Abstract
Background
Cancer-associated fibroblasts (CAFs) critically regulate tumor microenvironment remodeling, with exosomes (Exos) derived from CAFs serving as key mediators of intercellular communication. However, the functional significance of CAF-derived exosomal long non-coding RNA ANRIL (lncRNA ANRIL) in modulating non-small cell lung cancer (NSCLC) glycolytic metabolism and proliferation remains incompletely characterized. This study systematically investigated the lncRNA ANRIL/miR-186-5p/HIF-1α regulatory axis in NSCLC progression.
Methods
CAFs were isolated from fresh NSCLC surgical specimens, followed by Exo isolation through differential ultracentrifugation. The functional impacts of CAF-derived Exos on NSCLC cellular proliferation and glycolytic activity were comprehensively evaluated using CCK-8 assays, EdU incorporation tests, glucose consumption measurements, lactate production quantification, and extracellular acidification rate (ECAR) assessments. Molecular interactions within the ANRIL/miR-186-5p/HIF-1α axis were mechanistically validated through dual-luciferase reporter systems, RNA pull-down assays, and RT-qPCR profiling. Functional validation was achieved via targeted overexpression and siRNA-mediated knockdown approaches.
Results
CAF-derived Exos significantly enhanced NSCLC cell proliferation indices and glycolytic parameters. CRISPR-mediated ANRIL silencing in CAFs substantially attenuated these pro-tumorigenic effects, establishing exosomal ANRIL as a critical molecular effector. Integrated bioinformatics prediction and experimental validation confirmed direct binding between lncRNA ANRIL and miR-186-5p, with subsequent identification of HIF-1α as the downstream target of miR-186-5p. Functional perturbation experiments demonstrated that miR-186-5p overexpression or HIF-1α knockdown effectively suppressed NSCLC proliferation and glycolysis, while rescue assays confirmed HIF-1α as the terminal mediator of miR-186-5p regulatory effects.
Conclusions
This mechanistic study demonstrates that CAF-secreted exosomal lncRNA ANRIL drives NSCLC progression by enhancing glycolytic metabolism through competitive sponging of miR-186-5p, thereby derepressing HIF-1α expression. Our findings provide novel insights into exosome-mediated metabolic reprogramming in NSCLC and propose therapeutic targeting of the ANRIL/miR-186-5p/HIF-1α signaling axis as a potential precision medicine strategy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03109-7.
Keywords: Non-small cell lung cancer, Cancer-associated fibroblasts, Exosomes, LncRNA ANRIL, MiR-186-5p, HIF-1α, Glycolytic metabolism.
Introduction
Lung cancer persists as the most prevalent malignancy and primary contributor to global cancer-related mortality [1]. Non-small cell lung cancer (NSCLC), constituting approximately 80% of pulmonary malignancies, demonstrates escalating incidence rates with substantial detrimental impacts on patient quality of life [2]. While radiotherapy and chemotherapy remain cornerstone therapies for advanced NSCLC [3, 4], their clinical utility is frequently compromised by severe adverse effects and persistent challenges of tumor metastasis/recurrence. This underscores the imperative to develop more effective therapeutic regimens and identify sensitive molecular biomarkers for early diagnosis, treatment optimization, and prognostic assessment in NSCLC.
Metabolic reprogramming constitutes a fundamental cancer hallmark, with dysregulated glycolytic metabolism representing the most conspicuous feature of tumor energetics [5, 6]. Cancer-associated fibroblasts (CAFs), as dominant stromal components within tumor microenvironments, serve pivotal roles in tumor progression and metastatic dissemination [7]. Exosomes (Exos), extracellular vesicles transporting diverse molecular cargoes (including proteins, nucleic acids, and lipids), have emerged as critical mediators of intercellular signaling [8, 9]. Emerging evidence indicates CAFs can deliver long non-coding RNAs (lncRNAs) to neoplastic cells via Exos, thereby modulating oncogenic behaviors [10–12]. Functionally defined as > 200-nucleotide non-coding transcripts, lncRNAs orchestrate multilayered gene regulation [13]. Although accumulating studies implicate lncRNAs in tumor glycolytic modulation [14, 15], their precise mechanistic contributions remain largely uncharacterized.
LncRNA ANRIL has been established as an oncogenic driver across multiple malignancies, promoting tumor proliferation, migration, and glycolytic reprogramming [16, 17]. Nevertheless, the potential involvement of CAF-derived exosomal ANRIL in NSCLC glycolytic regulation remains unexplored. Through integrated bioinformatics analysis, we identified a putative regulatory network connecting ANRIL, miR-186-5p, and HIF-1α. The tumor-suppressive miR-186-5p has been demonstrated to regulate cancer cell glycolytic pathways [18–21], while hypoxia-inducible factor 1α (HIF-1α)—frequently overexpressed in NSCLC—similarly participates in tumor glycolytic metabolism [14, 22]. Previous validations confirm miR-186-5p directly targets HIF-1α [23, 24], yet the functional relevance of this axis in NSCLC metabolic regulation warrants systematic investigation.
This study mechanistically examines the ANRIL/miR-186-5p/HIF-1α signaling axis in NSCLC glycolytic metabolism. We validate the molecular interplay between ANRIL and miR-186-5p, and further elucidate miR-186-5p-mediated HIF-1α regulation. By delineating these regulatory connections, we aim to advance understanding of lncRNA-driven metabolic reprogramming in NSCLC, potentially informing novel diagnostic and therapeutic strategies.
Materials and methods
Isolation and culture of CAFs
Fresh surgical specimens were obtained from NSCLC patients undergoing curative resection, with ethics approval and informed consent. Tissues were immediately immersed in sterile phosphate-buffered saline (PBS) containing 1% penicillin-streptomycin (P/S) and maintained on ice during transport. Tumor specimens were minced into 1–2 mm³ fragments and enzymatically digested in collagenase type I (1 mg/mL; Sigma-Aldrich, C0130) and hyaluronidase (100 U/mL; Sigma-Aldrich, H3506) dissolved in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, 11965092) using a 2-hour incubation at 37 °C with orbital agitation (150 rpm). The enzymatic digestate was filtered through 70 μm nylon mesh to eliminate undigested debris. Filtered cells were pelleted by centrifugation (300 × g, 5 min) and resuspended in complete medium (DMEM with 10% FBS (Gibco, 10099141) and 1% P/S). The suspension was seeded into tissue culture-treated flasks and incubated under standard conditions (37 °C, 5% CO₂). Following 24-hour adherence, non-adherent cellular components were removed via PBS washing, and adherent fibroblasts were maintained in fresh complete medium.
Cell transfection
Lentiviral shRNAs targeting lncRNA ANRIL (sh-lncRNA ANRIL) were designed using BLOCK-iT™ RNAi Designer and cloned into pLKO.1 vectors, with scrambled shRNA (sh-NC) serving as non-targeting control. For HIF-1α overexpression, the full-length coding sequence (NCBI accession: NM_001530) was subcloned into pCDH vectors, with empty vector as control. Lentiviral packaging was performed by co-transfecting HEK293T cells (ATCC® CRL-3216™) with transfer vectors (shRNA/HIF-1α) and packaging plasmids (psPAX2/pMD2.G) using Lipofectamine 3000 (Invitrogen, L3000015). Viral supernatants were harvested at 48 h post-transfection, filter-sterilized (0.45 μm PVDF membrane), and concentrated via ultracentrifugation (70,000 × g, 4 °C, 2 h). Recipient cells were plated in 6-well plates (2 × 10⁵ cells/well) and transduced with viral particles in the presence of polybrene (8 µg/mL). Post-transduction (24 h), medium was replaced with complete growth medium, and stable pools were selected using puromycin (2 µg/mL, 7-day treatment).
For miRNA modulation, A549 and H1299 cells (ATCC® CCL-185™ and CRL-5803™) were seeded at 2 × 10⁵ cells/well in 6-well plates. At 70% confluency, cells were transfected with miR-186-5p mimic (50 nM), mimic negative control (miR-NC), miR-186-5p inhibitor (100 nM), or inhibitor control (anti-miR-NC) using Lipofectamine 3000 per manufacturer’s protocol. Post-transfection (6 h), medium was replaced with complete DMEM.
The specific sequences are as follows:
sh-lncRNA ANRIL: sense 5’-ACGGAGTCAACCGTTTCGGGA-3’, anti-sense 5’-TTCGCCAAAGGTTGACTCCGT-3’.
shRNA-NC: sense 5’-CGCTGAGTACTTCGAAATGTC-3’, anti-sense 5’-GACATTCGAAGTACTCAGCG-3’.
miR-186-5p mimic: sense 5’-CAAAGAAUUCUCCUUUUGGGCU-3’, 5’-AGCCCAAAAGGAGAAUUCUUUG-3’.
miR-NC: sense 5’-UUCUCCGAACGUGUCACGUTT-3’, 5’-ACGUGACACGUUCGGAGAATT-3’.
Anti-miR-186-5p: 5’-AGCCCAAAAGGAGAAUUCUUUG-3’.
Anti-miR-NC: 5’-UAGCUGACCGUGAUACGAUCU-3’.
Exos isolation
The cell supernatant was filtered through 0.22 μm polyethersulfone membranes (Millipore) to eliminate cellular debris and large vesicles. Exos were pelleted via sequential ultracentrifugation at 100,000 × g for 70 min at 4 °C (Optima XE-100, Beckman Coulter). The crude exosomal fraction was washed with ice-cold PBS and centrifuged under identical conditions. Purified Exos were resuspended in sterile PBS and cryopreserved at −80 °C. Exosomal quantification and size profiling were performed using Nanoparticle Tracking Analysis (NanoSight NS300). Exosome-specific markers CD63 and TSG101 were verified by western blotting. To validate exosome-dependent effects, CAFs were pre-treated with 20 µM GW4869 exosome secretion inhibitor (Selleck, S7609) for 24 h prior to supernatant collection [25].
Culture of NSCLC cells
A549 and H1299 cell lines (ATCC) were maintained in complete growth medium under standard culture conditions (37 °C, 5% CO₂ humidified atmosphere) with medium renewal every 48–72 h.
Exo treatment
Prior to intervention, NSCLC cells were washed twice with serum-free medium. Exosome-enriched PBS suspensions were diluted in fresh complete medium to achieve 50 µg/mL exosomal protein concentration. Cells were exposed to Exos for 24 h at 37 °C to permit cellular uptake, followed by three PBS washes to remove uninternalized vesicles.
Western blotting
Cellular and exosomal lysates were prepared using RIPA lysis buffer (Beyotime, P0013B) containing protease inhibitor cocktail (Roche). Post-centrifugation (12,000 × g, 15 min, 4 °C), protein quantification was performed with BCA assay (Beyotime, P0012). Equal protein aliquots (30 µg) were resolved by 10% SDS-PAGE (Bio-Rad) and transferred to PVDF membranes (0.45 μm, Millipore) via wet transfer (100 V, 90 min, 4 °C). Membranes were blocked with 5% non-fat dry milk/TBST and probed with primary antibodies including primary antibodies against CD63 (ab134045, 1:1000, abcam), TSG101 (ab125011, 1:1000, abcam), and HIF-1α (ab51608, 1/500) (4 °C overnight) followed by HRP-conjugated secondary antibodies (ab97051, 1:5000, Abcam). Signal detection employed ECL Prime (Cytiva) and ChemiDoc MP Imaging System (Bio-Rad).
CCK-8 assay
A549 and H1299 cells were seeded in 96-well plates at a density of 5 × 10³ cells per well using 100 µL of complete culture medium. The plates were incubated at 37 °C in a humidified atmosphere with 5% CO₂ for 24 h until cell attachment was achieved. Then, 10 µL of CCK-8 solution was added to each well, and the plates were incubated at 37 °C for 2 h. The absorbance at 450 nm was measured spectrophotometrically using a microplate reader. Cell viability was calculated as a percentage relative to the untreated control group using the formula: Cell viability (%) = (absorbance of treated group − absorbance of blank)/(absorbance of control group − absorbance of blank) × 100%.
Cell proliferation assay using edu
A549 and H1299 cells were seeded in 96-well plates at a density of 5 × 10³ cells per well using 100 µL of complete culture medium. After 24 h, the culture medium was replaced with fresh medium containing 10 µM EdU (RiboBio, China), and the cells were incubated for 2 h at 37 °C. The cells were then washed twice with PBS and fixed with 4% paraformaldehyde for 15 min. After fixation, the cells were washed twice with PBS and permeabilized with 0.5% Triton X-100 in PBS for 20 min. The cells were washed twice with PBS and incubated with Hoechst 33,342 (1 µg/mL dissolved in PBS) for 10 min at room temperature to stain the nuclei. The cells were washed twice with PBS, and images were captured using a fluorescence microscope.
Glucose consumption assay
Glucose and lactate concentrations in the culture medium were measured using a glucose assay kit (Nanjing Jiancheng Bioengineering) and a lactate assay kit (Nanjing Jiancheng Bioengineering) according to the manufacturer’s instructions, respectively. Briefly, 50 µL of the collected medium was mixed with 50 µL of glucose assay reagent and incubated at 37 °C for 30 min. The absorbance at 450 nm and 570 nm was measured using a microplate reader, respectively.
Measurement of extracellular acidification rate (ECAR)
The culture medium was replaced with XF assay medium (DMEM without bicarbonate, supplemented with 2 mM glutamine, 1 mM pyruvate, and 10 mM glucose, pH 7.4). The cells were incubated at 37 °C in a non-CO₂ incubator for 1 h to allow temperature and pH equilibration. The XF24 microplate was loaded into the Seahorse XF Analyzer. Baseline ECAR measurements were taken, followed by sequential injections of oligomycin (1 µM), 2-deoxy-D-glucose (2-DG, 50 mM), and rotenone/antimycin A (0.5 µM each) to assess glycolysis, glycolytic capacity, and non-glycolytic acidification, respectively. ECAR was measured in real-time under basal conditions and after each injection.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent following the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDrop spectrophotometer (A260/A280 ratio of 1.8–2.0). For lncRNA-ANRIL and HIF-1α mRNA, 1 µg of total RNA was reverse-transcribed into cDNA using a PrimeScript RT reagent kit with gDNA Eraser. For miR-186-5p, a miRNA-specific stem-loop RT primer and a miRNA reverse transcription kit were used. The reaction conditions were as follows: 37 °C for 15 min, followed by 85 °C for 5 s. qPCR was performed using SYBR Green Master Mix on a real-time PCR system. The reaction mixture (20 µL) contained 10 µL of SYBR Green Master Mix, 2 µL of cDNA, 0.8 µL of forward and reverse primers (10 µM each), and 7.2 µL of nuclease-free water. The PCR conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The primer sequences were as follows:
lncRNA-ANRIL: Forward 5′-TACATCCGTCACCTGACACG-3′, Reverse 5′-ACGAGGGGAGCCAGGAATAA-3′.
miR-186-5p: Forward 5′-GCCGGCAAAGAATTCTCCTTT-3′, Reverse 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCCCA-3′.
HIF-1α: Forward 5′-ATCCATGTGACCATGAGGAAATG-3′, Reverse 5′-TCGGCTAGTTAGGGTACACTTC-3′.
GAPDH: Forward 5′-AGTGCCAGCCTCGTCTCATA-3′, Reverse 5′-GGTAACCAGGCGTCCGATA-3′.
U6: Forward 5′-CTCGCTTCGGCAGCACA-3′, Reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Relative expression levels of lncRNA-ANRIL, miR-186-5p, and HIF-1α were calculated using the 2−ΔΔCt method, normalized to GAPDH (for lncRNA-ANRIL and HIF-1α) or U6 (for miR-186-5p).
Dual-luciferase reporter assay
The wild-type (WT) lncRNA ANRIL or HIF-1 A fragment containing the predicted miR-186-5p binding site was cloned into the pmirGLO dual-luciferase reporter vector. A mutant (MUT) lncRNA ANRIL or HIF-1 A fragment with the binding site mutated was also constructed. A549 cells were seeded in 24-well plates at a density of 5 × 10⁴ cells per well and cultured for 24 h. Cells were co-transfected with either the WT or MUT lncRNA ANRIL or HIF-1 A reporter plasmid and miR-186-5p mimic or miR-NC (negative control) using Lipofectamine 3000 as per the manufacturer’s instructions. After 48 h of transfection, cells were lysed, and luciferase activity was measured using the dual-luciferase reporter assay system. Firefly luciferase activity was normalized to Renilla luciferase activity for each sample.
RNA pull-down assay
Biotinylated miR-186-5p (Biotin-miR-186-5p) and a biotinylated negative control miRNA (Biotin-NC) were synthesized and used in the RNA pull-down assay. A549 cells were lysed in RNA immunoprecipitation (RIP) buffer supplemented with protease and RNase inhibitors. The lysate was centrifuged at 12,000 × g for 10 min at 4 °C, and the supernatant was collected. The cell lysate was incubated with Biotin-miR-186-5p or Biotin-NC at 4 °C for 2 h. Streptavidin-coated magnetic beads were added to the mixture and incubated for an additional 1 h at 4 °C to capture the biotinylated miRNA and its associated proteins. The beads were washed three times with RIP buffer to remove non-specifically bound molecules. The RNA–protein complexes were eluted from the beads using elution buffer, and RNA was extracted for RT-qPCR analysis.
Statistical analysis
Data were expressed as mean ± SD and analyzed by GraphPad Prism 9.0 software. Statistical differences were assessed by Student’s t-test or ANOVA. P < 0.05 was considered statistically significant.
Results
Characterization of exos derived from CAFs
Exosomes (Exos) were successfully isolated from the conditioned medium of cancer-associated fibroblasts (CAFs) using ultracentrifugation. Transmission electron microscopy (TEM) images revealed that the isolated Exos exhibited a typical circular-shaped morphology with clear edges, consistent with the characteristic structure of exosomes (Fig. 1A). Nanoparticle tracking analysis (NTA) was performed to determine the size distribution of the isolated Exos. The results showed that the Exos had a mean diameter of approximately 150 nm, which is within the expected size range for exosomes (Fig. 1B). Western blot analysis demonstrated strong expression of CD63 and TSG101, two well-established exosomal markers, in the isolated Exos (Fig. 1C). These findings confirm the successful isolation of Exos from CAFs.
Fig. 1.
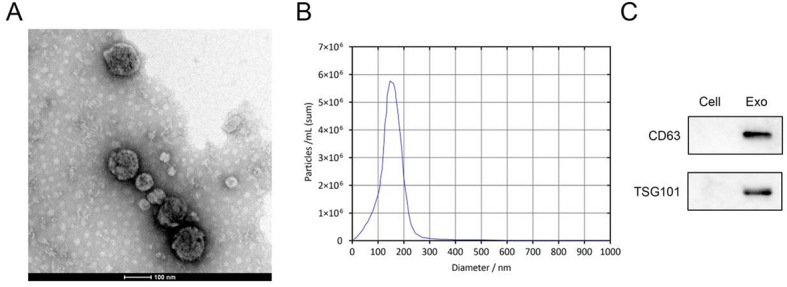
Characterization of Exos derived from CAFs. (A) TEM image showing the typical circular-shaped morphology of isolated Exos. Scale bar: 100 nm. (B) Size distribution of Exos as determined by NTA. (C) Western blot analysis confirming the expression of exosomal markers CD63 and TSG101 in the isolated Exos
CAF-derived exos promote lung cancer proliferation and glycolytic metabolism
Treatment with CAF-derived Exos significantly increased the viability and proliferation of both A549 and H1299 cells compared to the control group, as measured by CCK-8 and EdU assays, respectively (Fig. 2A–C). However, when cells were co-treated with GW4869, an inhibitor of exosome secretion, the Exos-induced effects were markedly attenuated, indicating that Exos play a crucial role in promoting lung cancer cell growth. Furthermore, CAF-derived Exos significantly enhanced glucose consumption (Fig. 2D), lactate levels (Fig. 2E), and extracellular acidification rate (ECAR) (Fig. 2F) in both A549 and H1299 cells. The addition of GW4869 significantly reduced these Exos-induced metabolic changes, suggesting that Exos contribute to the glycolytic activity and metabolic reprogramming of lung cancer cells. These findings collectively demonstrate that CAF-derived Exos play a critical role in enhancing the viability, proliferation, and glucose metabolism of lung cancer cells.
Fig. 2.

Effects of CAF-derived Exos and GW4869 on lung cancer cell viability, proliferation, and glucose metabolism. (A) Cell viability of A549 and H1299 cells treated with CAF-derived Exos (Exo) or CAF-derived Exos in the presence of GW4869 (Exo+GW4869) was measured using the CCK-8 assay. (B - C) Cell proliferation was assessed using the EdU assay. Exos enhanced proliferation, which was suppressed by GW4869. (D) Glucose consumption and (E) lactate levels were significantly increased in cells treated with Exos, and this effect was reduced by GW4869. (F) ECAR, a measure of glycolytic activity, was elevated in Exo-treated cells, and GW4869 treatment reversed this increase
Role of LncRNA ANRIL in CAF-derived Exo-mediated effects on lung cancer cells
LncRNA ANRIL is a potential therapeutic target for lung cancer [26, 27]. LncRNA ANRIL expression was significantly downregulated in CAFs, and exosomes (Exos) were subsequently isolated from their conditioned medium. Treatment with CAF-derived Exos significantly increased the viability and proliferation of both A549 and H1299 cells. However, this effect was markedly attenuated when cells were treated with Exos derived from CAFs following lncRNA ANRIL knockdown, indicating that lncRNA ANRIL plays a critical role in exosome-mediated enhancement of cell viability (Fig. 3B–D). Furthermore, CAF-derived Exos significantly promoted glycolytic metabolism in A549 and H1299 cells, as evidenced by increased glucose consumption, lactate production, and extracellular acidification rate (ECAR). The knockdown of lncRNA ANRIL in CAFs significantly suppressed the Exo-induced metabolic activation, suggesting that lncRNA ANRIL is essential for Exo-mediated metabolic reprogramming (Fig. 3E–G).
Fig. 3.

Role of lncRNA ANRIL in CAF-derived Exo-mediated effects on lung cancer cells. (A) Relative expression of lncRNA ANRIL in CAFs with lncRNA ANRIL knockdown. (B) Cell viability and (C - D) proliferation of A549 and H1299 cells treated with Exo or Exo+sh-lncRNA-ANRIL, measured using the CCK-8 assay and EdU assay, respectively. (E) Glucose consumption, (F) lactate levels, and (G) ECAR in A549 and H1299 cells treated with Exo or Exo+sh-lncRNA-ANRIL. Exosomes increased glucose consumption, which was attenuated by lncRNA ANRIL knockdown
Functional role of miR-186-5p in LncRNA ANRIL-mediated effects on lung cancer cells
Bioinformatics analysis predicted a binding site between lncRNA ANRIL and miR-186-5p (Fig. 4A), which was confirmed by a luciferase reporter assay. Co-transfection with the wild-type (WT) lncRNA ANRIL reporter plasmid and miR-186-5p mimic significantly reduced luciferase activity in A549 cells compared to the negative control (miR-NC), while no significant change was observed with the MUT lncRNA ANRIL reporter plasmid, confirming the direct interaction between lncRNA ANRIL and miR-186-5p (Fig. 4B). Further validation using an RNA pull-down assay demonstrated that lncRNA ANRIL was significantly enriched in the biotinylated miR-186-5p group compared to the biotinylated negative control, providing additional evidence for their direct binding (Fig. 4C). Additionally, knockdown of lncRNA ANRIL in A549 and H1299 cells remarkably upregulated miR-186-5p expression compared to the negative control (sh-NC) (Fig. 4D), indicating that lncRNA ANRIL negatively regulates miR-186-5p expression. These findings collectively demonstrate that lncRNA ANRIL directly interacts with miR-186-5p and acts as a negative regulator of miR-186-5p in lung cancer cells, highlighting its role in modulating miR-186-5p levels. Subsequently, miR-186-5p levels were downregulated in both NSCLC cells (Fig. 5A). Inhibition of lncRNA ANRIL significantly suppressed cell viability, proliferation, glucose consumption, lactate levels, and ECAR levels of NSCLC cells, and a miR-186-5p inhibitor partially reversed the effects of lncRNA ANRIL knockdown on cell proliferation and glycolytic metabolism (Fig. 5B–G).
Fig. 4.
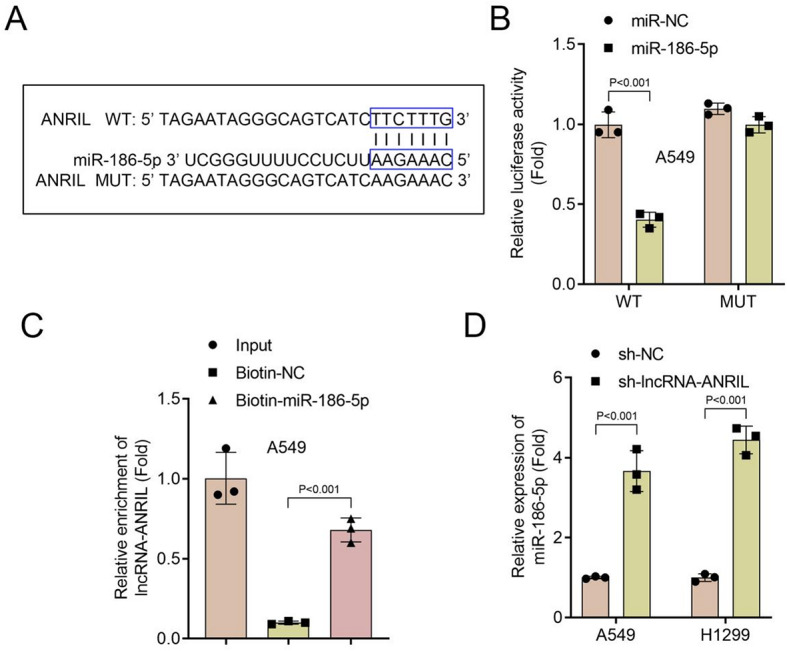
Interaction between lncRNA ANRIL and miR-186-5p in lung cancer cells. (A) Predicted binding site between lncRNA ANRIL and miR-186-5p, with WT and MUT sequences indicated. (B) Luciferase reporter assay showing that co-transfection of the WT lncRNA ANRIL reporter plasmid with miR-186-5p mimic significantly reduced luciferase activity in A549 cells compared to miR-NC. No significant change was observed with the MUT lncRNA ANRIL reporter plasmid. (C) RNA pull-down assay demonstrating significant enrichment of lncRNA ANRIL in the Biotin-miR-186-5p group compared to Biotin-NC. (D) Relative expression of miR-186-5p in A549 and H1299 cells with lncRNA ANRIL knockdown compared to the negative control. miR-186-5p expression was significantly upregulated in sh-lncRNA-ANRIL cells
Fig. 5.

Functional Role of miR-186-5p in lncRNA ANRIL-mediated effects on lung cancer cells. (A) Relative expression of miR-186-5p in A549 and H1299 cells with miR-186-5p knockdown. (B - D) Cell viability and proliferation of A549 and H1299 cells treated with sh-lncRNA-ANRIL or sh-lncRNA-ANRIL+Anti-miR-186-5p. lncRNA ANRIL knockdown reduced cell viability and proliferation, which was partially reversed by miR-186-5p inhibition. (E - F) Glucose consumption and lactate levels in A549 and H1299 cells treated with sh-lncRNA-ANRIL or sh-lncRNA-ANRIL+Anti-miR-186-5p. lncRNA ANRIL knockdown decreased glucose consumption and lactate levels, which was partially restored by miR-186-5p inhibition. (G) ECAR in A549 and H1299 cells treated with sh-lncRNA-ANRIL or sh-lncRNA-ANRIL+Anti-miR-186-5p. lncRNA ANRIL knockdown reduced ECAR, which was partially reversed by miR-186-5p inhibition
Functional role of miR-186-5p and HIF-1α in lung cancer cells
Bioinformatics analysis predicted the existence of a binding site between miR-186-5p and the 3’ untranslated region (3’UTR) of HIF-1α (Fig. 6A). To validate this interaction, WT and MUT HIF-1α 3’UTR sequences were cloned into a luciferase reporter vector. Co-transfection with the WT HIF-1α 3’UTR reporter plasmid and miR-186-5p mimic significantly reduced luciferase activity in A549 cells compared to the negative control (miR-NC). In contrast, no significant change in luciferase activity was observed with the MUT HIF-1α 3’UTR reporter plasmid, confirming the direct interaction between miR-186-5p and HIF-1α (Fig. 6B). The RNA pull-down assay demonstrated that HIF-1α was significantly enriched in the biotinylated miR-186-5p group compared to the biotinylated negative control (Biotin-NC) group, further confirming the direct interaction between miR-186-5p and HIF-1α (Fig. 6C). Overexpression of miR-186-5p markedly reduced HIF-1α expression in A549 and H1299 cells compared to miR-NC, indicating that miR-186-5p functions as a negative regulator of HIF-1α expression (Fig. 6D). Subsequently, miR-186-5p and HIF-1α were individually overexpressed in NSCLC cells (Fig. 7A–B). Overexpression of miR-186-5p significantly suppressed cell proliferation and glycolytic metabolism in A549 and H1299 cells. This effect was partially reversed when HIF-1α was co-expressed, indicating that HIF-1α acts as a critical downstream effector of miR-186-5p in modulating cell proliferation and glycolytic metabolism (Fig. 7C–H). The protein expression levels of HIF-1α in lung cancer cells following treatment with CAF-derived Exos were assessed via Western blotting. As shown in Figure S1, treatment with CAF-derived Exos significantly decreased the protein expression of HIF-1α in both A549 and H1299 cells compared to the control group. However, when cells were co-treated with GW4869, an exosome secretion inhibitor, the downregulatory effect of Exos on HIF-1α expression was notably attenuated (Figure S1) . This result further indicates that CAF-derived Exos play a critical role in modulating HIF-1α expression in lung cancer cells, which is in line with the proposed regulatory mechanism of the ANRIL/miR-186-5p/HIF-1α axis.
Fig. 6.

Interaction between miR-186-5p and HIF-1α in lung cancer cells. (A) Predicted binding site between miR-186-5p and the 3'UTR of HIF-1α, with WT and MUT sequences indicated. (B) Luciferase reporter assay showing that co-transfection of the WT HIF-1α 3'UTR reporter plasmid with miR-186-5p mimic significantly reduced luciferase activity in A549 cells compared to miR-NC. No significant change was observed with the MUT HIF-1α 3'UTR reporter plasmid. (C) RNA pull-down assay demonstrating significant enrichment of HIF-1α in the Biotin-miR-186-5p group compared to the Biotin-NC. (D) Relative expression of HIF-1α at the mRNA and protein levels in A549 and H1299 cells overexpressing miR-186-5p compared to the negative control. miR-186-5p overexpression significantly downregulated HIF-1α expression
Fig. 7.

Functional role of miR-186-5p and HIF-1α in lung cancer cells. (A, B) Relative expression of miR-186-5p and HIF-1α at the mRNA levels in A549 and H1299 cells overexpressing miR-186-5p or HIF-1α. (C - E) Cell viability and proliferation of A549 and H1299 cells treated with miR-186-5p or miR-186-5p+HIF-1α. miR-186-5p overexpression reduced cell viability and proliferation, which was partially reversed by HIF-1α co-expression. (F - G) Glucose consumption and lactate levels in A549 and H1299 cells treated with miR-186-5p or miR-186-5p+HIF-1α. miR-186-5p overexpression decreased glucose consumption and lactate levels, which was partially restored by HIF-1α co-expression. (H) ECAR in A549 and H1299 cells treated with miR-186-5p or miR-186-5p+HIF-1α. miR-186-5p overexpression reduced ECAR, which was partially reversed by HIF-1α co-expression
Discussion
This study investigated the role of exosomal lncRNA ANRIL derived from CAFs in regulating glycolytic metabolism and proliferation in NSCLC through the miR-186-5p/HIF-1α axis. We demonstrated that CAFs-derived exosomes promote NSCLC cell proliferation and glycolytic metabolism, and identified lncRNA ANRIL as a key mediator of these effects. Furthermore, we validated the direct interactions between lncRNA ANRIL, miR-186-5p, and HIF-1α, and elucidated their functional roles in NSCLC progression.
Glycolytic metabolism, a hallmark of cancer, plays a critical role in NSCLC progression by providing energy and biosynthetic precursors for rapidly proliferating tumor cells [28]. Recent studies have highlighted the importance of lncRNAs in regulating tumor metabolism, including glycolysis [29–31]. In this study, we found that lncRNA ANRIL, enriched in CAF-derived exosomes, significantly enhances glycolytic metabolism in NSCLC cells. This is consistent with previous reports demonstrating that lncRNAs can modulate metabolic pathways by interacting with miRNAs or proteins, thereby influencing tumor progression [32–34]. Our findings suggest that targeting lncRNA ANRIL represents a promising therapeutic strategy to disrupt glycolytic metabolism in NSCLC.
The competing endogenous RNA (ceRNA) mechanism has emerged as a key regulatory network in cancer metabolism [35, 36]. In this study, we identified lncRNA ANRIL as a ceRNA that sponges miR-186-5p, thereby upregulating HIF-1α expression. This ceRNA network not only highlights the complexity of lncRNA-mediated gene regulation but also provides a mechanistic link between lncRNA ANRIL and glycolytic metabolism in NSCLC. Our results are supported by previous studies [33, 37] showing that lncRNAs can act as molecular sponges for miRNAs, modulating the expression of downstream targets involved in tumor metabolism.
miR-186-5p and HIF-1α are well-established regulators of cancer metabolism [20, 38]. miR-186-5p has been shown to suppress tumor growth by targeting glycolytic genes, while HIF-1α functions as a master regulator of glycolytic metabolism under hypoxic conditions. In this study, we demonstrated that miR-186-5p directly targets HIF-1α, and its overexpression inhibits glycolytic metabolism in NSCLC cells. Furthermore, our rescue experiments confirmed that HIF-1α acts as a critical downstream effector of miR-186-5p in regulating glycolysis, elucidating the functional role of the miR-186-5p/HIF-1α axis in NSCLC.
Despite these significant findings, our study has some limitations. First, the in vivo effects of CAF-derived exosomal lncRNA ANRIL on NSCLC progression were not examined, and future studies using animal models are needed to validate these findings. Second, the specific mechanisms governing the packaging of lncRNA ANRIL into exosomes and its transfer to tumor cells remain unclear. Finally, the clinical relevance of the lncRNA ANRIL/miR-186-5p/HIF-1α axis in NSCLC patients requires further investigation through large-scale clinical studies.
In conclusion, our study reveals that CAF-derived exosomal lncRNA ANRIL promotes NSCLC proliferation and glycolytic metabolism by modulating the miR-186-5p/HIF-1α axis. These findings shed light on the role of lncRNAs in tumor metabolism and highlight the therapeutic potential of targeting the lncRNA ANRIL/miR-186-5p/HIF-1α pathway in NSCLC.
Electronic supplementary material
Supplementary Material 1. Figure S1. Protein expression of HIF-1α in lung cancer cells before and after CAF-derived Exos and GW4869 treatment
Acknowledgements
Not applicable.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. B Z drafted the work and revised it critically for important intellectual content; R H and Z T were responsible for the acquisition, analysis and interpretation of data for the work; B Z and Y H made substantial contributions to the conception or design of the work. All authors read and approved the final manuscript.
Funding
The study was supported by Wenzhou municipal basic scientific research project (Y20240945).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Wenzhou Central Hospital. This study was performed in line with the principles of the Declaration of Helsinki. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ. The effect of advances in Lung-Cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian Y, Xu L, Li X, Li H, Zhao M. SMARCA4: current status and future perspectives in non-small-cell lung cancer. CANCER LETT. 2023;554:216022. [DOI] [PubMed] [Google Scholar]
- 3.Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, Zhou Z, Liang J, Lv J, Feng Q, Xiao Z, Chen D, Wang Y, Li J, Wang J, Gao S, Wang L, He J. Effect of postoperative radiotherapy for patients with pIIIA-N2 Non-Small cell lung Cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA ONCOL. 2021;7(8):1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Lu S, Tian Y, Jiang L, Li L, Xie S, Li Q. Neoadjuvant low-dose radiotherapy plus durvalumab and chemotherapy for potentially resectable stage III NSCLC: A phase Ib dose-escalation study. RADIOTHER ONCOL. 2024;196:110316. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Li J, Zhang J, Feng W, Lu J, Ma X, Ding W, Ouyang S, Lu J, Yue P, Wan G, Liu P, Zhang X. Metabolic reprogramming driven by IGF2BP3 promotes acquired resistance to EGFR inhibitors in Non-Small cell lung Cancer. Cancer Res (Chicago Ill). 2023;83(13):2187–207. [DOI] [PubMed] [Google Scholar]
- 6.Arora S, Singh P, Tabassum G, Dohare R, Syed MA. miR-495–3p regulates sphingolipid metabolic reprogramming to induce Sphk1/ceramide mediated mitophagy and apoptosis in NSCLC. Free Radic Biol Med. 2022;189:71–84. [DOI] [PubMed] [Google Scholar]
- 7.Lavie D, Ben-Shmuel A, Erez N, Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. NAT CANCER. 2022;3(7):793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Teixeira AF, Zhu HJ, Ten DP. Cancer associated-fibroblast-derived exosomes in cancer progression. MOL CANCER. 2021;20(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Wang XY, Zhang P, He TC, Han JH, Zhang R, Lin J, Fan J, Lu L, Zhu WW, Jia HL, Zhang JB, Chen JH. Cancer-derived Exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. CELL DEATH DIS. 2022;13(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, Han C, Fang P, Ma Z, Wang X, Chen H, Wang S, Meng F, Wang C, Zhang E, Dong G, Zhu H, Yin W, Wang J, Zuo X, Qiu M, Wang J, Qian X, Shen H, Xu L, Hu Z, Yin R. Cancer-associated fibroblast-specific LncRNA LINC01614 enhances glutamine uptake in lung adenocarcinoma. J Hematol Oncol. 2022;15(1):1–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring Exosomal LncRNA H19. THERANOSTICS. 2018;8(14):3932–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Wang K, Lu X, Wang Y, Chen J. Cancer-associated fibroblasts-derived exosomes promote lung cancer progression by OIP5-AS1/ miR-142-5p/ PD-L1 axis. MOL IMMUNOL. 2021;140:47–58. [DOI] [PubMed] [Google Scholar]
- 13.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: LncRNA localization and function. J CELL BIOL. 2021;220(2):e202009045. [DOI] [PMC free article] [PubMed]
- 14.Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J, Huang G. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1alpha axis. THERANOSTICS. 2020;10(11):4762–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Wang H, Liu Q, Dai S, Tian G, Wei X, Li X, Zhao L, Shan B. LncRNA CCAT1 facilitates the progression of gastric cancer via PTBP1-mediated Glycolysis enhancement. J EXP CLIN CANC RES. 2023;42(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Zhao W, Zhang H, Chu Z, Liu H, Fang X, Tang D. Long non-coding RNA ANRIL promotes chemoresistance in triple-negative breast cancer via enhancing aerobic Glycolysis. LIFE SCI. 2022;306:120810. [DOI] [PubMed] [Google Scholar]
- 17.Mosaad H, Shalaby SM, Mahmoud NM, Ahmed MM, Fayed A, Ashour HR, Sarhan W. LncRNA ANRIL promotes glucose metabolism and proliferation of Colon cancer in a High-Glucose environment and is associated with worse outcome in diabetic Colon cancer patients. Asian Pac J cancer Prevention: APJCP. 2024;25(4):1371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Wang Y, Bai R, Yang K, Tian Z. MiR-186 inhibited aerobic Glycolysis in gastric cancer via HIF-1alpha regulation. ONCOGENESIS. 2017;6(4):e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun P, Hu JW, Xiong WJ, Mi J. miR-186 regulates Glycolysis through Glut1 during the formation of cancer-associated fibroblasts. Asian Pac J Cancer Prev. 2014;15(10):4245–50. [DOI] [PubMed] [Google Scholar]
- 20.Shen X, Zhu X, Hu P, Ji T, Qin Y, Zhu J. Knockdown circZNF131 inhibits cell progression and Glycolysis in gastric Cancer through miR-186-5p/PFKFB2 Axis. BIOCHEM GENET. 2022;60(5):1567–84. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Li Y, Zhong J, Wen G. circ-PRKCI targets miR-1294 and miR-186-5p by downregulating FOXK1 expression to suppress Glycolysis in hepatocellular carcinoma. MOL MED REP. 2021;23(6):464. [DOI] [PMC free article] [PubMed]
- 22.Huang Y, Chen Z, Lu T, Bi G, Li M, Liang J, Hu Z, Zheng Y, Yin J, Xi J, Lin Z, Zhan C, Jiang W, Wang Q, Tan L. HIF-1alpha switches the functionality of TGF-beta signaling via changing the partners of Smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J EXP CLIN CANC RES. 2021;40(1):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan H, Zhao L. LncRNA nuclear-enriched abundant transcript 1 promotes cell proliferation and invasion by targeting miR‐186‐5p/HIF‐1α in osteosarcoma. J CELL BIOCHEM. 2019;120(4):6502–14. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Zhao J, Chen J, Zheng Y, Mo R, Zhang L, Zhang B, Lin Q, He C, Li S, Lin L, Xie T, Ding Y. miR–186–5p regulates the inflammatory response of chronic obstructive pulmonary disorder by targeting HIF–1alpha. MOL MED REP. 2024;29(2):34. [DOI] [PMC free article] [PubMed]
- 25.Chen G, Pei Y, Jiang P, Ye Q, Xie Z, Gyawali L. Exosomal NEDD4L derived from HG + OxLDL-induced vascular endothelial cells accelerates macrophage M1 polarization and OxLDL uptake by ubiquitinating IkappaBalpha and PPARgamma. CELL BIOL TOXICOL. 2025;41(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Z, Zhang F, Liu L, Shen H, Liu T, Jin J, Yu N, Wan Z, Wang H, Hu X, Chen Y, Cai J. LncRNA ANRIL promotes HR repair through regulating PARP1 expression by sponging miR-7-5p in lung cancer. BMC Cancer. 2023;23(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Hashimoto RF. Dynamical analysis of a boolean network model of the oncogene role of LncRNA ANRIL and LncRNA UFC1 in Non-Small cell lung Cancer. BIOMOLECULES. 2022;12(3):420. [DOI] [PMC free article] [PubMed]
- 28.Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. PHARMACOL RES. 2019;150:104511. [DOI] [PubMed] [Google Scholar]
- 29.Hong J, Guo F, Lu SY, Shen C, Ma D, Zhang X, Xie Y, Yan T, Yu T, Sun T, Qian Y, Zhong M, Chen J, Peng Y, Wang C, Zhou X, Liu J, Liu Q, Ma X, Chen YX, Chen H, Fang JY. F. nucleatum targets LncRNA ENO1-IT1 to promote Glycolysis and oncogenesis in colorectal cancer. Gut. 2021;70(11):2123–37. [DOI] [PubMed] [Google Scholar]
- 30.Zhao S, Guan B, Mi Y, Shi D, Wei P, Gu Y, Cai S, Xu Y, Li X, Yan D, Huang M, Li D. LncRNA MIR17HG promotes colorectal cancer liver metastasis by mediating a glycolysis-associated positive feedback circuit. Oncogene. 2021;40(28):4709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Li Y, Yan S, Wang H, Shao X, Xiao M, Yang B, Qin G, Kong R, Chen R, Zhang N. Interactome analysis reveals that LncRNA HULC promotes aerobic Glycolysis through LDHA and PKM2. NAT COMMUN. 2020;11(1):3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y, Chen X, Wang X, Li H, Zhu Y, Li X, Xiao Z, Zi T, Qin X, Zhao Y, Yang T, Wang L, Wu G, Fang X, Wu D. Glycolysis related LncRNA SNHG3 / miR-139-5p / PKM2 axis promotes castration-resistant prostate cancer (CRPC) development and enzalutamide resistance. INT J BIOL MACROMOL. 2024;260(Pt 2):129635. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Chen X, Wang J, Liu B. Glycolysis-related LncRNA TMEM105 upregulates LDHA to facilitate breast cancer liver metastasis via sponging miR-1208. CELL DEATH DIS. 2023;14(2):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Duan W, Wu F, Yang D, Wang X, Wu J, Zhou D, Shen Y. LncRNA-HOTAIRM1 promotes aerobic Glycolysis and proliferation in osteosarcoma via the miR-664b-3p/Rheb/mTOR pathway. CANCER SCI. 2023;114(9):3537–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as CeRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/beta-catenin pathway. MOL CANCER. 2018;17(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. CeRNA in cancer: possible functions and clinical implications. J MED GENET. 2015;52(10):710–8. [DOI] [PubMed] [Google Scholar]
- 37.Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L, Liu J, Huang G. LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic Glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J HEMATOL ONCOL. 2019;12(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1alpha-mediated aerobic Glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Figure S1. Protein expression of HIF-1α in lung cancer cells before and after CAF-derived Exos and GW4869 treatment
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


