Abstract
A green fluorescent protein-based Pseudomonas fluorescens strain A506 biosensor was constructed and characterized for its potential to measure benzene, toluene, ethylbenzene, and related compounds in aqueous solutions. The biosensor is based on a plasmid carrying the toluene-benzene utilization (tbu) pathway transcriptional activator TbuT from Ralstonia pickettii PKO1 and a transcriptional fusion of its promoter PtbuA1 with a promoterless gfp gene on a broad-host-range promoter probe vector. TbuT was not limiting, since it was constitutively expressed by being fused to the neomycin phosphotransferase (nptII) promoter. The biosensor cells were readily induced, and fluorescence emission after induction periods of 3 h correlated well with toluene, benzene, ethylbenzene, and trichloroethylene concentrations. Our experiments using flow cytometry show that intermediate levels of gfp expression in response to toluene reflect uniform induction of cells. As the toluene concentration increases, the level of gfp expression per cell increases until saturation kinetics of the TbuT-PtbuA1 system are observed. Each inducer had a unique minimum concentration that was necessary for induction, with Kapp values that ranged from 3.3 ± 1.8 μM for toluene to 35.6 ± 16.6 μM for trichloroethylene (means ± standard errors of the means), and maximal fluorescence response. The fluorescence response was specific for alkyl-substituted benzene derivatives and branched alkenes (di- and trichloroethylene, 2-methyl-2-butene). The biosensor responded in an additive fashion to the presence of multiple inducers and was unaffected by the presence of compounds that were not inducers, such as those present in gasoline. Flow cytometry revealed that, in response to toxic concentrations of gasoline, there was a small uninduced population and another larger fully induced population whose levels of fluorescence corresponded to the amount of effectors present in the sample. These results demonstrate the potential for green fluorescent protein-based bacterial biosensors to measure environmental contaminants.
Contamination of soils and surface and subsurface water supplies with petroleum products is a serious environmental problem. Of particular concern for drinking water quality are water-soluble aromatic components (e.g., benzene, toluene, ethylbenzene, and xylenes) of petroleum products. Although many of these contaminants are readily biodegradable, they often persist in the environment. Our understanding of why they persist is poor, and explanations of persistence are undoubtedly complex. There are a variety of factors that can limit biodegradation, such as low availability of pollutants to biodegraders (2, 12) due to sorption of pollutants to soil constituents, poor degradative capabilities of organisms present, toxic pollutant concentrations, heterogeneity of degraders and bioavailable concentrations of pollutants, and physiochemical conditions that affect bacteria and the pollutants themselves (8, 9, 27, 30). There is a need to know whether conditions favorable for biodegradation occur in a contaminated site and whether pollutant availability limits biodegradation. In particular, we have a relatively poor understanding of bacterial degradation of pollutants in situ in soil systems and how habitat-specific features affect bioavailability and biodegradation at scales relevant to microorganisms.
Microorganisms are increasingly being used as specific and sensitive sensing devices for measuring biologically relevant concentrations of pollutants. These biosensors rely on analysis of gene expression, typically by creating transcriptional fusions between a promoter of interest and a reporter gene, and the extent of reporter gene expression serves as a measure of the available concentration of a pollutant. Commonly used reporter genes include lacZ, luxAB, and luxCDABE (1, 4, 19, 31, 38, 40, 41, 43). Bioluminescence has been very successful as a reporter for pollutant detection in part because of the sensitive instrumentation (fiber optic probes, integrated circuit chips) available for detecting light production (14, 35), and if the entire luxCDABE gene cassette is used the addition of an exogenous substrate for signal production is not required. Unfortunately, bioluminescence is not compatible with fluorescent in situ hybridization or scanning confocal laser microscopy, which is increasingly being used to characterize microbial community level processes and structure and function relationships at microbially relevant scales. The gene for green fluorescent protein (GFP) from Aequoria victoria (7, 13, 39) is increasingly being used to construct whole-cell biosensors (3, 6, 17, 21, 23), in part because it allows for in situ assessments of bioavailability, although it has not been used extensively as a reporter for measuring biologically relevant concentrations of pollutants.
In the present study we describe the construction and characterization of a GFP-based Pseudomonas fluorescens strain A506 whole-cell biosensor for the detection of toluene and other monosubstituted benzene derivatives. This biosensor was constructed by creating a transcriptional fusion between gfp and the toluene-benzene utilization (tbu) pathway promoter (PtbuA1) of Ralstonia pickettii PKO1. The tbu operon encodes a toluene-3-monooxygenase, and the pathway has a broad effector range, including many monosubstituted benzene derivatives and trichloroethylene (TCE) (5, 26). The biosensor strain also contained the PtbuA1 promoter transcriptional activator (TbuT) fused to the constitutive neomycin phosphotransferase (PnptII) promoter that was carried on a broad-host-range promoter probe vector (24, 25).
MATERIALS AND METHODS
Chemicals and media.
Unless stated otherwise, all chemicals were obtained from Fisher Scientific (Pittsburgh, Pa.) and were at least of American Chemical Society grade. Difco Inc. (Detroit, Mich.) supplied Luria-Bertani medium (LB), Casamino Acids, yeast extract, and agar. Yeast extract-succinate (YES) medium contains (per liter) 0.5 g of NH4Cl, 1.73 g of Na2HPO4 · 7H2O, 1.38 g of KH2PO4, 7.4 mM disodium succinate, 0.02% yeast extract, 10 ml of Hutner's mineral solution (36) (pH 6.8), and 50 μg of kanamycin (Km) ml−1. Modified M9 medium contained (per liter) 6.0 g of Na2HPO4, 3.0 g of KH2PO4, 0.5 g of NaCl, 0.535 g of NH4Cl, 1 mM MgSO4, 0.1 mM CaCl2, 7.4 mM disodium succinate, 1 mg of thiamine, 0.3% Casamino Acids, 50 μg of Km ml−1, and, when necessary, 15 g of Noble agar. All potential inducers were filter sterilized with a 0.2-μm-pore-size Teflon syringe filter (Pall Corp., Ann Arbor, Mich.) before use and were added undiluted or as aqueous solutions, depending on the desired final concentration. Saturated solutions of these compounds were prepared by mixing excess amounts with 50 ml of sterile distilled deionized water in sterile test tubes fitted with a Mini-nert cap (Alltech, Deerfield, Ill.). Solutions were incubated at 28°C for 24 to 48 h and then stored at room temperature. Solutions of catechol, benzoate, and salicylate (80 mg liter−1 in YES) were prepared in YES, and saturated solutions of phenanthrene (1 mg liter−1), naphthalene (10 mg liter−1), and biphenyl (7.5 mg liter−1) were prepared by adding excess amounts of each chemical to 250 ml of YES and stirring the solution for 18 h at room temperature prior to filter sterilization.
Molecular biology techniques.
Recombinant DNA techniques were performed according to published protocols (29). Plasmid minipreps were done with the Wizard Plus Minipreps DNA Purification System (Promega Inc., Madison, Wis.). When necessary, we gel purified DNA fragments from agarose gels with the Elu-Quick DNA purification kit (Schleicher & Schuell, Keene, N.H.). General DNA purification was performed with the Wizard DNA Purification Kit (Promega). All restriction endonucleases, T4 polynucleotide kinase, and T4 ligase were obtained from Promega. PCR and sequencing primers were synthesized at the Iowa State University DNA Sequencing and Synthesis Facility. Nucleotide sequences were determined at the same facility with an Applied Biosystems Model 377 Prism DNA Sequencer (Perkin-Elmer Inc., Foster City, Calif.).
pTS construction.
PtbuA1 and its upstream activation sequence were PCR amplified from pKRZ1-p352 X/S with primers PTBUA1 (5′-GCGCTCGGATCCATTCTTACCA-3′) and PTBUA2 (5′-ATATAAGGATCCGTCCAGTTGGTCG-3′) (engineered BamHI recognition sites are italicized and in bold). PCR conditions were as follows: 95°C for 5 min followed by 50 cycles of 95°C for 30 s, 68°C for 30 s, and 72°C for 2 min, followed by 72°C for 7 min. Pfu DNA polymerase and deoxynucleoside triphosphates were from Stratagene (La Jolla, Calif.). Purified PCR product was treated with T4 polynucleotide kinase and ligated into the SmaI site of pGEM-7 (Promega) in a nondirectional manner to create pG-7.1, and a BamHI fragment containing the promoter was subcloned into pGEM-7 to create pG-7.2 (Table 1). DNA sequencing and restriction digest analysis of the fragment verified proper sequence and orientation.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rk− mk+) PhoA supE44 λ− thi-1 gyrA96 relA1 | Gibco/BRL |
| DH5α(pTS) | Kmr | This work |
| XL1-Blue MRF′-Kan | mcrA183(mcrCB-hsdSMR-mrr) 173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB lacIqΔM15Tn5 (Kmr)] | Stratagene |
| P. fluorescens | ||
| A506 | Rfr | 22 |
| A506(pTS) | Pollutant biosensor used in this work; Rfr Kmr | This work |
| Plasmids | ||
| pKRZ1-p352 X/S | A 352-bp sequence containing PtbuA1 plus an upstream activator sequence fused to lacZ on pKRZ1 | 5 |
| pRO1614-tbuT | pRO1614 with a 3.1-kb EcoRI-PvuII fragment containing tbuT | 26 |
| pRK2073 | pRP4; Smr | 11 |
| pGEM-7 | Apr | Promega |
| pG-7.1 | pGEM-7 carrying nondirectionally oriented PtbuA1 cloned into SmaI site | This work |
| pG-7.2 | pGEM-7 carrying directionally oriented PtbuA1 cloned into BamHI site | This work |
| pG-7.3 | pG-7.2 carrying PnptII oriented in the opposite direction of PtbuA1 | This work |
| pG-7cass | pG-7.3 carrying tbuT driven by PnptII | This work |
| pPROBE-NT | Promoter-probe vector with promoterless gfp, pBBR1 ori | 24 |
| pTS | pPROBE-NT carrying the PtbuA1-PnptII-tbuT cassette (pG-7cass) | This work |
| pPnptII:gfp | pPROBE-NT carrying a PnptII-gfp fusion | C. Casavant |
The neomycin phosphotransferase promoter (PnptII) was amplified from pVSP61 (25) by using primers NPT1 (5′-GGTACCGTCAGGCTGTAACAGCTCAGA-3′) and NPT2 (5′-GAATTCATCCTGTCTCTTGATCAGATCTTG-3′) containing engineered KpnI and EcoRI recognition sites (restriction sites are italicized and in bold). PCR was performed with Taq DNA polymerase (Promega) under the following conditions: 94°C for 5 min followed by 25 cycles of 94°C for 30 s, 72°C for 30 s, and 72°C for 30 s followed by an extension cycle of 72°C for 5 min. The resulting PCR product was gel purified prior to being cloned into pGEM-T (Promega). DNA sequencing was performed to verify that we obtained the correct amplification product prior to digestion by KpnI and EcoRI and transference of the restriction digestion product into pG-7.2 to create plasmid pG-7.3. tbuT was amplified from pRO1614-3.1-kb (5) by using primer TBUT1 (5′-GAATTCACCCTTCTGGCCGCGAT-3′), containing an EcoRI site, and primer TBUT2 (5′-CTCGAGTGTCGTGTCTCAGCTTCCG-3′), containing an XhoI recognition site (engineered restriction sites are italicized and in bold). Pfu DNA polymerase (Stratagene) was used for amplification of tbuT under the following conditions: 94°C for 5 min, followed by 25 cycles of 94°C for 30 s, 72°C for 30 s, and 72°C for 4 min followed by 72°C for 10 min. PCR products were purified as described above before being cloned into pPCR-Script, which was then transformed into Escherichia coli XL1-Blue MRF′-Kan. After DNA sequence verification, an EcoRI-XhoI fragment containing the PCR product was subcloned into pG-7.3 to create pG-7-cass, and it was transformed into E. coli DH5α. An XbaI and SacI digest of pG-7cass was the source of DNA for directional cloning of the cassette into the multiple cloning site of pPROBE-NT (24) to create the biosensor plasmid pTS.
E. coli DH5α(pTS) was grown overnight in LB amended with 50 μg of Km ml−1 and diluted in phosphate-buffered saline prior to being plated on MM9-A. Plates were then placed in a desiccator and exposed to sufficient toluene vapor to yield an aqueous-phase toluene concentration of 20 μg ml−1, while a replicate plate was incubated in the absence of toluene. After 3 days of incubation, gfp activity was assessed visually with a 365-nm-wavelength UV box (UVP Inc., San Gabriel, Calif.). One colony that fluoresced green only in the presence of toluene was used to transfer pTS to P. fluorescens A506, and its presence was verified by exposing transconjugants to toluene. Constitutively expressed gfp was constructed by inserting the amplified PnptII promoter fragment described above into the multiple cloning site of pPROBE-NT.
Determination of organic compound concentrations in culture medium.
Saturated solutions of toluene, benzene, and ethylbenzene were prepared by mixing equal volumes of the organic compound with YES overnight in a 27°C incubator on a platform shaker. Aliquots of the saturated organic compounds in YES were transferred to fresh YES medium to achieve the desired solution-phase concentration of the organic compound based on Henry's Law and the appropriate constants as described below. The concentration of toluene in the YES solutions was determined by measuring the UV absorption at λmax and calculating the concentration from the extinction coefficient for each compound at λmax obtained from literature values measured in methanol (42). The toluene saturation concentrations were determined to be 6.0 ± 0.5 mM (mean ± standard deviation; n = 7), which is comparable with the published value for toluene saturation in water (5.8 mM) (16). We also independently verified the toluene concentrations in our experimental system by gas chromatography by using a Hewlett Packard 6890 gas chromatograph with a flame ionization detector as described previously (15). These values were within 4 to 13% of the targeted aqueous toluene concentrations based on the solubility of toluene in YES as determined by UV spectroscopy.
Induction experiments.
All experiments, unless stated otherwise, were conducted with the following protocol. A506(pTS) was cultivated overnight at 28°C on LB agar amended with 75 μg of Km ml−1. Flasks (377-ml triple-baffled sidearm; Bellco Inc., Vineland, N.J.) containing 50 ml of YES amended with Km (50 μg ml−1) were capped with a Teflon-lined screw cap and a Mini-nert cap on the side port. The appropriate amount of filter-sterilized effector was added through the Mini-nert cap to achieve the desired equilibrium aqueous-phase concentration based upon Henry's Law and appropriate constants adjusted for 28°C (37) and equilibrated for at least 12 h before use. A506(pTS) was resuspended in YES, and 1.5 ml of the cell suspension was transferred to the flasks through the Mini-nert caps. For assessing the effect of growth phase on A506(pTS) response, cells were grown in LB, harvested at various growth stages by centrifugation (2,800 × g for 10 min at room temperature), washed twice in YES, and resuspended in YES before the cells were exposed to toluene (217 μM). Optical density at 660 nm (OD660) and fluorescence were measured at the beginning of and after the desired incubation period. Cultures were incubated in an orbital shaker at 275 rpm at 28°C for various lengths of time.
Toluene exposure time necessary for initiating gfp expression.
A506(pTS) was exposed to 0.22 μM toluene, and at various times aliquots were removed, centrifuged, washed twice in YES, resuspended in 5 ml of YES, and then incubated for the duration of a 3-h incubation period. Measurements of samples following the 3- and 3.5-h exposure periods were conducted as described above, but there was no additional incubation time.
Effector range and simultaneous exposure to multiple effectors.
A506(pTS) was exposed to 80-μg ml−1 concentrations of TCE, chlorobenzene, phenol, p-xylene, benzoate, salicylate, catechol, styrene, methyl-tert butyl ether, 2-methyl-2-butene, ethanol, isopropanol, 2-methyl-1-propanol, butanol, and 1,1,1-TCE to assess their ability to induce gfp expression. We also examined equimolar concentrations (610 μM) of 1,1-dichloroethylene (1,1-DCE), 1,2-cis- and 1,2-trans-DCE, and TCE. For naphthalene, phenanthrene, biphenyl, n-hexane, and cyclohexane we used saturated solutions of each in YES. The biosensor was exposed to various concentrations of multicomponent mixtures of toluene and benzene, TCE, or gasoline to determine whether those compounds interfered with biosensor response. The density of the gasoline obtained from a local supplier was determined to be 1.73 ± 0.02 g ml−1. In these experiments, we used 22-ml screw cap tubes fitted with Mini-nert caps and 15 ml of YES medium, and tubes were incubated horizontally in a rotary shaker.
Flow cytometry.
For flow cytometric analysis of gfp expression the cells were diluted in filtered (0.2-μm-pore-size filters) YES to approximately 5 × 105 CFU ml−1 prior to analysis with a Coulter XL flow cytometer (Coulter Corp., Miami, Fla.) equipped with a 15-mW argon ion laser (488 nm excitation). Fluorescence emission intensities of 20,000 particles per sample were measured with a 525 BP filter to restrict the emission wavelength measured to 505 to 545 nm. Fluorescence intensity values are presented as the geometric mean of a distinct population of cells. The half-peak height coefficient of variation (HPCV) is defined as 42.46 multiplied by the width of the peak at half the peak height divided by peak position; peak position is the point with the highest cell count. Flow cytometry data were analyzed with WinMDI software (http://facs.scripps.edu).
Spectrofluorometry.
Fluorescence intensity was measured with a Fluoromax-2 spectrofluorometer with Datamax for Windows software interface (Instruments S.A. Inc., Edison, N.J.). Unless stated otherwise, emission and excitation wavelengths, bandpass, and integration times were 488 nm, 510 nm, 3 nm, and 0.5 s, respectively. Relative fluorescence unit (RFU) is defined as the culture fluorescence relative to culture biomass at OD660. Induction ratio is defined as the RFU of an effector-exposed sample divided by the RFU of a no-effector (0 μM) control.
Statistical analysis.
We performed statistical analysis with Systat version 7.0 (SPSS Inc., Chicago, Ill.), using the general linear model procedure for analysis of variance. Fisher's least significant difference test (P = 0.05) was calculated by Systat software for comparison of treatment means. We used SigmaPlot version 5.0 (SPSS Inc.) to generate nonlinear best-fit lines of the data to the hyperbolic equation and for linear regressions.
RESULTS
Description of the biosensor plasmid pTS and creation of the biosensor strain.
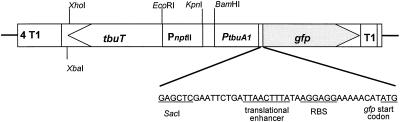
A diagram of key features of pTS is shown in Fig. 1. The position of the PtbuA1 promoter fragment was 150 bp upstream of the red-shifted gfp gene. The 344-bp promoter fragment contained the upstream regulatory sequences for TbuT binding, the −24 and −12 promoter sequences, the transcriptional start site, and 60 bp of tbuA1. The constitutively expressed PnptII-tbuT fusion was inserted so that transcription occurred in the direction opposite to that of the PtbuA1-gfp fusion (Fig. 1). In the absence of an effector, the presence of pTS in strain A506 did not result in a statistically significant increase in the fluorescence intensity relative to that of cells of A506 without pTS. This suggests that the PtbuA1 promoter is not leaky enough to result in a low-fluorescence background.
FIG. 1.
Schematic diagram of the XbaI-SacI cassette of pTS inserted into pPROBE-NT. Properties of plasmid pPROBE-NT containing the pGreenTir gfp cassette have been described elsewhere (24, 25). Abbreviations: T1, rrnB rRNA T1 terminator; PtbuA1, promoter of the tbu operon and the upstream activating sequences; RBS, ribosome binding site; PnptII, neomycin phosphotransferase promoter; tbuT, transcriptional activator gene. Arrows indicate the direction of transcription. The diagram is not drawn to scale.
Effect of growth stage and medium on biosensor response to toluene.
Toluene induced gfp expression in A506(pTS) in all media examined, but the level of induction varied among the different media. The induction ratio (mean ± standard deviation; n = 3) in response to 217 μM toluene after 3 h in the semidefined medium YES was 11.6 ± 1.1, whereas when succinate was replaced with citrate, glucose, or glycerol the induction ratio was 4.8 ± 1.0, 8.0 ± 0.3, or 9.0 ± 0.6, respectively. The biosensor also responded to toluene in quarter-strength LB and Trypticase soy broth, although the induction ratio was only 50% of that obtained in YES medium. Dilution of LB and Trypticase soy broth was necessary to reduce the high-fluorescence background of the media at the wavelength used to measure green fluorescence.
When exposed to 217 μM toluene for 3 h, a 2.5-h-old A506(pTS) culture harvested at an OD660 of 0.2 had an induction ratio (mean ± standard deviation; n = 3) of 13.27 ± 0.5, whereas a 17-h-old culture harvested at an OD660 of 1.7 had an induction ratio of 13.02 ± 1.1. Cells obtained from overnight LB agar plate cultures exhibited an induction ratio of 11.9 ± 0.3 (n = 4). These results suggest that growth phase does not affect induction of the PtbuA1-gfp fusion. For all subsequent experiments we used overnight plate cultures to generate biosensor biomass.
Time-dependent induction of green fluorescence with toluene.
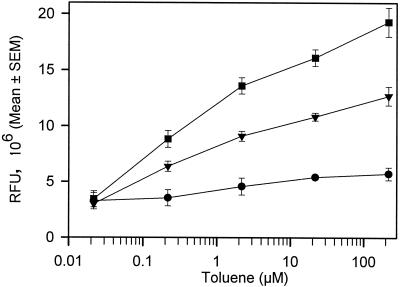
The background fluorescence response of the A506(pTS) negative control (no toluene) treatments after a 3-h incubation period was 1.78 × 106 ± 0.12 × 106 RFU, which was 1.1 ± 0.1 (n = 11)-fold greater than the fluorescence response at the beginning of the experiment. This fluorescence response was significantly lower than the response of A506(pTS) exposed to 0.022 μM toluene (3.44 × 106 ± 0.72 × 106 RFU), which was the lowest concentration at which we could detect gfp expression with a fluorometer. In general, the longer the toluene exposure time the greater the fluorescence intensity (Fig. 2). Although at high toluene concentrations there was a statistically significant (P < 0.05) increase in fluorescence intensity within 1 h, at low concentrations (<5 μM) the fluorescence intensity was not statistically different (P > 0.05) from that of the no-effector negative control. Three-hour incubation times resulted in reproducible separation in fluorescence intensities among the distinct toluene concentrations, and longer exposure times (up to 10 h) did not significantly increase resolution of the fluorescence signal among toluene concentrations (data not shown).
FIG. 2.
Fluorescence response of P. fluorescens A506(pTS) to various concentrations of toluene measured after different exposure periods. Fluorescence (in RFU) measured with a fluorometer is defined as culture fluorescence divided by culture OD660. •, 1-h toluene incubation; ▾, 2-h toluene incubation; ▪, 3-h toluene incubation. All points represent the means of 2 to 5 replicates, and error bars represent the standard errors of the means (SEM).
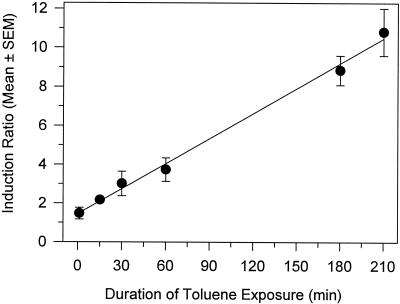
The minimum length of time necessary for sufficient toluene-mediated transcription of gfp to occur for fluorimetric detection of green fluorescence was determined as described in the legend to Fig. 3. A standardized length of time was used for GFP folding (180 min; 210 min for the 3.5-h treatment). There was a linear correlation between duration of exposure and induction ratio (r2 = 0.995). A statistically significant increase (P < 0.05) in fluorescence over that of the control occurred after 15 min of toluene exposure (Fig. 3), and the response was approximately 20% of the response measured at 180 min. These results suggest that the level of fluorescence measured after a 3-h incubation will be directly related to the level of gfp expression that occurred within the first 15 min of toluene exposure.
FIG. 3.
Effect of transient toluene exposure on fluorescence response of P. fluorescens A506(pTS). A506(pTS) was exposed to 0.22 μM toluene for the lengths of time indicated on the x axis, and cells were pelleted by centrifugation and washed twice to remove toluene. Cells were then reincubated without toluene in YES for the duration of a 3-h incubation period prior to fluorescence measurements. The 180- and 210-min treatments were handled similarly, except there was no additional incubation period following the washing step. All points represent the means of 3 replicates, and error bars represent the standard errors of the means (SEM).
Analysis of PtbuA1-gfp expression in individual cells.
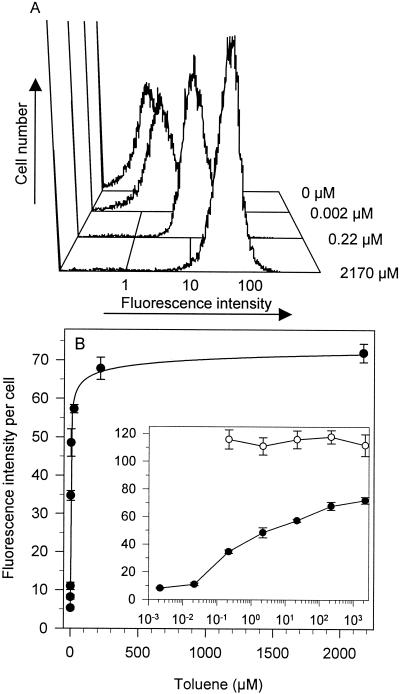
Populations of A506(pTS) cells exposed to a particular toluene concentration for 3 h exhibited a unimodal distribution of fluorescence intensity without significant tailing, except at 2,170 μM, at which cells exhibited a small amount of leftward (decreased emission) tailing (Fig. 4A). A similar distribution was observed in each population of A506(pTS) cells following a 1-h toluene exposure, although the fluorescence intensities for each treatment were lower than those for the 3-h exposure. Even a small shift in fluorescence intensity per cell could be detected by flow cytometry (compare 0 and 0.002 μM toluene treatments in Fig. 4A). At toluene concentrations above 0.2 μM only 2 to 4% of the population did not exhibit fluorescence intensities greater than those of cells in the 0 μM toluene treatment, indicating that most of the population was able to express gfp and that high toluene concentrations did not reduce or prevent gfp expression in a subpopulation of cells. The fluorescence intensities of populations of cells induced with ≥0.2 μM toluene exhibited a narrower distribution of fluorescence intensities than cells treated with 0 to 0.02 μM toluene (half-peak-height coefficient of variation [mean ± standard deviation] for 0 μM toluene was 39.2 ± 3.5, whereas for 0.2 μM toluene it was 20.4 ± 1.0).
FIG. 4.
Flow cytometric analysis of fluorescence intensity of individual cells of P. fluorescens A506(pTS) exposed to toluene. Cultures were exposed to various toluene concentrations, incubated for 3 h, and analyzed by flow cytometry as described in Materials and Methods. (A) Representative histograms showing the distribution of fluorescence intensity per cell upon exposure to 0, 0.002, 0.22, or 2,170 μM toluene. Fluorescence intensity is on a log scale. The y axis is the number of fluorescent cells detected. (B) Mean fluorescence intensity of P. fluorescens A506(pTS) (•) and A506(pPnptII-gfp) (○) exposed to various toluene concentrations. The line represents a nonlinear best fit of the data to the hyperbolic equation y = ymax − {(ymax − ymin)/(1 + K1/2 × [toluene]1/d}, where y is the fluorescence response at a given toluene concentration, ymax is the fluorescence response at saturating concentrations of toluene, ymin is the fluorescence response at 0 μM toluene, K1/2 is the concentration of toluene at which the half-maximal effect is observed, and d is the hyperbolic coefficient (d = 5.2 ± 1.6). The inset shows the same data, but it is plotted on a log10 toluene concentration scale. The regression equation for 0.002 to 2,170 μM toluene is y = {11.71 × (log10 [toluene])} + 38.8; r2 = 0.96. Points represent the means ± standard errors of the means of 3 to 5 replications.
Dose-dependent induction of green fluorescence with toluene.
The distribution of fluorescence intensities of individual cells was used to derive the geometric mean fluorescence intensity to compare the fluorescence response of the population of A506(pTS) cells to various toluene concentrations (Fig. 4B). In general the geometric mean and median fluorescence intensities per cell were similar. Fluorescence exhibited a hyperbolic dependence on toluene concentration (r2 > 0.99). The inset to Fig. 4B shows the same data plotted on a log toluene concentration scale to facilitate visualization of the fluorescence response over the broad range of concentrations we examined; fluorescence also exhibited a linear dependence on the log10 toluene concentration (0.002 to 2,170 μM; r2 = 0.96). Results from flow cytometry and fluorometry were in congruence with each other, and the apparent K (Kapp) values were 0.5 ± 0.7 to 3.3 ± 1.8 μM (means ± standard errors), with a maximal fluorescence increase of 14- to 15-fold, respectively (compare Fig. 4B with 5A). The fluorescence response of A506(pTS) cultures exposed to 0.022 μM toluene was statistically greater (P < 0.05) than that of the no-effector control, indicating that the biosensor's limit of toluene detection is at least 0.022 μM (Fig. 2, 4, and 5). We detected an increase in fluorescence intensity per cell of cultures exposed to 0.002 μM toluene (Fig. 4), although it was not statistically greater than the fluorescence intensity of A506(pTS) in the absence of toluene (P > 0.05). It is unlikely that the plateau in fluorescence intensity per cell at toluene concentrations above 100 μM is due to toluene-mediated impairment of gfp expression or GFP fluorescence, since toluene exposure had no statistically significant effect (P > 0.05) on fluorescence intensity of A506 (pPnptII-gfp) cells, which constitutively express gfp, compared to that of the 0 μM treatment (Fig. 4B). Toxic effects, as measured by a 5 to 12% reduction in OD660 during the 3-h incubation period, were observed at toluene concentrations greater than 800 μM for A506(pTS). High-level gfp expression did not affect A506 growth, since there was no difference in culture doubling times (mean ± standard deviation; n = 3) of A506(pTS) (68 ± 3 min) and A506(pPnptII-gfp) (64 ± 5 min) in LB medium.
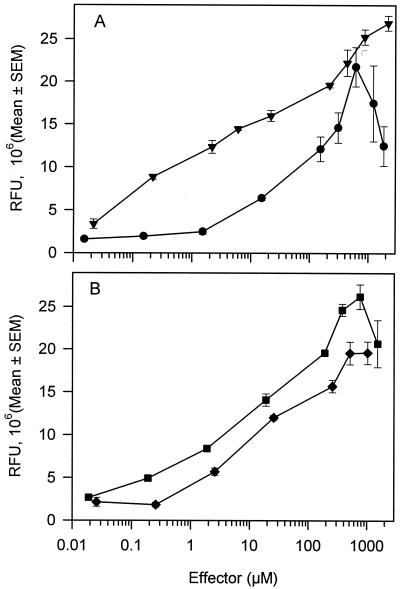
Dose-dependent induction of green fluorescence with benzene, ethylbenzene, and TCE.
The dose-response relationship of A506(pTS) was examined for three other compounds that are known inducers of the tbu operon. For all three compounds, fluorescence exhibited a hyperbolic curve dependent on effector concentration for induction times of 180 min (r2 > 0.93) (data not shown). Plots of the dose-response relationships of A506(pTS) to these effectors as measured by fluorometry are shown in Fig. 5. For ethylbenzene, the Kapp was 5.4 ± 1.9 μM, with a maximum induction of 15-fold, and 0.19 μM ethylbenzene was necessary to induce gfp expression. For benzene, the Kapp was 7.0 ± 2.5 μM, with a maximum induction of 10-fold, and 0.2 μM benzene was necessary to induce gfp expression. For TCE, the Kapp was 35.6 ± 16.6 μM, with a maximum induction of eightfold, and 1.5 μM TCE was necessary to induce gfp expression. There was a decrease in the biosensor response at TCE concentrations greater than 600 μM, which possibly reflected TCE toxicity (>25% reduction in growth as measured by OD660). There were also toxic effects caused by benzene and ethylbenzene as measured by a 13 to 19% and 7 to 13% reduction in growth at concentrations greater than 756 and 1,024 μM, respectively.
FIG. 5.
Fluorescence response of P. fluorescens A506(pTS) to various concentrations of toluene, ethylbenzene, benzene, or TCE. (A) ▾, toluene; •, TCE. (B) ⧫, benzene; ▪, ethylbenzene. Fluorescence was measured with a fluorometer. RFU represents the fluorescence of the effector-exposed treatments relative to the culture density (OD660). Points represent the mean fluorescence response, and error bars represent the standard errors of the means (SEM); n = 4 to 5. The regression equation for 0.02 to 2,170 μM toluene is y = {4.48 × (log10 [toluene])} + 10.91; r2 = 0.98; y is the fluorescence response at a given toluene concentration. The regression equation for 1.5 to 600 μM TCE is y = {6.6 × (log10 [TCE])} + −0.14; r2 = 0.90. The regression equation for 0.02 to 756 μM ethylbenzene is y = {5.23 × (log10 [ethylbenzene])} + 9.26; r2 = 0.95. The regression equation for 0.025 to 1,024 μM benzene is y = {4.28 × (log10 [benzene])} + 6.2; r2 = 0.94. The range of fluorescence for the 0 μM treatments was 1.48 × 106 to 1.82 × 106 RFU.
Structure activity analysis of chemically similar compounds.
A range of substituted monoaromatics, polyaromatics, chloroethylenes, aliphatic alcohols, aliphatic hydrocarbons, and several other compounds were examined for their inducing ability (Table 2). Concentrations of the compounds ranged from the aqueous solubility of the compound to 868 μM to ensure detection if the compound was an inducer; lower concentrations were used if there was significant growth inhibition at higher concentrations. Significant induction was found for the benzene and the alkyl-substituted benzene derivatives and TCE, which had previously been shown to be effectors of the tbu operon (5, 20, 26). The chlorinated ethylenes 1,2-cis-DCE and 1,1-DCE were able to induce gfp expression. The gasoline constituent isopentane was not an inducer (induction ratio, 1.5), whereas 2-methyl-2-butene had inducing capabilities. Isopropylbenzene and propylbenzene exhibited weak inducing capabilities, which indicates that the alkyl substitution of the benzene ring can be larger than that of an ethyl group. The polyaromatics, catechol, benzoate, salicylate, aliphatic hydrocarbons, aliphatic alcohols, 1,1,1-TCE, and methyl-tert-butyl ether did not induce gfp expression in the biosensor.
TABLE 2.
Induction of green fluorescence in P. fluorescens A506(pTS) by various compoundsa
| Compound | Induction ratio (mean ± SEM) |
|---|---|
| Benzene derivatives | |
| Ethylbenzene | 14.7 ± 0.8 |
| Toluene | 14.2 ± 0.5 |
| Chlorobenzene | 11.3 ± 3.0 |
| Benzene | 11.0 ± 0.7 |
| Styrene | 10.4 ± 1.0 |
| Phenol | 2.8 ± 0.1 |
| Isopropyl benzene | 2.6 ± 0.3 |
| Propyl benzene | 2.2 ± 0.4 |
| o-Xylene | 2.4 ± 0.2 |
| m-Xylene | 1.3 ± 0.1 |
| p-Xylene | 1.4 ± 0.3 |
| 2-Hydroxy benzoic acid | 1.5 ± 0.6 |
| Benzoate | 1.1 ± 0.1 |
| 2,4-Dinitrophenol | 0.6 ± 0.1 |
| Aliphatic alcohols | |
| Ethanol | 1.0 ± 0.1 |
| Isopropanol | 1.0 ± 0.1 |
| Butanol | 1.0 ± 0.1 |
| 2-Methyl-1-propanol | 0.9 ± 0.1 |
| Alkenes and alkanes | |
| TCE | 12.2 ± 1.3 |
| 1,2-cis-DCE | 8.7 ± 0.3 |
| 1,1-DCE | 6.7 ± 0.6 |
| 1,2-trans-DCE | 1.6 ± 0.3 |
| 1,1,1-TCE | 1.1 ± 0.2 |
| 2-Methyl-2-butene | 6.5 ± 1.0 |
| n-Hexane | 0.9 ± 0.1 |
| Ethylene glycol | 1.1 ± 0.1 |
| Cyclohexane | 1.2 ± 0.1 |
| Isopentane | 1.3 ± 0.3 |
| Polyaromatics | |
| Naphthalene | 1.2 ± 0.1 |
| Phenanthrene | 1.1 ± 0.1 |
| Biphenyl | 1.0 ± 0.3 |
| Other | |
| Catechol | 1.1 ± 0.1 |
| Methyl-tert-butyl ether | 0.8 ± 0.1 |
| No effector control | 1.0 |
Induction ratio is the RFU of an effector-exposed sample divided by the RFU of a no-effector control; no-effector control was arbitrarily set to 1.0. Assay concentrations of effectors were 80 mg ml−1, except for saturated aqueous solutions of phenanthrene (approximately 1 mg liter−1), naphthalene (approximately 10 mg liter−1), biphenyl (approximately 7.5 mg liter−1), n-hexane (approximately 1 mg ml−1), cyclohexane (approximately 55 mg liter−1), equimolar (610 μM) concentrations of the chloroethylenes, 2-methyl-2-butene, isopentane, isopropylbenzene, and propylbenzene (200 μM). All experiments were repeated 3 to 5 times. Values in bold indicate that there was a statistically significant increase (P < 0.05) in fluorescence above that of the no-effector control based on a paired t test.
Effect of multicomponent mixtures on biosensor response.
Since A506(pTS) responds to more than one effector, and since contaminated sites often contain multiple pollutants, we assessed the effect of the presence of multiple effectors on biosensor response. We exposed the biosensor to mixtures of various compounds that induce gfp expression 26 to 50% of the maximum fluorescence individually and approximately 36 to 54% when added as mixtures to test whether the individual effectors acted in an additive manner (Table 3). There was good agreement between the measured fluorescence and the predicted fluorescence in each of the multicomponent mixtures, which suggests that these components are acting in an additive manner. This additive response to multiple effectors was observed even when the nonaromatic compound TCE was mixed with toluene as well as when a gasoline sample was spiked with toluene (Table 3). All components were added individually and in combination at concentrations that did not inhibit growth of A506(pTS) cultures.
TABLE 3.
Additivity of induction of the toluene biosensor by multicomponent mixturesa
| Treatment mixture | Fluorescence (106; mean ± SEM) | Maximum toluene equivalent response
|
|
|---|---|---|---|
| % Measured | % Predicted | ||
| Toluene and benzene | |||
| Toluene (0.22 μM) | 9.84 ± 0.3 | 37 ± 2 | |
| Benzene (10 μM) | 13.3 ± 1.1 | 49 ± 4 | |
| Toluene (0.22 μM) and benzene (10 μM) | 15.3 ± 0.4 | 57 ± 2 | 51 |
| TCE and toluene | |||
| TCE (15 μM) | 7.0 ± 0.3 | 26 ± 2 | |
| Toluene (4 μM) | 13.6 ± 0.2 | 50 ± 1 | |
| TCE (15 μM) and toluene (4 μM) | 15.1 ± 0.4 | 56 ± 2 | 54 |
| Toluene and gasoline | |||
| Toluene (0.2 μM) | 8.8 ± 0.3 | 33 ± 1 | |
| Gasoline (0.37 ppm) | 7.5 ± 0.2 | 28 ± 2 | |
| Toluene (2 μM) and gasoline (0.37 ppm) | 9.7 ± 0.4 | 36 ± 1 | 36 |
The measured percentage of maximum toluene equivalent response represents the fluorescence response elicited by an effector relative to the maximum toluene fluorescence response (RFU = 26.9 × 106 ± 0.9 × 106). The predicted percentage of maximum toluene equivalent response was derived by converting the fluorescence response measurements of each effector to a toluene equivalent concentration with the hyperbolic equation derived from the toluene standard curve. The toluene equivalent concentration for each multicomponent mixture was then used to predict from the toluene standard curve what fluorescence response that toluene concentration would elicit. Values in parentheses are the effector concentrations used in the assay. Fluorescence responses (n = 3 to 5 per treatment) were measured after a 3-h incubation period.
Biosensor response to gasoline.
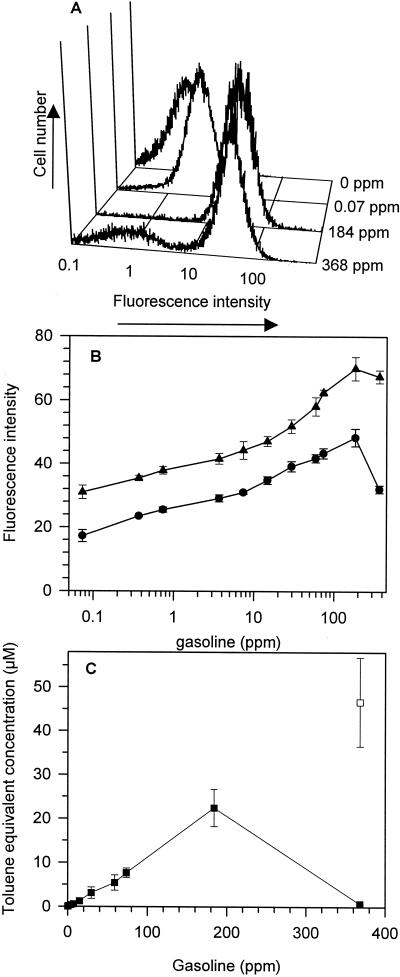
A506(pTS) was exposed to a range of gasoline concentrations (0.07 to 368 ppm). Concentrations of 184 ppm and above exceeded aqueous solubility in YES medium. Flow cytometry revealed that each population of cells exposed to gasoline concentrations of 0.07 to 184 ppm exhibited a unimodal distribution of fluorescence intensity without significant leftward tailing, except at a gasoline concentration of 368 ppm, where there was a bimodal distribution of fluorescence intensities (Fig. 6A). We gated our flow cytometric analysis so that the first peak comprised 98% of the population in the 0-ppm control treatment, and the second peak was comprised of cells that had fluorescent intensities greater than 98% of the untreated control treatment (0 ppm). This second peak will be referred to as the induced population. In the treatments of gasoline at 368 ppm, the first peak comprised 23.5% ± 0.8% of the population and had a mean fluorescence intensity of 5.2 ± 0.1, and the second peak comprised 76.5% ± 0.8% of the population and had a mean fluorescence intensity of 67.5 ± 2.0 (Fig. 6B). The mean fluorescence intensity of the second peak in the gasoline treatment at 184 ppm was 70.0 ± 3.6. Similarly, the fluorescence response of each population of A506(pPnptII-gfp) cells exposed to gasoline concentrations of 0.07 to 184 ppm exhibited a unimodal distribution of fluorescence intensity but exhibited a bimodal distribution of fluorescence intensities when exposed to gasoline at 386 ppm (data not shown). There were also toxic effects caused by gasoline, as measured by a 5% ± 2% and 8.0% ± 4% reduction in growth (OD660) at 184 and 368 ppm, respectively.
FIG. 6.
Fluorescence response of P. fluorescens A506(pTS) to various concentrations of gasoline. (A) Representative histograms showing the distribution of fluorescence intensities per cell when exposed to gasoline. (B) Mean fluorescence intensity per cell in response to gasoline. Error bars represent the standard errors of the means; n = 3. •, Whole-culture mean fluorescence intensity; ▴, mean fluorescence intensity of the induced population of cells. (C) Toluene equivalent concentration in the gasoline samples. ▪, Toluene equivalent concentration derived from whole-culture mean fluorescence intensity; □, toluene equivalent concentration in the 368-ppm samples of gasoline derived from the fluorescence intensity of the induced population. Toluene equivalent concentrations were derived from the linear regression equation of the log10 toluene concentration of the toluene standard curve that was performed simultaneously. The regression equation for 0.02 to 200 μM toluene for the whole culture was y = {13.6 × (log10 [toluene])} + 39.2; r2=0.96; y is the fluorescence response at a given toluene concentration. The regression equation for the induced population was y = {12.8 × (log10 [toluene])} + 46.1; r2 = 0.97.
The toluene equivalent concentrations of the gasoline samples were derived from the linear regression equation of the log10 toluene concentration of a standard curve (Fig. 6C). There was a linear relationship (r2 = 0.99) between 0.075- to 184-ppm concentrations of gasoline and the toluene equivalent concentration derived from the fluorescence response from that sample. The uninduced cells in the 368-ppm treatment resulted in a significant reduction in the mean fluorescence response of cells in the culture, and as a consequence there was an underestimation of the toluene equivalent concentration in that sample. However, the predicted toluene equivalent concentration in the 368-ppm sample of gasoline derived from the fluorescence intensity for the induced population was in good agreement with what would be expected in the sample (Fig. 6C).
DISCUSSION
In this study we describe the construction and characterization of a GFP whole-cell biosensor for the measurement of aqueous concentrations of various aromatic and nonaromatic organic pollutants, and we address the feasibility of using GFP as a reporter for pollutant biosensor systems. The use of gfp as a reporter gene gives this biosensor the advantages associated with GFP, such as the ability to fluoresce without the need for exogenous enzyme substrates, its stability, and the ability to use fluorometry, flow cytometry, and fluorescence microscopy to monitor gene expression to assess bioavailability. The basis of our biosensor system was P. fluorescens A506(pTS) containing the gene for the transcriptional activator TbuT and a transcriptional fusion between PtbuA1 and a promoterless gfp gene on a broad-host-range promoter probe vector. There was a very low level of background expression from PtbuA1 under uninduced conditions, possibly because of the transcriptional shielding of the PtbuA-gfp fusion. The ability to detect induction after short incubation periods and at low inducer concentrations may reflect the fact that the PtbuA-gfp fusion was carried on a multicopy plasmid which amplifies the signal as well as reflects the sensitivity of fluorescence detection instrumentation.
An advantage of gfp as a reporter of gene expression is its stability. The total amount of GFP in a cell will reflect the number of copies of the gene, duration of expression, and strength of the transcriptional promoter as well as the presence of toxic compounds that may interfere with gfp expression or folding. When using GFP derivatives with enhanced solubility mutations, which were used here, it takes about 1 to 2 h to complete posttranslational fluorophore formation once GFP is formed. Consequently, cells can be removed from an inducing environment (e.g., pollutants), and after allowing sufficient time for posttranslational fluorophore formation green fluorescence can be detected. Our results (Fig. 3) showed that there was a direct relationship between the duration of expression (i.e., minutes of exposure) and green fluorescence when there was a constant assay time for exposure and posttranslational fluorophore formation (i.e., 180 min). This feature of GFP has practical implications, since it provides flexibility in monitoring protocols and permits removal of the biosensor cells from the pollutant sample after as little as 30 min. This could reduce detrimental effects of pollutant toxicity on biosensor response if the biosensor is exposed to pollutants for longer periods of time.
The minimum effector concentration required for activity is effector dependent, ranging from 0.02 μM for toluene to approximately 1.5 μM for TCE, and these minimum concentrations closely parallel the maximum inducing capabilities of the individual effectors (Fig. 4 and 5). For toluene, the threshold concentration necessary for detecting gfp expression is equivalent to about 31 molecules of toluene within the cytoplasm of each cell, assuming an average cell volume of 1.6 μm3 and an equal distribution of toluene between cells and medium (38). This is in congruence with other reports indicating that 20 to 50 effector molecules per cell are sufficient to lead to activation of transcriptional regulators (10, 38) for biodegradative operons. The minimum effector concentration necessary for inducing gfp expression is also similar to those reported for inducing reporter gene expression in other pollutant biosensor systems (1, 32, 33, 35, 38). For all effectors tested, the TbuT-PtbuA1 system exhibits a saturation kinetic behavior in gfp expression at concentrations above 20 to 40 μM, and consequently the most accurate measurements of pollutants present in a sample would be at concentrations of 20 to 40 μM and below. It is unlikely that this apparent saturation kinetic behavior is due to pollutant toxicity, which would interfere with fluorescence, because 2,000 μM toluene had no effect on fluorescence in A506 (pPnptII-gfp), even though this concentration is slightly toxic and inhibits growth.
Although the hyperbolic curve (r2 > 0.99) best described the toluene dose-response data set, it was equally well fit by a linear regression (r2 = 0.96 to 0.98) of a log10 transformation of the aqueous toluene concentration. Similarly, the ethylbenzene, benzene, and TCE dose-response data set were equally well fit by both models (Fig. 5). Use of the linear regression equation to derive toluene equivalent concentrations from the fluorescence response of the multicomponent mixtures (Table 3 and Fig. 6) provided values similar to those derived from the hyperbolic equation (data not shown). Advantages of modeling the fluorescence response on a log10 transformation of the effector concentration include that the model is easier to use, the graphical presentation of the data set provides a more readily visible means for determining minimum inducer concentrations, and the fluorescence responses that provide accurate measurements of effector concentrations are more readily identified.
The promoter PtbuA1 was induced uniformly throughout the A506(pTS) population (Fig. 4A), in contrast to many inducible promoters that are induced to distinct levels in distinct subpopulations within a population (21, 34). Intermediate levels of gene expression in cultures do not always mean that gene expression is uniform with respect to individual cells. Either every cell in the culture makes the same percentage of the fully induced level as seen for the culture as a whole, or the culture consists of a mixture of fully induced and completely uninduced cells. In the latter case, the intermediate expression levels observed in the culture reflect the proportion of cells that are fully induced rather than the intermediate expression in individual cells. Our experiments with flow cytometry show that intermediate levels of gfp expression in a culture in response to toluene reflect uniform induction of cells (Fig. 4). As the toluene concentration increases, the level of gfp expression per cell increases until saturation kinetics of the TbuT-PtbuA1 system are observed. This is unlike many sugar-inducible promoter systems, such as the AraC arabinose-inducible promoter PBAD system (18, 34) and the FruR fructose-1-phosphate-inducible promoter PfruBKB system (21), where intermediate levels of expression reflect population averages of nonuniform mixtures of cells. Due to the intrapopulation variance in GFP intensity in response to a particular toluene concentration, there is overlap among populations of cells that experience different toluene concentrations (Fig. 4A). Consequently, the lowest-fluorescence-intensity cell in an induced population might be as bright as the highest-fluorescence-intensity cell in an uninduced population. This implies that a single cell's GFP fluorescence must be interpreted in the context of other cells within the same population. The uniformity in induction across a population adds to the probability that any given cell that contacts a bioavailable pollutant will in fact be induced, which is an extremely valuable feature for any biosensor that is used for in situ assessments of pollutant availability in soil.
Our results confirm earlier reports on the specificity of TbuT for monosubstituted benzenes that do not possess a carboxyl group (5, 20). We show that the effector range is broader than previously shown and that TbuT can accommodate propyl- and isopropyl-alkyl substitutions of the benzene ring and that a variety of substituted ethylene compounds (TCE, 1,2-cis-DCE, and 2-methyl-2-butene) can serve as inducers. It is unclear whether the branched alkenes bind to TbuT at the same site as the aromatic inducers. If different compounds bind TbuT at the same site and produce similar degrees of activation, responses to multiple inducing compounds should behave in an additive manner when the compounds are present at concentrations below those that induce maximal activity. However, if different compounds bind to TbuT simultaneously at different sites or if noninducing compounds bind to TbuT at sites that interfere with activation, then inhibitory effects may be observed. The good agreement between our predicted responses and our measured responses in the multicomponent mixtures and toluene-spiked-gasoline experiments indicate that the compounds are acting in an additive manner (Table 3). Furthermore, since A506(pTS) responded to a two-component mixture of TCE and toluene in an additive fashion (Table 3), our results suggest that even if toluene and branched alkenes bind TbuT at different sites simultaneously there was no apparent interference with transcriptional activity.
In our gasoline studies, gfp expression in A506(pTS) cultures reflected uniform induction of cells (Fig. 6A), except at gasoline concentrations of 384 ppm, where we observed two populations of cells: a small population of uninduced cells and a larger population of induced cells. Apparently, a subpopulation of A506(pTS) cells either were killed by toxic constituents present in the gasoline or were unable to respond to the effectors present in the gasoline. We observed a similar population level response in our experiments with A506(pPnptII-gfp) exposed to a gasoline concentration of 384 ppm; that is, there was a small population of uninduced cells and a larger fully induced population with fluorescence intensities comparable to those observed in the 0-ppm treatments. These results were unexpected, since it is reasonable to predict that if toxicity affected all cells equally within a population then there would be a uniform decrease in fluorescence intensity per cell for the whole population. Instead it appears that the toxic gasoline constituents affected only a subpopulation of cells and that the remaining cells were apparently able to respond to the effectors present in the gasoline at levels that reflect the concentration of effectors that we would predict to be present in the sample (Fig. 6B). It is unlikely that the toxic constituents in the gasoline sample interfered with GFP fluorescence, since gasoline had no effect on the fluorescence intensity of A506 (pPnptII-gfp) cells. Flow cytometry allowed us to account for the lack of gfp expression in a subpopulation of cells by gating the data set to examine only the induced population; this is not possible with reporter systems not based on fluorescence.
Gasoline is comprised of a variety of compounds that can function as inducers, including toluene, benzene, ethylbenzene, propyl- and isopropyl-benzene, xylene isomers, and 2-methyl-2-butene (Table 2), as well as a variety of other branched alkenes and aromatics that potentially could serve as inducers (28). Since we cannot differentiate between possible inducers, the data are expressed as toluene equivalents. There was a direct relationship between biosensor response (Fig. 6B) and the amount of gasoline present in the sample, except at saturating concentrations of gasoline (Fig. 6A). The linear relationship between the gasoline concentration and the toluene equivalent concentration (Fig. 6C) indicates that increasing the concentration of potential inhibitors present in the gasoline did not inhibit the biosensor's ability to detect the inducers present in the sample. Since we did not independently determine the concentration of inducers present in the gasoline sample, we are unable to state conclusively that the toluene equivalent concentrations detected with the biosensor were accurate. Due to the broad range of effectors that can induce gfp expression, the possibility that some related compounds in the gasoline sample added to the inductive effect cannot be excluded.
In summary, the results presented here outline the characterization of a GFP-based whole-cell biosensor for the detection of various aromatic and branched alkene pollutants that is fairly rapid and easy to use and that may be useful in determining pollutant bioavailability in environmental samples. The strengths of the A506(pTS) biosensor, including its sensitivity to low concentrations of effectors, its rapid induction times, its predictable response to a broad range of effector concentrations, and its uniformity in induction across a population, add to the probability that any given cell that contacts bioavailable pollutants will in fact be induced. The fact that the A506(pTS) biosensor is growth stage independent indicates that its effectiveness as a tool in soil studies should not be compromised by the physiological changes common to bacteria in soil. These features are particularly beneficial for using this biosensor to explore how soil properties (e.g., type and amount of organic matter in soil) and the presence or absence of roots affect expression of the TbuT-PtbuA1 system and, hence, biologically relevant pollutant concentrations in soil.
Acknowledgments
We thank Carol Casavant and Martijn van de Mortel for critically reading an earlier draft of this paper and Kristie Harkins and Donghui Cheng for assistance with flow cytometry.
This research was supported in part by the Agronomy Department and the Iowa Agriculture and Home Economics Experiment Station and was also supported by the Hatch Act and the State of Iowa.
Footnotes
Journal paper no. J-19197 of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, project no. IOW03944.
REFERENCES
- 1.Applegate, B. M., S. R. Kehrmeyer, and G. S. Sayler. 1998. A chromosomally based tod-luxCDABE whole-cell reporter for benzene, toluene, ethylbenzene, and xylene (BTEX) sensing. Appl. Environ. Microbiol. 64:2730-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn, J. W., and W. R. Hafker. 1993. The impact of biochemistry, bioavailability, and bioactivity on the selection of bioremediation techniques. Trends Biotechnol. 11:328-333. [DOI] [PubMed] [Google Scholar]
- 3.Brandl, M. T., B. Quinones, and S. E. Lindow. 2001. Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc. Natl. Acad. Sci. USA 98:3454-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burlage, R. S., G. S. Sayler, and F. Larimer. 1990. Monitoring of naphthalene catabolism by bioluminescence with nah-lux transcriptional fusions. J. Bacteriol. 172:4749-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne, A. M., and R. H. Olsen. 1996. Cascade regulation of the toluene-3-monooxygenase operon (tbuA1UBVA2C) of Burkholderia pickettii PKO1: role of the tbuA1 promoter (PtbuA1) in the expression of its cognate activator, TbuT. J. Bacteriol. 178:6327-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha, H. J., R. Srivastava, V. N. Vakharia, G. Rao, and W. E. Bentley. 1999. Green fluorescent protein as a noninvasive stress probe in resting Escherichia coli cells. Appl. Environ. Microbiol. 65:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalfie, M., and S. Kain (ed.). 1998. Green fluorescent protein: properties, applications, and protocols. Wiley-Liss, New York, N.Y.
- 8.Chung, N., and M. Alexander. 1998. Differences in sequestration and bioavailability of organic compounds aged in dissimilar soils. Environ. Sci. Technol. 32:855-860. [Google Scholar]
- 9.Daubaras, D., and A. M. Chakrabarty. 1992. The environment, microbes and bioremediation: microbial activities modulated by the environment. Biodegradation 3:125-135. [Google Scholar]
- 10.DiMarco, A. A., B. Averhoff, and L. N. Ornston. 1993. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J. Bacteriol. 175:4499-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figurski, D., and D. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harms, H., and T. N. P. Bosma. 1997. Mass transfer limitation of microbial growth and pollutant degradation. J. Indust. Microbiol. Biotechnol. 18:97-105. [Google Scholar]
- 13.Heim, R., D. C. Prasher, and R. Y. Tsien. 1994. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 91:12501-12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitzer, A., K. Malachowsky, J. E. Thonnard, P. R. Bienkowski, D. C. White, and G. S. Sayler. 1994. Optical biosensor for environmental on-line monitoring of naphthalene and salicylate bioavailability with an immobilized bioluminescent catabolic reporter bacterium. Appl. Environ. Microbiol. 60:1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden, P. A., L. J. Halverson, and M. K. Firestone. 1997. Water stress effects on toluene biodegradation by Pseudomonas putida. Biodegradation 8:143-151. [DOI] [PubMed] [Google Scholar]
- 16.Howard, P. H. 1989. Handbook of environmental fate and exposure data for organic chemicals, vol. II. Lewis Publishers, Chelsea, Mich.
- 17.Joyner, D. C., and S. E. Lindow. 2000. Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. Microbiology 146:2435-2445. [DOI] [PubMed] [Google Scholar]
- 18.Khlebnikov, A., O. Risa, T. Skaug, T. A. Carrier, and J. D. Keasling. 2000. Regulatable arabinose-inducible gene expression system with consistent control in all cells of a culture. J. Bacteriol. 182:7029-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layton, A. C., M. Muccini, M. M. Ghosh, and G. S. Sayler. 1998. Construction of a bioluminescent reporter strain to detect polychlorinated biphenyls. Appl. Environ. Microbiol. 64:5023-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leahy, J. G., G. R. Johnson, and R. H. Olsen. 1997. Cross-regulation of toluene monooxygenases by the transcriptional activators TbmR and TbuT. Appl. Environ. Microbiol. 63:3736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leveau, J. H., and S. E. Lindow. 2001. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 98:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindemann, J., and T. V. Suslow. 1987. Competition between ice-nucleation-active wild-type and ice-nucleation-deficient deletion mutant strains of Pseudomonas syringae and P. fluorescens biovar I and biological control of frost injury on strawberry blossoms. Phytopathology 77:882-886. [Google Scholar]
- 23.Miller, W. G., M. T. Brandl, B. Quinones, and S. E. Lindow. 2001. Biological sensor for sucrose availability: relative sensitivities of various reporter genes. Appl. Environ. Microbiol. 67:1308-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 25.Miller, W. G., and S. E. Lindow. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149-153. [DOI] [PubMed] [Google Scholar]
- 26.Olsen, R. H., J. J. Kukor, and B. Kaphammer. 1994. A novel toluene-3-monooxygenase pathway cloned from Pseudomonas pickettii PKO1. J. Bacteriol. 176:3749-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riser-Roberts, E. 1998. Remediation of petroleum contaminated soils. Lewis Publishers, Boca Raton, Fla.
- 28.Riser-Roberts, E. 1998. Remediation of petroleum contaminated soils: biological, physical and chemical processes. Lewis Publishers, Boca Raton, Fla.
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Scow, K. M. (ed.). 1993. Effect of sorption-desorption and diffusion processes on the kinetics of biodegradation of organic chemicals in soil. American Society of Agronomy, Madison, Wis.
- 31.Selifonova, O., R. Burlage, and T. Barkay. 1993. Bioluminescent sensors for detection of bioavailable Hg(II) in the environment. Appl. Environ. Microbiol. 59:3083-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selifonova, O., and R. Eaton. 1996. Use of an ipb-lux fusion to study regulation of the isopropylbenzene catabolism operon of Pseudomonas putida RE204 and to detect hydrophobic pollutants in the environment. Appl. Environ. Microbiol. 62:778-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingleton, J. T., B. M. Applegate, A. C. Nagel, P. R. Bienkowski, and G. S. Sayler. 1998. Induction of the tod operon by trichloroethylene in Pseudomonas putida TVA8. Appl. Environ. Microbiol. 64:5049-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegele, D. A., and J. C. Hu. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. USA 94:8168-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson, M. L., G. S. Sayler, B. M. Applegate, S. Ripp, D. E. Nivens, M. J. Paulus, and G. E. J. Jellison. 1998. Bioluminescent-bioreporter integrated circuits form novel whole-cell biosensors. Trends Biotechnol. 16:332-338. [Google Scholar]
- 36.Smibert, R. M., and N. R. Krieg. 1994. Phenotypic characterization, p. 611-654. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 37.Staudinger, J., and P. V. Roberts. 2001. A critical compilation of Henry's law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 44:561-576. [DOI] [PubMed] [Google Scholar]
- 38.Sticher, P., M. C. Jaspers, K. Stemmler, H. Harms, A. J. Zehnder, and J. R. van der Meer. 1997. Development and characterization of a whole-cell bioluminescent sensor for bioavailable middle-chain alkanes in contaminated groundwater samples. Appl. Environ. Microbiol. 63:4053-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509-544. [DOI] [PubMed] [Google Scholar]
- 40.Van der Meer, J. R., W. M. De Vos, S. Harayama, and A. J. B. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Dyk, T. K., D. R. Smulski, T. R. Reed, S. Belkin, A. C. Vollmer, and R. A. LaRossa. 1995. Responses to toxicants of an Escherichia coli strain carrying a uspA′::lux genetic fusion and an E. coli strain carrying a grpE′::lux fusion are similar. Appl. Environ. Microbiol. 61:4124-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weast, R. C., and J. G. Grasselli (ed.). 1989. Handbook of data on organic compounds, 2nd ed. CRC Press, Boca Raton, Fla.
- 43.Willardson, B. M., J. F. Wilkins, T. A. Rand, J. M. Schupp, K. K. Hill, P. Keim, and P. J. Jackson. 1998. Development and testing of a bacterial biosensor for toluene-based environmental contaminants. Appl. Environ. Microbiol. 64:1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]