Abstract
Escherichia coli NZN111, which lacks activities for pyruvate-formate lyase and lactate dehydrogenase, and AFP111, a derivative which contains an additional mutation in ptsG (a gene encoding an enzyme of the glucose phophotransferase system), accumulate significant levels of succinic acid (succinate) under anaerobic conditions. Plasmid pTrc99A-pyc, which expresses the Rhizobium etli pyruvate carboxylase enzyme, was introduced into both strains. We compared growth, substrate consumption, product formation, and activities of seven key enzymes (acetate kinase, fumarate reductase, glucokinase, isocitrate dehydrogenase, isocitrate lyase, phosphoenolpyruvate carboxylase, and pyruvate carboxylase) from glucose for NZN111, NZN111/pTrc99A-pyc, AFP111, and AFP111/pTrc99A-pyc under both exclusively anaerobic and dual-phase conditions (an aerobic growth phase followed by an anaerobic production phase). The highest succinate mass yield was attained with AFP111/pTrc99A-pyc under dual-phase conditions with low pyruvate carboxylase activity. Dual-phase conditions led to significant isocitrate lyase activity in both NZN111 and AFP111, while under exclusively anaerobic conditions, an absence of isocitrate lyase activity resulted in significant pyruvate accumulation. Enzyme assays indicated that under dual-phase conditions, carbon flows not only through the reductive arm of the tricarboxylic acid cycle for succinate generation but also through the glyoxylate shunt and thus provides the cells with metabolic flexibility in the formation of succinate. Significant glucokinase activity in AFP111 compared to NZN111 similarly permits increased metabolic flexibility of AFP111. The differences between the strains and the benefit of pyruvate carboxylase under both exclusively anaerobic and dual-phase conditions are discussed in light of the cellular constraint for a redox balance.
Succinic acid (succinate) and its derivatives are widely used as specialty chemicals for applications in foods, pharmaceuticals, and cosmetics (9, 19, 20, 49). An economically feasible biosynthetic process for succinate has not yet been developed, although a recent study concluded that the production of succinate by fermentation has potential to become economic with improvements in process design and strain development (43).
Numerous studies have focused on bacterial succinate production. Media and process improvements with the obligate anaerobe Anaerobiospirullum succiniciproducens resulted in succinate production to a mass yield of 0.87 (grams per gram), a productivity of 1.9 g/liter/h at a pH of 6.1, and a molar succinate-to-acetate ratio of 2.06 (8, 41). An atmosphere of 5% H2-95% CO2 in the headspace resulted in a higher succinate yield (0.91), a productivity of 1.8 g/liter/h, and a succinate-to-acetate ratio of 2.16 (26). Succinate production from renewable feedstocks such as whey has also been reported with A. succiniciproducens, with a yield of greater than 90% (27, 42). Only a few other bacteria have been studied for succinate production. Succinate yield with Actinobacillus sp. strain 130Z is markedly influenced by the CO2, N2, and H2 composition in the headspace, and this organism achieves a yield of 0.74 under an atmosphere of pure H2, indicating that the presence of an additional electron donor is favorable for succinate production (47). Recently, Enterococcus sp. strain RKY1 was observed to convert fumarate to succinate at a high yield (0.98) with glycerol as the carbon source (40).
Escherichia coli also produces succinate, although as a minor product of fermentation for wild-type strains. E. coli principally converts phosphoenolpyruvate (PEP) and pyruvate formed through the Embden-Meyerhof-Parnas pathway under anaerobic conditions to formate, lactate, and ethanol (6), and genetic manipulations are necessary to increase succinate production and reduce by-product formation. For example, increasing flux at the first step in the succinate branch by overexpressing PEP carboxylase results in an increase in succinate yield from 0.12 to 0.45 (34). Because PEP is also a required cosubstrate for glucose transport via the phosphotransferase system (PTS) in wild-type E. coli, another approach is to direct pyruvate to the succinate branch. A wild-type E. coli strain transformed with plasmid pTrc99A-pyc, which expresses Rhizobium etli pyruvate carboxylase, resulted in a succinate yield of 0.17 and a productivity of 0.17 g/liter/h (16).
Diversion of carbon to succinate alone is insufficient to prevent the accumulation of other undesired products; therefore, mutations in lactate- and formate-forming steps can further improve succinate production. Mutants of E. coli deficient only in fermentative lactate dehydrogenase (ldh) did not exhibit decreased anaerobic growth (31), while E. coli NZN111, which lacks both the pyruvate-formate lyase (pfl) and ldh genes, exhibited marginal anaerobic growth on glucose (3). NZN111 accumulated pyruvate to about 0.25 g/liter before metabolism ceased, even when supplied with acetate (1). However, when transformed with the mdh gene encoding malate dehydrogenase, E. coli NZN111 grew anaerobically (1). Similarly, when the gene encoding malic enzyme from Ascaris suum was transformed into NZN111, succinate yield was 0.39 and productivity was 0.29 g/liter/h (45, 46). Donnelly et al. (13) reported an unknown spontaneous chromosomal mutation in NZN111 which permitted anaerobic growth on glucose, and this strain was named AFP111. AFP111 grown anaerobically under 5% H2-95% CO2 resulted in a succinate yield of 0.70 and a molar succinate-to-acetate ratio of 1.97 (13). Moreover, this strain first grown aerobically for biomass generation and then subjected to anaerobic conditions (under CO2) (dual-phase fermentation) resulted in a succinate yield and a productivity as high as 0.99 and 0.87 g/liter/h, respectively (35). Recently, the causative mutation in AFP111 was mapped to the ptsG gene, which encodes an enzyme of the PTS (4).
Of the few studies concerning strains NZN111 and AFP111, only one (with NZN111 grown anaerobically) reported pyruvate accumulation (45). Since some carbon presumably flows from PEP to pyruvate, this central metabolite may accumulate in the absence of assimilating enzymes, and accumulation likely will depend on the mode of growth: exclusively anaerobic fermentations versus dual-phase fermentations. Furthermore, the pathways used by E. coli to generate high yields of succinate are ambiguous. Specifically, previous studies focused on the reductive arm of the tricarboxylic acid (TCA) cycle (i.e., oxaloacetate [OAA] → malate → fumarate → succinate) as the mode of succinate formation (4, 13, 45, 46). However, this route from glucose does not result in a balance between reduced and oxidized cofactors (NADH and NAD), raising the question of whether this pathway is exclusively involved in succinate production. No study has presented enzyme activities which might clarify the biochemical pathways to succinate in these two multiply mutated strains under these two distinct growth and product formation conditions. Since E. coli lacks the anaplerotic enzyme pyruvate carboxylase, pyruvate accumulation and succinate formation in these strains could be altered by directing pyruvate to the succinate branch with this enzyme. As already mentioned, wild-type E. coli expressing R. etli pyruvate carboxylase resulted in increased succinate production (17). The objectives of this study therefore were to elucidate the biochemical pathways used for succinate accumulation by examining differences in key enzyme activities between E. coli strains NZN111 and AFP111 and to determine for these strains the effect of R. etli pyruvate carboxylase on succinate accumulation in both exclusively anaerobic fermentations and aerobic-anaerobic dual-phase fermentations.
MATERIALS AND METHODS
Strains and plasmids.
All strains and plasmids used in this study are listed in Table 1. The ppc gene encodes the enzyme PEP carboxylase. To construct AFP111 Δppc, a P1 lysate from ALS804 was used to transduce AFP111 to Tetr. To verify that the ppc::Kan deletion had been introduced into AFP111, a P1 lysate was prepared from AFP111 Δppc and used to transduce MG1655 to Tetr. The MG1655 Tetr transductant colonies were then scored for Kanr to show that the ppc::Kan deletion was linked to the zii-510::Tn10 transposon as expected. To construct ALS804, a P1 lysate from CGSC6390 was used to transduce JCL1242 to Tetr on rich medium with tetracycline and kanamycin in order to preserve the ppc::Kan deletion.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| NZN111 | F+ λ−rpoS396(Am) rph-1 Δ(pflAB::Cam) ldhA::Kan | Bunch et al. (3) |
| AFP111 | NZN111 ptsG | Donnelly et al. (13) |
| Chatterjee et al. (4) | ||
| CGSC6390 | thr-1 araC14 leuB6 fhuA31 lacY1 tsx-78 Δ[galK-att (λ)]99 λ−eda-50 hisG4(Oc) rpsL136 (Strr) xylA5 mtl-1 zii-510::Tn10 metF159(Am)thi-1 | E. coli Genetic Stock Center |
| MG1655 | Wild type (F− λ−) | 20a |
| JCL1242 | F− λ− Δ (argF-lac)U169 ppc::Kan | 3a |
| ALS804 | JCL1242 zii-510::Tn10 | This study |
| AFP111 Δppc | AFP111 ppc::Kan | This study |
| Plasmid | ||
| pTrc99A-pyc | R. etli pyc bla lacIqtrc ColE1 | Gokarn et al. (18) |
Fermentation medium.
Anaerobic fermentations contained 25 g of Luria-Bertani broth/liter and 10 g of glucose/liter. The pH of the medium was maintained at between 6.7 and 7.3 by supplementing the medium with 40 g of MgCO3/liter.
Each dual-phase fermentation was performed with complex medium which contained (in grams per liter) the following: glucose, 20; yeast extract, 10; tryptone, 20; K2HPO4·3H2O, 0.90; KH2PO4, 1.14; (NH4)2SO4, 3.0; MgSO4·7H2O, 0.50; and CaCl2·2H2O, 0.25. At the onset of the anaerobic phase, 40 g of MgCO3/liter was added in a sterile manner to serum bottles to maintain the pH at between 6.4 and 7.2.
The medium was supplemented with 1.0 mg of biotin/liter and 1.0 mg of thiamine/liter, and 100 mg of ampicillin/liter was added for the strains that contained plasmid pTrc99A-pyc. Pyruvate carboxylase expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1.0 mM unless otherwise indicated.
Growth conditions.
Anaerobic fermentations (100 ml) were performed with serum bottles under an atmosphere of pure CO2 or pure H2 and agitated at 250 rpm. Serum bottles were inoculated with 10 ml of aerobically grown culture. Dual-phase fermentations (100 ml) comprised an aerobic growth phase followed by an anaerobic production phase. The aerobic phase occurred in baffled 500-ml flasks agitated at 250 rpm for 8 h. The anaerobic phase subsequently commenced when the culture was transferred to sterile serum bottles with oxygen-free CO2 sparged to displace air from the headspace. Samples were collected at the moment of phase transition and at the end of the anaerobic phase. All anaerobic and dual-phase fermentations were performed at 37°C in triplicate with independent inocula. Statistical analyses were completed using Student's t test, and a P value of <0.10 was considered the criterion for significance.
Analyses.
Cell growth during the aerobic phase was monitored by measuring the optical density (OD) at 550 nm (DU-650 UV-Vis spectrophotometer; Beckman Instruments, San Jose, Calif.). OD during the anaerobic phase was not measured due to interference by solid MgCO3. Samples were centrifuged (10,000 × g for 10 min at 25°C), and the supernatants were analyzed for sugars, organic acids, and ethanol by high-pressure liquid chromatography as previously described (14).
Enzyme assays.
Cell extracts of the E. coli strains were prepared by washing the cell pellets with an appropriate buffer and disrupting the suspended cells with an SLM-Aminco French pressure cell (Spectronic Instruments, Rochester, N.Y.) at a pressure of 14,000 lb/in2. Cell debris was removed by centrifugation (20,000 × g for 15 min at 4°C), and cell extracts were used to measure enzyme activities. The following enzymes were examined: acetate kinase (25), fumarate reductase (33), glucokinase (36), isocitrate dehydrogenase (44), isocitrate lyase (12), PEP carboxylase (29), and pyruvate carboxylase (37). For all of these enzymes, 1 U of enzyme activity is the quantity of enzyme that converts 1 μmol of substrate to product per min. Total protein in the cell extracts was determined with bovine serum albumin as the standard (28).
RESULTS
Substrate and products present during exclusively anaerobic growth.
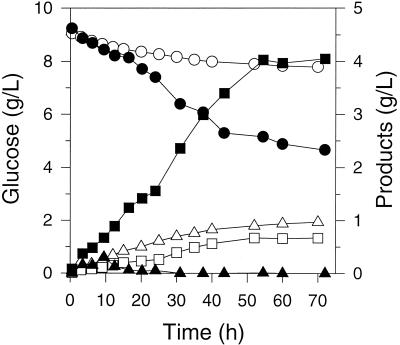
We first compared the products formed during exclusively anaerobic fermentations of E. coli NZN111 and AFP111 with and without pTrc99A-pyc. Fermentations of NZN111 without pTrc99A-pyc were terminated at 72 h after the rate of succinate production ceased (Fig. 1). For this strain, glucose was consumed very slowly (0.018 g/liter/h), and about 1.0 g of pyruvate/liter and 0.6 g of succinate/liter were the principal end products. AFP111 (Fig. 1) consumed glucose more quickly (0.057 g/liter/h) and generated succinate to a final concentration of 4.0 g/liter; pyruvate accumulated to about 0.4 g/liter by 10 h, before itself being consumed completely by about 30 h.
FIG. 1.
Concentrations of glucose (circles), succinate (squares), and pyruvate (triangles) in exclusively anaerobic fermentations of E. coli NZN111 (open symbols) and AFP111 (solid symbols) on glucose-rich media.
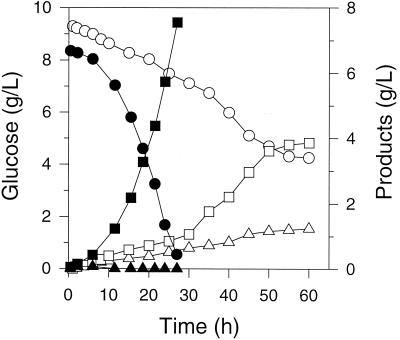
We studied two levels of pyruvate carboxylase expression for both strains; minimal pyruvate carboxylase expression was obtained by excluding IPTG, and a comparatively high level of pyruvate carboxylase expression was obtained by using 1.0 mM IPTG. Without IPTG induction, NZN111/pTrc99A-pyc consumed glucose and produced succinate four to six times faster than NZN111 (Fig. 2). Also, NZN111/pTrc99A-pyc yielded a final succinate concentration of 4.0 g/liter and a final pyruvate concentration of 1.2 g/liter. Without induction, AFP111/pTrc99A-pyc similarly consumed glucose and generated succinate more quickly than AFP111, yielding a succinate concentration of nearly 8.0 g/liter (Fig. 2).
FIG. 2.
Concentrations of glucose (circles), succinate (squares), and pyruvate (triangles) in exclusively anaerobic fermentations of E. coli NZN111/pTrc99A-pyc (open symbols) and AFP111/pTrc99A-pyc (solid symbols) on glucose-rich media. The strains were not induced with IPTG at the onset of the fermentations.
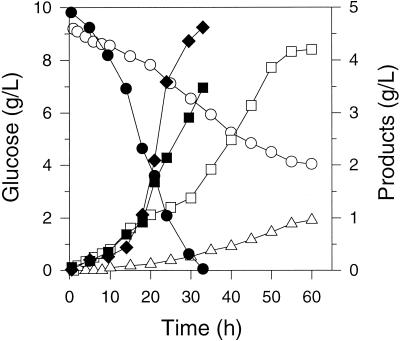
Fermentations with NZN111/pTrc99A-pyc in the presence of 1.0 mM IPTG were similar to fermentations with this strain in the absence of IPTG induction (Fig. 3). AFP111/pTrc99A-pyc with IPTG also consumed glucose at the same rate (0.28 g/liter/h) as this strain without IPTG. However, for AFP111/pTrc99A-pyc, both succinate and fumarate were significant products, with a molar succinate-to-fumarate ratio of 43:57 and a combined productivity of 0.25 g/liter/h. As in other AFP111 fermentations, pyruvate accumulated slightly by 10 h before being consumed. When hydrogen was used in the headspace instead of carbon dioxide for AFP111/pTrc99A-pyc fermentations (with IPTG), fumarate did not accumulate, and succinate productivity was 0.35 g/liter/h.
FIG. 3.
Concentrations of glucose (circles), succinate (circles), pyruvate (triangles), and fumarate (diamonds) in exclusively anaerobic fermentations of E. coli NZN111/pTrc99A-pyc (open symbols) and AFP111/pTrc99A-pyc (solid symbols) on glucose-rich media. The strains were induced with 1.0 mM IPTG at the onset of the fermentations.
Product yields in exclusively anaerobic fermentations are summarized in Table 2. For NZN111 strains, increasing the level of pyruvate carboxylase expression resulted in increased succinate and decreased pyruvate accumulations. For AFP111 strains, a low level of pyruvate carboxylase expression resulted in an insignificant increase in succinate accumulation compared to the results obtained when the pyc gene was absent. However, a high level of pyruvate carboxylase expression resulted in both succinate and fumarate generation. Replacement of carbon dioxide in the headspace with hydrogen restored the succinate yield. Acetate yield was much lower in NZN111 strains than in AFP111 strains without the pyc gene or with a low level of gene expression. With a high level of pyruvate carboxylase expression, however, acetate yield generated by AFP111/pTrc99A-pyc was reduced to the level generated by NZN111 strains. Ethanol yield did not appear to follow any trend and was no greater than 0.07 for any of the strains.
TABLE 2.
Mass yields of products during exclusively anaerobic growth on glucose-rich mediaa
| Strain | Headspace | IPTG (mM) | Yield (g of product/g of glucose) ofb:
|
||||
|---|---|---|---|---|---|---|---|
| Succinate | Pyruvate | Acetate | Ethanol | Fumarate | |||
| NZN111 | CO2 | 0.0 | 0.53 a | 0.76 a | 0.06 a | 0.06 a | 0.00 a |
| NZN111/pTrc99A-pyc | CO2 | 0.0 | 0.77 b | 0.25 b | 0.09 b | 0.03 b | 0.00 a |
| NZN111/pTrc99A-pyc | CO2 | 1.0 | 0.81 c | 0.19 c | 0.11 c | 0.05 c | 0.00 a |
| AFP111 | CO2 | 0.0 | 0.88 d | 0.00 d | 0.22 d | 0.07 d | 0.00 a |
| AFP111/pTrc99A-pyc | CO2 | 0.0 | 0.96 d | 0.00 d | 0.23 d | 0.06 ade | 0.07 b |
| AFP111/pTrc99A-pyc | CO2 | 1.0 | 0.35 e | 0.00 d | 0.09 abc | 0.06 ae | 0.47 c |
| AFP111/pTrc99A-pyc | H2 | 1.0 | 0.91 d | 0.00 d | 0.11 c | 0.07 ade | 0.00 a |
Serumbottles were under an atmosphere of CO2 or H2.
Yields followed by different letters were statistically significantly different at the 90% confidence level.
Enzyme activities present during exclusively anaerobic growth.
We also compared the enzyme activities during exclusively anaerobic fermentations of NZN111 and AFP111 with and without pTrc99A-pyc. Specific activities were measured for seven enzymes involved in the formation of the products (Table 3). In NZN111 and AFP111, PEP carboxylase is the only enzyme that directs carbon toward OAA for succinate production. When the strains were grown under exclusively anaerobic conditions, several significant differences in specific enzyme activities were observed between NZN111 and AFP111. AFP111 showed much higher activities than NZN111 for acetate kinase (about 5 times higher), fumarate reductase (2 times as high), and glucokinase (about 50 times higher). AFP111 was also observed to have slightly higher activity for PEP carboxylase.
TABLE 3.
Enzyme activities during exclusively anaerobic growth on glucose-rich mediaa
| Strain | Headspace | IPTG (mM) | Sp act (U/mg of protein) ofb:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ACK | FR | GK | ICDH | ICL | PPC | PYC | |||
| NZN111 | CO2 | 0.0 | 0.20 ab | 0.17 a | 0.018 a | 0.00 | 0.00 | 0.10 a | 0.00 a |
| NZN111/pTrc99A-pyc | CO2 | 0.0 | 0.15 a | 0.26 b | 0.025 a | 0.00 | 0.00 | 0.061 b | 0.050 b |
| NZN111/pTrc99A-pyc | CO2 | 1.0 | 0.27 b | 0.33 c | 0.11 b | 0.00 | 0.00 | 0.020 c | 0.086 bc |
| AFP111 | CO2 | 0.0 | 1.11 c | 0.45 cd | 0.98 c | 0.00 | 0.00 | 0.13 d | 0.00 ad |
| AFP111/pTrc99A-pyc | CO2 | 0.0 | 1.24 c | 0.52 d | 1.08 d | 0.00 | 0.00 | 0.11 ad | 0.056 bd |
| AFP111/pTrc99A-pyc | CO2 | 1.0 | 1.42 d | 0.68 e | 1.30 e | 0.00 | 0.00 | 0.057 b | 0.12 c |
| AFP111/pTrc99A-pyc | H2 | 1.0 | 1.79 e | 0.74 e | 1.58 f | 0.00 | 0.00 | 0.099 a | 0.17 e |
Serum bottles were under an atmosphere of CO2 or H2.
Enzyme activities followed by different letters were statistically significantly different at the 90% confidence level. Enzyme abbreviations: ACK, acetate kinase; FR, fumarate reductase; GK, glucokinase; ICDH, isocitrate dehydrogenase; ICL, isocitrate lyase; PPC, PEP carboxylase; PYC, pyruvate carboxylase.
As expected, full induction of the pyc gene with 1.0 mM IPTG resulted in the highest pyruvate carboxylase activities for both NZN111/pTrc99A-pyc and AFP111/pTrc99A-pyc. Lower but significant activities were observed in these strains without the addition of IPTG. The activities of acetate kinase, fumarate reductase, and glucokinase generally increased with increasing pyruvate carboxylase activity for both strains. In contrast, the activity of PEP carboxylase decreased with increasing pyruvate carboxylase activity. Indeed, the sums of PEP carboxylase and pyruvate carboxylase activities (about 0.11 U/mg of protein) were not significantly different for NZN111, NZN111/pTrc99A-pyc without IPTG, and NZN111/pTrc99A-pyc with IPTG. Except when hydrogen was used in the headspace, the sums of the activities of these two enzymes were 0.13 to 0.17 for AFP111, AFP111/pTrc99A-pyc without IPTG, and AFP111/pTrc99A-pyc with IPTG. Isocitrate lyase and isocitrate dehydrogenase activities were not detected during exclusively anaerobic fermentations for any of the strains. The use of hydrogen in the headspace instead of carbon dioxide for AFP111/pTrc99A-pyc resulted in the highest enzyme activities observed during anaerobic growth for acetate kinase, glucokinase, and pyruvate carboxylase.
Substrate and products present during dual-phase fermentations.
Exclusively anaerobic fermentations resulted in certain activities for key enzymes and relatively slow substrate consumption and product formation. Different enzyme activities would generally be expected during aerobic growth; therefore, an anaerobic phase following aerobic growth might lead to a product distribution vastly different from that observed during exclusively anaerobic growth. In order to elucidate how product formation might be different after aerobic growth, we studied dual-phase fermentations. Each strain was grown aerobically for 8 h, after which an anaerobic production phase commenced. Fermentations were terminated at 24 h or when the substrate was depleted. Cell growth at the end of the aerobic phase was determined by measuring the OD at 8 h. Each product yield was calculated as the mass of that product formed during the anaerobic phase divided by the mass of the substrate consumed during the anaerobic phase (Table 4). Average productivities and glucose consumption rates were also calculated for the anaerobic phase.
TABLE 4.
Mass yields of products during the anaerobic phase after aerobic growth on glucose-rich media
| Strain | IPTG (mM) | OD at 8 h | Yield (g of product/g of glucose) ofa:
|
||||
|---|---|---|---|---|---|---|---|
| Succinate | Pyruvate | Acetate | Ethanol | Fumarate | |||
| NZN111 | 0.0 | 6.5 a | 0.56 a | 0.50 a | 0.03 a | 0.05 a | 0.00 |
| NZN111/pTrc99A-pyc | 0.0 | 8.9 b | 0.81 b | 0.15 b | 0.24 b | 0.14 b | 0.00 |
| NZN111/pTrc99A-pyc | 1.0 | 9.3 c | 0.93 cd | 0.05 cd | 0.09 c | 0.00 c | 0.00 |
| AFP111 | 0.0 | 10.4 d | 0.85 be | 0.01 c | 0.12 d | 0.05 a | 0.00 |
| AFP111/pTrc99A-pyc | 0.0 | 10.3 d | 0.90 ce | 0.06 d | 0.19 e | 0.04 a | 0.00 |
| AFP111/pTrc99A-pyc | 1.0 | 15.5 e | 0.96 d | 0.06 cd | 0.21 be | 0.00 c | 0.00 |
| AFP111 Δppc | 0.0 | 9.9 bcd | 0.54 a | 0.20 b | −1.06 f | 0.15 b | 0.00 |
Yields followed by different letters were statistically significantly differentat the 90% confidence level.
In dual-phase fermentations, NZN111 again yielded a product distribution different from that of AFP111. NZN111 consumed glucose at 0.17 g/liter/h during the anaerobic phase, leading to final succinate and pyruvate concentrations of about 3 g/liter. In contrast, AFP111 consumed glucose at 0.71 g/liter/h and led to greater than 8 g of succinate/liter and less than 1 g of pyruvate/liter. AFP111 consistently grew more quickly than NZN111 during the 8-h aerobic phase, achieving a 60% higher cell density. The specific rate of glucose consumption was also about three times higher in AFP111 than in NZN111 during the subsequent anaerobic phase.
For NZN111/pTrc99A-pyc and AFP111/pTrc99A-pyc, two levels of pyc gene expression were again studied. When IPTG was excluded, NZN111/pTrc99A-pyc consumed glucose more quickly (0.26 g/liter/h) and generated more succinate (yield, 0.81) than NZN111. When IPTG was provided, NZN111/pTrc99A-pyc consumed glucose at about the same rate but generated more succinate (yield, 0.93). NZN111/pTrc99A-pyc achieved about a 30% higher cell density during the aerobic phase than NZN111 regardless of whether IPTG was added to the medium. In dual-phase fermentations without IPTG, AFP111/pTrc99A-pyc consumed glucose at 1.01 g/liter/h and generated succinate at a yield of 0.90. With IPTG, the succinate yield was slightly higher (0.96), although the glucose consumption rate and succinate-to-acetate ratio were statistically identical to those observed without IPTG. No fumarate was observed in any of these dual-phase fermentations. No ethanol was observed in NZN111/pTrc99A-pyc or AFP111/pTrc99A-pyc fermentations which included IPTG.
Since the presence of pyruvate carboxylase increases succinate yield in the two strains, we were interested in learning whether succinate production would be abolished in the absence of both pyruvate carboxylase and PEP carboxylase. We therefore constructed an AFP111 ppc mutant, AFP111 Δppc, and similarly performed a set of dual-phase fermentations. As expected, AFP111 Δppc grew much more slowly than the other strains (Table 4) and by 8.0 h had accumulated about 3.6 g of acetate/liter (data not shown). Interestingly, during the anaerobic production phase, both acetate and glucose were consumed, resulting in a succinate yield of 0.54, a pyruvate yield of 0.20, and an ethanol yield of 0.15. Significantly more pyruvate and ethanol and less succinate were produced by AFP111 Δppc than by AFP111.
We also grew the strains in dual-phase fermentations using the same medium but excluding glucose. In these experiments, the cells grew to about the same OD but did not generate any of these products during the anaerobic production phase (data not shown).
Enzyme activities present during dual-phase fermentations.
Specific activities were again measured for seven enzymes at the onset of the anaerobic phase (Table 5). Specific enzyme activities were consistently higher after 8 h of aerobic growth than at the end of exclusively anaerobic growth. Also, significant differences were observed between NZN111 and AFP111 after aerobic growth. The activities of acetate kinase and glucokinase were again much higher in AFP111 than in NZN111. However, the activities of fumarate reductase, PEP carboxylase, and isocitrate lyase (not detected under exclusively anaerobic conditions) were higher in NZN111 than in AFP111. Isocitrate dehydrogenase was not detected in NZN111 but was detected in AFP111.
TABLE 5.
Enzyme activities at the onset of the anaerobic phase after aerobic growth on glucose-rich media
| Strain | IPTG (mM) | Sp act (U/mg of protein) ofa:
|
||||||
|---|---|---|---|---|---|---|---|---|
| ACK | FR | GK | ICDH | ICL | PPC | PYC | ||
| NZN111 | 0.0 | 0.82 a | 1.30 a | 0.26 a | 0.00 a | 1.56 a | 0.44 a | 0.00 a |
| NZN111/pTrc99A-pyc | 0.0 | 0.70 b | 0.90 b | 0.30 a | 0.00 a | 0.33 b | 0.38 b | 0.17 b |
| NZN111/pTrc99A-pyc | 1.0 | 0.93 c | 1.35 c | 0.29 a | 0.00 a | 0.38 b | 0.26 c | 0.49 c |
| AFP111 | 0.0 | 1.41 d | 0.85 bc | 1.30 b | 6.20 b | 0.37 b | 0.35 b | 0.00 a |
| AFP111/pTrc99A-pyc | 0.0 | 1.54 e | 1.35 a | 1.49 c | 4.62 c | 0.93 c | 0.61 d | 0.72 d |
| AFP111/pTrc99A-pyc | 1.0 | 1.64 f | 0.77 bc | 1.51 c | 3.85 c | 2.31 d | 0.49 abd | 0.81 e |
| AFP111 Δppc | 1.0 | 2.18 g | 0.11 d | 2.68 d | 1.93 d | 4.48 e | 0.03 e | 0.00 a |
Enzyme activities followed by different letters were statistically significantly different at the 90% confidence level. See Table 3, footnote b, for enzyme abbreviations.
The highest activities of pyruvate carboxylase were obtained with 1.0 mM IPTG for both NZN111/pTrc99A-pyc and AFP111/pTrc99A-pyc, with lower activities observed for these strains in the absence of IPTG. The activities of acetate kinase consistently increased and the activities of PEP carboxylase generally decreased with increasing pyruvate carboxylase activities for both strains. No correlation was observed between pyruvate carboxylase activities and the activities of fumarate reductase, glucokinase, isocitrate lyase, and isocitrate dehydrogenase. For any level of pyruvate carboxylase activity, however, the activities of acetate kinase, glucokinase, and isocitrate dehydrogenase were always higher in AFP111 strains than in NZN111 strains.
We also measured the activities of these seven enzymes for AFP111 Δppc. The acetate kinase, glucokinase, and isocitrate lyase activities were much higher in this strain than under any other condition. The activities of fumarate reductase and isocitrate dehydrogenase were lower than those observed for other AFP111 strains in dual-phase fermentations. As expected, the activities of PEP carboxylase and pyruvate carboxylase were insignificant.
DISCUSSION
In this study, we compared two doubly mutated (ldh pfl) strains of E. coli, NZN111 and AFP111, in the absence and presence of the enzyme pyruvate carboxylase and with two different modes of growth. The synthesis of OAA is a key step in the production of succinate. In most eukaryotes and some prokaryotes, OAA is replenished both from PEP and from pyruvate by PEP carboxylase and pyruvate carboxylase, respectively. However, in wild-type E. coli, PEP carboxylase represents the principal anaplerotic reaction to replenish OAA. That portion of PEP not flowing to OAA is converted to pyruvate, and under anaerobic conditions for NZN111 and AFP111 (in the absence of the assimilating enzymes lactate dehydrogenase and pyruvate formate lyase), pyruvate was observed to accumulate. When these strains are transformed with pTrc99A-pyc, which expresses pyruvate carboxylase from R. etli, E. coli is provided with another anaplerotic route to OAA formation (17, 18), and the strains show decreased pyruvate accumulation and concomitant increased succinate production. Moreover, we observed increased cell growth rates and glucose consumption rates under anaerobic or aerobic conditions for either of these strains when this additional anaplerotic route was available.
NZN111 and AFP111 are different, and pyruvate carboxylase benefits each strain differently toward the goal of succinate accumulation. NZN111 has been reported to grow very slowly on glucose in the absence of oxygen (3, 45, 46), while AFP111 isolated as a result of a ptsG mutation in NZN111 grows more quickly (4, 46). Both strains have been reported to accumulate significant quantities of succinate during anaerobic growth (35, 45, 46). Since E. coli is a facultative anaerobe, another approach to generate succinate is dual-phase fermentation, that is, aerobic growth followed by an anaerobic production phase (22). Such dual-phase fermentations have the advantage of largely uncoupling growth and product formation; thus, unique operational conditions may be applied to each phase. Additionally, enzymes which carry out biotransformations in the production phase are expressed during the aerobic phase and remain active throughout the second phase. Because the activities of key enzymes generally will differ during anaerobic growth and aerobic growth, we measured enzyme activities and products formed in strains NZN111 and AFP111 under both conditions and in the presence of the additional anaplerotic route afforded by pyruvate carboxylase.
The significant findings in this study are the demonstration of enhanced glucokinase activity in AFP111 strains and the observation of isocitrate lyase activity when either strain is grown aerobically. Thus, our results show two means of glucose consumption and two paths from PEP to succinate. The two general routes which E. coli uses to transport and phosphorylate glucose differ in E. coli strains NZN111 and AFP111. One route involves two multienzyme systems, collectively termed the PTS, which concomitantly transport and phosphorylate glucose to intracellular glucose 6-phosphate by using PEP as a cosubstrate (32, 38, 39). This route ultimately leads to the formation of both PEP and pyruvate, and the resulting net reaction may be expressed as
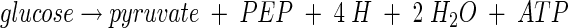 |
(1) |
The 1 mol of PEP formed in this reaction is available to PEP carboxylase to generate OAA or to pyruvate kinase to generate a second mole of pyruvate and ATP. In the absence of PEP synthase activity during growth on glucose (21), the 1 mol committed to pyruvate is not available for direct conversion to OAA. Wild-type E. coli can still grow in the absence of the PTS, and a mutation in the glk gene for glucokinase is necessary to eliminate growth on glucose completely (7). Thus, a second route for glucose uptake involves glucose transport uncoupled from phosphorylation, a route which generally appears to be insignificant compared to the PTS in wild-type E. coli. This route requires the action of glucokinase intracellularly to phosphorylate glucose with ATP as a cosubstrate and leads ultimately to the formation of PEP. The resulting net reaction may be expressed as
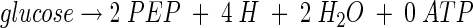 |
(2) |
In this situation, 2 mol of PEP is available to PEP carboxylase for OAA formation. Of course, 1 mol of PEP could form pyruvate via pyruvate kinase with the generation of ATP so that the ultimate equations for the two routes to pyruvate are equivalent. In this study, for both anerobically and aerobically grown cells, AFP111 showed markedly higher glucokinase activity than NZN111.
From three-carbon intermediates, succinate may be formed by two means: via the reductive arm of the TCA cycle or via the glyoxylate shunt. The reductive branch of the TCA cycle converts OAA to malate, fumarate, and then succinate. From a three-carbon precursor of OAA (PEP or pyruvate), this path requires the incorporation of four electrons and 1 mol of CO2. The net equation of this C3 + C1 pathway is
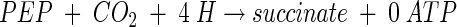 |
(3) |
The glyoxylate shunt operates as a cycle to convert 2 mol of acetyl coenzyme A (acetyl-CoA) to succinate. From 2 mol of the three-carbon precursor pyruvate, one cycle around the glyoxylate pathway generates six electrons and 2 mol of CO2. The net equation of this C2 + C2 pathway is
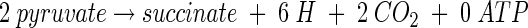 |
(4) |
Figure 4 shows the biochemical pathways leading from glucose consumption to succinate synthesis when each of these pathways is present (including the conversion of pyruvate to OAA by pyruvate carboxylase). The glyoxylate shunt has not been shown to be important in the formation of succinate, and it is most commonly associated with microbial growth on acetate (21, 24). However, the glyoxylate shunt appears to play an important role during high-cell-density aerobic fermentation with E. coli (23, 48). The genes for isocitrate lyase and malate synthase constitute an operon (2, 5) and are regulated by the fadR gene (30). In this study, the key glyoxylate shunt enzyme, isocitrate lyase, was not detected with either strain grown under anaerobic conditions but was observed with both NZN111 and AFP111 after 8 h of aerobic growth. Because the two strains differ in their modes of glucose uptake and growth conditions affect the expression of isocitrate lyase, one would expect differences in the distribution of end products between the two strains and during anaerobic growth and aerobic growth.
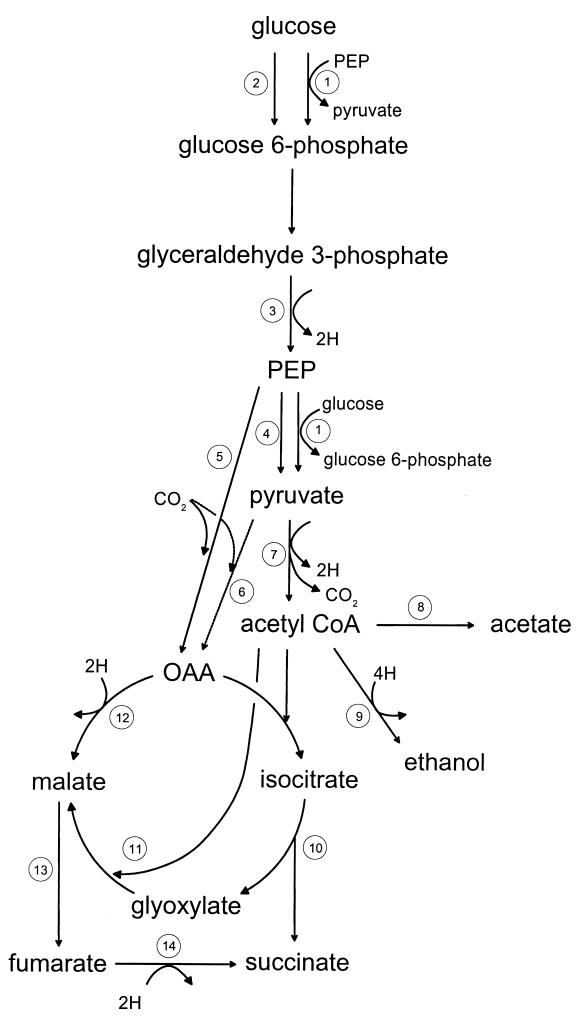
FIG. 4.
Biochemical pathways for the synthesis of succinate from glucose in E. coli. Not all enzymatic steps or intermediates are shown. Key enzymes in the pathways are as follows: 1, PTS; 2, glucokinase; 3, glyceraldehyde phosphodehydrogenase; 4, pyruvate kinase; 5, PEP carboxylase; 6, pyruvate carboxylase; 7, pyruvate dehydrogenase complex; 8, phosphoacetyltransferase and acetate kinase; 9, acetaldehyde dehydrogenase and alcohol dehydrogenase; 10, isocitrate lyase; 11, malate synthase; 12, malate dehydrogenase; 13, fumarase; and 14, fumarate reductase.
Both glucose consumption routes to three-carbon intermediates generate four electrons per glucose. Since the C3 + C1 pathway requires eight electrons per mol of glucose to form 2 mol of succinate and the C2 + C2 pathway generates six electrons to form 1 mol of succinate per mol of glucose, neither of these two succinate-producing pathways alone is sufficient to balance the electrons in the overall conversion of glucose to succinate. The maximum possible yield of succinate based solely on a carbon balance occurs when all the succinate is formed via the C3 + C1 pathway (46), providing a mass yield of 1.31 based on glucose. However, the maximum possible succinate yield based on the necessary additional constraint of a redox balance is 1.714 mol of succinate from 1 mol of glucose, providing a mass yield of 1.12. In the absence of an additional electron donor, this maximum theoretical yield necessitates that both pathways function from three-carbon intermediates to succinate and that specifically 71.4% of the carbon flows to OAA and 28.6% of the carbon flows to acetyl-CoA. If the glyoxylate shunt is not active, as we observed during exclusively anaerobic growth, then this maximal yield of succinate cannot be achieved.
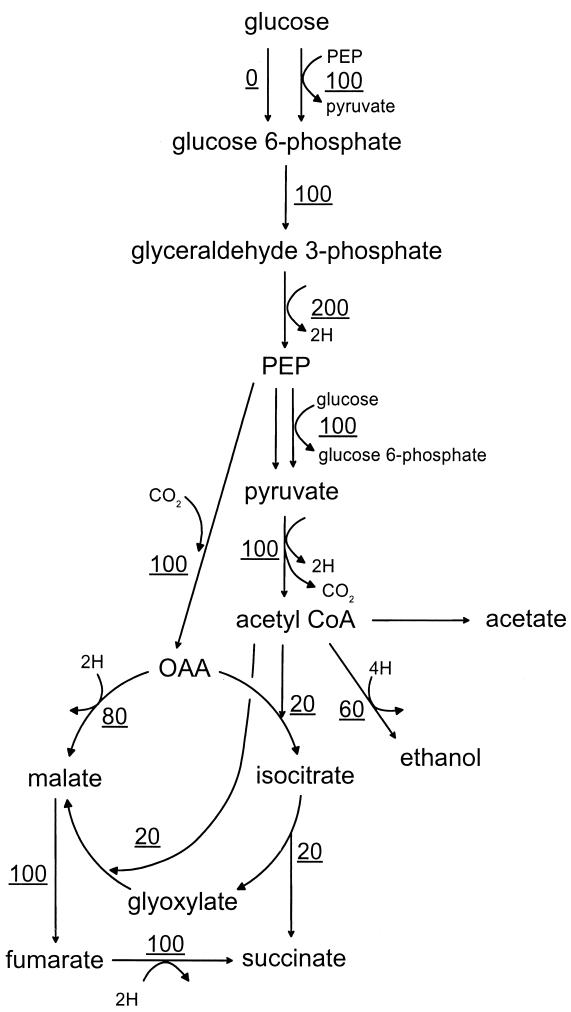
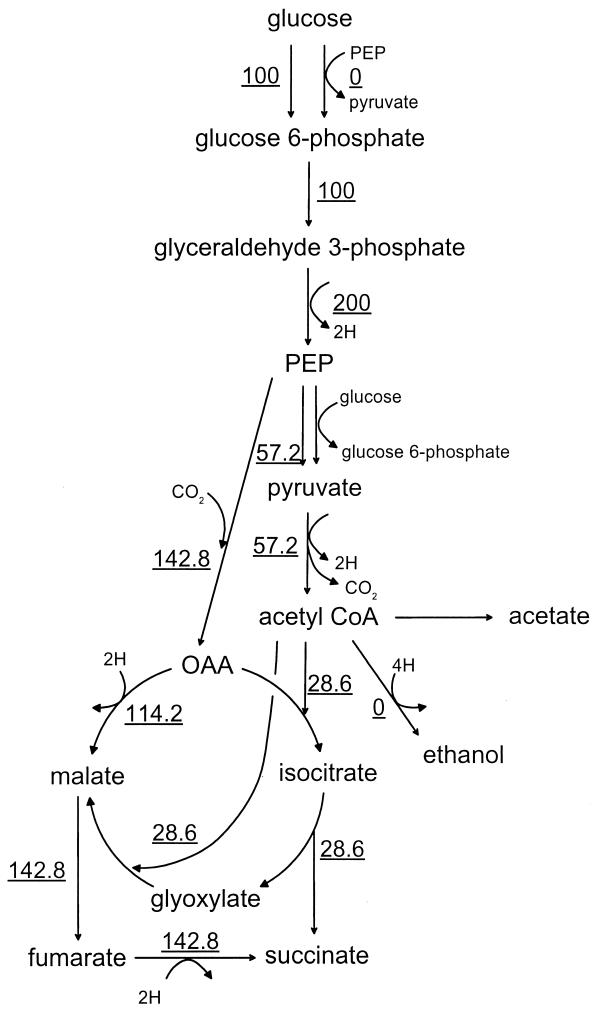
Without the pyc gene, NZN111 and AFP111 have only one means for PEP to flow directly to OAA, and that is via PEP carboxylase. For these strains, the two routes for glucose uptake result in vastly different maximal succinate yields, a fact which can be illustrated by considering the two extreme cases of glucose uptake—either exclusively via the PTS or exclusively via glucokinase. For a strain relying exclusively on the PTS for glucose uptake, because half of the carbon is committed to pyruvate by the PTS, only 50% of the carbon is available for subsequent conversion to succinate via the C3 + C1 pathway. This fraction is lower than the 71.4% needed for a maximum theoretical yield of 1.12. In this situation, the maximum succinate yield is attained when the 1 mol of PEP generated from glucose is entirely converted to OAA (and the flux from PEP to pyruvate via pyruvate kinase is zero). Such a scenario satisfying a redox balance could generate 1.20 mol of succinate per mol of glucose, with 17% of the succinate coming from the glyoxylate shunt, for a mass yield of 0.79. Figure 5 shows the carbon fluxes necessary to achieve this theoretical maximum succinate yield in a strain which exclusively uses the PTS for glucose uptake. For a strain relying exclusively on glucokinase for glucose uptake, all carbon from glucose is available for subsequent conversion to succinate via the C3 + C1 pathway. In this situation, 28.6% of the PEP can flow through pyruvate kinase to pyruvate to achieve the maximum succinate mass yield of 1.12, satisfying a redox balance. Figure 6 shows the carbon fluxes necessary to achieve this theoretical maximum succinate yield in a strain which exclusively uses glucokinase for glucose uptake. Note that in the present study, actual carbon fluxes could not be calculated because for NZN111 strains, the fractions of glucose consumed by glucokinase and the PTS cannot be distinguished because the presence of pyruvate carboxylase creates a mathematical singularity at the PEP-pyruvate-OAA nodes and because the fraction of carbon flowing through the glyoxylate shunt cannot be determined. The optimal ratio of molar flux through the reductive branch of the TCA cycle to the molar flux through glyoxylate is always 5.0.
FIG. 5.
Flux through biochemical pathways corresponding to the maximum theoretical yield of succinate in the absence of pyruvate carboxylase when the PTS is the exclusive means of glucose uptake. The data are based on 100 molar U of glucose uptake.
FIG. 6.
Flux through biochemical pathways corresponding to the maximum theoretical yield of succinate in the absence of pyruvate carboxylase when glucokinase is the exclusive means of glucose uptake. The data are based on 100 molar U of glucose uptake.
The differences between the observed activities of glucokinase in NZN111 and AFP111 demonstrate differences in the flexibility of the organisms in forming succinate. During exclusively anaerobic growth of NZN111, with the PTS dominating glucose uptake, nearly one-half of the carbon is committed to pyruvate. In the absence of isocitrate lyase activity, we observed pyruvate to accumulate to about twice the final molar concentration of succinate (Table 2). During exclusively anaerobic growth of AFP111, with glucose uptake occurring via glucokinase, glucose is converted to PEP and not committed to pyruvate. All carbon therefore potentially can be diverted to succinate via OAA, and we observed no pyruvate at the end of AFP111 fermentations. Pyruvate assimilation leading to acetate and ethanol formation in AFP111 was apparently just able to keep pace with pyruvate formation by pyruvate kinase. The higher level of acetate kinase activity in AFP111 than in NZN111 is consistent with a higher level of pyruvate assimilation under these anaerobic conditions.
Although NZN111 and AFP111 subjected to a dual-phase fermentation resulted in succinate and pyruvate yields similar to those observed for exclusively anaerobic growth, the rates at which these products were formed were much higher after aerobic growth, presumably because of the higher cell density and higher enzyme activities under these conditions. Calculation of carbon recovery in these fermentations is not possible because of the ambiguity in the relative amounts of carbon flowing through the C3 + C1 and C2 + C2 pathways and the fact that one pathway is carbon dioxide consuming and the other is carbon dioxide generating.
Strains with pyruvate carboxylase activity can more readily approach the maximum theoretical yield after aerobic growth because they can generate OAA from either PEP or pyruvate, regardless of whether PTS or glucokinase is the dominant means of glucose uptake. The addition of pyruvate carboxylase activity to NZN111 ameliorates PTS, which constrains succinate generation. The presence of high pyruvate carboxylase activity in NZN111 in the dual-phase fermentations resulted in a succinate yield (0.93) statistically equal to the yield observed with AFP111/pTrc99A-pyc (0.96). Table 6 summarizes the maximum theoretical succinate yields considering each of the constraints studied. The calculations demonstrate that the presence of both pyruvate carboxylase and isocitrate lyase activities is needed for optimal succinate production. The presence of both enzymes provides E. coli with greater flexibility to achieve the maximum theoretical succinate yield. Isocitrate lyase activity permits succinate to be the redox-balanced end product and prevents the accumulation of fumarate. Pyruvate carboxylase activity overcomes the commitment of carbon to pyruvate.
TABLE 6.
Maximum theoretical mass yields of succinate considering biochemical pathways available to E. colia
| Means of glucose uptake | Activity of:
|
Maximum theoretical succinate yield (g/g) | |
|---|---|---|---|
| PYC | ICL | ||
| PTS | + | + | 1.12 |
| + | − | 0.87 | |
| − | + | 0.79 | |
| − | − | 0.66 | |
| Glucokinase | + | + | 1.12 |
| + | − | 0.87 | |
| − | + | 1.12 | |
| − | − | 0.87 | |
These theoretical yields were calculated assuming that NADH and NAD are balanced as a result of central carbon metabolism. See Table 3, footnote b, for enzyme abbreviations. +, present; −, absent.
An important result is that the presence of pyruvate carboxylase provided via plasmid pTrc99A-pyc increased the rates of both glucose consumption and cell growth. This second result is contrary to common observations that the expression of heterologous cloned genes substantially reduces cell growth rate (reviewed recently by Diaz Ricci and Hernández [11]). Furthermore, increases in glucose uptake rate have been proposed to be due to enhanced expression of proteins involved in the PTS (10). In the current study, AFP111 with pyruvate carboxylase averaged a fivefold-higher glucose uptake rate under anaerobic conditions and achieved a 50% higher cell density after 8 h under aerobic conditions compared to AFP111 without pyruvate carboxylase. Since this organism appears not to have a significant PTS for glucose uptake, additional studies are needed to reconcile the reason why the growth and glucose uptake of this particular multiply mutated strain benefit from the additional anaplerotic reaction afforded by pyruvate carboxylase.
The level of pyruvate carboxylase activity affects the final product distribution, with fumarate being the redox-balanced end product. As noted above, 2 mol of NADH (4H) is produced for every mole of glucose consumed during glycolysis. NADH must be reoxidized to NAD for the fermentation to progress (6). This reoxidation is achieved by the reduction of OAA to either fumarate, which requires 1 mol of NADH, or succinate, which requires 2 mol of NADH. If all the carbon from PEP were to flow to OAA, we would expect fumarate to be the end product, a situation which would balance the NADH generated in glycolysis. In fact, if greater than 71.8% of the carbon from PEP were to flow to OAA (with a limitation of alcohol dehydrogenase activity), then a redox balance would necessitate fumarate to be present in addition to succinate. Thus, in those situations where both pyruvate carboxylase and PEP carboxylase activities are high and other pyruvate-assimilating enzyme activities are low, the large fraction of PEP expected to flow to OAA would result in some fumarate accumulation. For AFP111/pTrc99A-pyc grown anaerobically with IPTG (with no isocitrate lyase activity and hence limited pyruvate assimilation), we indeed did observe fumarate to accumulate to a molar fumarate-to-succinate ratio of 1.33. For this strain grown aerobically with IPTG, no fumarate was observed. The isocitrate lyase activity present under aerobic conditions may have thus been sufficient to balance the greater pyruvate carboxylase and PEP carboxylase activities by providing additional NADH (from the action of pyruvate dehydrogenase and malate dehydrogenase) for the fumarate-to-succinate step. Growing AFP111/pTrc99A-pyc anaerobically in the presence of hydrogen similarly prevented the accumulation of fumarate, suggesting that the strains have a mechanism for regenerating NAD by using hydrogen. Several previous studies reported the presence of lactate during fermentation of the ldh mutant NZN111 with malic enzyme or malate dehydrogenase (13, 22, 45). In the present study, we did not observe lactate but instead observed fumarate, a solute which commonly elutes at a retention time similar to that of lactate but which can readily be distinguished by UV absorbance (14).
One consistent observation was the higher activity of acetate kinase in AFP111 strains than in NZN111 strains for any set of conditions. This higher activity was correlated with a higher acetate yield in AFP111 than in NZN111 for either mode of fermentation, but not consistently for these strains with pyruvate carboxylase. When more carbon is diverted to succinate from PEP through OAA, this carbon, of course, does not flow to pyruvate via pyruvate kinase, an ATP-generating step. Without an additional ATP-generating step, one would therefore expect AFP111 to generate less ATP than NZN111, since the former generates more succinate and less pyruvate. Since the formation of acetate from acetyl phosphate also generates ATP, the higher yield of acetate in AFP111 than in NZN111 may be a result of the organism compensating for the reduced ATP generated via pyruvate formation. The first enzyme in the PTS can also be phosphorylated by acetate kinase and ATP (15), so that the heightened acetate kinase activity in AFP111 compared to NZN111 may also be related to the differences in glucose uptake in these two strains.
Strain AFP111 Δppc, lacking both pyruvate carboxylase and PEP carboxylase, was still able to accumulate succinate under dual-phase conditions. In this situation, the organism consumed acetate. Since the isocitrate lyase activity was over 10 times higher in AFP111 Δppc than in AFP111, it seems likely that a portion of the acetate yielded succinate. Also, since AFP111 Δppc appears to lack an effective pathway to regenerate NAD (the reductive arm of the TCA cycle is not available due to the absence of PEP carboxylase), the higher yield of ethanol that we observed with AFP111 Δppc (0.15) compared to AFP111 (0.05) is consistent with this strain meeting the demand for a redox balance. Finally, the accumulation of pyruvate in AFP111 Δppc (yield, 0.20) compared to AFP111 (yield, 0.01) is consistent with the former strain having limited means to regenerate NAD and the undesirability of converting pyruvate into acetyl-CoA via pyruvate dehydrogenase, a step which generates additional NADH.
In summary, the glyoxylate shunt is a key pathway for the accumulation of succinate by the two pfl ldh strains of E. coli that we studied. With the glyoxylate shunt not active during exclusively anaerobic growth, the inability to assimilate pyruvate leads to pyruvate accumulation in NZN111 and higher acetate formation in AFP111. Active during aerobic growth in these strains, the glyoxylate shunt provides a means for these organisms to sustain a redox balance under subsequent anaerobic conditions while converting carbon to succinate at yields of up to 0.96, based on glucose. Heightened glucokinase activity provides AFP111 with greater metabolic flexibility to form succinate optimally because less carbon is committed to pyruvate in the absence of an active PTS. Pyruvate carboxylase activity ameliorates the metabolic inflexibility of NZN111 and, for both strains, provides an alternate route to the reductive arm of the TCA cycle, a pathway through which the majority of carbon (71.4%) must flow to achieve a maximal succinate yield.
Acknowledgments
We thank the University of Georgia College of Agricultural and Environmental Sciences and the Georgia Agricultural Experiment Stations for financial support.
We acknowledge S. A. Lee, R. E. B. Ball, K. DeWitt, P. Reeves, and L. Sanderson for technical assistance and R. R. Gokarn for helpful discussions.
REFERENCES
- 1.Boernke, W. E., C. S. Millard, P. W. Stevens, S. N. Kakar, F. J. Stevens, and M. I. Donnelly. 1995. Stringency of substrate specificity of Escherichia coli malate dehydrogenase. Arch. Biochem. 322:43-52. [DOI] [PubMed] [Google Scholar]
- 2.Brice, C. G., and H. L. Kornberg. 1968. Genetic control of isocitrate lyase activity in Escherichia coli. J. Bacteriol. 96:2185-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldh gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187-195. [DOI] [PubMed] [Google Scholar]
- 3a.Chao, Y.-P., and J. C. Liao. 1993. Alteration of growth yield by overexpression of phosphoenolypyruvate carboxylase and phosphoenolpyruvate carboxykinase in Escherichia coli. Appl. Environ. Microbial. 59:4261-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee, R., C. S. Millard, K. Champion, D. P. Clark, and M. I. Donnelly. 2001. Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, T., D. J. Klumpp, and D. C. LaPorte. 1988. Glyoxylate bypass operon of Escherichia coli: cloning and determination of the functional map. J. Bacteriol. 170:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 63:223-234. [DOI] [PubMed] [Google Scholar]
- 7.Curtis, S. J., and W. Epstein. 1975. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucose phosphotransferase, mannose phosphotransferase, and glucokinase. J. Bacteriol. 122:1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta, R., D. A. Glassner, M. K. Jain, and J. R. Vick Roy. January1991. Fermentation and purification process for succinic acid. European patent 405,707.
- 9.Datta, R. September1992. Process for the production of succinic acid by anaerobic fermentation. U.S. patent 5,143,833.
- 10.Diaz Ricci, J. C., J. Bode, J. I. Rhee, and K. Schügerl. 1995. Gene expression enhancement due to plasmid maintenance. J. Bacteriol. 177:6684-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz Ricci, J. C., and M. E. Hernández. 2000. Plasmid effects on Escherichia coli metabolism. Crit. Rev. Biotechnol. 20:79-108. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, G. H., and H. L. Kornberg. 1959. Assay methods of key enzymes of the glyoxylate cycle. Proc. Biochem. Soc. 72:3P. [Google Scholar]
- 13.Donnelly, M. I., C. S. Millard, D. P. Clark, M. J. Chen, and J. W. Rathke. 1998. A novel fermentation pathway in an Escherichia coli mutant producing succinic acid, acetic acid, and ethanol. Appl. Biochem. Biotechnol. 70-72:187-198. [DOI] [PubMed] [Google Scholar]
- 14.Eiteman, M. A., and M. J. Chastain. 1997. Optimization of the ion-exchange analysis of organic acids from fermentation. Anal. Chim. Acta 338:69-75. [Google Scholar]
- 15.Fox, D. K., N. D. Meadow, and S. Roseman. 1986. Sugar-transport by the bacterial phosphotransferase system. 27. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J. Biol. Chem. 261:3498-3503. [PubMed] [Google Scholar]
- 16.Gokarn, R. R., E. Altman, and M. A. Eiteman. 1998. Metabolic analysis of Escherichia coli glucose fermentation in presence of pyruvate carboxylase. Biotechnol. Lett. 20:795-798. [Google Scholar]
- 17.Gokarn, R. R., M. A. Eiteman, and E. Altman. 2000. Metabolic analysis of Escherichia coli in the presence and absence of the carboxylating enzymes phosphoenolpyruvate carboxylase and pyruvate carboxylase. Appl. Environ. Microbiol. 66:1844-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokarn, R. R., J. D. Evans, J. R. Walker, S. A. Martin, M. A. Eiteman, and E. Altman. 2001. The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli. Appl. Microbiol. Biotechnol. 56:188-195. [DOI] [PubMed] [Google Scholar]
- 19.Guettler, M. V., M. K. Jain, and B. K. Soni. April1996. Process for making succinic acid, microorganisms for use in the process and methods of obtaining the microorganisms. U.S. patent 5,504,004.
- 20.Guettler, M. V., and M. K. Jain. May1996. Method for making succinic acid, Anaerobiospirillum succiniproducens variants for use in the process and methods for obtaining variants. U.S. patent 5,521,075.
- 20a.Guyer, M. S., R. R. Reed, J. A. Steitz, and K. B. Low. 1980.. Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harbor Symp. Quant. Biol. 45:135-140. [DOI] [PubMed] [Google Scholar]
- 21.Holms, W. H. 1986. The central metabolic pathways of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr. Top. Cell. Regul. 28:69-105. [DOI] [PubMed] [Google Scholar]
- 22.Hong, S. H., and S. Y. Lee. 2000. Metabolic flux distribution in a metabolically engineered Escherichia coli strain producing succinic acid. J. Microbiol. Biotechnol. 10:496-501. [Google Scholar]
- 23.Kleman, G. L., and W. R. Strohl. 1994. Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl. Environ. Microbiol. 60:3952-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kornberg, H. L., and N. B. Madsen. 1957. Synthesis of C4-dicarboxylic acids from acetate by a “glyoxylate bypass” of the tricarboxylic acid cycle. Biochim. Biophys. Acta 24:651-653. [DOI] [PubMed] [Google Scholar]
- 25.Lamed, R., and J. G. Zeikus. 1980. Glucose fermentation pathway of Thermoanaerobium brokii. J. Bacteriol. 141:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, P. C., W. G. Lee, S. Kwon, S. Y. Lee, and H. N. Chang. 1999. Succinic acid production by Anaerobiospirillum succiniproducens: effects of the H2/CO2 supply and glucose concentration. Enzyme Microb. Technol. 24:549-554. [Google Scholar]
- 27.Lee, P. C., W. G. Lee, S. Kwon, S. Y. Lee, and H. N. Chang. 2000. Batch and continuous cultivation of Anaerobiospirillum succiniproducens for the production of succinic acid from whey. Appl. Microbiol. Biotechnol. 54:23-27. [DOI] [PubMed] [Google Scholar]
- 28.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 29.Maeba, P., and B. D. Sanwal. 1969. Phosphoenolpyruvate carboxylase from Salmonella typhimurium strain LT2. Methods Enzymol. 13:283-288. [Google Scholar]
- 30.Maloy, S. R., M. Bohlander, and W. D. Nunn. 1980. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J. Bacteriol. 143:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mat-Jan, F., K. Y. Alam, and D. P. Clark. 1989. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J. Bacteriol. 171:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadow, N. D., D. K. Fox, and S. Roseman. 1990. The bacterial phosphoenolpyruvate:glycose phosphotransferase system. Annu. Rev. Biochem. 59:497-542. [DOI] [PubMed] [Google Scholar]
- 33.Melville, S. B., T. A. Michel, and J. M. Macy. 1988. Pathway and sites for energy conservation in the metabolism of glucose by Selenemonas ruminantium. J. Bacteriol. 170:5298-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millard, C. S., Y. P. Chao, J. C. Liao, and M. I. Donnelly. 1996. Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl. Environ. Microbiol. 62:1808-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nghiem, N. P., M. Donnelly, C. S. Millard, and L. Stols. February1999. U.S. patent 5,869,301.
- 36.Pakoskey, A. M., E. C. Lesher, and D. B. M. Scott. 1965. Hexokinase of Escherichia coli. Assay of enzyme activity and adapation to growth in various media. J. Gen. Microbiol. 38:73-80. [DOI] [PubMed] [Google Scholar]
- 37.Payne, J., and J. G. Morris. 1969. Pyruvate carboxylase in Rhodopseudomonas spheroides. J. Gen. Microbiol. 59:97-101. [DOI] [PubMed] [Google Scholar]
- 38.Postma, P. W., and J. W. Lengeler. 1985. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol. Rev. 49:232-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 40.Ryu, H.-W., K.-H. Kang, and J.-S. Yun. 1999. Bioconversion of fumarate to succinate using glycerol as a carbon source. Appl. Biochem. Biotechnol. 77-79:511-520. [DOI] [PubMed] [Google Scholar]
- 41.Samuelov, N. S., R. Lamed, S. Lowe, and J. G. Zeikus. 1991. Influence of CO2-HCO3− levels and pH on growth, succinate production, and enzyme activities of Anaerobiospirillum succiniciproducens. Appl. Environ. Microbiol. 57:3013-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuelov, N. S., R. Datta, M. K. Jain, and J. G. Zeikus. 1999. Whey fermentation by Anaerobiospirillum succiniciproducens for production of a succinate-based animal feed additive. Appl. Environ. Microbiol. 65:2260-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schilling, L. B. 1995. Chemicals from alternative feedstocks in the United States. FEMS Microbiol. Rev. 16:101-110. [Google Scholar]
- 44.Soundar, S., G. T. Jennings, McAllister-Henn, L., and Colman, R. F. 1996. Expression of pig heart mitochondrial NADP-dependent isocitrate dehydrogenase in Escherichia coli. Protein Expr. Purif. 8:305-312. [DOI] [PubMed] [Google Scholar]
- 45.Stols, L., G. Kulkarni, B. G. Harris, and M. I. Donnelly. 1997. Expression of Ascaris suum malic enzyme in a mutant Escherichia coli allows production of succinic acid from glucose. Appl. Biochem. Biotechnol. 63-65:153-158. [DOI] [PubMed] [Google Scholar]
- 46.Stols, L., and M. I. Donnelly. 1997. Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl. Environ. Microbiol. 63:2695-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Werf, M. J., M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1997. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch. Microbiol. 167:332-342. [DOI] [PubMed] [Google Scholar]
- 48.Van de Walle, M., and J. Shiloach. 1998. Proposed mechanism of acetate accumulation in two recombinant Escherichia coli strains during high density fermentation. Biotechnol. Bioeng. 57:71-78. [DOI] [PubMed] [Google Scholar]
- 49.Zeikus, J. G., M. K. Jain, and P. Elankovan. 1999. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 51:545-552. [Google Scholar]