Abstract
The temporal variation in archaeal diversity in vent fluids from a midocean ridge subseafloor habitat was examined using PCR-amplified 16S rRNA gene sequence analysis and most-probable-number (MPN) cultivation techniques targeting hyperthermophiles. To determine how variations in temperature and chemical characteristics of subseafloor fluids affect the microbial communities, we performed molecular phylogenetic and chemical analyses on diffuse-flow vent fluids from one site shortly after a volcanic eruption in 1998 and again in 1999 and 2000. The archaeal population was divided into particle-attached (>3-μm-diameter cells) and free-living fractions to test the hypothesis that subseafloor microorganisms associated with active hydrothermal systems are adapted for a lifestyle that involves attachment to solid surfaces and formation of biofilms. To delineate between entrained seawater archaea and the indigenous subseafloor microbial community, a background seawater sample was also examined and found to consist only of Group I Crenarchaeota and Group II Euryarchaeota, both of which were also present in vent fluids. The indigenous subseafloor archaeal community consisted of clones related to both mesophilic and hyperthermophilic Methanococcales, as well as many uncultured Euryarchaeota, some of which have been identified in other vent environments. The particle-attached fraction consistently showed greater diversity than the free-living fraction. The fluid and MPN counts indicate that while culturable hyperthermophiles represent less than 1% of the total microbial community, the subseafloor at new eruption sites does support a hyperthermophilic microbial community. The temperature and chemical indicators of the degree of subseafloor mixing appear to be the most important environmental parameters affecting community diversity, and it is apparent that decreasing fluid temperatures correlated with increased entrainment of seawater, decreased concentrations of hydrothermal chemical species, and increased incidence of seawater archaeal sequences.
Hydrothermal vent environments consist of multiple biotopes that span many of the physical and chemical conditions known to support life, including large ranges of temperature, pH, pressure, and oxygen concentrations and various forms of chemical energy, such as hydrogen sulfide, methane, and hydrogen (3, 27, 35). In all of these biotopes, microorganisms utilize either chemical energy derived from the reaction of water with rock at high temperatures or organic compounds derived from multiple sources (27). The microbial diversity varies from a single species of bacteria, as is the case for animal endosymbionts (52), to complex communities of different thermal groups of bacteria and archaea observed in hot vent fluids (42, 48, 49), sulfide structures (14, 20, 48, 50), and microbial mats (31, 36). The subseafloor biotopes are probably the most enigmatic, since they are characterized by variable porosity (22) and complex thermal and chemical gradients (6) due to mixing of seawater with hydrothermal fluids. The subseafloor is also one of the most difficult environments to sample. Studies of the subsurface have relied either on sediment cores from deep-sea drilling (9, 38, 45) or on fluids leaking out of the crust at stable vents or at new sea floor eruption sites (13, 23, 24, 44). Most of what is known about subseafloor microbial communities is based on cultured microorganisms (23, 24, 44, 46, 47) and microscopic observations of microbial communities ejected from the subsurface (8, 23) or is inferred from chemical characteristics of subseafloor hydrothermal fluids (2, 6, 30; D. A. Butterfield, M. D. Lilley, J. A. Huber, K. K. Roe, R. W. Embley, and G. J. Massoth, unpublished data). Thermophilic and hyperthermophilic methanogens and heterotrophs are viewed as indicator organisms of hot subseafloor habitats and usually have a minimum growth temperature that is higher than the temperature of the fluids sampled (23, 46). However, little is known about the overall phylogenetic diversity of microbial communities in the subseafloor and in particular the physiological diversity of microorganisms specifically adapted to this biotope.
In this study, we followed changes in archaeal diversity in hydrothermal fluids from a single vent over a 3-year period. Diffuse, low-temperature hydrothermal fluids show marked variations in thermal and chemical characteristics over periods of 1 to 3 years due to the evolution of hydrothermal systems (5, 6, 21, 53) and over minutes to hours due to changes in the degree of mixing with seawater and tidal cycles (5, 6). The effects these variations have on microbial communities are unknown. In an effort to understand how these variations in fluid properties affect the microbial communities, we performed molecular phylogenetic and chemical analyses on diffuse-flow vent fluids from one site 7 months after the January 1998 Axial Seamount eruption and again in 1999 and 2000. The archaeal diversity was divided into particle-attached (>3-μm-diameter cells) and free-living fractions to test the hypothesis that subseafloor microorganisms associated with active hydrothermal systems are adapted to a lifestyle that involves attachment to solid surfaces and the formation of biofilms (46). Our results indicate that archaeal diversity does vary with changes in temperature and chemistry and furthermore provides evidence that there are archaea specifically adapted to the subseafloor environments.
MATERIALS AND METHODS
Sampling.
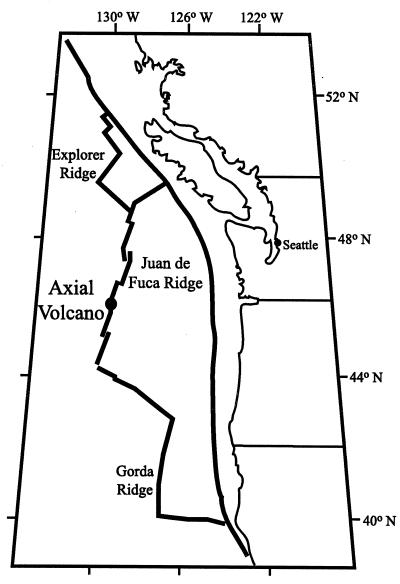
Axial Seamount (46°55′N; 130°00′W) is an active submarine volcano located on the Juan de Fuca Ridge, approximately 300 miles off the coast of Oregon (Fig. 1). The caldera of the volcano (3 by 8 km) rises 700 m above the mean ridge level; it lies between two rift zones to the north and south and is bordered on three sides by a boundary fault. Active long-term venting occurs along these rift zones and near the boundary fault (25). In January 1998, Axial experienced a week-long series of earthquakes, and subsequent water column and sea floor observations on the southeast portion of the caldera found temperature and chemical anomalies, extensive new sea floor lava flows, large “snowblower” type vents, and other characteristics commonly associated with diking-eruptive events (1, 16, 17).
FIG. 1.
Map of North Pacific spreading ridges, with study site, Axial Volcano, marked.
Diffuse fluids were collected at marker 33 on dive R473 (September 1998), dive R485 (July 1999), and dive R551 (July 2000) with the ROV ROPOS. Using the hydrothermal fluid and particle sampler (HFPS), filtered and unfiltered fluids were sampled at the vent after a steady temperature was found on the intake probe and fluid was pumped at a known rate. Fluid was pumped through a 47-mm-diameter (3-μm-pore-size) Millipore mixed cellulose ester filter followed by a Sterivex-GP (0.22-μm-pore-size) filter. Approximately 1 liter of fluid was pumped through the filters over a 10- to 15-min period. The temperature and volume of fluid collected were monitored throughout sampling. Once on deck, the filters were placed in 50-ml sterile Falcon tubes (BD Sciences Labware), frozen in liquid nitrogen, and stored at −20°C until they were processed. Unfiltered fluid samples were used for chemical analyses, including sulfide, magnesium, and silica content; epifluorescent microbial counts; and culturing. The HFPS records temperature throughout the sampling procedure, so the average temperature of the water collected can be calculated and compared with the average temperature of the water passed through the filters used for DNA analysis. Extreme microgradients in temperature and fluid flow make it difficult to achieve a steady temperature during diffuse fluid sampling in some vents, including marker 33. Repositioning the intake probe by a centimeter or less can result in significant changes in the sampled fluid temperature. Whenever possible, we collected multiple fluid and particle samples without changing position.
The collected fluids were inoculated into various anaerobic enrichment media for most-probable-number (MPN) semiquantitative enrichment of both heterotrophic and autotrophic hyperthermophiles (18, 23). Medium ingredients and methods for hyperthermophile enrichment were as previously described (23). The cultures were incubated at 90°C until they were turbid or for a week. Positive autotrophic cultures were checked for methanogens by examining the organisms for autofluorescence using a blue-violet 05 excitation filter in a fluorescence microscope (Zeiss). An 18-ml aliquot of each fluid sample was preserved in formaldehyde (3.7% final concentration) in duplicate, stored at 2°C, and counted by epifluorescence microscopy with DAPI (4′,6′-diamidino-2-phenylindole; Sigma) (41). A background (no detectable hydrothermal plume) filtered seawater sample from a depth of 1,275 m and approximately 700 m southeast of the active vent site was collected with a Niskin bottle mounted on a CTD instrument, which measures conductivity, temperature, and depth. The seawater was filtered through a sterile 47-mm-diameter, 0.22-μm-pore-size filter shipboard and processed for further molecular analyses.
Chemical analysis.
The analytical methods have been described by Butterfield et al. (6). The fluids collected with the HFPS were analyzed aboard ship for H2S, pH, and dissolved silica. On shore, the fluids were analyzed for major, minor, and trace elements. The levels of precision (±1 standard deviation) of the reported chemical analyses were as follows: pH, 0.05 U; H2S, 4%; Mg, 1%; Cl, 0.5%; silica, 0.5%; and Fe, 4%.
DNA extraction and purification.
DNA was extracted by the method of Crump et al. (10) with some modifications. The Sterivex and 47-mm-diameter filters were allowed to thaw, the 47-mm-diameter filter was cut into strips with a sterile razor, and the pieces were placed back in the Falcon tube. The ends of the Sterivex filter were sealed with the threaded plastic caps from 1-ml syringes to prevent leaking during extraction. DNA extraction buffer (0.1 M Tris-HCl [pH 8], 0.1 M Na-EDTA [pH 8], 0.1 M NaH2PO4 [pH 8], 1.5 M NaCl, 5% cetyltrimethylammonium bromide), and proteinase K (1%) were added to each filter (1.85 ml and 20 μl, respectively, to the Sterivex filter and 2.0 ml and 20 μl, respectively, to the cut-up 47-mm-diameter filter). Samples were frozen at −80°C and thawed at 65°C three times and then incubated on a rotating carousel for 30 min at 37°C. Sodium dodecyl sulfate (SDS; 20%) was added to each sample (60 μl), and the samples were incubated at 65°C on a rotating carousel for 2 h. The liquid from each filter was then removed using a 3-ml syringe and placed in a 2-ml microcentrifuge tube, which was centrifuged at room temperature (6,000 × g; 5 min). The supernatant from each microcentrifuge tube was then placed in separate 15-ml Falcon collection tubes. DNA extraction buffer, SDS, and proteinase K were then added to each filter (1 ml and 75 and 20 μl, respectively) and to each microcentrifuge tube containing spun-down particles (0.37 ml and 75 and 10 μl, respectively). Both the filter samples and the microcentrifuge tubes were incubated on a rotating carousel for 10 min. The microcentrifuge tubes were again centrifuged (6,000 × g; 5 min), and the supernatant was added to the appropriate collection tube. Liquid was then removed from the filters, placed in the microcentrifuge tubes, and centrifuged (6,000 × g; 5 min), and the supernatant was added to the collection tubes. The extraction buffer, SDS, and proteinase K were added to each filter and the particles again, and the extraction process was repeated. An equal volume of chloroform-isoamyl alcohol (24:1) was added to each collection tube of supernatant, and the tubes were vortexed and centrifuged (1,200 × g; 10 min). The aqueous (top) layer from each tube was drawn off into a 30-ml acid-washed sterile Corex (Corning) tube, and an equal volume of isopropanol was added to each tube and mixed gently. Often additional aliquots of isopropanol-water (1:1) were added to adequately dissolve the aqueous layer in the isopropanol. After the tubes were incubated for 1 h at room temperature, the precipitated DNA was centrifuged at room temperature (16,000 × g; 20 min), and the isopropanol supernatant was removed and replaced with 5 ml of 70% ethanol. After a final centrifugation (16,000 × g; 20 min), the ethanol was removed and the DNA was dried down and resuspended in 500 μl of TE buffer (10 mM Tris-HCl, 1 mM Na-EDTA; pH 8). The DNA was purified using Qiaquick PCR purification columns (Qiagen) according to the manufacturer's instructions and stored at −20°C.
PCR.
PCR was performed on environmental DNA with the universal archaeon-specific primers 21f (5′-TTC CGG TTG ATC CYG CCG GA-3′) and 958r (5′-YCC GGC GTT GAM TCC AAT T-3′). Each PCR mixture (20 μl) contained 3.0 mM MgCl2, 0.8 mM deoxynucleoside triphosphates, 0.25 μM (each) primer, 1× PCR buffer (Promega), and 1 U of Taq DNA polymerase (Promega). An initial denaturation step of 5 min at 94°C was followed by 22 to 24 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. The final extension step was 72°C for 10 min. To minimize bias in amplification, PCR cycles were stopped while the product concentration was still in the exponential phase, as visualized and quantified on 1% (wt/vol) agarose gels stained with SYBR green (Molecular Probes) at 15, 20, 25, and 30 cycles. To minimize PCR drift (40), 6 to 10 replicate amplifications were pooled and then concentrated and purified with Qiaquick PCR purification columns in accordance with the manufacturer's instructions.
Cloning and restriction fragment length polymorphism (RFLP) analysis.
The consolidated and cleaned PCR products were cloned with a TA cloning vector kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. A total of 100 to 150 white colonies for each library were selected and stored on agar plates. Approximately 20 to 25 randomly selected clones were grown in 100 μl of Luria-Bertani broth medium with shaking at 220 rpm for 1 h at 37°C and PCR amplified with primers M13F (5′-GTA AAA CGA CGG CCA G-3′) and M13R (5′-CAG GAA ACA GCT ATG AC-3′). Each 50-μl reaction mixture contained 5 μl of clone culture, 1 mM MgCl2, 0.8 mM deoxynucleoside triphosphates, 1 μM (each) primer, 1× PCR buffer (Promega), and 5 U of Taq DNA polymerase (Promega). The PCR conditions were as follows: 2 cycles of 1.5 min at 94°C, 45 s at 56°C, and 1.5 min at 72°C followed by 22 cycles of 30 s at 90°C, 30 s at 56°C, and 1 min at 72°C. The final step consisted of a 10-min extension at 72°C. PCR products were visualized on 1% (wt/vol) agarose gels stained with SYBR green.
To ensure we had sequenced a representative community from each library, approximately 100 clones from each library were analyzed by RFLP. The clones were inoculated into 100 μl of Luria-Bertani broth medium and incubated with shaking at 220 rpm for 1 h at 37°C. The plasmid inserts were PCR amplified with M13F and M13R (20-μl reaction volumes with 1 μl of clone culture, 3 mM MgCl2, 0.8 mM deoxynucleoside triphosphates, 1 ng of each primer/ml, 2.5 U of Taq DNA polymerase [Promega], and 1× PCR buffer [Promega]). PCR amplification began with a 1-min denaturation at 94°C followed by 30 cycles of 94°C for 30s, 50°C for 30s, and 72°C for 1.5 min and ended with a 5-min extension at 72°C. The PCR products were digested with HaeIII (Gibco BRL) and HhaI (New England BioLabs) according to the manufacturer's instructions and visualized on 2.5% (wt/vol) agarose gels stained with SYBR green. Different banding patterns were noted. RFLP was not performed on the marker 33 2000 or CTD background sample.
Sequencing and analysis.
Clones with unique RFLP patterns and 25 randomly selected clones were used in sequencing. The PCR products were cleaned, and 200 ng was sequenced with at least two of the primers 21f, 515r (5′-TTA CCG CGG CKG CTG RCA C-3′), and 958r using the Thermosequenase II dye terminator cycle sequencing kit (Amersham Pharmacia Biotech Inc.) and analyzed on a 373 A DNA sequencer (Applied Biosystems) or using the DYEnamic ET dye terminator kit (Amersham Pharmacia Biotech Inc.) and analyzed on a MegaBACE 1000 (Molecular Dynamics). The sequences were assembled using the Sequencher program (Gene Codes Corp.) and checked for chimeric sequences by examining the secondary structure and using the CHIMERA_CHECK program of the Ribosomal Database Project website (32). Nonchimeric sequences were submitted for alignment to the Ribosomal Database Project Sequence Alignment program, with common gaps conserved, and manually manipulated in the BioEdit version 4.7.8 program (19). Sequences were also submitted to the Advanced BLAST search program (available through the National Center for Biotechnology Information) to find closely related sequences to be used in subsequent analyses. Approximately 600 nucleotide bases were used in phylogenetic analyses, with only homologous positions included in the comparisons. The Phylip version 3.5 package (obtained from J. Felsenstein, University of Washington, Seattle) was used to construct distance trees (NEIGHBOR and FITCH) and maximum-likelihood trees (DNAML). Bootstrap analysis (SEQBOOT) was used to provide confidence estimates for tree topologies. Negative branch lengths were prohibited.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for the sequences in this study are AF355807 through AF355981.
RESULTS
Study site description.
Marker 33 is a unique diffuse vent due to both its location on an eruptive fissure and its high temperatures in 1998 and 1999. Before the eruption, there was a diffuse vent at the marker 33 site. During the 1998 eruption, the site was paved over with lava sheet flows, and a new vent formed. This vent is a long crack in basaltic sheet flows with a width of 30 cm and a length of several meters. Temperatures varied over the length of the crack, and it was probed to find the highest and steadiest temperatures for sampling. This often occurred at a point in the middle of the crack, where the flow appeared most focused. Over the 3 years of sampling marker 33, there were visible changes in the system after the initial disturbance of the eruption, such as colonization by limpets and worms and the formation of extensive microbial mats lining the crack. Obvious visually but hard to measure was the strength of the flow, or how vigorous it appeared. The most focused flow was apparent in 1999, when a maximum temperature of 78°C was measured.
The chemical, thermal, and microbiological characteristics of marker 33 and the background seawater are shown in Table 1. Cell counts of preserved fluids from marker 33 indicate populations elevated above those of the background seawater. Microscopic examination of the fluids revealed abundant free-living cells, as well as clumps of cells and large filamentous cells, likely members of the particle-attached fraction. Hyperthermophilic anaerobic heterotrophs and autotrophs were successfully cultured from marker 33 all 3 years, as well as from waters above the volcano 15 days after the eruption. The majority of autotrophic cultures autofluoresced at the wavelength indicative of methanogens.
TABLE 1.
Chemical, thermal, and microbial characteristics of marker 33 (1998, 1999, and 2000) and background seawater
| Characteristic | Value
|
|||
|---|---|---|---|---|
| Marker 33
|
Seawater | |||
| 1998 | 1999 | 2000 | ||
| Cells/ml | 2.53 × 105 | 1.17 × 105 | 1.15 × 105 | 5.0 × 104 |
| MPN results (cells/liter) | ||||
| YPSa (90°C) | ≥48,000 | 3,000-96,000 | ≥48,000 | NDc |
| YEb (90°C) | 280-4600 | 600-8,800 | 720-26,000 | ND |
| Temp range at vent (°C) | 30-55 | 45-78 | 20-32 | 2 |
| Avg temp (°C) during filtrationd | 36 ± 6 | 46 ± 6 | 26.4 ± 2 | 2 |
| Mg in cosampled fluid (mmol/kg) | 48.1 | 44.1 | 44.9 | 53 |
| No. of fluid samples analyzed | 3 | 5 | 2 | |
| Lowest Mg content in sampled fluids (mmol/kg) | 48.1 | 40.3 | 44.9 | |
| % Crustal seawatere | 90.8 | 76.0 | 84.7 | |
| pH of best sample | 5 | 4.6 | 5.8 | 7.8 |
| Max H2S (mmol/kg) | 2.1 | 2.4 | 0.2 | 0 |
| End member chloride (mmol/kg)d | 86 ± 11 | 250 ± 9 | 415 ± 5 | 542 |
| H2S/Si (mol/mol) | 1.5 | 0.76 | 0.18 | 0 |
| H2S/heat (nmol/J) | 11 | 8 | 1.2 | 0 |
| H2S/Fe (mol/mol) | 1,100 | 1,230 | 70 | 0 |
0.3% yeast extract and peptone with elemental sulfur; Ar headspace.
0.1% yeast extract; H2-CO2 headspace.
ND, none detected.
±1 standard deviation.
>100 × (lowest Mg content/seawater Mg content).
It is important to characterize the two primary aspects of change in the chemistry of marker 33 fluids over time. First, there were changes in the hot source fluid (or end member) at depth, and second, there were changes in the degree to which this fluid mixed with crustal seawater below the point of venting. In Table 1, the maximum temperature, lowest Mg content, pH of the best sample, maximum H2S content, and percent crustal seawater all describe the extent of subseafloor mixing at the marker 33 vent. All of these indicate that the fluids venting in 1999 contained the highest proportion of high-temperature source fluid. For example, the maximum measured temperature increased over 20°C from 1998 to 1999 and then dropped 40°C in 2000, while the proportion of crustal seawater in the fluids (based on zero Mg in the hot source fluid) decreased from 1998 to 1999 and then increased in 2000. The extent of subseafloor mixing, as indicated by the temperature and simple chemical properties, is the cause of the most significant changes in environmental variables at the point of venting and presumably in the subseafloor near the point of venting. The extent of mixing is the main factor that distinguishes a 70°C vent from a 10°C vent, and it makes a huge difference in the likely microbial metabolism that will predominate in a vent (6, 35).
Superimposed on the changes resulting from variation in mixing, there are changes in the source fluids that are seen in the mixing-independent properties (end member chloride concentration and H2S/Si and H2S/heat ratios). The end member chloride concentration (5, 6, 53) increased progressively, while the ratio of hydrogen sulfide to other hydrothermal components decreased, consistent with a decreasing vapor component over time in the hot source fluids (5, 6, 53). The chloride concentration of marker 33 fluids remained below that of seawater over the period of this study, the fluids maintained a high H2S/Fe ratio, and we did not see a transition to brine-like character. Although the evolution of the source fluids following the eruption is easily measured, it does not result in changes in the environmental variables at the point of venting that are as important as those caused by the degree of mixing.
Phylogenetic analyses.
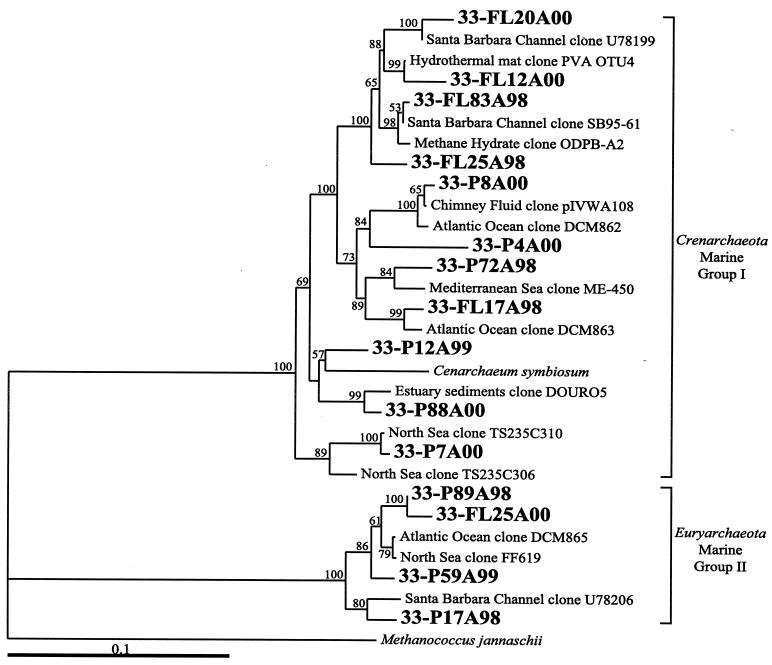
DNA was successfully extracted and amplified with archaeon-specific primers from all samples collected. A negative control of sterile frozen filters was extracted and showed no amplification products. Table 2 shows a summary of the different phylotypes (determined by distance matrices and 97% sequence similarity) obtained from each sample, the most closely related organism or clone, the percent similarity, and the number of clones found in each library for each phylotype based on sequencing. Over the three sampling periods, 14 different archaeal phylotypes were obtained that were considered unique to the vent environment, as well as 14 phylotypes belonging to Marine Group I and II Archaea (28) (Table 2). All sequenced clones from the background seawater sample belonged to either the Marine Group I or II Archaea (28, 33). Phylogenetic trees indicate the relationships between the sequences of clones from this study and other archaeal sequences for both representative seawater (Fig. 2) and likely hydrothermal vent (Fig. 3) populations. Those clones from marker 33 that fall into the Marine Groups I and II are closely related to clones from the Santa Barbara Channel (33), the digestive tracts of marine fish (51), a hydrothermal microbial mat (36), methane hydrate sediments (4), black smoker vent water (48), the Mediterranean Sea (34), and the subtropical North Atlantic (unpublished) (Fig. 2). In all years, the particle-attached fractions consistently had more different phylotypes than the free-living fractions, while all diffuse fluid samples had more different phylotypes than the background sample, which had only two.
TABLE 2.
Summary of 16S rRNA clone sequences from marker 33, 1998 to 2000, in the particle-attached and free-living populations
| Clonea | Phylogenetic group | Closest matchb | Similarityb | No. of clonesc
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FL98 | FL99 | FL00 | P98 | P99 | P00 | BG | ||||
| Euryarchaeota | ||||||||||
| 33-P127A99 | Thermoplasmales group I | VC2.1 Arc13 (AF068820) | 96 | 2 | ||||||
| 33-P23A98 | Thermoplasmales group II | VC2.1 Arc13 (AF068820) | 99 | 2 | ||||||
| 33-FL45A99 | Mesophilic methanogen I | “M. aeolicus” (U39016) | 94 | 20 | 3 | 8 | 4 | |||
| 33-FL96A98 | Mesophilic methanogen II | “M. aeolicus” (U39016) | 95 | 1 | 3 | |||||
| 33-FL27A98 | Hyperthermophilic methanogen | M. jannaschii (U67517) | 98 | 2 | 4 | 1 | ||||
| 33-FL1A00 | UE I | J4.75-24 (AF199337) | 71 | 7 | 1 | 1 | 7 | |||
| 33-P120A98 | UE II | pMC2A24 (AB019736) | 80 | 1 | ||||||
| 33-P74A98 | UE III | pMC2A24 (AB019736) | 84 | 1 | ||||||
| 33-P44A00 | UE IV | pMC2A211 (AB019746) | 88 | 1 | ||||||
| 33-FL18A98 | UE V | pISA35 (AB019748) | 96 | 1 | ||||||
| 33-P27A98 | UE VI | M. stadtmanae (M59139) | 77 | 1 | ||||||
| 33-FL31A00 | UE VII | 65B (AF268642) | 78 | 1 | ||||||
| 33-P73A98 | UE VIII | 2C25 (AF015971) | 94 | 1 | ||||||
| Marine Group II | ||||||||||
| 33-P59A99 | Marine Group IIa | DCM865 (AF121996) | 97 | 1 | ||||||
| 33-P17A98 | Marine Group IIb | U78206 (UEU78206) | 97 | 1 | 1 | |||||
| 33-FL25A00 | Marine Group IIc | DCM865 (AF121996) | 96 | 3 | 2 | 2 | 3 | 1 | 4 | |
| Crenarchaeota | ||||||||||
| 33-P92A98 | UC I | pMC2A36 (AB019720) | 97 | 1 | ||||||
| Marine Group I | ||||||||||
| 33-P88A00 | Marine Group Ia | DOURO5 (AF201359) | 96 | 1 | ||||||
| 33-P8A00 | Marine Group Ib | pIVWA108 (AB019726) | 99 | 1 | 1 | |||||
| 33-P12A99 | Marine Group Ic | TS235C306 (AF052948) | 94 | 1 | ||||||
| 33-P7A00 | Marine Group Id | TS235C310 (AF052949) | 93 | 1 | ||||||
| 33-P4A00 | Marine Group Ie | DCM862 (AF121995) | 94 | 1 | 3 | |||||
| 33-P72A98 | Marine Group If | ME-450 (AF223117) | 96 | 4 | 2 | 2 | ||||
| 33-FL17A98 | Marine Group Ig | DCM863 (AF121991) | 98 | 4 | 1 | 1 | ||||
| 33-FL12A00 | Marine Group Ih | PVA OTU4 (UHU46680) | 98 | 5 | 6 | 2 | ||||
| 33-FL20A00 | Marine Group Ii | U78199 (UCU78199) | 99 | 3 | 4 | 1 | 1 | |||
| 33-FL25A98 | Marine Group Ij | PVA OTU4 (UHU46680) | 96 | 1 | 1 | |||||
| 33-FL83A98 | Marine Group Ik | ODPB-A2 (AF121092) | 99 | 11 | 9 | 2 | 5 | 13 | 24 | |
| Total clones sequencedd | 30 | 30 | 22 | 33 | 30 | 30 | 28 | |||
Representative 16S rRNA sequence from this study used in phylogenetic analyses.
Based on BLAST search and similarity matrices. GenBank accession numbers are indicated in parentheses.
FL, free living; P, particle attached; BG, CTD background sample. The number is the last two digits of the year of sampling.
Total numbers of phylotypes were as follows: FL98, 9; FL99, 3; FL00, 6; P98, 18; P99, 10; P00, 12; BG, 2.
FIG. 2.
Phylogenetic tree as determined by neighbor-joining and maximum-likelihood analyses of archaeal 16S rRNA clones for likely seawater clones from marker 33 and other marine environments in Marine Groups I and II. Clones from this study are indicated in large boldface font and labeled with P (particle attached) or FL (free living) and the last two digits of the appropriate year (98, 99, or 00, respectively). Accession numbers for GenBank are provided if the clone or organism name is not unique. The percentage of 100 bootstrap resamplings above 50% is indicated. The scale bar represents the expected number of changes per nucleotide position.
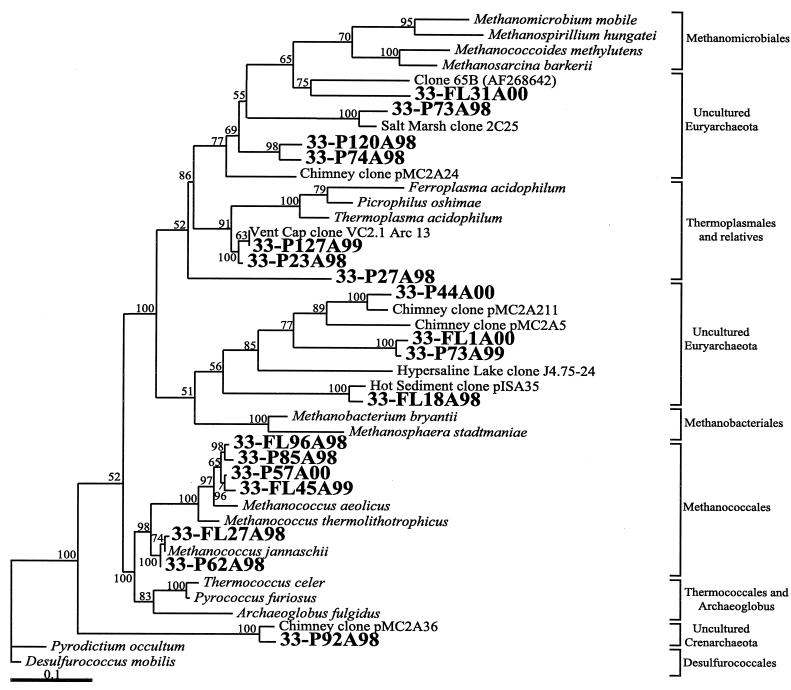
FIG. 3.
Phylogenetic tree as determined by neighbor-joining and maximum-likelihood analyses of archaeal 16S rRNA clones for likely subseafloor clones from marker 33 and other environmental clones and cultured organisms. Clones from this study are indicated in large boldface font and labeled with P (particle attached) or FL (free living) and the last two digits of the appropriate year (98, 99, or 00, respectively). Accession numbers for GenBank are provided if the clone or organism name is not unique. The percentage of 100 bootstrap resamplings above 50% is indicated. The scale bar represents the expected number of changes per nucleotide position.
Of the 14 vent phylotypes identified, 3 are closely related to known cultured organisms. Two phylotypes are 94 to 95% related to “Methanococcus aeolicus,” and 1 phylotype is 98% related to Methanococcus jannaschii. Members of the order Methanococcales utilize hydrogen and carbon dioxide as a substrate for methanogenesis and are strict anaerobes, and all described isolates are from marine environments (54). For all 3 years, in both the particle-attached and free-living fractions, with the exception of the free-living population in 2000, clones were obtained that are closely related to the mesophilic methanogen “M. aeolicus.” “M. aeolicus” is the most deeply branching mesophilic member of the order Methanococcales, suggesting a deep divergence of this microbe (29). The source, temperature, and pH optimum of “M. aeolicus” have not been reported, although it is described as a mesophile (35 to 40°C) (54). Clones closely related (98%) to the high-temperature methanogen M. jannaschii were obtained in both the free-living and particle-attached fractions from 1998 and 1999. No such clones were found in either fraction in 2000. M. jannaschii was first isolated from the base of a white smoker chimney on the East Pacific Rise in 1983 (26). This organism grows optimally at 85°C and pH 6.0 to 7.0 and can also grow rapidly with carbon dioxide as the sole carbon source. One unique clone, 33-P27A98 (unknown Euryarchaeota VI [UEVI]), is distantly (77%) related to Methanosphaera stadtmanae, a methanogenic mesophilic chemoorganotroph.
The remaining 10 phylotypes are related only to other environmental sequences, including those from a black smoker chimney and hot vent fluids >100°C (48), warm vent fluids <70°C (42), hypersaline lake water (12), estuarine sediments (unpublished), and coastal salt marsh sediments (37). The largest group, comprising 22% of the vent clones, is UEI (Fig. 3, 33-FL1A00 and 33-P73A99), members of which were found in all 3 years and are distantly related (71%) to environmental clone J4.75-24 from anaerobic, methane-rich, hypersaline lake sediments (12). In phylogenetic analyses, UEI clones consistently grouped with environmental clones from other hydrothermal habitats (Fig. 3), including clones from hot water in deep-sea sediments in the Okinawa trough and a black smoker chimney from the Izu-Ogasawara arc (48).
In the particle-attached fractions from 1998 and 1999, there were four clones that fell into two groups (Thermoplasmales I and II) that are closely related (96 and 99%, respectively) to clone VC2.1Arc13 from an in situ growth chamber study by Reysenbach et al. (42). The clone from that study, as well as the clones from this study (Fig. 3, 33-P23A98 and 33-P127A99), fell into the same group as cultured microorganisms in the Thermoplasmales group, including Thermoplasma acidophilium and Picrophilus oshimae. Examination of clear-water shimmering sediments from the Okinawa trough and a black smoker chimney from Izu-Ogasawara arc also found environmental sequences that grouped closely with the Thermoplasma-Picrophilus clade (48).
The unique clones found once over the 3-year sampling period are related to uncultured Crenarchaeota and Euryarchaeota. Clone 33-P92A98 was closely related (97%) to an environmental crenarchaeota found in a black smoker chimney and its high-temperature fluid (Fig. 3) (48). This clone belongs to a distinct group of uncultured crenarchaeota, to date found only in the hydrothermal vent environment. Also found were a number of unique clones related to other environmental euryarchaeotal sequences from the Takai and Horikoshi (48) study. These included clones 33-P120A98 (UEII) and 33-P74A98 (UEIII), both distantly related (80 and 84%, respectively) to a clone from a black smoker chimney (48). Phylogenetically, these clones formed a cluster of uncultured euryarchaeota within a larger group including the Methanomicrobiales and Thermoplasmales (Fig. 3). Two other clones, 33-FL31A00 (UEVII) and 33-P73A98 (UEVIII), also fell into this group, and clone 33-P73A98 was closely related (94%) to an environmental sequence from sediments in a salt marsh (37). Clone 33-P44A00 (UEIV) was related (88%) to a sequence from a black smoker chimney (48), although it fell into a different cluster rooted with the Methanobacteriales that also includes other hydrothermal vent sequences and those from hypersaline lake sediments (Fig. 3). 33-FL18A98 (UEV) from this study also fell into this cluster of uncultured euryarchaeota, along with its closely related match (96%), an environmental sequence from hot shimmering sediments (48).
DISCUSSION
All samples from marker 33 had levels of archaeal diversity higher than that in the surrounding seawater, suggesting the presence of a unique microbial community in the subsurface environment. If those clones considered to be part of the plankton community are removed (either temporarily living in the subsurface or in the water column), there remain 14 phylotypes found only in the vent samples. These include clones that were found in only 1 year and others that were found in all 3 years. Over 60% of the unique vent clones are closely related to members of the order Methanococcales. This is the first study showing evidence from environmental sequences for the presence of both mesophilic and thermophilic methanogens in hydrothermal vent fluids. These data suggest the presence of warm to hot (10 to 90°C) anaerobic habitats in the subsurface. While methanogens were not detected in the in situ growth chamber from the Mid-Atlantic Ridge (42), a clone closely related to M. jannaschii was detected from a smoker chimney at Izu-Ogasawara arc (48). Hyperthermophilic methanogens were also cultured from marker 33 using semiquantitative enrichments (Table 1). The isolation of methanogens and their detection by molecular methods point to the importance of methanogens in producing the high concentrations of methane often measured in vent fluids (2). In contrast, while relatively high numbers of the hyperthermophilic Thermococcales were enumerated by culturing techniques, they were not detected by phylogenetic methods (Table 1). Similarly, Takai and Horikoshi (48) did not detect Thermococcales in six different deep-sea vent habitats using molecular methods. Hyperthermophilic heterotrophs were detected in the in situ growth chamber at the Mid-Atlantic Ridge (42). However, this was an enrichment study and selected for organisms that grow rapidly and colonize surfaces. Thermococcales-related clones have been reported from shallow marine vent fluids (49), as well as in a black smoker chimney from Manus Basin in New Guinea (50).
The remaining vent phylotypes were related only to other uncultured environmental sequences, and the fact that they were found only in vent fluid samples and not in background seawater indicates that these phylotypes potentially represent uncultured organisms inhabiting the subsurface vent environment at marker 33. Additionally, many of the sequences were related to others from hydrothermal vent habitats, indicating that there may be a wide array of ubiquitous vent microorganisms that culturing efforts are missing. Based on this work and the study by Takai and Horikoshi (48), there is a great amount of novel diversity in the euryarchaeota of vent environments, and culturing efforts should continue to search out and describe these microbes. For example, this is the third study of hydrothermal vent fluids to find phylogenetic evidence of microbes related to the Thermoplasmales group (42, 48), suggesting the potential role of these organisms in the hydrothermal vent ecosystem and likely the subsurface oceanic crust. The cultured members of the genus Thermoplasma are anaerobic, thermoacidophilic heterotrophs, and they have never been isolated from the hydrothermal vent environment (43).
The group I marine Crenarchaeota dominated the clone libraries (with the exception of the 1999 free-living group) from both the vent and the background seawater, comprising half of all sequenced clones. As in other studies that have investigated hydrothermal vents (36, 42, 48), there was a significant contribution from the archaeoplankton community in the vent fluid samples. Because the background seawater sample was composed only of Marine Group I and II Archaea, the presence of these groups in the vent samples indicates a considerable seawater component in the hydrothermal system. A recent report shows that marine Crenarchaeota belonging to Marine Group I increase with depth in the Pacific Ocean, contributing significantly to the mesopelagic microbial community (28). We conclude that the group I Archaea found in marker 33 vent fluids are from background seawater. Since nothing is known about the physiology of these pelagic marine archaea, it cannot be determined if they are capable of growing in the hydrothermal vent environment, although it is unlikely that a pelagic group is capable of growing at temperatures in excess of 30°C. Another unknown is the residence time of the seawater that is getting mixed into the shallow crust, which will greatly influence whether seawater microbes could be growing and surviving in the subsurface. It is unlikely that seawater organisms present in hydrothermal fluids are due simply to entrainment of ambient seawater during sampling. If this were the only source of seawater crenarchaeota and euryarchaeota, these microbes should be seen in every year and every sample, which is not the case. Obviously, the seawater component of the diffuse-flow system is important to consider and should be incorporated into a model of what may be occurring in hydrothermal vents and the oceanic crust (7, 39).
We found more diversity in the particle-attached fraction at marker 33 than in the free-living population, which agrees with previous diversity studies of particle-associated bacteria in the Columbia River estuary (11) and on marine snow (15). However, with the exception of the Thermoplasmales group and most of the unique vent clones (UEII, UEIII, UEIV, UEVI, UEVIII, and unknown Crenarchaeota I [UCI]), the other phylotypes are distributed in both the particle-attached and the free-living populations. This suggests that there may be some interaction between particle-attached and free-living organisms that could include the release of organisms or clumps of organisms from biofilms. We hypothesize that biofilm formation is the common mode of existence for subsurface microbial communities. Many of the hyperthermophilic microbes isolated from the subseafloor have been found to attach to mineral surfaces and form copious amounts of carbohydrates (47; J. A. Huber, unpublished data). The only group unique to the particle fraction was that found in the Thermoplasmales group, which suggests that these cells might simply be larger than 3 μm or that their entire life cycle is maintained on particle surfaces or in biofilms.
The chemical properties of marker 33 have been changing since the 1998 eruption. There has been a gradual shift away from a vapor-dominated fluid, characterized by an increase in chlorinity and a decrease in the hydrogen sulfide content and the overall heat and fluid flux over the period of our observations. These changes are consistent with posteruptive fluid evolution models (5, 6, 53). However, the measured temperature at the marker 33 vent does not steadily decrease and implies variability in the degree of subsurface mixing of seawater and hydrothermal fluids, which depends on the details of plumbing and the flow rate of hydrothermal fluid from the deep subsurface through the mixing zone to the vent orifice.
The temperature and chemical indicators of the degree of subseafloor mixing at marker 33 appear to be the environmental variables correlated best with the composition of the microbial community. In 1999, there was less mixing of seawater with hydrothermal fluid, and this was reflected in the microbial community. There were no seawater-associated Marine Group I Archaea in the free-living fraction and a higher proportion of unique clones than in the other sampling periods. Clones related to M. jannaschii were found in 1998 and 1999 but not in 2000. This result may again reflect the greater extent of dilution of hydrothermal fluid with seawater in 2000 or may relate to the cooling and long-term evolution of fluid chemistry at the vent site (Butterfield et al., unpublished). Molecular methods generally detect only the numerically dominant phylotypes. While Thermococcales were cultured all 3 years, their numbers, based on MPNs, represent less than 1% of the total microbial population based on epifluorescent counts. The Thermoplasmales groups were also found in the 1998 and 1999 samples. In contrast, the putative mesophilic methanogens were found in all 3 years, indicating that the intermediate temperature zone of the crust may support high numbers of mesophilic microbes. While we are limited to what we can infer about the thermal properties of the unknown euryarchaeota group, their presence in all 3 years suggests they may also inhabit the intermediate anaerobic, mesophilic zone. Moreover, since exactly the same clones were detected in different years, it is likely that these euryarchaeota are growing in the subseafloor. In a separate study in preparation, a very high diversity of bacteria was detected in these samples. These include mesophilic sulfur and methane oxidizers and clones that cluster near thermophilic bacteria (J. A. Huber and J. A. Baross, unpublished data). At the present time, the proportion of the microbial communities that is archaea or bacteria is not known.
This study supports the existence of a subseafloor microbial biosphere and reports the distinct archaeal diversity belonging to the subseafloor community. The higher diversity of microbes in the particle-attached fraction supports the idea that the subseafloor microbial community could exist predominately as biofilms, and this is being further studied using microbes cultured from these environments. The focus of our future efforts also includes quantifying the proportion of archaea to bacteria in the vent fluids, as well as determining the actual numbers of indigenous subseafloor microbes compared to those from seawater. Additionally, while this study followed the changes in archaeal diversity and chemistry at one site, an examination of microbial and chemical characteristics at a variety of diffuse-flow vents will increase our understanding of the distribution and incidence of the microbial population beneath the sea floor.
Acknowledgments
We thank Bob Embley for his cooperation in sample collection as well as the crews of the R/V Thompson, R/V Brown, and ROV ROPOS for their assistance. Thanks also to Kevin Roe for analyzing vent fluids and Jonathan Kaye for thoughtful discussion.
This work was supported by a Washington Sea Grant (NA76RG0119), the National Science Foundation (OCE 9816491), NSF IGERT (DGE-9870713), the NASA Astrobiology Institute through the Carnegie Geophysical Institute, the NOAA/PMEL Vents Program (PMEL contribution no. 2401), the NOAA West Coast and Polar Undersea Research Center, an NSF predoctoral fellowship to J.A.H., and the Joint Institute for the Study of the Atmosphere and Ocean (JISAO contribution no. 868) under NOAA Cooperative Agreement no. NA117RJ1232.
REFERENCES
- 1.Baker, E. T., C. G. Fox, and J. P. Cowen. 1999. In situ observations of the onset of hydrothermal discharge during the 1998 submarine eruption of Axial Volcano, Juan de Fuca Ridge. Geophys. Res. Lett. 26:3445-3448. [Google Scholar]
- 2.Baross, J. A., M. D. Lilley, and L. I. Gordon. 1982. Is the CH4, H2, and CO venting from submarine hydrothermal systems produced by thermophilic bacteria? Nature 298:366-368. [Google Scholar]
- 3.Baross, J. A., and J. W. Deming. 1995. Growth at high temperatures: isolation, taxonomy, physiology, and ecology, p. 169-217. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Inc., Boca Raton, Fla.
- 4.Bidle, K. A., M. Kastner, and D. H. Bartlett. 1999. A phylogenetic analysis of microbial communities associated with methane hydrate containing marine fluids and sediments in the Cascadia margin (ODP site 892B). FEMS Microbiol. Lett. 177:101-108. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield, D. A., and G. J. Massoth. 1994. Geochemistry of north Cleft segment vent fluids: temporal changes in chlorinity and their possible relation to recent volcanism. J. Geophys. Res. 99:4951-4968. [Google Scholar]
- 6.Butterfield, D. A., I. R. Jonasson, G. J. Massoth, R. A. Feely, K. K. Roe, R. E. Embley, J. F. Holden, R. E. McDuff, M. D. Lilley, and J. R. Delaney. 1997. Seafloor eruptions and evolution of hydrothermal fluid chemistry. Phil. Trans. R. Soc. Lond. A 355:369-386. [Google Scholar]
- 7.Cooper, M. J., and H. Elderfield. 2000. Diffuse hydrothermal fluids from Lucky Strike hydrothermal vent field: evidence for a shallow conductively heated system. J. Geophys. Res. 105:19369-19375. [Google Scholar]
- 8.Cowen, J. P., R. Shackleford, D. McGee, and P. Lam. 1999. Microbial biomass in hydrothermal plumes associated with the 1998 Axial Volcano eruption. Geophys. Res. Lett. 26:3637-3640. [Google Scholar]
- 9.Cragg, B. A., and R. J. Parkes. 1994. Bacterial profiles in hydrothermally active deep sediment layers from Middle Valley (NE Pacific), sites 857 and 858. Proc. ODP Sci. Results 139:509-516. [Google Scholar]
- 10.Crump, B., J. A. Baross, and E. V. Armbrust. 1999. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl. Environ. Microbiol. 65:3192-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump, B. C., and J. A. Baross. 2000. Archaeaplankton in the Columbia River, its estuary and the adjacent coastal ocean, USA. FEMS Microbiol. Ecol. 31:231-239. [DOI] [PubMed] [Google Scholar]
- 12.Cytryn, E., D. Minz, R. S. Oremland, and Y. Cohen. 2000. Distribution and diversity of archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl. Environ. Microbiol. 66:3269-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaney, J. R., D. S. Kelley, M. D. Lilley, D. A. Butterfield, J. A. Baross, W. S. D. Wilcock, R. W. Embley, and M. Summit. 1998. The quantum event of oceanic crustal accretion: impacts of diking at mid-ocean ridges. Science 281:222-230. [PubMed] [Google Scholar]
- 14.Delaney, J. R., D. S. Kelley, E. A. Mathez, D. R. Yoerger, J. A. Baross, M. O. Schrenk, M. K. Tivey, J. Z. Kaye, and V. Robigou. 2001. “Edifice Rex” sulfide recovery project: analysis of submarine hydrothermal, microbial habitat. EOS Trans. 82:67-73. [Google Scholar]
- 15.DeLong, E. F., D. G. Franks, and A. L. Alldredge. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol. Oceanogr. 38:924-934. [Google Scholar]
- 16.Dziak, R. P., and C. G. Fox. 1999. The January 1998 earthquake swarm at Axial Volcano, Juan de Fuca Ridge: hydroacoustic evidence of seafloor volcanic activity. Geophys. Res. Lett. 26:3429-3432. [Google Scholar]
- 17.Embley, R. W., W. W. Chadwick, Jr., D. Clague, and D. Stakes. 1999. 1998 eruption of Axial Volcano: multibeam anomalies and seafloor observations. Geophys. Res. Lett. 26:3425-3428. [Google Scholar]
- 18.Greenberg, A. E., L. S. Clescen, and A. D. Eaton. 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 19.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 20.Harmsen, H. J. M., D. Prieur, and C. Jeanthon. 1997. Distribution of microorganisms in deep-sea hydrothermal vent chimneys investigated by whole-cell hybridization and enrichment culture of thermophilic subpopulations. Appl. Environ. Microbiol. 63:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haymon, R. M., D. J. Fornari, K. L. VonDamm, M. D. Lilley, M. R. Perfit, J. M. Edmond, C. Shanks-Wayne III, R. A. Lutz, J. M. Grebmeier, S. Carbotte, D. Wright, E. McLaughlin, M. Smith, N. Beedle, and E. Olson. 1993. Volcanic eruption of the mid-ocean ridge along the East Pacific Rise crest at 9 degrees 45-52′N; direct submersible observations of seafloor phenomena associated with an eruption event in April, 1991. Earth Planet. Sci. Lett. 119:85-101. [Google Scholar]
- 22.Hildebrand, J. A., J. M. Stevenson, P. T. C. Hammer, M. A. Zumberge, and R. L. Parker. 1990. A seafloor and sea surface gravity survey of Axial Volcano. J. Geophys. Res. 95:12751-12763. [Google Scholar]
- 23.Holden, J. F., M. Summit, and J. A. Baross. 1998. Thermophilic and hyperthermophilic microorganisms in 3-30°C hydrothermal fluids following a deep-sea volcanic eruption. FEMS Microbiol. Ecol. 25:33-41. [Google Scholar]
- 24.Huber, R., P. Stoffers, J. L. Cheminee, H. H. Richnow, and K. O. Stetter. 1990. Hyperthermophilic archaebacteria within the crater and open-sea plume of erupting Macdonald Seamount. Nature 345:179-181. [Google Scholar]
- 25.Johnson, H. P., and R. W. Embley. 1990. Axial Seamount: an active ridge axis volcano on the central Juan de Fuca Ridge. J. Geophys. Res. 95:12689-12696. [Google Scholar]
- 26.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254-261. [Google Scholar]
- 27.Karl, D. M. 1995. Ecology of free-living, hydrothermal vent communities, p. 35-124. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Inc., Boca Raton, Fla.
- 28.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 29.Keswani, J., S. Orkand, U. Premachandran, L. Mandeleco, M. J. Franklin, and W. B. Whitman. 1996. Phylogeny and taxonomy of mesophilic Methanococcus spp. and comparison of rRNA, DNA hybridization, and phenotypic methods. Int. J. Syst. Bacteriol. 46:727-735. [DOI] [PubMed] [Google Scholar]
- 30.Lilley, M. D., J. A. Baross, and L. I. Gordon. 1984. Reduced gases and bacteria in hydrothermal fluids: the Galapagos spreading center and 21°N East Pacific Rise, p. 411-449. In P. A. Rona, K. Bostrom, L. Laubier, and K. L. Smith, Jr. (ed.), Hydrothermal processes at seafloor spreading centers. Plenum Publishing Corp., New York, N.Y.
- 31.Longnecker, K., and A.-L. Reysenbach. 2001. Expansion of the geographic distribution of a novel lineage of epsilon-Proteobacteria to a hydrothermal vent site on the Southern East Pacific Rise. FEMS Microbiol. Ecol. 35:287-293. [DOI] [PubMed] [Google Scholar]
- 32.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massana, R., A. E. Murray, C. M. Preston, and E. F. DeLong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massana, R., E. F. DeLong, and C. Pedros-Alio. 2000. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 66:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCollom, T. M., and E. L. Shock. 1997. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta 61:4375-4391. [DOI] [PubMed] [Google Scholar]
- 36.Moyer, C. L., J. M. Tiedje, F. C. Dobbs, and D. M. Karl. 1998. Diversity of deep-sea hydrothermal vent Archaea from Loihi Seamount, Hawaii. Deep-Sea Res. II 45:303-317. [Google Scholar]
- 37.Munson, M. A., D. B. Nedwell, and T. M. Embley. 1997. Phylogenetic diversity of archaea in sediment samples from a coastal salt marsh. Appl. Environ. Microbiol. 63:4729-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkes, R. J., B. A. Cragg, S. J. Bale, J. M. Getliff, K. Goodman, P. A. Rochelle, J. C. Fry, A. J. Weightman, and S. M. Harvey. 1994. Deep bacterial biosphere in Pacific Ocean sediments. Nature 371:410-413. [Google Scholar]
- 39.Pascoe, A. R., and J. R. Cann. 1995. Modeling diffuse hydrothermal flow in black smoker vent fields, p. 159-173. In I. M. Parson, C. L. Walker, and D. R. Dixon (ed.), Hydrothermal vents and processes. Geological Society, London, United Kingdom.
- 40.Polz, M. F., and C. M. Cavanaugh. 1998. Bias in template-to-product ratios in multitemplate PCR. Appl. Environ. Microbiol. 64:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 42.Reysenbach, A.-L., K. Longnecker, and J. Kirshtein. 2000. Novel bacterial and archaeal lineages from an in-situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl. Environ. Microbiol. 66:3798-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segerer, A. H., and K. O. Stetter. 1992. The genus Thermoplasma, p. 712-718. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 44.Summit, M., and J. A. Baross. 1998. Thermophilic subseafloor microorganisms from the 1996 North Gorda Ridge eruption. Deep-Sea Res. II 45:2751-2766. [Google Scholar]
- 45.Summit, M., A. Peacock, D. Ringelberg, D. C. White, and J. A. Baross. 2000. Phospholipid fatty acid-derived microbial biomass and community dynamics in hot, hydrothermally influenced sediments from Middle Valley, Juan de Fuca Ridge. Proc. ODP Sci. Results 169:1-19. [Google Scholar]
- 46.Summit, M., and J. A. Baross. 2001. A novel microbial habitat in the mid-ocean ridge subseafloor. Proc. Natl. Acad. Sci. USA 98:2158-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summit, M. 2000. Ecology, physiology, and phylogeny of subseafloor thermophiles from mid-ocean ridge environments. Ph.D. thesis. University of Washington, Seattle.
- 48.Takai, K., and K. Horikoshi. 1999. Genetic diversity of archaea in deep-sea hydrothermal vent environments. Genetics 152:1285-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takai, K., and Y. Sako. 1999. A molecular view of archaeal diversity in marine and terrestrial hot water environments. FEMS Microbiol. Ecol. 28:177-188. [Google Scholar]
- 50.Takai, K., T. Komatsu, F. Inagaki, and K. Horikoshi. 2001. Distribution of archaea in a black smoker chimney structure. Appl. Environ. Microbiol. 67:3618-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van der Maarel, M. J. E. C., R. R. E. Artz, R. Haanstra, and L. J. Forney. 1998. Association of marine archaea with the digestive tracts of two marine fish species. Appl. Environ. Microbiol. 64:2894-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Dover, C. L. 2000. The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton, N.J.
- 53.Von Damm, K. L., S. E. Oosting, R. Kozlowski, L. G. Buttermore, D. C. Colodner, H. N. Edmonds, J. M. Edmond, and J. M. Grebmeier. 1995. Evolution of East Pacific Rise hydrothermal vent fluids following a volcanic eruption. Nature 375:47-50. [Google Scholar]
- 54.Whitman, W. B., T. L. Bowen, and D. R. Boone. 1992. The methanogenic bacteria, p. 719-767. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.