Abstract
The natural antimicrobial compound carvacrol shows a high preference for hydrophobic phases. The partition coefficients of carvacrol in both octanol-water and liposome-buffer phases were determined (3.64 and 3.26, respectively). Addition of carvacrol to a liposomal suspension resulted in an expansion of the liposomal membrane. Maximum expansion was observed after the addition of 0.50 μmol of carvacrol/mg of l-α-phosphatidylethanolamine. Cymene, a biological precursor of carvacrol which lacks a hydroxyl group, was found to have a higher preference for liposomal membranes, thereby causing more expansion. The effect of cymene on the membrane potential was less pronounced than the effect of carvacrol. The pH gradient and ATP pools were not affected by cymene. Measurement of the antimicrobial activities of compounds similar to carvacrol (e.g., thymol, cymene, menthol, and carvacrol methyl ester) showed that the hydroxyl group of this compound and the presence of a system of delocalized electrons are important for the antimicrobial activity of carvacrol. Based on this study, we hypothesize that carvacrol destabilizes the cytoplasmic membrane and, in addition, acts as a proton exchanger, thereby reducing the pH gradient across the cytoplasmic membrane. The resulting collapse of the proton motive force and depletion of the ATP pool eventually lead to cell death.
There is increasing interest in the use of plant-derived antimicrobial compounds as natural preservatives for foods. An example of such a natural antimicrobial compound is carvacrol, which is present in the essential oil fractions of oreganum (60 to 70% carvacrol) and thyme (45% carvacrol) (1, 13). While inhibition of the growth of several pathogens by carvacrol has been reported in various articles (6, 10, 12, 23), none of these articles has addressed the mode of action of carvacrol. Using the spore-forming, food-borne pathogen Bacillus cereus as a model organism, it has been shown that exposure of vegetative cells to carvacrol concentrations up to 1 mM leads to an extension of the lag phase, a lower maximum specific growth rate, and a lower final population density (24). At concentrations above 1 mM, the viability of B. cereus is decreased exponentially. At the same time, increases in the membrane fluidity and leakage of protons and potassium ions are observed, leading to a decrease in the pH gradient across the cytoplasmic membrane (ΔpH), a collapse of the membrane potential (Δψ), and the inhibition of ATP synthesis. Finally, these events are followed by cell death (25, 26). Leakage of essential ions during exposure to antimicrobial compounds is often observed in microorganisms (5, 8, 21, 22, 28). However, for many compounds, the underlying mechanism of this leakage is not known. The present study with carvacrol gives insight into the mode of action of this compound. Due to its hydrophobic character and the observed effects on cells of B. cereus (24-26), accumulation of carvacrol in the membrane is expected. The partition behavior of carvacrol in both the octanol-water and membrane-buffer phases is described, together with the effects of carvacrol and cymene, its biological precursor, on different membrane characteristics. Compounds with structures similar to that of carvacrol were compared for their activities towards B. cereus to obtain more knowledge about the relationship between the structure of carvacrol and its antimicrobial activity. Such knowledge may facilitate the application of novel natural antimicrobial compounds and provide optimal use for the preservation of foods.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
B. cereus IFR-NL94-25 (obtained from the Institute of Food Research, Norwich, United Kingdom) was used in all experiments. Cells were grown at 30°C in brain heart infusion (BHI) medium (Oxoid) supplemented with 0.5% (wt/vol) glucose (initial pH 6.7). Cell cultures were maintained at −80°C in 15% glycerol as a cryoprotectant.
Determination of the Po/w of carvacrol.
The partition coefficient (P) of carvacrol in octanol-water (Po/w) was determined by the following procedure. In an Eppendorf tube, 750 μl of 1-octanol and 750 μl of water were mixed for 30 min. After centrifugation (30 min, 412,000 × g), carvacrol was added to the (water-saturated) octanol phase at subsaturating concentrations (0 to 10 mM). Samples were mixed for 1 h (5 min of mixing and 10 min of rest, four times), followed by centrifugation (30 min, 412,000 × g). Both phases were analyzed by high-pressure liquid chromatography using a Nova Pak C18 column (250 by 4 mm; Waters Corporation, Milford, Mass.). Samples were eluted with acetonitrile-water-2% tetraethylammonium hydroxide-2 M citric acid (100:100:1:1) as the mobile phase. Peaks were analyzed spectroscopically at 283 nm with a UV-visible-light detector (Waters Corporation).
The Po/w was determined as follows:
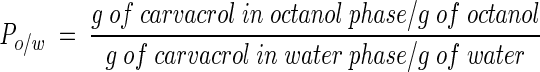 |
(1) |
Preparation of liposomes.
l-α-Phosphatidylethanolamine (PE) type III from egg yolk (approximately 98%, dissolved in chloroform) was obtained from Sigma (St. Louis, Mo.). The lipids were dried under vacuum with a vacuum evaporator (35°C), suspended in 50 mM HEPES-KOH buffer (pH 7.0), and dispersed by ultrasonic treatment in a bath sonicator. Single-membrane liposomes were obtained by a 240-s sonication that involved 15-s sonication and 45-s rest intervals. To determine the concentration of phospholipids, the amount of phosphate was analyzed as described by Rouser et al. (18).
Determination of the P of carvacrol in membrane-buffer (Pmembrane/buffer).
Single-membrane liposomes were prepared as described above (6.25 mg of PE/ml). Different amounts of carvacrol were added to a 0.5-ml suspension at subsaturating concentrations (0 to 10 mM). After incubation for 1 h at 20°C, the samples were centrifuged for 1 h at 412,000 × g. Carvacrol was extracted twice from the supernatants by addition of 0.5 ml of diethylether, mixing for 10 min, and centrifugation (10 min, 4,300 × g). The diethylether was evaporated, and both the residues and pelleted liposomes were dissolved in 50 μl of hexane containing 0.314 μg of nonanone/μl as an internal standard.
Samples were analyzed with a Carlo Erba (ThermoQuest, San Jose, Calif.) gas chromatograph equipped with a flame ionization detector and a CP-Sil 19 CB capillary column (25 m by 0.32 mm). The following temperature program was used: 1 min at 76°C, followed by an increase in temperature to 130°C at a rate of 30°C/min, which was held for 6 min, followed by heating to 220°C at a rate of 30°C/min. The carrier gas was nitrogen administered at a flow rate of 0.7 ml/min. The chromatograms were recorded with a Chromjet integrator (ThermoQuest).
The Pmembrane/buffer of carvacrol was determined as follows:
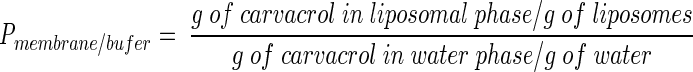 |
(2) |
Measurement of liposomal-membrane expansion.
The expansion of liposomal membranes in the presence of carvacrol or cymene was visualized by labeling liposomes with the fluorescent self-quenching fatty acid octadecyl rhodamine β chloride (R18; Molecular Probes, Leiden, The Netherlands). This method is based on the relief of fluorescence self-quenching of this probe as a result of dilution of the probe, which is caused by an expansion of the membrane (9, 15). R18 was incorporated in liposomal membranes (0.33 mg of PE/ml) during liposome preparation at an input concentration of 4 mol% phospholipid phosphorus. The fluorescence of a 2-ml liposome suspension was measured (excitation wavelength, 560 nm; emission wavelength, 590 nm; slit widths, 10 nm) in a Perkin-Elmer (Oosterhout, The Netherlands) model LS 50B spectrofluorometer in the absence or presence of carvacrol or cymene at 20°C. Maximum fluorescence was determined upon addition of 1% (vol/vol) Triton X-100. To discriminate between fluorescence increases due to expansion of the membrane and extraction of membrane constituents, incubation mixtures with different concentrations of the lipophilic compound were centrifuged (1 h, 412,000 × g) and fluorescence of the supernatant was determined relative to that of the supernatant of an incubation mixture without hydrophilic compound added. In addition, the phospholipid contents of the supernatants were measured by phosphate analysis as described by Rouser et al. (18).
Influence of carvacrol and cymene on membrane potential.
Exponentially growing cells were harvested at an optical density at 660 nm (OD660) of 3 to 4 and washed twice in a 50 mM HEPES-KOH buffer (pH 7.0) containing 1 mM MgSO4. Microscopic analysis indicated that spores were not present. The cell pellet was resuspended to an OD660 (light path, 1 cm) of approximately 10. The cell suspension (30 μl) was diluted in 2 ml of buffer containing 5 μM 3,3-dipropylthiacarbocyanine (DiSC3 [5]) (Molecular Probes). The membrane potential (Δψ) was monitored with a Perkin-Elmer model LS 50B spectrofluorometer at 20°C (excitation wavelength, 643 nm; emission wavelength, 666 nm). Following equilibration, 15 mM glucose was added to energize the cells. After a constant reading had been reached, 1 nM nigericin (Sigma) was added to rule out any effect on the pH gradient across the cytoplasmic membrane. After a steady fluorescence reading was reached, a selected concentration of carvacrol or cymene (0 to 0.5 mM) was added. Valinomycin (1 nM) was added as a control. Data were corrected for the effects of carvacrol and cymene on the fluorescence of the probe DiSC3 (5) in the absence of cells.
Intracellular pH measurements.
The determination of the intracellular pH was based on the method described by Breeuwer et al. (3a) with some adaptations. Exponentially growing cells were harvested, washed three times in a 50 mM HEPES-KOH buffer (pH 7.0), and diluted to an OD660 of approximately 1.0. Subsequently, cells were incubated in the presence of 1.5 μM carboxyfluorescein diacetate succinimidyl ester (cFDASE; Molecular Probes) for 10 min at 30°C. cFDASE was hydrolyzed to cFSE (carboxyfluorescein succinimidyl ester) in the cell and was subsequently conjugated to aliphatic amines. After the cells were washed with 50 mM potassium phosphate buffer (pH 7), they were incubated with 10 mM glucose for 30 min at 30°C to eliminate nonconjugated cFSE. In addition, they were washed twice, resuspended in 50 mM potassium phosphate, and kept on ice until further use. The analysis was started when 100 μl of the cell suspension was added to a quartz cuvette containing 3 ml of a 50 mM phosphate buffer (pH 5.81). The cuvette was placed in the cuvette holder of a spectrofluorometer (Perkin-Elmer model LS 50B), and the mixture was stirred continuously. Fluorescence intensities were measured at excitation wavelengths of 490 nm (pH sensitive) and 440 nm (pH insensitive) by rapidly alternating the monochromator between these two wavelengths. The emission was determined at 525 nm, and the excitation and emission slit widths were set at 5 and 10 nm, respectively. The intracellular pH was calculated from the ratio of the emissions at the 490- and 440-nm excitation wavelengths. A calibration curve was determined for buffers with pH values ranging from 3 to 10. Buffers contained 50 mM glycine, 50 mM citric acid, 50 mM Na2HPO4 · 2H2O, and 50 mM KCl. pH values were adjusted with NaOH or HCl. The intracellular (pHin) and extracellular (pHout) pHs were equilibrated by the addition of 1 nM valinomycin and 1 nM nigericin.
Influence of cymene on intracellular and extracellular ATP pools.
Cells of an overnight culture were washed three times in a 25 mM potassium phosphate buffer (pH 7), and a suspension with an OD660 of 1 (light path, 1 cm) was prepared. The experiment was started by the addition of glucose to a final concentration of 0.5% (wt/vol). Cymene was added 5 min after the addition of glucose. Samples of 200 μl were taken every 2 min, added to Eppendorf tubes containing 200 μl of a silicon oil mixture (AR200 and AR20, 2:1) (Wacker Chemicals, Munich, Germany) on top of 100 μl of trichloroacetic acid-EDTA buffer (10% trichloroacetic acid, 2 mM EDTA), and centrifuged directly (5 min, 12,000 × g). The extracellular (upper-layer) and the intracellular (lower-layer) ATP concentrations were measured with a BO1243-107 ATP assay kit (Labsystems Oy, Helsinki, Finland). Luminescence was recorded with a model 1250 luminometer (Bio-Orbit, Turku, Finland). The determination of the amount of protein in the cells of B. cereus was carried out as described by Lowry et al. (14), with bovine serum albumin as a standard.
Determination of antibacterial activity.
To determine the effects of carvacrol, thymol, menthol, carvacrol methyl ester, and cymene on the growth of B. cereus, cells were cultivated at 30°C, washed twice in BHI, and incubated at 30°C in microtiter plates containing BHI supplemented with different concentrations (0 to 10 mM) of the above-mentioned compounds. The initial OD660 of the cell suspensions was set at 0.02. The OD660 was measured at different time intervals until a constant reading was observed.
Chemicals.
Purified carvacrol, thymol, cymene, and carvacrol methyl ester were obtained from Fluka Chemie AG (Buchs, Switzerland), and menthol was obtained from Carl Roth GmbH & Co. (Karlsruhe, Germany). Stock solutions (1 M) were made in 95% ethanol. The final ethanol concentration in the experiments was always kept below 2% (vol/vol). This concentration of ethanol did not affect the growth of B. cereus. Stock solutions of R18 and DiSC3 (5) (Molecular Probes) were prepared in ethanol and stored at −20°C. An aliquot of this stock solution was added to phospholipid solutions in chloroform to give the desired lipid/R18 ratio. cFDASE was obtained from Molecular Probes. A stock solution was prepared in acetone.
RESULTS
P's of carvacrol in octanol-water and membrane-buffer systems.
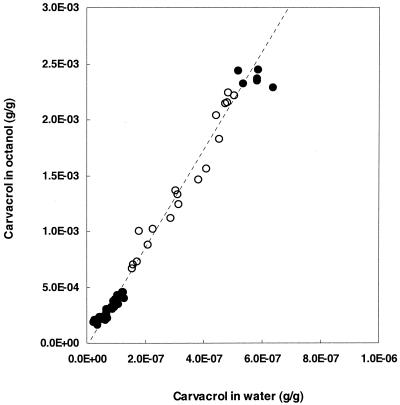
To obtain more knowledge about the lipophilic character of carvacrol, its partition behavior in a mixture of octanol and water was determined (Fig. 1). The Po/w was calculated from the linear part of the curve. Carvacrol had a stronger preference for the more hydrophobic octanol phase than for the water phase, and this resulted in a log Po/w of 3.64.
FIG. 1.
Relationship between the concentrations of carvacrol in the water and octanol phases. Carvacrol was added to the (water-saturated) octanol phase, and both phases were mixed (see Materials and Methods). From the linear part of the curve, indicated by the open symbols, a log P of 3.64 was calculated.
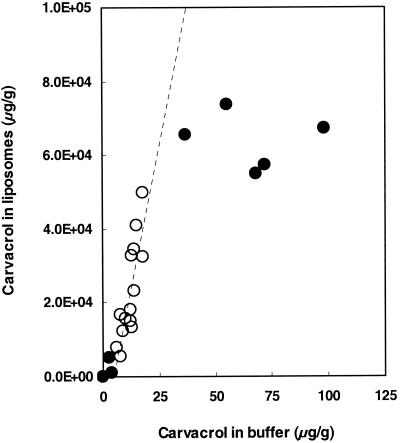
The P of carvacrol was also determined in a (liposomal) membrane-buffer system to gain insight into the preference of carvacrol for the membranes of bacterial cells. Because membranes of B. cereus are rich in PE, this lipid was used to prepare liposomes. Carvacrol showed a marked preference for the hydrophobic phase (Fig. 2), and this resulted in a log Pmembrane/buffer of 3.26. The maximum carvacrol concentration in the liposomal membranes was 6 × 104 μg/g.
FIG. 2.
Relationship between the concentrations of carvacrol in the buffer phase and in the liposomes (6.25 mg of phospholipid/ml). From the linear part of the curve, indicated by the open symbols, a log P of 3.26 was calculated.
Expansion of liposomal membranes.
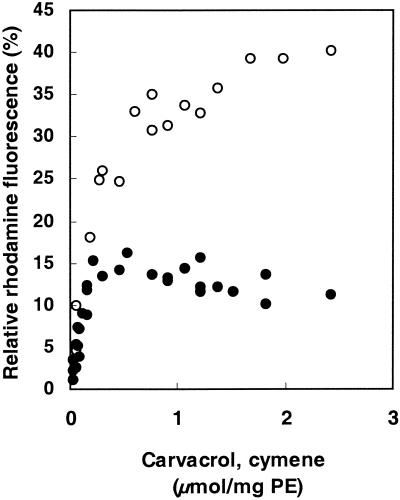
When carvacrol accumulates in the cytoplasmic membrane, changes in the membrane integrity occur, such as expansion (swelling) of the membrane. Therefore, the expansion of the liposomal membrane in the presence of carvacrol was studied (Fig. 3). Maximum fluorescence was determined in the presence of 1% Triton X-100. All data were related to the maximum expansion. Addition of carvacrol to liposomes led to expansion of the membrane, with a maximum expansion occurring at concentrations above 0.5 μmol/mg of PE. Higher carvacrol concentrations did not lead to a further increase in the expansion. Cymene also expanded the membrane; however, the degree of expansion with cymene was found to be 2.7 times higher than that with carvacrol. The maximum expansion produced by cymene was observed at approximately 2 μmol/mg of PE, a concentration four times higher than that for carvacrol.
FIG. 3.
Effects of carvacrol (filled circles) and cymene (open circles) on the relief of fluorescence self-quenching of R18-labeled liposomes. Maximum fluorescence (100%) was obtained by the addition of 10% Triton X-100.
Both carvacrol and cymene did not cause extraction of the fluorescent probe from the membrane since no increase in the fluorescence of the supernatant was observed in the presence of carvacrol or cymene and, in addition, no phosphate could be detected in the supernatant (data not shown).
In conclusion, cymene and carvacrol lead to an increase in the fluorescence of the R18-labeled liposomes as a result of swelling of the membrane and not as a result of extraction of the probe from the membrane.
Membrane potential.
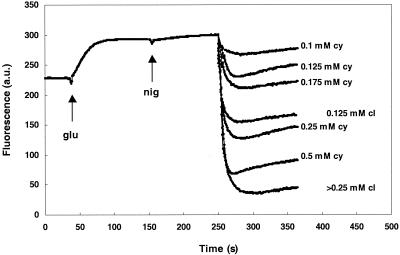
Insertion of carvacrol in the membranes of energized cells leads to a decrease in the membrane potential (26). To obtain more knowledge about the exact mode of action of carvacrol and the relationship between its structure and its activity, cymene, which lacks the hydroxyl group, was also tested for its effect on the membrane potential. Like carvacrol, cymene decreased the membrane potential (Fig. 4); however, higher concentrations were necessary to obtain the same reduction as that obtained with carvacrol. Therefore, the hydroxyl group seems to play an important role in the effect of carvacrol on the membrane potential.
FIG. 4.
Effect of carvacrol on the membrane potential of B. cereus in the presence of glucose (glu), nigericin (nig), and different concentrations of carvacrol (cl) or cymene (cy). Carvacrol and cymene were added at 250 s. The membrane potential was monitored with the fluorescent probe DiSC3 and measured in arbitrary units (a.u.).
Influence of cymene on intracellular pH.
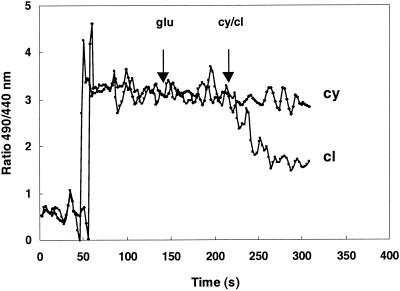
Because cymene expands the cytoplasmic membrane and causes a decrease in the membrane potential, its influence on the pH gradient across the membrane was studied in more detail. While 1.0 mM carvacrol completely eliminated the pH gradient (26), cymene had no effect on the ΔpH (Fig. 5) at the concentrations tested (0.5 to 2 mM).
FIG. 5.
Effect of cymene (2.0 mM) and carvacrol (1 mM) on the pH gradient across the cytoplasmic membrane. The pH was measured with the fluorescent probe cFDASE (see Materials and Methods) at an external pH of 5.81. The ratio was calculated from the fluorescence at 490 nm (pH dependent) and 440 nm (pH independent). glu, glucose; cy, cymene; cl, carvacrol.
Effect of cymene on ATP pools.
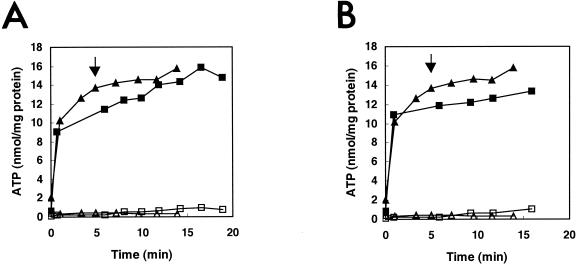
Destabilization of the cytoplasmic membrane and/or leakage of ions is expected to have an impact on the membrane-associated energy-transducing system. Exposure of B. cereus to carvacrol results in a decrease of the intracellular ATP pool, caused by a decrease in the membrane potential and/or pH gradient (26). Because cymene also had an effect on the membrane potential, the influence of cymene on the intra- and extracellular ATP pools was studied. Additions of both 1 and 2.4 mM cymene did not have an effect on the intracellular or extracellular amount of ATP (Fig. 6), indicating no leakage of ATP in the presence of cymene.
FIG. 6.
Intracellular (filled symbols) and extracellular (open symbols) ATP pools of glucose-energized vegetative cells of B. cereus in the absence (▴, ▵) or in the presence (▪, □) of 1 mM (A) or 2.4 mM (B) cymene. Cymene was added at 5 min (indicated by arrow).
Relationship between structure and inhibition of growth.
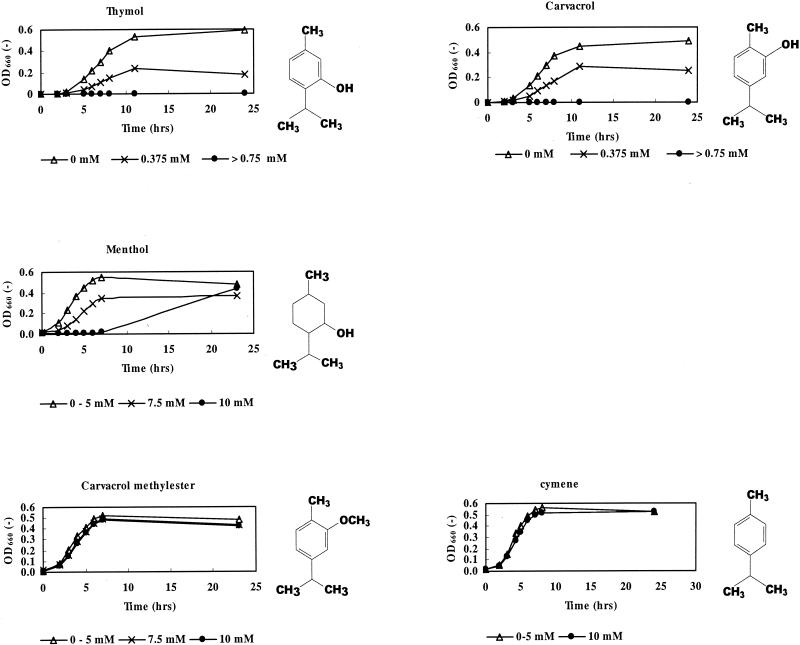
Carvacrol accumulates in the cytoplasmic membrane, thereby causing an expansion of the membrane. The hydroxyl group of carvacrol was shown to be important for its effect on membrane properties and probably for its antimicrobial activity. To test the importance of the hydroxyl group in relation to its antimicrobial activity, different compounds, all with structures comparable to that of carvacrol, were tested for their activity towards B. cereus (Fig. 7). The activity of thymol (with its hydroxyl group located at the meta position) is comparable to that of carvacrol, and no growth was observed at concentrations of 0.75 mM and above. Menthol, which lacks a system of delocalized electrons, was much less effective than carvacrol: growth was not completely suppressed at concentrations that were approximately 10 times higher than the carvacrol concentrations. Carvacrol methyl ester, containing a methyl ester group instead of a hydroxyl group, and cymene, which lacks a hydroxyl group, did not inhibit the growth of B. cereus at the concentrations tested. From these results, it can be concluded that the presence of the hydroxyl group and a system of delocalized electrons play an important role in the antimicrobial activity of carvacrol. The position of the hydroxyl group in the benzene ring does not affect the activity.
FIG. 7.
Growth of B. cereus in BHI in the presence of different concentrations of thymol, carvacrol, menthol, carvacrol methyl ester, and cymene (30°C). All data are means of triplicate measurements.
DISCUSSION
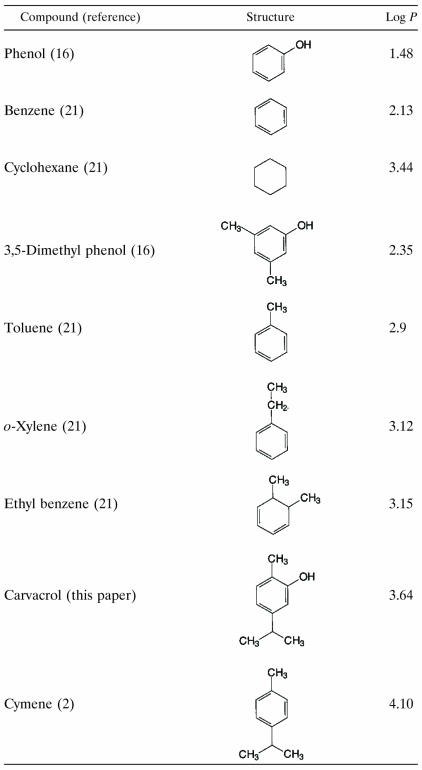
The present study shows that the antimicrobial compound carvacrol accumulates in hydrophobic phases. Comparison of the Po/w of carvacrol with those of other compounds (Table 1) shows that the determined value agrees with values found for similar compounds. The addition of alkyl groups to a molecule increases the P, while the addition of a hydroxyl group or a system of delocalized electrons to a molecule decreases the P. The Pmembrane/buffer of carvacrol is lower than its Po/w (3.26 versus 3.64). This result may be explained by the fact that PE liposomes are more hydrophilic than octanol due to the presence of hydrophilic head groups attached to the fatty acids. As was described by De Young and Dill (7), there is a difference between the partitioning of a compound into membranes, which are interfacial phases, and an octanol phase, which is uniform throughout. Sikkema et al. (21) and Machleidt et al. (16) observed Pmembrane/buffers for cyclic hydrocarbon compounds that were lower than their Po/ws.
TABLE 1.
Comparison of the observed P (expressed as log P) of carvacrol with those of different compounds in relation to their structures
Weber et al. (30) reported that compounds with a log Po/w value higher than 3 partition deeply in the membrane. As a result of this partitioning of carvacrol, interactions of components (lipids and proteins) in the membrane are affected. Accumulated carvacrol occupies more than the normal amount of space between fatty acid chains, resulting in conformational changes of the phospholipid bilayer. A disturbance of the van der Waals interactions between the lipid acyl chains in the membrane causes fluidization of membrane lipids. This fluidization by carvacrol was described earlier (25). In addition, an expansion of membranes was observed. The expansion did not increase further when we added carvacrol at concentrations above 0.50 μmol/mg of PE, which corresponded with 3 × 104 μg of carvacrol/g of PE (calculated on the basis of the P of carvacrol in the liposomes). The maximum concentration of carvacrol in the liposomal membrane (6 × 104 μg/g of PE) was higher, which indicates that the maximum expansion is not solely determined by the maximum possible concentration of carvacrol in the membrane.
Due to the higher P of cymene, more cymene is expected to accumulate in the membranes, which may explain the higher expansion of the membrane in the presence of cymene. This possibility agrees with the observations of Sikkema et al. (21), who found that the expansion of the membrane increased when the log Po/w of a compound increased. The marked preference of cymene (compared to that of carvacrol) for the hydrophobic phase also explains the observation that a maximum expansion is reached at a higher concentration of cymene than of carvacrol (2 and 0.5 μmol/mg of PE, respectively).
An expansion of the membrane may result in a destabilization of the membrane and, consequently, the leakage of ions. Both carvacrol and cymene cause a decrease in the membrane potential, suggesting leakage of ions. However, the addition of 0.125 mM cymene resulted in much higher concentrations of cymene in the membrane than the addition of 0.125 mM carvacrol as a result of the higher P of cymene. In the presence of cymene, the membrane potential is probably decreased mainly by leakage of ions other than H+, e.g., K+, because only a very small effect on the ΔpH was observed. This absence of an effect on the ΔpH may explain why ATP pools were not affected by cymene. Cells can probably cope with the effect on membrane potential, as was also observed by the absence of an effect of cymene on the growth of B. cereus. As described by Ultee et al. (26), decreasing the membrane potential alone did not decrease the viability of B. cereus (27), even though leakage of ions was observed. Vermuë et al. (29) showed that a higher P did not always result in the greater toxicity of a compound. Compounds with a log P value above a certain maximum (approximately 4, dependent on the kind of compound and the organism tested) are not toxic to organisms. These results clearly show that both carvacrol and cymene have an effect on membrane integrity. However, destabilization of the membrane does not explain the higher antimicrobial activity of carvacrol than that of cymene. This indicates that another factor must be involved.
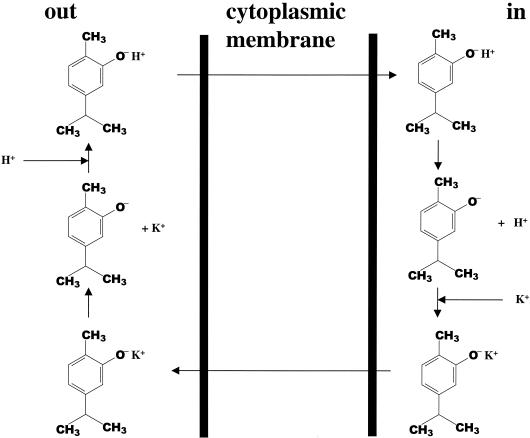
This factor is the presence of the hydroxyl group at the carvacrol molecule. The above-described observations (summarized in Table 2) support the following model for the antimicrobial action of carvacrol, which is shown schematically in Fig. 8. A characteristic feature of a phenolic hydroxyl group is its significantly greater acidity than that of an aliphatic hydroxyl group (4). We propose that carvacrol acts as a transmembrane carrier of monovalent cations by exchanging its hydroxyl proton for another ion such as a potassium ion. Undissociated carvacrol diffuses through the cytoplasmic membrane towards the cytoplasm and dissociates by releasing its proton to the cytoplasm. It may then return undissociated by carrying a potassium ion (or other cation) from the cytoplasm which is transported through the cytoplasmic membrane to the external environment. A proton is taken up, and carvacrol in the protonated form diffuses again through the cytoplasmic membrane and dissociates by releasing a proton to the cytoplasm. This hypothesis is supported by the observed efflux of K+ and influx of H+ in B. cereus during exposure to carvacrol (26). Given a pKa of phenolic compounds of 10 (16), 0.1% carvacrol will be dissociated at the hydroxyl group under the experimental conditions (pH 7). Cymene does not have this property of carvacrol because of the lack of a hydroxyl group. The large accumulation of cymene in the membrane probably causes sufficient expansion of the membrane and, consequently, spacing of phospholipids in the destabilized membrane through which ions can leak. However, the presence of the hydroxyl group seems to be more important for the antimicrobial activities of these compounds than the ability to expand and consequently to destabilize the membrane.
TABLE 2.
Summary of effects of carvacrol and cymene on different parameters in this studya
| Antimicrobial agent | Log Po/w | Log Pmembrane/buffer | Membrane expansion | Effect on:
|
|||
|---|---|---|---|---|---|---|---|
| Membrane potential | ΔpH | ATP | Growth | ||||
| Carvacrol | 3.64 | 3.26 | + | ++ | ++ | ++ | ++ |
| Cymene | 4.10 | ND | ++ | + | − | − | − |
ND, not determined; ++, strong effect, +, effect; −, no effect.
FIG. 8.
Schematic overview of the hypothesized activity of carvacrol. Undissociated carvacrol diffuses through the cytoplasmic membrane towards the cytoplasm and dissociates, thereby releasing its proton to the cytoplasm. It then returns undissociated by carrying a potassium ion (or other cation) from the cytoplasm, which is transported through the cytoplasmic membrane to the external environment. A proton is taken up, and carvacrol in the protonated form diffuses again through the cytoplasmic membrane and dissociates by releasing a proton to the cytoplasm.
The above-described hypothesis is supported by the results with menthol, carvacrol methyl ester, and thymol. Although menthol has a hydroxyl group, it does not possess high antimicrobial activity. This lack is caused by the absence of a system of delocalized electrons (double bonds) and, consequently, the inability of the hydroxyl group to release its proton. Although the log P value of menthol is not known, it is expected to be higher than the log P of carvacrol because introduction of a system of delocalized electrons (as in carvacrol) lowers the P. This finding indicates that the reduced antimicrobial activity of menthol is not caused by a reduced partition of menthol in the membrane compared to that of carvacrol. Like menthol, carvacrol methyl ester does not have the ability to release a proton and is therefore not antimicrobial. Similar to carvacrol, thymol contains both a hydroxyl group and a system of delocalized electrons and was found to possess strong antimicrobial activity. It was described earlier that the hydroxyl group (bound to a benzene ring) is important for the activities of some antimicrobial compounds and that these activities are enhanced by the presence of α-β double bonds (3, 11, 17, 20). Salih et al. (19) showed that hydroxycinnamic acids showed antimicrobial activities even though their esters were not active.
The synergistic activity between carvacrol and cymene has been described previously (27). Based on the knowledge obtained in the present study, we conclude that cymene probably acts synergistically with carvacrol by expanding the membrane, which results in the destabilization of the membrane. This supports the possible role of carvacrol as an exchanger of cations.
In conclusion, although both carvacrol and cymene cause destabilization of the membrane and a decrease in the membrane potential, the antimicrobial activity of carvacrol is most probably caused by an additional decrease in the ΔpH as a result of the presence of a hydroxyl group and a system of delocalized electrons. If we suppose that metabolism is aerobic, these events result in the absence of a proton motive force and, consequently, no more ATP can be synthesized. Depletion of ATP pools leads to impairment of essential processes in the cell and finally to cell death.
Acknowledgments
This work was financially supported by the Wageningen Centre for Food Sciences (WCFS).
We thank M. G. Sanders for his help with the high-pressure liquid chromatography measurements, J. Slotboom and D. Somhorst for their help with the gas chromatography measurements, and T. Abee for critically reading the manuscript.
REFERENCES
- 1.Arrebola, M. L., M. C. Navarro, J. Jiménez, and F. A. Ocaña. 1994. Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry 36:67-72. [Google Scholar]
- 2.Banerjee, S., S. H. Yalkowsky, and S. C. Valvani. 1980. Water solubility and octanol/water partition coefficients of organics. Limitations of the solubility-partition coefficient correlation. Environ. Sci. Technol. 14:1227-1229. [Google Scholar]
- 3.Bose, S. M., C. N. BhimaRao, and V. Subramanyan. 1949. Influence of organic matter on bactericidal efficiency of Indian essential oils. J. Sci. Ind. Res. 8:157. [Google Scholar]
- 3a.Breeuwer, P., J. L. Drocourt, F. M. Rombouts, and T. Abee. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5- (and 6-) carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, W. H. 1975. Introduction to organic chemistry, 3rd ed. Willard Grant Press, Boston, Mass.
- 5.Cartwright, C. P., J. R. Juroszek, M. J. Beavan, M. S. Ruby, S. M. F. de Morais, and A. H. Rose. 1986. Ethanol dissipates the proton-motive force across the plasma membrane of Saccharomyces cerevisiae. J. Gen. Microbiol. 132:369-377. [Google Scholar]
- 6.Conner, D. E. 1993. Naturally occurring compounds, p. 441-468. In P. M. Davidson and A. L. Branen (ed.), Antimicrobials in foods, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 7.De Young, L. R., and K. A. Dill. 1988. Solute partitioning into lipid bilayer membranes. Biochemistry 27:5281-5289. [DOI] [PubMed] [Google Scholar]
- 8.Heipieper, H. J., G. Meulenbeld, Q. Oirschot, and J. A. M. de Bont. 1996. Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl. Environ. Microbiol. 62:6665-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoekstra, D., T. de Boer, K. Klappe, and J. Wilschut. 1984. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 23:5675-5681. [DOI] [PubMed] [Google Scholar]
- 10.Juven, B. J., J. Kanner, F. Schved, and H. Weisslowicz. 1994. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 76:626-631. [DOI] [PubMed] [Google Scholar]
- 11.Katayama, T., and I. Nagai. 1960. Chemical significance of the volatile components of spices from the food preservative view point. IV. Structure and antibacterial activity of some terpenes. Nippon Suisan Gakkaishi 26:29. [Google Scholar]
- 12.Kivanç, M., and A. Akgul. 1988. Effect of some essential oil components on the growth of food-borne bacteria and synergism with some food ingredients. Flavour Fragr. J. 3:95-98. [Google Scholar]
- 13.Lagouri, V., G. Blekas, M. Tsimidou, S. Kokkini, and D. Boskou. 1993. Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z. Lebensm.-Unters.-Forsch. A 197:20-23. [Google Scholar]
- 14.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 15.MacDonald, R. I. 1990. Characteristics of self-quenching of the fluorescence of lipid-conjugated rhodamine in membranes. J. Biol. Chem. 265:13533-13539. [PubMed] [Google Scholar]
- 16.Machleidt, H., S. Roth, and P. Seeman. 1972. The hydrophobic expansion of erythrocyte membranes by the phenol anesthetics. Biochim. Biophys. Acta 255:178-189. [DOI] [PubMed] [Google Scholar]
- 17.Pruthi, J. S. 1980. Properties and uses, p. 16. In J. S. Pruthi (ed.), Spices and condiments: chemistry, microbiology, technology. Academic Press, New York, N.Y. [PubMed]
- 18.Rouser, G., S. Fleischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 19.Salih, A. G. Q., L. M. le Quéré, and J. F. Drilleau. 2000. Action des acides hydroxycinnamiques libres et esteréfiés sur la croissance des bactéries lactiques. Sci. Aliments 20:537-560. [Google Scholar]
- 20.Shelef, L. A. 1983. Antimicrobial effects of spices. J. Food Saf. 6:29-44. [Google Scholar]
- 21.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 11:8022-8028. [PubMed] [Google Scholar]
- 22.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivropoulou, A., E. Papanikolaou, C. Nikolaou, S. Kokkini, T. Lanaras, and M. Arsenakis. 1996. Antimicrobial and cytotoxic activities of oreganum essential oils. J. Agric. Food Chem. 44:1202-1205. [Google Scholar]
- 24.Ultee, A., L. M. G. Gorris, and E. J. Smid. 1998. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 85:211-218. [DOI] [PubMed] [Google Scholar]
- 25.Ultee, A., E. P. W. Kets, M. Alberda, F. A. Hoekstra, and E. J. Smid. 2000. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 174:233-238. [DOI] [PubMed] [Google Scholar]
- 26.Ultee, A., E. P. W. Kets, and E. J. Smid. 1999. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 65:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ultee, A., R. A. Slump, G. Steging, and E. J. Smid. 2000. Antimicrobial activity of carvacrol on rice. J. Food Prot. 63:620-624. [DOI] [PubMed] [Google Scholar]
- 28.Uribe, S., J. Ramirez, and A. Peña. 1985. Effects of β-pinene on yeast membrane functions. J. Bacteriol. 161:1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermuë, M., J. Sikkema, A. Verheul, and J. Tramper. 1993. Toxicity of homologous series of organic solvents for the gram-positive bacteria Arthrobacter and Nocardia sp. and the gram-negative bacteria Acinetobacter and Pseudomonas sp. Biotechnol. Bioeng. 42:747-758. [DOI] [PubMed] [Google Scholar]
- 30.Weber, F. J., and J. A. M. de Bont. 1996. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1286:225-245. [DOI] [PubMed] [Google Scholar]