Abstract
The soft rot bacteria Erwinia carotovora and Erwinia chrysanthemi are important pathogens of potato and other crops. However, the taxonomy of these pathogens, particularly at subspecies level, is unclear. An investigation using amplified fragment length polymorphism (AFLP) fingerprinting was undertaken to determine the taxonomic relationships within this group based on their genetic relatedness. Following cluster analysis on the similarity matrices derived from the AFLP gels, four clusters (clusters 1 to 4) resulted. Cluster 1 contained Erwinia carotovora subsp. carotovora (subclusters 1a and 1b) and Erwinia carotovora subsp. odorifera (subcluster 1c) strains, while cluster 2 contained Erwinia carotovora subsp. atroseptica (subcluster 2a) and Erwinia carotovora subsp. betavasculorum (subcluster 2b) strains. Clusters 3 and 4 contained Erwinia carotovora subsp. wasabiae and E. chrysanthemi strains, respectively. While E. carotovora subsp. carotovora and E. chrysanthemi showed a high level of molecular diversity (23 to 38% mean similarity), E. carotovora subsp. odorifera, E. carotovora subsp. betavasculorum, E. carotovora subsp. atroseptica, and E. carotovora subsp. wasabiae showed considerably less (56 to 76% mean similarity), which may reflect their limited geographical distributions and/or host ranges. The species- and subspecies-specific banding profiles generated from the AFLPs allowed rapid identification of unknown isolates and the potential for future development of diagnostics. AFLP fingerprinting was also found to be more differentiating than other techniques for typing the soft rot erwinias and was applicable to all strain types, including different serogroups.
The genus Erwinia is a member of the family Enterobacteriaceae and consists of 18 species that fall into two main groups, the necrogenic, or Amylovora, group and the soft rot, or Carotovora, group (15, 26, 34). Within the soft rot group, Erwinia carotovora and Erwinia chrysanthemi are the most commercially important soft rotting pathogens. Until 1981, E. carotovora contained only two subspecies, Erwinia carotovora subsp. carotovora (causing soft rot diseases, mainly in storage, on a wide variety of plant species, including potato) and Erwinia carotovora subsp. atroseptica (causing a vascular disease [blackleg] of potato plants and storage rot of potato tubers). More recently, a number of new subspecies have been included, namely, Erwinia carotovora subsp. betavasculorum (causing soft rot of sugar beet [37]), Erwinia carotovora subsp. wasabiae (originally isolated from Japanese horseradish but causing soft rot disease of various vegetables, including potato [16]), and Erwinia carotovora subsp. odorifera (isolated from and causing disease in chicory, leeks, and celery [15]). Hauben et al. (18) reclassified E. carotovora and E. chrysanthemi into the genus Pectobacterium based on 16S ribosomal DNA (rDNA) sequence analysis. Although both Erwinia and Pectobacterium are validly published names for this group of pathogens, throughout the remainder of this manuscript they will be referred to as the soft rot erwinias, E. carotovora (with subspecies E. carotovora subsp. atroseptica, E. carotovora subsp. carotovora, E. carotovora subsp. betavasculorum, E. carotovora subsp. odorifera, and E. carotovora subsp. wasabiae) and E. chrysanthemi.
Understanding the diversity within and relationships among pathogenic taxa is an important prerequisite to meaningful taxonomic classification, accurate identification, pathogen detection, and epidemiology studies. This is particularly important when a number of closely related subspecies cause disease on the same host, e.g., E. chrysanthemi, E. carotovora subsp. carotovora, E. carotovora subsp. atroseptica, and E. carotovora subsp. wasabiae on potato. Several taxonomic and identification studies of the soft rot erwinias have been undertaken using molecular techniques, such as DNA-DNA hybridization (3-5, 15), PCR-restriction fragment length polymorphism (RFLP) (2, 9, 20, 31), ribotyping (30), and 16S rDNA analyses (18, 27), and phenotypic techniques, including biochemistry (12-14) and API identification strips (11, 29, 41). Although a small number of studies have included the more recent subspecies E. carotovora subsp. betavasculorum, E. carotovora subsp. odorifera, and E. carotovora subsp. wasabiae (15, 18, 27), they have been limited by the number of strains used. Studies using the highly conserved 16S rDNA gene (18, 27) have confirmed the heterogeneity of the genus Erwinia, which forms four clusters intermixed with other genera. They have also shown that the soft rot erwinias fall into a single cluster, in one case leading to the renaming of members of this group as Pectobacterium (18). However, the technique approaches its limit of taxonomic resolution at subspecies level (40), and the accuracy of the groupings for the E. carotovora subspecies is thus questionable. Furthermore, one study (27) did not investigate all subspecies, and in both studies only one strain of each subspecies was used, offering no information about the genetic variation within each subspecies.
Amplified fragment length polymorphism (AFLP) is a genomic fingerprinting method first described by Vos et al. (42), with an effective taxonomic resolution from species to strain level (40), which has been used to study the taxonomy and genetic diversity of a number of organisms, including a growing list of bacteria (6, 7, 21, 23-25, 36, 38). The aims of this study were to investigate the utility of the AFLP technique for the taxonomic classification of the soft rot erwinias at species and subspecies levels and as a method of identification, to generate markers for the development of diagnostics, and to type strains for epidemiological investigations.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in this study are listed in Tables 1 and 2. They consist of over 130 well-characterized soft rot erwinias and other strains from both established and recent culture collections from around the world. In addition, over 200 recent isolates from potato, collected both locally and from Australia, were included in the study. The reference strains are species type strains obtained from the National Collection of Plant Pathogenic Bacteria (NCPPB), York, United Kingdom. In cases where type strains were not available from the NCPPB, strains identified by the NCPPB as belonging to certain species or subspecies were used. The bacterial strains were stored in freezing medium at −80°C (1), and all cultures were maintained on nutrient agar (code CM3; Oxoid) at 18°C. When required, Erwinia species were grown at 27°C, while other enterobacteria were grown at 37°C in Luria broth medium for 18 h with shaking.
TABLE 1.
Soft rot erwinia strains used in this study
| Bacterial strain | Host | Location | Source |
|---|---|---|---|
| E. carotovora subsp. carotovora | |||
| SCRI 108 | Potato | Finland | P. Harju |
| SCRI 112 (NCPPB 1746)a | Potato | Japan | D. C. Graham |
| SCRI 134 | Cyclamen | Israel | V. M. Lumb |
| SCRI 167 | Water | Israel | M. C. M. Pérombelon |
| SCRI 174 | Potato | Peru | E. French; J. G. Elphinstone |
| SCRI 193 | Potato | United States | M. P. Starr |
| SCRI 196 | Caladium | United States | D. C. Graham |
| SCRI 212 | Potato | Scotland | P. Harju |
| SCRI 244, SCRI 295 | Potato | Scotland | R. Lowe; L. J. Hyman |
| SCRI 258 | Potato | Israel | V. M. Lumb |
| AU3b, AU6b, AU8b, AU9b, AU12b, AU14b, AU43b, AU44b, AU55b, AU56b | Potato | Australia | T. Wicks |
| E. carotovora subsp. atroseptica | |||
| SCRI 13, SCRI 47, SCRI 53, SCRI 54, SCRI 59, SCRI 83, SCRI 93, SCRI 1035, SCRI 1039, SCRI 1040, G999P6b, G994A7b, G998F1b, G98292b, G998V8b, G991N8b, G98218b | Potato | Scotland | L. J. Hyman |
| SCRI 33 | Water | Scotland | L. J. Hyman |
| SCRI 37 | Soil | Scotland | L. J. Hyman |
| SCRI 49 | Insect | Scotland | L. J. Hyman |
| SCRI 135 | Potato | Arizona | M. Stanghellini |
| BSB 9202, BSB 9205, BSB 9207, BSB 9302, H1 | Potato | Germany | F. Neipold |
| IPO 161, N88.30, N88.37, N88.40, N88.41, N88.45, N88.46, N88.49, N88.52, J12, IPO 723, IPO 848, IPO 850, IPO 852, IPO 856, IPO 861, IPO 862, IPO 1005, IPO 1008 | Potato | Netherlands | J. M. Van der Wolf |
| Z141, Z144, Z147, Z148, Z149, Z150, Z153, Z406, Z413, Z414 | Potato | Sweden | P. Persson |
| 494, 495, 310 (88.33), 311 (88.45), 312 (87.7), 315 (86.14.11) | Potato | France | Y. Bertheau |
| 581-1.1, 1342-47, 1346-8, 1346-11, 1347-39, 1348-37, 1366-19 | Potato | Spain | M. Lopez |
| E. carotovora subsp. betavasculorum | |||
| SCRI 479 (NCPPB 2795)ac, SCRI 908 (NCPPB 2792)a, SCRI 909 (NCPPB 2793)a, SCRI 910 (NCPPB 2794)a, SCRI 911 (NCPPB 3075)a | Sugar beet | United States | NCPPB |
| E. carotovora subsp. odorifera | |||
| SCRI 482 (NCPPB 3840), SCRI 912 (NCPPB 3839), SCRI 913 (NCPPB 3841), SCRI 914 (NCPPB 3842), SCRI 915 (NCPPB 3843), SCRI 916 (NCPPB 3844) SCRI 487 | Chicory | France | NCPPB |
| E. carotovora subsp. wasabiae | |||
| SCRI 481 (NCPPB 3701), SCRI 917 (NCPPB 3702), SCRI 918 (NCPPB 3703), SCRI 919 (NCPPB 3704) | Horseradish | Japan | NCPPB |
| SCRI 488 | Horseradish | Japan | Y. Bertheau |
| E. chrysanthemi | |||
| pv, unspecified | |||
| SCRI 417, SCRI 4039 | Potato | Peru | E. French; J. G. Elphinstone |
| SCRI 4000 (IPO 645) | Potato | Netherlands | E. Cother |
| SCRI 4044 (IPO 502) (biovar 7) | Potato | Netherlands | J. M. Van der Wolf |
| SCRI 4076 (NCPPB 1125)ad | Pineapple | Malaysia | Y. Bertheau |
| E. chrysanthemi | |||
| pv. dianthicola | |||
| SCRI 401 (NCPPB 426), SCRI 409 (NCPPB 518)ad | Carnation | Denmark | D. C. Graham |
| E. chrysanthemi | |||
| pv. zeae | |||
| SCRI 4071 (NCPPB 2541) (biovar 7)ad | Maize | United States | NCPPB |
| SCRI 4072 (NCPPB 2547) (biovar 7)ad | Maize | India | NCPPB |
| SCRI 4078 (NCPPB 1065)ad | Maize | Egypt | D. C. Graham |
| E. chrysanthemi | |||
| pv. dieffenbachiae | |||
| SCRI 4074 (NCPPB 1514)ad | Dieffenbachia | Germany | Y. Bertheau |
Authenticity of culture verified by NCPPB.
Previously uncharacterized isolate.
Type strain.
Pathogenicity of culture confirmed by NCPPB.
TABLE 2.
Other strains used in this study
| Bacterial strain | Host | Location | Source |
|---|---|---|---|
| Pantoea agglomerans | |||
| SCRI 435 | Red deer; human | United States | D. C. Graham |
| SCRI 462 | Gypsophila | Israel | H. Vigodsky-Haas |
| Pantoea stewartii | |||
| SCRI 475 (NCPPB 449)ab | Maize | United States | NCPPB |
| Pantoea ananatis | |||
| SCRI 485 (NCPPB 1846)abc | Pineapple | Brazil | NCPPB |
| SCRI 922 (NCPPB 544) | Pineapple | Hawaii | NCPPB |
| Enterobacter cancerogenus | |||
| SCRI 489 (NCPPB 2176)c, SCRI 920 (NCPPB 2177)a | Carolina poplar | Not known | NCPPB |
| Enterobacter dissolvens | |||
| SCRI 921 (NCPPB 1850)ac | Maize | United States | NCPPB |
| Enterobacter nimipressuralis | |||
| SCRI 491 (NCPPB 2045)ac, SCRI 901 (NCPPB 440) | Elm | United States | NCPPB |
| Erwinia rhapontici | |||
| SCRI 421 (NCPPB 139)a | Rhubarb | United Kingdom | D. C. Graham |
| SCRI 423 (NCPPB 1578)ac | Rhubarb | United Kingdom | NCPPB |
| Erwinia uredovora | |||
| SCRI 432 (NCPPB 800)ac | Wheat | United States | D. C. Graham |
| Erwinia cypripedii | |||
| SCRI 478 (NCPPB 2636)b | Orchid | Germany | NCPPB |
| Erwinia quercina | |||
| SCRI 442, SCRI 477 | Oak | United States | D. C. Graham |
| Erwinia rubrifaciens | |||
| SCRI 445 (NCPPB 2020)ac, SCRI 446 (NCPPB 2021)a | Persian walnut | United States | D. C. Graham |
| Erwinia nigrifluens | |||
| SCRI 476 (NCPPB 564)ac | Persian walnut | United States | NCBBP |
| Erwinia amylovora | |||
| SCRI 444 | Pear | United States | D. C. Graham |
| SCRI 906 (NCPPB 595)ad | Pear | United Kingdom | NCPPB |
| SCRI 907 (NCPPB 686)a | Crataegus spp. | United Kingdom | NCPPB |
| Erwinia salicis | |||
| SCRI 474 (NCPPB 447)ac, SCRI 905 (NCPPB 2535)a | Cricket-bat willow | United Kingdom | NCPPB |
| Erwinia persicinus | |||
| SCRI 480 (NCPPB 3375) | French bean | United States | NCPPB |
| Erwinia cacticida | |||
| SCRI 484 (NCPPB 3849) | Giant cactus | United States | NCPPB |
| Erwinia mallotivora | |||
| SCRI 490 (NCPPB 2851)abc, SCRI 903 (NCPPB 2852)ab | Mallotus japonicus | Japan | NCPPB |
| Erwinia psidii | |||
| SCRI 492 (NCPPB 3555)a, SCRI 902 (NCPPB 3556)a | Guava | Brazil | NCPPB |
| Erwinia tracheiphila | |||
| SCRI 900 (NCPPB 2133) | Cucumber | United States | NCPPB |
Authenticity of culture verified by NCPPB.
Pathogenicity of culture confirmed by NCPPB.
Type strain.
Authenticity and virulence have been well confirmed.
AFLP.
Bacterial genomic DNA was extracted and purified using a DNeasy tissue kit (Qiagen) as described by the manufacturer. The DNA was stored at −20°C until it was required. The AFLP reactions were performed as described previously (42) with minor modification. Bacterial genomic DNA (0.5 μg) was digested with 5 U each of the restriction enzymes EcoRI and MseI in OnePhorAll buffer (Amersham Pharmacia Biotech) at 10 μg ml−1 for 3 h at 37°C. MseI (50 pmol; 5′-GACGATGAGTCCTGAG-3′ and 5′-TACTCAGGACTCAT-3′) and EcoRI (5 pmol; 5′-CTCGTAGACTGCGTACC-3′ and 5′-AATTGGTACGCAGTC-3′) double-stranded adapters were ligated to the digested DNA in a total volume of 35 μl using 1 U of T4 DNA ligase in OnePhorAll buffer (10 μg ml−1) plus 1 mM ATP for 3 h at 37°C. Following ligation, the DNA was first amplified by PCR using nonselective MseI (M00) (5′-GATGAGTCCTGAGTAA-3′) and EcoRI (E00) (5′-GACTGCGTACCAATTC-3′) primers in a 25-μl total volume. Each reaction mixture contained 2 μl of the ligation mixture, 2.5 μl of AmpliTaq LD buffer, 1 U of AmpliTaq LD (Perkin-Elmer), all four deoxynucleoside triphosphates at 200 μM, and 50 ng each of the MseI and EcoRI primers. The PCR was performed under the following conditions: 35 cycles of 30 s of denaturing at 94°C, 30 s of annealing at 60°C, and 1 min of extension at 72°C. All amplifications were performed in a PE-9600 thermocycler (Perkin-Elmer). The amplification products were then diluted threefold, and 0.5 μl was used as a template for selective PCR with primers M00 and E19 (an EcoRI primer with the extension GA). In each selective PCR, the EcoRI primers were radiolabeled for 1 h at 37°C. The labeling reaction contained 3.5 ng of primer, 0.125 U of T4 polynucleotide kinase (Invitrogen), 0.1 μl of 5× forward reaction buffer provided with the enzyme, and 0.5 μCi (18.5 kBq) of [γ-33P]ATP (Amersham), and the total volume was adjusted to 0.5 μl with sterile distilled water. The selective PCR mixture in a total volume of 11 μl contained 1 μl of Perkin-Elmer AmpliTaq LD buffer, all four deoxynucleoside triphosphates at 200 μM, 15 ng of M00 primer, 3.5 ng of labeled EcoRI primer, and 1 U of Taq polymerase (Invitrogen). The selective PCR was performed under the following conditions: (i) 1 cycle of 30 s of denaturing at 94°C, 30 s of annealing at 65°C, and 1 min of extension at 72°C; (ii) 11 cycles over which the annealing temperature was reduced from 65°C by 0.7°C each cycle; and (iii) 23 cycles of 30 s of denaturing at 94°C, 30 s of annealing at 56°C, and 1 min of extension at 72°C. To the completed reactions, 10 μl of gel loading buffer (94% formamide, 10 mM EDTA, 0.5 mg of xylene cyanol FF ml−1, 0.5 mg of bromophenol blue ml−1) was added. Samples were heated to 90°C for 5 min and cooled on ice. The AFLP products were electrophoresed through a 6% polyacrylamide denaturing gel (sequencing gel; Severn Biotech Ltd.) at 100 W. After being dried, the gels were exposed to autoradiographic film (Kodak) for 24 to 72 h to visualize the results. The autoradiographs were converted to TIF format, and the data were analyzed using GelCompar version 4.1 software (Applied Maths) as described by the manufacturer. E. carotovora subsp. atroseptica strain SCRI 1039 was used as a reference strain to normalize tracks from different gels.
Biochemical and phenotypic tests.
Biochemical tests, including production of phosphatase and indole; utilization of citrate; acid production from α-methyl glucoside, palatinose, sorbitol, melibiose, and lactose; reducing substances from sucrose; and growth in 5% NaCl and on nutrient agar at 37°C were performed as described previously (13). Cavity formation on crystal violet pectate medium at 27, 33.5, and 37°C was assessed as described previously (33).
Data analysis.
Following electrophoresis of polyacrylamide gels, the autoradiographs were digitized and band profiles were analyzed using GelCompar software. The Pearson product-moment correlation coefficient was used to estimate levels of similarity between densitometric profiles for each isolate. Unweighted pair-group method of averages (UPGMA) and neighbor-joining algorithms within GelCompar were then used to construct dendrograms from the similarity matrices (22).
RESULTS AND DISCUSSION
Reproducibility of AFLP and choice of restriction enzymes.
To determine the reproducibility of AFLP profiles, a standard strain (E. carotovora subsp. atroseptica SCRI 1039) was used for each AFLP amplification and on each electrophoresis gel. The AFLP profile of this strain was also used to normalize gels in GelCompar. Following normalization, the similarity between profiles from this strain was at least 90% within each gel and 85% between gels, using the Pearson product-moment correlation. This variation appeared to be in the intensities of the lanes and may be due to differences in exposure of the autoradiograph, as previously noted (6, 21, 24).
The restriction enzymes EcoRI and MseI, together with different combinations of 0-, 1-, and 2-bp primer extensions, were tested on representative strains of E. carotovora and other Erwinia and enterobacterial species. Following these tests, primers M00 (no extensions) and E19 (2-bp extensions), which generated between 30 and 50 clearly distinguishable bands for each strain tested (with the total number of bands for cluster analysis being considerably higher), were chosen for the remainder of the study.
AFLP fingerprinting of soft rot erwinias and other Erwinia and enterobacterial species.
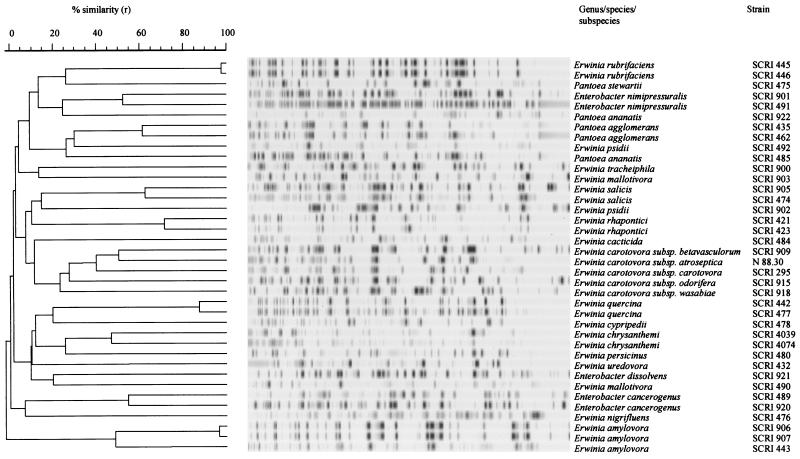
When different species and subspecies of Erwinia and other genera (selected as outgroups) were compared using AFLP fingerprinting under the conditions chosen for the study, a high level of heterogeneity was observed (Fig. 1). The infrequency of shared AFLP bands provided little information on the molecular relationships among species. However, this has not been the case for all genera, e.g., Acinetobacter (24), Bacillus (25), Burkolderia (6), Ralstonia (7), Stenotrophomonas (19), and Xanthomonas (36). Where bands of similar size were observed, this may simply have arisen due to comigration of unrelated bands on the gel (homoplasy), a possibility that is increasingly likely with more distantly related organisms (8). In the majority of cases where more than one strain of a species was analyzed, related but nonidentical profiles were obtained (Fig. 1), indicating the utility of AFLPs to differentiate within species. As expected, the soft rot erwinias did group, and thus, these conditions were used for a more detailed analysis of the group.
FIG. 1.
Dendrogram derived from the UPGMA linkage of correlation coefficients between AFLP profiles from soft rot erwinias, and other erwinias and enterobacterial species. The levels of linkage representing the Pearson product-moment correlation coefficient (r) are expressed as percentages for convenience. The banding profiles against each branch represent normalized and background-subtracted digitized gel strips processed in GelCompar.
AFLP fingerprinting of the soft rot erwinias.
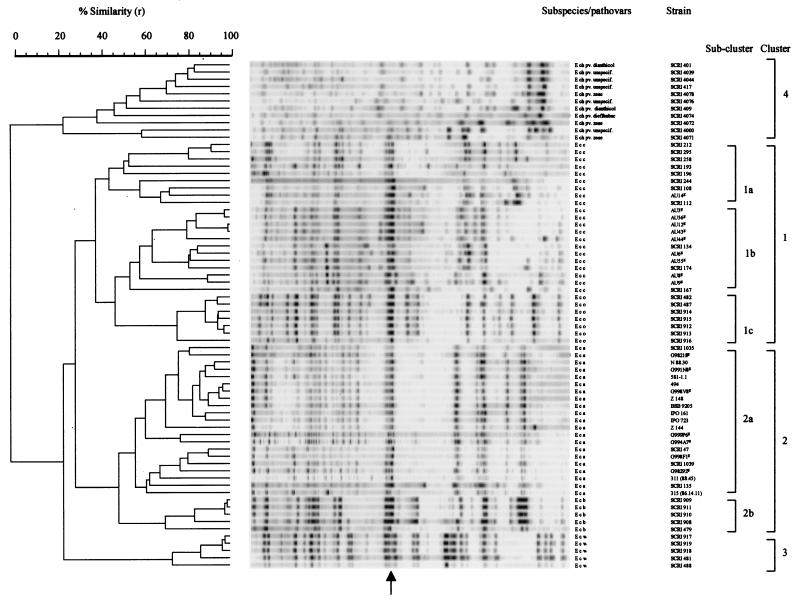
A selection of type strains and other well-characterized strains from the soft rot erwinias were investigated (Tables 1 and 2) and, in all cases, known species and subspecies were clearly distinguished by AFLP (Fig. 2). Following numerical analysis of the AFLP banding profiles, whether by UPGMA or neighbor joining (the results shown are for UPGMA only), the taxa clustered into four broad groups (clusters 1 to 4) (Fig. 2). Cluster 1 contained all E. carotovora subsp. carotovora and E. carotovora subsp. odorifera strains, which linked at a relatively low similarity (38.4% ± 12.6%). However, the cluster was further divided into three subclusters, 1a (n = 9), 1b (n = 12), and 1c (n = 7), with all E. carotovora subsp. carotovora strains in subclusters 1a and 1b (38.4% ± 12.6% similarity) and all E. carotovora subsp. odorifera strains in subcluster 1c (75.8% ± 2.8% similarity). Although E. carotovora subsp. carotovora appeared in two subclusters, this did not reflect any obvious subdivision in terms of host or geographic origin. Such intragroup diversity in E. carotovora subsp. carotovora had been noted previously with PCR-RFLP (9, 20) and may simply reflect the high molecular diversity within the subspecies. E. carotovora subsp. odorifera-specific banding profiles clearly distinguished E. carotovora subsp. odorifera from E. carotovora subsp. carotovora, supporting previous results using DNA-DNA hybridization (15) and 16S rDNA sequencing (18). However, the latter study, which included representatives from all subspecies, used only one strain of each, offering no information on diversity within these subspecies. API tests (11, 41) also led to the formation of a distinct cluster for all chicory strains of “atypical” E. carotovora subsp. atroseptica (“atypical” now E. carotovora subsp. odorifera), related to but separate from E. carotovora subsp. carotovora.
FIG. 2.
Cluster analysis of E. chrysanthemi and E. carotovora subspecies together with suggested species and subspecies clusters (clusters 1 to 4). ¥ indicates a previously uncharacterized strain. The dendrogram is derived from the UPGMA linkage of correlation coefficients between AFLP profiles. The levels of linkage representing the Pearson product-moment correlation coefficient (r) are expressed as percentages for convenience. The banding profiles against each branch represent normalized and background-subtracted digitized gel strips processed in GelCompar. The arrow indicates E. carotovora-specific AFLP bands. Ech, E. chrysanthemi; Ecc, E. carotovora subsp. carotovora; Eco, E. carotovora subsp. odorifera; Eca, E. carotovora subsp. atroseptica; Ecb, E. carotovora subsp. betavasculorum; Ecw, E. carotovora subsp. wasabiae.
Cluster 2 contained all E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum strains, with the two subspecies linking at 49.0% ± 7.5% similarity. This cluster was further divided into two subclusters, 2a (n = 21) and 2b (n = 5), which were composed of all E. carotovora subsp. betavasculorum strains, grouped at 70.2% ± 3.8% similarity, and all E. carotovora subsp. atroseptica strains, grouped at 56.6% ± 10.4% similarity, respectively. The results clearly showed that E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum were related but distinct taxa (Fig. 2). 16S rDNA sequencing (18) showed E. carotovora subsp. atroseptica, E. carotovora subsp. betavasculorum, and E. carotovora subsp. wasabiae to be more closely related to each other than to E. carotovora subsp. carotovora, E. carotovora subsp. odorifera, and E. chrysanthemi, while E. carotovora subsp. atroseptica was more closely related to E. carotovora subsp. wasabiae than to E. carotovora subsp. betavasculorum. Similarly, phenotypic tests (11, 41) showed that E. carotovora subsp. betavasculorum strains formed a group distinct from other subspecies. However, unlike AFLP and 16S rDNA analyses, these tests linked E. carotovora subsp. atroseptica more closely with E. carotovora subsp. carotovora than with E. carotovora subsp. betavasculorum (Fig. 2), showing a discrepancy between the molecular and phenotypic studies. No clustering data were obtained for E. carotovora subsp. betavasculorum by PCR-RFLP due to problems with amplification of E. carotovora subsp. betavasculorum DNA (9, 20).
Cluster 3 (n = 5) was composed of all E. carotovora subsp. wasabiae strains and grouped at 73.5% ± 3.5% similarity. It was distinct from all other subclusters, although marginally more closely related to clusters 1 (E. carotovora subsp. carotovora and E. carotovora subsp. odorifera) and 2 (E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum) than to cluster 4 (E. chrysanthemi). In both 16S rDNA sequence (18, 27) and RFLP (20) analyses, E. carotovora subsp. wasabiae was more closely related to E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum (subcluster 2) than to members of clusters 1 (E. carotovora subsp. carotovora and E. carotovora subsp. odorifera) and 4 (E. chrysanthemi) and, in the case of 16S rDNA sequencing, clustered between E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum (18). Given the close grouping between E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum using AFLP, the discrepancies between AFLP and 16S rDNA sequencing may be related to the number of strains used.
E. chrysanthemi, not unexpectedly, formed the most distantly related and genetically diverse cluster (cluster 4; n = 11) within the soft rot erwinias and grouped at 23.6% ± 10.3% (Fig. 2). API tests (11, 29, 41) found that E. chrysanthemi formed a cluster distinct from the E. carotovora subspecies. In addition, however, these tests (41) showed that E. chrysanthemi pv. dieffenbachia strains, which are known to constitute a well-defined group corresponding to DNA hybridization group II and biovar II (3, 12), clustered separately from other E. chrysanthemi strains. E. chrysanthemi strains have also been characterized using ribotyping (30, 31) and PCR-RFLP (2), which suggest that E. chrysanthemi clusters show some correlation with other intraspecific levels, such as pathovar, biovar, and, to a lesser extent, geographical origin, although these correlations are by no means universal. There were no such correlations in our study, although only 11 E. chrysanthemi strains were used.
Polymorphisms were demonstrated both among and within species and subspecies, with some amplified bands being shared among subspecies. In all isolates of E. carotovora subspecies, a characteristic pattern of two to three bands was clearly visible (Fig. 2), providing potential molecular markers for identification and targets for the development of diagnostics. For individual subspecies, other characteristic bands were present. In most cases, the species- and subspecies-specific bands appeared more intense than others (Fig. 2), perhaps reflecting their higher genomic copy numbers. It has been suggested that rDNA genes could be the sources of these bands (25).
To investigate the utility of AFLP fingerprinting as a method of identification, the dendrogram produced from well-characterized strains was used to compare profiles from over 200 additional unidentified soft rot erwinia strains, freshly isolated from potato material both locally and from Australia. To verify these identifications, all strains, including a number of different serogroups, were tested in parallel using biochemical and phenotypic methods (13, 33). In all cases, the unidentified strains grouped with either E. carotovora subsp. atroseptica or E. carotovora subsp. carotovora on the dendrogram, and these identifications were confirmed using the biochemical and phenotypic methods (only 17 of these strains are included in Fig. 2). In addition to showing the utility of AFLPs for identification, the results also show that the reproducibility of the method, in terms of comparing different gels produced at different times, had little effect on strain identification. It also showed that using entire banding profiles in GelCompar, a process much faster than marking individual bands, was sufficient for an accurate identification of the soft rot erwinias and could be used routinely. Finally, strain SCRI 135, originally identified by biochemical and phenotypic tests as E. carotovora subsp. carotovora, was identified as E. carotovora subsp. atroseptica by AFLP. On retesting by the former methods, the new identification was confirmed, again highlighting the utility of AFLP for identification purposes.
Investigating diversity within subspecies.
E. carotovora and E. chrysanthemi are phenotypically and genetically diverse, and this was reflected in the AFLP study, with mean similarities of ca. 23% for both species (Fig. 2). E. carotovora subsp. carotovora was the most diverse subspecies at 34% similarity, while E. carotovora subsp. odorifera, E. carotovora subsp. betavasculorum, E. carotovora subsp. atroseptica, and E. carotovora subsp. wasabiae were considerably more homogeneous, with mean similarities of between 59 and 75%. This homogeneity is well known for E. carotovora subsp. atroseptica (9-11, 17, 32, 35) and has been reported for E. carotovora subsp. odorifera (11), but it is less clear for E. carotovora subsp. betavasculorum (11) and is unknown for E. carotovora subsp. wasabiae. Such relatively low levels of genetic diversity may be due to a subspecies having more recent origins, limited population divergence, and/or limited host range, e.g., due to recent spread of a New World crop such as the potato. From our current knowledge of these subspecies, and including data from this study, all three possibilities may apply. This is not the case for E. chrysanthemi and E. carotovora subsp. carotovora, however, where an earlier divergence, wider geographical distribution, and wider host range (see the introduction) may explain their genetic diversity.
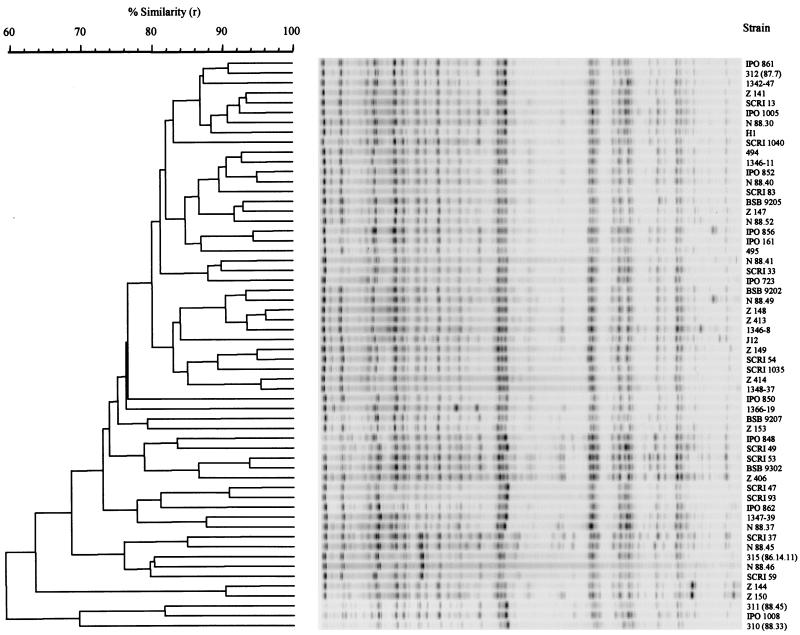
As detailed studies of the diversity within E. carotovora subsp. atroseptica have been carried out using a number of physiological and molecular methods (39), we chose E. carotovora subsp. atroseptica to investigate the applicability of AFLP fingerprinting for studying diversity within a subspecies. Fifty-nine strains of E. carotovora subsp. atroseptica were compared by AFLP fingerprinting, and a high level of diversity among these strains was seen (Fig. 3). Thus, AFLP fingerprinting is a powerful technique for determining genetic variation in a large number of E. carotovora subsp. atroseptica isolates and shows improvements over present methods, such as phage typing and randomly amplified polymorphic DNA (28, 39), i.e., the ability to work on all serogroups and an improved resolving power.
FIG. 3.
Dendrogram derived from the UPGMA linkage of correlation coefficients between AFLP profiles from strains of E. carotovora subsp. atroseptica. The levels of linkage representing the Pearson product-moment correlation coefficient (r) are expressed as percentages for convenience. The banding profiles against each branch represent normalized and background-subtracted digitized gel strips processed in GelCompar.
Conclusions. This is the first study to investigate the taxonomic and phylogenetic relationships among the soft rot erwinias using multiple strains from E. chrysanthemi and all E. carotovora subspecies. Although interspecific relationships among more distantly related taxa could not be determined using AFLP fingerprinting, at least under the conditions chosen, the method did generate subspecies-specific banding profiles that allowed four clusters to be delineated. In addition, the method was used to identify a number of unknown isolates, to discriminate between closely related strains for epidemiological investigations, and to provide species- and subspecies-specific banding profiles that are now being used in our laboratory to develop molecular diagnostics.
Acknowledgments
This work was funded by the Scottish Executive Environment and Rural Affairs Department and the British Potato Council.
We are grateful to Trevor Wicks and Barbara Morgan, The University of Adelaide, Adelaide, South Australia, for supplying isolates.
REFERENCES
- 1.Birch, P. R. J., L. Hyman, R. Taylor, A. F. Opio, C. Bragard, and I. K. Toth. 1997. RAPD PCR-based differentiation of Xanthomonas campestris pv. phaseoli and Xanthomonas campestris pv. phaseoli var. fuscans. Eur. J. Plant Pathol. 103:809-814. [Google Scholar]
- 2.Boccara, M., R. Vedel, D. Lalo, M. H. Lebrun, and J. F. Lafay. 1991. Genetic diversity and host range in strains of Erwinia chrysanthemi. Mol. Plant Microb. Interact. 4:293-299. [Google Scholar]
- 3.Brenner, D. J., G. R. Fanning, and A. G. Steigerwalt. 1977. Deoxyribonucleic acid relatedness among erwiniae and other enterobacteria. II. Corn stalk rot bacterium and Erwinia chrysanthemi. Int. J. Syst. Bacteriol. 27:211-221. [Google Scholar]
- 4.Brenner, D. J., and F. Falkow. 1971. Molecular relationships among members of the Enterobacteriaceae. Adv. Genet. 16:81-118. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, D. J., G. R. Fanning, and A. G. Steigerwalt. 1972. Deoxyribonucleic acid relatedness among species of Erwinia and between Erwinia species and other enterobacteria. J. Bacteriol. 110:12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coenye, T., L. M. Schouls, J. R. W. Govan, K. Kersters, and P. Vandamme. 1999. Identification of Burkholderia species and genomovars from cystic fibrosis patients by AFLP fingerprinting. Int. J. Syst. Bacteriol. 49:1657-1666. [DOI] [PubMed] [Google Scholar]
- 7.Coenye, T., E. Falsen, M. Vancanneyt, B. Hoste, J. R. W. Govan, K. Kersters, and P. Vandamme. 1999. Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int. J. Syst. Bacteriol. 49:405-413. [DOI] [PubMed] [Google Scholar]
- 8.Cooke, D. E. L., J. W. Forster, P. D. Jenkins, D. G. Jones, and D. M. Lewis. 1998. Analysis of intraspecific and interspecific variation in the genus Alternaria by the use of RAPD-PCR. Ann. Appl. Biol. 132:197-209. [Google Scholar]
- 9.Darrasse, A., S. Priou, A. Kotoujansky, and Y. Bertheau. 1994. PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato diseases. Appl. Environ. Microbiol. 60:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boer, S. H., and M. Sasser. 1986. Differentiation of Erwinia carotovora ssp. carotovora and E. carotovora ssp. atroseptica on the basis of cellular fatty acid composition. Can. J. Microbiol. 32:796-800. [Google Scholar]
- 11.De Boer, S. H., L. Verdonck, H. Vruggink, P. Harju, H. O. Bång, and J. De Ley. 1987. Serological and biochemical variation among potato strains of Erwinia carotovora subsp. atroseptica and their taxonomic relationship to other E. carotovora strains. J. Appl. Bacteriol. 63:487-495. [Google Scholar]
- 12.Dickey, R. S. 1979. Erwinia chrysanthemi: a comparative study of phenotypic properties of strains from several hosts and other Erwinia species. Phytopathology 69:324-329. [Google Scholar]
- 13.Dye, D. W. 1969. A taxonomic study of the genus Erwinia. II. The “Carotovora “ group. N.Z. J. Sci. 12:81-97. [Google Scholar]
- 14.Dye, D. W. 1981. A numerical taxonomy of the genus Erwinia. N.Z. J. Agric. Res. 24:223-229. [Google Scholar]
- 15.Gallois, A., R. Samson, E. Ageron, and P. A. D. Grimont. 1992. Erwinia carotovora subsp. odorifera subsp. nov., associated with odorous soft rot of chicory (Cichorium intybus L.). Int. J. Syst. Bacteriol. 42:582-588. [Google Scholar]
- 16.Goto, M., and K. Matsumoto. 1987. Erwinia carotovora subsp. wasabiae subsp. nov. isolated from diseased phizomes and fibrous roots of Japanese horseradish (Eutrema wasabi Maxim.). Int. J. Syst. Bacteriol. 37:130-135. [Google Scholar]
- 17.Gross, D. C., M. L. Powelson, K. M. Regner, and G. K. Radamaker. 1991. A bacteriophage typing system for surveying the diversity and distribution of strains of Erwinia carotovora in potato fields. Phytopathology 81:220-226. [Google Scholar]
- 18.Hauben, L., E. R. Moore, L. Vauterin, M. Steenackers, J. Mergaert, L. Verdonck, and J. Swings. 1998. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Syst. Appl. Microbiol. 21:384-397. [DOI] [PubMed] [Google Scholar]
- 19.Hauben, L., L. Vauterin, E. R. B. Moore, B. Hoste, and J. Swings. 1999. Genomic diversity of the genus Stenotrophomonas. Int. J. Syst. Bacteriol. 49:1749-1760. [DOI] [PubMed] [Google Scholar]
- 20.Helias, V., A.-C. Le Roux, Y. Bertheau, D. Andrivon, J.-P. Gauthier, and B. Jouan. 1998. Characterisation of Erwinia carotovora subspecies and detection of Erwinia carotovora subsp. atroseptica in potato plants, soil and water extracts with PCR-based methods. Eur. J. Plant Pathol. 104:685-699. [Google Scholar]
- 21.Huys, G., R. Coopman, P. Janssen, and K. Kersters. 1996. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int. J. Syst. Bacteriol. 46:572-580. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, D. A., K. M. Somers, and H. H. Harvey. 1989. Similarity coefficients: measures of co-occurrence and association or simply measures of occurrence. Am. Nat. 133:436-453. [Google Scholar]
- 23.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 24.Janssen, P., K. Maquelin, R. Coopman, I. Tjernberg, P. Bouvet, K. Kersters, and L. Dijkshoorn. 1997. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int. J. Syst. Bacteriol. 47:1179-1187. [DOI] [PubMed] [Google Scholar]
- 25.Keim, P., A. Kalif, J. Schupp, K. Hill, S. E. Travis, K. Richmond, D. M. Adair, M. Hugh-Jones, C. R. Kuske, and P. Jackson. 1997. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J. Bacteriol. 179:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, W.-S., L. Gardan, S.-L. Rhim, and K. Geider. 1999. Erwinia pyrifoliae sp. nov ., a novel pathogen that affects Asian pear trees (Pyrus pyrifolia Nakai). Int. J. Syst. Bacteriol. 49:899-906. [DOI] [PubMed] [Google Scholar]
- 27.Kwon, S.-W., S.-J. Go, H. W. Kang, J.-C. Ryu, and J.-K. Jo. 1997. Phylogenic analysis of Erwinia species based on 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 47:1061-1067. [DOI] [PubMed] [Google Scholar]
- 28.Maki-Valkama, T., and R. Karjalainen. 1994. Differentiation of Erwinia carotovora subsp. atroseptica and carotovora by RAPD-PCR. Ann. Appl. Biol. 125:301-309. [Google Scholar]
- 29.Mergaert, J., L. Verdonck, K. Kersters, J. Swings, J.-M. Boeufgras, and J. De Ley. 1984. Numerical taxonomy of Erwinia species using API systems. J. Gen. Microbiol. 130:1893-1910. [Google Scholar]
- 30.Nassar, A., Y. Bertheau, C. Dervin, J. P. Narcy, and M. Lemattre. 1994. Ribotyping of Erwinia chrysanthemi strains in relation to their pathogenic and geographic distribution. Appl. Environ. Microbiol. 60:3781-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassar, A., A. Darrasse, M. Lemattre, A. Kotoujansky, C. Dervin, R. Vedel, and Y. Bertheau. 1996. Characterisation of Erwinia chrysanthemi by pectolytic isozyme polymorphism and restriction fragment length polymorphism analysis of PCR-amplified fragments of pel genes. Appl. Environ. Microbiol. 62:2228-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parent, J.-G., M. Lacroix, D. Page, and L. Vezina. 1996. Identification of Erwinia carotovora from soft rot diseased plants by random amplified polymorphic DNA (RAPD) analysis. Plant Dis. 80:494-499. [Google Scholar]
- 33.Perombelon, M. C. M., V. M. Lumb, and L. J. Hyman. 1987. A rapid method to identify and quantify soft rot erwinias on seed potato tubers. EPPO Bull. 17:25-35. [Google Scholar]
- 34.Perombelon, M. C. M. 1992. The genus Erwinia, p. 2899-2921. In A. H. Balows, G. Truper, M. Dworking, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. III. Springer-Verlag, London, United Kingdom. [Google Scholar]
- 35.Persson, P., and A. Sletten. 1995. Fatty acid analysis for the identification of Erwinia carotovora subsp. atroseptica and E. carotovora subsp. carotovora. EPPO Bull. 25:151-156. [Google Scholar]
- 36.Rademaker, J. L. W., B. Hoste, F. J. Louws, K. Kerters, J. Swings, L. Vauterin, P. Vauterin, and F. J. de Bruijn. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. Evol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 37.Thomson, S. V., D. C. Hildebrand, and M. N. Schroth. 1981. Identification and nutritional differentiation of the Erwinia sugar beet pathogen from members of Erwinia carotovora and Erwinia chrysanthemi. Phytopathology 71:1037-1042. [Google Scholar]
- 38.Thyssen, A., S. Van Eygen, L. Hauben, J. Goris, J. Swings, and F. Ollevier. 2000. Application of AFLP for taxonomic and epidemiological studies of Photobacterium damselae subsp. piscicida. Int. J. Syst. Evol. Microbiol. 50:1013-1019. [DOI] [PubMed] [Google Scholar]
- 39.Toth, I. K., Y. Bertheau, L. J. Hyman, L. Laplaze, M. M. López, J. McNicol, F. Niepold, P. Persson, A. Sletten, J. M. van der Wolf, and M. C. M. Pérombelon. 1999. Evaluation of phenotypic and molecular typing techniques for determining diversity in Erwinia carotovora subspecies atroseptica. J. Appl. Microbiol. 87:770-781. [DOI] [PubMed] [Google Scholar]
- 40.Vandamme, P., B. Pot, M. Gillis, P. De Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdonck, J., J. Mergaert, J. Rijckaert, J. Swings, K. Kersters, and J. DeLey. 1987. The genus Erwinia: numerical analysis of phenotypic features. Int. J. Syst. Bacteriol. 37:4-18. [Google Scholar]
- 42.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]