Abstract
Inactivation of TPI1, the Saccharomyces cerevisiae structural gene encoding triose phosphate isomerase, completely eliminates growth on glucose as the sole carbon source. In tpi1-null mutants, intracellular accumulation of dihydroxyacetone phosphate might be prevented if the cytosolic NADH generated in glycolysis by glyceraldehyde-3-phosphate dehydrogenase were quantitatively used to reduce dihydroxyacetone phosphate to glycerol. We hypothesize that the growth defect of tpi1-null mutants is caused by mitochondrial reoxidation of cytosolic NADH, thus rendering it unavailable for dihydroxyacetone-phosphate reduction. To test this hypothesis, a tpi1Δ nde1Δ nde2Δ gut2Δ quadruple mutant was constructed. NDE1 and NDE2 encode isoenzymes of mitochondrial external NADH dehydrogenase; GUT2 encodes a key enzyme of the glycerol-3-phosphate shuttle. It has recently been demonstrated that these two systems are primarily responsible for mitochondrial oxidation of cytosolic NADH in S. cerevisiae. Consistent with the hypothesis, the quadruple mutant grew on glucose as the sole carbon source. The growth on glucose, which was accompanied by glycerol production, was inhibited at high-glucose concentrations. This inhibition was attributed to glucose repression of respiratory enzymes as, in the quadruple mutant, respiratory pyruvate dissimilation is essential for ATP synthesis and growth. Serial transfer of the quadruple mutant on high-glucose media yielded a spontaneous mutant with much higher specific growth rates in high-glucose media (up to 0.10 h−1 at 100 g of glucose · liter−1). In aerated batch cultures grown on 400 g of glucose · liter−1, this engineered S. cerevisiae strain produced over 200 g of glycerol · liter−1, corresponding to a molar yield of glycerol on glucose close to unity.
Glycerol is used to synthesize many products, ranging from cosmetics to lubricants. Its current annual production of ca. 600,000 tonnes is mainly recovered as a by-product of soap manufacturing or produced from propylene (55). Alternatively, glycerol can be produced by microbial fermentation, using sustainable carbohydrate feedstocks. Although this may involve a wide range of microorganisms, including algae and bacteria, research has mostly focused on yeasts (1, 55; M. J. Taherzadeh, L. Adler, and G. Lidén, submitted for publication). In yeasts, glycerol is produced by the reduction of dihydroxyacetone phosphate (DHAP) to glycerol-3-phosphate (G3P), a reaction catalyzed by cytosolic NAD+-dependent G3P dehydrogenase. G3P is subsequently dephosphorylated by a glycerol-3-phosphatase (1). Especially during anaerobic growth, glycerol production serves as a redox sink to maintain the cytosolic redox balance (5, 50). Glycerol also functions as an osmolyte, thus enabling yeast growth at high osmolarity (1, 36). Consistent with the latter role, the highest glycerol yields reported to date have been achieved with osmotolerant yeast strains (Table 1).
TABLE 1.
Some representative yeast processes used for glycerol production
| Strain and process | Glycerol concn in broth (g · l−1) | Yield of glycerol on glucose (g · g−1) | Avg productivity (g · liter−1 · day−1) | Reference or source |
|---|---|---|---|---|
| S. cerevisiae | ||||
| Sulfite batch | 45 | 0.23 | 9.0 | 15 |
| Sulfite; fed batch under vacuum | 82 | 0.25 | 32.5 | 27 |
| GPD1 overproduction; batch | 25 | 0.12 | 4.3 | 44 |
| ADH deletion; shake flask | 4.6 | 0.26 | 2.5 | 12 |
| pdc2Δ mutant; shake flask | 2.9 | 0.16 | 2.1 | 35 |
| tpi1Δ mutant; shake flask (0-24 h) with extra glucose (24-56 h) | 36 | 0.46 | 36 | 9 |
| 43 | 0.20 | 5.3 | ||
| tpi1Δ mutant; as above (0-44 h) | 63 | 0.44 | 35 | 10 |
| tpi1Δ nde1Δ nde2Δ gut2Δ mutant, aerated batch | 219 | 0.50 | 57.6 | This study |
| Osmotolerant yeasts | ||||
| Candida magnoliae I2B; batch | 80a | 0.32 | 15.6 | 46 |
| Saccharomyces strain LORRE Y8; fed batch | 260 | 0.47 | 29.8 | 19 |
| Pichia farinosa; fed batch with solid glucose | 300a | 0.46 | 37.5 | 53 |
| Candida glycerinogenes; batch | 127 | 0.64 | 40.6 | 56 |
Total polyol concentration.
The amounts of glycerol that are naturally produced by Saccharomyces cerevisiae as a response to anaerobiosis and/or osmotic stress are relatively small (42, 44, 51). Much effort has been invested in attempts to redirect sugar metabolism in this yeast towards glycerol production. The first successful attempt was the sulfite process, devised by Neuberg and Reinfurth (34). In this process, sulfite added to fermenting S. cerevisiae cultures forms an adduct with acetaldehyde, thus making the latter compound unavailable as an electron acceptor for the reoxidation of glycolytic NADH. Instead, NADH is reoxidized by glycerol production. This early example of redirection of metabolic fluxes has been called metabolic engineering avant la lettre (13). Theoretically, the sulfite process can lead to the formation of equimolar amounts of glycerol, carbon dioxide, and sulfite-acetaldehyde adduct. However, the theoretical yield of glycerol on glucose of 0.51 g · g−1 has not been achieved, not even in modern adaptations of the sulfite process (Table 1). Moreover, the presence of by-products (ethanol, acetate, sulfite-acetaldehyde adduct, and biomass) poses problems during glycerol recovery (1).
Over the past decade, research on glycerol production by S. cerevisiae has shifted to true metabolic engineering, i.e., the application of recombinant DNA technology for a rational reprogramming of cellular metabolism (4). Several approaches were aimed at minimizing the reduction of acetaldehyde to ethanol, thus mimicking the sulfite process. Indeed, reduced expression of pyruvate decarboxylase (35) and deletion of alcohol dehydrogenase genes (12) led to an increased production of glycerol, but the glycerol concentration did not exceed 5 g · liter−1 (Table 1). Other strategies focused on overexpression of the key enzymes of the glycerol pathway in S. cerevisiae (33, 39, 44). Overproduction of the GPD1-encoded cytosolic G3P dehydrogenase led to an increased production of glycerol (33, 44) (Table 1), whereas overproduction of glycerol phosphatase had no effect on glycerol production (39, 44). Increasing glycerol export by deregulation of the Fps1p channel protein increased glycerol production but, in combination with overproduction of G3P dehydrogenase, negatively affected growth (44).
The highest glycerol yield and productivity reported to date for metabolically engineered S. cerevisiae were observed with a tpi1Δ deletion mutant (9, 10) (Table 1). Apparently, in tpi1Δ mutants, which lack the glycolytic enzyme triose phosphate isomerase, accumulation of DHAP is prevented by its conversion to glycerol (Fig. 1). The maximum theoretical yield of this process is 1 mol of glycerol · mol of glucose−1 if all glucose is metabolized via glycolysis. However, S. cerevisiae tpi1Δ mutants are unable to grow on glucose as the sole carbon source (9-11). Therefore, biomass was pregrown on glucose-ethanol mixtures, followed by a bioconversion of glucose to glycerol (9, 10). During the bioconversion phase, glycerol productivity decreased strongly with time (Table 1).
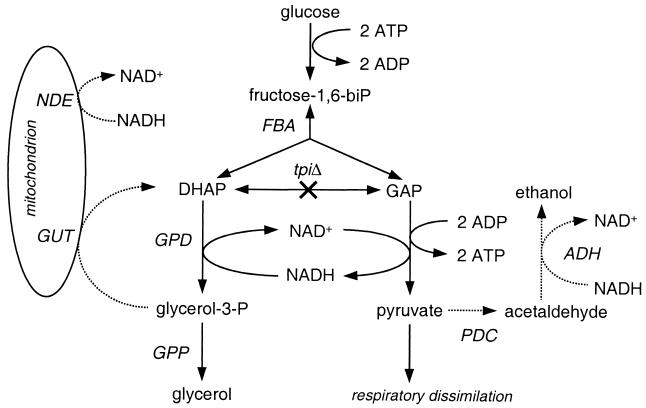
FIG. 1.
Hypothetical pathway (solid lines) for glucose dissimilation by triose phosphate isomerase-negative (tpi1Δ) S. cerevisiae. DHAP is detoxified by its reduction to G3P and subsequent dephosphorylation to glycerol. This requires that all NADH generated in the glyceraldehyde-3-phosphate dehydrogenase reaction be available for DHAP reduction. Oxidation of either cytosolic G3P or cytosolic NADH by other processes (dotted lines) will lead to accumulation of DHAP. Abbreviations: GAP, glyceraldehyde-3-phosphate; FBA, fructose-1,6-biphosphate aldolase; GPD, cytosolic G3P dehydrogenase; GPP, glycerol phosphatase; GUT, mitochondrial flavin adenine dinucleotide-dependent G3P dehydrogenase; NDE, external mitochondrial NADH dehydrogenase; PDC, pyruvate decarboxylase; ADH, alcohol dehydrogenase.
The biochemical mechanism responsible for the inability of S. cerevisiae tpi1Δ mutants to grow on glucose as the sole carbon source is unknown. Since conversion of glucose to equimolar amounts of pyruvate and glycerol is neutral in terms of ATP (Fig. 1), growth of tpi1Δ mutants should depend on the respiratory dissimilation of pyruvate. Glucose repression of the synthesis of key enzymes of respiratory glucose dissimilation (16, 50) may therefore prevent growth of tpi1Δ mutants on glucose in batch cultures. However, tpi1Δ mutants also failed to grow in aerobic, glucose-limited chemostat cultures, in which glucose repression is alleviated (11). As in batch cultures, growth on glucose in chemostat cultures required the inclusion of ethanol in the medium feed (11). This indicated that glucose repression of respiration is not the only factor that prevents growth of tpi1Δ mutants on glucose as the sole carbon source.
Complete conversion of DHAP to glycerol in a tpi1Δ mutant requires that all NADH generated in the glyceraldehyde-3-phosphate dehydrogenase reaction be used to reduce DHAP to glycerol (Fig. 1). If other reactions were to compete for NADH with glycerol production, this would lead to accumulation of DHAP, which in turn can be converted to methylglyoxal, a cytotoxic compound that can inhibit growth (25, 32). We hypothesize that oxidation of cytosolic NADH by the mitochondrial respiratory chain is such a competing mechanism and thus contributes to the inability of tpi1Δ mutants to grow on glucose as the sole carbon source (Fig. 1). Two mechanisms are involved in the mitochondrial oxidation of cytosolic NADH in S. cerevisiae: mitochondrial external NADH dehydrogenase and a G3P shuttle (29, 31, 37, 48). At low specific growth rates, either of these two systems is sufficient to sustain respiratory growth (37). The NDE1 and NDE2 genes encode two isoenzymes of the external NADH dehydrogenase (31, 48), whereas the GUT2 gene encodes a mitochondrial respiratory-chain-linked G3P dehydrogenase, a key enzyme of the G3P shuttle (29, 45). The latter enzyme might also directly affect growth of tpi1Δ mutants by reoxidizing G3P to DHAP (Fig. 1).
The aim of this study is to test the hypothesis that mitochondrial reoxidation of cytosolic NADH and/or G3P plays a role in the phenotype of tpi1Δ mutants and to investigate whether metabolic engineering of mitochondrial respiration can be used to improve glycerol production by S. cerevisiae.
MATERIALS AND METHODS
Yeast strains and maintenance.
The S. cerevisiae strains used and constructed in this study (Table 2) are prototrophic strains belonging to the CEN.PK family (49). Stock cultures were grown at 30°C in shake flasks on YPED medium (10 g of Bacto yeast extract · liter−1, 20 g of peptone · liter−1, 10 ml of ethanol · liter−1, and 0.5 g of glucose · liter−1) except the adaptation to high glucose concentration (AHG) strain, which was grown on 100 g of glucose · liter−1 in MMU medium (3.0 g of K2SO4 · liter−1, 3.0 g of KH2PO4 · liter−1, 3.0 g of urea · liter−1, 0.5 g of MgSO4 · 7H2O · per liter) with trace elements and vitamins prepared and sterilized as described previously (52). Urea was added to the medium after separate filter sterilization. When stationary phase was reached, 30% (vol/vol) sterile glycerol was added, and 2-ml aliquots were stored in sterile vials at −80°C.
TABLE 2.
S. cerevisiae strains used in this study
| Strain | Genotypea | Source or reference |
|---|---|---|
| CEN.PK113-7D | MATa | 31 |
| CEN.PK122 | MATa/MATα | 31 |
| CEN.PK167-2B | MATande1 (41-1659)::loxP-kanMX4-loxP nde2(51-100)::loxP-kanMX4-loxP | 31 |
| CEN.PK225-2C | MATagut2(41-2010)::loxP-kanMX4-loxP | 37 |
| CEN.PK536-5B | MATα tpi1(41-707)::loxP-kanMX4-loxP nde1(41-1659)::loxP-kanMX4-loxP nde2 (51-100)::loxP-kanMX4-loxP | This study |
| CEN.PK530-1B | MATα tpi1(41-707)::loxP-kanMX4-loxP | This study |
| CEN.PK530-1C | MATatpi1(41-707)::loxP-kanMX4-loxP | This study |
| CEN.PK546-12B | MATatpi1 (41-707)::loxP-kanMX4-loxP nde1 (41-1659)::loxP-kanMX4-loxP nde2(51-100)::loxP-kanMX4-loxP gut2(41-2010)::loxP-kanMX4-loxP | This study |
| CEN.PK546-AHG | Spontaneous mutant of CEN.PK546-12B, selected for AHG concn | This study |
The numbers in parentheses indicate the deleted nucleotides (ATG = 1).
Construction of null mutants.
Standard techniques and media for genetic modification of S. cerevisiae were used (3). Deletions in TPI1 were obtained by the short flanking homology method (54), using pUG6 as a template (21). PCR amplification, yeast transformation, and verification of the correct gene deletion, as well as determination of the mating type, were carried out as described by Luttik et al. (31). The loxP-kanMX4-loxP cassette amplified by PCR was used to transform diploid strain CEN.PK122. After tetrad analysis, the G418R segregants were checked by diagnostic PCR for the correct integration of the kanMX cassette (Table 3).
TABLE 3.
Oligonucleotides used for construction of the TPI1 deletion cassette (S1/S2) and for diagnostic PCR (A1/K1) and (K2/A4) of the deletion strains.a
| Oligonucleotide | DNA sequence |
|---|---|
| TPII-S1 | 5′-ATG GCT AGA ACT TTC TTT GTC GGT GGT AAC TTT AAA TTA ACA GCT GAA GCT TCG TAC GC-3′ |
| TPII-S2 | 5′-TTA GTT TCT AGA GTT GAT GAT ATC AAC AAA TTC TGG CTT CGC ATA GGC CAC TAG TGG ATC TG-3′ |
| TPII-A1 | 5′-CTT CTG CGG TAT CAC CCT AC-3′ |
| TPII-A4 | 5′-CAA TGC AGT CTT CGG TAC AC-3′ |
| K1 | 5′-GGA TGT ATG GGC TAA ATG TAC G-3′ |
| K2 | 5′-GTT TCA TTT GAT GCT CGA TGA G-3′ |
All further double, triple, or quadruple deletion strains were constructed by crossing of the corresponding single, double, or triple deletion strains. The resulting diploid strains were subsequently analyzed by tetrad analysis to obtain the respective segregants and further analyzed by diagnostic PCR to confirm the correct deletion of the corresponding genes. Strain CEN.PK536-5B (nde1Δ nde2Δ tpi1Δ) resulted from crossing of strains CEN.PK167-2B (nde1Δ nde2Δ) and CEN.PK530-1B (tpi1Δ). To obtain the quadruple deletion strain CEN.PK546-12B (nde1Δ nde2Δ tpi1Δ gut2Δ), the strains CEN.PK536-5B (nde1Δ nde2Δ tpi1Δ) and CEN.PK225-2C (gut2Δ), respectively, were crossed.
Shake flask cultivation.
Shake flask cultures were grown in an orbital incubator (200 rpm, 30°C) in spherical flat-bottom flasks (500 ml) containing 100 ml of medium. Precultures were grown on MMU medium supplemented with 5 ml of ethanol · liter−1 and 1 g of glucose · liter−1. During the exponential growth phase, the optical density at 660 nm (OD660) was measured with an Amersham Pharmacia Novaspec II spectrophotometer. Exponential-phase cultures were used as the inoculum for further shake flask experiments. Prior to inoculation, cells were centrifuged (4,500 × g; 3 min) and washed aseptically with sterile medium to remove residual substrates and metabolites. The washed cells were used to inoculate (initial OD660 of 0.1) fresh shake flasks containing MMU medium with 5 ml of ethanol · liter−1 and 1 g of glucose · liter−1. During the exponential growth phase, cells were harvested as described above, washed, and resuspended in the medium used for the final shake flask cultivation, which was MMU medium with glucose as the sole carbon source at a concentration of 1, 5, 10, 50, or 100 g · liter−1. Growth was monitored by regular OD660 measurements. When OD660 was above 0.3, samples were diluted in MMU medium containing the same glucose concentration as the culture, to avoid changes in OD660 due to osmotic effects.
Chemostat cultivation.
Chemostat cultures were grown in 1-liter working-volume laboratory fermentors as described previously (31). This reference also describes procedures for gas analysis and determination of biomass dry weight. One-hundred-milliliter precultures in shake flasks, inoculated with 2 ml of a frozen stock culture, were grown to stationary phase on YPED medium. These precultures were used to inoculate a fermentor containing 1 liter of MMA medium [5.0 g of (NH4)2SO4 · liter−1, 3.0 g of KH2PO4 · liter−1, 0.5 g of MgSO4 · 7H2O per liter, vitamins, and trace elements as in the MMU medium described above] supplemented with 1 g of glucose · liter−1 and 10 ml of ethanol · liter−1. The continuous medium supply was initiated when a steep increase of the dissolved-oxygen concentration indicated depletion of the carbon source. The synthetic medium used for continuous cultivation was MMA medium supplemented with 7.5 g of glucose · liter−1 (60% of total carbon) and 83 mM ethanol (40% of total carbon). The dilution rate was set at 0.05 h−1. For growth on glucose as a sole carbon source, the feed of the chemostat culture growing on the glucose-ethanol mixture was switched to the same medium without ethanol.
Batch cultivation in fermentors.
One milliliter of frozen stock culture of the glucose-adapted CEN.PK546-12B strain (CEN.PK546-AHG, where AHG is a designation for adapted to high glucose; see Results) was inoculated in shake flasks with double-strength MMU medium supplemented with 400 g of glucose · liter−1. After 4 days of incubation, 150 ml of this culture was transferred to a 2-liter laboratory fermentor (Applikon, Schiedam, The Netherlands). The fermentor was previously autoclaved (20 min at 110°C) while containing 1.2 liters of demineralized water with 600 g of glucose and 100 μl of silicone antifoam (BDH). Together with the inoculum, 45 ml of a solution containing 9 g of urea and 100 ml of 30-fold concentrated MM medium (MMU without urea) was added. This yielded an initial culture volume of 1.5 liters consisting of double-strength MMU medium and an initial glucose concentration of 400 g·liter−1. The pH was kept at 5.0 by an Applikon ADI 1030 biocontroller, via the automatic addition of 4 M KOH or 4 M H2SO4. The fermentor was aerated (0.75 liter · min−1) and stirred at 900 rpm. Additional antifoam was added manually when foaming occurred. Biomass growth was monitored by OD660 measurements (when necessary, diluted in a 400-g · liter−1 glucose solution) and via biomass dry-weight measurements (31). Metabolite concentrations were determined by high-performance liquid chromatography (30). High-biomass-density cultures were performed in the same way, but were inoculated with 200 ml of a stationary-phase fermentor culture grown as described above.
RESULTS
Growth on glucose in shake flask cultures.
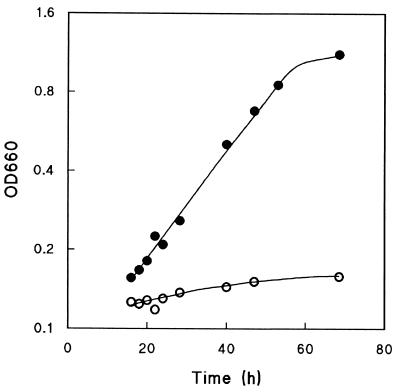
Previously published reports state that tpi1Δ mutants of S. cerevisiae are unable to grow on glucose as the sole carbon source, both in shake flasks (9, 38) and in chemostat cultures (11). This was confirmed in shake flask cultures containing 5 g of glucose · liter−1, in which the specific growth rate was below 0.01 h−1 (Fig. 2). To investigate whether this inability to grow on glucose was due to mitochondrial reoxidation of cytosolic NADH and/or G3P (Fig. 1), thus preventing the complete reduction of dihydroxyacetone phosphate, a tpi1Δ nde1Δ nde2Δ gut2Δ mutant was constructed. Indeed, this quadruple deletion mutant was able to grow on glucose with a specific growth rate of 0.045 h−1 (Fig. 2). Yet, this specific growth rate is still substantially lower than that of the isogenic wild-type strain CEN.PK113-7D, which under these conditions exhibits a ca. eightfold-higher specific growth rate (data not shown).
FIG. 2.
Growth on glucose of tpi1Δ S. cerevisiae (○) and an isogenic tpi1Δ nde1Δ nde2Δ gut2Δ strain (•). Both strains were grown in shake flasks on MMU medium with 5 g of glucose · liter−1 as the sole carbon source. Three independent replicate cultures gave the same results.
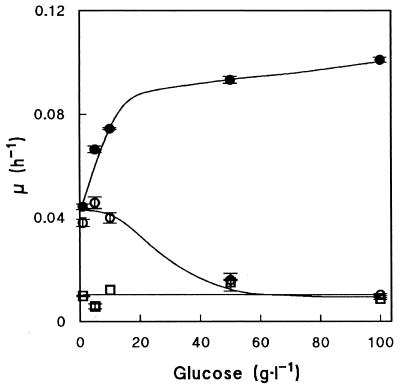
Specific growth rates of the tpi1Δ nde1Δ nde2Δ gut2Δ strain on glucose strongly depended on the glucose concentration in the medium (Fig. 3). At glucose concentrations of 50 g · liter−1 and higher, the specific growth rate of the quadruple deletion strain was very low and similar to that of the tpi1Δ single mutant. Conversion of glucose into equimolar amounts of glycerol and pyruvate is neutral in terms of ATP and redox metabolism. Therefore, growth of the tpi1Δ mutants on glucose critically depends on respiration for ATP production. The synthesis of many enzymes involved in the respiratory dissimilation of pyruvate is subject to glucose catabolite repression (16, 50), which may explain the glucose sensitivity of the tpi1Δ nde1Δ nde2Δ gut2Δ strain.
FIG. 3.
Effect of glucose concentration on the specific growth rate of isogenic S. cerevisiae tpi1Δ (□) and tpi1Δ nde1Δ nde2Δ gut2Δ (○) strains and a tpi1Δ nde1Δ nde2Δ gut2Δ-AHG (adapted to high glucose) strain (•) in shake flask cultures. All cultures were grown on MMU medium supplemented with different initial glucose concentrations. Data are presented as the average ± mean deviation of at least two independent replicate cultures for each glucose concentration.
Glucose-limited chemostat cultivation.
Analysis of fermentation products in glucose-grown shake flask cultures suggested that the tpi1Δ nde1Δ nde2Δ gut2Δ strain produced large amounts of glycerol. For a quantitative analysis of biomass and product yields, the tpi1Δ nde1Δ nde2Δ gut2Δ strain and the reference strain CEN.PK113-7D were grown in aerobic, glucose-limited chemostat cultures. In such cultures, glucose catabolite repression can be alleviated by the low-residual-glucose concentrations. The biomass yield of the tpi1Δ nde1Δ nde2Δ gut2Δ strain was more than twofold lower than the reference strain during growth at a dilution rate of 0.05 h−1 (Table 4). This was partly due to the conversion of glucose into glycerol. The molar yield of glycerol on glucose was 0.83 mol · mol−1. Under the same cultivation conditions, the isogenic reference strain CEN.PK113-7D did not produce detectable amounts of glycerol. The extensive production of glycerol is consistent with the metabolic scheme proposed in Fig. 1. This scheme predicts a glycerol yield of 1 mol · mol−1. The lower glycerol yield in the glucose-limited chemostat cultures can be explained from the assimilation of glucose-6-phosphate via metabolic pathways other than glycolysis, such as the pentose phosphate pathway, cell wall biosynthesis, and storage carbohydrate biosynthesis.
TABLE 4.
Growth of the reference strain S. cerevisiae CEN.PK113-7D and the isogenic tpi1Δ nde1Δ nde2Δ gut2Δ mutant CEN.PK546-12B.a
| Strain (relevant genotype) | Biomass yield (g·g glucose−1) | Glycerol yield (mol·mol glucose−1) | qO2 | qCO2 | Carbon recovery (%) |
|---|---|---|---|---|---|
| CEN PK113-7D (TPI1 NDE1 NDE2 GUT2) | 0.47 ± 0.01 | <0.01 | 1.3 ± 0.1 | 1.4 ± 0.1 | 95 |
| CEN.PK546-12B (tpi1Δ nde1Δ nde2Δ gut2Δ) | 0.20 ± 0.02 | 0.83 ± 0.01 | 2.2 ± 0.2 | 2.7 ± 0.3 | 98 |
Strains were grown in aerobic, glucose-limited chemostat cultures (30°C, pH 5.0, dilution rate = 0.05 h−1, 7.5 g of glucose·liter−1 in feed). Data are presented as the average ± mean deviation of two independent chemostat experiments for each strain. Values for qO2 and qCO2 are in millimoles per gram per hour.
Selection of a strain adapted to high-glucose concentrations.
The glycerol yield (0.83 mol · mol glucose−1, i.e., 0.42 g · g of glucose−1) in glucose-limited chemostat cultures of the tpi1Δ nde1Δ nde2Δ gut2Δ strain is among the highest reported for a growing, metabolically engineered S. cerevisiae strain, although it is matched by a bioconversion process with the nongrowing tpi1Δ strain (Table 1). However, the glucose sensitivity of the quadruple mutant is a drawback for any large-scale application in glycerol production. The tpi1Δ nde1Δ nde2Δ gut2Δ strain (CEN.PK 546-12B) was adapted to growth at high-glucose concentrations by serial transfer. This was started by transferring 10 ml of a steady-state glucose-limited chemostat culture to a shake flask containing MMA medium with 100 g of glucose · liter−1 (555 mM). This culture showed extremely slow growth accompanied by acetate production and a decrease of the culture pH to ca. 3. After 2 days, growth and acetate production ceased. After another 4 days, acetate consumption occurred and growth resumed. Eventually the low pH (ca. 2.5) caused by ammonia consumption abolished growth altogether, despite the presence of approximately 350 mM residual glucose. From this culture, 1 ml was transferred to a new shake flask with the same medium. No acetate production was observed, but acidification again prevented complete consumption of glucose. Use of urea instead of ammonium salts as the sole nitrogen source can sometimes prevent acidification of yeast cultures (24). Therefore, 1 ml of culture was transferred to a shake flask with MMU medium containing 100 g of glucose · liter−1. This indeed led to complete consumption of glucose. A total of 0.5 ml of culture was transferred to the next flask with the same medium. After two further transfers, the culture was streaked onto agar plates with MMU medium containing 50 g of glucose · liter−1. The culture was purified by three subsequent transfers of a single colony to a new plate. Finally, a single colony was transferred to a shake flask with MMU medium containing 100 g of glucose · liter−1. This glucose-adapted culture of the tpi1Δ nde1Δ nde2Δ gut2Δ strain was called CEN.PK546-AHG. Especially at high-glucose concentrations, the selected mutant exhibited much higher specific growth rates than the original, nonadapted tpi1Δ nde1Δ nde2Δ gut2Δ strain (Fig. 3).
Production of glycerol by engineered S. cerevisiae.
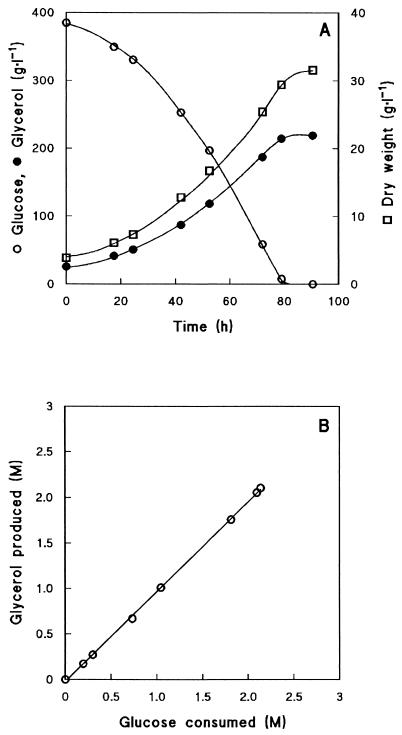
Glycerol production by S. cerevisiae CEN.PK546-AHG was investigated in aerated batch cultures with 400 g of glucose · liter−1, which were inoculated from glucose-grown shake flasks at 0.3 g (dry weight) · liter−1. Despite the high initial glucose concentration, these batch cultures exhibited exponential growth (μ = 0.027 h−1). The slow growth was accompanied by the accumulation of glycerol to a final concentration of over 200 g · liter−1 (data not shown). Glycerol was the major fermentation product. Concentrations of acetate and ethanol remained below 1.5 and 4 g · liter−1, respectively, and were completely consumed towards the end of fermentation (data not shown). Throughout the fermentation process, the glycerol yield on glucose was 1.0 mol · mol−1. Due to the low initial biomass concentration and the long start-up period, the overall glycerol production rate was 1.0 g glycerol · liter−1·h−1. When the experiment was repeated with an initial biomass dry weight of 3.9 g · liter−1 (Fig. 4A), all glucose was consumed in 80 h and the final concentration of glycerol was 219 g · liter−1. The molar ratio of glycerol produced per glucose consumed was 0.99 mol · mol−1 (Fig. 4B), and the overall glycerol production rate was 2.4 g glycerol · liter−1 · h−1. Glycerol consumption did not occur, even after glucose depletion. Most wild-type S. cerevisiae strains can consume glycerol, although it is a very poor carbon and energy source (43, 49). However, in the quadruple mutant, glycerol dissimilation is not possible as the deletion of the GUT2 gene and the absence of triose phosphate isomerase block the oxidation pathway.
FIG. 4.
Glycerol production in an aerated fermentor culture with an initial glucose concentration of 400 g · liter−1 by a spontaneous mutant of the tpi1Δ nde1Δ nde2Δ gut2Δ S. cerevisiae strain, isolated by serial transfer in high-glucose medium (AHG strain). An independent duplicate experiment gave identical results. (A) Concentrations of biomass (□), glucose (○), and glycerol (•). (B) The molar ratio between glucose consumed and glycerol produced during the fermentation.
DISCUSSION
Phenotype of tpi1Δ mutants.
In many organisms, null mutations in the structural gene encoding triose phosphate isomerase or mutations that lead to severely reduced activities of this enzyme result in growth deficiencies (7, 8, 23, 41). The complete inability of tpiΔ mutants to grow on glucose as the sole carbon source, as found with S. cerevisiae (9, 11) (Fig. 2), is not representative for all yeasts. A Kluyveromyces lactis Kltpi1Δ mutant did grow on glucose in shake flask cultures, albeit at a reduced growth rate (8). A similar difference has been observed for other glycolytic mutants of S. cerevisiae and K. lactis (17, 22). This has been attributed to the ability of K. lactis, but not S. cerevisiae, to couple the oxidation of cytosolic NADPH to the mitochondrial respiratory chain (20). This coupling enables glycolytic null mutants of K. lactis to use the pentose phosphate pathway, with its NADP+-linked dehydrogenases, as an alternative dissimilatory pathway (20, 26). In tpi1Δ mutants, this partial bypass of fructose-1,6-bisphosphate aldolase (6) generates sufficient NADH to prevent the accumulation of DHAP.
Even when glucose dissimilation cannot be rerouted via the pentose phosphate pathway, the metabolic network of S. cerevisiae should, at least in theory, be sufficiently flexible to allow for growth on glucose in the absence of an active triose phosphate isomerase. However, this demands that all NADH produced in the glyceraldehyde-3-phosphate dehydrogenase reaction be used for reduction of DHAP to glycerol (Fig. 1). The inability of the tpi1Δ mutant to grow on glucose as a sole carbon source and the partial restoration of growth in a quadruple tpi1Δ nde1Δ nde2Δ gut2Δ strain (Fig. 2), indicates that mitochondrial oxidation of NADH and/or G3P competes for the NADH formed in glycolysis and thus contributes to the phenotype of tpi1Δ mutants. This also provides an explanation for the restoration of growth by cofeeding ethanol or ethanol-formate mixtures to aerobic, glucose-limited chemostat cultures of tpi1Δ mutants (11). In these cultures, the cytosolic, NAD+-dependent alcohol and formate dehydrogenases can provide sufficient NADH for complete reduction of DHAP, even in the presence of an active mitochondrial respiratory chain. Interestingly, it has recently been proposed that the nonviability of a tpiΔ mutant of the bloodstream form of the parasite Trypanosoma brucei is due to a similar redox problem, caused in this case by the activity of a mitochondrial G3P oxidase (23).
Özcan et al. (38) and Schulte et al. (47) isolated and characterized suppressor mutants of a S. cerevisiae tpi1Δ mutant which had regained the ability to grow on glucose. Some of the suppressor mutants exhibited a strongly reduced activity of phosphoglycerate kinase. Reduced activity of phosphoglycerate kinase will cause a reduced flux through glyceraldehyde-3-phosphate dehydrogenase, which in turn affects the cytosolic NADH/NAD+ ratio. As a result of the different affinities of isolated mitochondria and NAD+-dependent G3P dehydrogenases for NADH (2, 40), a changed cytosolic NADH/NAD+ ratio may suppress the interference of mitochondrial respiration with redox metabolism in tpi1Δ mutants (Fig. 1). Another class of suppressor mutants exhibited reduced glucose uptake and reduced glucose repression of respiratory enzymes (38, 47). This appears to be more difficult to reconcile with our hypothesis. However, isolation of the suppressor mutants was done with glucose-containing complex medium (38). With such a medium, it is difficult to assess whether growth is due solely to glucose consumption or to consumption of other carbon sources present in the complex medium (14). To further study the phenotype of tpi1Δ mutants, it would be of interest to isolate and characterize tpi1Δ suppressor mutants that grow on glucose-containing synthetic medium.
Although the additional deletion of the NDE1, NDE2, and GUT2 genes restored growth of tpi1Δ S. cerevisiae (Fig. 2), the specific growth rate of the resulting quadruple mutant remained much lower than that of wild-type S. cerevisiae. Several factors may have contributed to this low specific growth rate. For example, the rate of glucose dissimilation may have been controlled by a limited capacity of enzymes involved in DHAP reduction and/or glycerol export (33, 44). Furthermore, the glucose sensitivity of the tpi1Δ nde1Δnde2Δ gut2Δ strain (Fig. 3) indicated that glucose repression of mitochondrial pyruvate dissimilation exerted control over the specific growth rate.
Metabolic engineering of S. cerevisiae for glycerol production.
Glycerol yields on glucose in chemostat cultures (Table 4) of the tpi1Δ nde1Δ nde2Δ gut2Δ strain were 83% of the theoretical maximum of the classical sulfite process. However, this mutant’s sensitivity to glucose and, to a lesser extent, its low specific growth rate are drawbacks for any application in glycerol production. In view of the many cellular reactions that may contribute to this phenotype, it was decided to use serial transfer in batch cultures, rather than targeted genetic engineering, for further optimization of glycerol production. The yield and volumetric productivity of the resulting AHG strain are the highest reported to date for S. cerevisiae (Table 1). With respect to glycerol concentration, the results are only surpassed by a fed-batch experiment with the addition of sulfite and CO2 sparging, which led to a final glycerol concentration of 230 g · liter−1 in 368 h (28). We have not attempted to further increase the glycerol concentration by, for example, feeding solid glucose (53).
The molar yield of glycerol on glucose of the AHG strain corresponded to the theoretical maximum of 1 mol · mol−1 that can be reached in tpi1Δ mutants or in the classical sulfite process. Still, this glycerol yield was higher than anticipated, as a significant fraction of the glucose was expected to be metabolized via assimilatory reactions (e.g., those of the pentose phosphate pathway [6, 18]), instead of via fructose-1,6-bisphosphate aldolase. In keeping with this, the glycerol yield in aerobic, glucose-limited chemostat cultures of the unadapted quadruple mutant was less than 1.0 mol · mol−1 (Table 4). The contribution of assimilatory glucose metabolism will decrease with decreasing specific growth rate due to the increasing impact of glucose dissimilation for maintenance processes. Thus, the low specific growth rate (0.027 h−1) in the high-glucose batch cultures may have contributed to the high glycerol yield. Furthermore, the glycerol yields may have been slightly overestimated due to the changing volumetric masses in the high-density cultures.
The glucose-adapted tpi1Δ nde1Δ nde2Δ gut2Δ strain is an interesting experimental platform to study factors involved in the kinetics of glycerol production and the further optimization of final glycerol concentrations. However, from a scientific point of view, the most important challenge is to increase the glycerol yield on glucose to values above 1 mol of glycerol·mol of glucose−1. In view of the constraints imposed by the tpi1Δ mutation (Fig. 1), this will require a fundamentally different approach from the one adopted in this study.
Acknowledgments
This work was financially supported by the Delft University DIOC-6 program “Mastering the Molecules of Manufacturing.”
We thank Arjen van Tuijl (Delft University of Technology) for his practical assistance.
REFERENCES
- 1.Agarwal, G. P. 1990. Glycerol, p. 95-128. In A. Fiechter (ed.), Advances in biochemical engineering/biotechnology 41: microbial bioproducts. Springer-Verlag, Berlin, Germany.
- 2.Albertyn, J., A. van Tonder, and B. A. Prior. 1992. Purification and characterization of glycerol-3-phosphate dehydrogenase of Saccharomyces cerevisiae. FEBS Lett. 308:130-132. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 4.Bailey, J. E. 1991. Toward a science of metabolic engineering. Science 252:1668-1675. [DOI] [PubMed] [Google Scholar]
- 5.Bakker, B. M., K. M. Overkamp, A. J. A. van Maris, P. Kötter, M. A. H. Luttik, J. P. van Dijken, and J. T. Pronk. 2001. Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:15-37. [DOI] [PubMed] [Google Scholar]
- 6.Bruinenberg, P. M., J. P. van Dijken, and W. A. Scheffers. 1983. A theoretical analysis of NADPH production and consumption in yeasts. J. Gen. Microbiol. 129:953-964. [Google Scholar]
- 7.Ciriacy, M., and I. Breitenbach. 1979. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J. Bacteriol. 139:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compagno, C., F. Boschi, A. Daleffe, D. Porro, and B. M. Ranzi. 1999. Isolation, nucleotide sequence, and physiological relevance of the gene encoding triose phosphate isomerase from Kluyveromyces lactis. Appl. Environ. Microbiol. 65:4216-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compagno, C., F. Boschi, and B. M. Ranzi. 1996. Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol. Prog. 12:591-595. [DOI] [PubMed] [Google Scholar]
- 10.Compagno, C., F. Boschi, and B. M. Ranzi. 1998. Factors affecting glycerol production by a bioconversion process with a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biocat. Biotransf. 16:135-143. [Google Scholar]
- 11.Compagno, C., L. Brambilla, D. Capitanio, F. Boschi, B. M. Ranzi, and D. Porro. 2001. Alterations of the glucose metabolism in a triose phosphate isomerase-negative Saccharomyces cerevisiae mutant. Yeast 18:663-670. [DOI] [PubMed] [Google Scholar]
- 12.Drewke, C., J. Thielen, and M. Ciriacy. 1990. Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J. Bacteriol. 172:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Mansi, E. M. T., C. F. A. Bryce, and B. S. Hartly. 1999. Fermentation biotechnology: an historical perspective, p. 1-8. In E. M. T. El-Mansi and C. F. A. Bryce (ed.), Fermentation microbiology and biotechnology. Taylor & Francis, Ltd., London, United Kingdom.
- 14.Flikweert, M. T., M. de Swaaf, J. P. van Dijken, and J. T. Pronk. 1999. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 174:73-79. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, G. G., and G. M. S. Donald. 1957. Fermentation processes leading to glycerol. I. The influence of certain variables on glycerol formation in the presence of sulfites. Appl. Microbiol. 5:197-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gancedo, J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goffrini, P., M. Wésolowski-Louvel, and I. Ferrero. 1991. A phosphoglucose isomerase gene is involved in the Rag phenotype of the yeast Kluyveromyces lactis. Mol. Gen. Genet. 228:401-409. [DOI] [PubMed] [Google Scholar]
- 18.Gombert, A. K., M. M. dos Santos, B. Christensen, and J. Nielsen. 2001. Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J. Bacteriol. 183:1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong, C. S., J. X. Du, N. J. Cao, and G. T. Tsao. 2000. Coproduction of ethanol and glycerol. Appl. Biochem. Biotechnol. 84-86:543-559. [DOI] [PubMed] [Google Scholar]
- 20.González Siso, M. I., M. A. Freire Picos, and M. E. Cerdán. 1996. Reoxidation of the NADPH produced by the pentose phosphate pathway is necessary for the utilization of glucose by Kluyveromyces lactis rag2 mutants. FEBS Lett. 387:7-10. [DOI] [PubMed] [Google Scholar]
- 21.Güldener, U., S. Heck, T. Fiedler, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinisch, J. J., L. Kirchrath, L. Liesen, K. Vogelsang, and C. P. Hollenberg. 1993. Molecular genetics of phosphofructokinase in the yeast Kluyveromyces lactis. Mol. Microbiol. 8:559-570. [DOI] [PubMed] [Google Scholar]
- 23.Helfert, S., A. M. Estévez, B. M. Bakker, P. Michels, and C. Clayton. 2001. Roles of triosephosphate isomerase and aerobic metabolism in Trypanosoma brucei. Biochem. J. 357:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hensing, M. C. M., K. Bangma, L. Raamsdonk, E. de Hulster, J. P. van Dijken, and J. T. Pronk. 1995. Effects of cultivation conditions on the production of heterologous α-galactosidase by Kluyveromyces lactis. Appl. Microbiol. Biotechnol. 43:58-64. [Google Scholar]
- 25.Inoue, Y., Y. Tsujimoto, and A. Kimura. 1998. Expression of the glyoxalase I gene of Saccharomyces cerevisiae is regulated by high osmolarity glycerol mitogen-activated protein kinase pathway in osmotic stress response. J. Biol. Chem. 273:2977-2983. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby, J., C. P. Hollenberg, and J. J. Heinisch. 1993. Transaldolase mutants in the yeast Kluyveromyces lactis provide evidence that glucose can be metabolized through the pentose phosphate pathway. Mol. Microbiol. 10:867-876. [DOI] [PubMed] [Google Scholar]
- 27.Kalle, G. P., and S. C. Naik. 1985. Continuous fed-batch vacuum fermentation system for glycerol from molasses by the sulfite process. J. Ferment. Technol. 63:411-414. [Google Scholar]
- 28.Kalle, G. P., S. C. Naik, and B. Z. Lashkari. 1985. Improved glycerol production from cane molasses by the sulfite process with vacuum or continuous carbon dioxide sparging during fermentation. J. Ferment. Technol. 63:231-237. [Google Scholar]
- 29.Larsson, C., I. L. Påhlman, R. Ansell, M. Rigoulet, L. Adler, and L. Gustafsson. 1998. The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast 14:347-357. [DOI] [PubMed] [Google Scholar]
- 30.Luttik, M. A. H., P. Kötter, F. A. Salomons, I. J. van der Klei, J. P. van Dijken, and J. T. Pronk. 2000. The Saccharomyces cerevisiae ICL2 gene encodes a mitochondrial 2-methylisocitrate lyase involved in propionyl-coenzyme A metabolism. J. Bacteriol. 182:7007-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttik, M. A. H., K. M. Overkamp, P. Kötter, S. de Vries, J. P. van Dijken, and J. T. Pronk. 1998. The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J. Biol. Chem. 273:24529-24534. [DOI] [PubMed] [Google Scholar]
- 32.Martins, A. M., C. A. Cordeiro, and F. Ponces. 2001. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 499:41-44. [DOI] [PubMed] [Google Scholar]
- 33.Michnick, S., J. L. Roustan, F. Remize, P. Barre, and S. Dequin. 1997. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783-793. [DOI] [PubMed] [Google Scholar]
- 34.Neuberg, C., and E. Reinfurth. 1918. Natürliche und erzwungene glycerinbildung bei der alkoholischen gärung. Biochem. Z. 92:234-266. [Google Scholar]
- 35.Nevoigt, E., and U. Stahl. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331-1337. [DOI] [PubMed] [Google Scholar]
- 36.Nevoigt, E., and U. Stahl. 1997. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:231-241. [DOI] [PubMed] [Google Scholar]
- 37.Overkamp, K. M., B. M. Bakker, P. Kötter, A. van Tuijl, S. de Vries, J. P. van Dijken, and J. T. Pronk. 2000. In vivo analysis of the mechanisms for oxidation of cytosolic NADH by Saccharomyces cerevisiae mitochondria. J. Bacteriol. 182:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Özcan, S., K. Friedel, A. Leuker, and M. Ciriacy. 1993. Glucose uptake and catabolite repression in dominant HTR1 mutants of Saccharomyces cerevisiae. J. Bacteriol. 175:5520-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Påhlman, A.-K., K. Granath, R. Ansell, S. Hohmann, and L. Adler. 2001. The yeast glycerol 3-phosphatases Gpp1p and Gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress. J. Biol. Chem. 276:3555-3563. [DOI] [PubMed] [Google Scholar]
- 40.Påhlman, I.-L., L. Gustafsson, M. Rigoulet, and C. Larsson. 2001. Cytosolic redox metabolism in aerobic chemostat cultures of Saccharomyces cerevisiae. Yeast 18:611-620. [DOI] [PubMed] [Google Scholar]
- 41.Pekrun, A., B. A. Neubauer, S. W. Eber, M. Lakomek, H. Seidel, and W. Schroter. 1995. Triosephosphate isomerase deficiency: Biochemical and molecular genetic analysis for prenatal diagnosis. Clin. Genet. 47:175-179. [DOI] [PubMed] [Google Scholar]
- 42.Petrovska, B., E. Winkelhausen, and S. Kuzmanova. 1999. Glycerol production by yeasts under osmotic and sulfite stress. Can. J. Microbiol. 45:695-699. [DOI] [PubMed] [Google Scholar]
- 43.Pronk, J. T., T. J. Wenzel, M. A. H. Luttik, C. C. Klaassen, W. A. Scheffers, H. Y. Steensma, and J. P. van Dijken. 1994. Energetic aspects of glucose metabolism in a pyruvate-dehydrogenase-negative mutant of Saccharomyces cerevisiae. Microbiology 140:601-610. [DOI] [PubMed] [Google Scholar]
- 44.Remize, F., L. Barnavon, and S. Dequin. 2001. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab. Eng. 3:301-312. [DOI] [PubMed] [Google Scholar]
- 45.Rønnow, B., and M. C. Kielland-Brandt. 1993. Yeast sequencing reports. IX. GUT2, a gene for mitochondrial glycerol 3-phosphate dehydrogenase of Saccharomyces cerevisiae. Yeast 9:1121-1130. [DOI] [PubMed] [Google Scholar]
- 46.Sahoo, D. K., and G. P. Agarwal. 2001. An investigation on glycerol biosynthesis by an osmophilic yeast in a bioreactor. Process Biochem. 36:839-846. [Google Scholar]
- 47.Schulte, F., R. Wieczorke, C. P. Hollenberg, and E. Boles. 2000. The HTR1 gene is a dominant negative mutant allele of MTH1 and blocks Snf3- and Rgt2-dependent glucose signaling in yeast. J. Bacteriol. 182:540-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small, W. C., and L. McAlister-Henn. 1998. Identification of a cytosolically directed NADH dehydrogenase in mitochondria of Saccharomyces cerevisiae. J. Bacteriol. 180:4051-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Dijken, J. P., J. Bauer, L. Brambilla, P. Duboc, J. M. Francois, C. Gancedo, M. L. Giuseppin, J. J. Heijnen, M. Hoare, H. C. Lange, E. A. Madden, P. Niederberger, J. Nielsen, J. L. Parrou, T. Petit, D. Porro, M. Reuss, N. van Riel, M. Rizzi, H. Y. Steensma, C. T. Verrips, J. Vindeløv, and J. T. Pronk. 2000. An inter-laboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 26:706-714. [DOI] [PubMed] [Google Scholar]
- 50.van Dijken, J. P., and W. A. Scheffers. 1986. Redox balances in the metabolism of sugars by yeast. FEMS Microbiol. Rev. 32:199-224. [Google Scholar]
- 51.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395-403. [DOI] [PubMed] [Google Scholar]
- 52.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 53.Vijaikishore, P., and N. G. Karanth. 1987. Glycerol production by fermentation: a fed-batch approach. Biotechnol. Bioeng. 30:325-328. [DOI] [PubMed] [Google Scholar]
- 54.Wach, A., A. Brachat, R. Poehlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 55.Wang, Z.-X., J. Zhuge, H. Fang, and B. A. Prior. 2001. Glycerol production by microbial fermentation: a review. Biotechnol. Adv. 19:201-223. [DOI] [PubMed] [Google Scholar]
- 56.Zhuge, J., H. Fang, Z.-X. Wang, D.-Z. Chen, H.-R. Jin, and H.-L. Gu. 2001. Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl. Microbiol. Biotechnol. 55:686-692. [DOI] [PubMed] [Google Scholar]