Abstract
Background
Traditional Chinese herbal medicines are frequently used to treat premenstrual syndrome (PMS) in China. Until now, their efficacy has not been systematically reviewed.
Objectives
To evaluate the effectiveness and safety of traditional Chinese herbal medicines in the treatment of women with premenstrual syndrome.
Search methods
We searched MEDLINE (January 1950 to December 2007), EMBASE (January 1980 to December 2007), Chinese Biomedical Database (CBM) (January 1975 to December 2007), China National Knowledge Infrastructure (CNKI) (January 1994 to December 2007), and the VIP Database (January 1989 to December 2007).
Selection criteria
Randomised controlled trials (RCTs) studying the efficacy of traditional Chinese herbal medicine(s) for treatment of premenstrual syndrome were included.
Data collection and analysis
Two review authors telephoned the original authors of the RCTs to confirm the randomisation procedure and extracted and analysed data from the trials that met the inclusion criteria.
Main results
Two RCTs involving 549 women were included. One trial which was identified to be of higher methodological quality demonstrated the therapeutic effectiveness of Jingqianping granule. The other study was considered of lower quality due to the inherent risk of various biases in it. The two studies showed statistically significant differences in favour of taking Jingqianping granule compared with Xiaoyaowan in the elimination of symptoms during the proliferative and premenstrual phases (RR 3.50, 95% CI1.74 to 7.06). Women treated with Cipher decoction had a higher rate of recovery than those taking co‐vitamin B6 capsules (RR 48.99, 95% CI 3.06 to 783.99).
Authors' conclusions
It is rare in PMS management that efficacy claims are substantiated by clinical trials. One of the identified trials was well designed and reported on the effectiveness of Jingqianping in the treatment of premenstrual syndrome. However, currently there is insufficient evidence to support the use of Chinese herbal medicine for PMS and further, well‐controlled trials are needed before any final conclusions can be drawn.
Keywords: Female; Humans; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Phytotherapy; Phytotherapy/methods; Premenstrual Syndrome; Premenstrual Syndrome/drug therapy; Randomized Controlled Trials as Topic; Vitamin B 6; Vitamin B 6/therapeutic use; Vitamin B Complex; Vitamin B Complex/therapeutic use
Plain language summary
Herbal treatment for premenstrual syndrome
Herbal medicines are sometimes used for treating premenstrual syndrome (PMS). However, the effectiveness of this type of therapy has not be rigorously evaluated in randomised controlled trials.
The authors identified two trials that evaluated herbal medicines in PMS. One of these was a higher quality study that tested the traditional Chinese medicine decoction Jingqianping granule. This was shown to increase the rate of recovery from PMS symptoms. Because the formula for this herbal medicine was provided by the trialists themselves, the review authors recommend further trials to ensure that the results are reproducible with other formulations. Strong evidence in support of other herbal formulae for the treatment of PMS is currently lacking.
Summary of findings
Summary of findings for the main comparison. New Summary of findings table.
Background
Description of the condition
Premenstrual syndrome (PMS) is a common cyclic disorder for young and middle‐aged women that consistently occurs during the luteal phase of the menstrual cycle. It is characterised by emotional and physical symptoms and can manifest with a wide variety of symptoms, including depression, mood lability, abdominal pain, breast tenderness, headache, and fatigue (Dickerson 2003; Tempel 2001). The symptoms of PMS may be sufficiently severe to disrupt women's normal functioning, quality of life, and interpersonal relationships. More severe, mainly psychological symptoms such as depression, anxiety, and other affective symptoms which recur in the luteal phase of the menstrual cycle are classified as premenstrual dysphoric disorder (PMDD) (Dickerson 2003; Sundstrom 2003).
As many as 75% of women with regular menstrual cycles experience some symptoms of PMS, PMDD affects only 3% to 8% of women in this group (Steiner 2000). In addition, PMS is more prevalent among white women, smokers, obese, and younger women (Masho 2005).
The Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) (APA 2005) requires at least five specified symptoms in order to diagnose premenstrual dysphoric disorder (PMDD); while the International Statistical Classification of Diseases, 10th Revision (ICD‐10) (WHO 2005) requires only one distressing symptom for a diagnosis of PMS. A diagnosis of PMS requires determining the timing of the symptoms in relation to menses, meaningful change between post‐ and premenstrual symptom severity, and clinically significant severity of the symptoms. The current diagnostic standard requires confirmation of subjective symptom reports by prospective daily diaries. Diagnostic criteria for PMS must recognise the broad range of symptoms, the temporal pattern of the symptoms, and the critical issue of symptom severity, which differentiates clinically significant PMS from normal menstrual cycle changes (Freeman 2003). The best tool to diagnose PMS is a daily symptoms rating calendar. To have the diagnosis of PMS, the symptoms must be severe enough to disrupt normal daily activities (Tempel 2001).
The pathogenesis of PMS remains uncertain; research suggests that altered regulation of neurohormones and neurotransmitters is involved (Dickerson 2003). Several biological mechanisms that underlie menstrually related symptoms have been proposed. They focus mostly on gonadal hormones, their metabolites, and interactions with neurotransmitters and neurohormonal systems such as serotonin, GABA, cholecystokinin, and the renin‐angiotensin‐aldosterone system. Altered responses of these systems to ovarian hormone fluctuations during the menstrual cycle, as well as increased sensitivity to changes in ovarian hormones, may contribute to menstrually related symptoms in vulnerable women. Core underlying mechanisms may be disrupted homeostasis and deficient adaptation (Halbreich 2003). Current research focusing on the biological background of premenstrual syndrome suggests that both psychological and physiological factors exert their effect through dysregulation of serotonergic function. The results of several studies point to a variation in the function of the serotonergic system throughout the menstrual cycle. Ovarian steroids have been found to profoundly influence the activity of the serotonergic system (Eriksson 1994; Gonda 2004; Joffe 1998; Rubinow 1998). Additionally, reduced serotonin uptake by platelets and reduced whole blood serotonin levels during the luteal phase have been demonstrated in women with PMS (Taylor 1984; Rapkin 1987; Steege 1992).
Hypomagnesaemia (low magnesium levels in the blood) has been implicated in the pathogenesis of PMS (Ventskivs'ka 2005). Moreover, high intake of fats and low intake of foods with high concentration of carbohydrate may be associated with premenstrual symptoms (Nagata 2004).
Description of the intervention
Many different treatments have been suggested as possible therapies for PMS. This is due to the uncertainty of its pathogenesis, the wide range of its manifestations, and a large placebo effect. As serotonin has been implicated in the pathogenesis of PMS, the therapeutic effectiveness of continuous and luteal phase dosing of selective serotonin reuptake inhibitors (SSRIs) has been evaluated (Dimmock 2000). SSRIs have emerged as first‐line therapy for PMS (Steiner 2006).
Ovarian function appears to play a fundamental role in PMS, accordingly treatment strategies designed to suppress ovulation have generally been found to be effective for treatment of menstrually related syndromes and symptoms. Gonadotrophin‐releasing hormone analogues (GnRHa) appear to provide an effective treatment of premenstrual syndrome (Backstrom 2003; Kouri 1998; Wyatt 2004).
In other studies, women with PMS who practised aerobic exercise reported fewer symptoms than participants in the control group (O'Brien 2000; Steege 1993). Dietary restrictions or supplements may also be useful in women with PMS (Kessel 2000; Moline 2000). Sodium restriction has been proposed to minimise bloating, fluid retention, breast swelling and tenderness. Caffeine restriction is recommended because caffeine intake is related to premenstrual irritability and insomnia. A systematic review of placebo‐controlled trials of evening primrose oil suggested lack of any benefit in PMS, although mild relief was demonstrated in women with breast tenderness (Budeiri 1996).
A randomised placebo‐controlled study reported there were significant improvements in the symptoms of negative feeling, pain, water retention, and total PMS symptoms in women receiving qigong therapy when compared to placebo controls (Jang 2004). Qigong consists primarily of meditation, relaxation, physical movement, mind‐body integration, and breathing exercises. Practitioners of qigong develop an awareness of qi sensations (energy) in their body and use their mind to guide the qi. When the practitioners achieve a sufficient skill level (master), they can direct or emit external qi for the purpose of healing others.
Some studies also indicate that acupuncture, homeopathy, aromatherapy, reflexology, Gingko biloba, kava kava, black cohosh, and agnus castus can relieve symptoms of PMS such as anxiety, depression, and irritability (Jones 2003; Tesch 2003; Yu 2005).
Other treatments for PMS, for which there is inconclusive evidence, include photic stimulation, cognitive behavioural therapy, relaxation therapy, vitamin B6, L‐tryptophan, stress reduction, spironolactone, and a complex carbohydrate drink. Although evidence for relief of PMS symptoms is inconclusive, it is reasonable to recommend these as healthy lifestyle changes which may have overall benefits (Douglas 2002; Girman 2003; Rapkin 2003).
How the intervention might work
Herbal medicines have long been used in traditional healing systems to treat conditions of particular interest to women, such as premenstrual syndrome (PMS) and menopausal symptoms. For a select number of phytomedicines, including evening primrose oil, black cohosh root extract, dong quai, and chaste tree berry, scientific investigation has identified the pharmacologically active constituents, mechanism of action, and clinical value. Based on the available evidence, chaste tree berry may be a reasonable treatment alternative for some women with PMS. Dong quai (in standard pinyin it is called dang gui) may be used to treat PMS when combined with traditional Chinese multiple‐herb formulations (Hardy 2000). The current literature supports the use of chaste berry for cyclical breast discomfort and premenstrual syndrome (Roemheld‐Hamm 2005).
Why it is important to do this review
Traditional Chinese medicine (TCM) has been used for thousands of years to treat PMS. The rule of TCM for PMS is 'Bian Zheng Lun Zhi': adding or reducing some herbs in a traditional preparation, so‐called 'demonstrated preparation', depending on the TCM 'Zheng' signs recognised by a TCM physician. At least eight types of 'Zheng' are recognised by TCM physicians, and various formulations can be used to treat the same type of 'Zheng'. Some new preparations for various 'Zheng xing' (TCM signs) have been developed, for example, 'gan yu qi ci' type PMS can be treated by using adjusted 'cai hu su gan san' (Lang 1996), 'pi shen yang xu' type PMS by adjusted 'fang ji huang qi tang' combined with 'you gui wan' (Zhu 1995). These formulations or preparations have been described as effective remedies and it has been suggested they could have clinical applications.
There is increasing public interest in, and use of, herbal medicine therapies which lie outside the 'mainstream' or traditional Western medical practice across the globe (HLSC 2000). There is evidence to indicate that not all herbs are risk‐free. There are concerns about adverse events, particularly allergic reactions, as well as Chinese herbal nephropathy (CHN) affecting the kidneys (Lampert 2002; Lord 2001; Nortier 2000). Hence, there is a need to systematically review herbal medicines for their effectiveness and safety.
Objectives
To evaluate the effectiveness and safety of traditional Chinese herbal medicines in the treatment of women with premenstrual syndrome.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included, without restriction on language or publication type. Pseudo‐RCTs were not be considered.
Types of participants
Women of reproductive age diagnosed with premenstrual syndrome who suffered with one or more symptoms occurring periodically during 0 to 14 days before menstruation, for three or more menstrual cycles. Women known to have medical problems such as hypothyroidism, hypoglycaemia, psychiatric disorders, or had bilateral oophorectomy or any type of cancer were excluded.
Types of interventions
Any form of herbal medicine was considered, including oral preparations, decoctions, injections, and tablets. Comparisons could consist of a placebo, no intervention, acupuncture, western medication, or any other intervention. Comparison of one kind of herbal medicine versus another herbal medicine was also included.
Types of outcome measures
Primary outcomes
Change of overall symptoms
The following tools were considered.
(1) PMS scoring systems Any validated objective scoring system used for evaluating premenstrual symptoms, such as the Moos Menstrual Distress Questionnaire or the Daily Symptom Report were considered.
(2) Improvement of overall symptoms (dichotomous data)
If the above two outcome measures were not used in a study, we considered the outcome measures as follows:
‐ records made by participants or doctors on a chart or using a visual analogue scale;
‐ severity of symptoms reported, judged in accordance with the definitions of the numerical scales used in the charts.
Secondary outcomes
1. Quality of life The Health Related Quality of Life (HRQOL) or other validated scales were considered in this review.
2. Adverse events Events such as functional injury of the liver or kidney, nausea, vomiting, diarrhoea, and skin rash were recorded.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or in progress) (Appendix 1).
We searched: (1) the Cochrane Menstrual Disorders and Subfertility Group Trials Register and the Cochrane Complementary Medicine Field Trials Register; (2) the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 4, 2007) (Appendix 2); (3) MEDLINE (January 1966 to December 2007), EMBASE (January 1980 to December 2007) (Appendix 4), CISCOM (to December 2007), Chinese Biomedical Database (CBM) (January 1975 to December 2007), China National Knowledge Infrastructure (CNKI) (January 1979 to December 2007), VIP Database (January 1989 to December 2007), AMED (Appendix 4);
(4) ongoing trials in the Meta‐register of Controlled Trials, which includes the Medical Research Council Clinical Trials Directory, National Research Register, Chinese Clinical Trial Register, as well as other registers for ongoing trials;
(5) reference lists of the relevant trials and reviews identified;
(6) unpublished and on‐going trials, through correspondence with authors;
(7) major herbal treatment and obstetrics and gynaecology conference proceedings and poster abstracts (about this disease) over the last five years;
(8) for side‐effect studies and contacted various adverse reaction‐reporting bodies.
Data collection and analysis
The study selection and data analysis were undertaken by two authors (ZJ and WTX).
Selection of studies
From the search results, ZJ and YXZ scanned the identified results abstracts and relevant records. Full articles were retrieved for all potentially relevant trials. Since all the publications were in Chinese, WTX and ZJ were independently able to interview the original authors by telephone to identify what method was used to generate the allocation sequence. WTX selected the trials for inclusion. All identified reports were scrutinised to check for multiple publications of the same trials.
Data extraction and management
Using a piloted data extraction form, we extracted data on study characteristics including methods, participants, interventions, and outcomes. There were no disagreements between the authors.
For the included studies we extracted the formulation contents and herb names in three languages (Table 2, Table 3).
1. Herbs names in different language.
| Pinying name | Latin name | English name |
| Baishao | Radix Paeoniae Alba | White Peony root |
| Baishu | Atractylodes macrocephala Koidz. | Largehead Atractylodes rhizome |
| Bohe | Herba Menthae | Field mint/peppermint |
| Chaihu | Radix Bupleuri | Chinese Thorowax root /Red Thorowax root |
| Chaozaoren | Stir‐baked Semen Ziziphi Spinosae | Stir‐baked Semen Ziziphi Spinosae |
| Chuanlianzi | Fructus Toosendan | Szechwan chinaberry fruit |
| Chuanxiong | Rhizoma Chuanxiong | Szechwan Lovage rhizome |
| Cuchaihu | Stir‐baked Radix Bupleuri with vinegar | Stir‐baked Radix Bupleuri with vinegar |
| Danggui | Radix Angelicae Sinensis | Angelica; angelica root |
| Fuling | Poria | Indian Bread/ Poria cocos/Poria cocos Wolf |
| Hehuanpi | Cortex Albizziae | Silktree albizzia bark |
| Juye | Citrus reticulata Blanco var. erythorosa H.H.Hu | Tangerine leaf |
| Shenglongchi | Dens Draconis | Dragon'sTeeth |
| Sigualuo | Retinervus Luffae Fructus | Retinerus Luffae Fructus |
| Xiangfu | Rhizoma Cyperi | Nutgrass Galingale rhizome |
| Yujin | Radix Curcumae | Aromatic Turmeric root‐tuber |
| Zhigancao | Radix Glycyrrhizae Preparata | Prepared Radix Glycyrrhizae |
| Zhike | Fructus Aurantii | Bitter Orange |
2. Contents of the formulae in included studies.
| Study ID | Contents |
| Qiao 2002 | Did not provide any information about the contents of interventions but the drugs were the same as Wei 2006a, including the types and drug manufacture. Jingqianping granule: baishao, xiangfu, chuanxiong, zhike, chuanlianzi, etc; Xiaoyaowan: chaihu, danggui, baishao, baishu, fuling, zhigancao, bohe. |
| Wei 2006a | Jingqianping granule: baishao, xiangfu, chuanxiong, zhike, chuanlianzi, etc; it did not provide any information about the contents of xiaoyaowan (the control). We had indexed and found the contents of xiaoyaowan: chaihu, danggui, baishao, baishu, fuling, zhigancao, bohe. |
| Zhu 2003 | Cipher Fang decoction: cuchaihu, baishao, yujin, juye, sigualuo, chaozaoren, hehuanpi, fuling, shenglongchi, etc. Vitamin B6 capsule: vitamin B6 20mg, vitamin B1 10mg, oryzanol 10mg. |
Assessment of risk of bias in included studies
Risks of bias were assessed independently by at least two authors using the criteria that are described in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 (Higgins 2008) and (Wu 2007).
The following characteristics were assessed.
Randomisation process: assessment for selection bias
A ‐ adequate sequence generation was reported using one of the following approaches: random number tables or computer‐generated random numbers, coin tossing or shuffling for generating the allocation sequence before the launch of the trial; trials considered eligible and with a low risk of selection bias.
B ‐ one of the adequate methods outlined in (A) was not specified and only mentioned 'random'; considered to have a moderate risk of selection bias.
C ‐ other methods of allocation, for example quasi‐randomisation, that appeared to have a high risk of bias; trials were excluded.
Allocation concealment process: assessment of selection bias
A ‐ adequate measures to conceal allocation: where the person who generated an allocation sequence did not attend to recruitment of the participants, and the allocation sequence was sealed in opaque envelopes or held in a locked computer, or another description that contained convincing elements of concealment; considered to have a low risk of selection bias.
B ‐ unclear: concealed trials in which the author did not report an approach to allocation concealment at all; considered as having a moderate risk of selection bias.
C ‐ inadequately‐concealed allocation that reported an approach that did not fall into one of the categories in (A).
D ‐ did not conceal allocation.
C and D were considered as having a high risk of selection bias.
Level of blinding: assessment for performance bias and detection bias
A ‐ double blinding: participants and outcome assessor were masked and were considered as having a low risk of both performance and detection bias.
B ‐ single blinding of the outcome assessor was considered to have a moderate risk of both performance and detection bias. If participants were blind to group allocation but not the outcome assessor, the study was considered to have a high risk of detection bias.
C ‐ non‐blinding was considered to have a high risk of both performance and detection bias.
Measures of treatment effect
We have analysed the data using Review Manager (Version 5.0). We compared outcome measures for continuous data using the mean difference with 95% confidence interval. For binary data we used relative risks with 95% confidence interval.
Dealing with missing data
The risk of bias of missing data was estimated according to following method (Higgins 2008).
A ‐ low risk of bias: trials where few dropouts and losses to follow up were noted and an intention‐to‐treat analysis was possible.
B ‐ moderate risk of bias: trials which reported the rate of exclusions as about 10% whatever intention‐to‐treat analysis was used.
C ‐ high risk of bias: the rate of exclusions was at least 15%, or wide differences in exclusions between groups, whatever intention‐to‐treat analysis was used.
Assessment of heterogeneity
In the future, we will analyse the clinical and methodological heterogeneity if the included studies are similar enough. We will assess heterogeneity amongst trials to decide whether the data could be pooled by using the Chi2 test and I2 statistic. An I2 < 25% means low heterogeneity, I2 between 25% and 50% is considered moderate heterogeneity, and I2 > 50% means notable heterogeneity. We will combine data using random‐effects model. If I2 > 75%, which means substantive heterogeneity existed, the data will not be combined (Higgins 2008).
Assessment of reporting biases
The risk of reporting bias was assessed: refer to the description of Cochrane Handbook of Systematic Reviews of Interventions 5.0.0 (Higgins 2008).
No ‐ low risk of reporting bias: all outcomes were reported in detail.
Probably yes ‐ moderate risk of reporting bias: at least one of the outcomes was mentioned but not in detail.
Yes ‐ high risk of reporting bias: at least one outcome was not reported.
Data synthesis
We did not perform a combined analysis due to the use of different TCM therapy formulations and comparators in the included studies.
Potential publication bias was not tested because there were only two studies in this review.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis based on the different traditional Chinese herbal medicine formulae used.
Sensitivity analysis
We did not conduct a sensitivity analysis as a combined analysis was not performed.
Results
Description of studies
A total of 17 trials that claimed to be randomised were identified. We successfully contacted seven of the trial authors by telephone. Of the seven trials, four were excluded because the trial authors misunderstood what was meant by random allocation and their trials were not real randomised controlled trials. Two remaining studies were identified as true RCTs (Qiao 2002; Zhu 2003).
Excluded studies
See 'Characteristics of excluded studies'.
Of the 17 published articles initially identified, nine articles were excluded as they were not randomised controlled comparisons (Li 2003; Pei 2005; Sun 2003; Wang 1997; Wang 2004; Yin 1999; Yue 2005; Zhang 2000; Zhang 2003). Outcome measures in Wei 2006 were not included in the criteria for this review. Excluded studies and the reasons for exclusion are listed in the 'Characteristics of excluded studies' table.
We were unable to contact the authors of five studies and these are listed in 'Studies awaiting classification' (Feng 1996; Guo 2004; Li 2002; Xie 1994; Yuan 2004).
Included studies
See 'Characteristics of included studies'.
Two RCTs fulfilled our inclusion criteria (Qiao 2002; Zhu 2003).
Design
Both trials used a parallel group design. One of the studies (Qiao 2002) had a multicentre, double‐dummy, double‐blind design.
Participants
The two studies recruited participants based on traditional Chinese medicine (TCM) signs. These studies included a total of 549 participants, age range 16 to 45 years. The trials authors of both studies reported that the diagnosis of PMS was made according to the "Regulation of clinical study of new TCM for PMS", however, there was no detailed description of this regulation.
Interventions
Qiao 2002 compared Jingqianping granule and Xiaoyaowan.
Zhu 2003 compared Cipher Fang decoction and vitamin B6 in a capsule.
Outcomes
The two studies (Qiao 2002; Zhu 2003) did not report outcomes using the tools defined in this review. Instead they reported on recovery, marked improvement, and no improvement based on TCM signs.
Risk of bias in included studies
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
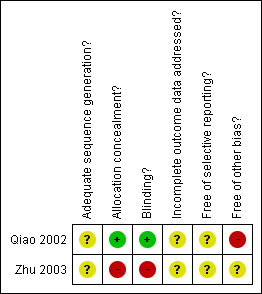
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Randomisation
Both included studies were RCTs that used random number tables.This was clearly stated in the study by Zhu 2003 while it was only confirmed following contact with the authors for the Qiao 2002 study.
Allocation concealment
Neither of the of the included trials mentioned allocation concealment.
Following contact with the study authors, concealment was considered adequate in one of the included trials (Qiao 2002) as the random number allocation sequence was generated by a statistician not involved in participant recruitment.
Blinding
Single blinding was used in one study (Zhu 2003) while the Qiao 2002 study used a double‐dummy, double‐blind methodology hence the risk of both performance bias and detection bias was deemed very low.
Withdrawals, dropout, loss to follow‐up and intention to treat
Neither of the included trials reported on withdrawals, dropouts, or loss to follow up of participants.
Effects of interventions
See: Table 1
1. Change in premenstrual syndrome symptoms
(1) Rate of recovery (defined as disappearance of symptoms during the trial)
The two studies (Qiao 2002; Zhu 2003) reported recovery rates and showed statistically significant differences in elimination of symptoms between the intervention and control groups.
The rate of recovery in the group using Jingqianping granule was 27.7% (56/202) while it was 8% (8/101) in the Xiaoyaowan group (RR 3.50, 95% CI 1.74 to 7.06). This indicated that Jingqiaping granule was more effective than Xiaoyaowan in eliminating PMS symptoms (Qiao 2002).
Participants who took the Cipher decoction had a recovery rate of 30% (50/166) while there were no reported recoveries in the vitamin B6 capsule group (0/80) (RR 48.99, 95% CI 3.06 to 783.99). This suggested that Cipher decoction was more effective than vitamin B6 in eliminating PMS symptoms (Zhu 2003).
(2) Rate of no improvement (defined as symptom severity that remained unchanged during the trial)
Both studies also reported the rate of 'no improvement' and showed statistically significant differences between the intervention and control groups. Eight participants reported no improvement (4%, 8/202) in the Jingqianping granule group compared to 18 in the Xiaoyaowan group (18%, 18/101) (Qiao 2002).
Two participants reported no improvement in TCM signs (1.2%, 2/166) in the Cipher Fang decoction group compared to 31 in the vitamin B6 group (38.8%, 31/80) (Zhu 2003).
2. Time between commencing treatment and BBT or endocrine levels returning to normal
One study (Zhu 2003) reported on the changes in prolactin, estradiol, and progestin concentrations, before and after treatment. However, only a very small number of participants contributed to the data (14/166 in the experiment group and 10/80 in the control group). We therefore did not include these results in the analysis of this review.
3. Development of adverse events
Neither study reported any toxic reactions or adverse events.
Discussion
Only two trials (Qiao 2002; Zhu 2003) fulfilled the inclusion criteria. One study (Qiao 2002) was considered to be of higher methodological quality than the other with regard to study design and trial processing because a double‐dummy technique was used. Due to the potential selection, detection, and attrition biases in the Zhu 2003, the study was considered to be of a lower methodological quality. However, the formula of the experimental drug (Jingqianping granule) was prepared by the authors themselves and the drug was made by the authors' university. Similarly, the experimental drug used in the study by Qiao 2002 was also prepared by authors themselves. Therefore, independent validation of the findings of these trials is advisable.
None of the included trials used a placebo control and active controls were used instead. PMS is a condition with a substantial placebo response rate, hence it is scientifically important to include a placebo group in trials evaluating the therapeutic effectiveness of new medications. Moreover, it has been suggested that, by making comparisons with external placebo, the need for placebo control groups in new studies on patients could be minimised assuming that the novel medication will perform in the same way in a study with only active controls as it would have in a placebo‐controlled trial. However, evidence from 32 RCTs involving 7264 participants showed that the degree of improvement was nearly double in active‐controlled trials than with the same drugs and dosages in placebo‐controlled studies Woods 2005. Therefore, the high RRs identified in this review could be secondary to the lack of use of placebo controls.
None of the trials provided sufficient information about allocation concealment. After contacting the authors, it was judged that Qiao 2002 used adequate methodology to ensure concealment at the time of randomisation. In addition, none of the studies reported on their justification for the required sample size. Although the included trials seem to have recruited a large sample size (Qiao 2002 included 303 participants, 202:101; and Zhu 2003 included 166:80) it is important to have formal sample size calculations to ensure that the trial is of sufficient power to identify a difference, if one exists.
Surprisingly, several trials claimed to be RCTs but, after contacting the trial authors to enquire about the method of randomisation used, we found that most of the authors misunderstood the concept of randomisation or had forgotten the details of the methodology they employed; they had conducted the studies several years ago. Therefore, most of the studies of Chinese herbal medicine in the management of premenstrual syndrome (PMS) were inadequate to provide reliable therapeutic effect estimates because of poor study design and methodological quality.
Finally, although the diagnostic standard was mentioned in two studies a detailed description of the standard was not provided. In the absence of this key parameter we were unable to judge the validity of the studies and how the diagnosis of PMS was made for the study participants.
Authors' conclusions
Implications for practice.
Currently there is insufficient evidence on the efficacy of traditional Chinese medicine in the treatment of PMS.
Implications for research.
Further randomised clinical trials are required of traditional Chinese herbal medicine for the treatment of premenstrual syndrome. Independent validation of the therapeutic effectiveness of Jingqianping granule is advisable. It is crucial that investigators give considerable attention to the methods of randomisation, blinding, determining sample sizes, and the use of placebo‐controlled trials. The information on trial methodology should also be reported in detail, according to both CONSORT (Moher 2001) and CONSORT for TCM (Wu 2007 CONSORT).
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 30 April 2008 | New search has been performed | Converted to new review format. |
| 8 September 2006 | New citation required and major changes | Substantive amendment |
Notes
Approved by all of the authors.
Acknowledgements
The authors thank Cindy Farquhar, Jane Marjoribanks, Marian Showell, X Zhu, Ying Cheong, Olive Ford, Sofia, Anne, and Helen for their important comments and the staff of Cochrane Menstrual Disorders and Subfertility Group for their help in writing this review.
Appendices
Appendix 1. Search strategy for electronic databases
Search strategy for the above electronic database.
1 Complementary Therapies 2 alternative medicine$.ti,ab. 3 Plant Extracts 4 plant extract$.ti,ab. 5 botanical extract$.ti,ab. 6 Plants, Medicinal 7 medicinal plant$.ti,ab. 8 Medicine, Kampo/ or Phytotherapy/ or Drugs, Chinese Herbal 9 Medicine, Chinese Traditional/ or Medicine, Oriental Traditional 10 (Chinese adj3 medicin$).ti,ab. 11 phytodrug$.mp. 12 phytomedicine$.ti,ab. 13 phytopharmaceutical$.ti,ab. 14 herbal medicine$.ti,ab. 15 (complementary adj3 medicine$).ti,ab. 16 non‐prescription drug$.mp. or Drugs, Non‐Prescription 17 (Chinese adj3 herb$).mp. 18 herbal remed$.mp. 19 herbal extract$.mp. 20 herbal preparation$.mp. 21 botanical preparation$.mp. 22 (herb$ adj3 mixture$).mp. 23 exp medicine, traditional/ or exp medicine, African traditional/ or exp medicine, arabic/ or exp medicine, unani/ or exp medicine, ayurvedic/ or exp medicine, kampo/ or exp medicine, oriental traditional/ or exp medicine, Chinese traditional/ or exp medicine, Tibetan traditional/ or exp shamanism 24 or/1‐23 25 premenstrual syndrome 26 ((premenstrual or pre‐menstrual or pre menstrual) adj syndrome$).tw. 27 ((premenstrual or pre‐menstrual or pre menstrual) adj tension).tw. 28 premenstrual dysphor$.tw. 29 late luteal.tw. 30 (luteal adj dysphor$).tw. 31 (pms or pmt or pmdd or llpd or llpdd).tw. 32 or/25‐31 33 24 and 32 34 randomised controlled trial.pt. 35 controlled clinical trial.pt. 36 Randomized controlled trials 37 random allocation 38 double‐blind method 39 single‐blind method 40 or/34‐39 41 clinical trial.pt. 42 exp clinical trials 43 (clin$ adj25 trial$).ti,ab,sh. 44 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. 45 placebos 46 placebo$.ti,ab,sh. 47 random$.ti,ab,sh. 48 Research design 49 or/41‐48 50 animal/ not (human/ and animal/) 51 40 or 49 52 51 not 50 53 33 and 52 54 from 53 keep 1‐39
Appendix 2. Key words
Keywords CONTAINS "premenstrual " or "premenstrual aggravation" or "premenstrual dysphoric disorder" or "premenstrual symptom scores" or "premenstrual symptoms" or "premenstrual syndrome" or "premenstrual syndrome‐symptoms" or "PMDD" or "PMS" or Title CONTAINS "premenstrual " or "premenstrual aggravation" or "premenstrual dysphoric disorder" or "premenstrual symptom scores" or "premenstrual symptoms" or "premenstrual syndrome" or "premenstrual syndrome‐symptoms" or "PMDD" or "PMS"
AND
Keywords CONTAINS "Chinese" or "Chinese herbal medicine" or "Chinese drugs" or "chinese herbal preparations" or "Chinese herbal remedy" or "Chinese traditional medicine" or "traditional Chinese medicine" or "traditional medicine" or "herbal preparations" or "herbal remedy" or "herbal supplement" or "herbal supplements" or Title CONTAINS "Chinese" or "Chinese herbal medicine" or "Chinese drugs" or "chinese herbal preparations" or "Chinese herbal remedy" or "Chinese traditional medicine" or "traditional Chinese medicine" or "traditional medicine" or "herbal preparations" or "herbal remedy" or "herbal supplement" or "herbal supplements"
Appendix 3. AMED
1 exp chinese medicine/ or exp herbal medicine/ or exp oriental medicine/ or exp tibetan medicine/ (0) 2 exp Phytotherapy/ (1070) 3 exp alternative medicine/ (0) 4 herb$.tw. (9836) 5 (chinese adj5 medicine$).tw. (4694) 6 plant$.tw. (19442) 7 botanical.tw. (294) 8 (traditional adj5 medicine$).tw. (6403) 9 or/1‐8 (27813) 10 exp Premenstrual Dysphoric Disorder/ (0) 11 exp Premenstrual Syndrome/ (93) 12 Premenstrual Dysphoric Disorder$.tw. (0) 13 (PMDD or PMS).tw. (55) 14 late luteal.tw. (1) 15 (luteal adj5 dysphor$).tw. (0) 16 (pmt or llpd$).tw. (20) 17 premenstrual.tw. (145) 18 pre‐menstrual.tw. (9) 19 or/10‐18 (185) 20 9 and 19 (45) 21 from 20 keep 1‐45 (45)
Appendix 4. EMBASE
1 exp chinese medicine/ or exp herbal medicine/ or exp oriental medicine/ or exp tibetan medicine/ (14672) 2 exp Phytotherapy/ (3716) 3 exp alternative medicine/ (12096) 4 herb$.tw. (27873) 5 (chinese adj5 medicine$).tw. (6058) 6 plant$.tw. (127358) 7 botanical.tw. (1523) 8 (traditional adj5 medicine$).tw. (7549) 9 or/1‐8 (173861) 10 exp Premenstrual Dysphoric Disorder/ (411) 11 exp Premenstrual Syndrome/ (2702) 12 Premenstrual Dysphoric Disorder$.tw. (407) 13 (PMDD or PMS).tw. (2324) 14 late luteal.tw. (919) 15 (luteal adj5 dysphor$).tw. (90) 16 (pmt or llpd$).tw. (677) 17 premenstrual.tw. (2733) 18 pre‐menstrual.tw. (99) 19 or/10‐18 (6576) 20 9 and 19 (191) 21 Controlled study/ or randomised controlled trial/ (2692903) 22 double blind procedure/ (69337) 23 single blind procedure/ (7559) 24 crossover procedure/ (20294) 25 drug comparison/ (81252) 26 placebo/ (113542) 27 random$.ti,ab,hw,tn,mf. (408483) 28 latin square.ti,ab,hw,tn,mf. (1102) 29 crossover.ti,ab,hw,tn,mf. (35014) 30 cross‐over.ti,ab,hw,tn,mf. (11844) 31 placebo$.ti,ab,hw,tn,mf. (163643) 32 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (114046) 33 (comparative adj5 trial$).ti,ab,hw,tn,mf. (15034) 34 (clinical adj5 trial$).ti,ab,hw,tn,mf. (566821) 35 or/21‐34 (3194701) 36 nonhuman/ (3067942) 37 animal/ not (human/ and animal/) (14468) 38 or/36‐37 (3071635) 39 35 not 38 (1885193) 40 20 and 39 (83) 41 from 40 keep 1‐83 (83)
Data and analyses
Comparison 1. Jinqianping granule versus Xiaoyaowan tablet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 No improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.

Comparison 1 Jinqianping granule versus Xiaoyaowan tablet, Outcome 1 Recovery.
1.2. Analysis.

Comparison 1 Jinqianping granule versus Xiaoyaowan tablet, Outcome 2 No improvement.
Comparison 2. Cipher decoction versus vitamin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 48.99 [3.06, 783.99] |
| 2 No improvement | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.13] |
2.1. Analysis.

Comparison 2 Cipher decoction versus vitamin B, Outcome 1 Recovery.
2.2. Analysis.

Comparison 2 Cipher decoction versus vitamin B, Outcome 2 No improvement.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Qiao 2002.
| Methods | Multicentre (6 hospitals) parallel design, double simulation. "Randomly allocated" was mentioned but no details about the method in original article. We telephoned the author: a random number table was used to generate the allocation sequence. Double blinding was used. | |
| Participants | Women with premenstrual syndrome (PMS), with "Gan Qi" invasion. Diagnosed as having TCM signs of 'premenstrual tension syndrome', aged 18 to 40 years. 403 cases were divided into the intervention group (n=202), a control group (n=101), and an open treatment group (n=100). Status of disease for the treatment groups: light (22), moderate (133), severe (147). | |
| Interventions | Jingqianping granule was used in the intervention group, oral 15 g tid, and xiaoyaowan placebo 9g bid; for two menstrual cycles. Xiaoyaowan for the control group, oral 9 g bid and Jingqianping granule placebo 15 g oral tid; for two menstrual cycles. | |
| Outcomes | Observations for symptoms, menstrual cycle, BBT, body weight, endocrine.
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No mention of the randomisation method. |
| Allocation concealment? | Low risk | The allocation sequence was generated by a statistician, and the sequences (four copies) were put in optic envelopes. |
| Blinding? All outcomes | Low risk | Double placebo technique was used. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not mentioned. |
| Free of selective reporting? | Unclear risk | Not mentioned. |
| Free of other bias? | High risk | A potential conflict of interest existed because the Jingqianping granule was made by the authors' university. |
Zhu 2003.
| Methods | Parallel design. The published article described that a random numbers table was used to generate the allocation sequence. The participants were blinded to the drugs. | |
| Participants | 246 women with premenstrual tension syndrome, according to the diagnostic criteria: Le Jie, Gynecology and Obstetrics, 4th edition, Beijing: People's Medical Publishing House, 1997:341. 166 women in intervention group, aged 16 to 42 years; 80 women in control group, aged 16 to 40 years. | |
| Interventions | Cipher Fang decoction was used in the intervention group, oral 20g tid, from the day BBT increased in the menstrual cycle for 12 days; repeated in the next menstrual cycle. Or, before menstruation oral 20g tid for 12 days; total of 3 menstrual cycles. Vitamin B6 capsule was given in the control group, oral 1 pill tid. The usage same as intervention. | |
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description about the method of randomisation. |
| Allocation concealment? | High risk | No description about allocation. |
| Blinding? All outcomes | High risk | No description. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not mentioned. |
| Free of selective reporting? | Unclear risk | Not mentioned. |
| Free of other bias? | Unclear risk | Potential conflict of interest existed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Li 2003 | Claimed as RCT. We telephone interviewed the original author and learned that the method of allocation was according to the order of patients coming to hospital. |
| Pei 2005 | Claimed as RCT. We telephone interviewed the original author and learned that the method of allocation was according to the order of patients coming to hospital. |
| Sun 2003 | Claimed as RCT. We telephone interviewed the original author and learned that the method of allocation was according to the order of patients coming to hospital. |
| Wang 1997 | Claimed as RCT. We telephone interviewed the original author and she said she had forgotten the method. |
| Wang 2004 | Claimed as RCT. We telephone interviewed the original author and learned that the method of allocation was according to the order of patients coming to hospital. |
| Wei 2006 | Hormone levels were used as outcomes in the study, which have very little relationship to PMS. |
| Yin 1999 | Claimed as RCT. We telephone interviewed the original author and learned that the allocation method was performed optionally. |
| Yue 2005 | Claimed as RCT. We telephoned the original author and learned that the patients were allocated according to the order of patients came to hospital. |
| Zhang 2000 | We telephoned the original author and learned that the patients were allocated by authors optionally. |
| Zhang 2003 | Claimed as RCT. We telephoned the original author and learned that this actually was a retrospective paper for a summary of the author's clinical experience. |
Characteristics of studies awaiting assessment [ordered by study ID]
Feng 1996.
| Methods | "Randomly allocated patients to two groups" mentioned. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The original author cannot be contacted by telephone for confirmation of the randomisation method. |
Guo 2004.
| Methods | "Randomly allocated patients to two groups" mentioned. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The original author cannot be contacted by telephone for confirmation of the randomisation method. |
Li 2002.
| Methods | "Randomly allocated patients to two groups" mentioned. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The original author cannot be contacted by telephone for confirmation of the randomisation method. |
Xie 1994.
| Methods | "Randomly allocated patients to two groups" mentioned. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The original author cannot be contacted by telephone for confirmation of the randomisation method. |
Yuan 2004.
| Methods | "Randomly allocated patients to two groups" mentioned. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The original author cannot be contacted by telephone for confirmation of the randomisation method. |
Differences between protocol and review
1. The outcomes measures were revised.
2. The standard of 'assessment of quality of included studies' had changed, given in the new version of the Cochrane Handbook of Systematic Reviews of Interventions 5.0.0.
Contributions of authors
Xiaoyan Chen, Taixiang Wu, and Khaled MK Ismail contributed to developing the protocol. Zheng Jing, Xunzhe Yang, Taixiang Wu, and Khaled MK Ismail contributed to developing the review.
Sources of support
Internal sources
Chinese Cochrane Center, West China Hospital of Sichuan University, China.
External sources
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Qiao 2002 {published data only}
- Qiao MQ, Zhang HY, Jiang K, Fu YF, Wang JY, Dai SZ, et al. A multicenter, random, double‐blind and double simulation controlled trial for efficacy and safety of Jingqianping granule in treatment of 403 cases of the liver˜qi invasion of premenstrual syndrome. Zhong Guo Xin Yao Za Zhi [Chinese Journal of New Drugs] 2002;11(5):389‐91. [Google Scholar]
Zhu 2003 {published data only}
- Zhu M, Xu X. Clinical study of Shugan anshen in the treatment of premenstrual tension syndrome. Zhong Guo Yi Yao Xue Bao [Chinese Journal of Medicine] 2006;18(5):286‐8. [Google Scholar]
References to studies excluded from this review
Li 2003 {published data only}
- Li ZP, Zhang Y, Tang Q. Analysis of Jingqianshuye in treating 50 cases with premenstrual syndrome. Zhong Yi Yao Xue Kan [Journal of Traditional Chinese Medicine] 2003;21(10):1749‐50. [Google Scholar]
Pei 2005 {published data only}
- Pei RL, Jiang LH. Clinical observation on Jingqianping granule in treating 168 cases with premenstrual syndrome. Zhong Guo Ji Ceng Yi Yao [Chinese Journal of Primary Medicine and Pharmacy] 2005;12(11):1631‐2. [Google Scholar]
Sun 2003 {published data only}
- Sun HL. Traditional Chinese Medicine in the treatment of 48 cases with premenstrual migraine. Xin Zhong Yi [New Journal of Traditional Chinese Medicine] 2003;35(2):52‐3. [Google Scholar]
Wang 1997 {published data only}
- Wang PY. Chinese and western medicine in treating 26 cases with premenstrual tension syndrome. Fujian Yi Yao Za Zhi [Fujian Medical Journal] 1997;19(5):83‐4. [Google Scholar]
Wang 2004 {published data only}
- Wang J, Yin HM. Jingqiankang in treating 120 cases with premenstrual tension syndrome. Shan Dong Zhong Yi Za Zhi [Shandong Journal of Traditional Chinese Medicine] 2004;23(9):524‐5. [Google Scholar]
Wei 2006 {published data only}
- Wei X, Zhang HY, Qiao MQ. The influence on Jingqianping granule on E2 and P peak in serum of PMS with liver`qi invasion. Shan Dong Zhong Yi Yao Da Xue Xue Bao [Journal of ShanDong University of TCM] 2006;30(3):198‐200. [Google Scholar]
Yin 1999 {published data only}
- Yin YY. Treatment of 45 cases of premenstrual tension syndrome with modified Ease powder. Nan Jing Zhong Yi Yao Da Xue Xue Bao [Journal of Nanjing University of Traditional Chinese Medicine] 1999;15(2):84‐6. [Google Scholar]
Yue 2005 {published data only}
- Yue XJ, Chen JL. Clinical study of Xiaoyaosan in the treatment of 300 cases with premenstrual syndrome. Shi Yong Yi Ji Za Zhi [Journal of Practical Medical Techniques] 2005;12(9B):2572‐3. [Google Scholar]
Zhang 2000 {published data only}
- Zhang L, Shen LF. Acupuncture and traditional Chinese medicine in treating 50 cases with premenstrual tension syndrome. Ning Xia Yi Xue Za Zhi [Ningxia Medical Journal] 2000;22(7):436. [Google Scholar]
Zhang 2003 {published data only}
- Zhang JM, Sun XF. Observation of effect of Traditional and Western Medicine in treating 84 cases with premenstrual tension syndrome.. Shan Dong Yi Yao [ShanDong Medical Journal] 2003;43(7):26. [Google Scholar]
References to studies awaiting assessment
Feng 1996 {published data only}
Guo 2004 {published data only}
Li 2002 {published data only}
Xie 1994 {published data only}
Yuan 2004 {published data only}
Additional references
APA 2005
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition: Text Revision (DSMIV‐TR). 4. Washington DC: RR Donnelley & Sons, 2005. [Google Scholar]
Backstrom 2003
- Backstrom T, Andreen L, Birzniece V, Bjorn I, Johansson IM, Nordenstam‐Haghjo M, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs 2003;17(5):325‐42. [DOI] [PubMed] [Google Scholar]
Budeiri 1996
- Budeiri D, Li Wan Po A, Dornan JC. Is evening primrose oil of value in the treatment of premenstrual syndrome?. Controlled Clinical Trials 1996;17:60‐8. [DOI] [PubMed] [Google Scholar]
Dickerson 2003
- Dickerson LM, Mazyck PJ, Hunter MH. Premenstrual syndrome. American Family Physician 2003;67(8):1743‐52. [PubMed] [Google Scholar]
Dimmock 2000
- Dimmock PW, Wyatt K M, Jones PW, O'Brien PM. Efficacy of selective serotonin‐reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet 2000;356:1131‐6. [DOI] [PubMed] [Google Scholar]
Douglas 2002
- Douglas S. Premenstrual syndrome. Evidence‐based treatment in family practice. Canadian Family Physician 2002;48:1789‐97. [PMC free article] [PubMed] [Google Scholar]
Eriksson 1994
- Eriksson E, Alling C, Andersch B, Andersson K, Berggren U. Cerebrospinal fluid levels of monoamine metabolites. A preliminary study of their relation to menstrual cycle phase, sex steroids, and pituitary hormones in healthy women and in women with premenstrual syndrome. Neuropsychopharmacology 1994;11:201‐13. [DOI] [PubMed] [Google Scholar]
Freeman 2003
- Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology 2003;28 Suppl 3:25‐37. [DOI] [PubMed] [Google Scholar]
Girman 2003
- Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. American Journal of Obstetrics and Gynecology 2003;188 Suppl 5:56‐65. [DOI] [PubMed] [Google Scholar]
Gonda 2004
- Gonda X, Bagdy G. Neurochemical background of the premenstrual syndrome: the role of the serotonergic system. Neuropsychopharmacologia Hungarica 2004;6(3):153‐62. [PubMed] [Google Scholar]
Halbreich 2003
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology 2003;28 Suppl 3:55‐99. [DOI] [PubMed] [Google Scholar]
Hardy 2000
- Hardy ML. Herbs of special interest to women. Journal of the American Pharmaceutical Association 2000;40(2):234‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Application of quality assessment criteria. Cochrane Handbook for Systematic Reviews of Interventions 2008.
HLSC 2000
Jang 2004
- Jang HS, Li MS. Effects of qi therapy (external qigong) on premenstrual syndrome: a randomized placebo‐controlled study. Journal of Alternative and Complementary Medicine 2004;10(3):456‐62. [DOI] [PubMed] [Google Scholar]
Joffe 1998
- Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge?. Biological Psychiatry 1998;44:798‐811. [DOI] [PubMed] [Google Scholar]
Jones 2003
- Jones A. Homeopathic treatment for premenstrual symptoms. Journal of Family Planning and Reproductive Health Care 2003;29(1):25‐8. [DOI] [PubMed] [Google Scholar]
Kessel 2000
- Kessel B. Premenstrual syndrome. Advances in diagnosis and treatment. Obstetrics and Gynecology Clinics of North America 2000;27:625‐39. [DOI] [PubMed] [Google Scholar]
Kouri 1998
- Kouri EM, Halbreich U. Hormonal treatments for premenstrual syndrome. Drugs of Today (Barcelona, Spain) 1998;34(7):603‐10. [DOI] [PubMed] [Google Scholar]
Lampert 2002
- Lampert N, Xu Y. Chinese herbal nephropathy. Lancet 2002;359(9308):796‐7. [DOI] [PubMed] [Google Scholar]
Lang 1996
- Lang XY. Chinese traditional medicine in the treatment of premenstrual nerves syndrome. Yunna Journal of TCM 1996;17(5):9. [Google Scholar]
Lord 2001
- Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Urothelial malignant disease and Chinese herbal nephropathy. Lancet 2001;358(9292):1515‐6. [DOI] [PubMed] [Google Scholar]
Masho 2005
- Masho SW, Adera T, South‐Paul J. Obesity as a risk factor for premenstrual syndrome. Journal of Psychosomatic Obstetrics and Gynaecology 2005;26(1):33‐9. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Moline 2000
- Moline ML, Zendell SM. Evaluating and managing premenstrual syndrome. Medscape Women's Health 2000;5(2):1‐16. [PubMed] [Google Scholar]
Nagata 2004
- Nagata C, Hirokawa K, Shimizu N, Shimizu H. Soy, fat and other dietary factors in relation to premenstrual symptoms in Japanese women. BJOG: British Journal of Obstetrics and Gynaecology 2004;111(6):594‐9. [DOI] [PubMed] [Google Scholar]
Nortier 2000
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). New England Journal of Medicine 2000;342(23):1686‐92. [DOI] [PubMed] [Google Scholar]
O'Brien 2000
- O'Brien PM, Wyatt K, Dimmock PW. Premenstrual syndrome is real and treatable. Practitioner 2000;224(1608):185‐9,191,194‐5. [PubMed] [Google Scholar]
Rapkin 1987
- Rapkin A J, Edelmuth E, Chang LC, Reading A E, McGuire MT, Su T P. Whole‐blood serotonin in premenstrual syndrome. Obstetrics and Gynecology 1987;70:533‐7. [PubMed] [Google Scholar]
Rapkin 2003
- Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology 2003;28 Suppl 3:39‐53. [DOI] [PubMed] [Google Scholar]
Roemheld‐Hamm 2005
- Roemheld‐Hamm B. Chasteberry. American Family Physician 2005;72(5):821‐4. [PubMed] [Google Scholar]
Rubinow 1998
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen‐serotonin interactions: implications for affective regulation. Biological Psychiatry 1998;44:839‐50. [DOI] [PubMed] [Google Scholar]
Steege 1992
- Steege J F, Stout A L, Knight D L, Nemeroff CB. Reduced platelet tritium‐labeled imipramine binding sites in women with premenstrual syndrome. American Journal of Obstetrics and Gynecology 1992;167:168‐72. [DOI] [PubMed] [Google Scholar]
Steege 1993
- Steege JF, Blumenthal JA. The effects of aerobic exercise on premenstrual symptoms in middle‐aged women: a preliminary study. Journal of Psychosomatic Research 1993;37:127‐33. [DOI] [PubMed] [Google Scholar]
Steiner 2000
- Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: pathophysiology and treatment. Journal of Clinical Psychiatry 2000;61 Suppl 12:17‐21. [PubMed] [Google Scholar]
Steiner 2006
- Steiner M, Pearlstein T, Cohen LS, Endicott J, Kornstein SG, Roberts C. Expert guidelines for the treatment of severe PMS, PMDD, and co‐morbidities: the role of SSRIs. Journal of Women's Health 2006;15(1):57‐69. [DOI] [PubMed] [Google Scholar]
Sundstrom 2003
- Sundstrom Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Archives of Women's Mental Health 2003;6(1):23‐41. [DOI] [PubMed] [Google Scholar]
Taylor 1984
- Taylor DL, Mathew RJ, Ho BT, Weinman M L. Serotonin levels and platelet uptake during premenstrual tension. Neuropsychobiology 1984;12:16‐8. [DOI] [PubMed] [Google Scholar]
Tempel 2001
- Tempel R. PMS in the workplace. An occupational health nurse's guide to premenstrual syndrome. AAOHN Journal 2001;49(2):72‐8. [PubMed] [Google Scholar]
Tesch 2003
- Tesch BJ. Herbs commonly used by women: an evidence‐based review. American Journal of Obstetrics and Gynecology 2003;188 Suppl 5:44‐55. [DOI] [PubMed] [Google Scholar]
Ventskivs'ka 2005
- Ventskivs'ka IB, Senchuk AIa. Role of magnesium in the pathogenesis of premenstrual disorders. Likarska Sprava 2005;8:62‐5. [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. International Statistical Classification of Diseases, 10th Revision. 2nd Edition. Geneva: WHO, 2005. [Google Scholar]
Woods 2005
- Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Archives of General Psychiatry 2005;62:961‐70. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu TX, Liu GJ. The concepts, design, practice and report of allocation concealment and blinding. Chinese Journal of Evidence‐Based Medicine 2007;7(3):219‐22. [Google Scholar]
Wu 2007 CONSORT
- Wu TX, Li YP, Bian ZX, Li TQ, Li J, S Dagenais, D Moher. Consolidated Standards for Reporting Trials of Traditional Chinese Medicine (CONSORT for TCM). Chinese Journal of Evidence‐Base Medicine 2007;7(8):601‐5. [Google Scholar]
Wyatt 2004
- Wyatt KM, Dimmock PW, Ismail KM, Jones PW, O'Brien PM. The effectiveness of GnRHa with and without 'add‐back' therapy in treating premenstrual syndrome: a meta analysis. British Journal of Obstetrics and Gynaecology 2004;111(6):585‐93. [DOI] [PubMed] [Google Scholar]
Yu 2005
- Yu JN, Liu BY, Liu ZS, Robinson V. Evaluation of clinical therapeutic effects and safety of acupuncture treatment for premenstrual syndrome. Zhongguo Zhenjiu 2005;25(6):377‐82. [PubMed] [Google Scholar]
Zhu 1995
- Zhu YF. TCM Bian Zhing Zhi Liao for 289 patients with premenstrual syndrome. TCM Forum 1995;10(2):20. [Google Scholar]


