Abstract
We examined the harmful side effects on indigenous soil microorganisms of two organic solvents, acetone and dichloromethane, that are normally used for spiking of soil with polycyclic aromatic hydrocarbons for experimental purposes. The solvents were applied in two contamination protocols to either the whole soil sample or 25% of the soil volume, which was subsequently mixed with 75% untreated soil. For dichloromethane, we included a third protocol, which involved application to 80% of the soil volume with or without phenanthrene and introduction of Pseudomonas fluorescens VKI171 SJ132 genetically tagged with luxAB::Tn5. For both solvents, application to the whole sample resulted in severe side effects on both indigenous protozoa and bacteria. Application of dichloromethane to the whole soil volume immediately reduced the number of protozoa to below the detection limit. In one of the soils, the protozoan population was able to recover to the initial level within 2 weeks, in terms of numbers of protozoa; protozoan diversity, however, remained low. In soil spiked with dichloromethane with or without phenanthrene, the introduced P. fluorescens VKI171 SJ132 was able to grow to a density 1,000-fold higher than in control soil, probably due mainly to release of predation from indigenous protozoa. In order to minimize solvent effects on indigenous soil microorganisms when spiking native soil samples with compounds having a low water solubility, we propose a common protocol in which the contaminant dissolved in acetone is added to 25% of the soil sample, followed by evaporation of the solvent and mixing with the remaining 75% of the soil sample.
When biodegradation of specific compounds with hydrophobic properties (e.g., polycyclic aromatic hydrocarbons) is to be examined on a laboratory scale in soil, it is a problem to get them evenly distributed in the test system. Soil can easily be amended with polycyclic aromatic hydrocarbons in low concentrations close to their water solubility, but their concentration in soil from contaminated sites is often considerably higher, in the range of thousands of milligrams of polycyclic aromatic hydrocarbons per kilogram of soil, and hence it is necessary to dissolve the hydrophobic compound in some kind of organic solvent to distribute the compound efficiently in the soil matrix. Most commonly dichloromethane (36) is used for this purpose (3, 4, 32, 35, 46), but acetone (19, 21), methanol (2), toluene (37), cyclohexane (33), N′,N′-dimethylformamide (6), and ethylacetate (27, 28) have also been used.
The side effects of these routine laboratory procedures on the indigenous microbial populations in the soil may severely influence conclusions based on experiments with soil spiked with specific compounds.
We thus decided to investigate the possible side effects of application of organic solvents to natural soils and, based on these investigations, suggest a protocol for introducing contaminants to soil samples that minimizes misleading side effects on the indigenous microbial community.
MATERIALS AND METHODS
Experiment 1: two solvents, no phenanthrene. (i) Soil.
Two soils were sampled in Bordeaux, France (Table 1). After sampling, the soil was passed through a 2-mm sieve, dried at 30°C for 24 h, and stored at 5°C in glass containers in the dark until experimental setup, for 1 month at a maximum.
TABLE 1.
Important characteristics of the soils used
| Soil | Composition (%)
|
Organic carbon (%) | pH | ||
|---|---|---|---|---|---|
| Sand (>20 μm) | Silt (2-20 μm) | Clay (<2 μm) | |||
| Podzolic agricultural, Bordeaux | |||||
| A1 (0-20 cm) | 91.1 | 2.2 | 1.5 | 2.0 | 5.8 |
| A2 (40-60 cm) | 95.6 | 1.1 | 0.9 | 0.8 | 5.7 |
| Sandy loam, Roskilde | 56.0 | 30.7 | 10.9 | 2.4 | 6.5 |
(ii) Contamination protocols.
The dried and sieved soil was transferred from storage, rewetted to a moisture content of 15% (dry weight), and incubated at 20°C. The effects of artificial contamination were tested on dried soils that had been rewetted and incubated for 5 days prior to the contamination procedure. An initial 20-day pilot experiment had shown that after rewetting, an increase in the number of bacteria occurred within the first 2 days and in the number of protozoa within the first 5 days. Thereafter a steady state was reached and maintained for 30 days. No significant differences in steady-state levels were observed between dried soils and soils that had been stored at natural moisture content (data not shown).
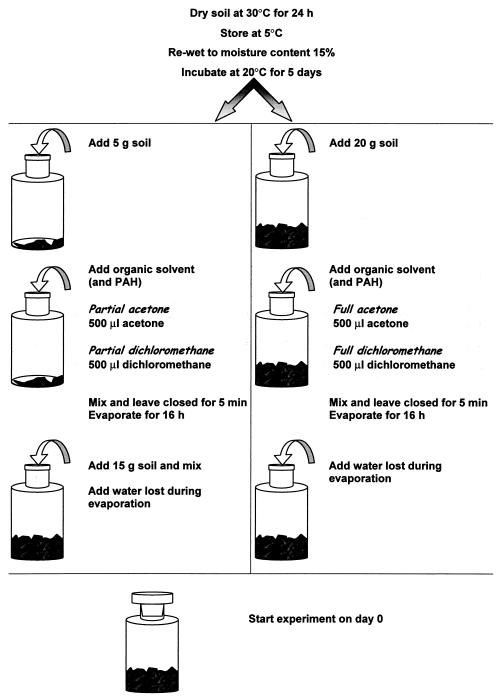
The soils were treated with the solvent acetone or dichloromethane according to two different protocols, partial and full treatment, giving four different treatments in all (Fig. 1). In the partial treatment protocol, 500 μl of solvent or contaminant is added to a 25% fraction (5 g) of the soil sample and the flasks are closed for 5 min to let the solvent disperse. Thereafter the solvent is evaporated for 16 h, and the subsample is mixed with the remaining 75% (15 g) of the soil sample. This yields a solvent concentration of 10% (vol/wt) in the treated fraction of the soil sample. With the full treatment protocol, the solvent or contaminant is added to the whole soil sample, the flasks are closed for 5 min to let the solvent disperse, and the solvent is then evaporated for 16 h. This protocol yields a solvent concentration of 2.5% (vol/wt) in the whole sample treated. All mixings were performed thoroughly in each separate flask for 1 min with a metal spatula.
FIG. 1.
Protocols for artificial contamination of soil with polycyclic aromatic hydrocarbons (PAH) by using acetone or dichloromethane as the solvent and adding the solvent-polycyclic aromatic hydrocarbon mixture to a fraction of the soil sample (partial treatment protocol) or to the whole soil sample (full treatment protocol).
We used a procedure with dry soil for two reasons: (i) to obtain standard conditions for the amendment (a dried rewetted soil provides a much better standard baseline than a soil that has contained an arbitrary water content for an arbitrary period) and (ii) to ensure that we observed effects on an active microbial community. Especially for the soil protozoa, it is generally accepted that they are only active for short periods just after soil rewetting (8, 9, 13, 15) and that active protozoa are more susceptible to the effect of xenobiotics than nonactive encysted forms (14). If a protocol can be proven to have only slight side effects under these worst-case conditions, it is a good argument that it will have only slight side effects under all conditions.
(iii) Indigenous bacteria and protozoa.
All sampling of soil for the determination of protozoan and bacterial numbers was performed as follows: 1 g of soil was sampled, dissolved in 9.5 ml of phosphate buffer, and then shaken vigorously for 20 s before further processing. Indigenous bacteria and protozoa were counted on days 0, 1, 4, 6, 8, 14, and 33 following the four different treatments. Soil samples were plated as dilution series on three different media. CFU were counted after 3 weeks on the oligotrophic 1/300 tryptic soy agar (TSA), after 24 h on the rich Luria-Bertani (LB) medium, and after 48 h on the Pseudomonas spp.-specific (25) Gould's S1 medium. The dilutions were plated by drop plating (23) in the case of LB and by standard plating in the case of 1/300 TSA and Gould's S1. Protozoa were estimated by using the most-probable-number (MPN) method as described by Rønn et al. (44). The soil samples were distributed in 96-well microtiter plates by using eight replicates and threefold dilutions with 1/300 TSA as the nutrient medium. The microtiter plates were incubated at 10°C in darkness. Individual wells were inspected for the presence or absence of protozoa after 1 and 3 weeks by inversion microscopy (Olympus CK40) at a magnification of ×200.
Flagellate diversity was determined 33 days after the four treatments; 5-g soil samples were wrapped in lens tissue and incubated in 5 ml of Neff's modified amoeba saline (38) in sterile 50-ml Nunc flasks. A sterilized wheat grain was added to the raw cultures to provide nutrients for bacterial growth, which would subsequently serve as a food source for the protozoa. After 5 and 8 days, the flagellates were identified to the lowest possible taxonomic level by a routine examination as described previously (12). A rough estimate of ciliate numbers 33 days after the four treatments was obtained by using an MPN approach as previously described for protozoa at year 1 (20), with the counts being made on 0.1-g, 1-g, and 10-g soil samples.
Experiment 2: dichloromethane with and without phenanthrene. (i) Soil.
Soil was sampled at Roskilde, Denmark (Table 1), and processed as described above except that it was dried at room temperature. The background level of indigenous phenanthrene degraders in the soil was determined by the spray plate technique (29), in which degraders produce clearing zones in a crystal layer of phenanthrene on the surface of hydrocarbon minimal medium (HCMM2) (42) Noble agar (Difco, Detroit, Mich.) plates.
(ii) Bacterial strains and media.
Phenanthrene-degrading wild-type strain VKI171 (26) was gram negative and oxidase positive, and the species was identified as Pseudomonas fluorescens by Analytical Profile Index 20NE (API 20NE) (BioMérieux SA, Marcy-l'Étoile, France). In addition, the isolates were fluorescent under UV light on Gould's S1 agar (18). P. fluorescens VKI171 proved to be resistant to chloramphenicol at 25 μg/ml and streptomycin at 50 μg/ml and sensitive to kanamycin, necessary for selection of mutants after transposon mutagenesis.
Bacterial cultures were stored in 50% (vol/vol) LB medium (Difco) and glycerol at −80°C. Prior to use they were grown overnight on LB. All incubations were undertaken at 30°C, with the liquid cultures being shaken at 150 rpm. The phosphate buffer used was 0.010 M and pH 7.4. HCMM2 and Davis minimal medium (DMM) (31) containing phenanthrene as the sole carbon source were prepared by stirring autoclaved medium with crystalline phenanthrene for 24 h. The solutions were filtered through Steritop filters (Millipore Corporation, Bedford, Mass.). The phenanthrene concentration was approximately 0.9 mg/liter, determined on a gas chromatograph HP3365 series II (Hewlett-Packard, Palo Alto, Calif.) by extraction with pentane by using hexadecane as an internal standard.
Bacteria for the soil experiments were acclimatized before inoculation so as to physiologically adapt the inoculum to the low-nutrient conditions in soil. One colony from an overnight culture on LB agar plates was inoculated into LB and incubated for 23 h. Cells were washed twice in DMM by centrifugation (8,000 × g, 15 min, 4°C). The pellet was resuspended in DMM with phenanthrene as the sole carbon source prepared as described above. After 22 h of incubation, the culture was centrifuged (8,000 × g, 15 min, 4°C), and the pellet was resuspended in phosphate buffer and used for soil inoculation. Cell number before and after acclimatization of inoculum was determined by microscopic counts on cells stained with 4′,6′-diamidino-2-phenylindole (DAPI) (39). This resulted in smaller, starved cells, as indicated by a 54% decrease in the average length of DAPI-stained cells and a 4.5-fold decrease in optical density at 600 nm (OD600) for the same number of cells.
Transposon mutagenesis.
The quantification of introduced bacteria in natural soil requires genetic labeling. The selected phenanthrene degrader P. fluorescens VKI171 was therefore labeled by luxAB::Tn5 transposon mutagenesis, essentially as described previously (30). In brief, plasmid pRL1063 (47) was mobilized into P. fluorescens VKI171 by using the helper plasmid pRK2013 (10). The transposon, which was promoterless, was thereby inserted randomly into the chromosome. A total of 202 mutants were isolated on LB agar plates with kanamycin (25 μg/ml) and chloramphenicol (10 μg/ml).
Selection of mutant.
As the performance of the selected luxAB::Tn5 transconjugant P. fluorescens VKI171 SJ132 was comparable to that of the wild type, it was selected for use in the present studies. This was done on the basis of a screening procedure encompassing tests for phenotype, growth, stability of genetic insert, single copy insertion, and phenanthrene-degrading abilities. Phenotype was established by using the API 20NE test kit. Growth in LB was examined by measuring the OD600 (Lambda Bio UV/VIS spectrophotometer; Perkin- Elmer Instruments Inc., Norwalk, Conn.) and determining the number of CFU once an hour for 42 h. Linear regression of log-phase growth in LB yielded slopes within the same confidence interval as the parent strain.
The genetic insert was shown to be stable by replica plating. Stationary-phase cultures obtained after 42 h in LB were plated on LB agar. After incubation for 16 h, the colonies were transferred from the original plates to LB agar plates devoid of kanamycin and to plates containing kanamycin (25 μg/ml). That only a single copy of the transposon had been inserted into the same chromosome was determined by DNA extraction and Southern blot DNA hybridization analysis performed by using standard techniques (34). The phenanthrene-degrading ability of the mutants and the wild type were confirmed as follows. Bacteria were transferred from an LB agar plate to phosphate buffer, and the batch was adjusted to an OD600 of 0.5. Then 1.0 ml of this was inoculated into 20 ml of HCMM2 containing phenanthrene, producing a final inoculum of approximately 107 cells/ml. Sampling was undertaken on days 0 and 7. The concentration of phenanthrene in 1.0-ml samples was determined by gas chromatography. Both modified and parent strains degraded phenanthrene from 0.89 to 0.03 mg/liter during the 7 days.
Soil microcosms.
The soil microcosms were prepared in glass-stoppered 100-ml flasks each containing soil equivalent to 20 g (dry weight) at a moisture content of 15% (dry weight). Dichloromethane with or without phenanthrene was added according to the modified partial treatment protocol described above, with the modification that the solvent was added to 80% of the soil sample. Controls received no dichloromethane. The remaining 4 g of soil was dried at 105°C for 20 h and inoculated by carefully mixing acclimatized cells suspended in phosphate buffer into the soil. The volume of cell suspension was equivalent to the water required to rewet the 4 g of dry soil plus the amount lost from microcosms during the evaporation of dichloromethane after the addition of phenanthrene. The resulting concentration was 105 cells/g of dry soil. Microcosms were incubated in the dark and aerated by removing the glass stopper for 1 min every day to avoid oxygen deprivation.
The number of P. fluorescens VKI171 SJ132 was counted in soil sampled from each soil system by plating on LB agar supplemented with streptomycin at 25 μg/ml, kanamycin at 25 μg/ml, and nystatin at 50/μg ml to suppress growth of indigenous bacteria and fungi. CFU were counted after incubation overnight. That all the resulting colonies originated from P. fluorescens VKI171 SJ132 was verified by the expression of the luxAB genes registered on X-ray film in the presence of decanal (Aldrich 12.577-6) as described before (30). The protozoan number was determined by using the MPN method as described above.
Data analysis.
All experiments were carried out in triplicate. Growth in liquid LB was plotted and statistically analyzed by using Fig. P. version 5 (Biosoft, Cambridge, United Kingdom). Linear regression was performed on four data points selected from the log growth phase. Differences in slope coefficients were tested by using an F test and considered significant at the 5% level. Protozoan MPN was calculated as described before (39). CFU and MPN data were analyzed by using two-way analysis of variance (ANOVA) combined with Tukey's multiple-comparison test on log10 transformed data, and a 5% significance level was used. For the ANOVAs we used SigmaStat (SPSS Inc., Chicago, Ill.).
RESULTS
Experiment 1: effects of contamination protocols.
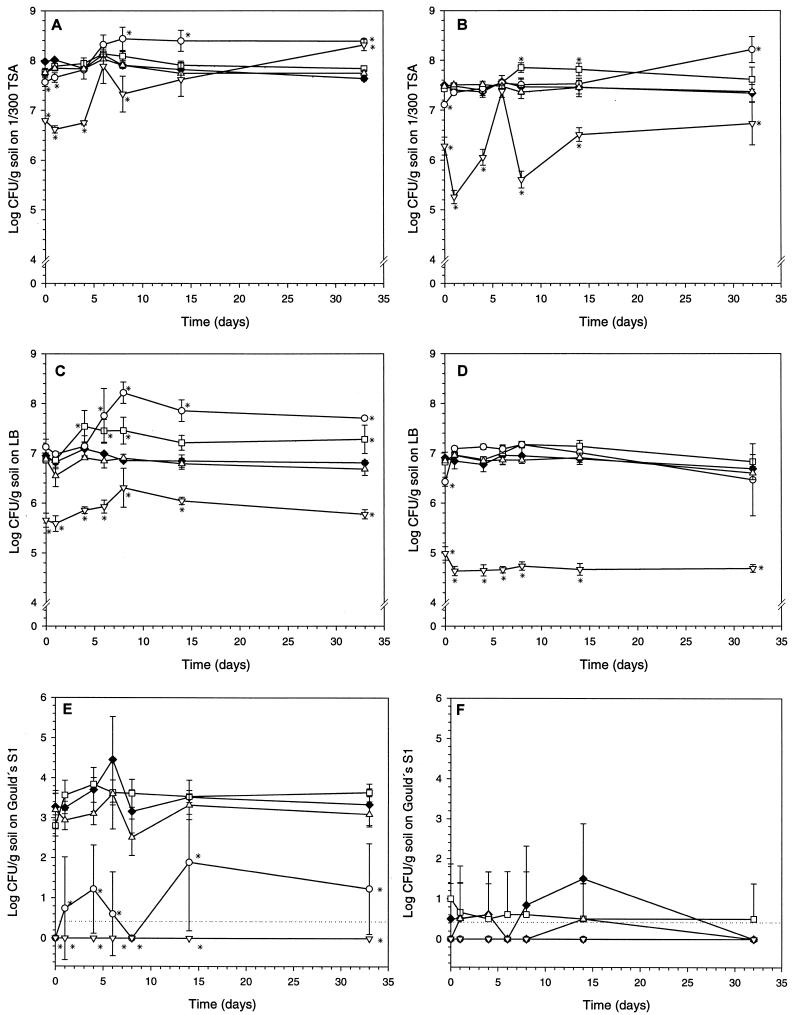
On the whole, we encountered more bacterial colonies on the oligotrophic medium 1/300 TSA (Fig. 2A and B) than on the rich LB medium (Fig. 2C and D). Generally the full treatment protocols with both acetone and dichloromethane resulted in markedly lower populations of bacteria than in the controls at the beginning of the experiment (Fig. 2A to F), except for the acetone treatment in the Bordeaux A1 topsoil on LB (Fig. 2C). In the Bordeaux A2 soil sampled 40 to 60 cm below the soil surface, the number of Pseudomonas spp. culturable on Gould's S1 was 100-fold lower than in Bordeaux A1 topsoil.
FIG. 2.
Culturable indigenous bacteria determined on days 0, 1, 4, 6, 8, 14, and 33 after treating the Bordeaux A1 and A2 soils with the organic solvents acetone and dichloromethane according to two different protocols: partial treatment protocol with acetone (□) or dichloromethane (▵) and full treatment protocol with acetone (○) or dichloromethane (▿). Controls (⧫) did not receive any solvents. Three different media were used to detect different groups of bacteria: the oligotrophic medium 1/300 TSA (A and B), the rich medium LB (C and D), and the Pseudomonas spp.-specific medium Gould's S1 (E and F). The detection limit is indicated by a stippled line. (A, C, and E) Bordeaux A1 (0- to 20-cm depth). (B, D, and F) Bordeaux A2 (40- to 60-cm depth). Each data point represents the mean (± SD) of three independent replicates. Significant differences from the control are indicated by asterisks (P < 0.05). To simplify panel F, SD is only indicated when positive.
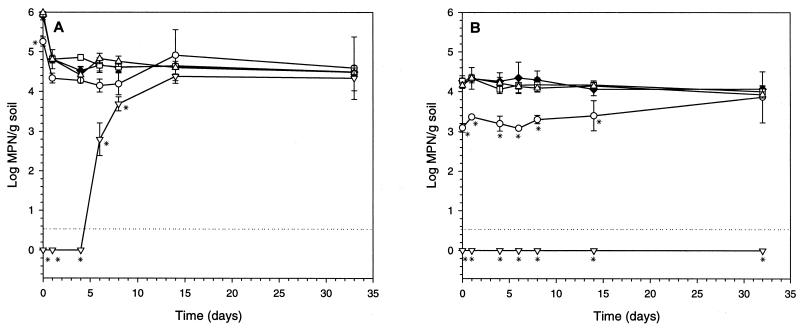
The pseudomonads were strongly affected by the full treatments, but statistical comparison in Bordeaux A2 was impossible due to the generally very small number of CFU (Fig. 2F). Likewise, numbers of protozoa were either severely depressed or below detection level for both full treatment protocols at the beginning of the experiments (Fig. 3). The initial toxic response to the full treatment protocols for both bacteria and protozoa was often followed by renewed growth, in some cases to levels significantly above the control level (Fig. 2, Fig. 3). It was clear that for both bacteria (Fig. 2) and protozoa (Fig. 3), the most severe effects were observed after full treatments with dichloromethane and neither bacterial nor protozoan numbers were significantly reduced by any of the partial treatment protocols.
FIG. 3.
MPN of protozoa determined on days 0, 1, 4, 6, 8, 14, and 33 after treating the Bordeaux A1 and A2 soils with the organic solvents acetone and dichloromethane according to two different protocols: partial treatment protocol with acetone (□) or dichloromethane (▵) and full treatment protocol with acetone (○) or dichloromethane (▿). Controls (⧫) did not receive any solvents. The detection limit is indicated by a stippled line. (A) Bordeaux A1 (depth, 0 to 20 cm). (B) Bordeaux A2 (depth, 40 to 60 cm). Each data point represents the mean (± SD) of three independent replicates. Significant differences from the control are indicated by asterisks (P < 0.05).
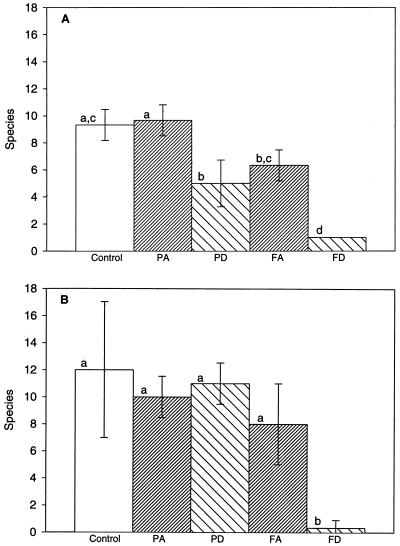
The diversity of protozoa 33 days after the full treatment protocol with dichloromethane in the Bordeaux A1 soil revealed that this population consisted of only one morphotype, a small flagellate with Spumella morphology (possibly Spumella elongata) (Fig. 4A, Table 2). This form was also observed in the Bordeaux A2 soil (Fig. 4B, Table 2), although the population here remained at a level below the detection limit of 10 individuals/g of soil as determined by the MPN method (Fig. 3B).
FIG. 4.
Diversity of flagellates in soil, measured as number of species 33 days after treating the Bordeaux A1 and A2 soils with the organic solvents acetone and dichloromethane according to two different protocols: partial treatment protocol with acetone (PA) or dichloromethane (PD) and full treatment protocol with acetone (FA) or dichloromethane (FD). Controls did not receive any solvents. (A) Bordeaux A1 (depth, 0 to 20 cm). (B) Bordeaux A2 (depth, 40 to 60 cm). Each column represents the mean (± SD) of three independent replicates. Differences between treatments are significant (P < 0.05) when the lowercase letters above the columns are not the same.
TABLE 2.
Protozoan types identified in experiment 1a
| Protozoan type | Bordeaux A1
|
Bordeaux A2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | PA | PD | FA | FD | Control | PA | PD | FA | FD | |
| Apusomonadida | ||||||||||
| Apusomonas proboscidea Aléxéieff 1924 | x | x | x | |||||||
| Kinetoplastida | ||||||||||
| Bodo designis Skuja 1948 | x | x | x | x | x | |||||
| Dimastigella trypaniformis Sandon 1928 | x | x | ||||||||
| Cercomonadida | ||||||||||
| Cercomonas small type | x | x | x | |||||||
| Cercomonas large type | x | x | x | x | x | x | x | x | ||
| Heteromita amoeboid type | x | x | x | x | x | x | x | |||
| Heteromita globosa (Stein 1878) Kent 1880 | x | x | x | x | x | x | x | x | ||
| Choanoflagellida | ||||||||||
| Codosiga botrytis (Ehrenberg 1838) Kent 1880 | x | x | ||||||||
| Cryptophyceae | ||||||||||
| Goniomonas truncata (Fresenius 1858) Stein 1878 | x | x | x | |||||||
| Euglenophyceae | ||||||||||
| Petalomonas minuta Hollande 1942 | x | x | ||||||||
| Petalomonas pusilla Skuja 1948 | x | x | x | |||||||
| Petalomonas punctato-striata Skuja 1939 | x | x | x | x | x | |||||
| Stramenopiles | ||||||||||
| Spumella type | x | x | x | x | x | x | x | x | x | |
| Thaumatomastigidae | ||||||||||
| Protaspis simplex Vørs 1992 | x | x | x | x | x | x | ||||
| Insertae sedis | ||||||||||
| Allantion tachyploon Sandon 1924 | x | x | x | x | x | x | x | x | ||
| Sciviamonas terricola Ekelund, Patterson & Vørs 1997 | x | x | x | |||||||
| Flagellate type A | x | x | x | x | x | |||||
| Flagellate type B | x | x | x | |||||||
| Naked amoebae | x | x | x | x | x | x | x | |||
| Heliozoan type | x | |||||||||
| Ciliates | x | x | x | x | x | x | x | x | ||
The x indicates that the type was found in at least one of three replicates of the treatment in which it occurred. Whenever possible, a species name was assigned to the heterotrophic flagellates; taxonomic species in the table are followed by the original publication. In some cases, however, it was necessary to apply a broader morphotype concept. Cercomonas spp. are virtually impossible to identify to species level, and we saw several different forms in the samples which we could not identify; hence, we assigned Cercomonas cells that were ≤ 10 μm long to “small type,” whereas cells of >10 μm were assigned to “large type.” Cells with great similarity to Heteromita globosa but with more pronounced amoeboid properties, which were often seen in the samples, were assigned to Heteromita amoeboid type. As with Cercomonas, Spumella spp. are virtually impossible to identify to species level, and hence they are referred to as Spumella type. A few characteristic types occurred in the samples which we were unable to associate with any taxonomic unit; these are included in the table as flagellate type A and type B. Besides the flagellate morphotypes, we also included the ciliates as a morphotype as well as the groupings naked amoebae and heliozoan type. PA and PD, partial treatment with acetone and dichloromethane, respectively; FA and FD, full treatment with acetone and dichloromethane, respectively.
The diversity was still significantly lower after 33 days in Bordeaux A1 soil subjected to the partial treatment protocol with dichloromethane (Fig. 4A, Table 2), even though no effect on the numbers of protozoa was detected (Fig. 3A). The number of recognizable taxa after applying the partial treatment protocol by using acetone was not significantly different from that in the controls. We found relatively high numbers of protozoa in terms of both numbers and diversity in the microcosms prepared from soil originating from the 40- to 60-cm depth. This is unusual and may be due to agricultural activity at the site having caused mixing from the topsoil to the A2 horizon, which was originally devoid of organic matter.
The number of ciliates was lower than in the control 33 days after the full treatment protocol with acetone and was below the detection limit after the full treatment protocol with dichloromethane (Table 3).
TABLE 3.
Number of ciliates in microcosmsa
| Treatment | No. of ciliates per 20 g of soil
|
|
|---|---|---|
| A1 | A2 | |
| Control | 30 | >600b |
| PA | 92 | >600b |
| PD | 14 | >600b |
| FA | 8 | 30 |
| FD | <0.8c | <0.8c |
The number was determined 33 days after treating the Bordeaux A1 and A2 soils with the organic solvents acetone and dichloromethane according to two different protocols: partial treatment protocol with acetone (PA) or dichloromethane (PD) and full treatment protocol with acetone (FA) or dichloromethane (FD) Controls did not receive any solvents.
Above detection range.
Below detection range.
Experiment 2: dichloromethane with and without phenanthrene.
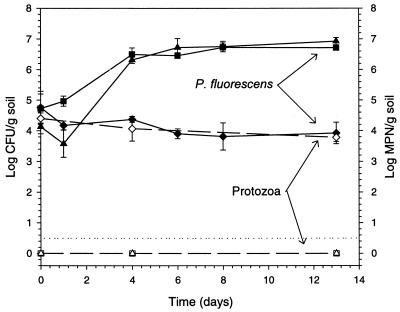
Quantification of indigenous phenanthrene degraders in the natural Roskilde soil revealed that there were 440 clearing-zone-forming colonies/g of soil on phenanthrene spray plates. Acclimatized P. fluorescens VKI171 SJ132 inoculated into soil that had been treated with dichloromethane grew from the inoculation level of between 104 and 105 CFU/g of soil to 107 CFU/g of soil (Fig. 5). The number of P. fluorescens VKI171 SJ132 organisms inoculated in soils treated with 500 ppm of phenanthrene in dichloromethane was slightly lower than in soils without phenanthrene (104 CFU/g of soil), but still grew to 107 CFU/g of soil.
FIG. 5.
Number of indigenous protozoa and survival of P. fluorescens VKI171 SJ132 inoculated after artificial contamination with the modified partial treatment with dichloromethane as the solvent with (▴) or without (▪) phenanthrene. Controls (⧫) did not receive any dichloromethane. Solid symbols indicate CFU (▴, ▪, ⧫), and open symbols indicate protozoan MPN (▵, □, ◊). The detection limit is indicated by a stippled line. Each data point represents the mean (± SD) of three independent replicates.
In the soils treated with dichloromethane with and without phenanthrene, the number of protozoa was below the detection limit. In untreated control soils, the number of P. fluorescens VKI171 SJ132 organisms decreased from the inoculation level of 105 CFU/g of soil to 104 CFU/g of soil within the first day after inoculation and remained at this level for the remainder of the experimental period. The number of protozoa in the untreated soil was stable at 104 protozoa/g of soil during the whole experimental period. Two-way ANOVA confirmed significant effects of the treatments on both P. fluorescens VKI171 SJ132 and protozoa.
DISCUSSION
Despite the fact that many organic solvents are lethal to microorganisms, they are nevertheless commonly used as solvents when artificially contaminating soil samples with compounds of low water solubility (45). We thus examined the response of the indigenous microorganisms to organic solvents of two different classes, the halogenated methane dichloromethane and the aliphatic ketone acetone. Both are often used for artificially contaminating soil with polycyclic aromatic hydrocarbons in laboratory experiments. When dichloromethane was applied to the total soil samples by using the full treatment protocol, it had direct toxic effects on the indigenous population of bacteria and protozoa. Acetone applied by using the full treatment protocol also affected these groups of organisms, though less severely.
Toxicity tests on bacteria are usually conducted in solution. Acetone is reported not to have growth-inhibiting (16) or mutagenic (17) effects on Vibrio fischeri, while several authors have reported negative effects of dichloromethane, including growth inhibition (7, 22), mutagenicity, and lethal toxicity (43). However, as bacterial tolerance to toluene is higher in soil than in liquid media (24), it is questionable to extrapolate solvent toxicity data directly from toxicity tests in liquid cultures to the actual effects in soil. Nevertheless, our results demonstrate that the toxic effects of dichloromethane seen in solution also apply to microorganisms in the soil. To our knowledge, the toxic effects of acetone on soil microorganisms have not been reported previously. In the present study, the number of both bacteria and protozoa decreased immediately after application of the solvent, although in some cases only transiently.
With the partial treatment protocol, only 25% of the soil sample was exposed to the organic solvent. The resulting solvent concentration was higher than with the corresponding full treatment protocol, however, since the same amount of organic solvent was used in each case. The treated fraction of the soil was mixed with the untreated fraction 16 h after treatment. Immediately after mixing, the total number of bacteria was 61 to 82% of the control value in the partial treatment protocol with acetone and 60 to 95% of the control in the partial treatment protocol with dichloromethane. The decrease in total numbers could be explained at least partly by a simple dilution effect. After the initial decline caused by the solvents, we often observed regrowth of the populations to levels higher than in the controls. The same effect has also been demonstrated after toluene exposure of Pseudomonas putida inoculated into natural soil (24). This could be attributable to the fact that when the unaffected microorganisms in the untreated 75% of the soil sample are mixed into the treated 25% of the sample, they are supplied with nutrients from the dead organisms in the treated fraction.
In the Bordeaux A2 soil exposed to the full treatment protocol with dichloromethane, the protozoan number never exceeded the detection limit of the MPN technique. With the other three treatments in both soils, the protozoan number had returned to a level not significantly different from that in the control by day 33. However, by comparing Fig. 3 and Fig. 4, it becomes evident that despite the recovery of numbers of protozoa after the dichloromethane treatment of Bordeaux A1, the protozoan community structure was irreversibly damaged. The “Spumella sp.” that was able to survive the full treatment protocol with dichloromethane was frequently observed, especially in the Bordeaux A1 soil, and is a species commonly found in soil (11). Not all species are sufficiently abundant to allow their absence to indicate a toxic effect of the solvent, but when 10 of 11 were missing in the Bordeaux A1 soil samples following the full treatment protocol with dichloromethane and 17 of 18 species were absent in the Bordeaux A2 soil samples (Fig. 4, Table 2), this suggests a strong toxic effect. A more careful examination of protozoan diversity than that applied here would probably have revealed even larger discrepancies in recovery in terms of numbers and diversity.
Bacteria inoculated in soil usually serve as an easily available food resource for the indigenous protozoa (13). Thus, it is commonly accepted that bacteria inoculated into natural soil in high numbers progressively decline and that their survival can be improved by prior sterilization of the soil (41). In the dichloromethane-treated soils, protozoa were absent (Fig. 5) and the inoculated P. fluorescens VKI171 SJ132 organisms grew to a level 1,000 times higher than in the untreated controls. The number of protozoa was constant in controls, while the number of P. fluorescens VKI171 SJ132 organisms stabilized after a 10-fold decrease. Even though P. fluorescens VKI171 SJ132 was able to grow on phenanthrene as the sole carbon source, additional growth was not observed in the phenanthrene-contaminated soil compared to in the soil treated with dichloromethane alone. Thus, although the MPN method of counting protozoa is based on a culture technique and hence does not provide any indication of the activity of the protozoa in the undisturbed soil, our results strongly suggest that protozoan grazing was responsible for the decline in the number of introduced bacteria in the control soils and that predation pressure in the soil at least partly explains the lower level of bacterial carrying capacity.
The fact that the lower number of bacterial grazers leads to less predation (1) is only one of three factors that might enhance the survival or growth of an inoculated bacterial strain, however. Others factors are that an impeded indigenous population of bacteria leads to less competition (40) and that lysed cells of both bacteria and protozoa supply the soil with a higher level of available substrate. All three factors probably act synergistically in our experiments, providing the introduced P. fluorescens VKI171 SJ132 with an advantage in the soil treated with dichloromethane.
Findings based on inoculated bacteria in artificially contaminated soils may thus be misinterpreted in some cases. For example, Møller et al. found that an Alcaligenes sp. inoculated to soil amended with phenanthrene and dichloromethane grew to 109 CFU/g of soil, compared to 107 CFU/g of soil in unamended soil (31). From this it was concluded that the phenanthrene-degrading abilities of this strain provide the inoculate with a selective advantage over the indigenous microflora in the presence of phenanthrene. An alternative explanation, however, is that the findings could be partly attributable to reduced predation and competition and enhanced nutrient availability resulting from exposure to the toxic organic solvent.
Survival levels are usually much lower than 109 CFU/g of soil, even when specialized degraders are inoculated into soil from contaminated sites. For example, in an experiment in which two pentachlorophenol-degrading P. fluorescens strains were inoculated into soils from a polycyclic aromatic hydrocarbon- and pentachlorophenol-contaminated site, the strains either survived at a level lower than the inoculation level of 106 to 108 CFU/g of soil or vanished within the 50-day experimental period. Similar results were obtained with the uncontaminated control soil (5). Our findings thus show that the effects of artificial contamination protocols per se need to be critically evaluated.
In conclusion, the present study demonstrates toxic and in some cases irreversible effects of dichloromethane and acetone on soil bacteria and microfaunal populations. Experimental set-ups for the artificial contamination of soils involving the application of toxic solvents to the whole soil sample followed by inoculation of the sample with specific degrading organisms or communities are thus likely to result in overestimation of their degradation potential due to better survival than would be the case in soil contaminated in situ and possibly also to underestimation of the degradation potential of the indigenous microbial population.
Adding solvent and polycyclic aromatic hydrocarbons to only 25% of the total soil sample entails the risk of uneven distribution of the polycyclic aromatic hydrocarbons in the final soil microcosms. Thorough mixing of the amended and nonamended fractions of the soil samples is therefore important. The polycyclic aromatic hydrocarbons would probably still not cover all surfaces equally well, however, and the perfect means of artificially contaminating soil with polycyclic aromatic hydrocarbons and other compounds having a low water solubility remains to be found. Nevertheless, for studies involving living organisms, the protocol suggested here in which only a fraction of the soil sample is exposed to acetone is suitable, since it had only slight effects on the indigenous bacterial and microfaunal communities. This protocol could thus serve as a common protocol for the introduction of low-water-solubility compounds such as polycyclic aromatic hydrocarbons into natural soils in a manner that least affects the natural microbial population.
Acknowledgments
This work was supported by the EU 4th Environment and Climate Program, contract number ENV4 CT97-0617, and by the Center for Biological Processes in Contaminated Soil and Sediments (BIOPRO) under the Danish Environmental Research Program.
We thank François Bartoli of the Centre de Pédologie Biologique, Vandoeuvre-lès-Nancy, France, for sampling and characterization of the Bordeaux soils, Claus Jørgensen of the Water Quality Institute, Hørsholm, Denmark, for assistance with the gas chromatography, and Anita Løve Nielsen for technical assistance.
REFERENCES
- 1.Acea, M. J., C. R. Moore, and M. Alexander. 1988. Survival and growth of bacteria introduced into soil. Soil Biol. Biochem. 20:509-515. [Google Scholar]
- 2.Al-Bashir, B., T. Cseh, R. Leduc, and R. Samson. 1990. Effect of soil/contaminant interactions on the biodegradation of naphthalene in flooded soil under denitrifying conditions. Appl. Microbiol. Biotechnol. 34:414-419. [DOI] [PubMed] [Google Scholar]
- 3.Aprill, W., and R. Sims. 1990. Evaluation of the use of prairie grasses for simulating polycyclic aromatic hydrocarbon treatment in soil. Chemosphere 20:253-265. [Google Scholar]
- 4.Banerjee, D. K., P. M. Fedorak, A. Hashimoto, J. H. Masliyah, M. A. Pickard, and M. R. Gray. 1995. Monitoring the biological treatment of anthracene-contaminated soil in a rotating-drum bioreactor. Appl. Microbiol. Biotechnol. 43:521-528. [Google Scholar]
- 5.Blackburn, N. T., A. G. Seech, and J. T. Trevors. 1994. Survival and transport of lac-lux marked Pseudomonas fluorescens strain in uncontaminated and chemically contaminated soils. Syst. Appl. Microbiol. 17:574-580. [Google Scholar]
- 6.Boonchan, S., M. L. Britz, and G. A. Stanley. 2000. Degradation and mineralization of high-molecular-weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl. Environ. Microbiol. 66:1007-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byers, H. K., and L. I. Sly. 1993. Toxic effects of dichloromethane on the growth of methanotrophic bacteria. FEMS Microbiol. Ecol. 12:35-38. [Google Scholar]
- 8.Clarholm, M. 1981. Protozoan grazing of bacteria in soil impact and importance. Microb. Ecol. 7:343-350. [DOI] [PubMed] [Google Scholar]
- 9.Clarholm, M. 1989. Effects of plant-bacterial-amoebal interactions on plant uptake of nitrogen under field conditions. Biol. Fertil. Soils 8:373-378. [Google Scholar]
- 10.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekelund, F. 1996. Growth kinetics of five common heterotrophic soil flagellates. European J. Soil Biol. 32:15-24. [Google Scholar]
- 12.Ekelund, F. 1999. The impact of the fungicide fenpropimorph (Corbel) on bacterivorous and fungivorous protozoa in soil. J. Appl. Ecol. 36:233-243. [Google Scholar]
- 13.Ekelund, F., and R. Rønn. 1994. Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol. Rev. 15:321-353. [DOI] [PubMed] [Google Scholar]
- 14.Ekelund, F., R. Rønn, and S. Christensen. 1994. The effect of three different pesticides on soil protozoan activity. Pestic. Sci. 42:71-78. [Google Scholar]
- 15.Foissner, W. 1987. Soil protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Prog. Protistol. 2:69-212. [Google Scholar]
- 16.Gellert, G. 2000. Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol. Environ. Saf. 45:87-91. [DOI] [PubMed] [Google Scholar]
- 17.Genouw, G., F. De Naeyer, P. Van Meenen, H. Van De Werf, W. De Nijs, and W. Verstraete. 1994. Degradation of oil sludge by landfarming: a case-study at the Ghent harbour. Biodegradation 5:37-46. [Google Scholar]
- 18.Gould, W. D., C. Hagedorn, T. R. Bardinelli, and R. M. Zablotowitcz. 1985. New selective media for enumeration and recovery of fluorescent pseudomonads from various habitats. Appl. Environ. Microbiol. 49:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramss, G., K.-D. Voigt, and B. Kirsche. 1999. Degradation of polycyclic aromatic hydrocarbons with three to seven aromatic rings by higher fungi in sterile and unsterile soils. Biodegradation 10:51-62. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, B. S., K. Ritz, R. Bardgett, R. Cook, S. Christensen, F. Ekelund, S. J. Sørensen, E. Bååth, J. Bloem, P. C. de Ruiter, J. Dolfing, and B. Nicolardot. 2000. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: an examination of the biodiversity-ecosystem function relationship. Oikos 90:279-294. [Google Scholar]
- 21.Guerin, W. F., and G. E. Jones. 1988. Two-stage mineralization of phenanthrene by estuarine enrichment cultures. Appl. Environ. Microbiol. 54:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, J. I., S. Lontoh, and J. D. Semrau. 1999. Degradation of chlorinated and brominated hydrocarbons by Methylomicrobium album BG8. Arch. Microbiol. 172:393-400. [DOI] [PubMed] [Google Scholar]
- 23.Hoben, H. J., and P. Somasegaran. 1987. Comparison of the pour, spread and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl. Environ. Microbiol. 44:1246-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huertas, M.-J., E. Duque, S. Marques, and J. L. Ramos. 1998. Survival in soil of different toluene-degrading Pseudomonas strains after solvent shock. Appl. Environ. Microbiol. 64:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnsen, K., and P. Nielsen. 1999. Diversity of Pseudomonas strains isolated with King's B and Gould's S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy characterisation. FEMS Microbiol. Lett. 173:155-162. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen, C., J. Aamand, B. K. Jensen, S. D. Nielsen, and C. S. Jacobsen. 1995. Microbial properties governing the microbial degradation of polycyclic aromatic hydrocarbons, p. 178-192. In M. Moo-Young, W. A. Anderson, and A. M. Chakrabarty (ed.), Environmental biotechnology: principles and applications. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 27.Kästner, M., M. Breuer-Jammali, and B. Mahro. 1998. Impact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil. Appl. Environ. Microbiol. 64:359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kästner, M., and B. Mahro. 1996. Microbial degradation of polycyclic aromatic hydrocarbons in soil affected by the organic matrix of compost. Appl. Microbiol. Biotechnol. 44:668-675. [DOI] [PubMed] [Google Scholar]
- 29.Kiyohara, H., K. Nagao, and K. Yana. 1982. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl. Environ. Microbiol. 43:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kragelund, L., B. Christoffersen, O. Nybroe, and F. J. de Bruijn. 1995. Isolation of lux reporter gene fusions in Pseudomonas fluorescens DF57 inducible by nitrogen or phosphorus starvation. FEMS Microbiol. Ecol. 17:95-106. [Google Scholar]
- 31.Kragelund, L., and O. Nybroe. 1994. Culturability and expression of outer membrane proteins during carbon, nitrogen, or phosphorus starvation of Pseudomonas fluorescens DF57 and Pseudomonas putida DF14. Appl. Environ. Microbiol. 60:2944-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löser, C., H. Seidel, P. Hoffman, and A. Zehnsdorf. 1999. Bioavailability of hydrocarbons during microbial remediation of a sandy soil. Appl. Microbiol. Biotechnol. 51:111.. [DOI] [PubMed] [Google Scholar]
- 33.Maliszewska-Kordybach, B. 1993. The effect of temperature on the rate of disappearance of polycyclic aromatic hydrocarbons from soils. Environ. Pollut. 79:15-20. [DOI] [PubMed] [Google Scholar]
- 34.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Manilal, V. B., and M. Alexander. 1991. Factors affecting the microbial degradation of phenanthrene in soil. Appl. Microbiol. Biotechnol. 35:401-405. [DOI] [PubMed] [Google Scholar]
- 36.Møller, J., and H. Ingvorsen. 1993. Biodegradation of phenanthrene in soil microcosms stimulated by an introduced Alcaligenes sp. FEMS Microbiol. Ecol. 102:271-278. [Google Scholar]
- 37.Novotný, C., P. Erbanova, V. Sasek, A. Kubátová, T. Cajtham, E. Lang, J. Krahl, and F. Zadrazil. 1999. Extracellular oxidative enzyme production and polycyclic aromatic hydrocarbons removal in soil by exploratory mycelium of white rot fungi. Biodegradation 10:159-168. [DOI] [PubMed] [Google Scholar]
- 38.Page, F. C. 1988. A new key to freshwater and soil gymnamoebae. Freshwater Biological Association, Ambleside, United Kingdom.
- 39.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 40.Postma, J., C. H. Hok A Hin, and J. A. van Veen. 1990. Role of microniches in protecting introduced Rhizobium leguminosarum biovar trifolii against competition and predation in soil. Appl. Environ. Microbiol. 56:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Providenti, M. A., C. A. Flemming, H. Lee, and J. T. Trevors. 1995. Effect of addition of rhamnolipid biosurfactants or rhamnolipid-producing Pseudomonas aeruginosa on phenanthrene mineralization in soil slurries. FEMS Microbiol. Ecol. 17:15-26. [Google Scholar]
- 42.Ridgway, H. F., J. Safarik, D. Phipps, P. Carl, and D. Clark. 1990. Identification and catabolic activity of well-derived gasoline-degrading bacteria from a contaminated aquifer. Appl. Environ. Microbiol. 56:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roldan, A. T., and C. Pueyo. 1993. Mutagenic and lethal effects of halogenated methanes in the Ara test of Salmonella typhimurium: quantitative relationship with chemical reactivity. Mutagenesis 8:127-131. [DOI] [PubMed] [Google Scholar]
- 44.Rønn, R., F. Ekelund, and S. Christensen. 1995. Optimizing of soil extract and broth media for MPN-enumeration of naked amoebae and heterotrophic flagellates in soil. Pedobiologia 39:10-19. [Google Scholar]
- 45.Shuttleworth, K. L., and C. E. Cerniglia. 1997. Practical methods for the isolation of polycyclic aromatic hydrocarbon (PAH)-degrading microorganisms and the determination of PAH mineralization and biodegradation intermediates, p. 766-775. In C. J. Hurst, G. R. Knudsen, A. D. McLaren, L. D. Stetzenbach, and M. V. Walter (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 46.Wischmann, H., and H. Steinhart. 1997. The formation of polycyclic aromatic hydrocarbons oxidation products in soils and soil/compost mixtures. Chemosphere 35:1681-1698. [Google Scholar]
- 47.Wolk, C. P., Y. Cai, and J.-M. Panoff. 1991. Use of a transposon with luciferase as a reporter to identify environmentally responsive genes in a cyanobacterium. Proc. Natl. Acad. Sci. USA 88:5355-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]