Abstract
The transport and attachment behaviors of Spumella guttula (Kent), a nanoflagellate (protist) found in contaminated and uncontaminated aquifer sediments in Cape Cod, Mass., were assessed in flowthrough and static columns and in a field injection-and-recovery transport experiment involving an array of multilevel samplers. Transport of S. guttula harvested from low-nutrient (10 mg of dissolved organic carbon per liter), slightly acidic, granular (porous) growth media was compared to earlier observations involving nanoflagellates grown in a traditional high-nutrient liquid broth. In contrast to the highly retarded (retardation factor of ∼3) subsurface transport previously reported for S. guttula, the peak concentration of porous-medium-grown S. guttula traveled concomitantly with that of a conservative (bromide) tracer. About one-third of the porous-medium-grown nanoflagellates added to the aquifer were transported at least 2.8 m downgradient, compared to only ∼2% of the broth-grown nanoflagellates. Flowthrough column studies revealed that a vital (hydroethidine [HE]) staining procedure resulted in considerably less attachment (more transport) of S. guttula in aquifer sediments than did a staining-and-fixation procedure involving 4′,6′-diamidino-2-phenylindole (DAPI) and glutaraldehyde. The calculated collision efficiency (∼10−2 for porous-medium-grown, DAPI-stained nanoflagellates) was comparable to that observed earlier for the indigenous community of unattached groundwater bacteria that serve as prey. The attachment of HE-labeled S. guttula onto aquifer sediment grains was independent of pH (over the range from pH 3 to 9) suggesting a primary attachment mechanism that may be fundamentally different from that of their prey bacteria, which exhibit sharp decreases in fractional attachment with increasing pH. The high degree of mobility of S. guttula in the aquifer sediments has important ecological implications for the protistan community within the temporally changing plume of organic contaminants in the Cape Cod aquifer.
Recent findings regarding the role of protists in natural attenuation of organically contaminated aquifers 20; N. E. Kinner, R. W. Harvey, D. M. Shay, D. W. Metge, and, A. Warren, submitted for publication), in groundwater quality (25, 36, 37, 41), and in controlling the flux of unattached bacteria in flowthrough column studies (5, 19) are leading to an increasing interest in their subsurface mobility. Although a number of recent studies examined the transport potential of oocysts of the obligate protozoan pathogen Cryptosporidium parvum through saturated porous media in laboratory microcosms (2, 9), little is known about the transport potential of protists that are indigenous to aquifers. In 1991, the groundwater nanoflagellate Spumella guttula (Kent) (18) was used in a small-scale injection-and-recovery study (12) involving a sandy, treated-sewage-contaminated aquifer. The contaminated aquifer harbors a large (up to 105/g [dry weight] [gdw]) (17) and diverse (29, 30) protistan community, dominated by 2- to 3-μm nanoflagellates that are largely associated with grain surfaces. The 1991 study suggested rather modest transport potentials for the liquid-broth-grown, vitally stained nanoflagellates. However, because the laboratory-grown nanoflagellates were much larger (4 to 5 μm) than their in situ size (2 to 3 μm) and because their transport parameters changed with time and distance in the aquifer, important questions remained about their true subsurface transport potential, including the effects of culturing in liquid broth and staining procedures upon mobility, retardation, and propensity for attachment.
The overall objective of the present study was to better determine the transport behavior of S. guttula in organically contaminated aquifer sediments. In particular, we were interested in knowing whether nanoflagellates, which are highly efficient predators of groundwater bacteria (18), have sufficient transport potential to respond rapidly to the spatial changes in unattached bacterial abundance that occur downgradient from sources of organic contamination. To better address these questions, a porous-medium, low-nutrient, low-pH culturing procedure was developed (3) that better preserves in situ cell size (17) by simulating some of the salient conditions in the aquifer. We hypothesized that porous-medium-grown, in situ-sized S. guttula should be readily transported within the aquifer based upon colloid filtration theory (39), a measured buoyant density of ∼1.02 g/cm3 (14), and previously observed trends of increasing mobility with decreasing cell size as the protists became acclimated to in situ conditions (12). Because we need to label the cultured nanoflagellates in order to differentiate them from the indigenous population before reintroduction into the aquifer, we also wanted to know what effect the staining procedure had upon the apparent transport behavior. In particular, we believed the use of a vital versus a lethal staining procedure could have a significant effect upon subsurface transport behavior.
To better assess the subsurface transport behavior of groundwater nanoflagellates, we conducted laboratory and field studies that assessed the ability of porous-medium-grown S. guttula to be advected through aquifer sediments. In 1994, a small-scale, natural-gradient injection-and-recovery experiment with porous-medium-grown S. guttula was conducted at the site used in a 1991 test. Breakthroughs of the porous-medium- and liquid-broth-grown nanoflagellates were compared at sampling points from 1.0 to 3.6 m downgradient from the point of injection. A conservative tracer (bromide) was used for comparative purposes to facilitate calculating the degrees of immobilization and retardation and to account for differences in hydrology between the 1991 and 1994 tests. Comparisons of transport behavior between porous-medium- and liquid-broth-grown nanoflagellates were made under more controlled conditions in laboratory flowthrough columns. The effect of staining procedure upon advective transport was assessed by comparing live (hydroethidine [HE]-stained) and dead (4′,6′-diamidino-2-phenylindole [DAPI]-stained, glutaraldehyde-fixed) S. guttula in flowthrough columns of sieved aquifer sediments. Finally, the pH-dependent attachment behavior of porous-medium-grown S. guttula was compared to that of groundwater bacteria using static minicolumns filled with sieved aquifer sediments.
MATERIALS AND METHODS
Nanoflagellates.
Nanoflagellates were recovered from aquifer sediments taken aseptically from a treated-sewage contaminated zone in the Cape Cod aquifer using a wireline piston-type coring device (40) in conjunction with a hollow-stem auger drill without the use of drilling fluids (12). Nanoflagellates recovered in the culturing procedure were grown in acidic, low-nutrient porous media as described by Kinner et al. (18). Briefly, the procedure involved growth in 500 g of sterile sieved (grain size, 0.5 to 1.0 mm) aquifer sediments saturated with dilute Cerophyl-Prescott medium to give a final dissolved organic carbon concentration of ∼10 mg/liter in the pore fluid. The pH of the sediment was adjusted to 6.0 to match conditions in the contaminated zone of the aquifer where the injection-and-recovery test was performed (32). The jars holding the sterile porous media, which also contained ∼1 cm of free-standing liquid, were each inoculated with 1 g of core material, gently swirled, and held at room temperature (20°C). Nanoflagellates, which grazed upon the groundwater bacteria that also grew in the cultures, were harvested at peak abundance (4 to 10 days after inoculation) using a suction device described by Mayberry (27). Nanoflagellates in the pore fluid of the porous media reached concentrations between 104 and 105 · ml−1. Although the community of nanoflagellates in the Cape Cod contaminant plume consists of several genera, including Bodo, Cercomonas, Cryptaulax, Cyanthomomonas, Goniomonas, and Spumella, and some undescribed species (30), most of the nanoflagellates harvested from the porous media culture were 2- to 3-μm S. guttula organisms (18).
Blue-fluorescing (DAPI-labeled) and red-fluorescing (HE-labeled) trophic and encysted nanoflagellates were enumerated with an epifluorescence microscope using the direct-count-scanning procedure described by Bunn (3). Aqueous samples containing DAPI- or HE-labeled S. guttula were filtered onto black, 25-mm-diameter, 0.8-μm-pore-size polycarbonate membrane filters (Poretics Corporation, Livermore, Calif.) atop a 0.45-μm-pore-size Metricel backing filter (Gelman Science, Inc., Ann Arbor, Mich.). A mild vacuum (<126 mm Hg) was employed to avoid rupturing the nanoflagellates during filtration. The black membrane filters and low-fluorescence (type A) immersion oil (Cargille Laboratories, Cedar Grove, N.J.) were then placed between glass slides and coverslips. The slides were observed under a ×600 magnification with an Optiphot microscope (Nikon Inc., Garden City, N.Y.) equipped with an EF-D mercury lamp and UV-2A (400-nm dichroic mirror, 330- to 380-nm excitation, and 400-nm barrier) and UV-1A (400-nm dichroic mirror, 330- to 380-nm excitation, and 420-nm barrier) exciter filter combinations for HE and DAPI, respectively. A metal stage brace that allowed the objective to travel a known distance (11.1 mm) across the filter was used. Scanning fields of 2.3 mm2 were chosen on the microscope vernier by using a random number generator. Scans (typically three to five) were made until at least 100 nanoflagellates had been counted. The limit of detection with this procedure is about seven nanoflagellates per ml. Enumeration did not differentiate between trophic and encysted forms.
Staining procedures.
The nanoflagellates used in the column and field experiments were stained either with the vital fluorescent dye HE or with the fluorochrome DAPI. Nanoflagellates to be labeled with DAPI were first fixed in a solution containing 100 μM glutaraldehyde and 16 mM cacodylic acid (final concentrations) for 15 min to ensure that the dead cells would remain structurally intact during the experiments. The fixed nanoflagellates were subsequently stained for 10 min with DAPI (Sigma Chemical, St. Louis, Mo.) at a final concentration of 860 μM. HE stock solutions consisting of 22 mM HE (Polysciences, Warrington, Pa.) in N,N-dimethylacetamide (Sigma Chemical) were made and stored at 0°C in volatile organic vials fitted with Teflon septa in order to retard oxidation and volatilization. Nanoflagellates were stained for 15 min at a final HE concentration of 44 μM, followed by a 1:10 dilution with groundwater.
Injection-and-recovery study.
The field tracer study was conducted at the U.S. Geological Survey (USGS) Cape Cod groundwater hydrology study site (Falmouth, Mass.). The nanoflagellate injection-and-recovery experiment was performed concurrently with a virus transport test (32) at USGS injection well M4-15, which is within a large (50- by 200-m) array of multilevel samplers (MLS) constructed for the purpose of studying advective transport (22). The zone of the aquifer utilized in the nanoflagellate transport study is moderately contaminated by a plume of secondarily treated domestic effluent discharged ∼0.5 km upgradient and was reasonably consistent with respect to groundwater chemistry, at least prior to the cessation of the source in 1995. It was employed in a number of earlier microbial transport studies (for example, see references 1, 13, and 32). Although subject to variations in the quantity of iron oxides on the grain surfaces, the effects of the geochemical heterogeneity upon subsurface microbial transport within the plume appears to have been largely masked by the sorbing organic matter within the plume (34).
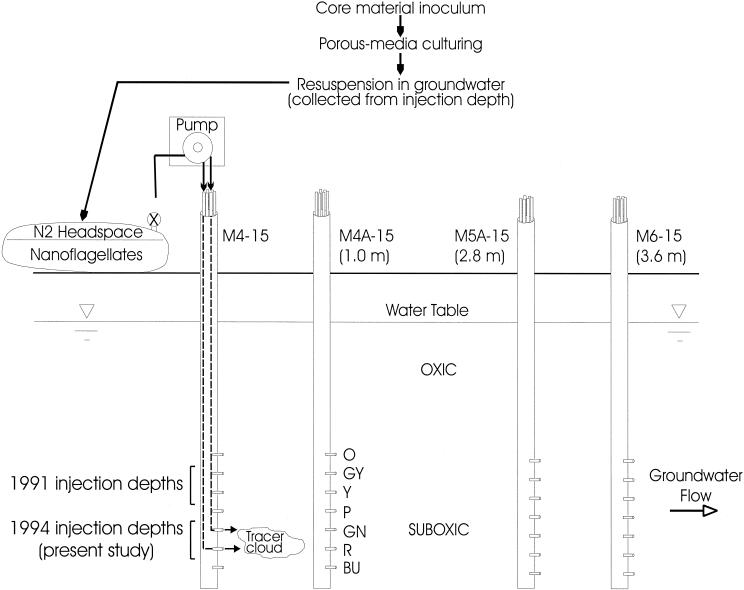
The protocol for the field study is depicted in Fig. 1. The nanoflagellates harvested from pore fluid of the porous media were stained and fixed as described above and added, with bromide (1.9 mM, final concentration), to groundwater collected from the injection depths. To avoid introduction of atmospheric oxygen, the injectate was pumped into a collapsed, acid-rinsed, nitrogen-sparged, gas-impermeable fuel bladder (Aero Tech Lab, Ramsey, N.J.) under nitrogen headspace. The 100-liter contents of the fuel bladder were mixed by agitation and pumped back (∼1 liter · min−1) into the suboxic zone. M4-14 sampling ports GN and R, which are, respectively, 9.8 and 10.0 m below land surface (mbs), served as injection ports for the introduction of the injectate into the aquifer. The injection ports chosen in the 1994 injection study were somewhat deeper than those used in the 1991 test, in case the hydraulic conductivity or grain surfaces within the strata used in the 1991 study (at 9.0 and 9.3 mbs) had been altered by the injectate used in that test (Fig. 1). The concentrations of labeled nanoflagellates and bromide were monitored as they were advected downgradient by the natural flow of groundwater past MLS (M4A-15, M5A-15, and M6-15) that lay along the trajectory of the injectate cloud at 1.0, 2.8, and 3.6 m downgradient, respectively. Breakthrough curves (dimensionless concentration histories) were obtained at each of the three aforementioned MLS at depths exhibiting substantive breakthrough (levels: GN [9.8 mbs], R [10.0 mbs], and BU [10.3 mbs]). The concentration of S. guttula in the injectate was 1,660 · ml−1.
FIG. 1.
Schematic depiction of the 1994 injection-and-recovery experiment with the porous-medium-grown groundwater nanoflagellate S. guttula. Shown are the injection well and the MLS that lay along the trajectory of the injectate cloud. The injection depths for a similar experiment performed in 1991 using liquid-broth-grown S. guttula are also shown. The water table is ∼6 mbs. Relative distances on the diagram are not to scale.
Flowthrough column studies.
Four flowthrough column studies, were conducted to examine the effects of staining upon the transport behavior of S. guttula in aquifer sediments. Glass chromatography columns, 60 by 4.8 cm (inside diameter [i.d.]) (Kontes, Vineland, N.J.) were modified for this purpose by replacing the polyethylene bed supports with less-hydrophobic stainless steel screens (40 and 325 mesh size). Aquifer sediments were collected in the contaminant plume near USGS well site F230, dried (110 ± 5°C), sieved (0.5 to 1.0 mm), autoclaved, and packed into the columns by the sequential homogenized layering method of Johnson (16) to minimize grain size segregation within the columns. The columns were run at 10 ± 2°C and ∼2 ml · h−1 in an up-flow mode at 10° from horizontal within an incubator (model 816; Precision Scientific, Chicago, Ill.). A modified Mariott bottle reservoir system (24) and small-diameter (0.5-mm-i.d.), 30-cm-long autoanalyzer tubing (Cole Parmer, Chicago, Ill.) were used to provide constant head and flow rates through the columns. The columns were connected to the constant-head reservoir and to a fraction collector with 3-mm-i.d. Tygon tubing. The column was saturated by initially displacing the air by flushing with medical-grade CO2 (Merriam-Graves Corp., Pembroke, N.H.) and then displacing the highly soluble CO2 by flushing with a degassed 0.005 M CaSO4 solution, followed by flushing with degassed, MilliQ-purified water (Millipore Corp. Bedford, Mass.). Column effluent was collected in 20-ml Wheaton (Millville, N.J.) glass scintillation vials placed in a universal fraction collector (model U-1A; Eldex Laboratories, San Carlos, Calif.). The setup of the columns is described in more detail by Mayberry (27).
Hydrologic and transport parameters.
To better interpret differences in the advective transport results of S. guttula between column studies in which different staining procedures were employed, between field and laboratory experiments, and between different conductive layers at the field site, hydrologic parameters for the experimental systems were calculated and compared. The physical parameters determined for the column experiments were linear velocity, volumetric flow rate, longitudinal dispersion, hydraulic conductivity, and effective porosity. Methods for calculating these parameters are described in detail elsewhere (27) and are given briefly here. Hydraulic conductivity was estimated by repacking the dried sand from each column into a falling head permeameter. Volumetric flow rate was measured over the experimental time course by monitoring the amount of column effluent collected in a fraction collector per unit time. Effective porosity (θ) for the columns (porosity subject to fluid flow) was calculated based upon breakthrough of the conservative tracer as t1/2Q/AL, where t1/2 is the time required for the conservative tracer in the column effluent to reach 50% of its concentration in the influent (C0), Q is the volumetric flow rate, A is the cross-sectional area of the column, and L is the length of the column. Apparent longitudinal dispersivity (αL) within each packed column was calculated as DL/v, where v is the fluid velocity (calculated as the column length [L] divided by t1/2) and DL is the coefficient of longitudinal dispersion. The latter parameter was calculated by fitting observed breakthrough of the conservative tracer to a one-dimensional advection-dispersion equation (6): C/C0 = 0.5{erfc[(1 − tR)/2(tRDL/vL)1/2]}, where C/C0 is the dimensionless concentration of the conservative tracer, erfc is the complementary error function, and tR is the number of effluent pore volumes collected (i.e., vt/L).
Hydrologic parameters for the field studies were those that could be estimated in the absence of destructive sampling (coring) and included v and DL. For each conductive layer (depth) and travel distance (1.0, 2.8, and 3.6 m), apparent longitudinal dispersion (AL) in the field was calculated by a graphical method (11) for small-scale natural-gradient tests involving pulse injection: x1(Δt/tpeak)2/16 ln 2, where x1 is the distance from point of injection, Δt is the duration of breakthrough when the concentration of the conservative tracer is at least 50% of the peak concentration, and tpeak is the time to peak concentration. Corresponding average groundwater velocities were calculated based upon the time required for the peak concentration of conservative tracer to reach the appropriate sampling point of known distance from point of injection.
Dimensionless concentration histories of labeled nanoflagellates (P/P0 versus t) and bromide (C/C0 versus t) were determined for the effluent of each column and for each of the aforementioned MLS sampling points in the small-scale injection-and-recovery experiments. The breakthrough data were used to calculate transport parameters for the nanoflagellates, including the maximum dimensionless concentrations of the nanoflagellates (Pmax), the fraction immobilized within the sediments (percent retention), the collision efficiency (α), and the retardation factor (RF). The methods and equations used to calculate (P/P0)max, percent retention, α, and RF for nanoflagellate transport parameters in the field study are the same as those used in the 1991 study (12). For the column experiments, the collision efficiency (α) was calculated as follows: [−dm ln (P/P0)]/[1.5(1 − θ)ηL], where dm is the median grain diameter, P/P0 is the steady-state dimensionless concentration of the labeled nanoflagellates, θ is the aforementioned effective porosity, and η is the single collector efficiency. The latter parameter was calculated by the method of Yao et al. (39), which ignores close approach effects, so that the resulting values of α would be directly comparable to those calculated in earlier studies at Cape Cod.
Static minicolumns.
The pH dependencies of porous-medium-grown S. guttula and the indigenous bacteria that serve as their prey were assessed by using static minicolumns, which were designed to simulate the physicochemical conditions present in aquifer sediments. Static columns consisted of 20-ml glass syringes (Popper & Sons) according to procedures modified from those of Scholl and Harvey (35). Each minicolumn was filled with 15 g of aquifer sediment. The sediments were sieved (0.5- to 1.0-mm grain size) a priori in order to preclude the possibility of straining during pore water withdrawal. The pH of each static minicolumn was adjusted by sequential equilibrations with NaOH or HCl. Three replicate minicolumns were constructed to test each pH adjustment effect. A consistent number of DAPI-labeled bacteria (4.7 × 106 bacteria/gdw) or HE-labeled nanoflagellates (2.5 × 103 nanoflagellates/gdw) were loaded into each static minicolumn. After 4-h incubations, the pore water (filter-sterilized groundwater collected from an uncontaminated zone in the aquifer) was collected, and the labeled microorganisms left in solution were enumerated. Fractional attachment was determined from the differences between what was added and what was recovered. Because of the potential importance of physiological condition for S. guttula attachment behavior, we used a vital (HE) stain to label them and limited the variation of pH values to a “moderate” range (3.2 to 8.6) and their exposure to 4 h. Structural integrity and intensity of fluorescence were monitored to ensure nanoflagellate viability during these tests.
RESULTS
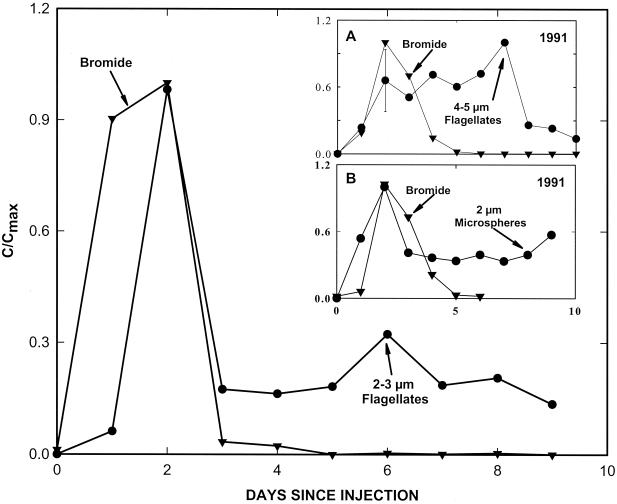
Peak abundance of the porous-medium-grown S. guttula at MLS M4A-15 occurred concomitantly with that of the bromide tracer (Fig. 2). In contrast, peak abundance of liquid-broth-grown S. guttula took more than three times as long to reach MLS M4A-15 as did the conservative tracer (Fig. 2, inset A). The pattern of breakthrough exhibited by the 2-μm microspheres during the 1991 injection-and-recovery study was similar to that observed for 2- to 3-μm, porous-medium-grown nanoflagellates, i.e., a peak abundance that was coincident with that of bromide, followed by a protracted tail (Fig. 2, inset B). However, the pattern of microsphere breakthrough was quite different from that of the 4- to 5-μm liquid-broth-grown nanoflagellates, which exhibited multiple peaks and a retarded center of mass relative to that of bromide (Fig. 2, inset A).
FIG. 2.
Dimensionless concentration histories (expressed as the concentration in samples collected daily at 1 m downgradient from point of injection normalized to the maximum concentration) for DAPI-stained, porous-medium-grown S. guttula and for bromide in the 1994 tracer test. The sampling port is level R (10.0 mbs) of MLS M4A-15. The average counting error for the nanoflagellates was ±15%. (Inset A) Dimensionless concentration histories for liquid-broth-grown S. guttula and bromide at level GY (12.0 mbs) of MLS M4A-15 in the 1991 test. (Inset B) Dimensionless concentration histories for 2-μm carboxylated microspheres and bromide at level GY (9.6 mbs) of MLS M7-15 in the 1991 test. Insets are redrawn from reference 12 with permission and are shown for comparison.
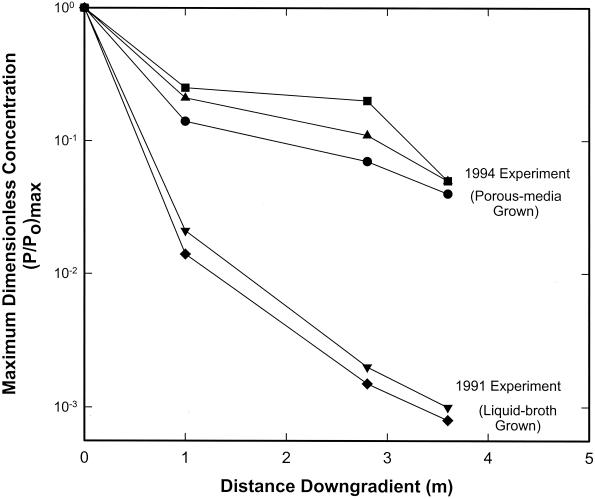
Computed groundwater velocities varied with depth (Table 1). However, the average groundwater velocity for the two sampled depths in the 1991 transport test was the same as that for the three depths sampled in the 1994 tests over the first 1 m but slightly lower at 3.6 m downgradient. Depth-averaged longitudinal dispersivities, estimated from observed spreading of the conservative tracer, were somewhat higher in the 1994 test but always within a factor of 2 of those estimated for the 1991 test. In contrast, the magnitude of nanoflagellate transport was very different for the two tests. For example, at 1 m downgradient from point of injection, the average maximum dimensionless concentration of the porous-medium-grown nanoflagellates was ∼10-fold higher than that observed earlier for the liquid-broth-grown nanoflagellates (Fig. 3). The difference in dimensionless concentrations between the porous-medium- and liquid-broth-grown nanoflagellates increased to 2 log units by 2.8 and 3.6 m downgradient.
TABLE 1.
Depths, groundwater velocities, and dispersivities for the small-scale injection-and-recovery tests
| Yr | Injection point
|
1.0 m downgradient (MLS M4A-15)
|
2.8 m downgradient (MLS M5A-15)
|
3.6 m downgradient (MLS M6-15)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Depth (mbs) | Altitude (masa) | Designation | vb (m · day−1) | ALc (cm) | vb (m · day−1) | ALc (cm) | vb (m · day−1) | ALc (cm) | |
| 1991 | 9.0 | 12.0 | GY | 0.5 | 7.2 | 0.7 | 7.8 | 0.3 | 1.8 |
| 9.3 | 11.7 | Y | 0.5 | 9.0 | 0.5 | 2.8 | 0.5 | 3.4 | |
| Avg | 0.5 | 8.1 | 0.6 | 5.3 | 0.4 | 2.6 | |||
| 1994 | 9.8 | 11.2 | GN | 0.5 | 5.1 | 0.6 | 11.4 | 0.5 | 9.7 |
| 10.0 | 11.0 | R | 0.5 | 16.0 | 0.6 | 4.9 | 0.6 | 2.9 | |
| 10.3 | 10.7 | BU | 0.5 | 10.9 | 0.4 | 5.4 | 0.3 | 2.3 | |
| Avg | 0.5 | 10.7 | 0.5 | 7.2 | 0.5 | 5.0 | |||
Meters above mean sea level.
Groundwater velocity based upon bromide transport.
Longitudinal dispersivity of bromide tracer.
FIG. 3.
Maximum dimensionless concentration histories for liquid-broth-grown S. guttula in the 1991 test and for porous-medium-grown S. guttula in the 1994 tests at MLS M4A-15 (1 m downgradient from point of injection), M5A-15 (2.8 m downgradient) and M6-15 (3.6 m downgradient). Squares, breakthrough at level R (10.0 mbs); triangles, level BU (10.7 mbs); circles, level GN (9.8 mbs); inverted triangles, level GY (12.0 mbs); diamonds, level Y (11.7 mbs). The average counting error for the nanoflagellates was ±15%.
The roles of culturing and staining protocols upon the ability of S. guttula to advect through columns of aquifer sediment were studied in the laboratory. Because transport behavior depends, in part, upon the hydraulic properties of the medium, the volumetric flow rates, hydraulic conductivities, bulk densities, travel distance, and porosities must be calculated for each individual column (Table 2). Modest variations in flow rates and porosity were observed among the columns due to the use of large columns and natural aquifer sediments. However, for the porous-medium-grown nanoflagellates, the maximum dimensionless concentrations, (P/P0)max, in the effluent end of the columns were, on average, about eightfold greater for the vitally stained (HE-stained) than the DAPI-stained, glutaraldehyde-fixed nanoflagellates. Unfortunately, the physical properties of the 1991 column experiment are not available. However, it is reasonable to assume that the physical parameters were roughly comparable to those in the later column studies, because the column dimensions, porous media, and method of packing were similar. For the vitally stained nanoflagellates, culturing in liquid broth resulted in a maximum dimensionless concentration in the eluent that was at least 3 log units lower than that obtained with the porous-medium culturing procedure. In general, (P/P0)max was inversely related to the initial concentrations, although not in a predictable manner.
TABLE 2.
Nanoflagellate transport and physical parameters in flowthrough columns of aquifer sediment as a function of growth medium and staining procedure
| Yr | Columna |
S. guttula
|
Physical column parameters
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stain | Culture medium | Size (μm) | P0b (105 · ml−1) | (P/P0)maxc | Qd (ml · min−1) | Ke (cm · s−1) | θf | Bulk density (g·cm3) | ||
| 1994 | D1 | DAPI | Porous media | 2-β | 3.3 | 0.08 | 2.3 | 0.024 | 0.37 | 1.62 |
| D2 | DAPI | Porous media | 2-3 | 1.1 | 0.39 | 2.1 | 0.024 | 0.42 | 1.62 | |
| H1 | HE | Porous media | 2-3 | 0.48 | 2.47 | 2.1 | 0.021 | 0.34 | 1.62 | |
| H2 | HE | Porous media | 2-3 | 0.55 | 1.40 | 2.0 | 0.021 | 0.38 | 1.62 | |
| 1991g | HE | Liquid broth | 4-5 | 6.3 | 0.001 | NAh | NA | NA | NA | |
Columns were 0.6 m long and contained sieved aquifer sediments (0.5- to 1-mm grain size).
Initial concentration.
Maximum dimensionless nanoflagellate concentration (effluent).
Volumetric flow rate.
Hydraulic conductivity.
Effective porosity.
Data calculated from information provided in reference 12 with permission.
NA, not available.
For the field tests, fractional attachment (percent retained), collision efficiency, and retardation were considerably greater for the liquid-broth-grown nanoflagellates than the porous-medium-grown nanoflagellates (Table 3). Collision efficiency, which represents the chemical and biological factors affecting attachment of microorganisms to grain surfaces, was ∼10−2 for porous-medium-grown nanoflagellates being advected over the first 2.8 m. Approximately one-third of the porous-medium-grown nanoflagellates were advected at least 2.8 m downgradient, with little apparent retardation. In contrast, only ∼2% of the liquid-broth-grown nanoflagellates were transported over the same distance. The liquid-broth-grown nanoflagellates also were subject to collision efficiencies and retardation factors that were ∼5- and 2-fold higher, respectively. The apparent collision efficiencies for nanoflagellates being advected through sediment columns were at least 1 log unit higher than what was observed for the field studies. However, HE-stained S. guttula exhibited a collision efficiency that was ∼3-fold lower than that observed for DAPI-stained S. guttula, indicating that the vitally stained nanoflagellates had a lower propensity for attachment to grain surfaces.
TABLE 3.
Retardation and immobilization of stained nanoflagellates in column and field tests
| Study type | Yr | Stain | Culture type | Flagellate size (μm) | Distance (m) | Immobilization
|
RFa | |
|---|---|---|---|---|---|---|---|---|
| % Retained | αb | |||||||
| Columnc | 1994 | DAPI | Porous media | 2-3 | 0.6 | 1.0d | 1.0 | |
| Columnc | 1994 | HE | Porous media | 2-3 | 0.6 | 0.3 | 1.4 | |
| Column | 1991e | HE | Liquid broth | 4-5 | 0.6 | 1.0d | 3.0 | |
| Field | 1994 | DAPI | Porous media | 2-3 | 1.0 | 59 | 3.0 × 10−2 | 2.5 |
| 2.8 | 68 | 1.1 × 10−2 | 0.9 | |||||
| 3.6 | 81 | NA | 0.9 | |||||
| Field | 1991e | HE | Liquid broth | 4-5 | 1.0 | 87 | 5.3 × 10−2 | 4.7 |
| 2.8 | 98 | 3.4 × 10−2 | 2.5 | |||||
| 3.6 | 99 | NA | 2.4 | |||||
Retardation factor, calculated on the basis of the centers of mass.
Collision efficiency.
Values are averages for two replicate columns.
Calculated values for α were greater than 1. The value is listed here as 1, because α must be between 0 and 1, by definition (values of 1.0 correspond to a sticking efficiency of 100%).
1991 data were calculated from information provided in reference 12 with permission.
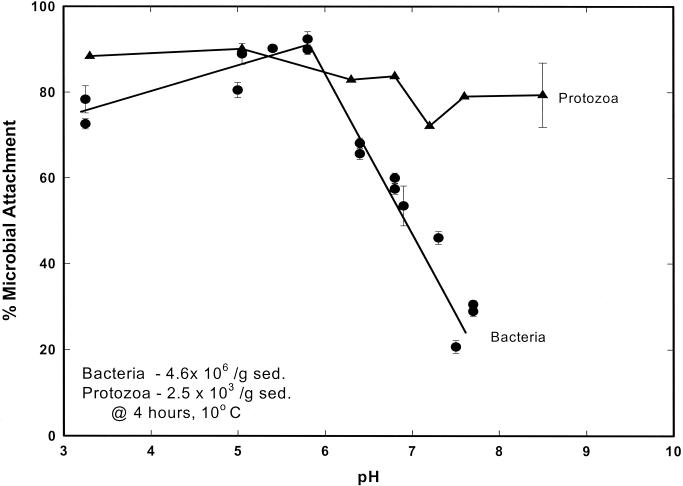
Attachment of S. guttula to sediment surfaces appeared to be relatively independent of pH (Fig. 4). Fractional attachment of nanoflagellates was consistently high (70 to 90%) over the pH range evaluated (pH 3.3 to 8.5); fractional attachments at the lowest and highest pHs evaluated were within the error of the measurement (∼13%) for nanoflagellate enumerations. In contrast, bacteria collected from wells sampling the Cape Cod aquifer exhibited a sharp decrease in propensity for attachment with increasing pH under neutral to slightly alkaline conditions. The pattern of pH dependency of bacterial attachment was consistent with a right-facing “adsorption edge” typical of negatively charged organics sorbing a mineral surface. At pH 5.5 to 6.0, both S. guttula and the indigenous community of groundwater bacteria were 80 to 90% attached. However, at pH 7.5, fractional bacterial attachment decreased to ∼20%, whereas nanoflagellate attachment remained high (∼80%).
FIG. 4.
Fractional attachment of microorganisms onto aquifer sediment surfaces versus pH in static minicolumns containing sieved (0.5- to 1.0-mm) aquifer sediment. Triangles, fractional attachment of vitally stained (HE-stained), porous-medium-grown S. guttula; circles, fractional attachment of DAPI-stained unattached bacteria that are indigenous to the Cape Cod aquifer. Error bars represent the standard errors for three replicate static minicolumns.
DISCUSSION
Effects of culture methods.
Substantial differences in transport behavior for porous-medium-grown versus liquid-medium-grown S. guttula in both field and column studies (Fig. 2 and 3; Table 3) suggest that the manner in which these nanoflagellates are grown substantively affects how they move through aquifer sediments. Not only were smaller (2- to 3-μm) porous-medium-grown nanoflagellates transported in much greater abundance than their larger (4- to 5-μm) broth-grown counterparts, but also their transport behavior was much closer to that of the conservative tracer (bromide) and similar-sized microspheres in an earlier test (Fig. 2). For bacteria, it is clear that a number of transport-determining characteristics, including cell size, motility, exopolymer production, and hydrophobicity can be affected by nutrient availability prior to their introduction into granular media (15). Important physicochemical considerations for nanoflagellate transport include the effect of the culturing protocol upon their size, buoyant density (specific gravity), and surface characteristics.
Greater transport for 2- to 3-μm porous-medium-grown S. guttula than for 4- to 5-μm liquid-broth-grown S. guttula (Fig. 2 and 3) is predicted based upon differences in cell size. Clean-bed filtration theory (33) suggests that the 2- to 3-μm S. guttula are near the optimal size for transport in the Cape Cod aquifer (11). This prediction is based on a measured S. guttula buoyant density of 1.02 g/cm3 (14), a median grain size of 0.59 mm (22), a porosity of 0.35 (22), and an average linear velocity of 0.5 m/day (23). In contrast, filtration theory predicts that transport of 4- to 5-μm nanoflagellates would be much less substantial due to increased settling and physical interception. The predicted size dependence of microbial transport is consistent with earlier observations obtained with flowthrough columns of aquifer sediments and well-defined carboxylated microspheres (13). In a polydispersed population of 0.5-, 0.7-, 1.7-, 2.8-, and 4.8-μm microspheres, the 1.7-μm size class was transported the best, whereas very little transport occurred for the 4.8-μm size class.
Judging from the observed increases in cell size for indigenous groundwater bacteria removed from the Cape Cod aquifer and cultured in high-nutrient liquid broth (14), it is not surprising that the 2- to 3-μm S. guttula that inhabit the Cape Cod aquifer (17) also grow to larger sizes in high-nutrient liquid broth. Indeed, a strong linear correlation between bacterial abundance in the growth medium and the size of a bacterivorous groundwater nanoflagellate has been reported (29). Therefore, the small size (2 to 3 μm) of the porous-medium-grown S. guttula seems reasonable, given that the nanoflagellates had a diminished abundance of prey bacteria because of sorptive losses and a lower concentration of dissolved organic carbon. Also, the nanoflagellates may have been physically constrained by the smallest pore throat diameters within the granular growth medium.
Buoyant density is another important factor affecting the ability of the nanoflagellates to be advected through the aquifer sediments. It is particularly important for the transport behavior of larger protists, because sedimentation rates in granular media are related to the square of a microorganism's diameter (14). As is the case with a microorganism's size, its buoyant density is affected by nutrient and physicochemical conditions present during growth. For bacteria, changes in nutrient conditions can influence intracellular amounts of density-determining storage molecules, such as poly-β-hydroxybutyrate (31) and glycogen (26). Uncultured microorganisms from Cape Cod typically exhibit very low buoyant densities, 1.01 to 1.02 g/cm3 (14). For microorganisms ≤2 μm in cell length, buoyant densities in the aforementioned range would result in a minimal amount of settling during an injection-and-recovery experiment. However, groundwater microorganisms grown in liquid broth often exhibit buoyant densities that are much higher (1.04 to 1.12 g/cm3) (14, 38). Because of their larger size, nanoflagellates with high buoyant densities (e.g., 1.09 g/cm3) would predictably settle at rates that are significant in small-scale injection-and-recovery experiments (14). However, S. guttula cultured in low-nutrient porous media had buoyant densities less than 1.02 g/cm3 (14). The low buoyant densities of this nanoflagellate are comparable to those of the unattached groundwater bacteria that serve as its primary food source (18) and, undoubtedly, contribute to its subsurface transport potential.
A third biological factor determining the subsurface transport behavior is the nature of the microbial surface. Determining the surface characteristics of S. guttula grown in the two different media was beyond the scope of this study. However, differences in retardation between the 1991 and 1994 field studies suggest that the surface characteristics of S. guttula were affected by the culturing procedure used. A comparison of transport between the liquid-broth- and porous-medium-grown HE-stained nanoflagellates through aquifer sediment columns (Table 3) indicates that the former were subject to greater retardation and immobilization. Increased immobilization for the broth-grown nanoflagellates would be expected because of the increased settling that accompanies the ∼2-fold, broth-culturing-induced increase in cell length. However, the observed increase in retardation of broth-grown S. guttula can be explained only by increased surface residence time. This suggests that growth in liquid broth leads to an increased affinity of S. guttula for grain surfaces. There is a dearth of information on the effects of growth conditions upon the attachment behavior of protists to mineral surfaces. However, for bacteria, even the growth phase can affect their surface characteristics and attachment behavior. For example, Pseudomonas aeruginosa Olin in stationary phase exhibited an increased zeta potential and increased attachment to dolomite compared with cells harvested in logarithmic growth or decay phases (8).
Effect of staining and fixation procedure.
The greater transport of the HE-stained S. guttula compared to the DAPI-stained S. guttula in aquifer sediment columns (Table 3) may be due to the a priori fixation with glutaraldehyde. Fixation with glutaraldehyde was necessary to prevent lysis of the nanoflagellates. It is doubtful that DAPI itself affected the surface chemistry of S. guttula. In a recent study assessing the effects of various fluorochromes upon the attachment and transport behavior of bacteria in porous media, it was determined that DAPI had no measurable effect upon the cell surfaces (4, 21) and was a suitable fluorescent tag in transport studies, at least for bacteria in short-term tests. However, the suitability of the DAPI labeling procedure for S. guttula in the month-long injection-and-recovery test is unknown. In our DAPI-staining protocol, the a priori fixation with glutaraldehyde likely affected surface structure (28). Given the negative effect that the fixation protocol had upon S. guttula transport, the difference in transport potential between porous-medium- and liquid-broth cultures is probably even greater than what is depicted in Fig. 3.
The use of HE to label S. guttula in the 1991 test is the first known use of a vital stain to label microorganisms that were subsequently injected into an aquifer and tracked as they moved downgradient. More recently, a vital-staining procedure involving 5,6-carboxylfluorescein diacetate succinimidyl ester (CFDA-SE) was developed for labeling bacteria that were to be tracked in sandy aquifer sediments in Virginia. The CFDA-SE stain allowed tracking of a groundwater isolate, Comamonas sp. strain DA001, for 28 days without a noticeable adverse effect upon the bacterium's physiology or transport (7). Because of the fragile nature of nanoflagellates, the use of vital stains may be critical in assessing their transport potential in in situ studies.
Ecological significance of nanoflagellate transport and attachment behavior.
Collision efficiency is an important predictive parameter of transport potential, because the ability of microorganisms to move through sandy aquifer sediments is strongly related to their propensity for attachment to grain surfaces. The collision efficiency for DAPI-stained nanoflagellates over the first 2.8 m of transport in the field was 1.1 × 10−2. This value is close to the upper end of the range of collision efficiencies (5.4 × 10−3 to 9.7 × 10−3) reported for a morphologically diverse community of free-living groundwater bacteria from the Cape Cod aquifer (11). The earlier small-scale (7-m) natural-gradient injection-and-recovery study involving DAPI-labeled bacteria was performed at an adjacent array of MLS. Because the glutaraldehyde fixation procedure led to increases in immobilization of nanoflagellates within the sediments, it is more likely that the true collision efficiency of S. guttula inhabiting the aquifer sediments in Cape Cod is between 10−3 and 10−2, similar to what was observed for the unattached bacteria that serve as their prey. The ability of S. guttula to be easily advected through sediments may give this predator an advantage, particularly in the contaminant plume, where changes in loading of dissolved organic matter can lead to redistributions of unattached bacteria within the contaminated aquifer (10).
The mechanism(s) of attachment of porous-medium-grown nanoflagellates may be fundamentally different from that of the free-living bacteria that serve as their prey (Fig. 4). The lack of a pH dependency in nanoflagellate attachment to aquifer sand grains, in contrast to the well-defined “adsorption edge” exhibited by the bacteria, suggests that attachment is likely to occur even at slightly to moderately alkaline pH, where the nanoflagellate and sand grain surfaces should be electrostatically repulsive. It has been documented that S. guttula organisms isolated from Cape Cod aquifer sediments often anchor themselves to solid surfaces by means of a filament arising from the posterior end and use the long flagellum inserted at the anterior end to create a capture zone (microeddy) in which unattached bacteria are trapped (18, 30). The ability of the posterior filament to attach itself to a heterogeneous surface would occur at pHs that would be unfavorable to the attachment of most bacteria. The pH of uncontaminated zones within the Cape Cod aquifer is ∼5.5. However, a more neutral pH may be found within the contaminant plume due to addition of lime to the treated sewage before discharge and to heterotrophic activities. The ability of S. guttula to attach regardless of the variability in groundwater pH is likely a significant ecological advantage, because this protist must be in the attached state to feed efficiently.
In summary, the ability to grow in situ-sized (2- to 3-μm) nanoflagellates allowed experiments that improved our understanding of their subsurface transport behavior. Their high subsurface mobility allows them to redistribute within the aquifer in response to changes in nutrient conditions that affect their prey. Because the prey of S. guttula are the unattached bacteria that contribute to degradation of organic contaminants (18), the ability of these nanoflagellates to be advected readily through aquifer sediments may have important implications for organically contaminated groundwater habitats. However, more research on the response of the subsurface protistan communities to changes in loading of organic contaminants is needed. Finally, the effect of S. guttula's swimming behavior upon its ability to move through aquifer sediment was not assessed but it may be significant and is worthy of further investigation.
Acknowledgments
The advice and assistance of Denis LeBlanc and Kathy Hess of the Massachusetts/Rhode Island District of the USGS are gratefully acknowledged. We also thank Annie Pieper, David Kinner, and Joseph Ryan (University of Colorado) for help with sampling and Karen Blakeslee (University of New Hampshire) for assistance with enumerations of S. guttula. We thank Jennifer Rogers (University of Kansas), Gil Barth (USGS), Larry Barber (USGS), and the two anonymous reviewers for all their useful comments and suggestions on the manuscript.
The laboratory and field studies were funded by National Science Foundation grant BSC 9012183, awarded to the University of New Hampshire.
REFERENCES
- 1.Bales, R. C., S. M. Li, K. M. Maguire, M. T. Yahya, C. P. Gerba, and R. W. Harvey. 1995. Virus and bacteria transport in a sandy aquifer, Cape Cod, MA. Ground Water 33:653-661. [Google Scholar]
- 2.Brush, C. F., W. C. Ghiorse, L. J. Anguish, J. Y. Parlange, and H. G. Grimes. 1999. Transport of Cryptosporidium parvum oocysts through saturated columns. J. Environ. Qual. 28:809-815. [Google Scholar]
- 3.Bunn, A. L. 1992. Ph.D. thesis. University of New Hampshire, Durham.
- 4.Chen, J., and B. Koopman. 1997. Effect of fluorochromes on bacterial surface properties and interaction with granular media. Appl. Environ. Microbiol. 63:3941-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenmann, H., H. Harms, R. Meckenstock, E. I. Meyer, and A. J. B. Zehnder. 1998. Grazing of a Tetrahymena sp. on adhered bacteria in percolated columns monitored by in situ hybridization with fluorescent oligonucleotide probes. Appl. Environ. Microbiol. 64:1264-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fetter, C. 1993. Contaminant hydrogeology. Macmillan Publishing Co., New York, N.Y.
- 7.Fuller, M. E., S. H. Streger, R. K. Rothmel, B. J. Mailloux, J. A. Hall, T. C. Onstott, J. K. Fredrickson, D. L. Balkwill, and M. F. DeFlaun. 2000. Development of a vital fluorescent staining method for monitoring bacterial transport in subsurface environments. Appl. Environ. Microbiol. 66:4486-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasso, D., B. F. Smets, K. A. Strevett, B. D. Machinist, C. J. VanOss, R. F. Giese, and W. Wu. 1996. Impact of physiological state on surface thermodynamics and adhesion of Pseudomonas aeruginosa. Environ. Sci. Technol. 30:3604-3608. [Google Scholar]
- 9.Harter, T., S. Wagner, and E. R. Atwill. 2000. Colloid transport and filtration of Cryptosporidium parvum in sandy soils and aquifer sediments. Environ. Sci. Technol. 34:62-70. [Google Scholar]
- 10.Harvey, R. W., and L. B. Barber. 1992. Associations of free-living bacteria and dissolved organic compounds in a plume of contaminated groundwater. J. Contam. Hydrol. 9:91-103. [Google Scholar]
- 11.Harvey, R. W., and S. P. Garabedian. 1991. Use of colloid filtration theory in modeling movement of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 25:178-185. [Google Scholar]
- 12.Harvey, R. W., N. E. Kinner, A. Bunn, D. Macdonald, and D. Metge. 1995. Transport behavior of groundwater protozoa and protozoan-sized microspheres in sandy aquifer sediments. Appl. Environ. Microbiol. 61:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey, R. W., N. E. Kinner, D. Macdonald, D. W. Metge, and A. Bunn. 1993. Role of physical heterogeneity in the interpretation of small-scale laboratory and field observations of bacteria, microbial-sized microsphere, and bromide transport through aquifer sediments. Water Resour. Res. 29:2713-2721. [Google Scholar]
- 14.Harvey, R. W., D. W. Metge, N. Kinner, and N. Mayberry. 1997. Physiological considerations in applying laboratory-determined buoyant densities to predictions of bacterial and protozoan transport in groundwater: results of in-situ and laboratory tests. Environ. Sci. Technol. 31:289-295. [Google Scholar]
- 15.Heise, S., and G. Gust. 1999. Influence of the physiological status of bacteria on their transport into permeable sediments. Mar. Ecol. Prog. Ser. 190:141-153. [Google Scholar]
- 16.Johnson, M. J. 1990. M.S. thesis. University of New Hampshire, Durham.
- 17.Kinner, N. E., A. L. Bunn, R. W. Harvey, A. Warren, and L. D. Meeker. 1991. Preliminary evaluation of the relations among protozoa, bacteria, and chemical properties in sewage-contaminated ground water near Otis Air Base, Massachusetts, p. 141-143. In USGS WRI report 91-4034. USGS Toxic Substances Hydrology Program Technical Meeting, Monterey, Calif. U.S. Geological Survey, Reston, Va.
- 18.Kinner, N. E., R. W. Harvey, K. Blakeslee, G. Novarino, and L. D. Meeker. 1998. Size-selective predation on groundwater bacteria by nanoflagellates in an organic-contaminated aquifer. Appl. Environ. Microbiol. 64:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinner, N. E., R. W. Harvey, and M. Kazmierkiewicz-Tabaka. 1997. Effect of flagellates on free-living bacterial abundance in an organically contaminated aquifer. FEMS Microbiol. Rev. 20:249-259. [DOI] [PubMed] [Google Scholar]
- 20.Kota, S., R. C. Borden, and M. A. Barlaz. 1999. Influence of protozoan grazing on contaminant biodegradation. FEMS Microbiol. Ecol. 29:179-189. [Google Scholar]
- 21.Kucukcolak, E., B. Koopman, G. Bitton, and S. Farrah. 1998. Validity of fluorochrome-stained bacteria as tracers of short-term microbial transport through porous media. J. Contam. Hydrol. 31:349-357. [Google Scholar]
- 22.LeBlanc, D. R. 1984. Digital modeling of solute transport in a plume of sewage-contaminated ground water, p. 11-46. In D. R. LeBlanc (ed.), Movement and fate of solutes in a plume of sewage-contaminated ground water. USGS open file report 84-475. U.S. Geological Survey, Reston, Va.
- 23.Leblanc, D. R., S. P. Garabedian, K. M. Hess, L. W. Gelhar, R. D. Quadri, K. G. Stollenwerk, and W. W. Wood. 1991. Large-scale natural gradient tracer test in sand and gravel, Cape-Cod, Massachusetts. 1. Experimental-design and observed tracer movement. Water Resour. Res. 27:895-910. [Google Scholar]
- 24.Macdonald, D. G. 1992. Senior honors thesis. University of New Hampshire, Durham.
- 25.Madsen, E. L., J. L. Sinclair, and W. C. Ghiorse. 1991. In-situ biodegradation--microbiological patterns in a contaminated aquifer. Science 252:830-833. [DOI] [PubMed] [Google Scholar]
- 26.Mas, J., C. Pedrosalio, and R. Guerrero. 1989. Variations in cell-size and buoyant density of Escherichia coli K-12 during glycogen accumulation. FEMS Microbiol. Lett. 57:231-236. [DOI] [PubMed] [Google Scholar]
- 27.Mayberry, N. A. 1996. M.S. thesis. University of New Hampshire, Durham.
- 28.Millonig, G., and V. Marinozzi. 1968. Fixation and embedding in electron microscopy, p. 251-326. In R. Barer and V. E. Cosslett (ed.), Advances in optical and electron microscopy, vol. 2. Academic Press, London, United Kingdom. [Google Scholar]
- 29.Novarino, G., A. Warren, H. Butler, G. Lambourne, A. Boxshall, J. Bateman, N. E. Kinner, R. W. Harvey, R. A. Mosse, and B. Teltsch. 1997. Protistan communities in aquifers: a review. FEMS Microbiol. Rev. 20:261-275. [DOI] [PubMed] [Google Scholar]
- 30.Novarino, G., A. Warren, N. E. Kinner, and R. W. Harvey. 1994. Protists from a sewage-contaminated aquifer on Cape-Cod, Massachusetts. Geomicrobiol. J. 12:23-36. [Google Scholar]
- 31.Pedrosalio, C., J. Mas, and R. Guerrero. 1985. The influence of poly-beta-hydroxybutyrate accumulation on cell-volume and buoyant density in Alcaligenes eutrophus. Arch. Microbiol. 143:178-184. [Google Scholar]
- 32.Pieper, A. P., J. N. Ryan, R. W. Harvey, G. L. Amy, T. H. Illangasekare, and D. W. Metge. 1997. Transport and recovery of bacteriophage PRD1 in a sand and gravel aquifer: effect of sewage-derived organic matter. Environ. Sci. Technol. 31:1163-1170. [Google Scholar]
- 33.Rajagopalan, R., and C. Tien. 1976. Trajectory analysis of deep-bed filtration with the sphere-in-cell porous media model. J. Am. Inst. Chem. Eng. 22:523-533. [Google Scholar]
- 34.Ryan, J. N., M. Elimelech, R. A. Ard, R. W. Harvey, and P. R. Johnson. 1999. Bacteriophage PRD1 and silica colloid transport and recovery in an iron oxide-coated sand aquifer. Environ. Sci. Technol. 33:63-73. [Google Scholar]
- 35.Scholl, M. A., and R. W. Harvey. 1992. Laboratory investigations on the role of sediment surface and groundwater chemistry in transport of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 26:1410-1417. [Google Scholar]
- 36.Sinclair, J. L., D. H. Kampbell, M. L. Cook, and J. T. Wilson. 1993. Protozoa in subsurface sediments from sites contaminated with aviation gasoline or jet fuel. Appl. Environ. Microbiol. 59:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strauss, E. A., and W. K. Dodds. 1997. Influence of protozoa and nutrient availability on nitrification rates in subsurface sediments. Microb. Ecol. 34:155-165. [DOI] [PubMed] [Google Scholar]
- 38.Wan, J. M., T. K. Tokunaga, and C. F. Tsang. 1995. Bacterial sedimentation through a porous medium. Water Resour. Res. 31:1627-1636. [Google Scholar]
- 39.Yao, K. M., M. T. Habibian, and C. R. O'Melia. 1971. Water and waste water filtration: concepts and applications. Environ. Sci. Technol. 5:1105-1112. [Google Scholar]
- 40.Zapico, M. M., S. Vales, and J. A. Cherry. 1987. A wireline piston core barrel from sampling cohesionless sand and gravel below the water table. Ground Water Monit. Rev. 7:74-87. [Google Scholar]
- 41.Zarda, B., G. Mattison, A. Hess, D. Hahn, P. Hohener, and J. Zeyer. 1998. Analysis of bacterial and protozoan communities in an aquifer contaminated with monoaromatic hydrocarbons. FEMS Microbiol. Ecol. 27:141-152. [Google Scholar]