Abstract
A mutant strain (39E H8) of Thermoanaerobacter ethanolicus that displayed high (8% [vol/vol]) ethanol tolerance for growth was developed and characterized in comparison to the wild-type strain (39E), which lacks alcohol tolerance (<1.5% [vol/vol]). The mutant strain, unlike the wild type, lacked primary alcohol dehydrogenase and was able to increase the percentage of transmembrane fatty acids (i.e., long-chain C30 fatty acids) in response to increasing levels of ethanol. The data support the hypothesis that primary alcohol dehydrogenase functions primarily in ethanol consumption, whereas secondary alcohol dehydrogenase functions in ethanol production. These results suggest that improved thermophilic ethanol fermentations at high alcohol levels can be developed by altering both cell membrane composition (e.g., increasing transmembrane fatty acids) and the metabolic machinery (e.g., altering primary alcohol dehydrogenase and lactate dehydrogenase activities).
Microorganisms such as Saccharomyces or Zymomonas strains that are used for industrial ethanol production from glucose or sucrose have high alcohol tolerance for growth (i.e., >6% [vol/vol]). Other species that produce ethanol from cheaper substrates such as cellulose or starch, like Clostridium thermocellum or Thermoanaerobacter ethanolicus, generally have a low alcohol tolerance for growth (<2% [vol/vol]). In general, alcohol-producing microbes respond to increasing solvent concentrations by increasing the percentage of unsaturated versus saturated fatty acids, long-chain fatty acids, and hopanes into their cytoplasmic membranes (2, 8, 9). These structural changes prevent the loss of membrane function from fluidization caused by a high solvent concentration.
Thermophilic ethanol fermentations offer the potential of direct degradation of cellulose or starch and direct recovery of ethanol at fermentation temperatures under reduced pressure (5, 16, 17, 18, 21, 23). This potential has not been demonstrated because of low-end product concentrations caused by bacterial ethanol inhibition. Thermophilic bacteria employ two different pathways for ethanol production, using either a primary alcohol dehydrogenase (ADH), as in C. thermocellum, or primary and secondary ADHs, as in T. ethanolicus (13, 15). Herrero and coworkers (3, 4) studied ethanol tolerance in C. thermocellum and concluded that the low tolerance to ethanol (<2% [vol/vol]) was a combined result of general solvent effects on membrane fluidity and a specific inhibition of enzymes involved in sugar metabolism. Work in the labs of Ljundahl, Wiegel, Demain, Zeikus, and others has showed that thermophilic anaerobic bacteria can adapt their tolerance to about 4% (vol/vol) ethanol (for a review, see reference 16).
We previously demonstrated (15) that moderate ethanol tolerance (<4% [vol/vol]) of a T. ethanolicus mutant strain was related to enzymatic prevention of metabolic inhibition caused by ethanol overreducing the pyridine nucleotide pool and inhibiting glycolysis. The ethanol-tolerant mutant 39EA lacked primary ADH, aldehyde-NAD reductase, and ferredoxin NAD(P) reductase activities that were found in the wild-type strain.
We later purified and characterized the primary ADH, secondary ADH, and acetaldehyde reductase (1) from wild-type cells and showed that the secondary ADH was very unique and could directly reduce acetyl coenzyme A (acetyl-CoA) to ethanol. Analysis of the enzymes' kinetic parameters led us (1) to hypothesize that the secondary ADH functions primarily to produce ethanol, whereas the primary ADH functions in ethanol consumption for nicotinamide cofactor recycling. Jung, Zeikus, and Hollingsworth also demonstrated (11) that T. ethanolicus contained a new family of very long α,ω-dicarboxylic acids as a major structural component of its membrane which might allow the organism to adapt to high temperature or solvent stress.
The purpose of the present investigation was to develop a mutant of T. ethanolicus with high ethanol tolerance (8% [vol/vol]) and to evaluate the physiological functions of the organism's unique transmembrane lipids and ADHs in relation to high alcohol tolerance.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals were of reagent grade or better. Gases were purchased from AGA Specialty Gases (Cleveland, Ohio), and oxygen was removed by passage through hot copper filings. Anaerobic work was performed inside a glove box (Coy Lab Products, Ann Arbor, Mich.). Acetyl-CoA (lot no. 72H7801) and coenzyme A (lot no. 20H7075) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Both were determined by the manufacturer to be free of aldehyde, alcohol, and ketone solvent contaminants. Porapak T and Super Q gas-liquid chromatography resins were obtained from Alltech Associates Inc. (Deerfield, Ill.). Protein concentrations were measured using the bicinchoninic acid (BCA) procedure (Pierce, Rockford, Ill.).
Organisms.
Cell cultivation and media preparation were performed under anaerobic conditions (20) with the specified headspace gases at 1 atm unless otherwise indicated. T. ethanolicus 39E (ATCC 33223) (1, 12), and a mutant strain derived from it, T. ethanolicus 39E-H8, were grown in tryptone-yeast extract-glucose (TYEG) medium (18) unless otherwise indicated.
T. ethanolicus 39E mutagenesis.
The 39E-H8 mutant strain was derived from strain 39E by a modification of existing chemical mutagenesis procedures (6). Exponential-phase T. ethanolicus 39E cells were treated with nitrosoguanidine as previously described (6). Treated cells were washed with TYE medium and then transferred to TYE medium with 0.5% starch and 350 mM ethanol. These cultures were incubated at 60°C for 36 h prior to plating on TYE-starch agar media. Plates were incubated anaerobically at 60°C using a modified paint tank (7). After 4 days, individual colonies were transferred to TYE medium containing 0.5% starch and 350 mM ethanol. This process yielded mutants resistant to 350 mM ethanol. This mutagenesis process was then repeated three times, increasing the ethanol concentration by 350 mM each time until the media contained 1.4 M ethanol. T. ethanolicus 39E-H8 was isolated from this final enrichment and grew in the presence of 1.4 M ethanol.
Batch cultures.
T. ethanolicus 39E and 39E-H8 cultures (10 ml) were grown under N2 at 60°C in 27-ml pressure-sealed tubes (Baxter, McGraw Park, Ill.). Glucose (27 mM unless otherwise indicated), ethanol, propan-2-ol, and propanone were added prior to media reduction and culture inoculation. Media were reduced with 0.06% (vol/vol) Na2S·9H2O added as 200 μl of 15% stock per 50 ml of medium just prior to culture inoculation. Exponential-phase cells used for inoculation (2% [vol/vol]) were transferred using Glaspak sterile syringes (Becton Dickinson and Co., Rutherford, N.J.) flushed with N2. Growth was measured in sealed tubes (11-mm path length) without liquid sampling by the change in optical density at 660 nm (OD660), measured using a Spectronic 20 spectrophotometer (Bausch & Lomb, Rochester, N.Y.). Media supplements never exceeded 10% of the total TYEG volume. Log-phase growth rates (μ) were derived from the OD data using the standard equation ln(X/X0) = (μ)(t), where X is the measured culture OD, X0 is the initial culture OD, and t is the elapsed time. All growth rate values represent the average of at least three replicates, with the errors reported as standard deviations.
Preparation of cell extracts.
Cells were pelleted and resuspended (1g [wet weight] to 5 ml of 50 mM Tris-HCl [pH 7.0]). Lysozyme (0.1 mg/ml), Triton X-100 (0.1% [vol/vol]), and 0.01 mg of DNase I/ml were added to the cell suspension, and this suspension was incubated at room temperature for 30 min. All buffers were degassed prior to use, stored under N2, and prepared in an anaerobic glove bag. Centrifugation steps were performed in polycarbonate tubes with gas-tight seals at 15,000 × g, and supernants were stored at 4°C in pressure vials under N2.
Enzyme assays.
The standard assay for secondary ADH activity was established as the reduction of NADP+ during the oxidation of butan-2-ol at 60°C under anaerobic conditions. One unit of activity was defined as the amount of enzyme which reduces 1 μmol of NADP+ (with butan-2-ol as a substrate) per min under the above conditions. The reaction mixture (total volume, 1 ml) contained 50 mM Tris-HCl buffer (pH 8.0; adjusted to temperature). Enzyme solutions were maintained at 40°C prior to addition to the assay solution at 60°C. The assay buffer solution was maintained at 60°C in a constant-temperature water bath. The reaction progress in all cases was measured as the loss of absorbance of NADPH upon oxidation to NADP+ or as the gain in absorbance of NADP+ upon reduction to NADPH at 340 nm (extinction coefficient = 6.22 mM−1 cm−1) using a Cary model 219 spectrophotometer. Primary ADH activity was determined by NADH oxidation during the reduction of ethanal. One unit of primary ADH activity was defined as the amount of enzyme which reduces 1 μmol of NAD (with ethanol as the substrate) per min at 60°C under anaerobic conditions in 1 ml of 50 mM Tris-HCl (pH 8.0). Enzyme activities were not detected in the presence of cofactor without substrate addition. In all cases, cofactor and substrate concentrations were 10 times the apparent Km value (1). Error values are reported as the larger of either the standard error of measurement or the standard deviation among measurements.
Fermentation product analysis.
Aqueous samples (2.5 ml) were extracted from pressure-sealed vials using a 5-ml syringe. Subsamples (1.25 ml) were placed in 1.5-ml Eppendorf tubes and centrifuged for 5 min at 14,000 × g. Portions (1.0 ml) of the clarified supernatants were transferred to fresh Eppendorf tubes, acidified with 0.1 ml of 10 N phosphoric acid, and then recentrifuged. Supernatants from these samples were transferred to 1-ml gas chromatography (GC) sample vials (Alltech Associates Inc.) for analysis. Soluble fermentation products were quantitated by gas-liquid chromatography on a Super Q column in a Hewlett-Packard model 5890 gas chromatograph (Avondale, Pa.) under conditions previously described (20). Propanone was separated from propan-2-ol by GC analysis using a Porapak-T column with the Hewlett-Packard 5890 gas chromatograph. The Porapak-T separation was performed at 160°C with 25 ml of N2 as carrier gas/min. The injector temperature was 180°C and the detector temperature was 190°C. Samples were analyzed by flame ionization.
Gaseous products were sampled from the culture headspace using 1-ml Glaspak syringes fitted with gas-tight valves (Alltech Associates Inc.). Methanol was quantitated from 0.4-ml samples with a Gow-Mac model 750 gas-liquid chromatograph (Bridgewater, N.J.) under conditions previously described (20). The methods used to purify and analyze membrane fatty acids were as we have described previously (11).
RESULTS
Solvent tolerance.
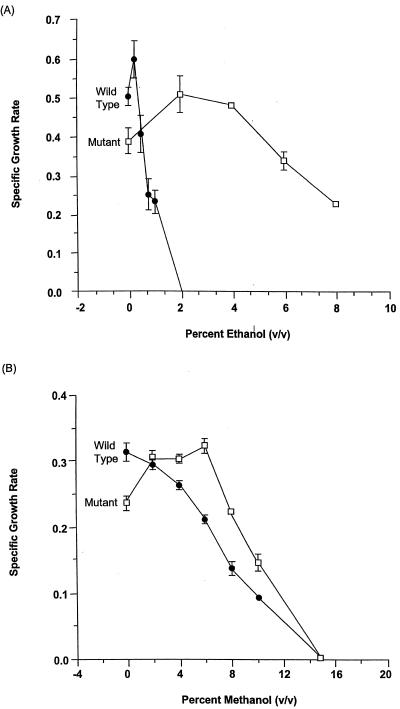
To identify physiological factors involved in ethanol inhibition of T. ethanolicus, the mutant strain 39E-H8 was developed. It was isolated from the wild-type strain after four successive incubations with nitrosoguanidine followed by enrichment in TYEG medium containing successively higher ethanol concentrations. Figure 1A compares the effect of increasing ethanol concentration on the growth rate of the wild-type 39E strain versus that of the mutant 39E-H8 strain. The mutant strain was capable of sustained growth in ethanol concentrations up to 8% (vol/vol). Multiple serial transfers of 39E-H8 cultures in medium containing 8% ethanol had no effect on culture growth, whereas growth of the wild type was stopped at concentrations above 1.5%. Figure 1B compares the quite different effect of increasing methanol concentration on the growth rate of the two strains. Methanol was selected as a solvent because it is neither metabolized by these strains nor is it a substrate of the primary or secondary ADH. Both strains were much more tolerant of increasing methanol concentration, and the mutant strain had a 50% inhibitory concentration (IC50) of 10% (vol/vol) methanol, which was higher than the IC50 of 8% measured for the wild-type strain. Notably, the maximal growth rate of the mutant strain required either 2 to 6% methanol or 2% ethanol, in contrast to the wild-type strain, which grew optimally in the absence of added solvent. Neither abnormal growth lag phases nor log phases were observed for either strain under the conditions tested.
FIG. 1.
Effect of alcohol concentration on T. ethanolicus wild-type strain 39E versus mutant strain 39E-H8 growth rates in the presence of ethanol (A) or methanol (B).
Because ethanol and methanol are similar polar solvents, their different effects on the growth rate of T. ethanolicus strains would seem to indicate that metabolic or regulatory properties and not just cell structural differences account for some of the IC50 value differences observed for ethanol and methanol. Growth and inhibition of T. ethanolicus by ethanol have been linked to overreduction of the nicotinamide cofactor pool (15). Consequently, the effect of solvents which are metabolized by its unique secondary ADH (1) were tested on growth of the T. ethanolicus wild-type versus mutant 39E-H8 strain (Table 1.) It was proposed that propanone when added to glucose-grown cells may rescue end product inhibition of cell growth due to ethanol by acting as an alternative electron acceptor for nicotinamide cofactor oxidation (15). Table 1 shows that growth of the wild-type strain was significantly inhibited by 130 mM (0.75%, vol/vol) ethanol addition but significantly stimulated by 50 mM propanone or a mixture of 130 mM ethanol-50 mM propan-2-ol, whereas 50 mM propan-2-ol alone had no effect on growth. However, growth of the mutant strain 39E-H8 was significantly stimulated by addition of all the solvent combinations tested. Consequently, growth inhibition for wild-type strain 39E is specific to ethanol, whereas growth of the mutant 39E-H8 is stimulated by all solvents tested. The enhanced mutant strain growth rate in propan-2-ol relative to no solvent suggests that the mutant may also have a structurally altered membrane that accounts for its higher tolerance to solvents than the wild type.
TABLE 1.
Effect of solvent addition on the growth rate of T. ethanolicus wild-type versus mutant strains
| Strain | Growth rate (μ)a in the presence of solvent (concn [mM])
|
||||
|---|---|---|---|---|---|
| None | Ethanold | Ethanol, propanone | Propanone | Propan-2-ol | |
| Wild typeb (39E) | 0.52 ± 0.01 | 0.17 ± 0.02 (130) | 0.90 ± 0.01 (130, 50)e | 1.0 ± 0.01 (50) | 0.50 ± 0.01 (50) |
| Mutantc (39E-H8) | 0.27 ± 0.02 | 0.59 ± 0.02 (770) | 0.54 ± 0.01 (770, 50) | 0.55 ± 0.03 (50) | 0.52 ± 0.05 (50) |
Mean ± standard deviation.
All cells were grown under nitrogen in 10 ml of TYEG culture medium with a 15-ml headspace.
Growth at 65°C was monitored by absorbance at 660 nm. All values represent averages of at least three separate cultures.
Ethanol concentrations used (130 mM [0.75%, vol/vol] for the wild type and 770 mM [4.5%, vol/vol] for the mutant) were in the middle of the respective tolerance ranges of the strains.
The first value corresponds to the ethanol concentration; the second value corresponds to the propanone concentration.
Membrane lipid composition and alcohol metabolism.
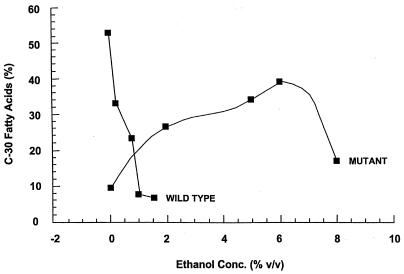
The membrane of T. ethanolicus contains a new family of very-long-chain α, ω-dicarboxylic acids (11). We examined if the synthesis of these unique lipids in the wild type versus ethanol-tolerant mutant differed in response to growth at a range of ethanol concentrations. Figure 2 shows that the amount of C30 fatty acid lipids in the wild type decreased from about 55 to 5% of total, at 0 versus 1.5% ethanol, whereas in the ethanol-tolerant mutant the amount of C30 fatty acid lipids increased from about 10 to 40% at 0 versus 6% added ethanol. This suggests that the regulation of long-chain fatty acid synthesis is related to high alcohol tolerance. Table 2 compares the levels of primary ADH, secondary ADH, and ALDH expressed in the wild-type and the ethanol-tolerant mutant strains. The secondary ADH is unique in T. ethanolicus because it reduces acetyl-CoA directly to ethanol (a primary alcohol), and the organism does not make any secondary alcohols when fermenting glucose alone. The enzyme levels are reported for cells grown on TYEG medium in batch culture; however, the same activity levels were observed in continuous culture (data not shown). The mutant differed from the wild-type strain in having higher levels of the secondary ADH and in lacking detectable levels of primary ADH. This finding lead us to examine if the primary ADH functions in ethanol consumption as previously hypothesized (1).
FIG. 2.
Comparison of T. ethanolicus strain 39E versus 39E-H8 transmembrane fatty acid levels produced during growth in the presence of increasing ethanol levels.
TABLE 2.
Alcohol-metabolizing enzyme levels in T. ethanolicus wild type versus mutant strainsa
| Enzyme | Specific enzyme activity (U/mg of protein [mean ± SD])
|
|
|---|---|---|
| Wild type (39E) | Mutant (39E-H8) | |
| ALDHb | 0.32 ± 0.02 | 0.27 ± 0.02 |
| Primary ADHc | 0.26 ± 0.01 | NDe |
| Secondary ADHd | 1.7 ± 0.09 | 2.8 ± 0.18 |
Cultures were grown at 60°C in TYE with 10 mM glucose and all extracts were prepared anaerobically.
ALDH activity was measured anaerobically by the oxidation of NADH during acetyl-CoA reductive thioester cleavage.
Primary ADH activity was measured anaerobically by the oxidation of NADH during ethanol reduction to ethanol.
Secondary ADH activity was measured anaerobically by the reduction of NADP+ during butan-1-ol oxidation. The corresponding ethanol-reducing activity, reported here, was calculated based on the ratio of 1.75, for ethanol reduction-to-butan-1-ol oxidation, determined for the purified enzyme (1).
ND, no activity detected.
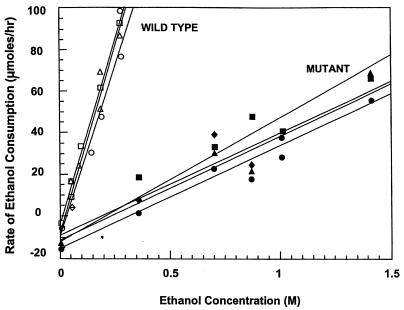
Figure 3 compares the rate of ethanol consumption by wild-type versus mutant cells. Wild-type cells, which contain primary ADH, consumed ethanol at a significantly higher rate than the ethanol-tolerant mutant, which lacked primary ADH. Table 3 compares the amount of ethanol consumed by wild-type versus ethanol-tolerant strains in the presence versus the absence of propanone, an exogenous electron acceptor reduced by the secondary ADH. In the absence of propanone, wild-type cells consumed four times more ethanol than the mutant strain at a sixfold-lower level of added ethanol. When propanone was added in the presence of ethanol, both the mutant and the wild type consumed the same amount of propanone and ethanol. The propan-2-ol generated accounted for all of the propanone consumed by both strains. Controls with neither ethanol nor propanone showed that the mutant strain makes greater than twofold more lactate than does the wild-type strain. Lactate production by both the wild-type and the mutant strains was also significantly reduced in the presence of solvents.
FIG. 3.
Effect of exogenous ethanol addition on the rates of ethanol consumption by T. ethanolicus strains 39E and 39E-H8.
TABLE 3.
Effect of solvent addition on end product formation in T. ethanolicus wild-type versus ethanol-tolerant mutant strainsa
| Solvent added (mM) and strain | Substrate or product amount (μmol)b
|
||||
|---|---|---|---|---|---|
| Propanone consumed | Ethanol consumed | Acetate produced | Lactate produced | Propanol produced | |
| Controls (none) | |||||
| Wild type 39E | 8.0 ± 0.6 | 6.5 ± 0.6 | |||
| Mutant 39E H8 | 6.8 ± 0.8 | 14 ± 1.0 | |||
| Ethanol | |||||
| Wild type 39E (130) | 26 ± 5.0 | 4.7 ± 0.5 | 0.54 ± 0.04 | ||
| Mutant 39E H8 (770) | 6.0 ± 2.0 | 2.6 ± 0.2 | 0.74 ± 0.06 | ||
| Ethanol + propanone | |||||
| Wild type 39E (130, 50) | 38 ± 2.0 | 38 ± 1.0 | 20 ± 0.7 | 0.30 ± 0.02 | 38 ± 0.08 |
| Mutant 39E H8 (770, 50) | 40 ± 0.7 | 38 ± 6.0 | 25 ± 3.0 | 0.46 ± 0.02 | 40 ± 0.07 |
Cultures were examined at the end of growth in 10 ml of TYEG medium in anaerobic test tubes. The wild-type and mutant control cultures consumed 12 and 14 μmol of glucose, respectively.
Data are means ± standard deviations.
DISCUSSION
We provide evidence here that ethanologenic thermophiles can display high tolerance to alcohols, including 8% (vol/vol) ethanol. In order for this to occur in T. ethanolicus the organism requires the ability to synthesize transmembrane lipids at high ethanol concentrations and must lose its primary ADH to prevent ethanol consumption. These results suggest that the utility of thermophilic ethanol fermentations should be reexamined with mutated or genetically engineered strains.
It appears that the C30 fatty acids may be required for high ethanol tolerance in T. ethanolicus because increasing ethanol concentrations were associated with high levels of C30 fatty acids in the ethanol-tolerant mutant and very low levels of these fatty acids were detected in the wild-type strain at moderate ethanol concentrations (i.e., 1.5%). The drop in transmembrane lipid concentration seen for strain 39E-H8 at 8% ethanol may reflect a secondary, nonlethal effect of high ethanol concentrations on membrane composition. Nonetheless, these findings support the hypothesis that transmembrane lipids are involved in alcohol tolerance, as first reported for Sarcina ventriculi (10). S. ventriculi overcomes the increase in membrane fluidity caused by increasing solvent or temperature by increasing the ratio of very-long-chain fatty acids to regular fatty acids (10-14).
Alcohol metabolism by the highly ethanol-tolerant strain 39E-H8 was similar to that reported previously for the moderately ethanol-tolerant mutant 39EA in that both mutants lacked primary ADH. The rate of ethanol consumption was drastically reduced in the mutant 39E-H8 versus that in wild-type strain 39E. Notably, propanone addition dramatically increased both the rate of ethanol consumption and the growth rate of the wild-type strain, whereas the growth rate of 39E-H8 was not altered. This suggests that the metabolic production of electron acceptors is limiting growth in the wild type but not in the mutant. These findings support the hypothesis presented elsewhere (1), based on enzyme kinetic analysis, that the primary ADH functions in alcohol consumption preventing growth at moderate or high ethanol concentrations. Thus, the primary ADH is believed to be involved in maintaining the redox balance between the NAD(P)(H) cofactor pools.
By and large, the results presented here suggest that the practicality of thermophilic ethanol fermentations should be reevaluated because of their newly demonstrated high tolerance to ethanol (8% [vol/vol]) (see reference 16). New mutants of T. ethanolicus that can synthesize long-chain fatty acids but that do not make lactate should be developed and examined for ethanol production. Perhaps these strains could make high enough ethanol concentrations to be recovered by conventional distillation technology or by vacuum fermentation recovery techniques. Alternatively, the genes for transmembrane fatty acid synthesis appear to be a target for metabolically engineering C. thermocellum to enable the direct fermentation of cellulose into practical concentrations of ethanol.
Acknowledgments
We gratefully acknowledge Maris Laivenicks for his assistance in all aspects of this work and John Kemner for helpful discussions.
This research was supported by a grant from the Cooperative State Research Service, U.S. Department of Agriculture, under the agreement 94-34189-0067.
REFERENCES
- 1.Burdette, D. S., and J. G. Zeikus. 1994. Purification of acetaldehyde dehydrogenase and alcohol dehydrogenases from Thermoanaerobacter ethanolicus 39E and characterization of the secondary alcohol dehydrogenase (2° Adh) as a bifunctional alcohol dehydrogenase-acetyl-CoA reductive thioesterase. Biochem. J. 302:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Amore, T., C. J. Panchal, I. Russell, and G. G. Stewart. 1990. A study of ethanol tolerance in yeast. Crit. Rev. Biotechnol. 9:287-304. [DOI] [PubMed] [Google Scholar]
- 3.Herrero, A. A., and R. F. Gomez. 1980. Development of ethanol tolerance in Clostridium thermocellum: effect of growth temperature. Appl. Environ. Microbiol. 40:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrero, A. A., R. F. Gomez, and M. F. Roberts. 1982. Ethanol induced changes in the membrane lipid composition of C. thermocellum. Biochem. Biophys. Acta 693:195-204. [DOI] [PubMed] [Google Scholar]
- 5.Hyun, H. H., and J. G. Zeikus. 1985. Simultaneous and enhanced production of thermostable amylases and ethanol from starch by co-cultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl. Environ. Microbiol. 49:1174-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyun, H. H., and J. G. Zeikus. 1985. Regulation and genetic enhancement of β-amylase production in Clostridium thermosulfurogenes. J. Bacteriol. 164:1163-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyun, H. H., and J. G. Zeikus. 1985. General biochemical characterization of thermostable extracellular β-amylase from Clostridium thermosulfurogenes. Appl. Environ. Microbiol. 49:1162-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram, L. O. 1976. Adaptation of membrane lipids to alcohols. J. Bacteriol. 125:670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram, L. O. 1990. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 9:305-319. [DOI] [PubMed] [Google Scholar]
- 10.Jung, S., E. S. Lowe, R. I. Hollingsworth, and J. G. Zeikus. 1993. Sarcina ventriculi synthesizes very long chain dicarboxylic acids in response to different forms of environmental stress. J. Biol. Chem. 268:2828-2835. [PubMed] [Google Scholar]
- 11.Jung, S., J. G. Zeikus, and R. I. Hollingsworth. 1994. A new family of very long chain α, ω-dicarboxylic acids is a major structural fatty acyl component of membrane lipids of Thermoanaerobacter ethanolicus 39E. J. Lipid Res. 35:1057-1065. [PubMed] [Google Scholar]
- 12.Lamed, R., and J. G. Zeikus. 1980. Ethanol production by thermophilic bacteria: relationship between fermentation product yield and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J. Bacteriol. 144:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, J. S., S. Jung, S. Lowe, J. G. Zeikus, and R. I. Hollingsworth. 1998. A dynamically regulated transformation of a bacterial bilayer membrane to a cross-linked 2-dimensional sheet during adaptation to unfavorable environmental pressures. J. Am. Chem. Soc. 29:5855-5863. [Google Scholar]
- 14.Lee, Y.-E., M. K. Jain, C. Lee, S. E. Lowe, and J. G. Zeikus. 1993. Taxonomic distinction of saccharolytic thermophilic anaerobes; description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E 100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobium thermosulfurigenes comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int. J. Syst. Bacteriol. 43:41-45. [Google Scholar]
- 15.Lovitt, R. W., G.-J. Shen, and J. G. Zeikus. 1988. Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J. Bacteriol. 170:2809-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovitt, R. W., B. H. Kim, and J. G. Zeikus. 1988. Solvent production by microorganisms. Crit. Rev. Biotechnol. 7:107-186. [Google Scholar]
- 17.Lowe, S. E., M. K. Jain, and J. G. Zeikus. 1993. Biology, ecology, and biotechnological applications of anaerobic bacteria adapted to environmental stresses in temperature, pH, salinity or substrates. Microbiol. Rev. 57:451-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng, T. K., A. Ben-Bassat, and J. G. Zeikus. 1981. Ethanol production by thermophilic bacteria: fermentation of cellulosic substrates by cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl. Environ. Microbiol. 41:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segel, I. H. 1976. Biochemical calculations, 2nd ed., p. 414-415. John Wiley & Sons, Inc., New York, N.Y.
- 20.Zeikus, J. G., P. W. Hegge, and M. A. Anderson. 1979. Thermoanaerobacterium brockii gen. nov. and sp. nov., a new chemoorganotrophic, caldoactive anaerobic bacterium. Arch. Microbiol. 122:41-48. [Google Scholar]
- 21.Zeikus, J. G. 1979. Thermophilic bacteria: ecology, physiology, and technology. Enzyme Microb. Technol. 1:243-252. [Google Scholar]
- 22.Zeikus, J. G., A. Ben-Bassat, and P. W. Hegge. 1980. Microbiology of methanogenesis in thermal, volcanic environments. J. Bacteriol. 143:432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeikus, J. G., A. Ben-Bassat, T. K. Ng, and R. J. Lamed. 1981. Thermophilic ethanol fermentation. Arch. Microbiol. 128:441-461. [DOI] [PubMed] [Google Scholar]