Abstract
Differences in the soluble protein fraction between the freshly isolated cyanobiont of lichen Peltigera membranacea, the corresponding free-living strain, and Nostoc punctiforme were analyzed. One protein, which was among the most prominent proteins of the freshly isolated cyanobiont, was expressed at a lower level in the corresponding free-living strain and was not detected at all on the two-dimensional gels of N. punctiforme. This protein was partially sequenced, and the corresponding open reading frame (ORF) in the N. punctiforme genome was identified. This ORF contains a fasciclin domain typical of a class of surface-associated proteins involved in cell adhesion. Similar fasciclin motif-containing genes have previously been shown to be symbiotically induced in other symbiotic systems.
Cyanobacteria of the genus Nostoc are photosynthetic and N2-fixing organisms capable of forming symbiotic associations with many hosts from different organism groups such as bryophytes, pteridophytes (Azolla), gymnosperms (cycads), angiosperms (Gunnera), and fungi (lichens) (1, 13). The type of structures where the symbiont is housed include root (cycad), leaf (Azolla), stem (Gunnera), and thallus (bryophyte and lichen).
There are different types of physiological roles of the symbiont. In the association with plants, Nostoc generally lives heterotrophically and provides fixed nitrogen to the host (13). In lichens, on the other hand, the fungal host is heterotrophic and thus needs to obtain photosynthate in addition to nitrogen from the symbiont.
The factors responsible for the symbiotic success of Nostoc as a symbiont are not known, but the fact that hosts, symbiotic structures, and physiological roles of the symbiont are so diverse makes specific coevolved adaptations less likely.
The nearly completed genome sequence of Nostoc punctiforme (ATCC 29133/PCC 73102) provides a reference for studies on symbiotic Nostoc strains (DOE Joint Genome Institute, http://www.jgi.doe.gov/JGL_microbial/html/Nostoc/Nostoc_homepage.html). This Nostoc strain has a broad symbiotic competence. It was originally isolated from a symbiotic association with a cycad and in reinfection studies readily associates with both the hornwort Anthoceros (4) and angiosperm Gunnera (6) in the laboratory. It has also been shown to belong to the same group of Nostoc as found in lichens (3, 10).
We have previously examined the genetic diversity and specificity of Nostoc symbionts in lichens using the tRNALeu(UAA) intron as a genetic marker (10). From these studies we established that there is great specificity in the examined lichen species (11, 12). One Nostoc type is found not only in one thallus, but in many cases in thalli collected from remote areas (11, 12). The fact that there is no Nostoc heterogeneity in one thallus makes it possible to perform experiments on freshly isolated symbionts without artifacts caused by mixed populations of Nostoc cells in the isolated cells.
In the present study, we have initiated the investigation of the modifications found in the Nostoc symbiont compared to the symbiotically competent free-living strain N. punctiforme PCC 73102 using two-dimensional gel electrophoresis in combination with mass spectrometry.
Biological material.
The lichen species used in this study was the bipartite Nostoc-containing lichen Peltigera membranacea (Ach.) Nyl. It was harvested from its natural habitat in Lunsen, Uppsala, Sweden. The free-living cyanobacterial strains used included the laboratory strain N. punctiforme PCC 73102 and a free-living culture of the Nostoc symbiont from the lichen described above.
Purification of the cyanobacterial symbiont was performed according to a protocol modified from Wastlhuber and Loos (16). No breaks were used in the centrifugations, and all work was performed at 4°C. About 15 lichen thalli, each around 20 cm2, were used. Thalli were freed from apothecia and rhizines and then submerged in water and rinsed. Thallus pieces were washed in extraction buffer (50 mM HEPES-NaOH buffer, pH 7.0, containing 0.25 M sorbitol) with the addition of 1% polyvinylpyrrolidone 40,000) and 0.25% bovine serum albumin. Homogenization was performed in a cold mortar in 15 ml of the same buffer, with the addition of sand.
To sediment larger fragments, the homogenate was brought to 700 × g, and then the centrifuge was turned off. Grinding in new buffer and centrifugation were repeated in total four times, and all supernatants were pooled and centrifuged (1,200 × g, 10 min). The pellet was resuspended in extraction buffer and then centrifuged twice (700 × g and 1,200 × g). The resulting pellet was resuspended in 1 ml of extraction buffer and added to a two-phase system containing 20 ml of 0.2-g/ml polyethylene glycol 4,000 and 20 ml of 0.2-g/ml dextran T500, before being inverted 25 times and centrifuged (10 min, 1,200 × g). The Nostoc cells, which become enriched at the interphase, were carefully removed, washed once in extraction buffer, and used in a second two-phase separation. The Nostoc layer was taken out and washed twice. The pellet obtained contained only a few traces of fungal hyphae.
The complete isolation procedure took about 3 h. Microscopic examination revealed that the purified Nostoc consisted mostly of single cells, but short filaments were also seen. The purified symbiont was still viable, as free-living Nostoc cultures readily grew from the fresh isolation when placed on agar plates (BG11o, 0.8% agar) (10).
Analytical procedures.
Nostoc cells were suspended in 150 μl of electrophoresis buffer (8 M urea, 2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 20 mM dithiothreitol [DTT], 0.001% bromophenol blue, and 0.5% IPG buffer [pH range 3 to 10; Amersham Pharmacia]). About 50 μl of glass beads (diameter, 200 μm) was added, and the cells were broken using a FastPrep 120 (Bio 101, Savant) shaker four times (15 s each, with intermittent cooling in ice). Two centrifugations (14,000 × g for 3 and 10 min) resulted in a soluble protein fraction.
Between 20 and 40 μl of the obtained sample was diluted to 350 μl in electrophoresis buffer and applied to 18-cm Immobiline dry strips with an immobilized pH nonlinear gradient, pH 3 to 10. The first dimension was performed on an IPGphor isoelectric focusing unit (Amersham Pharmacia) starting with a rehydration step at 50 V for 12 h at 20°C. The following steps were done at 500 V for 1 h, 1,000 V for 1 h, and 8,000 V until a total of 38,000 Vh was reached after, in total, about 19 h. The strips were equilibrated (15 min in 10 ml of 50 mM Tris-HCl [pH 8.8], 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, 0.001% bromophenol blue, and 65 mM DTT) followed by a 15-min alkylation step in 10 ml of the same solution except replacing DTT with 135 mM iodoacetamide. The second dimension was performed in 12.5% polyacrylamide gels (18 by 18 cm) at a constant current of 24 mA. Molecular weight markers were applied on a small piece of filter paper beside the strip before starting the second dimension. Finally, the gels were stained with silver (15).
The protein was excised from the two-dimensional gel and subjected to in-gel tryptic cleavage (18). Resulting peptides were separated on a SMART high-pressure liquid chromatography (HPLC) system and analyzed in a Q-TOF electrospray instrument (Micromass, Manchester, United Kingdom) (2).
Fasciclin motif protein.
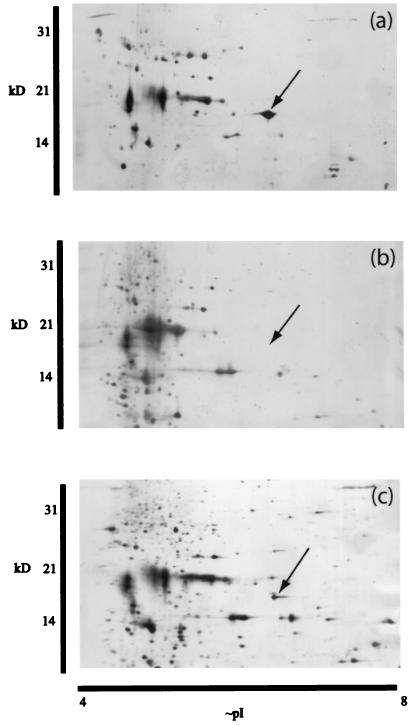
One of the dominating proteins in the soluble fraction from the Nostoc symbiont of the lichen Peltigera membranacea (Fig. 1a) was not detected on the two-dimensional gel of Nostoc punctiforme (Fig. 1b) and was present at a lower level in the free-living isolate of the P. membranacea symbiont (Fig. 1c). A dominating protein was also found in this position in a preparation from the lichen Peltigera neopolydactyla (data not shown).
FIG. 1.
Silver-stained gels obtained by two-dimensional gel electrophoresis showing the soluble protein fraction from different Nostoc strains. (a) Freshly isolated Nostoc symbiont from the lichen Peltigera membranacea. (b) N2-fixing culture of N. punctiforme PCC 73102. (c) Free-living Nostoc strain from the freshly isolated symbiont of P. membranacea. Arrows indicate a position in the gels where a highly expressed protein is present in the freshly isolated lichen cyanobiont (a). This protein was analyzed using mass spectrometry, and the corresponding ORF in the genomic sequence of N. punctiforme ATCC 29133 was identified.
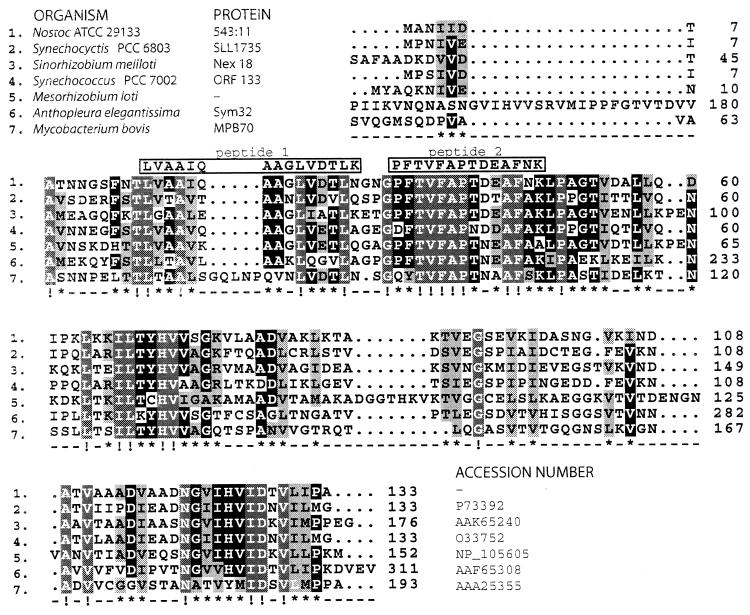
From this protein, two peptide sequences were obtained. Both peptides identified the same protein sequence when used in a Blast search against the Nostoc punctiforme genome sequence (JGI website cited above); one shared all 14 amino acids with the predicted N. punctiforme protein, whereas the other had 1 deviating amino acid out of 15 (Fig. 2). The identified open reading frame (ORF) encodes a putative protein with homology to the fasciclin I domain, found in a family of vertebrate and invertebrate proteins that mediate cell adhesion (7). This domain is also well known from the major mycobacterial antigen MPB70. The five most similar sequences are shown in Fig. 2 together with the sequence of the mycobacterial protein MPB70.
FIG. 2.
Deduced amino acid sequence of the identified ORF in the genomic sequence of N. punctiforme compared to homologous sequences from the databases. The Nostoc protein is identified as 543:11 (contig 543, gene 11). Obtained amino acid sequences from the lichen cyanobiont are shown in boxes above the alignment. Below the alignment the degree of conservation is indicated; !, all identical (white letters on grey); ∗, at least five of seven identical (white on black) or similar (black on grey).
Among these sequences, all bacterial proteins contain a single fasciclin domain, whereas the Anthopleura sequence contains two. The third sequence in the alignment comes from a symbiotically induced gene from the Rhizobium (Sinorhizobium)-legume symbiosis. This rhizobial gene is not essential for the symbiotic competence of this strain. However, a mutation in this locus causes an intermediate phenotype resulting in normal nodulation but a drastic decrease in the percentage of N2-fixing nodules (8, 9). The sequence from Anthopleura elegantissima, a marine cnidarian living in symbiosis with eukaryotic algae, was isolated as a symbiosis-enhanced mRNA (14). The corresponding protein was originally detected as one of the more prominent soluble proteins from symbiotic anemones. Aposymbiotic samples, from anemones not containing the algal symbiont, showed no expression of this protein (17).
The fact that homologues of this fasciclin domain protein are found to be symbiotically relevant in three separate cases in completely different symbiotic systems (Nostoc-lichen, Rhizobium-legume, and alga-cnidarian) is significant and worth further examination. The function of these proteins is not known, but the role of the fasciclin domain in cell adhesion in other organisms could suggest a similar function. The effect of the mycobacterial MPB70 protein expressed in Escherichia coli could indicate an additional function. It was previously shown that when this protein was expressed in E. coli, it was exported to the periplasmic space with the help of a signal peptide. However, it also caused an increased leakage of proteins, both MPB70 and other periplasmic proteins, from the periplasmic space to the culture medium (5). This was suggested to be caused by nonspecific hydrophobic interactions between the MPB70 protein and the outer membrane. An increase in membrane permeability could indeed be relevant in both pathogenicity (Mycobacterium) and symbioses (Nostoc, Sinorhizobium, and Anthopleura).
Acknowledgments
Håkan Larsson and Bo Ek at the Department of Plant Biology, SLU (Uppsala), are thanked for invaluable help with analytical procedures.
This work was funded by the Swedish Natural Science Research Council (NFR), Anna och Gunnar Vidfelts fund, and Oscar och Lilli Lamms fund.
REFERENCES
- 1.Adams, D. G. 2000. Symbiotic interactions, p. 523-561. In B. A. Whitton and M. Potts (ed.), The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 2.Apoga, D., B. Ek, and A. Tunlid. 2001. Analysis of proteins in the extracellular matrix of the plant pathogenic fungus Bipolaris sorokiniana using two-dimensional gel electrophoresis and MS/MS. FEMS Microbiol. Lett. 197:145-150. [DOI] [PubMed] [Google Scholar]
- 3.Besendahl, A., Y.-L. Qiu, J. Lee, J. D. Palmer, and D. Bhattacharya. 2000. The cyanobacterial origin and vertical transmission of the plastid tRNALeu group-I intron. Curr. Genet. 37:12-23. [DOI] [PubMed] [Google Scholar]
- 4.Enderlin, C. S., and J. C. Meeks. 1983. Pure culture and reconstitution of the Anthoceros and Nostoc symbiotic association. Planta 158:157-165. [DOI] [PubMed] [Google Scholar]
- 5.Hewinson, G., and W. P. Russel. 1993. Processing and secretion by Esherichia coli of a recombinant form of the immunogenic protein MPB70 of Mycobacterium bovis. J. Gen. Microbiol. 139:1253-1259. [DOI] [PubMed] [Google Scholar]
- 6.Johansson, C., and B. Bergman. 1994. Reconstitution of the symbiosis of Gunnera manicata Linden: cyanobacterial specificity. New Phytol. 126:643-652. [Google Scholar]
- 7.McAllister, L., C. S. Goodman, and K. Zinn. 1992. Dynamic expression of the cell adhesion molecule fasciclin I during embryonic development in Drosophila. Development 115:267-276. [DOI] [PubMed] [Google Scholar]
- 8.Oke, V., and S. R. Long. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol. Microbiol. 32:837-849. [DOI] [PubMed] [Google Scholar]
- 9.Oke, V., and S. R. Long. 1999. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 2:641-646. [DOI] [PubMed] [Google Scholar]
- 10.Paulsrud, P., and P. Lindblad. 1998. Sequence variation of the tRNALeu (UAA) intron as a marker for genetic diversity and specificity of symbiotic cyanobacteria in some lichens. Appl. Environ. Microbiol. 64:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulsrud, P., J. Rikkinen, and P. Lindblad. 1998. Cyanobiont specificity in some Nostoc-containing lichens and in a Peltigera aphthosa photosymbiodeme. New Phytol. 139:517-524. [Google Scholar]
- 12.Paulsrud, P., J. Rikkinen, and P. Lindblad. 2000. Spatial patterns of photobiont diversity in some Nostoc-containing lichens. New Phytol. 146:291-299. [DOI] [PubMed] [Google Scholar]
- 13.Rai, A. N., E. Söderback, and B. Bergman. 2000. Cyanobacterium-plant symbioses. New Phytol. 147:449-481. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds, W. S., J. A. Schwarz, and V. M. Weis. 2000. Symbiosis-enhanced gene expression in cnidarian-algal associations: cloning and characterization of a cDNA, sym32, encoding a possible cell adhesion protein. Comp. Biochem. Physiol. 126:33-44. [DOI] [PubMed] [Google Scholar]
- 15.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 16.Wastlhuber, R., and E. Loos. 1996. Differences between cultured and freshly isolated cyanobiont from Peltigera -- is there symbiosis-specific regulation of a glucose carrier. Lichenologist 28:67-78. [Google Scholar]
- 17.Weis, V. M., and R. P. Levine. 1995. Differential protein profiles reflect the different lifesyles of symbiotic and aposymbiotic Anthopleura elegantissima, a sea anemone from temperate waters. J. Exp. Biol. 199:883-892. [DOI] [PubMed] [Google Scholar]
- 18.Wilm, M., A. Shevchenko, T. Houthaeve, S. Breit, L. Schweigerer, T. Fotsis, and M. Mann. 1996. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379:466-468. [DOI] [PubMed] [Google Scholar]