Abstract
Genomic DNAs from human Cryptosporidium isolates previously typed by analysis of the 18S ribosomal DNA locus (Cryptosporidium parvum bovine genotype, C. parvum human genotype, Cryptosporidium meleagridis, and Cryptosporidium felis) were used to amplify the diagnostic fragment described by Laxer et al. (M. A. Laxer, B. K. Timblin, and R. J. Patel, Am. J. Trop. Med. Hyg., 45:688-694, 1991). The obtained 452-bp amplified fragments were sequenced and aligned with the homologous Cryptosporidium wrairi sequence. Polymorphism was exploited to develop a restriction fragment length polymorphism method able to discriminate Cryptosporidium species and C. parvum genotypes.
Cryptosporidium spp. are Apicomplexan parasites that infect the gastrointestinal or respiratory tract of humans and animals. In immunocompetent hosts, the infection is typically acute and self limiting, whereas in immunocompromised individuals, such as persons receiving immunosuppressive drugs and AIDS patients, cryptosporidiosis is often a chronic disease. Since drug therapy to control or eliminate these organisms is not yet available, persistent infections in these patients are therefore especially severe and can be life threatening. The potential of Cryptosporidium as an opportunistic parasite and the recent reports of major outbreaks of cryptosporidiosis in the United States, United Kingdom, and Australia due to contamination of drinkable water supplies indicate that Cryptosporidium should be considered a major public health problem (12, 25).
To date, eight Cryptosporidium species have been regarded as valid on the basis of host specificity, pathogenesis, and oocyst morphology (13). These included Cryptosporidium parvum in mammals, Cryptosporidium muris in rodents and ruminants, Cryptosporidium felis in domestic cats, Cryptosporidium wrairi in guinea pigs, Cryptosporidium baileyi and Cryptosporidium meleagridis in birds, Cryptosporidium serpentis in reptiles, and Cryptosporidium nasorum in fishes. According to this classification, the causative agent of cryptosporidiosis in humans and a range of mammalian species is the species C. parvum. Numerous PCR-based assays have been described for detection of Cryptosporidium parasites. The primers of these PCR assays are based on either undefined genomic sequences (2, 3, 22, 27, 46) or specific genes (4, 27-29, 35, 40, 41, 43, 45, 48, 49). Most PCR assays have led to the confirmation of C. parvum as the major cause of cryptosporidiosis in humans and to the identification of two genotypes within this species: the “human” genotype (genotype 1), which has so far been found exclusively in humans, with the exception of a single nonhuman primate (39) and a dugong (33), and the “bovine” genotype (genotype 2), found in domestic livestock such as cattle, sheep, and goats, etc., which can also infect humans. Additional genotypes have then been distinguished in C. parvum (32). However, most of the available genotyping tools were designed to analyze clinical specimens, and their specificities for other C. parvum genotypes or other Cryptosporidium species were not always established.
Laxer et al. were among the first authors to publish primers for detection of Cryptosporidium (22). These primers, which were not known to target a specific gene but to amplify a 452-bp fragment of an unidentified region, as well as the reported 452-bp sequence, have been widely used (1, 6-11, 14, 15, 17, 18, 20, 21, 23, 37, 38). The purpose of the present study was to investigate the extent of sequence heterogeneity for this diagnostic DNA fragment among human isolates of Cryptosporidium.
Sample analysis.
Fecal samples used in this study were obtained from infected bovine (named B1 isolate) or humans (named H isolates) (Table 1) and were identified as genotype 1 of C. parvum, genotype 2 of C. parvum, C. meleagridis, and C. felis, on the basis of 18S ribosomal DNA (rDNA) (16). Genomic DNA samples were prepared as described before (16) and were stored at −20°C until they were used. The 452-bp Laxer fragment was amplified by PCR using a pair of 26-mer primers previously reported (22). The reaction mixtures were prepared in 1× PCR buffer (75 mM Tris [pH 9], 20 mM [NH4]2SO4, and 0.01% Tween 20) and contained per 50-μl reaction 2 mM MgCl2, 0.5 μM concentrations of both primers (Eurogentec, Seraing, Belgium), a 200 μM concentration of each deoxynucleoside triphosphate, 1 U of DNA Goldstar Polymerase (Eurogentec), and 10 μl of the purified DNA at the 1/10 dilution. A negative control, consisting of a reaction mixture with water instead of DNA template, was included in each amplification run. The amplification reactions were initiated by denaturation of the DNA at 94°C for 5 min and were then subjected to 40 cycles of denaturation at 94°C for 30 s, annealing of the primer at 50°C for 30 s, and extension at 72°C for 30 s, with an additional 5-min extension at 72°C (PTC 200 thermocycler; MJ Research, Prolabo, France). The PCR product was analyzed by electrophoresis in a 2% agarose gel and was visualized after ethidium bromide staining. Amplified products were sequenced in both directions on an ABI 377 automated sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.).
TABLE 1.
Isolates and genotypes of Cryptosporidium used in this study
| Isolate codea | HIV statusc | Sourceb | Geographical location | Species and genotype by analysis of
|
|
|---|---|---|---|---|---|
| 18S rDNA locus | Laxer locus | ||||
| C1 | INRAT | France | Cattle | Cattle (L1 subgenotype) | |
| H1 | Neg. | CHD | France | Cattle | Cattle (L1 subgenotype) |
| H5 | Neg. | CHD | France | Cattle | Cattle (L1 subgenotype) |
| H4 | Neg. | CHRUL | France | Human | Human |
| H7 | Pos. | HSLP | France | Human | Human |
| H8 | Pos. | HSLP | France | Human | Human |
| H9 | Pos. | HSLP | France | Human | Human |
| H11 | Pos. | HSLP | France | C. meleagridis | C. meleagridis |
| H14 | Pos. | HSLP | France | Cattle | Cattle (L2 subgenotype) |
| H34 | Pos. | HSLP | France | C. meleagridis | C. meleagridis |
| H37 | Neg. | HSLP | France | Human | Human |
| H67 | Pos. | HSLP | France | C. felis | No amplification |
| H78 | Pos. | HSLP | France | C. felis | No amplification |
| H81 | Neg. | HSLP | France | C. felis | No amplification |
| H83 | Pos. | HSLP | France | C. felis | No amplification |
| H16 | Neg. | HHMC | France | Cattle | Cattle (L1 subgenotype) |
| H15 | Pos. | CHRUR | France | Cattle | Cattle (L2 subgenotype) |
| H17 | Neg. | CHUA | France | C. meleagridis | C. meleagridis |
| H18 | Pos. | CHUN | France | Cattle | Cattle (L2 subgenotype) |
| H22 | Pos. | CHUN | France | C. felis | Cattle (L2 subgenotype) |
| H53 | Neg. | CHRUN | France | Cattle | Cattle (L1 subgenotype) |
| H43 | Pos. | GHESKIO | Haiti | C. felis | Cattle (L2 subgenotype) |
C, cattle isolate; H, human isolate.
INRAT, Institut National de Recherche Agronomique de Tours; CHD, Centre Hospitalier de Dunkerque; CHRUL, Centre Hospitalier Régional Universitaire de Lille; HSLP, Hôpital Saint Louis de Paris; HHMC, Hôpital Henri Mondor de Créteil; CHRUR, Centre Hospitalier Régional Universitaire de Rennes; CHUA, Centre Hospitalier Universitaire d'Angers; CHUN, Centre Hospitalier Universitaire de Nantes; CHRUN, Centre Hospitalier Régional Universitaire de Nice; and GHESKIO, Groupe Haïtien d'Etudes sur le Sarcome de Kaposi et des Infections Opportunistes.
HIV, human immunodeficiency virus; Neg., negative; Pos., positive.
As our sequences, when aligned against the homologous C. wrairi sequence, showed polymorphism, a restriction fragment length polymorphism (RFLP)-based assay was developed. Purified PCR product was digested in a 20-μl mixture consisting of 1 U of MwoI (New England Biolabs, Beverly, Mass.), 1 U of MluI (New England Biolabs), 1 U of BpmI (New England Biolabs), 0.2 μl of 100× bovine serum albumin, and 2 μl of the appropriate 10× restriction buffer (NE buffer 3) under the conditions recommended by the supplier. The digestion mixture was incubated at 37°C for 2 h followed by 60°C for 2 h. The digest products were fractioned by 2% agarose gel electrophoresis and were visualized by ethidium bromide staining.
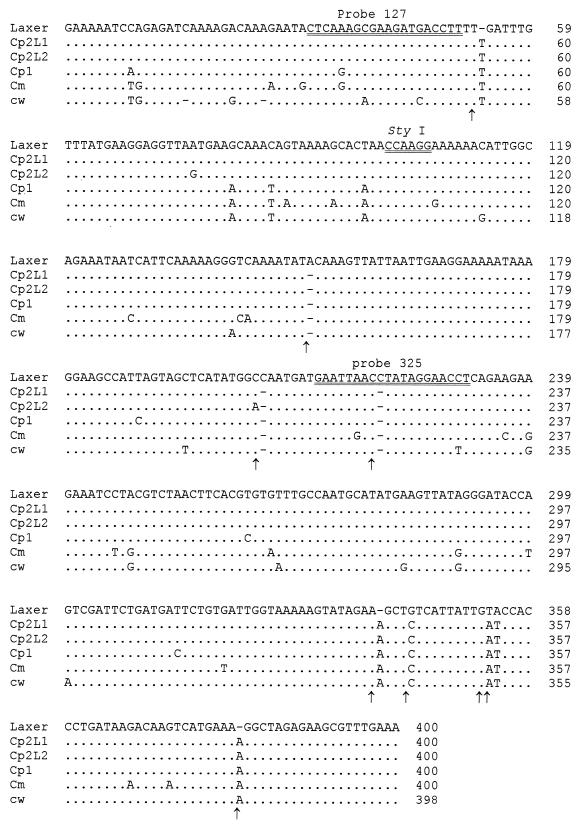
The DNA diagnostic fragment characterized by Laxer et al. (22) was amplified by PCR from 1 bovine and 21 human isolates of Cryptosporidium previously typed by analysis of the 18S rDNA locus (Table 1). The expected size of the amplified product was 452 bp, and all isolates except C. felis isolates produced a single amplicon. PCR products were sequenced on both strands, and a multiple alignment was performed with the obtained sequences (Fig. 1). The alignment obtained with our sequences defined four groups of genotypes: one of these matched genotype 1, while another matched C. meleagridis. The third and fourth ones matched genotype 2. Two subgenotypes for genotype 2 of C. parvum were therefore found at the Laxer locus. They were designated L1 and L2 subgenotypes. Alignment against the original Laxer sequence (22) (accession number M59419) revealed other sequence variations (Fig. 1). Nine common bases of the newly reported sequences were different from the one previously reported. This included three insertions (T at position 54, A at position 341, and A at position 383), three deletions (at positions 152, 206 and 221), a single base mutation (G to C change at position 345) and a 2-base mutation (TA-to-AT change at positions 355 and 356). Most differences between Laxer's sequence and ours did not occur at critical internal diagnostic sites (probe or restriction site). However, two sequences out of the four reported here had a substitution at probe 127 and all had a deletion at probe 325 compared to Laxer's sequence (Fig. 1).
FIG. 1.
Sequence alignment of the original sequence obtained by Laxer et al. (22) (Laxer), the C. wrairi sequence reported by Chrisp and Legendre (6) (cw), and the Cryptosporidium PCR diagnostic fragments obtained in this study for C. parvum genotype 2 (L1 subgenotype [Cp2L1] and L2 subgenotype [Cp2L2]), C. parvum genotype 1 (Cp1), and C. meleagridis (Cm). Dots denote nucleotides identical to those from the Laxer sequence, and dashes indicate deletions. Black arrows indicate mismatched nucleotides or gaps with the original sequence reported by Laxer. Double-underlined nucleotides on the sequence of Laxer et al. correspond to diagnostic probe 127, a StyI restriction site, and diagnostic probe 325. Numbering is arbitrary.
The four sequences presented here were also aligned with the C. wrairi sequence reported by Chrisp and LeGendre (6) (Fig. 1). DNA heterogeneity was exploited to identify polymorphic restriction enzyme sites in order to develop an RFLP assay for assessing polymorphisms. Genotyping was made by performing a triple digestion with MwoI, MluI, and BpmI. The sizes of the restriction fragments are given in Table 2. RFLP profiles (Fig. 2) were confirmed on 28 other human isolates: 12 were identified as genotype 1, while 16 were identified as genotype 2 (10 L1 and 6 L2 subgenotypes) (results not shown). All were in agreement with typing at the 18S rRNA gene locus (16).
TABLE 2.
RFLP (in base pairs) in the 452-bp PCR fragment of various Cryptosporidium organisms
| Species | Sizes of restriction fragments
|
|||
|---|---|---|---|---|
| Single digestion
|
Triple digestion | |||
| MwoI | MluI | BpmI | ||
| C. parvum cattle L1 subgenotype | 226, 188, 38 | 226, 188, 38 | ||
| C. parvum cattle L2 subgenotype | 414, 38 | 414, 38 | ||
| C. parvum human genotype | 226, 188, 38 | 284, 168 | 226, 130, 58, 38 | |
| C. meleagridis | 226, 188, 38 | 397, 55 | 188, 171, 55, 38 | |
| C. wrairi | 412, 38 | 395, 55 | 357, 55, 38 | |
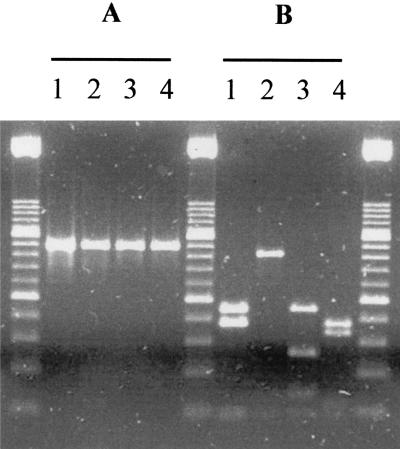
FIG. 2.
Ethidium bromide-stained agarose gel of the 452-bp PCR product amplified from C. parvum genotype 2/L1 subgenotype (lane 1), C. parvum genotype 2/L2 subgenotype (lane 2), C. parvum genotype 1 (lane 3), and C. meleagridis (lane 4) before (A) and after (B) triple digestion with MwoI, MluI, and BpmI. Size markers are DNA molecular weight marker XIII (Boehringer Mannheim).
In the present study, six human isolates identified as C. felis on the basis of 18S rDNA sequences were included. A 452-bp fragment was amplified from two isolates (H22 and H43). For both, sequencing revealed a genotype matching genotype 2 (L2 subgenotype). By using Laxer's primers, we did not succeed in obtaining a positive PCR result from the DNA of the four remaining isolates, in spite of repeated attempts and in the absence of inhibitor.
Detection of Cryptosporidium spp. by Laxer's PCR-based assay.
Numerous PCR methods for the detection of Cryptosporidium have been reported (12, 31, 47). The method of Laxer et al., which uses primers specific for an unidentified region, was among the first to be published (22). In their study, the authors selected a clone containing a 2.3-kb insert of C. parvum DNA according to its specificity for Cryptosporidium spp. upon screening the Escherichia coli transformants with DNA of C. parvum, Giardia lamblia, Plasmodium falciparum, Toxoplasma gondii, and Trichomonas vaginalis by colony hybridization. After sequencing, they designed a primer set allowing the amplification of a 452-bp segment of the sequence containing a unique StyI site. Two internal oligonucleotide probes, designed as probes 127 and 325, were also defined for confirming specificity by hybridization (Fig. 1). Following this work, other authors have used Laxer's primer set or the reported 452-bp sequence in order to develop other PCR-based diagnostic tests for C. parvum (1, 6-11, 14, 15, 17, 18, 20, 21, 23, 37, 38). These assays proved useful on a routine basis for detecting C. parvum parasites in livestock and humans. However, the utility of such methods seems now limited, since C. meleagridis, C. felis, and C. muris have been recognized in human infections (16, 19, 24, 26, 34, 36, 50).
In the present study, distinct sequences were obtained at Laxer's locus for C. parvum (for both genotypes 1 and 2) and C. meleagridis. Alignment of these sequences against the original one reported by Laxer et al. revealed nine differences between the newly reported sequences and Laxer's (Fig. 1). The likeliest explanation is that Laxer's sequence contains some errors. This hypothesis is supported by three previous studies in which the same differences were reported (6, 7, 11). In the first one, which targeted species differentiation, Chrisp and LeGendre attempted to adapt the PCR assay of Laxer et al. (22) for the specific detection of C. wrairi DNA and demonstrated variations between the two species (6). The internal probe used in the previously assay reported by Laxer et al. (probe 127) could not detect the amplified C. wrairi DNA. A new probe based on a sequence homologous to C. parvum and C. wrairi was successful in detecting both species, but their attempt to specifically recognize C. wrairi DNA using a specific oligonucleotide probe based on sequence differences was not successful. In the second study, das Graças C. Pereira et al. sequenced the Laxer locus for 11 animal-derived Cryptosporidium isolates and one environmental sample (7). As obtained sequences were very similar, the authors concluded that the Laxer marker did not appear to be sufficiently polymorphic to allow a reliable discrimination between C. parvum isolates. Alternatively, they proposed that all their isolates could belong to the same strain of C. parvum. The results presented in our study support the last hypothesis. In the third study, Deng et al. reported the existence of C. parvum in California sea lions (11).
In the present study, restriction analysis revealed different electrophoresis band patterns for genotype 1, genotype 2, and C. meleagridis. The sequence also predicts that C. wrairi generates a different band pattern. Results from human isolate genotyping for this locus were in agreement with those for the 18S rDNA locus (Table 1). Moreover, intragenotype variation was detected in the genotype 2 isolates. Two subgenotypes of C. parvum of cattle origin were identified. Both subgenotypes were retrieved in France, whereas only the L2 subgenotype was retrieved among 25 Haitian human isolates identified as genotype 2 of C. parvum at the 18S rDNA locus (data not shown).
Specificity of Laxer's protocol.
The primer pair originally described by Laxer et al. produced no PCR fragment with C. muris (5, 7, 10, 37) or C. baileyi (5, 37) DNA. Champliaud et al. (5) reported that this primer pair and other seven primer pairs previously proposed to detect C. parvum cross-reacted with C. meleagridis. These authors found that C. parvum and C. meleagridis could not be differentiated even after RFLP of the 452-bp PCR product. However, Morgan and Thompson (30) suggested that the bird isolate used in the referenced study could in fact be an isolate of C. parvum. This hypothesis was probably right because (i) the study of Champliaud et al. (5) was the first attempt at molecular characterization of C. meleagridis; for this reason, the authors did not have any molecular data as reference, and (ii) further studies have confirmed the existence of a genotype specific to C. meleagridis at the 18S rDNA locus (42, 52), at the Cryptosporidium oocyst wall protein locus (34, 51), at the locus of thrombospondin-related adhesive protein of Cryptosporidium 1(34), and at the heat shock protein 70 locus (44). In none of these studies was C. felis DNA included to assess PCR.
The C. felis case.
We succeeded in detecting Cryptosporidium DNA only in two isolates out of six found positive by PCR at the 18S rDNA locus. Curiously, genotyping revealed the identification of genotype 2 (L2 subgenotype). Our conclusion was that Laxer's primers were not specific of C. felis and that the amplification of genotype 2 DNA could be explained by coinfection. Actually, since the primer pair of Laxer was unable to recognize the hybridization site on the C. felis DNA, only C. parvum DNA was amplified. According to the hypothesis of C. parvum and C. felis cocarriage, C. parvum should be present at a low level compared to C. felis. In fact, the proportion between the two species could be such that only C. felis DNA was amplified by PCR at the 18S rDNA locus. A competitive phenomenon with generic primers can explain this result. In consequence, direct sequencing of the 18S rDNA C. felis fragment produced a beautiful electrophoregram without any reading ambiguity. Contamination risks can practically be excluded as we routinely perform PCR experiments in our laboratory and have therefore developed strict procedures to avoid cross-contamination. Procedures included physical separation of the rooms set aside for DNA extraction, PCR medium preparation, and electrophoresis. In addition, individual samples are treated in independent experiments.
Conclusion.
Findings of this study have practical implications on the use of Laxer's tool for detecting Cryptosporidium parasites. A positive PCR result with Laxer's primer pair does not necessarily imply the presence of C. parvum in the analyzed sample, as C. meleagridis DNA is also amplified at this locus. RFLP of PCR products should be performed to differentiate C. parvum and C. meleagridis (and C. wrairi) species as well as genotypes 1 and 2 of C. parvum. Furthermore, the specificity of Laxer's primers should be tested with the other genotypes of C. parvum as well as with the species C. serpentis and C. nasorum. As the used primers are not generic, the utility of this tool in the analysis of environmental or even clinical samples is therefore severely limited because of the narrow spectrum of Cryptosporidium species or genotypes that it can effectively detect.
Nucleotide sequence accession number.
The sequences determined in this study have been published in the GenBank database under accession numbers AF400130 to AF400133.
Acknowledgments
A. Follet-Dumoulin was supported by a grant from the Catholic University of Lille. This work was developed in part in the framework of the “Agence Nationale de Recherche sur le SIDA”-supported VIH-PAL program.
REFERENCES
- 1.Balatbat, A. B., G. W. Jordan, Y. J. Tang, and J. Silva, Jr. 1996. Detection of Cryptosporidium parvum DNA in human feces by nested PCR. J. Clin. Microbiol. 34:1769-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnin, A., M. N. Fourmaux, J. F. Dubremetz, R. G. Nelson, P. Gobet, G. Harly, M. Buisson, D. Puygauthier-Toubas, G. Gabriel-Pospisil, M. Naciri, and P. Camerlynck. 1996. Genotyping human and bovine isolates of Cryptosporidium parvum by polymerase chain reaction-restriction fragment length polymorphism analysis of a repetitive DNA sequence. FEMS Microbiol. Lett. 137:207-211. [DOI] [PubMed] [Google Scholar]
- 3.Caccio, S., W. Homan, R. Camilli, G. Traldi, T. Kortbeek, and E. Pozio. 2000. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology 120:237-244. [DOI] [PubMed] [Google Scholar]
- 4.Carraway, M., S. Tzipori, and G. Widmer. 1997. A new restriction fragment length polymorphism from Cryptosporidium parvum identifies genetically heterogeneous parasite populations and genotypic changes following transmission from bovine to human hosts. Infect. Immun. 65:3958-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champliaud, D., P. Gobet, M. Naciri, O. Vagner, J. Lopez, J. C. Buisson, I. Varga, G. Harly, R. Mancassola, and A. Bonnin. 1998. Failure to differentiate Cryptosporidium parvum from C. meleagridis based on PCR amplification of eight DNA sequences. Appl. Environ. Microbiol. 64:1454-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrisp, C. E., and M. LeGendre. 1994. Similarities and differences between DNA of Cryptosporidium parvum and C. wrairi detected by the polymerase chain reaction. Folia Parasitol. 41:97-100. [PubMed] [Google Scholar]
- 7.das Graças C. Pereira, M., E. R. Atwill, M. R. Crawford, and R. B. Lefebvre. 1998. DNA sequence similarity between California isolates of Cryptosporidium parvum. Appl. Environ. Microbiol. 64:1584-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, M. Q., and D. O. Cliver. 1998. Cryptosporidium parvum development in the BS-C-1 cell line. J. Parasitol. 84:8-15. [PubMed] [Google Scholar]
- 9.Deng, M. Q., and D. O. Cliver. 1999. Improved immunofluorescence assay for detection of Giardia and Cryptosporidium from asymptomatic adult cervine animals. Parasitol. Res. 85:733-736. [DOI] [PubMed] [Google Scholar]
- 10.Deng, M. Q., D. O. Cliver, and T. W. Mariam. 1997. Immunomagnetic capture PCR to detect viable Cryptosporidium parvum oocysts from environmental samples. Appl. Environ. Microbiol. 63:3134-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, M. Q., R. P. Peterson, and D. O. Cliver. 2000. First findings of Cryptosporidium and Giardia in California sea lions (Zalophus californianus). J. Parasitol. 86:490-494. [DOI] [PubMed] [Google Scholar]
- 12.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 13.Fayer, R., C. A. Speer, and J. P. Dubey. 1997. The general biology of Cryptosporidium, p. 1-41. In R. E. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 14.Filkorn, R., A. Wiedenmann, and K. Botzenhart. 1994. Selective detection of viable Cryptosporidium oocysts by PCR. Zentbl. Hyg. Umweltmed. 195:489-494. [PubMed] [Google Scholar]
- 15.Gobet, P., J. C. Buisson, O. Vagner, M. Naciri, M. Grappin, S. Comparot, G. Harly, D. Aubert, I. Varga, P. Camerlynck, and A. Bonnin. 1997. Detection of Cryptosporidium parvum DNA in formed human feces by a sensitive PCR-based assay including uracil-N-glycosylase inactivation. J. Clin. Microbiol. 35:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyot, K., A. Follet-Dumoulin, E. Lelièvre, C. Sarfati, M. Rabodonirina, G. Nevez, J. C. Cailliez, D. Camus, and E. Dei-Cas. 2001. Molecular characterization of Cryptosporidium obtained from humans in France. J. Clin. Microbiol. 39:3472-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallier-Soulier, S., and E. Guillot. 2000. Detection of cryptosporidia and Cryptosporidium parvum oocysts in environmental water samples by immunomagnetic separation-polymerase chain reaction. J. Appl. Microbiol. 89:5-10. [DOI] [PubMed] [Google Scholar]
- 18.Hallier-Soulier, S., and E. Guillot. 1999. An immunomagnetic separation polymerase chain reaction assay for rapid and ultra-sensitive detection of Cryptosporidium parvum in drinking water. FEMS Microbiol. Lett. 176:285-289. [DOI] [PubMed] [Google Scholar]
- 19.Katsumata, T., D. Hosea, I. G. Ranuh, S. Uga, T. Yanagi, and S. Kohno. 2000. Short report: possible Cryptosporidium muris infection in humans. Am. J. Trop. Med. Hyg. 62:70-72. [DOI] [PubMed] [Google Scholar]
- 20.Laberge, I., A. Ibrahim, J. R. Barta, and M. W. Griffiths. 1996. Detection of Cryptosporidium parvum in raw milk by PCR and oligonucleotide probe hybridization. Appl. Environ. Microbiol. 62:3259-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laxer, M. A., M. E. D'Nicuola, and R. J. Patel. 1992. Detection of Cryptosporidium parvum DNA in fixed, paraffin-embedded tissue by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 47:450-455. [DOI] [PubMed] [Google Scholar]
- 22.Laxer, M. A., B. K. Timblin, and R. J. Patel. 1991. DNA sequences for the specific detection of Cryptosporidium parvum by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 45:688-694. [DOI] [PubMed] [Google Scholar]
- 23.Leng, X., D. A. Mosier, and R. D. Oberst. 1996. Simplified method for recovery and PCR detection of Cryptosporidium DNA from bovine feces. Appl. Environ. Microbiol. 62:643-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLauchlin, J., C. Amar, S. Pedraza-Diaz, and G. L. Nichols. 2000. Molecular epidemiological analysis of Cryptosporidium spp. in the united kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J. Clin. Microbiol. 38:3984-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meinhardt, P. L., D. P. Casemore, and K. B. Miller. 1996. Epidemiologic aspects of human cryptosporidiosis and the role of waterborne transmission. Epidemiol. Rev. 18:118-136. [DOI] [PubMed] [Google Scholar]
- 26.Morgan, U., R. Weber, L. Xiao, I. Sulaiman, R. C. Thompson, W. Ndiritu, A. Lal, A. Moore, and P. Deplazes. 2000. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J. Clin. Microbiol. 38:1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan, U. M., C. C. Constantine, D. A. Forbes, and R. C. Thompson. 1997. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 83:825-830. [PubMed] [Google Scholar]
- 28.Morgan, U. M., P. Deplazes, D. A. Forbes, F. Spano, H. Hertzberg, K. D. Sargent, A. Elliot, and R. C. Thompson. 1999. Sequence and PCR-RFLP analysis of the internal transcribed spacers of the rDNA repeat unit in isolates of Cryptosporidium from different hosts. Parasitology 118:49-58. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, U. M., K. D. Sargent, P. Deplazes, D. A. Forbes, F. Spano, H. Hertzberg, A. Elliot, and R. C. Thompson. 1998. Molecular characterization of Cryptosporidium from various hosts. Parasitology 117:31-37. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, U. M., and R. C. Thompson. 1999. The importance of genotyping isolates before assigning species. Parasitol. Today 15:80-81. [DOI] [PubMed] [Google Scholar]
- 31.Morgan, U. M., L. Xiao, R. Fayer, A. A. Lal, and R. C. Thompson. 2000. Epidemiology and strain variation of Cryptosporidium parvum. Contrib. Microbiol. 6:116-139. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, U. M., L. Xiao, R. Fayer, A. A. Lal, and R. C. Thompson. 1999. Variation in Cryptosporidium: towards a taxonomic revision of the genus. Int. J. Parasitol. 29:1733-1751. [DOI] [PubMed] [Google Scholar]
- 33.Morgan, U. M., L. Xiao, B. D. Hill, P. O'Donoghue, J. Limor, A. Lal, and R. C. Thompson. 2000. Detection of the Cryptosporidium parvum “human” genotype in a dugong (Dugong dugon). J. Parasitol. 86:1352-1354. [DOI] [PubMed] [Google Scholar]
- 34.Pedraza-Diaz, S., C. Amar, and J. McLauchlin. 2000. The identification and characterisation of an unusual genotype of Cryptosporidium from human faeces as Cryptosporidium meleagridis. FEMS Microbiol. Lett. 189:189-194. [DOI] [PubMed] [Google Scholar]
- 35.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. Ong, W. R. MacKenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieniazek, N. J., F. J. Bornay-Llinares, S. B. Slemenda, A. J. da Silva, I. N. Moura, M. J. Arrowood, O. Ditrich, and D. G. Addiss. 1999. New Cryptosporidium genotypes in HIV-infected persons. Emerg. Infect. Dis. 5:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochelle, P. A., R. De Leon, M. H. Stewart, and R. L. Wolfe. 1997. Comparison of primers and optimization of PCR conditions for detection of Cryptosporidium parvum and Giardia lamblia in water. Appl. Environ. Microbiol. 63:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sluter, S. D., S. Tzipori, and G. Widmer. 1997. Parameters affecting polymerase chain reaction detection of waterborne Cryptosporidium parvum oocysts. Appl. Microbiol. Biotechnol. 48:325-330. [DOI] [PubMed] [Google Scholar]
- 39.Spano, F., L. Putignani, A. Crisanti, P. Sallicandro, U. M. Morgan, S. M. Le Blancq, L. Tchack, S. Tzipori, and G. Widmer. 1998. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J. Clin. Microbiol. 36:3255-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spano, F., L. Putignani, S. Guida, and A. Crisanti. 1998. Cryptosporidium parvum: PCR-RFLP analysis of the TRAP-C1 (thrombospondin-related adhesive protein of Cryptosporidium-1) gene discriminates between two alleles differentially associated with parasite isolates of animal and human origin. Exp. Parasitol. 90:195-198. [DOI] [PubMed] [Google Scholar]
- 41.Spano, F., L. Putignani, J. McLauchlin, D. P. Casemore, and A. Crisanti. 1997. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 150:209-217. [DOI] [PubMed] [Google Scholar]
- 42.Sretér, T., G. Kovács, A. J. Da Silva, N. J. Pieniazek, Z. Széll, M. Dobos-Kovács, K. Márialigeti, and I. Varga. 2000. Morphologic, host specificity, and molecular characterization of a Hungarian Cryptosporidium meleagridis isolate. Appl. Environ. Microbiol. 66:735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stinear, T., A. Matusan, K. Hines, and M. Sandery. 1996. Detection of a single viable Cryptosporidium parvum oocyst in environmental water concentrates by reverse transcription-PCR. Appl. Environ. Microbiol. 62:3385-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulaiman, I. M., U. M. Morgan, R. C. A. Thompson, A. A. Lal, and L. Xiao. 2000. Phylogenetic relationships of Cryptosporidium parasites based on the 70-kilodalton heat shock protein (HSP70) gene. Appl. Environ. Microbiol. 66:2385-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasquez, J. R., L. Gooze, K. Kim, J. Gut, C. Petersen, and R. G. Nelson. 1996. Potential antifolate resistance determinants and genotypic variation in the bifunctional dihydrofolate reductase-thymidylate synthase gene from human and bovine isolates of Cryptosporidium parvum. Mol. Biochem. Parasitol. 79:153-165. [DOI] [PubMed] [Google Scholar]
- 46.Webster, K. A., J. D. Pow, M. Giles, J. Catchpole, and M. J. Woodward. 1993. Detection of Cryptosporidium parvum using a specific polymerase chain reaction. Vet. Parasitol. 50:35-44. [DOI] [PubMed] [Google Scholar]
- 47.Widmer, G. 1998. Genetic heterogeneity and PCR detection of Cryptosporidium parvum. Adv. Parasitol. 40:223-239. [DOI] [PubMed] [Google Scholar]
- 48.Widmer, G., L. Tchack, C. L. Chappell, and S. Tzipori. 1998. Sequence polymorphism in the β-tubulin gene reveals heterogeneous and variable population structures in Cryptosporidium parvum. Appl. Environ. Microbiol. 64:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Widmer, G., S. Tzipori, C. J. Fichtenbaum, and J. K. Griffiths. 1998. Genotypic and phenotypic characterization of Cryptosporidium parvum isolates from people with AIDS. J. Infect. Dis. 178:834-840. [DOI] [PubMed] [Google Scholar]
- 50.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, L., J. Limor, U. M. Morgan, I. M. Sulaiman, R. C. Thompson, and A. A. Lal. 2000. Sequence differences in the diagnostic target region of the oocyst wall protein gene of Cryptosporidium parasites. Appl. Environ. Microbiol. 66:5499-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]