Abstract
The genus Colletotrichum is widely known for its phytopathological significance, especially as the causative agent of anthracnose in diverse agricultural crops. However, recent studies have unveiled its ecological versatility and biotechnological potential, particularly among endophytic species. These fungi, which asymptomatically colonize plant tissues, stand out as high-yielding producers of bioactive secondary metabolites. Given their scientific and economic relevance, this review critically examines endophytic Colletotrichum species, focusing on the chemical diversity and biological activities of the metabolites they produce, including antibacterial, antifungal, and cytotoxic activity against cancer cells, and antioxidant properties. This integrative review was conducted through a structured search of scientific databases, from which 39 relevant studies were selected, highlighting the chemical and functional diversity of these compounds. The analyzed literature emphasizes their potential applications in pharmaceutical, agricultural, and industrial sectors. Collectively, these findings reinforce the promising biotechnological potential of Colletotrichum endophytes not only as sources of bioactive metabolites but also as agents involved in ecological regulation, plant health promotion, and sustainable production systems.
Keywords: Colletotrichum, endophytes, secondary metabolites, biological activity, biotechnology
1. Introduction
Fungal endophytes are increasingly recognized for their abilities to produce a wide range of secondary metabolites with ecological and biotechnological significance. Among them, the genus Colletotrichum has gained attention due to its dual roles as a phytopathogen and as a symbiotic endophyte capable of synthesizing bioactive compounds, including polyketides, terpenoids, alkaloids, and sterols like ergosterol and β-sitosterol [1].
Fungal endophytes were first described by August Carl Joseph Corda in 1831 and formally published in 1837. Colletotrichum is ranked among the top ten most agriculturally and ecologically significant phytopathogenic fungal genera [2,3]. These genera belong to the phylum Ascomycota, class Sordariomycetes, order Glomerellales, and family Glomerellaceae, with the sexual morph named as Glomerella cingulata [4,5,6]. They are globally distributed and exhibit a striking diversity of lifestyles, including necrotrophic, hemibiotrophic, endophytic, latent, and saprophytic forms [2,7,8,9].
Colletotrichum species are widely known as etiological agents of anthracnose—a disease producing sunken necrotic lesions on leaves, stems, flowers, fruits, and roots [7,10,11,12]. This disease may occur both pre- and post-harvest, with especially damaging effects during fruit production. The economic burden of Colletotrichum infections is substantial, with losses reported across tropical, subtropical, and temperate crops, including cereals, legumes, fruit trees, vegetables, ornamentals, and grasses [7,10,13,14,15].
The genus was estimated to comprise hundreds of species associated with more than 2200 plant hosts. Some, like C. orbiculare, are highly host-specific (infecting cucurbits), whereas others, such as C. gloeosporioides, are generalists infecting over 470 hosts. Their geographical distribution varies: C. kahawae is regionally restricted, while C. acutatum and C. simmondsii are more common in Oceania, and C. godetiae predominates in Europe [16,17].
Although predominantly recognized as pathogens, many Colletotrichum species have been isolated as symptomless endophytes, especially in tropical regions. This dual behavior underscores the ecological complexity of the genus, as some species act as destructive pathogens, while others confer benefits to their host plants. Such functional plasticity presents both opportunities and challenges for biotechnological exploitation. Recent advances in omics-based tools, particularly genomics and metabolomics, have proven essential for deciphering the genetic and metabolic basis of this variability, enabling a more accurate discrimination between pathogenic and beneficial strains. Accurate strain selection is crucial for distinguishing beneficial from pathogenic lifestyles and preventing harm to non-target organisms or ecosystems [9,18,19,20,21].
Their ecological plasticities allow the same fungal isolate to behave as a mutualist or a parasite depending on host genotype and environmental conditions [22,23]. Under certain conditions, these fungi may promote plant growth, enhance drought tolerance, and increase resistance to biotic and abiotic stresses [24,25,26]. These benefits are often mediated by the production of indole-3-acetic acid (IAA), antifungal metabolites, and lytic enzymes and the induction of systemic resistance, supporting their role as effective biocontrol agents. Altogether, these attributes highlight the potential of endophytic Colletotrichum as sustainable alternatives to synthetic pesticides and fertilizers [21,27].
Among the most promising traits of endophytic Colletotrichum species is the production of bioactive secondary metabolites. Numerous studies have reported antifungal, antibacterial, phytotoxic, and cytotoxic compounds, highlighting their biotechnological values [13,20,28]. Notable compounds include colletotrichins A–C, colletotric acid, bisabolanoic acid A, and coletonic acid, with reported activities against pathogens like Staphylococcus aureus, Klebsiella pneumoniae, and Cladosporium spp. [29,30,31]. Additionally, the C. dematium isolate has shown activity against Shigella flexneri, S. boydii, Salmonella enteritidis, S. paratyphi, and Pseudomonas aeruginosa [25].
Beyond their pharmacological relevance, Colletotrichum endophytes also play important ecological and biotechnological roles by protecting host plants from pathogens, producing bioactive compounds, and mediating trophic interactions in ecosystems [32,33,34]. Despite their known roles and presence in tropical regions, research on endophytic fungi has focused mainly on temperate zones, leading to a geographical sampling bias. This limits our understanding of the true taxonomic and metabolic diversity of Colletotrichum, especially in biodiversity-rich tropical ecosystems that remain underexplored despite their potential to harbor novel metabolically diverse strains [21,35,36,37].
In this context, Colletotrichum emerges not only as a phytopathogen but also as a model organism for understanding endophytic fungal biology and plant–microbe interactions [20,38,39]. This review focuses on bioactive secondary metabolites produced by endophytic Colletotrichum species, and it is based on 39 selected studies. It highlights their chemical diversities and biosynthetic potential while discussing ecological relevance and prospective applications in agriculture, medicine, and the environment. By compiling evidence from diverse sources, including scientific articles, theses, and dissertations, this work also addresses current limitations and explores future directions to unlock the biotechnological potential of this genus.
2. Materials and Methods
This integrative review aimed to compile and synthesize current knowledge on bioactive secondary metabolites produced by endophytic Colletotrichum species. The strategy was designed to include a broad spectrum of sources relevant to chemical and biotechnological aspects. Literature research was conducted using four scientific databases: Google Scholar, ScienceDirect, PubMed, and SciFinder.
The search terms included combinations of “Colletotrichum”, “endophytic fungi”, “secondary metabolites”, and “bioactive compounds”. No date limits were imposed, allowing the inclusion of both historical and recent studies. Only full-text documents written in English or Portuguese were considered.
The inclusion criteria comprised original research, dissertations, and theses that reported the isolation or identification of biologically active secondary metabolites from Colletotrichum strains with endophytic behavior. These non-peer-reviewed sources were included to provide coverage of relevant data from underexplored regions and to compensate for gaps in the formal literature, particularly in tropical ecosystems. The exclusion criteria involved studies focused solely on the phytopathogenic role of Colletotrichum without reference to its endophytic activity or metabolite production, as well as duplicate records, restricted-access documents, or those unrelated to the research focus.
From an initial pool of 4639 records, 3321 remained after removing duplicates and incomplete or restricted-access materials. All studies were screened manually by the author, and this was carried out based on individual reading of titles, abstracts, and full texts. No automation tools or software were used. Ultimately, 39 documents met all inclusion criteria and were selected for full analysis. The selection process is summarized in Figure 1.
Figure 1.
Flowchart of the article selection process.
The selected studies were organized according to metabolite class, biological activities, and fungal species involved. This manual categorization allowed the identification of patterns and knowledge gaps in the bioprospecting of endophytic Colletotrichum species.
3. Results
A total of 10 unidentified isolates and 13 distinct species of Colletotrichum were reported in biological and chemical studies. These included Colletotrichum sp., C. fragariae, C. gloeosporioides, C. acutatum, C. capsici, C. dematium, C. siamense, C. taiwanense, C. fructicola, C. tropicicola, C. alatae, C. crassipes, C. brevisporum, and C. queenslandicum.
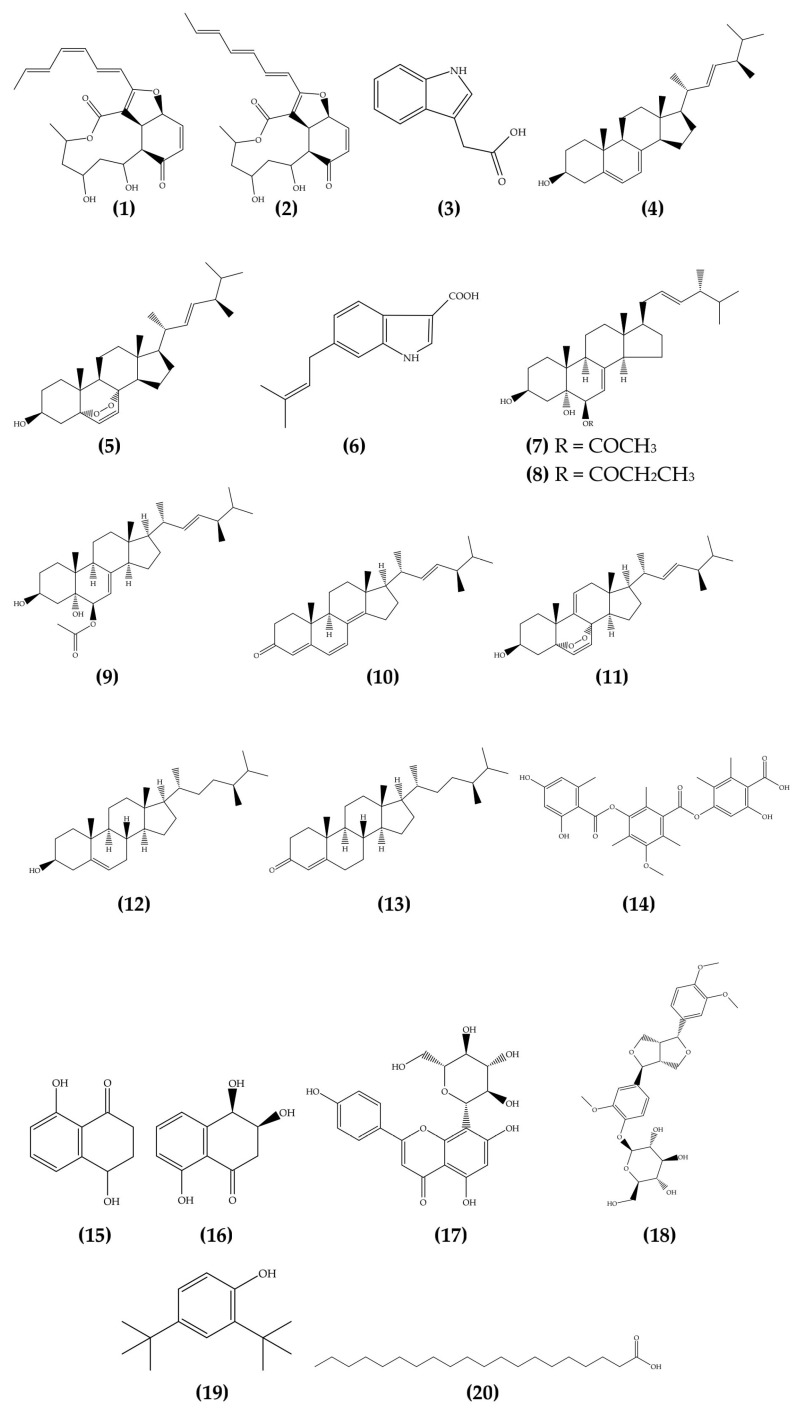
These species were found to produce a wide range of bioactive compounds, including some also known to be synthesized by their host plants. The metabolites belong to diverse chemical classes and exhibit various biological activities, such as antimicrobial, cytotoxic, and antioxidant effects. Table 1 summarizes the endophytic species, host plants, identified metabolites, chemical classes, and associated biological activities. The chemical structures of selected compounds are presented in Figure 2. For clarity and historical context, the references in Table 1 are organized chronologically from older to more recent studies.
Table 1.
Bioactive compounds identified from endophytic Colletotrichum species.
| Endophytic Fungus | Host Plant(s) | Identified Compound(s) | Chemical Class | Biological Activity | References |
|---|---|---|---|---|---|
| C. fragariae | Fragaria spp. | Colletofragarones; A1 (1) e A2 (2) | Polyketides | Self-germination inhibitory activity | [40] |
| Colletotrichum sp. (unresolved taxon); C. gloeosporioides; C. fructicola; C. siamense; C. queenslandicum | Artemisia annua; Piper nigrum; Vincetoxicum hirsutum; Cymbidium aloifolium; Vernonia amygdalina; Morus australis | Indole-3-acetic acid (IAA) * (3) | Indole alkaloid | Plant growth-promoting activity | [29,41,42,43,44,45,46] |
| Colletotrichum sp. (unresolved taxon); C. gloeosporioides; C. queenslandicum | Artemisia annua; Virola Michelii; Uncaria rhynchophylla; Morus australis | Ergosterol * (4) | Sterol | Anti-inflammatory activity; cytotoxic activity | [29,46,47,48] |
| Colletotrichum sp. (unresolved taxon); C. gloeosporioides | Artemisia annua; Virola michelli; Uncaria rhynchophylla | Ergosterol peroxide * (5) | Sterol | Anti-inflammatory activity; PI3Kα inhibitory activity | [29,47,48] |
| Colletotrichum sp. (unresolved taxon) | Artemisia annua | Isoprenylindole-3-carboxylic acid (6); 3,5-Dihydroxy-6-acetoxyergosta-7,22-dienoic acid (7); 3,5-Dihydroxy-6-phenylacetoxyergosta-7,22-dienoic acid (8); 3β,5α,6β-Trihydroxyergosta-7,22-diene (9); 3-Oxoergosta-4,6,8(14),22-tetraene (10); 3β-Hydroxy-5α,8α-epidioxyergosta-6,9(11),22-triene (11); 3β-Hydroxyergosta-5-ene (12); 3-Oxoergosta-4-ene (13) | Indole alkaloid; Sterol derivative; Sterol derivative; Sterol derivative; Sterol derivative; Sterol derivative; Sterol; Sterol derivative | Antimicrobial activity | [29] |
| C. gloeosporioides | Vincetoxicum hirsutum | Coletotric acid (14) | Polyketide | PTP1B inhibitory activity; anti-inflammatory activity | [43] |
| C. gloeosporioides; C. acutatum | Cryptocaryamandioccana; Fragaria × ananassa | (4R)-4,8-Dihydroxy-α-tetralone * (15); cis-4-Hydroxy-6-deoxyscytalone * (16) | Polyketides | Antifungal activity | [30,49] |
| Colletotrichum sp. (unresolved taxon) (NTB-2) | Ginkgo biloba | Apigenin-8-C-β-D-glucopyranoside (17) | Flavonoid glycoside | Anti-inflammatory activity; antioxidant activity; antihypertensive activity; antihepatotoxic activity; Antiarteriosclerotic activity | [50] |
| C. gloeosporioides | Forsythia suspensa | Phillyrin (18) | Lignan glycoside | Antioxidant activity; anti-inflammatory activity; antipyretic activity | [51] |
| C. gloeosporioides | Phlogacanthus thyrsiflorus | 2,4-Bis(1,1-dimethylethyl)phenol (19); Hexadecanoic acid (20); Methyl octadecanoate (21) | Phenolic compound; Fatty acid; Fatty acid ester | Antimicrobial activity; antioxidant activity | [52] |
| Colletotrichum sp. (unresolved taxon); C. gloeosporioides | Pandanus amaryllifolius | Colletotriolide (22); Tyrosol C (23) | Macrolide; Phenolic compound | Antibacterial activity | [53] |
| C. gloeosporioides C. dematium CBP2; | Tectona grandis; Not specified (KACC) | Taxol (Paclitaxel) * (24) | Diterpenoid | Cytotoxic activity | [54,55] |
| C. siamense; C. gloeosporioides | Piper nigrum; Magnolia champaca | Uracil * (25) | Pyrimidine derivative | Acetylcholinesterase (AChE) inhibitory activity; antifungal activity | [41,56] |
| Colletotrichum sp. (unresolved taxon) (HCCB03289) | Ludwigia prostrata | Pyrenocine N (26); Pyrenocine O (27); Macommelin-9-acetate (28); Pyrenocine A (29); Pyrenocine B (30); Pyrenocine E (31); Novaezelandine A (32) | Polyketide; Polyketide; Diterpenoid; Polyketide; Polyketide; Polyketide; Sesquiterpenoid | Cytotoxic activity | [57] |
| C. gloeosporioides | Piper nigrum | Piperine (33) | Alkaloid | Antimicrobial activity; antioxidant activity; cytotoxic activity | [58] |
| C. gloeosporioides XSXY05 | Camptotheca acuminata | 10-Hydroxycamptothecin (34) | Alkaloid | Cytotoxic activity | [59] |
| C. gloeosporioides | Magnolia champaca | 2-Phenylethyl 1H-indol-3-ylacetate (35); Cyclo-(S-Pro-S-Tyr) (36); Cyclo-(S-Pro-S-Val) (37); 2-(2-Aminophenyl)acetic acid (38); 2-(4-Hydroxyphenyl)acetic acid (39); 2-(2-Hydroxyphenyl)acetic acid (40); 4-Hydroxybenzamide (41) | Indole derivative; Diketopiperazines; Aromatic amino acid derivative; Phenolic acid; Phenolic acid; Benzamide derivative; | Antifungal activity; acetylcholinesterase (AChE) inhibitory activity | [56] |
| Colletotrichum sp. (unresolved taxon) | Huperzia serrata | Huperzine A (42) | Alkaloid | Antioxidant activity; acetylcholinesterase (AChE) inhibitory activity | [60] |
| C. gloeosporioides | Lannea corammendalica | 9-Octadecenamide (43); Hexadecenamide (44); Diethyl phthalate (45) | Fatty acid amide; Fatty acid amide; Phthalate ester | Antimicrobial activity | [61] |
| Colletotrichum sp. (unresolved taxon) (BS4) | Buxus sinica | Colletotrichone A (46); Colletotrichone B (47); Colletotrichone C (48); Chermesinone B (49) | Polyketides | Antibacterial activity; cytotoxic activity | [62] |
| C. capsici | Siegesbeckia pubescens | Citrinal A (50); Citrinal B (51) | Polyketides | Cytotoxic activity | [63] |
| C. gloeosporioides | Virola michelii | β-Sitosterol; (52); Stigmasterol; (53); Sitostenone (54) | Sterol; Sterol; Sterol derivative | Anti-inflammatory activity | [47] |
| C. gloeosporioides | Cymbidium aloifolium; Virola michelii | Squalene (55) | Triterpenoid | Antimicrobial activity; antioxidant activity; cytotoxic activity | [44,47] |
| C. capsici KT37396; C. taiwanense PI-3 KX580307 | Passiflora incarnata | Chrysin (56) | Flavonoid | Cytotoxic activity | [64] |
| Colletotrichum sp. (unresolved taxon) (JS-0367) | Morus alba | Evariquinone (57) | Anthraquinone | Neuroprotective activity | [65] |
| C. gloeosporioides | Centella asiatica | Asiaticoside (58) | Triterpenoid glycoside | Immunomodulatory activity; antidepressant activity; | [66] |
| C. crassipes | Casearia sylvestris | Cyclo-(D-Pro-D-Phe) (59); N-(2-Phenylethyl) acetamide; (60) | Diketopiperazine; Aromatic amide | Antioxidant activity; antifungal activity | [67] |
| C. gloeosporioides GT-7 | Uncaria rhynchophylla | Cyclo-(L-Leu-L-Leu) (61); Brevianamide F (62) | Diketopiperazine; Indole alkaloid | PI3Kα inhibitory activity | [48] |
| C. gloeosporioides B12 | Illigera rhodantha | Colletolides A (63) e B (64) | Polyketides | Antibacterial activity | [68] |
| C. gloeosporioides | Carica papaya | Aureonitol (65); Protocatechuic acid (66); Glucobrassicin (67) | Lignan; Phenolic acid; Indole glucosinolate | Antiviral activity; antibacterial activity; cytotoxic activity | [69] |
| C. gloeosporioides | Vincetoxicum hirsutum | Lumichrome (68); β-Acetyltryptamine (69); Cyclo-(Trp-Phe) (70); (Z)-2-(2-(2-(4-hydroxyphenyl)acetoxy)ethyl)but-2-enoic acid (71); | Flavin derivative; Indole derivative; Diketopiperazine; Phenolic acid derivative | PTP1B inhibitory activity; anti-inflammatory activity | [43] |
| C. acutatum | Angelica sinensis | 5-(1-Hydroxybutyl)-4-methoxy-3-methyl-2H-pyran-2-one (C-HMMP) (72) | Pyrone derivative | Antimicrobial activity; antibiofilm activity; antioxidant activity; antimalarial activity; antiproliferative activity | [70] |
| C. tropicicola F10154 | Native plants from Singapore (not specified) | Tropicicolide (73) | Polyketide | Antifungal activity | [71] |
| Colletotrichum sp. (unresolved taxon) (AP-4) | Andrographis paniculata | Andrographolide (AD) (74); Neandrographolide (NAD) (75); 14-Deoxyandrographolide (DAD) (76); 14-Deoxy-11,12-didehydroandrographolide (DDAD) (77) | Diterpenoids | Antioxidant activity; antibacterial activity | [28] |
| C. alatae LCS1 | Lycopodium clavatum | Bisabolol (78); Oxalic acid (79); 7-Isopropyl-1-methylphenanthrene (80); Pterine-6-carboxylic acid (81); Dimethylamine (82); 2-(2-Aminopropyl)phenol (83); Phthalic acid (84); Naphthalene (85) | Sesquiterpenoid; Dicarboxylic acid; Polycyclic aromatic hydrocarbon; Pteridine derivative; Amine; Aromatic amine derivative; Dicarboxylic acid; Polycyclic aromatic hydrocarbon | Antibacterial activity; antioxidant activity | [72] |
| Colletotrichum sp. (unresolved taxon) | Vernonia amygdalina | Palitantin (86); Cladosporin (87); p-Hydroxybenzaldehyde (88); Desmethyldichloro-diaportin (89); p-Hydroxybenzoic acid (90) | Alkaloid; Polyketide; Phenolic aldehyde; Polyketide; Phenolic acid; | Antimicrobial activity; antioxidant activity | [73] |
| C. gloeosporioides | Cymbidium aloifolium | Farnesol (91); Tryptophan (92); 4-Hydroxybenzyl alcohol (93) | Sesquiterpenoid alcohol; Aromatic amino acid; Phenolic alcohol | Antimicrobial activity | [44] |
| C. brevisporum JPSK19 | Bergenia ciliata | 4-(1,1-Dimethylpropyl)phenol (94); 1-Docosene (95) | Phenolic compound; Alkene | Antibacterial activity; antioxidant activity | [74] |
| Colletotrichum sp. (unresolved taxon) | Vernonia amygdalina | Acropyrone (96); Beauvericin (97); Indole-3-carbaldehyde (98); Rocaglamide A (99) | Polyketide; Cyclic hexadepsipeptide; Indole derivative; Flavagline | Antimicrobial activity; | [45] |
| C. taiwanense BPSRJ3 | Vanda cristata | Cyclobarbital (100); Phenanthrene (101); 2,6-Di-tert-butylphenol (102); 3-Carene (103); Camphene (104); 1-Fluorododecane (105); 17-Octadecenoic acid (106); 2,6-Dihydroxyacetophenone (107) | Barbiturate; Polycyclic aromatic hydrocarbon; Phenolic compound; Monoterpene; Monoterpene; Fluoroalkane; Unsaturated fatty acid; Phenolic ketone; | Antioxidant activity; antimicrobial activity; anti-inflammatory activity; cytotoxic activity | [75] |
| C. queenslandicum | Morus australis | Morucolletotricin (108); Tryptophol (109); Phomopyronol (110); 2-(3-Aminophenyl)acetic acid (111) | Polyketide; Indole derivative; Polyketide; Aromatic amino acid derivative | Cytotoxic activity | [46] |
* The same compound was identified from multiple Colletotrichum species associated with distinct host plants, as consolidated in this table.
Figure 2.
Chemical structures of bioactive compounds identified from endophytic Colletotrichum species.
Among the 111 compounds reported across 39 studies, polyketides (24), terpenoids (13), and phenolic compounds (13) were the most frequently identified classes of secondary metabolites produced by endophytic Colletotrichum species. These chemical classes also exhibited the widest range of biological activities, particularly antimicrobial and antioxidant effects. Sterols and alkaloids followed closely, while flavonoids were the least represented. Notably, many compounds exhibited multitarget activity profiles, especially within the polyketides and terpenoids classes. This distribution underscores the strong biotechnological potential of these fungi, revealing consistent correlations between chemical classes and bioactivity profiles. To better illustrate these trends, a stacked column chart was constructed (Figure 3), summarizing the distribution of compound classes in relation to their most frequently reported biological activities, based on the data presented in Table 1.
Figure 3.
Stacked column chart depicting the distribution of major biological activities across chemical classes of secondary metabolites based on data extracted from Table 1.
Based on the tabulated data, beyond their antimicrobial profile, polyketides also exhibited cytotoxic activity, with nine compounds reported, reinforcing their potential interest for anticancer research. Anti-inflammatory and other biological effects were less frequently reported for this class. Terpenoids were primarily associated with antimicrobial activity (five compounds), followed by examples of cytotoxic and antioxidant properties, reflecting their pharmacological diversity. Phenolic compounds showed a predominant antioxidant profile with five compounds, alongside occasional reports of antimicrobial and cytotoxic activities. Sterols were mainly linked to antimicrobial and antioxidant effects, while alkaloids were divided between antimicrobial and cytotoxic activities, both of which are relevant for anti-infective and anticancer research. Flavonoids, although the least represented, demonstrated a varied biological profile, including antimicrobial, cytotoxic, anti-inflammatory, and antioxidant activities. These data emphasize the chemical classes and bioactivities of greatest scientific interest, particularly those with potential for pharmacological development.
In addition to the predominant classes, a variety of structurally diverse compounds were identified, albeit in smaller numbers. These include metabolites such as fatty acids, macrolides, lignans, diketopiperazines, and polycyclic aromatic hydrocarbons, among others. Although less numerous, some of these compounds also exhibited biological activities that are mainly antimicrobial, with occasional reports of cytotoxic or antioxidant effects. This highlights the broad chemical and functional diversity of secondary metabolites produced by endophytic Colletotrichum species.
To provide a biosynthetic perspective on the chemical diversity observed, Figure 4 illustrates the main biosynthetic pathways that could potentially account for the production of the major classes of secondary metabolites identified in this review, namely, alkaloids, flavonoids, phenolics, polyketides, sterols, and terpenoids. Although the precise biosynthetic origins of all 111 compounds remain unconfirmed, these pathways, including PKS, NRPS, TPS, mevalonate, and shikimate/phenylpropanoid routes, represent plausible metabolic routes for the predominant chemical classes identified. Notable examples include ergosterol and derivatives (4, 5, 9, 11), apigenin-8-C-β-D-glucopyranoside (17), pyrenocines (26, 27, 29–31), macommelin-9-acetate (28), piperine (33), 10-hydroxycamptothecin (34), and phenolic acids such as 2-(4-hydroxyphenyl)acetic acid (39) and 2-(2-hydroxyphenyl)acetic acid (40) [29,46,47,48,56,57,58,59]. This biosynthetic overview underscores the metabolic adaptability of Colletotrichum endophytes and their promise as sources of novel compounds with pharmacological potential.
Figure 4.
Schematic representation of the main biosynthetic pathways potentially involved in the production of major secondary metabolites identified in endophytic Colletotrichum species. Polyketides and peptides are synthesized via polyketide synthase (PKS) and nonribosomal peptide synthetase (NRPS) systems, respectively. Terpenoids and sterols derive from terpene synthases (TPSs) and the mevalonate pathway. Shikimate and phenylpropanoid pathways contribute to phenolics, flavonoids, and some alkaloids.
4. Discussion
The data reviewed indicated that endophytic species of Colletotrichum are prolific producers of a wide array of bioactive metabolites. Among them, C. gloeosporioides is the most frequently investigated, and it is commonly found across a wide range of host plants and ecological niches. Its broad host adaptability likely contributes to its extensive metabolic output, which includes several compounds with potent antimicrobial, antioxidant, and cytotoxic activities [30,47]. Other species, such as C. capsici and C. queenslandicum, also contribute significantly to the diversity of Colletotrichum-derived natural products. However, many studies report only Colletotrichum sp., without species-level identification, which may be attributed to the high morphological similarity within the genus and the limited use of molecular methods. Even when molecular analyses are performed, phylogenetic resolution often reveals close genetic proximity and clustering among species, leading many identifications to remain at the genus level [29,46,50,53,57,63].
Numerous metabolites from these species have been characterized, predominantly belonging to polyketides, terpenoids, phenolic compounds, sterols, and alkaloids, with flavonoids being less common. This chemical profile reflects the genus’s biosynthetic adaptability and its relevance for pharmaceutical and agricultural applications [30,43,52,61,69]. Polyketides and terpenoids, in particular, exhibit a broad range of biological activities, including antimicrobial, antioxidant, cytotoxic, and anti-inflammatory effects. Sterols, especially ergosterol and its oxygenated derivatives, are frequently associated with anti-inflammatory properties, while tryptophan-derived compounds, such as indole-3-acetic acid (3), a known plant growth promoter, have been observed in species associated with medicinal plants like C. fructicola and C. siamense. Additional compounds like apigenin, piperine, and various cyclic peptides further expand the genus’s chemical diversity [29,41,42,50,58].
Available evidence suggests that specific structural features in metabolites derived from Colletotrichum are associated with distinct biological functions. For instance, peroxide groups in sterols are frequently linked to enhanced antifungal activity, as these moieties facilitate oxidative stress that disrupts fungal membrane integrity. Similarly, prenylated alkaloids often display intensified antimicrobial or neuroactive properties, likely due to increased lipophilicity that promotes membrane interaction and cellular uptake. Polyketides bearing aromatic rings and hydroxyl substitutions have demonstrated cytotoxic effects against tumor cell lines, potentially through mechanisms such as DNA intercalation, topoisomerase inhibition, or induction of apoptosis via oxidative pathways. These correlations highlight the relevance of structure–activity relationships (SARs), although systematic SAR studies within this genus remain scarce and fragmented. Improved characterization of these molecular features may support future strategies for compound optimization and the development of drug candidates [76,77,78].
Current biosynthetic data for endophytic Colletotrichum species remain limited; however, the presence of polyketides, terpenes, and alkaloids in previously reported compounds suggests the involvement of canonical biosynthetic gene clusters (BGCs), such as those encoding polyketide synthases (PKSs), nonribosomal peptide synthetases (NRPSs), and terpene synthases. Some molecules, such as morucolletotricin and isoprenylindole-3-carboxylic acid, may originate from hybrid PKS-NRPS pathways or the shikimate route, though these hypotheses lack experimental validation. Functional mapping of BGCs in endophytic Colletotrichum remains incomplete, yet the genus’s considerable chemical diversity reinforces the importance of integrated omics-based studies. Phylogenetic analysis of metabolite distribution has also been proposed as a strategy to identify clade-specific biosynthetic capacities, necessitating future comparative genomic investigations to elucidate the evolutionary dynamics of metabolite production [46,79,80,81].
Despite these insights, direct correlations between genes and secondary metabolites in Colletotrichum spp. remain poorly characterized. Evidence from related taxa using predictive genome mining suggests the presence of numerous silent or cryptic BGCs with the potential for novel chemical scaffolds. The integration of in silico pathway reconstruction with transcriptomic and metabolomic analyses is recommended for advancing the identification and functional annotation of these clusters [82]. Comparative genomic and transcriptomic studies have also provided insights into genomic plasticity and infection strategies, particularly regarding host adaptation. For example, analyses of C. higginsianum and C. graminicola reveal distinct secreted effectors and pectin-degrading enzymes, with stage-specific transcriptional shifts during infection. Comparisons between the endophytic C. tofieldiae and pathogenic C. incanum demonstrate differences in secondary metabolism gene expression and carbohydrate-active enzyme (CAZyme) repertoires, shedding light on lifestyle-related genomic traits. Although these datasets were not designed for metabolite discovery, they offer valuable frameworks for identifying candidate genes within BGCs and exploring their functional relevance [34,83,84].
This metabolic versatility is reflected in a range of biological activities observed in the isolated compounds. As illustrated in the quantitative analysis presented in this review, antimicrobial activity was the most frequently reported, followed by antioxidant, cytotoxic, and anti-inflammatory effects. For instance, compounds obtained from C. gloeosporioides associated with Cymbidium aloifolium exhibited inhibitory effects against Escherichia coli, S. aureus, and Candida albicans. In parallel, various unidentified Colletotrichum strains have been described as sources of antimicrobial, anticancer, antioxidant, cytotoxic, neuroprotective, and acetylcholinesterase-inhibitory properties [50,57,60].
In one study [29], metabolites from a Colletotrichum strain isolated from the stem of Artemisia annua included steroidal structures and three novel compounds with strong antimicrobial activity. These compounds, (6), (7), (8), (10), and (12), showed significant inhibition of both Gram-positive bacteria (Bacillus subtilis, S. aureus, and Sarcina lutea) and Gram-negative bacteria (Pseudomonas spp.), with minimum inhibitory concentrations (MICs) ranging from 25 to 75 µg/mL and 50–100 µg/mL against opportunistic fungi like C. albicans and A. niger. They also inhibited phytopathogens such as Gaeumannomyces graminis, Rhizoctonia cerealis, Helminthosporium sativum, and Phytophthora capsici at 200 µg/mL. Such results reinforce the genus’s biosynthetic potential and highlight medicinal plants as valuable sources of endophytic fungi with promising biotechnological applications [28,45,48,63,65,73]. In comparison, tropicicolide (73), isolated from C. tropicicola, exhibited IC50 values of 1.8 µg/mL against A. fumigatus and 7.1 µg/mL against C. albicans, which are substantially lower than the MICs recorded for the steroidal derivatives from the fungus isolated from A. annua [71].
Other species, such as C. crassipes isolated from Casearia sylvestris, have been reported to produce Cyclo-(D)-Pro-(D)-Phe (59) and N-(2-phenylethyl)acetamide (60), compounds with distinct bioactivities. N-(2-phenylethyl)acetamide demonstrated potent antifungal activity at 50 µg/mL against Cladosporium cladosporioides and moderate activity against C. sphaerospermum when tested using the thin-layer chromatography (TLC) diffusion method. Cyclo-(D)-Pro-(D)-Phe exhibited antioxidant capacity at 1 mg/mL, with structure–activity relationship analysis indicating that the presence of electron-donating groups enhances the efficiency of DPPH radical reduction, supporting the idea that the antifungal efficacy of Colletotrichum-derived metabolites can vary markedly across species and chemical classes [67].
C. gloeosporioides and C. dematium have also been identified in the literature as taxol producers, a compound of high relevance in anticancer therapy [54,55]. Similarly, studies on C. acutatum from Angelica sinensis described the production of C-HMMP, a multifunctional molecule with antimicrobial, antibiofilm, antioxidant, antimalarial, antiproliferative, antimutagenic, and antidiabetic activities [70]. C. taiwanense, C. alatae, and C. brevisporum have also been associated with antioxidant and antimicrobial properties. Collectively, the findings consolidate the role of Colletotrichum species as prolific sources of structurally diverse metabolites, for which their bioactivities are modulated by specific molecular frameworks [72,74,75].
Despite promising in vitro outcomes, few studies assess pharmacokinetic parameters, in vivo efficacy, or toxicity of these metabolites. Most reports are confined to screening assays using crude extracts or purified compounds at the cellular level, leaving the translational potential unclear. Evaluating metabolic stability, bioavailability, and off-target effects is essential for pharmaceutical development. Additionally, the ecological duality of the Colletotrichum genus, encompassing both endophytic and phytopathogenic species, raises further safety considerations, particularly for applications involving live cultures or minimally processed extracts. Clarifying the genetic and environmental triggers that mediate the shift between mutualism and pathogenicity is critical to ensure safe and reliable biotechnological applications [9,85].
Beyond safety concerns, poor reproducibility and lack of methodological standardization across studies significantly hinder the biotechnological exploitation of Colletotrichum spp. Variations in culture conditions, extraction protocols, and host plant associations directly affect metabolite profiles and yields, with considerable fluctuations even among strains of the same species. This scenario is further complicated by the structural complexity, chemical instability, and labor-intensive purification processes required for crude extracts, all of which hinder scalability and industrial application. Additionally, low yields (often <1 mg/mL), long fermentation periods (typically 14–30 days), and variability in biomass and metabolite production influenced by culture media composition and environmental parameters further compromise process consistency. To overcome these limitations, strategies such as culture medium optimization, co-culture systems to activate silent biosynthetic pathways, the use of bioreactors, and metabolic engineering have been proposed; however, their specific application to Colletotrichum still requires further investigation through targeted pilot-scale studies [86,87,88].
Although not included in the core analysis of this review, several other studies, even without compound isolation, have reported relevant bioactivities from crude extracts. These references are provided solely to reinforce the biotechnological relevance of the genus Colletotrichum. For instance, C. lindemuthianum ethyl acetate extracts showed broad-spectrum antimicrobial activity using the well diffusion method. Inhibition zones were reported as 8 mm against S. aureus, 6 mm against Proteus vulgaris, and 4 mm against K. pneumoniae [89]. Similarly, crude extracts of Colletotrichum sp. isolated from Rauvolfia serpentina demonstrated antibacterial activity against E. coli and S. aureus, with inhibition zones of 16 mm and 14 mm, respectively, indicating greater antibacterial activity than C. lindemuthianum and emphasizing species-specific differences in potency [90].
In previous studies, extracts from C. siamense, C. jiangxiense, and C. karstii exhibited moderate antibacterial activity, with MICs ranging from 500 to 1000 μg/mL against both Gram-positive and Gram-negative bacteria. Notably, activity was observed against clinically relevant Gram-negative strains such as K. pneumoniae, S. enteritidis, and S. flexneri, which are often associated with multidrug resistance. Although the MICs were relatively high, these findings highlight the potential of Colletotrichum species as sources of metabolites with activity against hard-to-treat Gram-negative pathogens [91]. In the context of anticancer and antioxidant potential, the ethyl acetate extract of C. gloeosporioides exhibited cytotoxic effects against the HCT116 and HeLa cancer cell lines, with IC50 values of 76.6 and 176.2 μg/mL, respectively, while also showing strong antioxidant activity against DPPH radicals (EC50 = 22.2 μg/mL). This combination of bioactivities is particularly relevant considering the role of oxidative stress in cancer development and progression [92].
Additional reports, such as that of Subbulakshmi et al. [93], have described that methanol extracts of C. gloeosporioides demonstrated antimicrobial activity against key pathogens such as S. aureus (12 mm), E. coli (12 mm), and C. albicans (22 mm). A notable exception in terms of potency is the work of Bin et al. [94], who explored an endophytic Colletotrichum species isolated from Aegiceras corniculatum. The ethyl acetate extract exhibited potent antibacterial activity against multidrug-resistant pathogens, with MICs of 4 μg/mL against K. pneumoniae and 0.5 μg/mL against Acinetobacter baumannii, indicating a noteworthy therapeutic potential even prior to compound purification.
Collectively, these studies indicate that the bioactivity of Colletotrichum crude extracts varies depending on species identity, host plant origins, and the solvent used for extraction. The highest antibacterial potency was observed in the ethyl acetate extract from the mangrove-derived Colletotrichum sp., which inhibited multidrug-resistant bacteria, while terrestrial species displayed broader but less potent antimicrobial spectra. Compared to purified compounds, crude extracts are valuable for initial screenings but are limited by their complex composition, which hinders precise attribution of bioactivity. Isolated compounds, in turn, offer higher potency and allow the identification of active chemical classes, enabling deeper pharmacological insights. Progress in harnessing the therapeutic potential of Colletotrichum requires bioassay-guided fractionation, structural characterization, and assessments of species and ecological diversity to better link metabolites to bioactivities [91,92,93,94].
Despite their biosynthetic potential, real-world applications of Colletotrichum-derived compounds face industrial and regulatory challenges, including fermentation scalability, batch consistency, regulatory approval, and environmental impacts. Addressing such barriers early in the discovery process is critical for commercial feasibility [95]. Omics-based tools, particularly genomics and metabolomics, are essential to unravel Colletotrichum’s metabolic complexity. Advances in genome sequencing and transcriptomics reveal gene expression patterns under different hosts or environments. These tools also facilitate studies on metabolic host–endophyte interplay, co-metabolite production, and biosynthetic regulation, which are pivotal for enhancing compound discovery [96].
Studies have demonstrated that some metabolites produced by endophytic fungi may arise from co-metabolic pathways or are influenced by host-derived compounds. For instance, precursors or inducers present in host plant tissues can activate otherwise silent biosynthetic pathways in the fungus, resulting in the production of unique metabolites. Certain biosynthetic routes are expressed only under symbiotic conditions or in response to plant stress signals, reflecting functional co-evolution. In planta-based approaches, comparative metabolomics, and co-culture experiments could uncover biosynthetic potentials that are not observable under axenic culture conditions [96,97].
In this context, co-culture strategies, whether involving host plants, other microorganisms, or even competing fungi, have been increasingly recognized as effective tools for eliciting the expression of cryptic or silent biosynthetic gene clusters (BGCs). By simulating natural ecological interactions, these approaches can induce metabolic exchanges or competitive responses that activate novel biosynthetic pathways that are otherwise dormant in monocultures. This approach could be particularly valuable for Colletotrichum, as genomic analyses have identified numerous biosynthetic gene clusters for which their full expression profiles under different culture conditions remain largely unexplored. Additionally, the integration of multi-omics approaches, combining genomics, transcriptomics, and metabolomics, provides a comprehensive framework to map and link these BGCs to specific chemical outputs. Such strategies not only aid in deciphering the molecular basis of metabolite production but also facilitate the discovery of novel compounds with high biotechnological potential [79,96,97].
The presence of oxygenated sterols, flavonoids, terpenoids, and alkaloids in these endophytic fungi aligns with the genus’s chemical profile, further substantiating its biosynthetic richness and adaptive metabolic capacity. These findings emphasize the importance of expanding research on endophytic Colletotrichum species, particularly through the integration of advanced isolation techniques, structural elucidation, and multi-target biological screening. Such efforts are essential to fully harness their chemical potential and to uncover novel compounds with applications in the pharmaceutical, agricultural, and industrial sectors [98].
5. Conclusions
This review highlights that the endophytic species of the genus Colletotrichum represent a promising source of bioactive metabolites with a wide spectrum of biological activities. The compounds identified to date have demonstrated considerable potential for applications in the pharmaceutical, agricultural, and industrial sectors, largely due to their structural and functional diversity. Importantly, even in the absence of isolated pure compounds, crude extracts from these fungi frequently exhibit significant bioactivity, suggesting possible synergistic interactions among secondary metabolites.
Despite this promise, these fungi remain underexplored, particularly in tropical ecosystems. Expanding research efforts on their ecological diversity, strain isolation, and comprehensive metabolite profiling is crucial to unlock their potential. Future research should prioritize the following concrete steps: (i) systematic bioprospecting in under-sampled tropical and subtropical regions; (ii) rigorous dereplication and identification of novel metabolites through integrated omics (genomics, metabolomics, and transcriptomics); and (iii) development of strain libraries with taxonomic validation and biosynthetic potential assessments.
Additionally, researchers should aim to (iv) characterize biosynthetic gene clusters (BGCs) through genome mining and expression studies; (v) investigate structure–activity relationships (SARs) using targeted synthetic modifications, cheminformatics, and molecular modeling; and (vi) evaluate bioactive candidates in validated in vivo models to assess pharmacokinetics, toxicity, and efficacy.
Moreover, the establishment of sustainable and scalable production methods will be essential for the industrial application of these metabolites. Optimizing bioprocesses, including culture media, fermentation parameters, co-cultivation strategies, and bioreactor configurations, can significantly enhance yield and cost-effectiveness. Functional validation through in vitro and in vivo assays is also necessary to confirm the therapeutic or agricultural efficacy of promising metabolites. Fostering interdisciplinary collaboration across microbiology, natural product chemistry, pharmacology, and biotechnology will be fundamental to translating these discoveries into real-world solutions.
Overall, advancing research on endophytic Colletotrichum species could contribute significantly to the discovery of novel natural products and sustainable solutions for crop protection and health care. This aligns with the broader goals in global health, sustainable agriculture, and biodiversity conservation, reinforcing the value of this fungal group in addressing contemporary scientific and societal challenges. Importantly, the ecological duality of the Colletotrichum genus, encompassing both endophytic and phytopathogenic species, should be carefully considered in future studies to ensure the safe and responsible development of these applications. Understanding the factors that regulate this dual behavior may enhance the biotechnological exploitation of these fungi while minimizing potential risks.
Acknowledgments
We thank PPGMBT-UEA and FAPEAM for providing a Master’s scholarship to M.V.N.d.S.
Author Contributions
Conceptualization: M.V.N.d.S. and C.V.N.; methodology: M.V.N.d.S.; original draft preparation: M.V.N.d.S.; writing—review and editing: A.d.S.A. and C.V.N.; funding acquisition: C.V.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Council for Scientific and Technological Development—Brazil (CNPq) (309704/2022-7); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) (001); and Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) (research support and scholarships).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wijesekara T., Xu B. Health-promoting effects of bioactive compounds from plant endophytic fungi. J. Fungi. 2023;9:997. doi: 10.3390/jof9100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corda A.K.J. Die Pilze Deutschlands. In: Sturm J., editor. Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen. Volume 3. Gedruckt auf kosten des verfassers; Nuremberg, Germany: 1817. [(accessed on 20 March 2025)]. pp. 1–144. Available online: https://books.google.com.br/books?id=iTF6vuXQ6FsC. [Google Scholar]
- 3.Dean R., Van Kan J.A.L., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrenk H., Spaulding P. The bitter-rot fungus. Science. 1903;17:750–751. doi: 10.1126/science.17.436.750.b. [DOI] [PubMed] [Google Scholar]
- 5.Damm U., Cannon P.F., Crous P.W., editors. Colletotrichum: Complex Species or Species Complexes? Vol. 73. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2012. pp. 1–213. [DOI] [Google Scholar]
- 6.Index Fungorum Partnership Landcare Research–NZ and RBG Kew: Mycology and Institute of Microbiology, Chinese Academy of Science. 2025. [(accessed on 20 March 2025)]. Available online: www.indexfungorum.org.
- 7.Freeman S., Katan T., Shabi E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998;82:596–605. doi: 10.1094/PDIS.1998.82.6.596. [DOI] [PubMed] [Google Scholar]
- 8.Münch S., Lingner U., Floss D.S., Ludwig N., Sauer N., Deising H.B. The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 2008;165:41–51. doi: 10.1016/j.jplph.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Jayawardena R.S., Bhunjun C.S., Hyde K.D., Gentekaki E., Itthayakorn P. Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere. 2021;12:519–669. doi: 10.5943/mycosphere/12/1/7. [DOI] [Google Scholar]
- 10.Bailey J.A., Jeger M.J. Colletotrichum: Biology, Pathology and Control. CAB International; Wallingford, UK: 1992. [(accessed on 20 March 2025)]. Available online: https://archive.org/details/colletotrichumbi0000unse/page/n15/mode/2up. [Google Scholar]
- 11.Agrios G.N. Plant Pathology. 5th ed. Elsevier Academic Press; San Diego, CA, USA: 2005. [(accessed on 20 March 2025)]. Available online: https://pt.slideshare.net/slideshow/agrios-2005-plant-pathology-5-edpdf/257736607. [Google Scholar]
- 12.Noireung P., Phoulivong S., Liu F., Cai L., Mckenzie E.H., Chukeatirote E., Jones E.B.G., Bahkali A.H., Hyde K.D. Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogam. Mycol. 2012;33:347–362. doi: 10.7872/crym.v33.iss3.2012.347. [DOI] [Google Scholar]
- 13.García-Pajón C.M., Collado I.G. Secondary metabolites isolated from Colletotrichum species. Nat. Prod. Rep. 2003;20:426–431. doi: 10.1039/B302183C. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Agriculture USDA Fungal Databases. [(accessed on 20 March 2025)];2024 Available online: https://fungi.ars.usda.gov/
- 15.Zakaria L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture. 2021;11:297. doi: 10.3390/agriculture11040297. [DOI] [Google Scholar]
- 16.Hyde K.D., Cai L., McKenzie E.H.C., Yang Y.L., Zhang J.Z., Prihastuti H. Colletotrichum—A catalogue of confusion. [(accessed on 23 March 2025)];Fungal Divers. 2009 39:1–17. Available online: https://www.researchgate.net/publication/274385803. [Google Scholar]
- 17.Talhinhas P., Baroncelli R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021;110:109–198. doi: 10.1007/s13225-021-00491-9. [DOI] [Google Scholar]
- 18.Petrini O. Fungal endophytes of tree leaves. In: Andrews J.H., Hirano S.S., editors. Microbial Ecology of Leaves. Springer; New York, NY, USA: 1991. pp. 179–197. [DOI] [Google Scholar]
- 19.Photita W., Taylor P.W.J., Ford R., Hyde K.D., Lumyong S. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. [(accessed on 23 March 2025)];Fungal Divers. 2005 18:117–133. Available online: https://www.academia.edu/download/34259306/18-9.pdf. [Google Scholar]
- 20.Aly A.H., Debbab A., Proksch P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011;90:1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- 21.Muhammad M., Basit A., Ali K., Ahmad H., Li W.J., Khan A., Mohamed H.I. A review on endophytic fungi: A potent reservoir of bioactive metabolites with special emphasis on blight disease management. Arch. Microbiol. 2024;206:129. doi: 10.1007/s00203-023-03828-x. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez R., Redman R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008;59:1109–1114. doi: 10.1093/jxb/erm342. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira-Silva A., Aliyeva-Schnorr L., Wirsel S.G.R., Deising H.B. Fungal pathogenesis-related cell wall biogenesis, with emphasis on the maize anthracnose fungus Colletotrichum graminicola. Plants. 2022;11:849. doi: 10.3390/plants11070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold A.E., Mejia L.C., Kyllo D., Rojas E.I., Maynard Z., Robbins N., Herre E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gond S.K., Mishra A., Sharma V.K., Verma S.K., Kumar J., Kharwar R.N., Kumar A. Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience. 2012;53:113–121. doi: 10.1007/S10267-011-0146-Z. [DOI] [Google Scholar]
- 26.Santos S.S., Silva A.A., Polonio J.C., Polli A.D., Orlandelli R.C., Oliveira J.A.S., Brandão-Filho J.U.T., Azevedo J.L., Pamphile J.A. Influence of plant growth-promoting endophytes Colletotrichum siamense and Diaporthe masirevici on tomato plants (Lycopersicon esculentum Mill.) Mycology. 2022;13:257–270. doi: 10.1080/21501203.2022.2050825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhammad M., Basit A., Ali K., Li W.J., Li L., Mohamed H.I. Endophytic fungi as potential bio-control agents of soil-borne pathogen. J. Crop Health. 2024;76:617–636. doi: 10.1007/s10343-024-00975-z. [DOI] [Google Scholar]
- 28.Li N., Xu D., Huang R.-H., Zheng J.-Y., Liu Y.-Y., Hu B.-S., Gu Y.-Q., Du Q. A New Source of Diterpene Lactones From Andrographis paniculata (Burm. f.) Nees—Two Endophytic Fungi of Colletotrichum sp. with Antibacterial and Antioxidant Activities. Front. Microbiol. 2022;13:819770. doi: 10.3389/fmicb.2022.819770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H., Zou W.X., Meng J.C., Hu J., Tan R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000;151:67–73. doi: 10.1016/S0168-9452(99)00199-5. [DOI] [Google Scholar]
- 30.Inácio M.L., Silva G.H., Teles H.L., Trevisan H.C., Cavalheiro A.J., Bolzani V.S., Young M.C.M., Pfenning L.H., Araújo A.R. Antifungal metabolites from Colletotrichum gloeosporioides, an endophytic fungus in Cryptocarya mandioccana Nees (Lauraceae) Biochem. Syst. Ecol. 2006;34:822–824. doi: 10.1016/j.bse.2006.06.007. [DOI] [Google Scholar]
- 31.Siqueira V.M., Conti R., Araújo J.M., Souza-Motta C.M. Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis. 2011;53:89–95. doi: 10.1007/s13199-011-0113-7. [DOI] [Google Scholar]
- 32.Kusari S., Hertweck C., Spiteller M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Santos L.S., Rhoden S.A., Barros I.T., Tonini R.C.G., Marques R.M., Souza V.H.E., Pamphile J.A. A interação harmônica entre fungos e plantas: Aspectos da relação endófito/hospedeiro. [(accessed on 23 March 2025)];SaBios Rev. Saúde Biol. 2013 8:92–101. Available online: http://periodicos.grupointegrado.br/revista/index.php/sabios/article/view/1335. [Google Scholar]
- 34.Newfeld J., Ujimatsu R., Hiruma K. Uncovering the Host Range–Lifestyle Relationship in the Endophytic and Anthracnose Pathogenic Genus Colletotrichum. Microorganisms. 2025;13:428. doi: 10.3390/microorganisms13020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon P.F., Johnston P.R., Weir B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012;73:181–213. doi: 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawksworth D.L., Lücking R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017;5:10-1128. doi: 10.1128/microbiolspec.FUNK-0052-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boufleur T.R., Ciampi-Guillardi M., Tikami I., Rogério F., Thon M.R., Sukno S.A., Massola Júnior N.S., Baroncelli R. Soybean anthracnose caused by Colletotrichum species: Current status and future prospects. Mol. Plant Pathol. 2021;22:393–409. doi: 10.1111/mpp.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azevedo J.L. Microrganismos endofíticos. In: Melo I.S., Azevedo J.L., editors. Ecologia microbiana. EMBRAPA; Jaguariúna, Brazil: 1998. [(accessed on 23 March 2025)]. pp. 117–137. Available online: https://pt.scribd.com/doc/114865324/Micro-organismos-endofiticos-Joao-Lucio-Azevedo. [Google Scholar]
- 39.Faria C.M.X., Inácio C.A. Considerações Sobre o Gênero Colletotrichum. Rev. Anu. Patol. Plantas. 2023;29:131–147. doi: 10.31976/0104-038321v290006. [DOI] [Google Scholar]
- 40.Inoue M., Takenaka H., Tsurushima T., Miyagawa H., Ueno T. Colletofragarones A1 and A2, novel germination self-inhibitors from the fungus Colletotrichum fragariae. Tetrahedron Lett. 1996;37:5731–5734. doi: 10.1016/0040-4039(96)01212-9. [DOI] [Google Scholar]
- 41.Munasinghe M.V.K., Kumar N.S., Jayasinghe L., Fujimoto Y. Indole-3-acetic acid production by Colletotrichum siamense, an endophytic fungus from Piper nigrum leaves. J. Biol. Act. Prod. Nat. 2017;7:475–479. doi: 10.1080/22311866.2017.1408429. [DOI] [Google Scholar]
- 42.Numponsak T., Kumla J., Suwannarach N., Matsui K., Lumyong S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola CMU-A109. PLoS ONE. 2018;13:e0205070. doi: 10.1371/journal.pone.0205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun S.F., Zhu S., Cao H.Y., Liu Y.B., Yu S.S. Tridepsides from the endophytic fungus Colletotrichum gloeosporioides associated with a toxic medicinal plant Tylophora ovata. Frigid Zone Med. 2021;1:45. doi: 10.2478/fzm-2021-0006. [DOI] [PubMed] [Google Scholar]
- 44.Wary S., Sarma A., Talukdar R., Tayung K. Leaf endophytic fungi of Cymbidium aloifolium L. produces antimicrobials and indole-3-acetic acid. S. Afr. J. Bot. 2022;149:381–388. doi: 10.1016/j.sajb.2022.06.035. [DOI] [Google Scholar]
- 45.Okoli J.T., Okolo C.C., Anyanwu O.O., Oranu E.C., Ezeagha C.C., Obidiegwu O.C., Okoye N.N., Okoye F.B.C. Antimicrobial activity of secondary metabolites produced by Colletotrichum species, an endophytic fungus on Vernonia amygdalina Del (fam. Asteraceae) World J. Biol. Pharm. Health Sci. 2023;14:031–042. doi: 10.30574/wjbphs.2023.14.1.0135. [DOI] [Google Scholar]
- 46.Ningsih K.N., Hakim E.H. New indole alkaloid morucolletotricin from endophytic fungus Colletotrichum queenslandicum associated with Morus australis Poir. Leaf. Nat. Prod. Res. 2025;39:202–207. doi: 10.1080/14786419.2023.2250520. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho J.M., Paixão L.K.O.D., Dolabela M.F., Marinho P.S.B., Marinho A.M.D.R. Phytosterols isolated from endophytic fungus Colletotrichum gloeosporioides (Melanconiaceae) Acta Amaz. 2016;46:7–12. doi: 10.1590/1809-4392201500072. [DOI] [Google Scholar]
- 48.Yang Z.D., Li Z.J., Zhao J.W., Sun J.H., Yang L.J., Shu Z.M. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol. 2019;76:904–908. doi: 10.1007/s00284-019-01707-7. [DOI] [PubMed] [Google Scholar]
- 49.Mancilla G., Jiménez-Teja D., Femenia-Rios M., Macías-Sánchez A.J., Collado I.G., Hernández-Galán R. Novel macrolide from wild strains of the phytopathogen fungus Colletotrichum acutatum. Nat. Prod. Commun. 2009;4:316. doi: 10.1177/1934578X0900400316. [DOI] [PubMed] [Google Scholar]
- 50.Zhou S.L., Zhou S.L., Wang M.X., Chen S.L. Two compounds from the endophytic Colletotrichum sp. of Ginkgo biloba. Nat. Prod. Commun. 2011;6:1131–1132. doi: 10.1177/1934578X1100600821. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q., Wei X., Wang J. Phillyrin produced by Colletotrichum gloeosporioides, an endophytic fungus isolated from Forsythia suspensa. Fitoterapia. 2012;83:1500–1505. doi: 10.1016/j.fitote.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Devi N.N., Singh M.S. GC-MS analysis of metabolites from the endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus thyrsiflorus Nees. [(accessed on 2 April 2025)];Int. J. Pharm. Sci. 2013 23:392–395. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=5d3b28ec90dd69b109fce5258c9497a47548f789. [Google Scholar]
- 53.Bungihan M.E., Tan M.A., Takayama H., Dela Cruz T.E.E., Nonato M.G. A new macrolide isolated from the endophytic fungus Colletotrichum sp. Philipp. [(accessed on 2 April 2025)];Sci. Lett. 2013 6:57–73. Available online: https://scienggj.org/2013/2013n1.7.pdf. [Google Scholar]
- 54.Senthilkumar N., Govindasamy V., Raguchander T., Samiyappan R. Taxol producing fungal endophyte, Colletotrichum gloeosporioides (Penz.) from Tectona grandis L. [(accessed on 2 April 2025)];Int. J. Eng. Sci. Technol. 2013 7:8–15. Available online: http://www.academia.edu/15213664/Taxol_producing_fungal_endophyte_Colletotrichum_gleospoiroides_Penz_from_Tectona_grandis_L. [Google Scholar]
- 55.Choi J., Park J.G., Ali M.S., Choi S.-J., Baek K.H. Systematic analysis of the anticancer agent taxol-producing capacity in Colletotrichum species and use of the species for taxol production. Mycobiology. 2016;44:105–111. doi: 10.5941/MYCO.2016.44.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapla V.M., Zeraik M.L., Leptokarydis I.H., Silva G.H., Bolzani V.S., Young M.C.M., Pfenning L.H., Araújo A.R. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules. 2014;19:19243–19252. doi: 10.3390/molecules191119243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang S.W., Li X., Lu Z., Sun L., Yang B. Pyrenocines from Colletotrichum sp. HCCB03289. J. Antibiot. 2014;67:343–346. doi: 10.1038/ja.2014.59. [DOI] [PubMed] [Google Scholar]
- 58.Chithra S., Jasim B., Anisha C., Mathew J., Radhakrishnan E.K. Piperine production by endophytic Colletotrichum gloeosporioides isolated from Piper nigrum and its antimicrobial properties. Braz. J. Microbiol. 2014;21:1137–1146. doi: 10.1016/j.phymed.2013.10.020. [DOI] [Google Scholar]
- 59.Su H., Kang J.C., Cao J.J., Mo L., Hyde K.D. Medicinal plant endophytes produce analogous bioactive compounds. [(accessed on 3 April 2025)];Chiang Mai J. Sci. 2014 41:1–13. Available online: https://www.thaiscience.info/journals/Article/CMJS/10905185.pdf. [Google Scholar]
- 60.Dong L.H., Fan S.W., Ling Q.Z., Huang B.B., Wei Z.J. Identification of huperzine A-producing endophytic fungi isolated from Huperzia serrata. World J. Microbiol. Biotechnol. 2014;30:1011–1017. doi: 10.1007/s11274-013-1519-6. [DOI] [PubMed] [Google Scholar]
- 61.Premjanu N., Jaynthy C. Identification and characterization of antimicrobial metabolite from an endophytic fungus, Colletotrichum gloeosporioides isolated from Lannea coromandelica. [(accessed on 3 April 2025)];Int. J. ChemTech Res. 2015 7:369–374. Available online: https://www.researchgate.net/publication/282275569_Identification_and_characterization_of_antimicrobial_metabolite_from_an_endophytic_fungus_Colletotrichum_gloeosporioides_isolated_from_Lannea_corammendalica. [Google Scholar]
- 62.Wang F.W., Jiao R.H., Cheng A.B., Tan S.H., Song Y.C. Azaphilones Colletotrichum sp. Isol. Buxus sinica. J. Nat. Prod. 2016;79:14–19. doi: 10.1021/acs.jnatprod.5b00436.s001. [DOI] [Google Scholar]
- 63.Wang F., Zhu H., Ma H., Jiang J., Sun W., Cheng L., Zhang G., Zhang Y. Citrinal B, a new secondary metabolite from endophytic fungus Colletotrichum capsici and structure revision of citrinal A. Tetrahedron Lett. 2016;57:4250–4253. doi: 10.1016/j.tetlet.2016.08.029. [DOI] [Google Scholar]
- 64.Seetharaman P., Gnanasekar S., Chandrasekaran R., Chandrakasan G., Kadarkarai M., Sivaperumal S. Isolation and characterization of anticancer flavone chrysin (5,7-dihydroxyflavone)-producing endophytic fungi from Passiflora incarnata L. leaves. Ann. Microbiol. 2017;67:321–331. doi: 10.1007/s13213-017-1263-5. [DOI] [Google Scholar]
- 65.Song J.H., Lee C., Lee D., Kim S., Bang S., Shin M.-S., Lee J., Kang K.S., Shim S.H. Neuroprotective compound from an endophytic fungus, Colletotrichum sp. JS-0367. J. Nat. Prod. 2018;81:1411–1416. doi: 10.1021/acs.jnatprod.8b00033. [DOI] [PubMed] [Google Scholar]
- 66.Gupta S., Bhatt P., Chaturvedi P. Determination and quantification of asiaticoside in endophytic fungus from Centella asiatica (L.) Urban. World J. Microbiol. Biotechnol. 2018;34:111. doi: 10.1007/s11274-018-2493-9. [DOI] [PubMed] [Google Scholar]
- 67.Chapla V.M., Zeraik M.L., Cafeu M.C., Silva G.H., Cavalheiro A.J., Bolzani V.S., Young M.C.M., Pfenning L.H., Araujo A.R. Griseofulvin, diketopiperazines and cytochalasins from endophytic fungi Colletotrichum crassipes and Xylaria sp., and their antifungal, antioxidant and anticholinesterase activities. J. Braz. Chem. Soc. 2018;29:1707–1713. doi: 10.21577/0103-5053.20180045. [DOI] [Google Scholar]
- 68.Li Y., Wei W., Wang R.L., Liu F., Wang Y.K., Li R., Ye Y.H. Colletolides A and B, two new γ-butyrolactone derivatives from the endophytic fungus Colletotrichum gloeosporioides. Phytochem. Lett. 2019;33:90–93. doi: 10.1016/j.phytol.2019.08.004. [DOI] [Google Scholar]
- 69.Abonyi D.O., Eze P.M., Abba C.C., Chukwunwejim C.R., Ejikeugwu C.P., Okoye F.B.C., Esimone C.O. Metabolites of endophytic Colletotrichum gloeosporioides isolated from leaves of Carica papaya. [(accessed on 4 April 2025)];Am. J. Essent. Oils Nat. Prod. 2019 7:39–46. Available online: http://95.179.195.156/bitstream/123456789/437/1/ABBA%20CHIKA%20C.%207.pdf. [Google Scholar]
- 70.Yehia R.S. Multi-function of a new bioactive secondary metabolite derived from endophytic fungus Colletotrichum acutatum of Angelica sinensis. J. Microbiol. Biotechnol. 2023;33:806–822. doi: 10.4014/jmb.2206.06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gakuubi M.M., Omosa L.K., Cheplogoi P.K., Wansi J.D., Muthee J.K., Wekesa C.S., Koorbanally N.A. Enhancing the discovery of bioactive secondary metabolites from fungal endophytes using chemical elicitation and variation of fermentation media. Front. Microbiol. 2022;13:898976. doi: 10.3389/fmicb.2022.898976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santra H.K., Banerjee D. Bioactivity study and metabolic profiling of Colletotrichum alatae LCS1, an endophyte of club moss Lycopodium clavatum L. PLoS ONE. 2022;17:e0267302. doi: 10.1371/journal.pone.0267302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eboh C.V., Okolo C.C., Anyanwu O.O., Okoye N.N., Ezeagha C.C., Okoye F.B.C. Metabolites of Colletotrichum species, an endophytic fungus isolated from Vernonia amygdalina Del possess antimicrobial and antioxidant activities. GSC Biol. Pharm. Sci. 2022;20:148–159. doi: 10.30574/gscbps.2022.20.3.0323. [DOI] [Google Scholar]
- 74.Prasai J.R., Sureshkumar S., Ahmad W., Ashraf M., Gopi C., Rajapriya P., Aloufi A.S., Natarajan N., Pandi M. Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata. Open Chem. 2023;21:20230158. doi: 10.1515/chem-2023-0158. [DOI] [Google Scholar]
- 75.Pun B., Joshi S.R. Bioprospection unveils the bioactive potential of Colletotrichum taiwanense BPSRJ3, an endophytic fungus of an ethnomedicinal orchid, Vanda cristata Wall. Ex Lindl. Syst. Microbiol. Biomanuf. 2024;5:754–771. doi: 10.1007/s43393-024-00276-6. [DOI] [Google Scholar]
- 76.Dembitsky V.M., Ermolenko E., Savidov N., Gloriozova T.A., Poroikov V.V. Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules. 2021;26:686. doi: 10.3390/molecules26030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hossain R., Sultana A., Nuinoon M., Noonong K., Tangpong J., Hossain K.H., Rahman M.A. A Critical Review of the Neuropharmacological Effects of Kratom: An Insight from the Functional Array of Identified Natural Compounds. Molecules. 2023;28:7372. doi: 10.3390/molecules28217372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du X., Liu D., Huang J., Zhang C., Proksch P., Lin W. Polyketide Derivatives from the Sponge Associated Fungus Aspergillus europaeus with Antioxidant and NO Inhibitory Activities. Fitoterapia. 2018;130:190–197. doi: 10.1016/j.fitote.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 79.Mózsik L., Iacovelli R., Bovenberg R.A., Driessen A.J. Transcriptional activation of biosynthetic gene clusters in filamentous fungi. Front. Bioeng. Biotechnol. 2022;10:901037. doi: 10.3389/fbioe.2022.901037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stroe M.C., Gao J., Pitz M., Fischer R. Complexity of fungal polyketide biosynthesis and function. Mol. Microbiol. 2024;121:18–25. doi: 10.1111/mmi.15192. [DOI] [PubMed] [Google Scholar]
- 81.González-Hernández R.A., Valdez-Cruz N.A., Macías-Rubalcava M.L., Trujillo-Roldán M.A. Overview of fungal terpene synthases and their regulation. World J. Microbiol. Biotechnol. 2023;39:194. doi: 10.1007/s11274-023-03635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu W., Pei R., Zhou J., Zeng B., Tu Y., He B. Molecular Regulation of Fungal Secondary Metabolism. World J. Microbiol. Biotechnol. 2023;39:204. doi: 10.1007/s11274-023-03649-6. [DOI] [PubMed] [Google Scholar]
- 83.O’Connell R.J., Thon M.R., Hacquard S., Amyotte S.G., Kleemann J., Torres M.F., Damm U., Buiate E.A., Epstein L., Alkan N., et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012;44:1060–1065. doi: 10.1038/ng.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hacquard S., Kracher B., Hiruma K., Münch P.C., Garrido-Oter R., Thon M.R., Weimann A., Damm U., Dallery J.-F., Hainaut M., et al. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 2016;7:11362. doi: 10.1038/ncomms11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiffmann S., Gunne S., Ulshöfer T., Henke M., Roser L.A., Schneider A.K., Cinatl J., Thomas D., Schreiber Y., Wagner P.V., et al. In Vitro Safety, Off-Target and Bioavailability Profile of the Antiviral Compound Silvestrol. Pharmaceuticals. 2022;15:1086. doi: 10.3390/ph15091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jha P., Kaur T., Chhabra I., Panja A., Paul S., Kumar V., Malik T. Endophytic Fungi: Hidden Treasure Chest of Antimicrobial Metabolites—Interrelationship of Endophytes and Metabolites. Front. Microbiol. 2023;14:1227830. doi: 10.3389/fmicb.2023.1227830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian D.S., Zhang X., Cox R.J. Comparing Total Chemical Synthesis and Total Biosynthesis Routes to Fungal Specialized Metabolites. Nat. Prod. Rep. 2025;42:720–738. doi: 10.1039/D4NP00015C. [DOI] [PubMed] [Google Scholar]
- 88.Rufino M.P. Master’s Thesis. Instituto de Química, Universidade Estadual Paulista “Júlio de Mesquita Filho”; Araraquara, Brazil: 2011. [(accessed on 6 April 2025)]. Avaliação Química e Biológica do Fungo Endofítico Colletotrichum sp. Isolado de Senna spectabilis. Available online: https://repositorio.unesp.br/bitstreams/3224f16f-eb22-4c94-8f04-32072dd6abc7/download. [Google Scholar]
- 89.Ningaraju S., Kalyani M.I. Endophytic fungi inhabitants of Hultholia mimosoides—Isolation, identification and antimicrobial activity. Asian J. Microbiol. Biotechnol. Environ. Sci. 2024;26:497–506. doi: 10.53550/AJMBES.2024.v26i04.009. [DOI] [Google Scholar]
- 90.Singh S.K., Verma M., Ranjan A., Singh R.K. Antibacterial activity and preliminary phytochemical screening of endophytic fungal extract of Rauvolfia serpentina. Open Conf. Proc. J. 2016;7:104–113. doi: 10.2174/2210289201607010104. [DOI] [Google Scholar]
- 91.Fruet T.K., Polonio J.C., Ramos A.V.G., Golias H.C., Malaco N.S., Baldoqui D.C., Pamphile J.A., Vicentini V.E.P. Prospection and antibacterial screening of metabolic extracts from endophytic fungi isolated from Tibouchina granulosa (Desr.) Cogn. (Melastomataceae) Ciência Nat. 2024;46:e74647. doi: 10.5902/2179460X74647. [DOI] [Google Scholar]
- 92.Rai N., Keshri P.K., Gupta P., Verma A., Kamble S.C., Singh S.K., Gautam V. Bioprospecting of fungal endophytes from Oroxylum indicum (L.) Kurz Antioxid. Cytotoxic activity. PLoS ONE. 2022;17:e0264673. doi: 10.1371/journal.pone.0264673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Subbulakshmi G.K., Thalavaipandian A., Ramesh V., Bagyalakshmi B., Rajendran A. Bioactive endophytic fungal isolates of Biota orientalis (L.) Endl., Pinus excelsa Wall. and Thuja occidentalis L. [(accessed on 6 April 2025)];Int. J. Adv. Life Sci. 2012 4:9–15. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20123374734. [Google Scholar]
- 94.Bin G., Yanping C., Hong Z., Zheng X., Yanqiu Z., Huaiyi F., Qiupin Z., Chenxiao Z. Isolation, characterization and anti-multiple drug resistant (MDR) bacterial activity of endophytic fungi isolated from the mangrove plant, Aegiceras corniculatum. Trop. J. Pharm. Res. 2014;13:593–599. doi: 10.4314/tjpr.v13i4.16. [DOI] [Google Scholar]
- 95.Garg S. The importance of fungal biotechnology for sustainable applications. Trends Biotechnol. 2025 doi: 10.1016/j.tibtech.2025.06.010. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z., Kim W., Wang Y.W., Yakubovich E., Dong C., Trail F., Townsend J.P., Yarden O. The Sordariomycetes: An Expanding Resource with Big Data for Mining in Evolutionary Genomics and Transcriptomics. Front. Fungal Biol. 2023;4:1214537. doi: 10.3389/ffunb.2023.1214537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao Y., Xu Y., Dong Z., Guo Y., Luo J., Wang F., Yan L., Zou X. Endophytic fungal diversity and its interaction mechanism with medicinal plants. Molecules. 2025;30:1028. doi: 10.3390/molecules30051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karpinski L. Bachelor’s Thesis. Universidade Tecnológica Federal do Paraná; Curitiba, Brazil: 2021. [(accessed on 8 April 2025)]. Bioprospecção de Fungos Endofíticos da Planta Medicinal Kalanchoe daigremontiana e Avaliação do Potencial Biotecnológico Aplicado à Agricultura. Available online: http://repositorio.utfpr.edu.br/jspui/handle/1/30465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.