Abstract
Phylogenetic analysis of tetracycline resistance genes, which confer resistance due to the efflux of tetracycline from the cell catalyzed by drug:H+ antiport and share a common structure with 12 transmembrane segments (12-TMS), suggested the monophyletic origin of these genes. With a high degree of confidence, this tet subcluster unifies 11 genes encoding tet efflux pumps and includes tet(A), tet(B), tet(C), tet(D), tet(E), tet(G), tet(H), tet(J), tet(Y), tet(Z), and tet(30). Phylogeny-aided alignments were used to design a set of PCR primers for detection, retrieval, and sequence analysis of the corresponding gene fragments from a variety of bacterial and environmental sources. After rigorous validation with the characterized control tet templates, this primer set was used to determine the genotype of the corresponding tetracycline resistance genes in total DNA of swine feed and feces and in the lagoons and groundwater underlying two large swine production facilities known to be impacted by waste seepage. The compounded tet fingerprint of animal feed was found to be tetCDEHZ, while the corresponding fingerprint of total intestinal microbiota was tetBCGHYZ. Interestingly, the tet fingerprints in geographically distant waste lagoons were identical (tetBCEHYZ) and were similar to the fecal fingerprint at the third location mentioned above. Despite the sporadic detection of chlortetracycline in waste lagoons, no auxiliary diversity of tet genes in comparison with the fecal diversity could be detected, suggesting that the tet pool is generated mainly in the gut of tetracycline-fed animals, with a negligible contribution from selection imposed by tetracycline that is released into the environment. The tet efflux genes were found to be percolating into the underlying groundwater and could be detected as far as 250 m downstream from the lagoons. With yet another family of tet genes, this study confirmed our earlier findings that the antibiotic resistance gene pool generated in animal production systems may be mobile and persistent in the environment with the potential to enter the food chain.
Until recently, antibiotic resistance studies have been primarily confined to cultivable clinical isolates and were mostly phenotype based. Because of these, critical information with regard to the circulation of antibiotic resistance genes in commensal microbiota and in the environment is lacking. A phylogeny-aided molecular ecology approach to examination of tetracycline resistance genes implemented in our previous work proved to be very useful in accessing the pool of antibiotic resistance genes without cultivation (1). Based on this approach, we investigated the presence of eight tetracycline resistance genes encoding the ribosomal protection proteins (RPP) in swine production facilities and in the waste lagoons and groundwater underlying these facilities (1, 8). These studies demonstrated the occurrence of RPP genes in the environment as a direct impact of agriculture and suggested that groundwater may serve as a potential source of antibiotic resistance entering the food chain.
In the present study, we extended this genotyping approach to another group of tetracycline resistance genes, which are found almost exclusively in gram-negative bacteria and confer the resistance due to the efflux of tetracycline from the cell catalyzed by drug:H+ antiport (22, 23). These transporters share a common structure with 12 transmembrane segments (12-TMS) and belong to family 2 (23) or, according to the other classification, to family 3 (22) within the major facilitator superfamily (MFS). Since these 12-TMS permeases uniformly catalyze drug:H+ antiport, they are also referred to as the DHA12 family (22). Phylogenetically, besides the tetracycline efflux pumps of gram-negative bacteria, the DHA12 family includes multidrug permeases and a number of other uncharacterized transporters (23).
Historically, the genotyping of tetracycline resistance genes in bacterial isolates has been performed by hybridization with probes generated from known tetracycline resistance determinants (9). Implementation of this approach to detect classes A to E in marine sediment community DNA has demonstrated, however, that the method is not sufficiently sensitive for environmental studies, with no hybridization signals associated with the samples actually containing the corresponding resistant bacteria (2). More recently, a PCR-based approach has been developed for detection of tetracycline efflux pumps of gram-negative bacteria in the environmental samples and in bacterial isolates (10). This set of primers, however, targets only five determinants (Tet A to E), while the conservative estimates bring the number of Tet determinants in this group to at least 11 (9). Consequently, there is a need to develop a set of primers targeting a wider range of tet efflux pumps of gram-negative bacteria.
In this study, we performed phylogenetic analysis of genes belonging to the DHA12 family. From the inferred tree, 11 genes encoding tet efflux pumps formed a phylogenetically coherent cluster, which included tet(A), tet(B), tet(C), tet(D), tet(E), tet(G), tet(H), tet(J), tet(Y), tet(Z), and tet(30). The tree-based alignments were used to design PCR primers targeting these genes. After validation with the characterized control tet genes, this primer set was used to detect the corresponding tetracycline resistance genes in swine feed and feces and in both the waste lagoons and groundwater underlying two large swine production facilities known to be impacted by waste seepage (15).
MATERIALS AND METHODS
Strains, plasmids, and culture techniques.
Escherichia coli strains and plasmids used in this study for validation and control purposes are listed in Table 1 (kindly provided by M. C. Roberts, Department of Pathobiology, University of Washington, Seattle; J.-M. Collard, Section of Biosafety and Biotechnology, Scientific Institute of Public Health, Brussels, Belgium; B. A. Castilho, Departamento de Microbiologia, Imunologia e Parasitologia, Universidade Federal de Sao Paulo, Sao Paulo, Brazil; E. Tietze, Robert Koch Institut, Wernigerode, Germany; A. Tauch, Fakultat fur Biologie, Universitat Bielefeld, Bielefeld, Germany; and S. K. Farrand, Department of Crop Sciences, University of Illinois at Urbana-Champaign, Urbana). E. coli strains were grown on Luria-Bertani medium at 37°C with aeration. Media were solidified when necessary with 1.8% (wt/vol) agar (Difco Laboratories, Detroit, Mich.). Tetracycline (10 μg/ml) or ampicillin (50 μg/ml) (Sigma Chemical Co., St. Louis, Mo.) was added to maintain the corresponding recombinant plasmids harboring tet genes. Plasmids were isolated with a QIAprep Spin Miniprep kit (Qiagen, Inc., Valencia, Calif.).
TABLE 1.
Characteristics of bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| HB101(RP1) | tet(A) | 26 |
| HB101(pRT11) | tet(B), 2.7-kb HpaI fragment from lambda::Tn10 cloned into pVH51 | 11, 13 |
| DO-7(pBR322) | tet(C) | 5 |
| JM109(pUC119D) | tet(D), 4.3-kb HindIII fragment from pSP9350 of P. piscida in pUC119 | 14 |
| HB101(pSL1504) | tet(E), pSL1456 subcloned into pACYC177 | 20 |
| HB101(pUC119G) | tet(G), 9-kb HindIII fragment from pJA8122 of V. anguillarum in pUC119 | 3, 27 |
| DH5α(pVM111) | tet(H) | 12 |
| DH5α(pZLE4.5) | tet(30), 4.5-kb EcoRI fragment cloned into pJB3 | 16 |
| Plasmids | ||
| pVM6 | tet(J), 4.5-kb Sau3A fragment of P. mirabilis cloned into pACYC177 | 18 |
| pIE1122 | tet(Y), 4.2-kb EcoRV fragment of pIE1120 cloned into pBluescriptKS+ | E. Tietze |
| pAGHD1 | tet(Z), 7.9-kb HindIII fragment of pAG1 cloned into pUC19 | 24 |
Phylogenetic analysis and primer design.
All currently available nucleotide sequences encoding tetracycline efflux pumps of gram-negative bacteria were downloaded from the GenBank database (4). These included the following tet genes and bacterial hosts (with GenBank accession numbers in parentheses): (i) tet(A) from E. coli plasmid RP1 (Tn1721) and Pseudomonas aeruginosa plasmid RP4 (X00006 and X75761); (ii) tet(B) from E. coli and Shigella flexneri Tn10 (J01830, K00615, K01493, and X00694); (iii) tet(C) from E. coli and Salmonella enterica serovar Typhimurium Tn3 (J01749, K00005, L08654, M10282, M10283, M10286, M10356, M10784, M10785, M10786, M33694, and V01119); (iv) tet(D) from Salmonella enterica serovar Ordonez, Photobacterium aerogenes, and Pasteurella piscicida (X65876, L06798, and D16172); (v) tet(E) from E. coli (L06940); (vi) tet(G) from Vibrio anguillarum, Pseudomonas sp. plasmid pPSTG1, Pseudomonas sp. plasmid pPSTG2 and Salmonella enterica serotype Typhimurium DT104 (S52437, AF133139, AF133140, and AF071555); (vii) tet(H) from Pasteurella aerogenes plasmid pPAT1, Pasteurella haemolytica plasmid pPHT1, and Pasteurella multocida plasmid pVM111 (AJ245947, Y16103, and U00792); (viii) tet(J) from Proteus mirabilis (AF038993); (ix) tet(Y) from E. coli plasmid pIE1120 (AF070999); (x) tet(Z) from Corynebacterium glutamicum plasmid pAG1 (AF121000); and (xi) tet(30) from Agrobacterium tumefaciens (AF090987). The sequences of the following other phylogenetically similar genes within the 12-TMS family of the MFS were downloaded from GenBank: norA from Staphylococcus aureus (M97169) and S. aureus strain TK2566 (D90119), blt (L32599) and bmrU (L25604) from Bacillus subtilis, cml from Streptomyces lividans (X59968), cmlA from P. aeruginosa (M64556), and cmlB from Rhodococcus fascians (Z12001). No sequence information for tet(I) was available in the databases, and it was not included in our analysis.
Phylogenetic analysis was performed essentially as described previously (1). In addition, the maximum-likelihood and parsimony analyses implemented in DNAML and DNAPARS programs of the PHYLIP package (distributed by J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle; PHYLIP [PHYLogeny Inference Package], version 3.6; http://evolution.genetics.washington.edu/phylip.html) were used for phylogenetic tree construction. PCR primers were designed to satisfy specificity and amplification of as many tet genes as possible with the fewest optimal PCR regimes to aid in a more rapid analysis of large sample sets. The set of 11 primers with the corresponding annealing and extension temperatures and expected amplicon sizes is shown in Table 2.
TABLE 2.
PCR primers targeting tet efflux pumps of gram-negative bacteria
| Primer pair | Class targeted | Sequence | PCR annealing and extension temp (°C)a | Amplicon size (bp) |
|---|---|---|---|---|
| Tet A-FW | A | GCGCGATCTGGTTCACTCG | 61 | 164 |
| Tet A-RV | AGTCGACAGYRGCGCCGGC | |||
| Tet B-FW | B | TACGTGAATTTATTGCTTCGG | 61 | 206 |
| Tet B-RV | ATACAGCATCCAAAGCGCAC | |||
| Tet C-FW | C | GCGGGATATCGTCCATTCCG | 68 | 207 |
| Tet C-RV | GCGTAGAGGATCCACAGGACG | |||
| Tet D-FW | D | GGAATATCTCCCGGAAGCGG | 68 | 187 |
| Tet D-RV | CACATTGGACAGTGCCAGCAG | |||
| Tet E-FW | E | GTTATTACGGGAGTTTGTTGG | 61 | 199 |
| Tet E-RV | AATACAACACCCACACTACGC | |||
| Tet G-FW | G | GCAGAGCAGGTCGCTGG | 68 | 134 |
| Tet G-RV | CCYGCAAGAGAAGCCAGAAG | |||
| Tet H-FW | H | CAGTGAAAATTCACTGGCAAC | 61 | 185 |
| Tet H-RV | ATCCAAAGTGTGGTTGAGAAT | |||
| Tet J-FW | J | CGAAAACAGACTCGCCAATC | 61 | 184 |
| Tet J-RV | TCCATAATGAGGTGGGGC | |||
| Tet Y-FW | Y | ATTTGTACCGGCAGAGCAAAC | 68 | 181 |
| Tet Y-RV | GGCGCTGCCGCCATTATGC | |||
| Tet Z-FW | Z | CCTTCTCGACCAGGTCGG | 61 | 204 |
| Tet Z-RV | ACCCACAGCGTGTCCGTC | |||
| Tet 30-FW | 30 | CATCTTGGTCGAGGTGACTGG | 68 | 210 |
| Tet 30-RV | ACGAGCACCCAGCCGAGC |
Two-step PCR conditions are given in Materials and Methods.
Sampling, DNA extraction, and PCR.
The description of site U (Swine Research Farm of the University of Illinois) and the sampling and DNA isolation procedures from pig feed components and feces were described previously (1). Briefly, fecal samples were collected from six randomly chosen sows, 1 to 2 years old, with an average weight of between 160 and 170 kg. Total DNA from the fecal and feed samples was isolated with the Soil DNA Purification kit (Mo Bio, Solana Beach, Calif.) according to the manufacturer's protocol. The description of two commercial swine production facilities (sites A and C), together with the details of sampling and DNA extraction, was also specified previously (8). Briefly, groundwater samples (2 liters) in triplicate were collected into sterile plastic bottles and stored on ice in the field. The lagoon sample was a composite of eight subsamples. Approximately 2-liter subsamples were collected from two locations on each side of the lagoon. The subsamples were composited into one sample, and then samples of the appropriate size were collected from the composite. Lagoon samples were collected from the berm of the lagoon. A 3-m pole with a 1-liter beaker attached to the end of the pole was used to collect samples from a depth of approximately 1 m and about 2.5 m from the manure-berm interface. The manure in the sample area was mixed by rapidly moving the pole and beaker back and forth prior to sampling. Samples were refrigerated at 4°C in the laboratory until analyzed. Groundwater samples (250 ml) were centrifuged at 17,700 × g for 20 min at 4°C. Supernatants were discarded, and the pellets were washed three times with 1/10 volume of phosphate-buffered saline (120 mM NaH2PO4 [pH 8.0], 0.85% NaCl) before extraction of total DNA by the method of Tsai and Olsen (25). Lagoon samples (50 ml) were centrifuged at 10,000 × g for 10 min at 4°C before DNA extraction as described above. DNA with a final concentration of 125 ng/μl was stored in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) at −20°C. A typical PCR mixture contained 25 pmol of each primer, 1× ExTaq reaction buffer (PanVera Corporation, Madison, Wis.), 100 μM each deoxynucleoside triphosphate, and 1.0 U of ExTaq DNA polymerase (PanVera Corporation), adjusted to a total volume of 25 μl. Purified DNA (125 ng) or one-half of a 1- to 2-mm-diameter individual colony resuspended in sterile water was used as the PCR template. PCR amplification was performed with either a GeneAmp PCR system 2400 thermocycler (Perkin-Elmer, Norwalk, Conn.) or a DNA Engine Thermocycler (MJ Research, Waltham, Mass.). A rapid two-step PCR cycling protocol was developed for the current set of primers shown in Table 2. The first PCR regimen, which was used for amplification of tet(C), tet(D), tet(G), tet(Y), and tet(30), consisted of initial denaturation at 94°C for 5 min followed by 25 cycles at 94°C for 5 s and 10 s of annealing and extension at 68°C, with a final extension at 68°C for 7 min. The second PCR regimen, which was used for amplification of tet(A), tet(B), tet(E), tet(H), tet(J), and tet(Z), consisted of initial denaturation at 94°C for 5 min, followed by 25 cycles at 94°C for 5 s and 30 s of annealing and extension at 61°C, with a final extension at 61°C for 7 min. A second, nested PCR was performed with 1 μl of the first PCR mixture as a template and amplification for 25 cycles, as described above, if PCR failed due to the presence of unidentified PCR-inhibiting substances. Aliquots of 5 μl were analyzed by electrophoresis on a 2.5% (wt/vol) agarose gel (NuSieve; FMC Bioproducts, Rockland, Maine) containing the fluorescent dye GelStar (FMC Bioproducts) or ethidium bromide.
Cloning and sequencing of PCR amplicons.
PCR products were cloned with a TOPO-TA cloning kit (Invitrogen, Carlsbad, Calif.). White colonies of ampicillin-resistant transformants were screened for the presence of tet fragments by PCR with the same primer set used for amplification. DNA sequence analysis of recombinant plasmids was performed for both strands (primers M13F and M13R) by the University of Illinois Biotechnology Center. Online similarity searching was performed with the BLAST (Basic Local Alignment Search Tool) family of programs in GenBank (17).
RESULTS
Phylogenetic analysis.
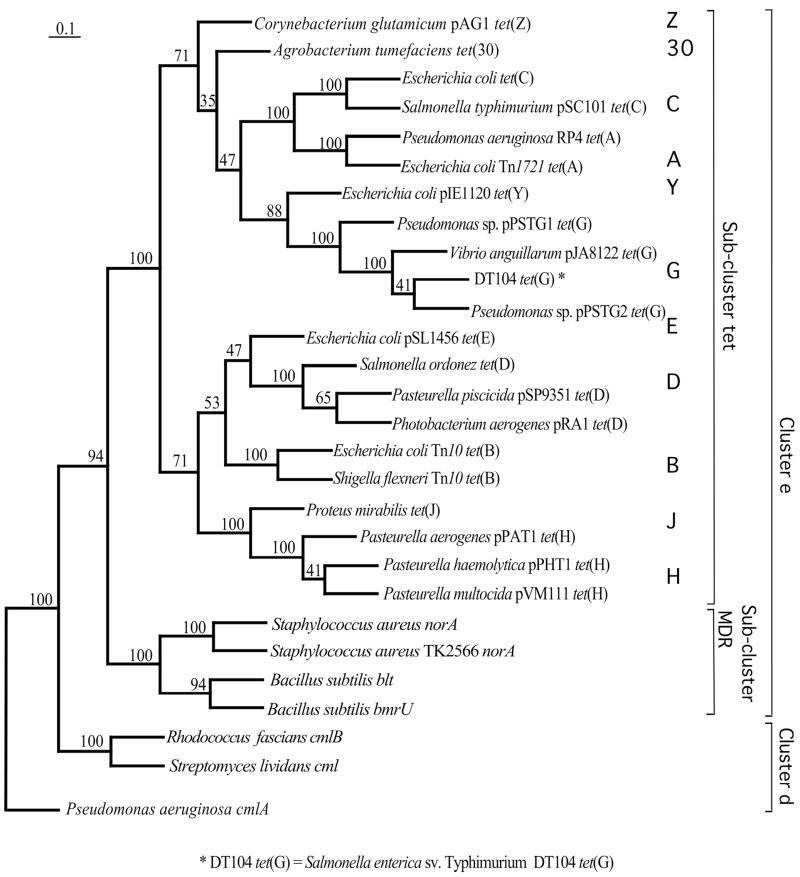
Phylogenetic analysis was performed with 21 complete nucleotide sequences encoding tetracycline efflux pumps of gram-negative bacteria (TEPGNB), with four sequences encoding multidrug efflux pumps (norA, blt, and bmrU), and with three sequences encoding chloramphenicol resistance proteins (cml, cmlA, and cmlB) (Fig. 1). In the previous protein-based phylogenetic analyses, TEPGNB and multidrug efflux pumps have been shown to form cluster e, while the chloramphenicol efflux pumps belong to cluster d (23). Our nucleotide sequence-based phylogenetic analysis essentially confirmed this topology and supported the corresponding clustering with high bootstrap values (Fig. 1). With 100% bootstrap support, this analysis confirmed the monophyletic origin of genes encoding TEPGNB, with the branching event separating them from the other subcluster, MDR, in cluster e (Fig. 1). The monophyletic origin of TEPGNB also suggests that the high drug specificity in this group appeared only once—probably at the time of separation from the MDR subcluster—and was selectively maintained in this group without changing or broadening the substrate specificity despite the profound divergence, which, at the protein level, is estimated to be in the range of 41 to 78% of amino acid identity (9). In contrast, members of the multidrug resistance (MDR) subcluster possess much broader specificity and can extrude structurally diverse compounds (21). The phylogenetically coherent tet subcluster includes 11 genes, tet(A), tet(B), tet(C), tet(D), tet(E), tet(G), tet(H), tet(J), tet(Y), tet(Z), and tet(30).
FIG. 1.
Maximum-likelihood tree of tetracycline resistance genes, encoding tet efflux pumps of gram-negative bacteria (subcluster tet), and other genes of the DHA12 family. The same overall topology was obtained by neighbor-joining and parsimony analyses. Numbers at nodes represent the percentages of occurrence of nodes in 100 bootstrap trials. The scale bar is in fixed nucleotide substitutions per sequence position. The tree is arbitrarily rooted with the P. aeruginosa cmlA gene. The set of PCR primers, presented in Table 2, targets various classes of tet genes, which are shown in boldface.
Design and validation of PCR primers targeting tet genes.
Despite the monophyletic appearance of the tet genes, their profound diversity precluded the possibility of designing a universal PCR primer pair targeting all genes in the tet subcluster. Thus, the designed set was limited to the primer pairs specific for the individual classes, A, B, C, D, E, G, H, J, Y, Z, and 30, and this set, with the expected amplicon sizes and annealing and extension temperatures, is shown in Table 2. As described in Materials and Methods, we also elaborated a two-step PCR cycling procedure for these primers. This set of primers was rigorously tested in PCR amplifications with DNA and colony biomass of control strains In all cases, the amplicons of expected size were produced with positive controls, and no product was detected in incongruent primer-template combinations (data not shown). Validation also included sequence analysis of amplicons from the environmental samples (see below).
Detection of tet efflux genes in swine feed.
In our previous work, we detected a considerable diversity of tetracycline resistance genes, encoding the alternative elongation factors, in the swine feed components (1). This suggested that antibiotic resistance gene contamination might already exist at the feed preparation stage. With the current set of primers, however, only four feed components tested were found to be contaminated with the tet efflux genes. These were whey powder [tet(C)]; plasma protein product [tet(E), tet(H), and tet(Z)]; the commercial preparation of Tylan (a macrolide that is used for prophylaxis in animals 6 weeks to 6 months old), which contained tet(D); and the growth-promoting antibiotic mix CSP (chlortetracycline, sulfonamide, and penicillin) used in group of animals 3 to 6 weeks old, in which DNA of tet(H) was detected (data not shown). Thus, the tet fingerprint of the animal feed with all components combined is tetCDEHZ, which does not match the fingerprints of the swine gut microbiota (tetBCGHYZ) or the waste lagoon (tetBCEHYZ) (see below). Similar to our previous experiments, the presence of bacterial DNA in all premix and mixed pig feed samples at site U was confirmed by amplification of the V3 region of bacterial 16S ribosomal DNA, and the identity of the tet amplicons was validated by sequencing (data not shown).
Detection of tet efflux genes in swine feces.
Total DNA preparations from swine fecal samples of six pigs at site U were genotyped with the current primer set (Table 3), and some amplicons were sequenced. tet(B), tet(C), tet(G), tet(H), tet(Y), and tet(Z) were found, but not consistently, in all animals. For example, animal 1 had just two tet genes circulating in the gut microbiota, while animals 4, 5, and 6 were genotyped with four tet genes (Table 3). Only two animals, 4 and 5, had similar profiles of tet genes in the total fecal DNA. Despite the fact that all animals were subjected to the uniform tetracycline selection and that the influx of tetracycline resistance genes with feed was also similar in the repertoire of genes ingested, the profiles of tet genes circulating in microbiota of individual animals differ significantly (Table 3). This suggests the differential proliferation of tetracycline-resistant bacteria carrying the distinct tet genes in individual animals. Integrated at the animal population level, the swine herd microbiota produces the overall tetBCGHYZ fingerprint, which is different from the feed fingerprint, tetCDEHZ, but still, these two share three common Tet determinants, C, H, and Z. Interestingly, these three determinants were among the most commonly encountered in the animal production systems, from animal feed, to animal waste, to groundwater (see below).
TABLE 3.
tet efflux genes detected in total DNA from pig feces, lagoons, and groundwater
| Sample |
tet genesa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| tet(A) | tet(B) | tet(C) | tet(E) | tet(G) | tet(H) | tet(Y) | tet(Z) | |
| Site U (fecal) | − | +b (2/6)c | + (3/6) | − | + (4/6) | + (5/6) | + (1/6) | + (5/6) |
| Site A (lagoon) | − | +d | +d | +d | − | +d | +d | + |
| A7 bkge | − | − | − | − | − | − | − | − |
| A10 | − | − | − | − | − | − | − | − |
| A8 | − | + | + | + | − | +d | − | + |
| A9 | − | − | + | − | − | − | − | − |
| A16 | − | − | + | − | − | + | − | + |
| A13 | − | − | + | − | − | + | − | − |
| A15 | − | − | − | − | − | + | − | − |
| A6 | − | − | + | − | − | − | − | − |
| A5 | − | − | + | − | − | + | − | + |
| A12 | − | − | + | − | − | − | − | − |
| A14 | − | + | − | − | − | + | − | + |
| A11 | − | − | + | − | − | + | − | − |
| A3 | − | + | + | − | − | + | − | + |
| A4 | − | − | − | − | − | + | − | − |
| Site C (lagoon) | − | + d | +d | +d | − | +d | +d | +d |
| C1 bkge | − | − | − | − | − | − | − | − |
| C3 | + | − | − | − | − | − | − | − |
| C2 | − | + | − | − | − | + | − | − |
| C4 | − | + | − | − | − | + | − | − |
| C6 | − | − | + | − | − | + | − | − |
| C7 | − | − | + | − | − | + | − | − |
No tet(D), tet(J), or tet(30) was detected in any sample.
PCR product detected in the nested reaction.
Number of animals positive for tet/total number of animals.
PCR product detected in the first reaction.
Background control well located upstream of the lagoon.
Detection of tet genes in lagoon and groundwater samples.
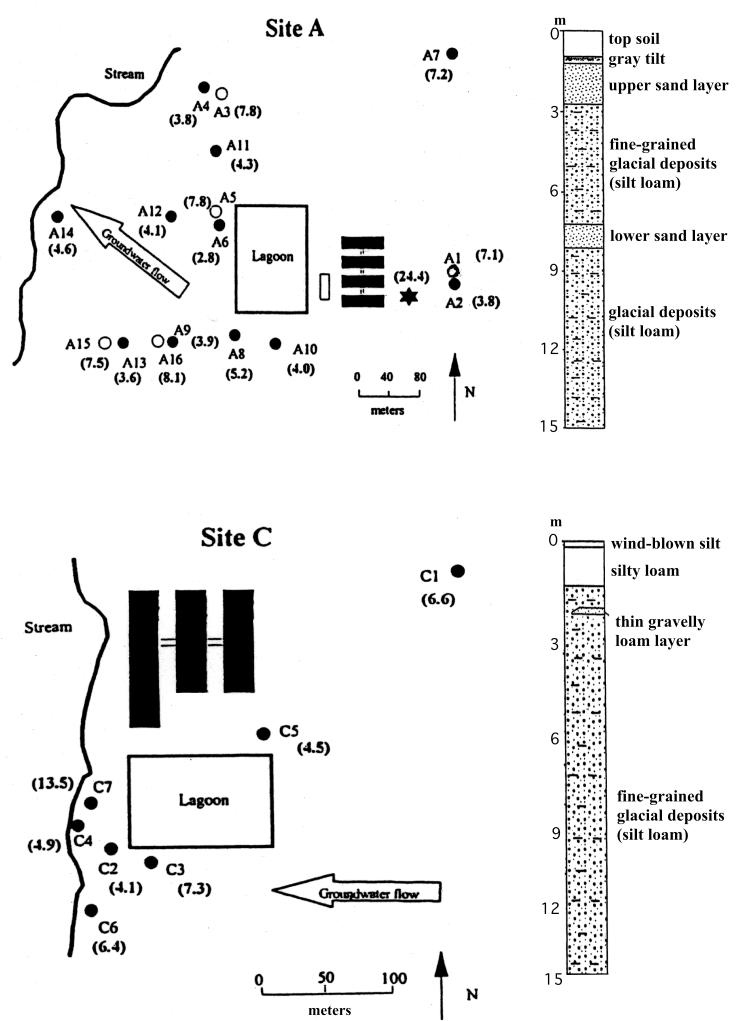
In our previous work, we detected all of the known ribosomal protection tetracycline resistance determinants in the lagoon samples of two study sites (8). In contrast, the general diversity of the tet efflux pump genes at these two sites was substantially lower, and only 6 determinants out of 11 were detected (Table 3). tet fingerprints from both waste lagoon samples were identical (tetBCEHYZ) and were similar to the overall swine herd fecal fingerprint determined at site U (tetBCGHYZ). None of the lagoon samples had detectable tet(A), tet(D), tet(G), tet(J), or tet(30). The most frequently detected determinants in groundwater samples from sites A and C were Tet B (in 25% of samples), Tet C (in 55% of samples), Tet H (in 60% of samples), and Tet Z (in 25% of samples) (Table 3). The highest number of tet genes was uncovered in the groundwater sample from the adjacent well A8 [tet(B), tet(C), tet(E), tet(H), and tet(Z)] and from one of the most distant wells, A3 [tet(B), tet(C), tet(H), and tet(Z)] at site A (Table 3 and Fig. 2). Similar to the occurrence of the RPP genes at these two sites (8), the nested wells in deeper sand layers again demonstrated the elevated numbers of tet efflux pump genes in comparison with wells in shallow sand layers (Fig. 2 and Table 3). Consistent with the lagoon position and the general direction of groundwater flow at site A, no tet efflux pump genes were found in groundwater from background wells A7 and A10 (Fig. 2). Although the lagoon sample at site C displayed a repertoire of the six tet genes similar to that at site A, a comparatively limited diversity of Tet determinants (B, C, and H) was revealed in the groundwater samples from that site (Fig. 2). Interestingly, tet(A), which was undetectable in the waste lagoons, was found in groundwater from well C3 at site C. The site C background well C1 did not contain any of the tet efflux pumps (Fig. 2).
FIG. 2.
Maps of sites A and C and corresponding stratigraphic columns indicating the location and characteristics of sand layers. Large open arrows indicate groundwater flow. Circles indicate the locations of monitoring wells. Open circles represent nested wells screened in deeper sand layers. Well depths in meters are in parentheses. Black rectangles represent confinement buildings.
DISCUSSION
The tet genes in this study, together with several multidrug efflux pumps, belong to cluster e in the DHA12 family (Fig. 1) (23). The branching event leading to the appearance of the tet subcluster (Fig. 1) most probably relates to the evolvement of specificity for drugs, in this case for tetracyclines. The efflux pumps of this subcluster have a relatively narrow substrate range, essentially limited to various tetracyclines, while the members of the MDR subcluster can extrude structurally diverse compounds, including rhodamine 6G and acridine dyes, ethidium bromide, tetraphenylphosphonium compounds (TPP), puromycin, chloramphenicol, doxorubicin, and fluoroquinolones (21). The proof of the monophyletic origin of the DHA12 tet efflux genes opens the opportunity for evolutionarily meaningful classification of these genes. For example, the unclear positioning of the tet(Y) gene within other efflux pumps (9) can be easily resolved in our phylogenetic analysis, suggesting that the gene belongs to the tet subcluster within cluster e of the 12-TMS efflux antiporter genes. Similarly, the tet(31) gene does not group with the tet subcluster and perhaps needs to be removed from group 1 (9).
The primer set that was based on the phylogeny-aided design was used to detect the occurrence of the tet efflux genes in animal production systems, from the feed component of animals, to waste material, to groundwater underlying the waste lagoons. Only a few tet efflux pumps can be detected in feed samples and, importantly, the main components of the feed mix, corn and soybean, are free of these contaminants. The tet genes were discovered only in the minor components of animal feed, whey powder, plasma protein, and antibiotics. These components are produced industrially, and this may facilitate the exploration of the potential sources of tet genes contaminating the final commercial product.
The broader diversity of tet genes in the gut microbiota in comparison with the animal feed (tetBCGHYZ versus tetCDEHZ) is consistent with the selective pressure imposed by the tetracycline use at site U (1) than with the transient inflow of tet genes with feed. This selective pressure produces the overall (but not uniform in all animals) tetBCGHYZ fingerprint in the swine herd microbiota. It is not clear yet how specific the fingerprint is in terms of reflecting the antibiotic usage regimen, animal host microbiota, or the combination of both factors. But if it is specific for the specialized animal production systems, which usually house one type of animal, with a specific antibiotic use regimen, then this type of molecular fingerprinting may be useful for tracking the sources of fecal pollution. Our preliminary results substantiate this possibility: tet fingerprints (tetBCEHYZ), which are very similar to the fecal fingerprint at site U (tetBCGHYZ), were found in lagoons of the distant swine production facilities at sites A and C.
In our previous work (8), the question remains of whether the antibiotic resistance genes in the environment (groundwater) are the result of the gene mobility or because of the in situ selection by tetracycline, which is leaking from the wastewater lagoons. In the present study, we analyzed the lagoon and groundwater samples for a number of antibiotics. Only the lagoon sample at site A contained 14 mg of free chlortetracycline per liter, but all other samples, including the site C lagoon water and groundwater from both sites, had levels of various tetracyclines below the detection limit of 0.5 mg/liter or contained unidentified interfering substances making the determination impossible (our unpublished data). Second, with a single exception [tet(A) in well C3], the diversity of the tet efflux pump genes detected in groundwater downstream of the waste lagoons was well within the diversity detected in swine lagoons (Table 3). These observations suggest that the antibiotic resistance genes detected in groundwater are the result of gene mobility, not of the in situ tetracycline selection.
In general, a higher occurrence of the tet genes was detected in wells proximal to both lagoons in the direction of groundwater flow, and several Tet determinants (B, H, and Z) were identified from well A14, which is located more than 250 m downstream of the lagoon at site A (Fig. 2). Two of the locations at site A were nested wells (Fig. 2, wells A4 and A6), with second wells screened in deeper layers of sand (wells A3 and A5). Again, similar to our previous results with the RPP genes, a higher diversity of tet efflux genes was detected in the deeper wells than in the corresponding shallow wells, demonstrating the possibility of vertical mobility of contaminants, depending on the hydrogeology of the location. Consistent with the location of the wells relative to the lagoons and direction of groundwater flow, the background wells A7, A10, C1, and C3 did not contain any of the tetracycline resistance determinants. Collectively with our earlier study (8), the data suggest once more that the presence of the tetracycline resistance genes is due to seepage and movement of groundwater underlying the lagoons.
Two genes, tet(C) and tet(H), demonstrated a broader presence in groundwater samples than other tet genes and were detected in 55 and 60% of wells, respectively. While the broad ecological presence of tet(C) is not surprising, since it has been described in 10 different bacterial genera, tet(H) seems to be confined to the representatives of two genera within the family Pasteurellaceae, Mannheimia and Pasteurella (9). These bacteria are important pathogens of food-producing animals and, in particular, swine (7). If, based on the currently available data, tet(H) is really specific only to Pasteurella, then finding tet(H) signatures in very distant groundwater samples may imply that the pathogenic microbiota emanating from swine has the potential to travel substantial distances with the groundwater flow. In laboratory experiments, Pasteurella strains may survive and maintain the infectivity and pathogenic potential for extended periods of time: for over 1 year in some samples of water (6, 19).
REFERENCES
- 1.Aminov, R. I., N. Garrigues-Jeanjean, and R. I. Mackie. 2001. Molecular ecology of tetracycline resistance: development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 67:22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, S. R., and R.-A. Sandaa. 1994. Distribution of tetracycline resistance determinants among gram-negative bacteria isolated from polluted and unpolluted marine sediments. Appl. Environ. Microbiol. 60:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, T., T. Satoh, and T. Kitao. 1987. New tetracycline resistance determinant on R plasmids from Vibrio anguillarum. Antimicrob. Agents Chemother. 31:1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, D. A., M. S. Boguski, D. J. Lipman, J. Ostell, B. F. Ouellette, B. A. Rapp, and D. L. Wheeler. 1999. GenBank. Nucleic Acids Res. 27:12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, H. W. Boyer, J. H. Crosa, and S. Falkow. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 6.Bredy, J. P., and R. G. Botzler. 1989. The effects of six environmental variables on Pasteurella multocida populations in water. J. Wildl. Dis. 25:232-239. [DOI] [PubMed] [Google Scholar]
- 7.Chanter, N., and J. M. Rutter. 1989. Pasteurellosis in pigs and the determinants of virulence of toxigenic Pasteurella multocida, p. 161-195. In C. Adlam and J. M. Rutter (ed.), Pasteurella and pasteurellosis. Academic Press, San Diego, Calif.
- 8.Chee-Sanford, J. C., N. Garrigues-Jeanjean, R. I. Aminov, I. J. Krapac, and R. I. Mackie. 2001. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 67:1494-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillaume, G., D. Verbrugge, M. Chasseur-Libotte, W. Moens, and J. Collard. 2000. PCR typing of tetracycline resistance determinants (Tet A-E) in Salmonella enterica serotype Hadar and in the microbial community of activated sludges from hospital and urban wastewater treatment facilities in Belgium. FEMS Microbiol. Ecol. 32:77-85. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen, R. A., and W. S. Reznikoff. 1979. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J. Bacteriol. 138:705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, L. M., L. M. McMurry, S. B. Levy, and D. C. Hirsh. 1993. A new tetracycline resistance determinant, Tet H, from Pasteurella multocida specifying active effux of tetracycline. Antimicrob. Agents Chemother. 37:2699-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillen, W., and K. Schollmeier. 1983. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 11:525-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, E. H., and T. Aoki. 1994. The transposon-like structure of IS26-tetracycline, and kanamycin resistance determinant derived from transferable R plasmid of fish pathogen, Pasteurella piscicida. Microbiol. Immunol. 38:31-38. [DOI] [PubMed] [Google Scholar]
- 15.Krapac, I. G., W. S. Dey, C. A. Smyth, and W. R. Roy. 1998. Impacts of bacteria, metals, and nutrients on groundwater at two hog confinement facilities, p. 29-50. In Proceedings of the National Ground Water Association. Animal feeding operations and groundwater. Issues, impacts, and solutions: a conference for the future. National Ground Water Association, St. Louis, Mo.
- 16.Luo, Z.-Q., and S. K. Farrand. 1999. Cloning and characterization of a tetracycline resistance determinant present in Agrobacterium tumefaciens C58. J. Bacteriol. 181:618-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden, T. L., R. L. Tatusov, and J. Zhang. 1996. Application of network BLAST server. Methods Enzymol. 266:131-141. [DOI] [PubMed] [Google Scholar]
- 18.Magalhaes, V. D., W. Schuman, and B. A. Castilho. 1998. A new tetracycline resistance determinant cloned from Proteus mirabilis. Biochim. Biophys. Acta 1443:262-266. [DOI] [PubMed] [Google Scholar]
- 19.Magariños, B., J. L. Romalde, J. L. Barja, and A. E. Toranzo. 1994. Evidence of a dormant but infective state of the fish pathogen Pasteurella piscicida in seawater and sediment. Appl. Environ. Microbiol. 60:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall, B., S. Morrissey, P. Flynn, and S. B. Levy. 1987. A new tetracycline-resistance determinant, class E, isolated from Enterobacteriaceae. Gene 50:111-117. [DOI] [PubMed] [Google Scholar]
- 21.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauch, A., A. Puhler, J. Kalinowski, and G. Thierbach. 2000. TetZ, a new tetracycline resistance determinant discovered in gram-positive bacteria, shows high homology to gram-negative regulated efflux systems. Plasmid 44:285-291. [DOI] [PubMed] [Google Scholar]
- 25.Tsai, Y.-L., and B. H. Olson. 1991. Rapid method for direct extraction of DNA from soil and sediments. Appl. Environ. Microbiol. 57:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters, S. H., J. Grinsted, P. Rogowsky, J. Altenbuchner, and R. Schmitt. 1983. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 11:6089-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao, J., and T. Aoki. 1992. Nucleotide sequence analysis of the class G tetracycline resistance determinant from Vibrio anguillarum. Microbiol. Immunol. 36:1051-1060. [DOI] [PubMed] [Google Scholar]