Abstract
In this study, we examined the effect that magnetic materials and pH have on the recoveries of Cryptosporidium oocysts by immunomagnetic separation (IMS). We determined that particles that were concentrated on a magnet during bead separation have no influence on oocyst recovery; however, removal of these particles did influence pH values. The optimal pH of the IMS was determined to be 7.0. The numbers of oocysts recovered from deionized water at pH 7.0 were 26.3% higher than those recovered from samples that were not at optimal pH. The results indicate that the buffers in the IMS kit did not adequately maintain an optimum pH in some water samples. By adjusting the pH of concentrated environmental water samples to 7.0, recoveries of oocysts increased by 26.4% compared to recoveries from samples where the pH was not adjusted.
The ability of immunomagnetic separation (IMS) to separate Cryptosporidium oocysts from sediments in concentrated samples is better than that of Percoll-sucrose flotation. Oocyst recoveries by Percoll-sucrose flotation of 10 to 96% have been observed when various sediment matrices originating from environmental water samples were spiked with oocysts (1, 6). IMS has shown more-consistent recovery efficiencies and has produced more-reproducible recoveries from higher-turbidity matrices than has flotation (3, 4). Although IMS has marked advantages over flotation methods, variation in IMS results or recovery efficiency by U.S. Environmental Protection Agency method 1622 has been observed with some environmental samples (1, 2, 5, 9, 10).
The objectives of this study were to identify characteristics of resuspended packed pellets from concentrated water samples that adversely affect the recovery efficiency of a commercially available IMS system and develop modifications of the IMS methodology that would provide greater efficiency and consistency in oocyst recovery. The two IMS variables that were examined were the possible interference of iron-like materials (sediments that bind to magnets) and the effect of pH on the efficiency of oocyst capture during the IMS process.
Oocysts.
Mouse feces containing Cryptosporidium parvum oocysts (human or mouse strain AZ-1) were purchased from Parasitology Research Laboratories, LLC (Neosho, Mo.). The oocysts were purified from the mouse feces by sucrose enrichment (8) and then with a cesium chloride (CsCl) gradient (11). The oocysts were enumerated via flourescent-antibody (FA) assay prior to use. The FA assay was performed as previously described (7). Oocyst dilution and enumeration were performed according to U.S. Environmental Protection Agency method 1622. The oocysts were used within 3 months.
Removal of magnetic material.
Ten-liter samples of Rio Grande (Las Cruces, N. Mex.) and Fountain River (Pueblo, Colo.) water were concentrated by hollow-fiber ultrafiltration (AHP-1010 Microzoa; Pall Corp., Glen Cove, N.Y.). Also, a 100-ml sample of each water was collected and tested for total iron content (inductively coupled plasma method) by an independent lab. The concentrated particulates in the retentate were pelleted via centrifugation at 1,200 × g for 20 min. The resulting pellet was resuspended in enough deionized (DI) water (10 ml) to produce 2 volumes of packed pellet (≤0.5 ml). The solutions were transferred to two screw-cap Leighton tubes. One tube (without IMS beads) was placed on an MPC-1 magnet (Dynal, Oslo, Norway). The tube was rocked back and forth on the magnet for 2 min, and then with the tube still on the magnet, the contents of the tube were poured into a clean 50-ml centrifuge tube. The material concentrated by the magnet was suspended in 1 ml of water and then pipetted from the Leighton tube into a 2-ml centrifuge tube. The 2-ml centrifuge tube containing the magnetic material was then placed back on the magnet, and the water was removed. The accumulated magnetic materials from the five magnet treatments were pooled into a 2-ml centrifuge tube and pelleted, and the wet weight was recorded. The suspended sediment sample that was treated with the magnet was then transferred back to the Leighton tube from the 50-ml centrifuge tube. The tube was placed back on the magnet and the steps described above were repeated five times. Each of the Leighton tubes (one was a concentrated control with no treatment and the other had its iron-like material removed) was then spiked with 1,000 oocysts. Then the kit buffers and magnetic beads were added. The IMS was then carried out with a 1-h capture step by following the manufacturer's instructions. The oocysts were disassociated from the beads, and the recovered oocysts were enumerated via FA assay. The pH values of the samples were also recorded before and after the magnet treatment prior to the addition of SL buffers provided with the IMS kit (Dynal).
The materials collected from magnet-treated concentrates originating from 10 liters of Rio Grande and Fountain River water weighed 15 and 52 mg, respectively. The materials varied in color from dark black to a rust color and completely covered most of the side of the tube where the magnet was placed. Magnetic material was removed each time the sample was placed on the magnet (five treatments); however, the majority of the magnetic material was recovered during the first two treatments. Samples from which the magnetic material was removed and which were then spiked with oocysts prior to purification by IMS showed mean oocyst recoveries of 31.3% (standard deviation [SD] = 2.4%) for the Rio Grande samples and 36.5% (SD = 3.6%) for the Fountain River samples, while the unmanipulated samples (no magnetic pretreatment) from the same sites demonstrated mean oocyst recoveries of 58.9% (SD = 2.8%) and 65.4% (SD = 1.6%), respectively (three samples were used for each calculation). When a Wilcoxon test was performed to compare the recovery values for the treated and nontreated samples, the differences were found to be not significant (P = 0.19). The iron content of the Rio Grande sample was 0.59 mg/liter, while the Fountain River sample had a much higher iron content of 3.59 mg/liter. The pH of the Rio Grande sample changed from 7.95 to 8.38 when the magnetic material was removed and that of the Fountain River sample changed from 7.66 to 8.12 following removal of the magnetic material.
During our experiments with spiked oocyst challenges, we have encountered occasional samples in which IMS recoveries have produced poor recovery efficiencies (data not shown). In some water samples, we have also observed a large amount of iron or iron-like material being concentrated with the magnetic beads during IMS. In experiments to examine the effects of iron on oocyst recovery, magnetic material did not appear to have an adverse effect on IMS recovery efficiencies. In fact, the opposite was true; removal of the magnetic material resulted in lower oocyst recovery efficiencies. This may be due to a pH increase when the iron-like material was removed. Also, Rio Grande and Fountain River water samples produced similar oocyst recovery efficiencies even when the Fountain River sample contained almost six times the amount of iron as the Rio Grande sample, indicating that total iron concentration may have little effect on oocyst recoveries.
Effect of pH on suspended-oocyst recovery in DI water.
Anti-Cryptosporidium kits (Dynal) were used for all IMS experiments. Ten milliliters of DI water and 1 ml of each SL buffer supplied with the IMS kit were added to three 50-ml centrifuge tubes. The pH of one tube was adjusted to 6.5 by the addition of 1 N hydrochloric acid (HCl), the second tube was adjusted to pH 7.5 by the addition of 1 N sodium hydroxide (NaOH), and the third tube was left at pH 7.0. The solutions were then transferred to screw-cap Leighton tubes, and each was spiked with 226 oocysts. The IMS was then carried out with a 1-h capture step by following the manufacturer's instructions. The preparation of concentrated oocysts was disassociated from the beads, and the recovered oocysts were enumerated via FA assay.
The mean rate of recovery of oocysts in the neutral-pH solutions was 96.2% (SD = 1.5%). The mean recovery efficiency decreased to 51.2% (SD = 5.8%) when the pH was raised to 7.5 and to 49.5% (SD = 7.2%) when the pH was lowered to 6.5 (n = 3).
Preparation of sample concentrates for pH experiments.
Forty-liter samples of surface water were collected from the Rio Grande (Las Cruces, N. Mex.) on 8 and 11 November 2000. These samples were used because low oocyst recoveries were produced previously with Rio Grande Water from seededsample experiments by the standard IMS procedure. Ten-liter aliquots from each sample were concentrated by using a hollow-fiber ultrafilter (AHP-1010 Microzoa; Pall Corp.) and an Envirochek capsule (Pall Gelman Laboratory, Ann Arbor, Mich.). The oocysts and particulate matter were then pelleted by centrifugation at 1,200 × g for 20 min. The resulting pellet was resuspended in an equivalent amount of DI water to produce a 10% sediment stock solution. Stock pellet samples were designated 11-8 and 11-11 on the basis of the water collection dates. From this 10% stock solution, samples were either diluted further or recentrifuged, depending on the desired packed-pellet volume. A single 0.5-ml packed-pellet volume was used to determine endogenous oocyst concentrations. In addition to the Rio Grande samples, we tested concentrated pellets from Canadian water sources supplied by Andrew Campbell (AUREON Biosystems, Vienna, Austria).
Effect of the SL buffers on pellet pH.
Packed-pellet volumes of 0.1, 0.5, 1.0, 1.5, and 2.0 ml in 10 ml of DI water were produced with the 11-8 Rio Grande stock solution. The pH of each solution was measured prior to the addition of the SL buffers. One milliliter of each SL buffer was added to each of the samples; the samples were then mixed, and the pHs of the samples were again measured while they were stirred. The pH was then measured every 30 min for 120 min. The initial pH of the unconcentrated Rio Grande water samples used for this experiment was 8.5.
The 0.1-, 0.5-, 1.0-, and 2.0-ml pellets suspended in 10 ml of DI water exhibited mean pH values of 8.01, 8.02, 8.50, and 8.61, respectively. With the addition of the SL buffers, the pH of each solution was changed to 7.00, 7.05, 7.09, and 7.11, respectively, at time zero. However, the pH slowly increased at each 0.5-h time increment for the entire 120-min sampling period. The final pH values recorded for each sample were 7.08, 7.15, 7.26, and 7.35, respectively (one sample for each pellet volume).
Stability of adjusted pellet pH over time.
To determine if a stable and optimal pH could be maintained during the capture stage, SL buffers were added to a 0.5-ml pellet that was produced from the 11-11 stock. The pH was then further adjusted to 7.0 and measured every 30 min for 150 min.
Since the pH of the pellet was adjusted after the addition of the SL buffers, it remained closer to the optimum range than that of a nonadjusted sample after the 150-min incubation. The initial pH of the nonadjusted 0.5-ml packed pellet following the addition of the SL buffers was 7.04. The pH for each sample continued to increase slightly at each 0.5-h increment. The final pH of the adjusted sample was 7.11 and that of the nonadjusted sample was 7.18 after the 150-min incubation period.
Recovery of oocysts from pH-adjusted pellets from concentrated water samples.
Two equivalent 0.5-ml packed-pellet solutions were prepared for each water sample tested. One sample from the 11-8 stock and two concentrated Canadian water samples were used. The SL buffers were added to each of the tubes, and the solutions were mixed. The pH of one solution was further adjusted to 7.0 by the addition of 1 N HCl or 1 N NaOH. The pH of the second solution was not adjusted. The pH of each solution was then recorded, and the samples were then transferred to screw-cap Leighton tubes and spiked with oocysts. The IMS was then carried out with a 1-h capture step according to the manufacturer's instructions. The oocysts were disassociated from the beads, and recovered oocysts were enumerated via FA assay.
The percentage of oocysts recovered from the concentrated Rio Grande sample was 33.6% when the pH of the 10-ml concentrate was not adjusted and 63.2% when it was adjusted. The pH of the nonadjusted Rio Grande sample was 7.48 following the addition of the SL buffers. The first Canadian water sample was acidic, with a pH of 6.46 following addition of the SL buffers. Adjustment of the pH to 7.00 produced an oocyst recovery efficiency of 62.8%, while the recovery efficiency for the nonadjusted sample was 44.9%. The second Canadian water sample had a pH of 6.89 following the addition of the SL buffers. The adjustment of the pH produced a small improvement, with a 61.3% recovery efficiency compared to 59.2% for the unadjusted sample (Table 1). The mean recovery from the pH-adjusted pellets from the three sites was 62.4% (SD = 0.81%), and it was 45.9% (SD = 10.4%) from the unadjusted samples (n = 3).
TABLE 1.
Oocyst recovery efficiencies from pH-adjusted and nonadjusted Rio Grande, Canada 1, and Canada 2 pellets
| Water sample | Initial pH | pH during capture step | Oocyst seed densitya | Recovery efficiency (%)d |
|---|---|---|---|---|
| Rio Grandeb | 8.38 | 7.48 | 126,000 | 33.6 |
| 8.38 | 7.00c | 126,000 | 63.2 | |
| Canada 1 | 5.00 | 6.46 | 563 | 44.9 |
| 5.00 | 7.00c | 563 | 62.8 | |
| Canada 2 | 5.18 | 6.89 | 423 | 59.2 |
| 5.18 | 7.00c | 423 | 61.3 |
The pellets were spiked with oocysts prior to IMS. Values are numbers of oocysts per 10 liters.
Stock pellet sample 11-8.
The pH was further adjusted after the addition of the SL buffers.
The mean recovery efficiencies were 62.4% (SD = 0.81%) for the pH-adjusted pellets and 45.9% (SD = 10.4%) for the unadjusted pellets.
Seeded 10-liter-sample oocyst challenges.
The water samples used for oocyst challenge experiments were from the Rio Grande (Las Cruces, N.Mex.) and Arkansas (Pueblo, Colo.) Rivers and raw water from the following utilities: Cobb CountyMarietta Water Authority (Marrietta, Ga.), the Authority of Charleroi (Charleroi, Pa.), Nottingham Water (Cleveland, Ohio), and Hetch Hetchy Water and Power (Moccasin, Calif.). The samples were concentrated by hollow-fiber ultrafiltration or with a Gelman Envirochek capsule. The retentate was further concentrated by centrifugation at 1,200 × g for 20 min. The resulting pellet volume was measured and resuspended in enough DI water so that two equivalent samples of packed pellets (≤0.5 ml) could be obtained from each water sample. The SL buffers were added, and the pH was measured. The pH of one sample was adjusted to 7.0 by the addition of either 1 N HCl or 1 N NaOH. The pH of the second sample was unmanipulated. The IMS was then carried out with a 1-h capture step according to the manufacturer's instructions. The oocysts were disassociated from the beads, and the recovered oocysts were enumerated via FA assay.
Concentration of the Rio Grande sample by use of the Envirochek cartridge resulted in oocyst recovery efficiencies of 0.5% for the nonadjusted pellet (pH 7.42) and 27.9% for the adjusted pellet. The recovery efficiencies were 27.5 and 59.8%, respectively, for the nonadjusted and adjusted pellets of the Rio Grande sample concentrated by hollow-fiber ultrafiltration. The efficiencies of recovery of oocysts from the Arkansas River sample were 32.8% for the nonadjusted (pH 7.28) sample and 53.7% for the adjusted sample. The oocyst recovery efficiency for the unadjusted Cobb County sample (pH 7.12) was 19.8%, while that for the adjusted sample was 51.9%. The percentage of oocysts recovered from the untreated Charleroi sample (pH 7.24) was 43.0%, while that from the adjusted sample was 60.3%. Adjustment of the pH produced less of an effect on the Nottingham and Hetch Hetchy samples. The recovery efficiencies for the unadjusted samples (pH 7.28 and 7.51, respectively) from these two sources were 6.8 and 14.1%, respectively, and those for the the adjusted samples were 15.6 and 29.4%, respectively (Table 2). The mean percentage of oocysts recovered from seeded 10-liter samples with pH adjustment was 47.4% (SD = 11.5%), and it was 21.0% (SD = 13.0%) for unadjusted pellets (n = 8). The difference in the oocyst recovery efficiencies between adjusted and unadjusted samples (including concentrated samples and 10-liter spiked samples) was statistically significant (Wilcoxon P value, 0.01).
TABLE 2.
Oocyst recovery efficiencies from seeded 10-liter samples with and without pellet adjustment prior to oocyst capture
| Water sample | pH during oocyst capture | Oocyst seed densitye | Turbidity (NTU)c | Recovery efficiency (%)d |
|---|---|---|---|---|
| Rio Grande 1a | 7.28 | 1,280 | 106.0 | 24.7 |
| 7.00 | 1,280 | 106.0 | 59.2 | |
| Rio Grande 2b | 7.42 | 127,000 | 159.0 | 0.4 |
| 7.00 | 127,000 | 159.0 | 27.9 | |
| Rio Grande 2a | 7.42 | 127,000 | 159.0 | 27.5 |
| 7.00 | 127,000 | 159.0 | 59.8 | |
| Arkansas 1a | 7.28 | 1,063 | 1.7 | 32.8 |
| 7.00 | 1,063 | 1.7 | 55.8 | |
| Cobb Countya | 7.12 | 131 | 9.9 | 19.8 |
| 7.00 | 131 | 9.9 | 51.9 | |
| Charleroia | 7.24 | 105 | 6.8 | 43.0 |
| 7.00 | 105 | 6.8 | 60.3 | |
| Nottinghama | 7.28 | 205 | 21.6 | 6.8 |
| 7.00 | 205 | 21.6 | 15.6 | |
| Hetch Hetchya | 7.51 | 156 | 0.2 | 14.1 |
| 7.00 | 156 | 0.2 | 29.4 |
The sample was concentrated by hollow-fiber ultrafiltration.
The sample was concentrated by using the Envirochek filter.
NTU, nephelometric turbidity units.
The mean recovery efficiencies were 47.4% (SD = 11.5%) for adjusted samples (including four samples with pH 7.0 after the addition of the SL buffers [Table 3]) and 21.0% (SD = 13.0%) for nonadjusted samples (excluding the Rio Grande sample concentrated by use of the Envirochek filter).
Values are numbers of oocysts per 10 liters.
A linear-regression analysis was used to determine the effects of turbidity and oocyst seed density on oocyst recoveries. Values for both variables were found to be not significant (P values = 0.210 and 0.604, respectively).
The impact of pH on oocyst recovery may be particularly important in the southwestern United States because water tends to be more basic in this region, as irrigation and storm runoff can accentuate the alkalinity of the samples. Our observations with the Rio Grande samples indicate that the pellet pH was more basic during the irrigation season than during the nonirrigation season. Changes in recovery efficiencies were likely due in part to changes in antibody affinity that can occur when the pH moves away from the optimal pH for antigen-antibody interactions.
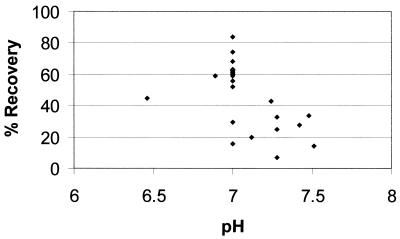
The experiments also showed that the pH of the suspended pellet may not remain constant during the course of oocyst capture from environmental samples. The SL buffers can buffer the IMS sample to an approximately neutral pH; however, the pH did not remain neutral but slowly became increasingly basic. The influence of pH on oocyst recovery was verified when improved recoveries were obtained when the pellet pH was adjusted back to a neutral pH after the addition of the SL buffers (Fig. 1). In some water samples, adjustment of the pellet pH did not keep the sample pH neutral; however, it did allow for the pH of the sample to remain closer to neutral for the duration of the capture step than it would have been if the pH was never adjusted.
FIG. 1.
Oocyst recovery efficiencies at various pH values of concentrated pellets following addition of IMS SL buffers.
Some of the recovery efficiencies did not increase as much in some samples as in others after adjustment of the pH. The Nottingham and Hetch Hetchy adjusted samples demonstrated slight improvements in oocyst recovery over that of the unadjusted samples but still produced poor overall oocyst recoveries. These results indicate that pH does play an important role in oocyst recovery for many samples that had previously shown poor recovery efficiencies; however, pH alone may not be the only factor. Connell et al. reported a combined mean recovery of 20.6% from Cobb-Marietta, Charleroi, Nottingham, and Hetch Hetchy water samples (5). Our combined means for these samples were 21% for nonadjusted samples and 39.3% for pH-adjusted samples. The Canadian water samples with an acidic pH demonstrated improved recovery efficiencies with pH adjustment (Table 2). We observed that if the pH was near neutral after the addition of the SL buffers, consistent and efficient oocyst recoveries were observed without any adjustment of pH (Table 3).
TABLE 3.
Oocyst recovery efficiencies from seeded 10-liter samples that required no pH adjustment prior to IMS capture
| Water sample source | pH during oocyst capture | Oocyst seed densitya | Turbidity (NTU)b | Recovery efficiency (%)c |
|---|---|---|---|---|
| San Juan River | 7.00 | 983 | 5.7 | 68.3 |
| Fountain River | 7.00 | 146 | 6.9 | 62.0 |
| Arkansas River | 7.00 | 7,079 | 12.0 | 84.0 |
| Rio Grande | 7.00 | 83 | 3.9 | 74.1 |
Values are numbers of oocysts per 10 liters.
NTU, nephelometric turbidity units.
The mean oocyst recovery efficiency was 72.1% (SD = 8.0%).
If the SL buffers can buffer a sample to a neutral pH and prevent changes in pH, it is conceivable that pellet size may play less of a role in oocyst recovery. This may account for the reported recovery efficiencies of over 50% seen in some large pellets (3 ml) (1). Adjustment of pellet pH may allow for larger pellet volumes to be tested, since greater pH variation can be generated with larger pellet size.
The results of this study indicate that the buffering capacity of the SL buffers may not be enough for all water matrices and that improved buffers may be needed in more acidic or basic samples. Overall, the buffers seemed to be better at buffering the more acidic samples than the more basic samples, since samples with pH values of 5.18 and higher were buffered to a neutral pH. This large buffering capacity was not seen in alkaline samples. It is also possible, as has been reported in this study, to adjust the pH manually after the addition of the SL buffers.
In conclusion, iron or iron-like materials concentrated with oocysts do not seem to influence oocyst recovery efficiencies. The pH of the solution during the oocyst capture step plays a more important role in affecting oocyst recovery than does the presence of particulate iron or other magnetic particles. In fact, we have been able to predict lower IMS recoveries by checking the pH of the pellet prior to the capture step. It is possible to enhance the performance of the IMS in some matrices by adjusting the pH of the capture step. When the pH of the 10-ml suspension was adjusted to 7.0, the mean recoveries increased an average of 26.3% compared to recoveries from samples that were not adjusted. These results indicate the need for an improved buffering system for water samples for which oocyst recovery is not possible with the currently used buffers.
Acknowledgments
This research was supported by the Southwest Center for Environmental Research & Policy (1-4-22328) and the New Mexico Water Research Institute (1-4-23949).
We thank Jennifer Scheller and Kevin Connell for providing us with contact information and water history data for the utility-provided water samples that we tested. We thank CobbCounty-Marietta Water Authority, Nottingham Water, Hetch Hetchy Water and Power, and the Authority of Charleroi for supplying us with water from their utilities.
REFERENCES
- 1.Bukhari, Z., R. M. McCuin, C. R. Fricker, and J. L. Clancy. 1998. Immunomagnetic separation of Cryptosporidium parvum from source water samples of various turbidities. Appl. Environ. Microbiol. 64:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell, A., and H. Smith. 1997. Immunomagnetic separation of Cryptosporidium oocysts from water samples: round robin comparison of techniques. Water Sci. Technol. 35:397-401. [Google Scholar]
- 3.Campbell, A. T., B. Gron, and S. E. Johnsen. 1997. Immunomagnetic separation of Cryptosporidium oocysts from high turbidity water sample concentrates, p. 91-96. In Proceedings of the International Symposium on Waterborne Cryptosporidium. American Water Works Association, Denver, Colo.
- 4.Clancy, J. L., C. R. Fricker, and W. Telliard. 1997. New USEPA standard method for Cryptosporidium analysis in water. In Proceedings of the AWWA Water Quality Technology Conference. American Water Works Association, Denver, Colo. [CD-ROM].
- 5.Connell, K., J. Scheller, K. Miller, and C. C. Rodgers. 2000. Performance of methods 1622/23 in the ICR supplemental surveys. In Proceedings of the AWWA Water Quality Technology Conference, Salt Lake City, Utah. American Water Works Association, Denver, Colo. [CD-ROM].
- 6.Fricker, C. R. 1995. Detection of Cryptosporidium and Giardia in water, p. 91-96. In W. B. Betts, D. P. Casemore, C. R. Fricker, H. V. Smith, and J. Watkins (ed.), Protozoan parasites and water. The Royal Society of Chemistry, Cambridge, England.
- 7.Kuhn, R. C., and K. H. Oshima. 2001. Evaluation and optimization of a reusable hollow fiber ultrafilter as a first step in concentrating Cryptosporidium oocysts from water. Water Res. 35:2779-2783. [DOI] [PubMed] [Google Scholar]
- 8.Nesternko, M. V. 1997. Sucrose suspension technique, p. 186. In J. M. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 9.Rochelle, P. A., R. De Leon, A. Johnson, M. H. Stewart, and R. L. Wolf. 1999. Evaluation of immunomagnetic separation for recovery of infectious Cryptosporidium parvum oocysts from environmental samples. Appl. Environ. Microbiol. 65:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons, O. D., III, M. D. Sobsey, C. D. Heaney, F. W. Schaefer III, and D. S. Francy. 2001. Concentration and detection of Cryptosporidium oocysts in surface water samples by method 1622 using ultrafiltration and capsule filtration. Appl. Environ. Microbiol. 67:1123-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taghi-Kilani, R., and L. Sekla. 1987. Purification of Cryptosporidium oocysts and sporozoites by cesium chloride and Percoll gradients. Am. J. Trop. Med. Hyg. 36:505-509. [DOI] [PubMed] [Google Scholar]