Abstract
Transposon insertional mutants of Listeria monocytogenes were constructed to identify genes involved in osmotolerance, and one mutant that showed reduced growth under high osmotic pressure was obtained. The cloned gene from the transposon insertion site of the mutant, named rel, was 2,214 bp in length and had very high homology to relA of Bacillus subtilis, which encodes guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) [collectively designated (p)ppGpp] synthetase during stringent response. The mutant showed a deficiency in (p)ppGpp accumulation. In the parental strain, the amount of intracellular (p)ppGpp was not increased after an osmotic upshift but was slightly decreased compared with the level before the upward shift. The reduced osmotolerance of the mutant was restored to a level almost equal to that of the parent strain when the chromosomal region that included rel of L. monocytogenes was introduced into the mutant. After exposure to methyl glucoside, the rel mutant accumulated (p)ppGpp at a higher level than the basal level and partially restored the ability to grow in NaCl-supplemented brain heart infusion broth. The mutant was found to grow in chemically defined minimal medium supplemented with glycine betaine or carnitine, so-called compatible solutes, and 4% NaCl. Our results suggest that the appropriate intracellular concentration of (p)ppGpp is essential for full osmotolerance in L. monocytogenes and that its mechanism is different from that for the accumulation of compatible solutes.
Listeria monocytogenes is the causative agent of listeriosis and is transmitted mainly by food to humans (6). Its characteristics, such as ubiquitous distribution in the environment (33) and strong tolerances to NaCl and refrigeration temperatures (26), make it difficult to prevent food contamination with L. monocytogenes. Recent studies have shown that Listeria accumulates so-called compatible solutes, such as glycine betaine (betaine), carnitine, and proline, under hyperosmotic stress to counteract the outward flow of water (2, 12). Such accumulations have also been observed in a wide range of other organisms, including Bacillus subtilis (3) and Escherichia coli (4). In L. monocytogenes, the betL gene and gbu operon have been identified as betaine transporter-encoding genes (27, 13), and opuC has been identified as a carnitine transporter-encoding operon (7). Disruption of these genes reduces the osmotolerance of L. monocytogenes (7, 13, 27). Both betL (7) and opuC (27) have σB-dependent promoters, and the sigB (the σB-encoding gene) mutant strain also shows a reduced ability to accumulate betaine, resulting in lower osmotolerance than that in the parent strain (1). On the other hand, there are some reports about the influence of disruption of osmotic stress-related genes to virulence of L. monocytogenes; sigB (32) and proBA (proline transporter-encoding gene) (28) mutants showed no differences from the parental strain in rates of recovery from organs of mice. But strains with mutations in opuC (29) and clpC (22), which encodes ClpC ATPase, showed reduced virulence compared to the parental strain. To further analyze the mechanism of osmotolerance, we made transposon insertional mutants of L. monocytogenes to obtain mutants with decreased NaCl resistance and succeeded in obtaining one clone that showed less resistance than the parental strain. We report the identification of the rel gene in L. monocytogenes derived from the mutant, its analysis, and the involvement of (p)ppGpp, which is synthesized by the rel gene product on growth in NaCl-supplemented broth. We also examined whether the rel mutant can use extracellular compatible solutes as osmoprotectants. Finally, we examined the recovery of rel mutants from mice to determine the influence of rel gene disruption on the virulence of L. monocytogenes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are shown in Table 1. L. monocytogenes strains were grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.), and E. coli was cultured in L broth (24). Plasmid pUC18 (Toyobo, Osaka, Japan) was used to clone fragments for sequencing. pMK4 (provided by A. Bubert) (30) is an E. coli-B. subtilis shuttle vector. pMK4Em was a derivative of pMK4 created by the insertion of an approximately 1.8-kb BamHI fragment that contained the erythromycin (EM) resistance genes from pBR322::MudEm at the same site. Where appropriate, antibiotics were used at the following concentrations: ampicillin (AP), 150 μg/ml for E. coli; chloramphenicol (CM), 5 μg/ml for L. monocytogenes and 10 μg/ml for E. coli; EM, 5 μg/ml for L. monocytogenes and 150 μg/ml for E. coli. To examine the efficacy of compatible solutes in L. monocytogenes strains, chemically defined minimal medium (CDM medium) (2) supplemented with 4% NaCl and with 1 mM betaine (Sigma, St. Louis, Mo.), 1 mM dl-carnitine (Sigma), or 10 mM l-proline (Sigma) was used. For in vivo (p)ppGpp measurements, CDM medium was modified as follows: KH2PO4 and Na2HPO4 concentrations were reduced to 1/200 of those in the original medium composition (low-phosphate CDM medium).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source |
|---|---|---|
| Strains | ||
| L. monocytogenes EGD | Laboratory stock | |
| L. monocytogenes ED1 (rel::Tn917 lx) | This study | |
| L. monocytogenes ED1/pMK4Em | This study | |
| L. monocytogenes ED1/pMK4Emrel | This study | |
| E. coli DH5α | Toyobo | |
| E. coli MC1061 | Laboratory stock | |
| Plasmids | ||
| pDlux917 | Apr Cmr Emr in E. coli and Cmr Emr in gram-positive bacteria | Shih-Tung Liu |
| pMK4 | Apr Cmr in E. coli and Cmr in gram-positive bacteria | A. Bubert (28) |
| pUC19 | Apr | Takara |
| pMK4Em | Apr Cmr Emr in E. coli and Cmr Emr in gram-positive bacteria; constructed with pMK4 BamHI digest and Emr fragment from pBR322::MudEm BamHI digest | This study |
Transposon mutagenesis.
Transposon Tn917 lx, a derivative of Tn917ac1 (5) which contains luxA-luxB genes for generating transcriptional fusion and ColE1ori for gene cloning, was used for transposon mutagenesis. For transposon mutagenesis, the temperature-sensitive delivery vector pDlux917, provided by Shih-Tung Liu (Chang-Gung Medical College, Kwei-Shan, Taoyuan, Taiwan), which carried Tn917 lx was introduced into L. monocytogenes strain EDG by electroporation with a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.), according to the method described by Park and Stewart (19). A single colony of a strain harboring the donor plasmid pDlux917 was used to inoculate medium containing CM (5 μg/ml) and a reduced concentration (0.15 μg/ml) of EM to keep the thermosensitive plasmid, and the inoculated bacterium was incubated at 25°C. For the induction of transposition, cells were cultured at 42°C overnight in 5 ml of BHI medium containing 0.15 μg of EM per ml. To cure the episomal form of pDlux917, cells were subsequently incubated at 42°C overnight. Cultures were then plated on BHI agar containing CM at 37°C overnight. CM-resistant (Cmr) and EM-sensitive (Ems) colonies were isolated from each colony and purified once on the same selective plates. These colonies were streaked on BHI agar plates supplemented with 6% NaCl. Mutants whose growth was markedly reduced, as assessed by colony diameter, only on the NaCl-supplemented plates but not on normal BHI agar plates were selected. Mutants that had the single transposon in their chromosome were selected by Southern hybridization with pDlux917 as the probe and a DNA labeling kit and a digoxigenin luminescent detection kit (Boehringer Mannheim, Mannheim, Germany).
Cloning of Tn917 lx-flanking regions from Tn917 lx mutants.
Tn917 lx has ColE1ori, the replication origin in E. coli, and an AP resistance gene. There are no EcoRI, BamHI, or PvuII sites in the region of ColE1ori and the AP resistance gene in Tn917 lx. Thus, chromosomal DNAs from NaCl-sensitive mutants were digested with either EcoRI, BamHI, or PvuII and self-ligated. The resulted plasmids were transformed into E. coli strain DH5α (Toyobo) and selected with AP resistance. After purification of the plasmids, the flanking region was subcloned into plasmid pUC18 and then sequenced.
DNA sequence and database screen.
Purified plasmid templates created using a Qiagen plasmid mini-kit (Qiagen, Valencia, Calif.) were used in cycle-sequencing reactions with a Thermo Sequenase fluorescent labeling kit (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom) and fluorescein isothiocyanate-labeled M13 forward and reverse primers (Shimadzu, Kyoto, Japan) according to the manufacturer's instructions. All sequence samples were run on a DSQ-2000L sequencer (Shimadzu). Open reading frames (ORFs) were initially identified with DNASIS software (Hitachi, Tokyo, Japan). For subsequent analysis, each ORF was compared to the current nonredundant protein database of the GenomeNet Database Service (http://www.genome.ad.jp.) by using BLAST and FASTA software through the Internet.
PCR and cloning of the rel region from L. monocytogenes.
To amplify the intact gene of rel, we designed a pair of primers, rel-A1 (5′-ATGGAGATAAGAGGGTGAA-3′) and rel-B1 (5′-AATCAGTAACAGACGGCCT-3′), based on the sequencing results. For PCR, TaKaRa Ex Taq DNA polymerase (Takara Shuzo, Otsu, Japan) was used. The PCR product from the chromosomal region of L. monocytogenes including rel and ORF1 was cloned into SmaI-digested pMK4Em shuttle vector in E. coli strain MC1061 and introduced into the rel mutant, named ED1, by electroporation. Transformants were selected for EM resistance, and their genotype was confirmed by PCR with primers rel-A1 and rel-B2 (5′-TCTCAACTAAATCTCGGCC-3′) and TaKaRa LA Taq DNA polymerase (Takara Shuzo). ED1/pMK4Em was also constructed.
Detection of in vivo (p)ppGpp synthesis in L. monocytogenes.
The (p)ppGpp accumulation patterns in L. monocytogenes were assessed by the modified method of stringent response in B. subtilis (31). Overnight cultures in BHI were washed twice with an equal volume of low-phosphate CDM medium and resuspended in the same medium, following incubation for 1 h at 37°C with vigorous agitation. H332PO4 (150 mCi/ml; Amersham Pharmacia Biotech) was added to the bacterial suspension, which was adjusted at a fixed optical density (OD) and incubated at 37°C for 2 h. After labeling with isotope, an equal volume (100 μl) of bacterial suspension was used for the following experiment. For the stringent response, serine hydroxamate (Sigma), a serine metabolic inhibitor, was added at a 1.5-mg/ml final concentration. For osmotic upshift and carbon source starvation, NaCl and α-methyl-d-(+)-glucoside (Wako, Osaka, Japan), glucose metabolic inhibitor, was added at 8 and 1% (wt/vol) final concentrations, respectively. After incubation at 37°C for 30 min, stressed cells were washed twice with low-phosphate CDM medium and resuspended in 50 μl of the same medium. Labeled bacterial cell suspensions were then mixed with an equal volume of 13 M formic acid. Samples were subjected to three cycles of freezing and thawing and centrifuged at 7,500 × g for 5 min. Supernatants (10 μl) were spotted on polyethyleneimine-cellulose plates F (Merck, Darmstadt, Germany) and air dried. Thin-layer chromatography was performed in 1.5 M non-pH-adjusted KH2PO4. Relative amounts of (p)ppGpp were calculated with a GT8700 scanner (Epson, Suwa, Japan) and NIH image software (http://rsb.info.nih.gov/nih-image/).
Growth in BHI under high osmotic pressure.
To assess the osmotic sensitivity of the wild-type and mutant strains of L. monocytogenes, aliquots of overnight cultures (100 μl) were added to 10 ml of BHI containing 7.5% NaCl or 10 ml of BHI without NaCl. Cultures were incubated at 37°C with agitation, and growth was monitored by measurement of OD at 660 nm (OD660) with a mini-photo OD meter (Taitec, Koshigaya, Japan) for the intervals indicated in the figure legends. Similar assays were performed in BHI supplemented with 8% KCl or 42% sucrose. To investigate the effect of accumulated (p)ppGpp on the growth in BHI supplemented with NaCl, the rel mutant was grown in BHI with 3.5% NaCl after exposure to methyl glucoside. After washing and resuspension in CDM medium, bacterial cells were incubated with 1% (wt/vol) methyl glucoside for 30 min and inoculated in BHI with and without 3.5% NaCl at 37°C, followed by measurement of OD660 every 1 h.
Growth in CDM medium supplemented with 4% NaCl and compatible solutes.
To examine the efficacy of compatible solutes in L. monocytogenes strains growing under hyperosmotic conditions, the OD660 values of the parental strain and the rel mutant cultured in CDM medium were measured. Bacteria were grown in BHI for 24 h at 37°C with agitation, washed twice with CDM medium to remove BHI broth, and then inoculated in CDM medium, CDM medium supplemented with 4% NaCl, or CDM medium with 4% NaCl that was supplemented either with 1 mM glycine betaine (Sigma) or 1 mM dl-carnitine (Sigma). The cultures were incubated with shaking at 37°C, and growth was monitored by measurement of OD660 as described above. The concentration of NaCl was reduced to 4% in this medium, while the BHI medium was supplemented with 7.5% NaCl, since the growth of Listeria in CDM medium supplemented with NaCl at concentrations above 5% is extremely limited even when compatible solutes are added (2).
Mouse virulence assay.
Seven-week-old female BALB/c mice (Charles River, Kanagawa, Japan) were used for all infection experiments and housed at the National Institute of Public Health according to Institutional Animal Committee guidelines. The experiments were reviewed and approved by the Institutional Animal Committee. Approximately 2 × 108 Listeria cells were suspended in 0.1 ml of saline and intragastrically inoculated into five mice. The animals were sacrificed at day 3 after infection, and numbers of CFU in the spleen and liver were determined on Luria-Bertani agar plates (24). The mean values and standard deviations were calculated with StatView software (Abacus Concepts, Berkeley, Calif.). P values of <0.05 were considered to indicate statistical significance.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper are registered in the DDBJ/EMBL/GenBank nucleotide sequence database under accession no. AB051847.
RESULTS
Isolation of the osmotolerance-deficient mutant of L. monocytogenes by random Tn917 lx insertion into the chromosome.
A total of 2,000 transposon-insertional mutants were streaked on BHI agar with and without 6% NaCl, resulting in 13 mutants on the BHI agar plates with 6% NaCl that showed reduced growth. However, Southern hybridization using EcoRI or BamHI-digested chromosomal DNA with a digoxigenin-labeled DNA probe specific for pDlux917 suggested that 12 of these mutants had the transposon in the same fragment of the chromosome (data not shown). The remaining mutant possessed two copies of the transposon in its chromosome. Thus, only one of these 12 mutants, strain ED1, was chosen for further detailed characterization.
Cloning and sequencing analysis of the rel region of L. monocytogenes.
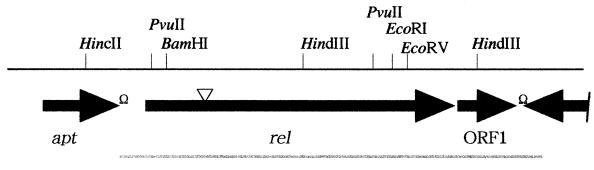
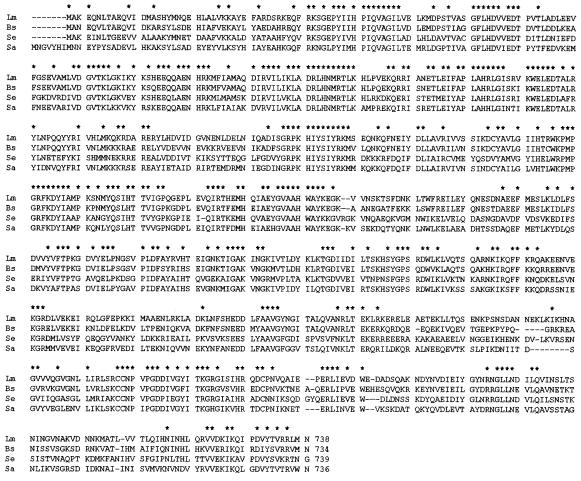
To investigate the cause of reduced osmotolerance, the Tn917 lx-flanking DNA sequence was determined and the insertion site for the mutant was assigned as described in Materials and Methods. A homology search in the DNA database revealed that the mutant possessed Tn917 lx within a gene encoding a protein homologous to the RelA, a ppGpp synthetase, of B. subtilis (31) (GenBank accession no. U68377). To analyze this region in L. monocytogenes, an approximately 4-kb DNA fragment inserted by Tn917 lx from mutant strain ED1 was sequenced, and three ORFs, including a relA homolog and a partial ORF, were identified. The 519-bp coding region of a putative protein sequence 205 bp upstream of the relA homolog and transcribed in the same orientation showed 73.8% identity with adenosine phosphoribosyltransferase from B. subtilis encoded by apt (accession no. U68377). A third ORF, located 15 bp downstream of the relA homolog and oriented similarly, encoded a 150-amino-acid putative protein with 54.7% identity to YrvI from B. subtilis, the function of which is unknown (accession no. U68377). A fourth partial ORF, located downstream of the YrvI homolog and transcribed divergently, encoded a putative protein with 41.4% identity to the C-terminal half of the YrvJ protein (similar to N-acetylmuramoyl-l-alanine amidase) from B. subtilis (accession no. U68377). Based on the significant similarity between these ORFs in L. monocytogenes and B. subtilis genes, and on their conserved order on the chromosome, we designated these ORFs apt, rel, and ORF1 (Fig. 1). To clone the intact L. monocytogenes rel gene, chromosomal DNA from the EGD strain was used as a template for PCR with primers rel-A1 and rel-B1. Nucleotide sequencing of the cloned rel region revealed that a putative ribosome-binding site, GAGGG, was present 9 bp upstream of the rel initiation codon, and the rel ORF of 2,217 bp was predicted to encode a protein of 738 amino acids with a calculated Mr of 84,731. The insertion of the transposon Tn917 lx in ED1 was found to have occurred between A484 and G485 of the rel structural gene, resulting in deletion of the C-terminal 567 amino acid residues of the rel gene product. The deduced amino acid sequence of Rel had a very high degree of similarity to the equivalent gene products of B. subtilis (79.6% identity), Streptococcus equisimilis (17) (58.7% identity; GenBank accession number X72832), and Staphylococcus aureus (8) (63.3% identity; DDBJ accession number D76414) (Fig. 2).
FIG. 1.
Organization of the rel region in L. monocytogenes EGD. Ω, potential transcriptional terminator; ▿, transposon insertional site; solid line, chromosome region sequenced in this study and its restriction sites; black arrows, size and orientation of putative genes; gray line (bottom), chromosomal region amplified and used for complementation.
FIG. 2.
Alignment of the predicted amino acid sequence of L. monocytogenes Rel with RelA of B. subtilis, Rel of S. equisimilis, and RelA of S. aureus. Asterisks indicate amino acids present in all sequences.
The rel mutant showed reduced (p)ppGpp synthetic activity.
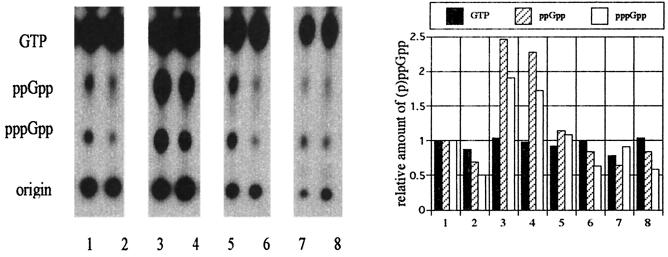
To determine whether the rel homologue was functional in L. monocytogenes, the (p)ppGpp-synthetic activities of the wild-type strain and the Tn917 lx mutant ED1 were measured. The wild type was found to accumulate large amounts of (p)ppGpp in response to amino acid deprivation by treatment with serine hydroxamate (Fig. 3, lane 3). A similar but weaker pattern was observed when L. monocytogenes cells were transferred to medium depleted of glucose as a carbon source by addition of methyl glucoside (Fig. 3, lane 5). In contrast, the mutant strain ED1 could synthesize smaller amounts of (p)ppGpp than the wild-type strain under the conditions of amino acid depletion (Fig. 3, lane 4) and carbon starvation (lane 6), even though the amount of (p)ppGpp was increased in the mutant grown under these starvation conditions.
FIG. 3.
(p)ppGpp accumulation patterns of EGD (odd lanes) and the rel mutant ED1 (even lanes). Lanes: 1 and 2, no stimulation; 3 and 4, after 30 min of incubation with serine hydroxamate (1.5 mg/ml); 5 and 6, after 30 min of incubation with α-methyl-d-(+)-glucoside (1% wt/vol); 7 and 8, after 30 min of incubation with NaCl (final concentration, 8%). The experiments were performed three times with similar results.
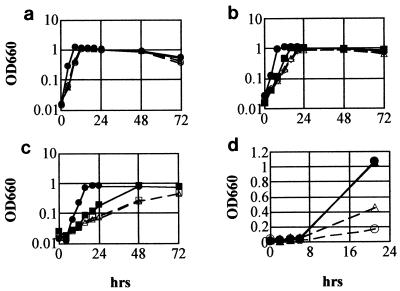
Effect of rel mutation on osmotolerance in L. monocytogenes.
The osmotolerance of the rel mutant cultured in BHI broth was compared with that of the parental strain. The rel mutant and its vector-transformed derivative had reduced growth rates at the exponential phase of growth in BHI with and without 3.5% NaCl (final concentration, 4%), but cell density of the stationary phase of the mutant culture reached a level similar to that of the parental strain and rel-complemented mutant after 24 h (Fig. 4b). But in BHI supplemented with 7.5% NaCl (final concentration, 8%), the rel mutant and its vector-transformed derivative showed limited growth, while the growth of the complemented mutant was partially restored (Fig. 4c). The growth rate of the mutant was much lower than that of the parental strain, and the cell density of the mutant culture did not reach the level of the parental strain even after 72 h. The growth of all strains was maximum at 72 h, and the OD660 of each strains was decreased when the incubation was extended for 96 h (data not shown). Similar results were obtained when these strains grew in BHI supplemented with KCl or sucrose (data not shown). In BHI supplemented with 12% NaCl (final concentration, 12.5%), the OD660 of the rel mutant was about one-seventh of that of the parental strain even at 72 h (data not shown). The rel mutant and its derivatives were grown normally in BHI broth without additional NaCl (Fig. 4a). These results showed that inactivation of rel caused reduction of osmotolerance in this organism. Since the rel mutation was suggested to affect synthesis of (p)ppGpp, we examined the effect of high concentrations of NaCl on intracellular amount of (p)ppGpp and compared the amount of (p)ppGpp in the mutant with that in the parental strain. Both the parental and mutant strains showed slightly reduced or nearly identical levels of (p)ppGpp after osmotic upshift (Fig. 3, lanes 7 and 8). Next, we examined the effect of elevated intracellular (p)ppGpp on the growth of the rel mutant in NaCl-supplemented BHI. When the rel mutant was exposed to methyl glucoside for 30 min, it accumulated a small amount of (p)ppGpp; as a result, the intracellular concentration of (p)ppGpp of the mutant came close to the basal level of the parental strain (Fig. 3, lane 6). In BHI supplemented with 3.5% NaCl, the (p)ppGpp-elevated mutant grew faster than the nonstimulated mutant, whereas no differences in growth were observed between the simulated and nonstimulated mutant in BHI (Fig. 4d).
FIG. 4.
(a to c) Growth of EGD (•), rel mutant ED1 (○), ED1/pMKEm rel (▴), and ED1/pMKEm (▵). Cultures were grown in BHI (a), BHI with 3.5% NaCl (final concentration, 4%) (b), and BHI with 7.5% NaCl (final concentration, 8%) (c) at 37°C. (d) Growth of the rel mutant in BHI after 60 min of incubation in CDM medium (•) or CDM medium with methyl glucoside (1%, wt/vol) (▴) and BHI with 3.5% NaCl after 60 min of incubation in CDM medium (○) or in CDM medium with methyl glucoside (1% wt/vol) (▵). The experiments were performed five times with similar results.
The rel mutant can use compatible solutes as osmoprotectants in CDM medium under 4% NaCl.
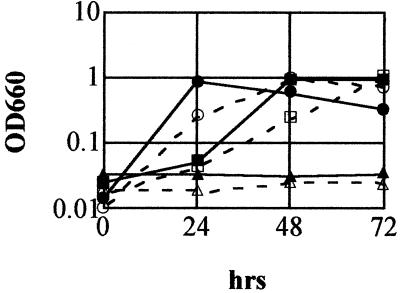
Since L. monocytogenes can use compatible solutes, such as betaine and carnitine, as osmoprotectants (2), we further tested the ability of strain ED1 to grow in NaCl-supplemented CDM medium in the presence of betaine (Fig. 5). Inoculation of overnight cultures of both the mutant and wild-type strains inoculated into fresh CDM medium with a low NaCl concentration showed that bacterial cells were capable of growing and reaching maximum growth after 24 (wild type) or 48 (mutant) h of incubation at 37°C. However, the addition of NaCl to CDM medium at a final concentration of 4% resulted in limited growth of both strains. When the bacterial cells were inoculated into the same medium supplemented with 1 mM betaine, the growth capability of the wild-type strain was restored, although a prolonged growth lag was observed. Similarly, ED1 was able to grow to wild-type levels after 72 h of incubation in the presence of betaine. The osmotolerance of both strains was also enhanced by another osmoprotectant, carnitine (data not shown).
FIG. 5.
Growth of EGD in CDM medium (•), CDM medium with 4% NaCl (▴), or CDM medium with 4% NaCl and 1 mM betaine (▪) and ED1 in CDM medium (○), CDM medium with 4% NaCl (▵), or CDM medium with 4% NaCl and 1 mM betaine (□) at 37°C. The experiments were performed three times with similar results.
The rel mutant has a level of virulence similar to that of the parental strain.
The virulence phenotype in the rel mutant strain was assessed by means of a mouse infection model. At 3 days postinoculation, the log10 CFU of strains EGD and ED1 recovered from livers were 2.989 ± 0.419 and 3.005 ± 0.496 (means ± standard deviations), respectively, and the log10 CFU recovered from spleens were 3.781 ± 0.470 and 2.795 ± 0.883, respectively. There was no significant difference in the accumulation of bacterial cells in the spleen or liver between the parental strain and rel mutant under the conditions used in this study, showing that mutation of the rel gene does not affect virulence in mouse infection. The experiments were performed twice, with similar results.
DISCUSSION
In this study, we identified rel of L. monocytogenes EGD as a gene involved in osmotolerance. rel shared very high homology to its homologues in B. subtilis, S. equisimilis, and S. aureus (Fig. 2). In E. coli, relA encodes the (p)ppGpp synthetase (18) and spoT encodes (p)ppGpp-synthetic and (p)ppGpp-degradative enzymes (25). But in Streptomyces coelicolor, RelA has bifunctional [(p)ppGpp-synthetic and -degradative] activities (16). Also, in gram-positive organisms, spoT is not identified, and Rel is thought to be such a bifunctional enzyme. Wendrich and Marahiel (31) found highly conserved residues, RKSGEPYI, common to the N-terminal region in RelA in B. subtilis, SpoT in E. coli, and Rel in S. equisimilis but not in RelA in E. coli, meaning that this residue is a putative motif responsible for the (p)ppGpp-degradative activity. We did not examine the (p)ppGpp-degradative activity of Rel in L. monocytogenes, but we also found the same motif at its N terminus (Fig. 2). Transposon inserted between 162 to 163 amino acids, and the mutant showed reduction of (p)ppGpp accumulation upon amino acid or carbon source starvation or osmotic shift up, but did not have a (p)ppGpp-zero phenotype (Fig. 3). This result suggests that the 162 amino acids from the N-terminal end of Rel in L. monocytogenes have weak (p)ppGpp synthetic activity.
In many organisms, (p)ppGpp is known as a nutritional alarmone and is accumulated in bacterial cells under nutrient-limited conditions, such as depletion of amino acid or carbon source or aminoacylated tRNA. (p)ppGpp is thought to be a stress response-related factor, because its accumulation induces inhibition of stable RNA synthesis, and the so-called stringent response occurs (4). For example, (p)ppGpp is the positive regulator of rpoS, which encodes the stress-responsive RNA polymerase subunit σS in E. coli (9, 15), and both induces the synthesis of proteins necessary for near-UV (300 to 400 nm) resistance in Salmonella enterica serovar Typhimurium (14) and increases acid tolerance in Lactococcus lactis (21). In addition, ppGpp accumulates in B. subtilis after an osmotic upshift (11), in contrast with our results in L. monocytogenes. In E. coli, mutation of relA affects the salt-induced acid sensitivity (23) and the suppression of temperature sensitivity by salt in the ftsZ (an essential gene for cell division) mutant (20).
In this study, we detected the intracellular (p)ppGpp of the L. monocytogenes rel mutant and its parent during stringent response and obtained the following results. (i) Like other organisms, L. monocytogenes accumulates (p)ppGpp under amino acid depletion induced by addition of the serine metabolic inhibitor serine hydroxamate and under carbon source depletion induced by addition of the glucose metabolic inhibitor methyl glucoside (Fig. 3, lanes 3 and 5). (ii) The amount of intracellular (p)ppGpp was reduced after an osmotic upshift (Fig. 3, lane 7). (iii) In the rel mutant, production of (p)ppGpp was reduced to less than that of the parental strain and became still lower after an osmotic downshift (Fig. 3, lanes 5 and 6). (iv) The growth of the rel mutant in the presence of a high concentration of NaCl became faster when the amount of intracellular (p)ppGpp was brought near the basal level of the parental strain by the addition of methyl glucoside (Fig. 4d). (v) The mutant showed partial restoration of osmotolerance by reinduction of the chromosomal region including rel (Fig. 4). From these results, we concluded that (p)ppGpp is involved in the growth of L. monocytogenes under high osmotic pressure and that the appropriate intracellular concentration of (p)ppGpp might also be essential to respond to osmotic stress.
The accumulation of compatible solutes to maintain cell turgor is well characterized as a bacterial osmoadaptation system (4). Disruption of the genes which encode compatible solute transporters and σB, which is related to promoters of these transporter genes, causes decrease of growth under high osmotic pressure (1, 7, 13). We showed that the rel mutant could grow at a reduced rate, but nonetheless, it reached a level nearly identical to that of the parental strain in CDM medium supplemented with NaCl and compatible solutes (Fig. 5), meaning that the rel mutant could use extracellular compatible solutes in 4% NaCl and that the intracellular accumulation of (p)ppGpp might be controlled by mechanisms distinct from compatible solute accumulation.
In this study, the rel mutant of L. monocytogenes was pathogenic to mice at the same level as its parent (see Results), demonstrating that the amount of (p)ppGpp accumulated via the rel gene is not involved in virulence, although the intracellular accumulation of ppGpp initiates the conversion from replicative to virulent forms of Legionella pneumophila (10).
Acknowledgments
This study was supported in part by grants from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Becker, L. A., M. H. Cetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beumer, R. R., M. C. Te Giffel, L. J. Cox, F. M. Rombouts, and T. Abee. 1994. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl. Environ. Microbiol. 60:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cashel. M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 5.Chang, L. K., C. L. Chen, Y. S. Chang, J. S. Tschen, Y. M. Chen, and S. T. Liu. 1994. Construction of Tn917ac1, a transposon useful for mutagenesis and cloning of Bacillus subtilis genes. Gene 150:129-134. [DOI] [PubMed] [Google Scholar]
- 6.Donelly, C. W. 2001. Listeria monocytogenes: a continuing challenge. Nutr. Rev. 59:183-194. [DOI] [PubMed] [Google Scholar]
- 7.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimura, T., and K. Murakami. 1997. Increase of methicillin resistance in Staphylococcus aureus caused by deletion of a gene whose product is homologous to lytic enzymes. J. Bacteriol. 179:6294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary-phase sigma factor ss is positively regulated by ppGpp. J. Bacteriol. 175:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 11.Hecker, M., U. Volker, and C. Heim. 1989. RelA-independent (p)ppGpp accumulation and heat shock protein induction after salt stress in Bacillus subtilis. FEMS Microbiol. Lett. 58:125-128. [Google Scholar]
- 12.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer, G. F., J. C. Baker, and B. N. Ames. 1988. Near-UV stress in Salmonella typhimurium: 4-thiouridine in tRNA, ppGpp, and ApppGpp as components of an adaptive response. J. Bacteriol. 170:2344-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Costa, O. H., M. A. Fernandez-Moreno, and F. Malpartida. 1998. The relA/spoT-homologous gene in Streptomyces coelicolor encodes both ribosome-dependent (p)ppGpp-synthesizing and -degrading activities. J. Bacteriol. 180:4123-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechold, U., K. Steiner, S. Vettermann, and H. Malke. 1993. Genetic organization of the streptokinase region of the Streptococcus equisimilis H46A chromosome. Mol. Gen. Genet. 241:129-140. [DOI] [PubMed] [Google Scholar]
- 18.Metzger, S., I. B. Dror, E. Aizenman, G. Schreiber, M. Toone, J. D. Friesen, M. Cashel, and G. Glaser. 1988. The nucleotide sequence and characterization of the relA gene of Escherichia coli. J. Biol. Chem. 263:15699-15704. [PubMed] [Google Scholar]
- 19.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 20.Powell, B. S., and D. L. Court. 1998. Control of ftsZ expression, cell division, and glutamine metabolism in Luria-Bertani medium by the alarmone ppGpp in Escherichia coli. J. Bacteriol. 180:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517-528. [DOI] [PubMed] [Google Scholar]
- 22.Rouquette, C., C. de Chastellier, S. Nair, and P. Berche. 1998. The ClpC ATPase of Listeria monocytogenes is a general stress protein required for virulence and promoting early bacterial escape from the phagosome of macrophage. Mol. Microbiol. 27:1235-1245. [DOI] [PubMed] [Google Scholar]
- 23.Rowbury, R. J., M. Goodson, Z. Lazim, and T. J. Humphrey. 1996. Sensitization to acid induced by sodium ions in Escherichia coli: dependence of (p)ppGpp and cAMP and suppression of the relA-associated defect by mutations in envZ. Microbios 85:161-177. [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Sarubbi, E., K. E. Rudd, H. Xiao, K. Ikehara, M. Kalman, and M. Cashel. 1989. Characterization of the spoT gene of Escherichia coli. J. Biol. Chem. 264:15074-15082. [PubMed] [Google Scholar]
- 26.Seeliger, H. P. R., and D. Jones. 1986. Listeria, p. 1235-1245. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams and Wilkins Co., Baltimore, Md. [Google Scholar]
- 27.Sleator, R. D., C. G. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2001. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl. Environ. Microbiol. 67:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sleator, R. D., J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21-26. [DOI] [PubMed] [Google Scholar]
- 31.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65-79. [DOI] [PubMed] [Google Scholar]
- 32.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida, T., T. Sugimoto, M. Sato, and K. Hirai. 2000. Incidence of Listeria monocytogenes in wild animals in Japan. J. Vet. Med. Sci. 62:673-675. [DOI] [PubMed] [Google Scholar]