Abstract
Objective
Cirrhotic patients with bacterial infections (BI) face high risks of acute-on-chronic liver failure (ACLF) and mortality. This study assessed the diagnostic value of serum complement component 3 (C3) for predicting 90-day ACLF and mortality in this population.
Methods
We prospectively analyzed clinical data from 105 cirrhotic patients with BI (mean age 57.2 ± 11.6 years; 57 male) admitted to the Second Hospital of Nanjing between September 2023 and March 2024. Primary outcomes were ACLF development and mortality within 90 days.
Results
Thirty-one patients (29.5%) developed ACLF within 90 days. Lower C3 levels independently predicted both ACLF [hazard ratio (HR): 0.14, 95% confidence interval (CI): 0.02–0.93; P = 0.04) and mortality (HR: 0.10, 95% CI: 0.00–0.89; P = 0.01). Time-dependent receiver operating characteristic analysis showed C3 predicted ACLF with AUROCs of 0.76 (30 day), 0.73 (60 day), and 0.72 (90 day). For mortality, areas under the time-dependent receiver operating characteristic curves (AUROCs) were 0.76 (30 day), 0.69 (60 day), and 0.68 (90 day). A cutoff of 0.66 g/L was established using etiology-adjusted restricted cubic spline. C3 correction improved the predictive AUROCs of Child–Turcotte–Pugh, Model of End-Stage Liver Disease, and the Chronic Liver Failure Consortium Acute Decompensation scores for mortality (all P > 0.05). Random forest regression identified C3 among the top 10 risk factors for ACLF development.
Conclusion
Serum C3 demonstrates significant prognostic value as a predictor for 90-day ACLF and mortality in cirrhotic patients with bacterial infections, offering potential clinical utility in risk stratification.
Keywords: acute-on-chronic liver failure, bacterial infections, cirrhosis, complement C3, survival
Introduction
Acute-on-chronic liver failure (ACLF) is a unique syndrome observed in patients with chronic liver disease [1] and is characterized by systemic inflammatory responses, single or multiple organ system failure, and high 28-day mortality [1,2]. Bacterial infection (BI) is the most common triggering event of ACLF [3]. The 28-day mortality in infected patients without ACLF was 5%, but the mortality in ACLF was 37% [4–6]. Therefore, it is particularly important to identify cirrhotic patients complicated with BI at a high risk of developing ACLF.
The PREDICT study pointed out that the C-reactive protein (CRP) and white blood cells (WBC) were higher in pre-ACLF patients than in unstable or stable decompensated cirrhotic (DC) patients [7]. The neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio showed satisfactory power in predicting ACLF and mortality [8]. It was further observed that elevated levels of interleukin-6 were independently associated with the development of ACLF within 28 days [9,10]. The area under the receiver operating characteristic (AUROC) of CRP and WBC, however, was still less than 0.6 [6]. The existing studies have failed to identify accurate clinical biomarkers that can predict the onset of ACLF.
The diagnostic performances of composite index scores were more satisfactory [11]. The Model of End-Stage Liver Disease (MELD) score was defined as one of the predictors of developing ACLF [12]. The European Association for the Study of Liver-Chronic-Liver Failure Group modified the intensive care Sequential Organ Failure Assessment score into CLIF-SOFA to recognize the presence of ACLF in short term [5]. The Chronic Liver Failure Consortium Acute Decompensation (CLIF-C AD) score was established to predict 3‐month mortality in patients with acute decompensation [13].
Complement component 3 (C3) activation, which induces membrane-attack complex formation, subsequently activates various immune cells and enhances proinflammatory cytokine secretion, thus playing an important role in host defense and wound repair [14]. Activation of the complement system plays a key role in the progression of liver injury [15], and it has been pointed out that complement C3 could be used as a prognostic marker for mortality in patients with ACLF [16], and low C3 levels indicated the development of ACLF in patients with DC [17].
On the basis of previous studies, we aimed to assess the diagnostic performance of complement C3 to predict ACLF and mortality in patients with DC complicated with BI within 90 days.
Methods
Patient selection
A prospective observational cohort study of cirrhotic inpatients was conducted at the Second Hospital of Nanjing from September 2023 to March 2024. The inclusion criteria of the study were as follows: (1) patients aged ≥18 years and ≤80 years; (2) cirrhosis diagnosed based on histological, biochemical, ultrasonic, or radiological findings; (3) patient with previous decompensation event was admitted unelectively due to BI, BI was diagnosed following the guideline [18]; (4) informed consent was obtained. The exclusion criteria of the study were as follows: (1) patient was admitted for elective therapy or acute decompensation without BI; (2) HIV infection; (3) treatment with corticosteroids, immunosuppressants, or cytotoxic drugs; (4) hepatocellular carcinoma beyond Milan criteria; (5) concomitant nonliver-related malignant tumors; (6) previous liver lobectomy or liver transplantation; (7) severe heart failure (New York Heart Association class 4), chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease grade 3–4), or kidney dysfunction (chronic kidney disease level 5) diagnosed before cirrhosis; (8) pregnancy or lactation; and (9) missing follow-up data.
Study design
Patients were evaluated on the inclusion and exclusion criteria upon admission. Patients meeting the criteria were introduced to the program, and those who agreed to be included in the study signed informed consent. Patients underwent routine physical examinations and routine laboratory tests on the day of admission or the day after admission. Patients underwent routine imaging exams (ultrasound and computed tomography). Demographic, clinical, laboratory, imaging, and treatment data were collected (antibiotic use was recorded in detail). Baseline indicators were recorded on the day of admission or the day after admission.
The detection time of blood samples was calculated as day 0. The follow-up was performed at 1 week, 1 month, and 3 months. Phone and WeChat follow-up was performed at any time. During the follow-up, any condition requiring active management was treated actively.
The endpoints of the study were ACLF and mortality. ACLF was diagnosed following the European Association for the Study of the Liver guideline [1].
Two researchers regularly reviewed the data to detect errors. Two researchers assessed the accuracy of the data. After verification of the collected clinical variables, statistical analysis was performed.
Ethics
The research followed both the Declaration of Helsinki and the Declaration of Istanbul and was approved by the Second Hospital of Nanjing Hospital Ethics Committee. All patients included gave their written consent.
Statistical analysis
Clinical indexes with missing values of less than 10% were imputed with the use of multiple imputations. The data are described as frequencies and percentages, means and standard deviations, or medians and interquartile ranges, as appropriate. Baseline characteristics were compared using Fisher’s exact test for categorical variables, Student’s t test for continuous variables, and the Wilcoxon rank sum test for ordinal and continuous variables. Spearman correlation analyses were performed to assess the relationships between C3 and clinical indexes. The univariable and multivariable Cox proportional hazards regression models were performed to recognize the risk factors with the rates of ACLF and mortality, and forward regression (Wald) analysis was used to select the variables included in the multivariable model. The time-dependent receiver operating characteristic (ROC) curves for ACLF and mortality were plotted, and the areas under the ROC curves (AUROC) were calculated. The optimal cutoff value for the complement C3 was calculated by plotting a 5-knot restricted cubic spline (RCS). The patients were grouped according to the cutoff value of complement C3 and the Kaplan–Meier curves between the high and low-risk groups are shown. The models used to predict ACLF were built by random forest regression. The predictive performance of each model was evaluated. Visualization and index calculation showed the model’s performance on the training set and the internal validation set. Statistical analyses were performed using GraphPad software (version 9.0; GraphPad Software, Inc., San Diego, California, USA), the Statistical Package for Social Sciences (version 22.0; IBM Corporation, Chicago, Illinois, USA), RStudio (version 4.2.1; RStudio, PBC, Boston, Massachusetts, USA), and Python (version 3.10; Python Software Foundation, Wilmington, Delaware, USA). The level of significance was the two-sided 5% level.
Results
Cohort baseline characteristics
According to the inclusion and exclusion criteria, 105 cirrhotic patients were enrolled in the study (mean age: 57.16 ± 11.59 years, 57 males) (Supplementary Figure 1, Supplemental digital content 1, http://links.lww.com/EJGH/B160). Forty-eight patients were transferred to our hospital after active therapy from other hospitals. The initial acute decompensation event was a BI in 34 patients (32.38%), and the remaining patients had a further BI complicated by another acute decompensation event. The most common BI site was the respiratory system (55/105, 52.38%). Notably, 34 patients had sufficient etiological evidence (12 had multiple BIs) and 27 of them were treated according to drug susceptibility test results. Seven patients were infected with drug-resistant bacteria (all seven patients developed ACLF within 90 days and four patients died), and antibiotic use was adjusted according to experience. Seventy-one patients were lack of etiological evidence (clinical index diagnosis), and appropriate antibiotics were selected empirically according to clinical conditions, infection site, and region common pathogens.
Twenty-six (24.76%) patients developed ACLF within 30 days and 31 (29.52%) in 90 days. The top three types of infections that progressed to ACLF were bacterial pneumonia, spontaneous peritonitis, and biliary tract infection. Thirteen patients died during the follow-up period, including 12 patients who died of ACLF and one patient who died of severe infection.
The patient baseline characteristics are shown in Table 1. There were significant differences in the complement C3 among different disease etiologies (P = 0.005) and was significantly higher in autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), or AIH-PBC overlap syndrome [AIH: 0.90 ± 0.43; PBC: 0.94 ± 0.43; AIH-PBC: 1.18 ± 0.31; alcoholic: 0.57 ± 0.30; hepatitis C viral (HCV) liver disease: 0.58 ± 0.15; hepatitis B viral (HBV) liver disease: 0.77 ± 0.33]. There was no correlation between other clinical indicators and complement C3 (Supplementary Figure 1, Supplemental digital content 1, http://links.lww.com/EJGH/B160). No correlation was found between C3 and the rest of the collected clinical indicators statistically (all P > 0.05, Supplementary Figure 2, Supplemental digital content 1, http://links.lww.com/EJGH/B160).
Table 1.
Baseline characteristics of the full cohort
| Patients developing ACLF in 90 days (n = 31) | Patients not developing ACLF in 90 days (n = 74) | P value | |
|---|---|---|---|
| Basic characteristics | |||
| Age (years) | 60.84 ± 12.67 | 55.62 ± 11.44 | 0.04 |
| Sex (males/females) | 21/10 | 36/38 | 0.09 |
| Etiology (AIH/PBC-AIH overlapping syndrome/PBC/alcoholic/HCV/HBV) | 3/0/4/3/2/19 | 8/6/7/8/7/38 | 0.63 |
| Complications | |||
| Ascites (yes/no) | 29/2 | 48/26 | 0.002 |
| EGV (yes/no) | 14/17 | 30/44 | 0.67 |
| HE (yes/no) | 16/15 | 9/65 | <0.001 |
| Splenectomy (yes/no) | 5/26 | 12/62 | 1.00 |
| TIPS (yes/no) | 1/30 | 2/72 | 1.00 |
| Diabetes (yes/no) | 7/24 | 13/61 | 0.59 |
| Cardiovascular disease (yes/no) | 6/25 | 21/53 | 0.46 |
| Chronic kidney diseases (yes/no) | 3/28 | 3/71 | 0.36 |
| Chronic lung disease (yes/no) | 0/31 | 1/73 | 1.00 |
| Etiological treatment | |||
| Receiving the etiological treatment (yes/no) | 18/13 | 52/22 | 0.26 |
| Etiological control or biochemical responsea (yes/no) | 6/25 | 26/48 | 0.16 |
| Clinical characteristics | |||
| Higher than ULN of CRP (yes/no) | 12/19 | 9/65 | 0.003 |
| White blood cells (×109/L) | 5.16 ± 2.36 | 4.62 ± 2.25 | 0.27 |
| Neutrophils percentage (%) | 66.69 ± 12.95 | 57.60 ± 14.60 | <0.001 |
| Hemoglobin (g/L) | 98.74 ± 25.50 | 117.38 ± 25.47 | <0.001 |
| Platelet (×1012/L) | 77.48 ± 47.04 | 106.36 ± 75.43 | 0.02 |
| Prothrombin time (s) | 20.70 ± 6.89 | 13.34 ± 2.80 | <0.001 |
| International normalized ratio | 1.97 ± 0.72 | 1.26 ± 0.23 | <0.001 |
| Total bilirubin (μmol/L) | 193.00 (56.20–314.90) | 26.90 (13.53–48.53) | <0.001 |
| Albumin (g/L) | 29.50 (26.30–34.00) | 35.75 (31.48–39.38) | <0.001 |
| Globulin (g/L) | 31.26 ± 9.55 | 29.86 ± 6.79 | 0.46 |
| Alanine transaminase (U/L) | 35.20 (22.10–174.30) | 35.05 (21.05–52.80) | 0.51 |
| Aspartate aminotransferase (U/L) | 91.30 (33.20–232.10) | 39.40 (27.48–84.13) | 0.02 |
| Gamma-glutamyl transpeptidase (U/L) | 52.00 (19.00–80.00) | 62.00 (28.40–131.83) | 0.14 |
| Alkaline phosphatase (U/L) | 125.00 (75.00–154.00) | 123.50 (86.50–185.25) | 0.30 |
| Creatinine (μmol/L) | 65.00 (55.00–93.00) | 64.00 (52.00–76.25) | 0.09 |
| Blood urea nitrogen (mmol/L) | 6.38 ± 4.20 | 5.58 ± 5.60 | 0.47 |
| Serum sodium (mmol/L) | 136.33 ± 5.39 | 139.72 ± 3.09 | <0.001 |
| C3 (g/L) | 1.13 ± 0.67 | 0.79 ± 0.32 | <0.001 |
| C4 (g/L) | 0.54 ± 0.43 | 0.15 ± 0.10 | 0.06 |
| IgA (g/L) | 2.50 ± 1.17 | 4.19 ± 2.71 | 0.04 |
| IgG (g/L) | 14.95 ± 4.80 | 18.41 ± 6.75 | 0.01 |
| IgM (g/L) | 2.01 ± 1.93 | 2.17 ± 1.76 | 0.75 |
| Composite index scores | |||
| CTP score | 10.13 ± 1.80 | 8.62 ± 1.47 | <0.001 |
| MELD | 11.09 ± 8.73 | 4.94 ± 7.36 | <0.001 |
| CLIF-C AD | 21.78 ± 3.55 | 19.09 ± 3.48 | <0.001 |
ACLF, acute-on-chronic liver failure; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; AST, aspartate aminotransferase; C3, complement component 3; C4, complement component 4; CLIF, chronic liver failure; CLIF-C AD, CLIF Consortium Acute Decompensation; CRP, C-reactive protein; CTP, Child–Turcotte–Pugh score; EGV, esophageal and gastric varices; HAI, histological activity index; HBV, hepatitis B virus; HCV, hepatitis C virus; HE, hepatic encephalopathy; MASH, metabolic dysfunction-associated steatohepatitis; MELD, Model of End-Stage Liver Disease; PBC, primary biliary cholangitis; TIPS, transjugular intrahepatic portosystemic stent-shunts; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Etiological control or biochemical response: for AIH: biochemical remission (normalization of serum aminotransferase and IgG levels) and liver histological remission (Ishak system HAI score <4 or Scheuer grading system g ≤ 1); for PBC: after 1 year of UDCA treatment, ALP ≤ 3*ULN, AST ≤ 2*ULN, and total bilirubin ≤ 1 mg/dl; for AIH-PBC: the above conditions for AIH and PBC were satisfied at the same time; for alcoholic liver cirrhosis: abstinence more than 6 months; for liver cirrhosis associated with HCV or HBV: the viral load was less than the minimum detectable amount for more than 1 year; for MASH: weight loss and recovery of the metabolic syndrome were noted.
Complement component 3 as a prognostic biomarker for the prediction of acute-on-chronic liver failure and mortality
The complement C3 was an independent predictor of ACLF occurrence in univariate and multivariate analyses [univariate analysis: hazard ratio (HR): 0.08, 95% confidence interval (CI): 0.02–0.32, P < 0.001; multivariate analysis: HR: 0.14, 95% CI: 0.02–0.93, P = 0.04]. Other important risk factors for developing ACLF were hepatic encephalopathy (HE) (HR: 4.00, 95% CI: 1.4343–1111.11, P = 0.01), hemoglobin (HR: 0.98, 95% CI: 0.96–1.00, P = 0.02), total bilirubin (TB) (HR: 1.01, 95% 1.00–1.01, P < 0.001), and creatinine (HR: 1.01, 95% CI: 1.01–1.01, P < 0.001) (Table 2). Subgroup analyses were performed based on etiology (autoimmune-related liver disease or not), which showed the great diagnostic power of predicting ACLF for complement C3 ignoring the etiology (patients with AIH, AIH-PBC and PBC: HR: 0.11, 95% CI: 0.02–0.56, P = 0.007; patients with alcoholic liver disease and viral liver disease: HR: 0.04, 95% CI: 0.00–0.53, P = 0.02).
Table 2.
Cox regression evaluation of factors associated with acute-on-chronic liver failure
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| Age (years) | 1.04 (1.00–1.07) | 0.03 | 1.07 (1.02–1.13) | 0.01 |
| Gender (male/female) | 2.13 (0.98–4.63) | 0.06 | ||
| Ascites (no/yes) | 6.21 (1.48–26.03) | 0.01 | ||
| HE (no/yes) | 4.61 (2.27–9.38) | <0.001 | ||
| Higher than ULN of CRP (yes/no) | 2.91 (1.41–6.01) | 0.004 | ||
| Neutrophils percentage (%) | 1.05 (1.02–1.08) | 0.002 | ||
| Hemoglobin (g/L) | 0.98 (0.96–0.99) | <0.001 | ||
| International normalized ratio | 3.98 (2.59–6.09) | <0.001 | ||
| Total bilirubin (μmol/L) | 1.01 (1.00–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.04 |
| Aspartate aminotransferase (U/L) | 1.00 (1.00–1.00) | 0.01 | ||
| Creatinine (μmol/L) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.02) | 0.03 |
| Serum sodium (mmol/L) | 0.84 (0.78–0.91) | <0.001 | ||
| C3 (g/L) | 0.08 (0.02–0.32) | <0.001 | 0.16 (0.01–0.98) | 0.047 |
| C4 (g/L) | 0.01 (0.00–0.89) | <0.001 | ||
| IgG (g/L) | 1.06 (1.02–1.11) | 0.006 | ||
| CTP score | 2.27 (1.79–2.88) | <0.001 | ||
| MELD | 1.18 (1.12–1.23) | <0.001 | 1.02 (1.00–1.04) | 0.03 |
ACLF, acute-on-chronic liver failure; C3, complement component 3; C4, complement component 4; CRP, C-reactive protein; CTP, Child–Turcotte–Pugh score; HE, hepatic encephalopathy; MELD, Model of End-Stage Liver Disease; ULN, upper limit of normal.
We then performed the Cox analyses in predicting mortality. In the univariate analysis, 12 factors were included as candidate variables in the multivariate model (Table 3), including HE, neutrophils percentage, hemoglobin, international normalized ratio (INR), TB, albumin (ALB), creatinine, blood urea nitrogen (BUN), serum sodium, C3, C4, and IgG. The Cox regression model showed that the complement C3 was an independent risk factor for mortality (univariate analysis: HR: 0.20, 95% CI: 0.00–0.71, P = 0.01; multivariate analysis: HR: 0.10, 95% CI: 0.00–0.89, P = 0.01). Other important risk factors for developing ACLF were HE (HR: 9.04, 95% CI: 1.61–50.62, P = 0.01), neutrophils percentage (HR: 1.11, 95% CI: 1.01–1.23; P = 0.04), hemoglobin (HR: 0.93, 95% CI: 0.87–0.98, P = 0.01), TB (HR: 1.01, 95% CI: 1.00–1.01, P = 0.02), creatinine (HR: 1.01, 95% CI: 1.00–1.03, P = 0.02), BUN (HR: 1.17, 95% CI: 1.04–1.31, P = 0.01), and C4 (HR: 0.00, 95% CI: 0.001–0.01, P = 0.01).
Table 3.
Cox regression evaluation of factors associated with mortality
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| HE (no/yes) | 3.56 (1.25–10.15) | 0.02 | 6.83 (1.02–45.87) | 0.048 |
| Neutrophils percentage (%) | 1.06 (1.01–1.11) | 0.02 | ||
| Hemoglobin (g/L) | 0.96 (0.94–0.98) | <0.001 | 0.93 (0.88–0.99) | 0.01 |
| International normalized ratio | 1.95 (0.96–3.95) | 0.06 | ||
| Total bilirubin (μmol/L) | 1.00 (1.00–1.01) | 0.04 | ||
| Albumin (g/L) | 0.88 (0.80–0.97) | 0.01 | ||
| Creatinine (μmol/L) | 1.01 (1.00–1.01) | 0.002 | 1.01 (1.00–1.04) | 0.04 |
| Blood urea nitrogen (mmol/L) | 1.04 (1.00–1.09) | 0.08 | 1.16 (1.03–1.31) | 0.01 |
| Serum sodium (mmol/L) | 0.88 (0.81–0.96) | 0.003 | ||
| C3 (g/L) | 0.20 (0.00–0.71) | 0.01 | 0.10 (0.00–0.89) | 0.01 |
| C4 (g/L) | 0.00 (0.00–0.81) | 0.04 | 0.00 (0.00–0.01) | 0.01 |
| IgG (g/L) | 1.07 (1.00–1.13) | 0.04 | ||
| CTP score | 1.49 (1.12–1.98) | 0.006 | ||
| MELD | 1.11 (1.04–1.18) | 0.001 | 1.19 (1.01–1.40) | 0.04 |
| CLIF-C AD | 1.19 (1.00–1.41) | 0.048 | ||
C3, complement component 3; C4, complement component 4; CLIF, chronic liver failure; CLIF-C AD, CLIF Consortium Acute Decompensation; CTP, Child–Turcotte–Pugh score; MELD, the model of end-stage liver disease; HE, hepatic encephalopathy; ULN, upper limit of normal.
The AUROC of C3 in ACLF was 0.76 for 30 days, 0.73 for 60 days, and 0.72 for 90 days, respectively (Fig. 1). The time-dependent AUROC of C3 in predicting survival was 0.76 for 30 days, 0.69 for 60 days, and 0.68 for 90 days, respectively (Fig. 1).
Fig. 1.
Time-dependent ROC curves to predict ACLF and mortality of C3. (a) Time-dependent ROC curves to predict ACLF of C3. (b) Time-dependent ROC curves to predict mortality of C3. ACLF, acute-on-chronic liver failure; AUROC, the areas under the ROC curves; C3, complement component 3; ROC, receiver operator characteristic.
The cutoff value of complement C3 as the biomarker to predict acute-on-chronic liver failure and mortality
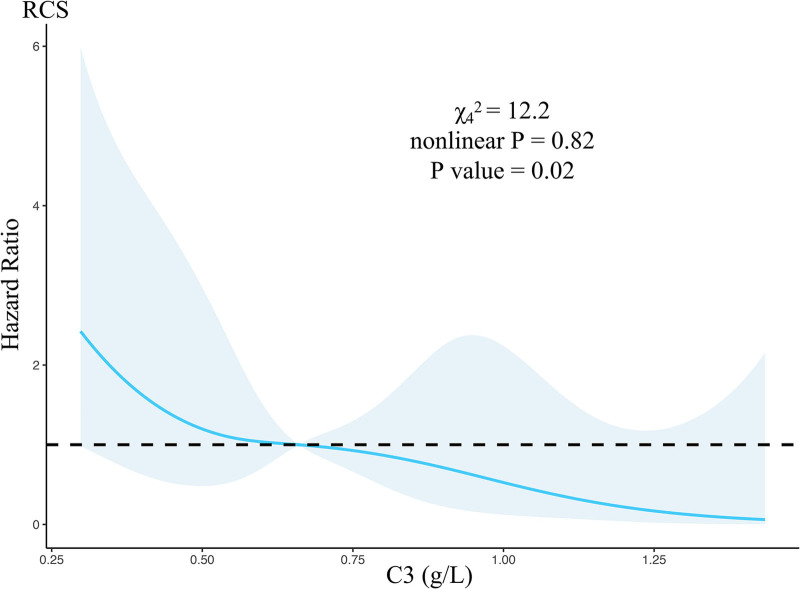
The risk of ACLF was linearly related to C3 levels (Fig. 2). The etiology-adjusted RCS was used to determine the cutoff value of C3, 0.66 g/L, for identifying a high risk of ACLF within 90 days (Fig. 2). According to the cutoff value, the cohort was divided into two groups, and there were significant differences between the two groups in the incidence of ACLF (HR: 2.69, 95% CI: 1.31–5.52, P = 0.007) and mortality (HR: 3.11, 95% CI: 1.05–9.24, P = 0.04) (Fig. 3). Figure 3 showed that the difference between the high-risk and low-risk groups was mainly reflected in the first 30 days after the onset of infection.
Fig. 2.
Continuous association between the complement C3 and incident risk of ACLF adjusted with etiology. ACLF, acute-on-chronic liver failure; C3, complement component 3; HR, hazard ratio; RCS, restricted cubic spline.
Fig. 3.
Comparison of ACLF-free survival rates and mortality between patients with high and low C3. (a) Comparison of ACLF-free survival rates between patients with high and low C3. (b) Comparison of liver-transplant-free survival between patients with high and low C3. ACLF, acute-on-chronic liver failure; C3, complement component 3.
C3-adjusted traditional model to predict mortality
In our cohort, CTP score, MELD, and CLIF-C AD score were not independent risk factors for predicting the development of ACLF (all P > 0.05) but were independent risk factors for predicting mortality (CTP: HR: 1.49, 95% CI: 1.12–1.98, P = 0.01; MELD: HR: 1.11, 95% CI: 1.04–1.18, P = 0.001; CLIF-C AD: HR: 1.19, 95% CI: 1.00–1.41, P = 0.048). The time-dependent AUROCs of the three models and the three models adjusted for C3 were plotted, suggesting that C3 correction could improve the AUROCs of the three models for predicting mortality in 30 days (CTP: 0.73–0.78; MELD: 0.81–0.84; CLIF-C AD: 0.65–0.83), 60 days (CTP: 0.76–0.78; MELD: 0.79–0.80; CLIF-C AD: 0.59–0.72), and 90 days (CTP: 0.76–0.78; MELD: 0.77–0.79; CLIF-C AD: 0.55–0.75) (Fig. 4).
Fig. 4.
Time-dependent ROC curves to predict mortality of traditional models and C3 combined with traditional models. (a) Time-dependent ROC curves to predict mortality of CTP scores. (b) Time-dependent ROC curves to predict mortality of MELD. (c) Time-dependent ROC curves to predict mortality of CLIF-C ADs. (d) Time-dependent ROC curves to predict mortality of C3 combined with CTP scores. (e) Time-dependent ROC curves to predict mortality of C3 combined with MELD. (f) Time-dependent ROC curves to predict mortality of C3 combined with CLIF-C ADs. AUROC, the areas under the ROC curves; C3, complement component 3; CLIF, chronic liver failure; CLIF-C ADs, CLIF Consortium Acute Decompensation score; CTP, Child–Turcotte–Pugh score; MELD, Model of End-Stage Liver Disease; ROC, receiver operator characteristic.
Prognostic model to predict acute-on-chronic liver failure
When we carried out feature selection, the top 10 risk factors to develop ACLF in cirrhotic patients were the INR, TB, high-sensitivity CRP, creatinine, BUN, C3, serum sodium, neutrophils percentage, age, ALB, and C4 (Fig. 5a). A new random forest model was built from the top five factors. Excellent goodness of fit was demonstrated in both the training set (R2 = 0.90, Fig. 5b) and the testing set (R2 = 0.89, Fig. 5c).
Fig. 5.
Ranking the importance of BI predictors by the random forest model. (a) Bar chart of importance ranking. (b) Actual value in the training set compared with the predicted value. (c) Actual value in the testing set compared with the predicted value. ALB, albumin; BI, bacterial infections; BUN, blood urea nitrogen; C3, complement component 3; C4, complement component 4; INR, international normalized ratio; NC percent, neutrophils percentage; TB, total bilirubin.
Discussion
The complement system, as a part of the innate immune system, plays a role in the immune response to infections [19,20]. Patients with C3 deficiency experienced recurrent purulent infections, primarily caused by Streptococcus pneumoniae and Neisseria meningitidis [19,21,22]. C3 could cause widespread tissue damage by increasing the production of proinflammatory cytokines by T lymphocytes [23]. The complement system undergoes changes at different stages of different causes of liver disease [24,25]. Previous studies have indicated that C3 complement content was elevated in the hepatitis stage, including fatty liver disease (alcohol related or metabolic disorder related) [14,26–28], HBV-related liver disease [15–17], and autoimmune liver disease [29,30]. The complement system activation is one of the characteristics of the hepatitis stage [31]. However, when the liver disease progresses to cirrhosis, liver synthesis function impairment and excessive inflammatory response lead to a decrease in the synthesis and an increase in consumption of C3 complement, resulting in a marked decrease in serum C3 complement content in end-stage liver disease patients compared with those in the hepatitis stage [17,32], which could partially explain the immune dysfunction in end-stage liver disease patients. For patients who progress to ACLF, further reduction in C3 levels might suggest that immune dysfunction could be particularly pronounced in those with co-existing BI in cirrhotic patients.
In this study, we aimed to explore the impact of low C3 levels in the peripheral blood on ACLF and mortality in the context of infection and to define its role as a prognostic biomarker for cirrhotic patients complicated with BI, as one of the acute decompensated events. We demonstrated for the first time that C3 was an independent predictor of the occurrence of ACLF and mortality in cirrhotic patients complicated with BI. Multivariable analyses showed that components of traditional scoring systems, namely TB and creatinine, remained important for predicting ACLF and mortality. The coexistence of HE with BI was an independent predictor of ACLF, which may be explained by HE being one of the diagnostic criteria for ACLF. Multivariable analysis suggested that hemoglobin was one of the predictors for ACLF and mortality, which was consistent with the previous findings from an Italian cohort study [12]. Unexpectedly, the data from this cohort did not suggest that the count of WBC was an independent predictor of ACLF and mortality for cirrhotic patients with BI, as an acute decompensated event, which was inconsistent with the findings of the study establishing CLIF-C AD score. This might be related to the fact that some patients included in this study had severe hypersplenism. However, C3 was independently associated with adverse outcomes. Our data suggested that C3 levels greater than 0.66 g/L were significantly more strongly associated with the risk of developing ACLF and death within 90 days.
Compared with complement C3, traditional models did not demonstrate the advantage as the independent predictor in predicting ACLF within 90 days. In predicting mortality, CTP and MELD demonstrated excellent predictive ability, while the CLIF-C AD score showed limited predictive ability in this study, which might be related to the fact that the low weight of WBC in predicting ACLF and mortality in our cohort. The predictive powers of all three traditional models (CTP, MELD, and CLIF-C AD score) adjusted by C3 were improved, in which the improvement was the most marked in CLIF-C AD score. This might suggest that the influence of hypersplenism on WBC could be partially corrected by C3 for predicting mortality in cirrhotic patients complicated with BI. From C3 dependently to C3 combined with traditional models to predict ACLF and mortality, the aforementioned results suggested that C3 should be more used as a short-term prognostic marker, and its predictive power decreased with time.
The limitations of this study are as follows: (1) due to the nature of single-center observational studies, selection bias was inevitable; (2) according to the diagnostic criteria for BI selected in the study, no etiological evidence was collected for some patients, and our results cannot be interpreted from the perspective of etiology in this study; (3) our study cannot be extended to patients who are receiving hormonal or immunosuppressive or cytotoxic drugs or who have concurrent immunodeficiency, of whom the complement system is abnormally activated or depleted.; (4) some of the enrolled patients had received care at a subordinate medical center before admission, and the content of this care was largely unknown; (5) the cohort was not established with the concept of recompensation in mind; and (6) our findings cannot be extended to patients with liver cancer and other organ failure.
In short, the complement C3 was an independent predictor of ACLF occurrence and mortality. C3 correction could improve the AUROCs of the CTP score, MELD, and CLIF-C AD score for predicting the mortality. Complement C3 was one of the top 10 risk factors recognized by the random forest regression to develop ACLF in cirrhotic patients. In conclusion, we identified the complement C3 as a prognostic predictor for the early identification of ACLF and mortality in cirrhotic patients complicated with BI within 90 days.
Acknowledgements
Most critically, the authors would like to thank all the patients who accepted to be enrolled in this cohort and with whose help this study was made possible the most deeply. The authors would like to thank clinical doctors and nurses from 113 ward and 111 ward in the Department of Hepatology at the Second Hospital of Nanjing for their help in patient screening, Dr Hao Zhang in Nanjing Drum Tower Hospital for his guidance on visualization, Dr Yang Wang and Mr. Chuan Zhou for support in patient follow-up, and Dr Wei Zhang in Nanjing Drum Tower Hospital for providing suggestions.
This work was supported by the Innovation Center for Infectious Disease of Jiangsu Province (No. CXZX202232) and Key Projects of Jiangsu Provincial Health Commission (ZD2021061).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Shuling Chen and Ruiqi Li contributed equally to the writing of this article and shared cofirst authorship.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023; 79:461–491. [DOI] [PubMed] [Google Scholar]
- 2.Moreau R, Gao B, Papp M, Bañares R, Kamath PS. Acute-on-chronic liver failure: a distinct clinical syndrome. J Hepatol. 2021; 75:S27–S35. [DOI] [PubMed] [Google Scholar]
- 3.Wong F, Piano S, Singh V, Bartoletti M, Maiwall R, Alessandria C, et al. ; International Club of Ascites Global Study Group. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J Hepatol. 2021; 74:330–339. [DOI] [PubMed] [Google Scholar]
- 4.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. ; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018; 67:1870–1880. [DOI] [PubMed] [Google Scholar]
- 5.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. ; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144:1426–37, 1437.e1. [DOI] [PubMed] [Google Scholar]
- 6.Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E, et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta-blocker therapy translates into improved clinical outcomes. Gut. 2021; 70:1758–1767. [DOI] [PubMed] [Google Scholar]
- 7.Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. ; PREDICT Study Group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020; 73:842–854. [DOI] [PubMed] [Google Scholar]
- 8.Cai YJ, Dong JJ, Dong JZ, Chen Y, Lin Z, Song M, et al. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017; 45:1413–1426. [DOI] [PubMed] [Google Scholar]
- 9.Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, et al. ; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016; 64:1249–1264. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021; 70:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashed E, Soldera J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: a systematic review. World J Hepatol. 2022; 14:2025–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017; 67:1177–1184. [DOI] [PubMed] [Google Scholar]
- 13.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, et al. ; CANONIC Study Investigators; EASL-CLIF Consortium. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015; 62:831–840. [DOI] [PubMed] [Google Scholar]
- 14.Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006; 3:333–340. [PubMed] [Google Scholar]
- 15.Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, et al. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009; 50:1809–1817. [DOI] [PubMed] [Google Scholar]
- 16.Zhang GL, Zhang T, Ye YN, Liu J, Zhang X-H, Xie C, et al. Complement factor 3 could be an independent risk factor for mortality in patients with HBV related acute-on-chronic liver failure. Biomed Res Int. 2016; 2016:3524842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Yuan Z, Li W, Fei L, Ji L, Huang Q, et al. Complement C3 facilitates stratification of stages of chronic hepatitis B and signifies development of acute-on-chronic liver failure in acute decompensated cirrhosis. Adv Ther. 2023; 40:1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. ; North American Consortium for the Study of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014; 60:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarantonello A, Revel M, Grunenwald A, Roumenina LT. C3-dependent effector functions of complement. Immunol Rev. 2023; 313:120–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisbrecht BV, Lambris JD, Gros P. Complement component C3: a structural perspective and potential therapeutic implications. Semin Immunol. 2022; 59:101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, et al. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008; 22:3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsoni SV, Kerrigan AM, Marakalala MJ, Srinivasan N, Duffield M, Taylor PR, et al. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect Immun. 2009; 77:3679–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannam A, Pernollet M, Fauquert JL, Monnier N, Ponard D, Villiers M-B, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008; 181:5158–5166. [DOI] [PubMed] [Google Scholar]
- 24.Potter BJ, Trueman AM, Jones EA. Serum complement in chronic liver disease. Gut. 1973; 14:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz LE, De Villiers D, Markham D, Whaley K, Thomas HC. Complement activation in chronic liver disease. Clin Exp Immunol. 1982; 47:548–554. [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Huang L, Yang A, Feng X, Mo Z, Zhang H, Yang X. Causal relationship between complement C3, C4, and nonalcoholic fatty liver disease: bidirectional Mendelian randomization analysis. Phenomics. 2021; 1:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng L, Zhao Y, Wang WL. Association between complement C3 and the prevalence of metabolic-associated fatty liver disease in a Chinese population: a cross-sectional study. BMJ Open. 2021; 11:e051218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Q, Li C, Xia Y, Zhang Q, Wu H, Du H, et al. Association between complement C3 and prevalence of fatty liver disease in an adult population: a cross-sectional study from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIHealth) cohort study. PLoS One. 2015; 10:e0122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasei M, Monabbati A, Alizadeh-Naeeni M, Houshmand S, Azarpira N, Lankarani KB, et al. Immunoglobulin and complement depositions in the liver of chronic hepatitis patients. Hepatogastroenterology. 2008; 55:1066–1070. [PubMed] [Google Scholar]
- 30.Biewenga M, Farina Sarasqueta A, Tushuizen ME, de Jonge-Muller ESM, van Hoek B, Trouw LA. The role of complement activation in autoimmune liver disease. Autoimmun Rev. 2020; 19:102534. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008; 47:729–736. [DOI] [PubMed] [Google Scholar]
- 32.Copenhaver M, Yu CY, Hoffman RP. Complement components, C3 and C4, and the metabolic syndrome. Curr Diabetes Rev. 2019; 15:44–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.