autofluorescence guided oral squamous cell carcinoma surgery. Diagnostics (Basel). 2023;13:3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White HW, Naveed AB, Campbell BR, Lee Y-J, Baik FM, Topf M, et al. Infrared Fluorescence-guided surgery for tumor and metastatic lymph node detection in head and neck cancer. Radiol Imaging Cancer. 2024;6:e230178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makouei F, Frehr TD, Agander TK, Lelkaitis G, Hyldig Dal M, Kaltoft M, et al. Feasibility of a novel 3D ultrasound imaging technique for intraoperative margin assessment during tongue cancer surgery. Curr Oncol. 2024;31:4414–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Makouei F, Ewertsen C, Agander TK, Olesen MV, Pakkenberg B, Todsen T. 3D ultrasound versus computed tomography for tumor volume measurement compared to gross Pathology-A pilot study on an animal model. J Imaging. 2022;8:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miles BA, Patsias A, Quang T, Polydorides AD, Richards-Kortum R, Sikora AG. Operative margin control with high-resolution optical microendoscopy for head and neck squamous cell carcinoma. Laryngoscope. 2015;125:2308–16. [DOI] [PubMed] [Google Scholar]
- 127.Vila PM, Park CW, Pierce MC, Goldstein GH, Levy L, Gurudutt VV, et al. Discrimination of benign and neoplastic mucosa with a high-resolution microendoscope (HRME) in head and neck cancer. Ann Surg Oncol. 2012;19:3534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn Ther. 2016;13:211–7. [DOI] [PubMed] [Google Scholar]
- 129.Sunny SP, Agarwal S, James BL, Heidari E, Muralidharan A, Yadav V, et al. Intra-operative point-of-procedure delineation of oral cancer margins using optical coherence tomography. Oral Oncol. 2019;92:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jerjes W, Hamdoon Z, Yousif AA, Al-Rawi NH, Hopper C. Epithelial tissue thickness improves optical coherence tomography’s ability in detecting oral cancer. Photodiagnosis Photodyn Ther. 2019;28:69–74. [DOI] [PubMed] [Google Scholar]
- 131.Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44:1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucchese A, Gentile E, Romano A, Maio C, Laino L, Serpico R. The potential role of in vivo reflectance confocal microscopy for evaluating oral cavity lesions: a systematic review. J Oral Pathol Med. 2016;45:723–9. [DOI] [PubMed] [Google Scholar]
- 133.Ramani RS, Tan I, Bussau L, Angel CM, McCullough M, Yap T. Confocal microscopy in oral cancer and oral potentially malignant disorders: A systematic review. Oral Dis. 2023;29:3003–15. [DOI] [PubMed] [Google Scholar]
- 134.Yadav GS, Donoghue M, Tauro DP, Yadav A, Agarwal S. Intraoperative imprint evaluation of surgical margins in oral squamous cell carcinoma. Acta Cytol. 2013;57:75–83. [DOI] [PubMed] [Google Scholar]
- 135.Naveed H, Abid M, Hashmi AA, Edhi MM, Sheikh AK, Mudassir G, et al. Diagnostic accuracy of touch imprint cytology for head and neck malignancies: a useful intra-operative tool in resource limited countries. BMC Clin Pathol. 2017;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loncar B, Pajtler M, Milicić-Juhas V, Kotromanović Z, Staklenac B, Pauzar B. Imprint cytology in laryngeal and pharyngeal tumours. Cytopathology. 2007;18:40–3. [DOI] [PubMed] [Google Scholar]
- 137.Zafar A, Sherlin HJ, Jayaraj G, Ramani P, Don KR, Santhanam A. Diagnostic utility of touch imprint cytology for intraoperative assessment of surgical margins and Sentinel lymph nodes in oral squamous cell carcinoma patients using four different cytological stains. Diagn Cytopathol. 2020;48:101–10. [DOI] [PubMed] [Google Scholar]
- 138.Holler S, Mansley E, Mazzeo C, Donovan MJ, Sobrero M, Miles BA. Raman spectroscopy of head and neck cancer: separation of malignant and healthy tissue using signatures outside the fingerprint region. Biosens (Basel). 2017;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cals FLJ, Koljenović S, Hardillo JA, Baatenburg de Jong RJ, Bakker Schut TC, Puppels GJ. Development and validation of Raman spectroscopic classification models to discriminate tongue squamous cell carcinoma from non-tumorous tissue. Oral Oncol. 2016;60:41–7. [DOI] [PubMed] [Google Scholar]
- 140.Weber A, Enderle-Ammour K, Kurowski K, Metzger MC, Poxleitner P, Werner M, et al. AI-Based detection of oral squamous cell carcinoma with Raman histology. Cancers (Basel). 2024;16:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barroso EM, Ten Hove I, Bakker Schut TC, Mast H, van Lanschot CGF, Smits RWH, et al. Raman spectroscopy for assessment of bone resection margins in mandibulectomy for oral cavity squamous cell carcinoma. Eur J Cancer. 2018;92:77–87. [DOI] [PubMed] [Google Scholar]
- 142.Steybe D, Poxleitner P, Metzger MC, Rothweiler R, Beck J, Straehle J, et al. Stimulated Raman histology for histological evaluation of oral squamous cell carcinoma. Clin Oral Investig. 2023;27:4705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ogrinc N, Attencourt C, Colin E, Boudahi A, Tebbakha R, Salzet M, et al. Mass Spectrometry-Based differentiation of oral tongue squamous cell carcinoma and nontumor regions with the spidermass technology. Front Oral Health. 2022;3:827360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J, Sans M, Garza KY, Eberlin LS, MASS SPECTROMETRY TECHNOLOGIES, TO ADVANCE CARE FOR CANCER PATIENTS IN CLINICAL AND INTRAOPERATIVE USE. Mass Spectrom Rev. 2021;40:692–720. [DOI] [PubMed] [Google Scholar]
- 145.Vaysse P-M, Demers I, van den Hout MFCM, van de Worp W, Anthony IGM, Baijens LWJ, et al. Evaluation of the sensitivity of metabolic profiling by rapid evaporative ionization mass spectrometry: toward more radical oral cavity cancer resections. Anal Chem. 2022;94:6939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ifa DR, Eberlin LS. Ambient ionization mass spectrometry for cancer diagnosis and surgical margin evaluation. Clin Chem. 2016;62:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paul A, Srivastava S, Roy R, Anand A, Gaurav K, Husain N, et al. Malignancy prediction among tissues from oral SCC patients including neck invasions: a 1H HRMAS NMR based metabolomic study. Metabolomics. 2020;16:38. [DOI] [PubMed] [Google Scholar]
- 148.Kumar BS. Recent advances and applications of ambient mass spectrometry imaging in cancer research: an overview. Mass Spectrom (Tokyo). 2023;12:A0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Santilli AML, Jamzad A, Janssen NNY, Kaufmann M, Connolly L, Vanderbeck K, et al. Perioperative margin detection in basal cell carcinoma using a deep learning framework: a feasibility study. Int J Comput Assist Radiol Surg. 2020;15:887–96. [DOI] [PubMed] [Google Scholar]
- 150.Tzafetas M, Mitra A, Paraskevaidi M, Bodai Z, Kalliala I, Bowden S, et al. The intelligent knife (iKnife) and its intraoperative diagnostic advantage for the treatment of cervical disease. Proc Natl Acad Sci U S A. 2020;117:7338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alexander J, Gildea L, Balog J, Speller A, McKenzie J, Muirhead L, et al. A novel methodology for in vivo endoscopic phenotyping of colorectal cancer based on real-time analysis of the mucosal lipidome: a prospective observational study of the iKnife. Surg Endosc. 2017;31:1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Hese L, De Vleeschouwer S, Theys T, Larivière E, Solie L, Sciot R, et al. Towards real-time intraoperative tissue interrogation for REIMS-guided glioma surgery. J Mass Spectrom Adv Clin Lab. 2022;24:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.St John ER, Balog J, McKenzie JS, Rossi M, Covington A, Muirhead L, et al. Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery. Breast Cancer Res. 2017;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang H, Li T, Shen Q, Liu Z, Chen Y-W, Huang D, et al. Lipidomic study and diagnosis of hepatocellular carcinoma tumor with rapid evaporative ionization mass spectrometry. Electrophoresis. 2023;44:1057–67. [DOI] [PubMed] [Google Scholar]
- 155.Phelps DL, Balog J, Gildea LF, Bodai Z, Savage A, El-Bahrawy MA, et al. The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Br J Cancer. 2018;118:1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balog J, Sasi-Szabó L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med. 2013;5:194ra93. [DOI] [PubMed] [Google Scholar]
- 157.Vaysse P-M, van den Hout MFCM, Engelen SME, Keymeulen KBMI, Bemelmans MHA, Heeren RMA, et al. Lipid profiling of electrosurgical vapors for real-time assistance of soft tissue sarcoma resection. J Surg Oncol. 2024;129:499–508. [DOI] [PubMed] [Google Scholar]
- 158.Behrmann J, Etmann C, Boskamp T, Casadonte R, Kriegsmann J, Maaß P. Deep learning for tumor classification in imaging mass spectrometry. Bioinformatics. 2018;34:1215–23. [DOI] [PubMed] [Google Scholar]
- 159.Chiosea SI. Intraoperative margin assessment in early oral squamous cell carcinoma. Surg Pathol Clin. 2017;10:1–14. [DOI] [PubMed] [Google Scholar]
- 160.Ransohoff A, Wood D, Solomon Henry A, Divi V, Colevas A. Third party assessment of resection margin status in head and neck cancer. Oral Oncol. 2016;57:27–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.