Abstract
Colletotrichum trifolii is the causative organism of alfalfa anthracnose. We previously cloned and characterized the small prototypical G protein, Ras, of C. trifolii, which is involved in the signaling pathways that mediate interaction between the pathogen and its host. Transformants expressing constitutively active forms of Ras have growth medium-dependent phenotypes. In nutrient-rich media (e.g., yeast extract and peptone), the phenotype of the transformants was indistinguishable from that of the wild type. However, during nutrient starvation, the transformants lose polarity, have distended hyphae, and fail to sporulate and produce appressoria. Since peptone caused the phenotype to revert, amino acids were tested singly and in combination to identify the responsible amino acid(s). We found that 1.6 mM proline in the medium reverses the constitutively active Ras phenotype.
Pathogenic development of filamentous fungi requires perception of the environment and response to appropriate cues. In a few cases, such signals have been identified, but the responsible pathways have not been worked out. Physical signals include plant surface topography, hydrophobicity, and rigidity (9, 14, 26). These physical signals generally are associated with conidial adhesion and appressorium differentiation. Chemical signals revolve around nutrient status, such as starvation or nitrogen deficiency (11, 15, 16). In all cases, perception of the appropriate stimulus triggers signals that eventually alter gene expression, producing the desired cellular response. Identification of the signals and the response pathways is important for understanding the underlying mechanisms responsible for fungal development, as well as suggesting targets for interference when such pathways are required for effective pathogenesis.
Colletotrichum trifolii is an important fungal pathogen of alfalfa which causes anthracnose (8). The infection process relies on a tightly coordinated series of developmental transitions that begins with spore attachment to the plant surface, appressorium formation, penetration of the plant cuticle and cell wall, acquisition of nutrients from plant tissue, and the production of asexual spores in acervuli (8).
Recently, we found that constitutively activated forms of Ras, the prototypical member of the superfamily of small G proteins, when expressed in C. trifolii yielded a nutrient-dependent response. The activated form of Ras was made by site-directed mutagenesis that substituted either valine for glycine at position 17 or leucine for glutamine at position 66. Both of these amino acid changes inhibit the activity of the GTPase-activating proteins that hydrolyze the GTP-bound active Ras to form the inactive GDP-bound form. Such inhibition ensures that Ras remains in the activated GTP-bound state and is a common mutation found in mammalian tumors.
The nutrient-dependent response of active Ras when grown on minimal medium was phenotypically expressed as aberrant hyphal morphology, virtually no conidiation, and failure to produce appressoria (24). The addition of 0.05% yeast extract causes the phenotype observed in minimal medium to revert to wild type. Various carbon sources (sucrose, glucose, and maltodextrin), nitrogen sources (nitrate, nitrite, hypoxanthine, and urea), vitamins, osmoticum, heat, pH, and combinations of these factors all failed to cause the phenotype to revert.
Subsequent studies suggested the involvement of amino acids in phenotype reversion. The evidence for this conclusion was based on supplementing minimal medium with 0.2% peptone, which caused the phenotype to revert. Thus, addition of peptone to minimal medium containing C. trifolii strains harboring activated Ras resulted in a wild-type phenotype, including restoration of normal hyphal morphology and conidiation. We were therefore interested in determining the component(s) in peptone involved in rescuing these mutants. Such information may be important for determining what C. trifolii perceives in the external (nutritional) environment to induce appropriate pathways for normal growth and development. Our objective in this study was to identify the nutritional factor(s) responsible for causing the phenotype to revert. In this paper, we report that proline can restore the wild-type phenotype in C. trifolii.
MATERIALS AND METHODS
Fungal strains and growth conditions.
The wild-type C. trifolii race 1 was isolated from alfalfa cultivar Saranac (8). C. trifolii transformants expressing constitutively active ras were obtained as previously described (24). Transformants with the prefix Val contain the G17V Ras mutation, and those with the prefix Leu contain the Q66L mutation. The stocks of all strains were maintained at −70°C in 25% glycerol. For spore production, the strains were first grown from glycerol stocks on YPSS agar (8) supplemented with hygromycin B (150 μg/ml) as necessary. After incubation at 25°C for 7 days, mycelial plugs were used as inocula for YPSS liquid cultures, which were grown for 4 to 5 days at 200 rpm and 24°C. Spores were collected by filtration through Miracloth (Calbiochem, La Jolla, Calif.) and washed three times in sterile distilled water. The medium for microscopic evaluation of growth was water agar, containing 1.6% Bacto Agar (Difco, Detroit, Mich.). The media were supplemented with 0.2% peptone (Difco) or with either a single amino acid or a combination of amino acids (Sigma-Aldrich, St. Louis, Mo.). We tested each amino acid individually and then tested mixtures of all amino acids from which one amino acid had been omitted in water agar plates inoculated with the transformed fungus. The following amino acids were used at the indicated concentrations in 0.2% peptone: aspartic acid-monosodium salt (0.8 mM), threonine-free acid (0.3 mM), serine-free acid (0.5 mM), glutamic acid-monosodium salt (1.3 mM), proline-free acid (1.6 mM), glycine-HCl (4 mM), alanine-free acid (2 mM), cysteine-HCl (30 μM), valine-free acid (40 μM), methionine-free acid (0.2 mM), isoleucine-free acid (0.2 mM), leucine-free acid (0.5 mM), tyrosine-HCl (70 μM), phenylalanine-free acid (0.2 mM), histidine-HCl (50 μM), lysine-HCl (0.5 mM), arginine-HCl (0.6 mM), and tryptophan-free acid (30 μM). To further investigate the role of proline in reversing aberrant Ras phenotypes, the following proline analogs (22) were tested; d-proline, 2-azetidinecarboxylic acid (AZC), thiazolidine-2-carboxylic acid, and thiazolidine-4-carboxylic acid (thioproline) (all from Sigma-Aldrich). All analogs were used at the same molar concentration (1.6 mM) used for proline.
Microscopy.
Microscopy was performed as described previously (24). Sterile glass microscope slides were overlaid with a thin (approximately 2-mm) layer of molten water agar with appropriate supplements. A 20-μl aliquot of a 5 × 105-spore/ml suspension was inoculated at one end of each slide and streaked across the agar to ensure an even distribution of spores. The inoculated slides were then placed in 150-mm-diameter plastic petri dishes that were in turn placed in a plastic box containing a water-saturated sponge. The box was sealed with Saran Wrap to ensure a humid environment. The cultures were incubated for 6 days at 25°C, after which a coverslip was placed over the resulting colonies and they were examined using a Zeiss (Göttingen, Germany) Axioskop microscope with differential interference contrast optics. Images were captured via a charge-coupled device camera and processed using Image-Pro Plus 3.0 for Windows (Media Cybernetics, Silver Spring, Md.).
Amino acid analyses.
Amino acid extracts were prepared as previously described (8). Briefly, mycelia were isolated after 6 days of growth in liquid YPSS medium. To obtain sufficient material for analysis from cultures grown under minimal nutritional conditions, cultures were first grown in YPSS medium for 3 days, washed three times in sterile distilled water, and then cultured in sterile distilled water for 3 days at 25°C with shaking to simulate the nutritional state of mycelia grown on water agar on the microscope slides.
Mycelia were harvested, freeze-dried, and ground with a mortar and pestle. The dry weight was determined, and samples were placed in boiling methanoic acid-ethanol-water (1:15:4) and sonicated for 1 min (5). The samples were centrifuged at 20,000 × g for 5 min, and the resulting pellet was washed twice with 5 N methanoic acid-50% ethanol, with heating at 70°C between centrifugations. All liquid fractions were combined and evaporated at 60°C overnight. Following rehydration with 1 ml of distilled water, the samples were placed on a cation-exchange column (AG 50W-X 200 to 400 mesh [H+ form]; Bio-Rad, Richmond, Calif.), eluted with 2 N ammonium hydroxide, and evaporated to dryness in a 60°C oven. The samples were resuspended in distilled water and processed through a Microcon centrifugal filter with a 3,000-molecular-weight cutoff (Millipore Corp., Bedford, Mass.) for 100 min to remove contaminating proteins. The samples were analyzed by high-performance liquid chromatography as described by Chastain and Chollet (5).
RESULTS
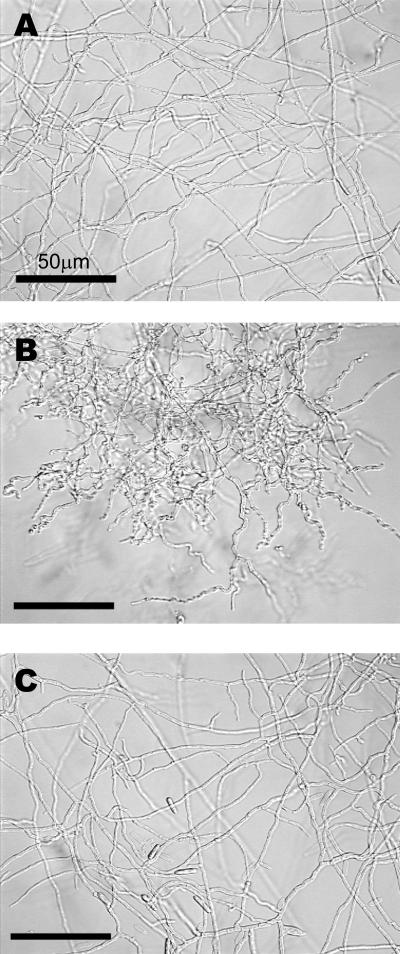
All studies (with the exceptions noted below) were performed on the mutant designated Val2 (RasG17V). Restoration of the wild-type phenotype required only proline (Fig. 1C). There was no detectable difference between the hyphal morphology of the wild type on minimal medium and that of Val2 on minimal medium supplemented with proline. Similar results were observed for sporulation of the wild type grown on agar plates containing minimal medium and that of Val2 on proline-supplemented medium (Fig. 1). Interestingly, the amount of sporulation of wild-type strains on minimal medium was also enhanced by proline supplementation. When the Val2 mutant was cultured in medium containing all of the amino acids except proline, normal hyphal morphology was partially restored. This might be due to the presence of metabolic precursors to proline in the medium. However, this supplementation did not restore sporulation, and the abnormal spore formation observed under these conditions was comparable to that observed on minimal medium.
FIG. 1.
Addition of proline restores wild-type phenotype of C. trifolii expressing activated Ras (Val2) when grown on water agar. (A) Wild-type C. trifolii on water agar. (B) Val2 mutant on water agar. (C) Val2 mutant on water agar containing 1.6 mM proline. Note the restoration of hyphal morphology and sporulation. All colonies were photographed 7 days postinoculation at ×400 magnification.
A possible explanation for the restoration of the wild-type phenotype by proline was that the transforming vector had coincidentally disrupted a gene involved in proline synthesis. The restoration of hyphal morphology in medium containing all of the amino acids except proline argues against this hypothesis. More importantly, other constitutively active Ras transformants resulting from different independent integration events as determined by DNA blots were restored by proline (23, 24). We tested two other G17V (Val3 and Val10) and three Q66L (Leu3, Leu4, and Leu5) ras transformants, all of which had different integration patterns, and found that proline caused the aberrant phenotype to revert in all cases (data not shown). All further studies were performed on the mutant designated Val2.
To determine if the mechanism of proline-mediated reversion was due to biological and/or physiochemical processes, proline analogs were tested. d-Proline treatment had no effect on the wild type and also failed to cause Val2 to revert. AZC was toxic to the wild type; germination was severely reduced, and the few colonies grew very slowly, with distorted hyphae. The Val2 phenotype was similar but more extreme. Thiazolidine B2-carboxylic acid appeared to reduce mycelial density in the wild type, but the hyphal morphology was unaltered with respect to controls. Thiazolidine B2-carboxylic acid partially restored the Val2 phenotype. Thiazolidine-4-carboxylic acid, however, did restore the wild-type phenotype effect of l-proline.
Amino acid analysis.
Having established that proline was capable of causing the aberrant phenotype to revert, we determined the minimal and maximal concentrations of proline required for phenotype reversion. The Val2 strain was plated on water agar containing 10- and 100-fold-higher and -lower concentrations of 1.6 mM proline (16 μM, 0.16 mM, 16 mM, and 160 mM). The results from three independent experiments all showed that 10-fold differences in either direction restored the wild-type phenotype. A 16 μM concentration had no effect on the mutant phenotype, and 160 mM also restored the wild-type phenotype, with the exception that was a large increase in sporulation similar to that observed in wild-type strains. The hyphal morphology at this concentration was only partially restored: polarity was regained, but hyphae were slightly thicker and shorter. We also compared the proline levels in both strains under both nutrient-rich and nutrient-limiting conditions. In order to set such values in context, a complete amino acid profile was constructed in each case. The central assumption in this analysis was that mycelia grown under nutrient-limited conditions are analogous, in terms of nutrient status, to those growing on the minimal medium used for microscopic observation.
There were small but consistent differences between the cytosolic amino acid profiles with different strains and culture conditions (Table 1). Val2 had a slightly elevated, or at least equivalent, amino acid level under conditions of nutrient excess compared to that of the wild type. Under nutrient-limited conditions, Val2 had lower amino acid concentrations than the wild type. When Val2 was placed in minimal medium, the concentrations of all amino acids decreased. Although some amino acid concentrations for the wild type decreased in minimal medium, it appears that the wild type has a greater capacity for maintaining amino acid concentrations in minimal medium. The concentration of total amino acids decreased by 6% in the wild type and by 45% in Val2 following transfer to minimal medium. Under starvation conditions, the proline concentration dropped by 47% in the wild type but by only 19% in Val2. With the exception of lysine, this decrease is the smallest for any amino acid in Val2 when transferred to starvation conditions.
TABLE 1.
Amino acid concentrations for wild-type and Val2 strains of C. trifolii under different nutritional conditionsa
| Amino acid | Concn (ng/mg [dry weight] of mycelia)b
|
|||
|---|---|---|---|---|
| Wild type on YPSS | Wild type on MM | Val2 on YPSS | Val2 on MM | |
| Aspartic acid | 140 ± 4 | 150 ± 9 | 130 ± 2 | 280 ± 37 |
| Threonine | 230 ± 7 | 220 ± 27 | 640 ± 33 | 140 ± 26 |
| Serine | 97 ± 2 | 120 ± 8 | 150 ± 3 | 66 ± 8 |
| Glutamic acid | 760 ± 51 | 360 ± 14 | 1000 ± 98 | 490 ± 16 |
| Proline | 65 ± 3 | 35 ± 38 | 99 ± 2 | 62 ± 33 |
| Glycine | 110 ± 4 | 97 ± 5 | 160 ± 5 | 81 ± 27 |
| Alanine | 440 ± 16 | 190 ± 9 | 440 ± 15 | 180 ± 11 |
| Cysteine | 120 ± 16 | 10 ± 4 | 120 ± 51 | 51 ± 27 |
| Valine | 87 ± 4 | 83 ± 7 | 91 ± 6 | 43 ± 12 |
| Methionine | 32 ± 1 | 27 ± 2 | 130 ± 6 | 37 ± 17 |
| Isoleucine | 53 ± 2 | 56 ± 6 | 64 ± 6 | 29 ± 7 |
| Leucine | 60 ± 1 | 82 ± 11 | 64 ± 7 | 31 ± 10 |
| Tyrosine | 60 ± 2 | 120 ± 12 | 89 ± 15 | 50 ± 27 |
| Phenylalanine | 44 ± 1 | 59 ± 6 | 67 ± 6 | 28 ± 5 |
| Histidine | 240 ± 4 | 130 ± 7 | 200 ± 1 | 100 ± 35 |
| Lysine | 320 ± 2 | 560 ± 39 | 400 ± 10 | 260 ± 14 |
| Arginine | 370 ± 8 | 370 ± 8 | 370 ± 11 | 200 ± 70 |
Results are means of two replicates
YPSS, rich medium; MM, water agar.
In summary, the expression of constitutively active Ras in C. trifolii affects the cytosolic amino acid profile in both nutrient-rich and nutrient-limited cultures. Transfer to minimal medium induces preferential loss of amino acids from the cytosol compared to the wild type. Proline and lysine levels are least affected, suggesting either preferential retention or increased biosynthesis.
DISCUSSION
Activating mutations in Ras have played an important role in the functional analysis of Ras signaling in a number of organisms. This importance is particularly well illustrated in mammalian systems, where Ras was originally discovered, and has led to considerable understanding of growth factor signaling proliferation, development, and disease. Signaling molecules and pathways are conserved across considerable evolutionary distances (2). As Ras is central to cellular growth control and response to external stimuli, proper regulation of this small G protein is expected to be important in filamentous fungi as well. The mode of action of Ras is to act as a molecular rheostat, alternating between active (GTP-bound) and inactive (GDP-bound) forms. This switching is mediated through GTP exchange factors and GTPase-activating proteins. Ras and its subtypes have been found in diverse eukaryotes, including Saccharomyces cerevisiae (18), Aspergillus nidulans (20), Drosophila melanogaster (12), and humans (3). Examples of the range of cellular responses mediated by Ras include switching from cellular to filamentous growth in Candida albicans (10), mating and filamentation in Cryptococcus neoformans (1), regulation of embryonic development in D. melanogaster (17), and the induction of tumors in mammalian cells (3).
Mutations in C. trifolii Ras that result in constitutive activity confer oncogenic properties, i.e., cause tumors, when inserted into mouse fibroblasts and also exhibit a nutrition-dependent phenotype in C. trifolii (24). This phenotype is observed only under conditions of starvation and causes aberrant hyphal morphology and severe defects in the ability of the fungus to conidiate and differentiate appressoria. In this paper, we have identified proline as an essential component of rich medium for the reversion of the activated Ras phenotype. The biochemical basis for this reversion is not known.
Proline is a compatible osmolyte in salt-stressed plants (13). There is as yet no evidence that constitutively active fungal Ras isolates are osmotically compromised. The addition of osmoticum did not reverse the phenotype in minimal medium (24). There is an apparent need for increased concentrations of amino acids in Val2 to maintain normal growth even in rich (YPSS) medium. Proline levels in Val2 growing on minimal medium are much higher than those found in the wild type, yet growth is normal for the wild type despite its lower proline concentration. Perhaps the reduced proline loss in Val2 with respect to the wild type is due to increased proline biosynthesis, as seen in salt-stressed prokaryotes (6) and plants (13). However, such synthesis is insufficient to reverse the phenotype. Proline is known to enhance germ tube formation in the pathogenic yeast C. albicans (7), indicating a role in morphogenesis for this amino acid.
Amino acid levels significantly decreased in the Val2 mutant when it was grown on minimal medium in comparison to the levels when it was grown on rich medium and to the levels in wild-type C. trifolii on minimal medium. However, proline (and lysine) exhibited the smallest decrease in amino acid levels when Val2 was grown with limiting nutrition. This suggests that Val2 has difficulty maintaining cystolic amino acid levels in minimal medium or that it is more rapid turnover of the amino acids because of increased metabolism due to activated Ras during cell division. This could indicate a tendency to retain proline preferentially or increased proline synthesis. It should be noted that some of the changes in amino acid concentrations could be due to amino acid interconversion and incorporation into proteins, as there are no free amino acids available in minimal medium. Alternatively, protein catabolism under starvation conditions may also play a role.
To identify the structural characteristics of l-proline that were involved in reversion, proline analogs were tested for their effects on C. trifolii mutants. From the effects of proline analogs, it is clear that steric interactions are important in the reversal of phenotype. This is evidenced by the failure of d-proline to cause the phenotype to revert and partial reversion by thiazolidine-2-carboxylic acid. AZC proved toxic to C. trifolii, which could be a direct effect of an AZC metabolite. Only thiazolidine-4-carboxylic acid truly mimics the effect of l-proline, suggesting the importance of the carboxyl group position.
The ability of thiazolidine-2-carboxylic acid to partially reverse the Val2 phenotype to wild type in minimal medium and that of thiazolidine-4-carboxylic acid to completely reverse the phenotypes are of interest. Both molecules are structurally very similar to proline; thiazolidine-2-carboxylic acid has a sulfur substitution for the methylene group of proline, whereas in thiazolidine-4-carboxylic acid, the gamma methylene is substituted by a sulfur atom. Such differences may be important in the efficiency of steric interactions and thus affect the efficiency of the reversion event. Both analogs are competitive inhibitors of proline (4). It is unclear why these analogs do not inhibit growth, since thiazolidine-2-carboxylic acid prevents protein elongation. At the concentrations used, there was no effect on hyphal morphology, as the wild type and Val2 grew normally.
A number of specific proline permeases have been identified in a broad range of organisms, including Staphylococcus aureus (19), S. cerevisiae (25), and A. nidulans (21). The existence and nature of such systems will require further investigation in C. trifolii. It is possible that active permease systems are stereospecific and thus prevent the uptake of d-proline into the cell. If passive uptake systems exist and d-proline is taken up by such systems, it seems probable that stereospecificity prevents its metabolism. This leads to the conclusion that if reversion of activated Ras strains is due to a simple diffusion-osmosis model, then d-proline should be effective.
Signaling during microbial pathogenic development is often mediated by the nutritional status of the local environment. It is well documented that fungi utilize environmental cues to trigger appropriate response pathways. These studies were designed to better understand the biochemical basis for the activated Ras phenotype and also to identify nutritional cues sensed by fungi, in this case C. trifolii, which are required for proper growth and development. We have identified the amino acid proline as being one such determinant. Although the underlying mechanisms behind the remarkable ability of proline to rescue activated Ras mutants remain unresolved, future studies will investigate uptake and transport and the generation of mutants which are either insensitive to or bypass the requirement for proline.
Acknowledgments
We thank Cynthia Stryker and Gina Truesdell for initial nutrient studies and Laura Steinkey, University of Nebraska Medical Center, for amino acid analysis.
This work was supported by the United States-Israel Binational Agriculture Research and Development Fund (BARD no. 2814-96).
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Birchmeier, C., D. Broek, T. Toda, S. Powers, T. Kataoka, and M. Wigler. 1985. Conservation and divergence of RAS protein function during evolution. Cold Spring Harbor Symp. Quant. Biol. 50:721-725. [DOI] [PubMed] [Google Scholar]
- 3.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49:4682-4689. [PubMed] [Google Scholar]
- 4.Busiello, V., M. di Girolamo, C. Cini, and C. De Marco. 1979. Action of thiazolidine-2-carboxylic acid, a proline analog, on protein synthesizing systems. Biochim. Biophys. Acta 564:311-321. [DOI] [PubMed] [Google Scholar]
- 5.Chastain, C. J., and R. Chollet. 1988. Incorporation of 14CO2 into C4 acids by leaves of C3-C4 intermediate and C3 species of Moricandia and Panicum at the CO2 compensation concentration. Planta 173:411-418. [DOI] [PubMed] [Google Scholar]
- 6.Csonka, L. N. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. 182:82-88. [DOI] [PubMed] [Google Scholar]
- 7.Dabrowa, N., S. S. Taxer, and D. H. Howard. 1976. Germination of Candida albicans induced by proline. Infect. Immun. 13:830-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickman, M. B., T. L. Buhr, V. Warwar, G. M. Truesdell, and C. Huang. 1995. Molecular signals during the early stages of alfalfa anthracnose. Can. J. Bot. 73:1169-1177. [Google Scholar]
- 9.Fang, E. G., and R. A. Dean. 2000. Site-directed mutagenesis of the magB gene affects growth and development in Magnaporthe grisea. Mol. Plant Microbe Interact. 13:1214-1227. [DOI] [PubMed] [Google Scholar]
- 10.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett, J. M. 1997. The control of morphogenesis in Saccharomyces cerevisiae by Elm1 kinase is responsive to RAS/cAMP pathway activity and tryptophan availability. Mol. Microbiol. 26:809-820. [DOI] [PubMed] [Google Scholar]
- 12.Gasperini, R., and G. Gibson. 1999. Absence of protein polymorphism in the Ras genes of Drosophila melanogaster. J. Mol. Evol. 49:583-590. [DOI] [PubMed] [Google Scholar]
- 13.Hare, P. D., and W. A. Cress. 1997. Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul. 21:79-102. [Google Scholar]
- 14.Kolattukudy, P. E., L. M. Rogers, D. Li, C. S. Hwang, and M. A. Flaishman. 1995. Surface signaling in pathogenesis. Proc. Natl. Acad. Sci. USA 92:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuman-Silberberg, F. S., S. Bhattacharya, and J. R. Broach. 1995. Nutrient availability and the RAS/cyclic AMP pathway both induce expression of ribosomal protein genes in Saccharomyces cerevisiae but by different mechanisms. Mol. Cell. Biol. 15:3187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrimon, N. 1994. Signaling pathways initiated by receptor protein tyrosine kinases in Drosophila. Curr. Opin. Cell Biol. 6:260-266. [DOI] [PubMed] [Google Scholar]
- 18.Powers, S., T. Kataoka, O. Fasano, M. Goldfarb, J. Strathern, J. Broach, and M. Wigler. 1984. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell 36:607-612. [DOI] [PubMed] [Google Scholar]
- 19.Schwan, W. R., S. N. Coulter, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, L. L. Brody, S. Westbrock-Wadman, A. S. Bayer, K. R. Folger, and C. K. Stover. 1998. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect. Immun. 66:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Som, T., and V. S. Kolaparthi. 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 14:5333-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sophianopoulou, V., and C. Scazzocchio. 1989. The proline transport protein of Aspergillus nidulans is very similar to amino acid transporters of Saccharomyces cerevisiae. Mol. Microbiol. 3:705-714. [DOI] [PubMed] [Google Scholar]
- 22.Tan, E. M., L. Ryhanen, and J. Uitto. 1983. Proline analogues inhibit human skin fibroblast growth and collagen production in culture. J. Investig. Dermatol. 80:261-267. [DOI] [PubMed] [Google Scholar]
- 23.Truesdell, G. M. 1998. Ph.D. thesis. University of Nebraska, Lincoln.
- 24.Truesdell, G. M., C. Jones, T. Holt, G. Henderson, and M. B. Dickman. 1999. A Ras protein from a phytopathogenic fungus causes defects in hyphal growth polarity, and induces tumors in mice. Mol. Gen. Genet. 262:46-54. [DOI] [PubMed] [Google Scholar]
- 25.Vandenbol, M., J. C. Jauniaux, and M. Grenson. 1989. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline-permease-encoding gene: similarities between CAN1, HIP1 and PUT4 permeases. Gene 83:153-159. [DOI] [PubMed] [Google Scholar]
- 26.Xu, J. R., and J. E. Hamer. 1996. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10:2696-2706. [DOI] [PubMed] [Google Scholar]