Abstract
Atrial fibrillation is a common cardiac arrhythmia that significantly increases the risk of morbidity and mortality in critically ill patients. This study aimed to investigate the associations between glycemic control indicators—hemoglobin A1c (HbA1c), hemoglobin glycation index (HGI), stress hyperglycemia ratio (SHR), and glycemic variability (GV)—and clinical outcomes in AF patients admitted to intensive care unit (ICU). We conducted a retrospective analysis utilizing data from the Critical Care Medicine Information Marketplace (MIMIC)-IV database and a cohort from Binhai County People’s Hospital, comprising patients with AF. Key endpoints included all-cause mortality during ICU stay and at 28-day post-ICU. Statistical analyses involved Kaplan-Meier survival curves, univariable and multivariable Cox regression models, smooth fitting curve, sensitivity analysis and weighted quantile sum (WQS) modeling. Finally, a total of 952 AF patients from MIMIC-IV database and 286 patients of Binhai People’s Hospital due to AF were included for external verification. The results indicated that GV was the strongest predictor of mortality, with higher levels correlating with an increased risk of death (area under curve (AUC) = 0.620 for ICU mortality; AUC = 0.607 for 28-day post-ICU mortality). HGI and SHR also demonstrated significant associations with mortality outcomes, particularly in specific subgroups. Notably, lower HGI levels were linked to increased ICU mortality risk (log-rank P = 0.006). GV was the dominant factor in both outcomes and had a higher weight for death at 28 days after ICU (62.7% for ICU mortality vs. 47.3% for 28-day post-ICU mortality). These findings suggest that glycemic control is critical for improving outcomes in critically ill AF patients, highlighting the need for targeted interventions in managing hyperglycemia and its variability. Future studies should explore the mechanisms underlying these associations and assess the impact of glycemic management strategies on patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-16724-9.
Keywords: Atrial fibrillation, Glycemic variability, Mortality, MIMIC, ICU
Subject terms: Endocrinology, Cardiology, Cardiovascular biology
Introduction
Atrial fibrillation is a common arrhythmia with a significantly increased incidence, especially in severely ill patients. The management of patients with atrial fibrillation in the intensive care unit (ICU) is challenging because it is often accompanied by multiple organ dysfunction and other complications1. The occurrence of atrial fibrillation not only increases the risk of cardiovascular events in patients, but also may lead to prolonged hospital stay and increased medical costs2. The incidence of AF in ICU patients ranges from 4.5 to 29.5%, and the all-cause mortality of AF patients within 30 days after admission to ICU is about 30%, emphasizing the need to determine the risk factors leading to such high mortality3.
Hemoglobin A1c (HbA1c) is an important biomarker in diabetes management and reflects average blood sugar levels over the past three months. One retrospective study showed that for every 1% increase in HbA1c levels, the risk of death in patients with atrial fibrillation increased by about 14%4. In ICU, monitoring of HbA1c is considered an important indicator for assessing blood glucose control in patients with diabetes. Studies have shown that elevated HbA1c levels are associated with in-hospital mortality and complication rates in ICU patients5. HbA1c ≥ 6.5% is considered as a high-risk marker for critically ill patients, suggesting the need for more stringent glycemic control strategies6. Hemoglobin glycation index (HGI) is a measure of HbA1c assessed by comparing the difference between the actual measured hemoglobin a1C and the predicted HbA1c based on blood glucose levels. HGI can reflect the variation of individual blood glucose fluctuation and glycation degree6. Changes in HGI are also associated with long-term survival in patients with atrial fibrillation, suggesting that clinicians should pay attention to monitoring HGI in the management of patients with atrial fibrillation7. Stress hyperglycemia ratio (SHR) is the ratio of blood sugar levels to HbA1c levels in a stressed state, and is designed to more accurately assess the severity of stress hyperglycemia. Studies have shown that SHR is associated with poor prognosis in a variety of diseases, including heart disease and stroke8. It has been found that an increase in SHR is associated with an increase in all-cause mortality at 30 and 90 days, and this relationship is particularly strong in patients with diabetes9,10. Glycemic variability (GV) refers to the fluctuation of blood glucose levels, especially in patients with diabetes, and its fluctuation is considered to be an important factor affecting clinical outcomes11. Studies have shown that GV is significantly associated with in-hospital mortality in patients with AF, especially in ICU patients, and the increase of GV is closely related to poor prognosis12.
Current literature indicates that poor glycemic control is linked to increased mortality rates among patients with AF, especially in ICU13. Elevated glucose levels and fluctuations have been associated with higher rates of complications, including cardiovascular events and overall mortality14. However, there is still a knowledge gap regarding how these glycemic metrics specifically correlate with the prognosis of AF patients in critical care environments. This gap necessitates a focused exploration of the interplay between glycemic parameters and clinical outcomes in this vulnerable population. HbA1c, HGI, SHR, and GV are important in the ICU management of patients with atrial fibrillation, and current studies should continue to explore the application potential of these biomarkers. This study utilized the MIMIC-IV database, a rich and open critical care data repository, to examine the relationship between atrial fibrillation and measures of glycemic control. External validation cohorts were included to attempt to identify critical thresholds for glycemic control that might serve as viable targets for therapeutic intervention in patients with critical AF.
In summary, this investigation aims to fill the existing research gaps by systematically analyzing the impact of glycemic control on the survival of AF patients within critical care settings. The insights gained from this study could potentially inform clinical guidelines and improve management strategies, ultimately enhancing patient care and outcomes in this high-risk population. The overarching goal is to establish a clearer understanding of how glycemic metrics relate to mortality risk, thereby paving the way for better-targeted interventions in critically ill patients with AF.
Method
Data source
Data for this study were derived from the Critical Care Medicine Information Marketplace (MIMIC)-IV database15 (https://mimic.mit.edu/), a comprehensive, open-access, and de-identified medical critical care data repository that includes patient personal information, laboratory metrics, prognostic scores, and surgical information. Medication information, etc. The shared nature of the repository has been reviewed by the Institutional Review Board of Beth Israel Deaconess Medical Center. A member of the research team was trained and approved to access the database and be responsible for data extraction (Certification number: 44274909). This study was conducted in accordance with the Declaration of Helsinki, for data information from the MIMIC-IV database, and the analyzed data was anonymous and therefore exempt from informed consent.
In order to better validate the MIMIC-IV database analysis results and make some additions, we retrospectively included the electronic medical records of patients admitted to the intensive care unit (ICU) and emergency intensive care Unit (EICU) due to atrial fibrillation (AF) in Binhai County People’s Hospital from January 2021 to January 2025. This study was reviewed and approved by the Ethics Committee of Binhai County People’s Hospital (2025-BYKYLL-016). All patients were followed up for 28 days, and the data itself was de-identified.
Study population
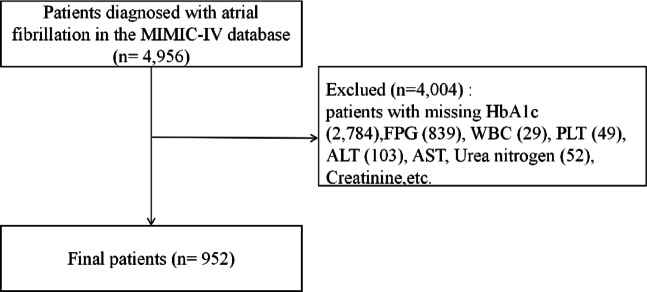
Patients diagnosed with AF upon admission to the ICU were identified according to the International Classification of Diseases (ICD) version 9 code (427.31) and ICD version 10 code (I48.X). In addition, we excluded patients based on the following criteria: (1) age below 18 years; (2) Patients with missing laboratory indicators and other information; (3) Patients with less than 4 days in ICU. The final number of patients enrolled in the study was 952. The same inclusion and exclusion criteria were applied to the single-center data collected in Binhai County People’s Hospital, and a total of 286 patients admitted to ICU and EICU due to AF were finally included for external verification (Fig. 1).
Fig. 1.
The inventory flow chart of patients with atrial fibrillation in the MIMIC database center.
Data extraction
The team used structured query language (SQL) and PostgreSQL 15 tools for data extraction and management. The extracted data included admission number, gender, age, concomitant diseases, laboratory test indicators, fasting blood glucose (FPG) five times after admission, mechanical ventilation, length of stay and date of death of AF patients. Pre-existing medical conditions include high blood pressure, diabetes, stroke, heart failure and kidney damage. Laboratory measures included white blood cells (WBC), hemoglobin (HGB), platelets, albumin, lactic acid (Lac), alanine aminotransferase (ALT), aspartate aminotransferase (AST), electrolyte level, FPG five times, glycated hemoglobin, urea nitrogen (BUN) and serum creatinine. The primary outcomes of the study were in-ICU and 28-day survival in patients with AF. The data information of Binhai County People’s Hospital was consistent with that extracted from MIMIC-IV data. For other laboratory measures, data were extracted 24 h before admission to the ICU.
Calculation of HGI, SHR and GV
Hemoglobin glycation index (HGI) calculation formula16: Based on the data of all patients in this study, a linear regression model between FPG and Hemoglobin A1c (HbA1c) was established. With HbA1c as the dependent variable and FPG as the independent variable, the linear regression equation was established. The predicted HbA1c value is calculated from the regression equation, and the difference between the actual HbA1c value and the predicted value is defined as HGI. HbA1c reflected the blood glucose control status of the patients in the past 3 months. The study group mainly measured HbA1c for the first time after the patients were admitted to the intensive care unit, and FPG was based on the above 5 test results. Stress Hyperglycemia Ratio (SHR) calculation formula: SHR = FPG/ (28.7 × HbA1c-46.7)17. Glucose variability (GV) calculation formula: GV is evaluated by calculating the coefficient of variation of blood glucose. The coefficient of variation is a measure of the variability of the data, obtained by dividing the standard deviation by the mean. Therefore, the formula is GV = standard deviation of blood glucose/mean blood glucose × 100%18.
Study outcome
The primary outcomes of our study were all-cause mortality in the ICU and all-cause mortality 28 days after ICU. All-cause mortality in the ICU refers to the deaths that occur during a patient’s stay in the intensive care unit. The all-cause mortality rate 28 days after ICU is death within 28 days from the date of admission to the ICU. Two survival outcomes were confirmed in the following ways: in-hospital deaths were recorded in real time through an electronic medical record system, and discharged patients were verified by the research team through standardized telephone follow-ups (data from Binhai County People’s Hospital).
Statistical analysis
According to the distribution trend of HbA1c, HGI, SHR and GV, they were divided into four groups: HbA1c: Q1 (≤ 5.4), Q2 (5.50–5.80), Q3 (5.90–6.30), Q4 (≥ 6.40); At HGI: Q1 (≤-0.60), Q2 (-0.60- -0.16), Q3 (0.16–0.29), Q4 (≥ 0.29); SHR: Q1 (≤ 0.94), Q2 (0.94–1.12), Q3 (1.12–1.36), Q4 (≥ 1.36); GV: Q1 (≤ 10.43), Q2 (10.44–15.66), Q3 (15.66–21.50), Q4 (21.51–83.67). In this analysis, continuous variables with baseline characteristics conforming to normal distribution are expressed as mean ± standard deviation; Variables that do not conform to the normal distribution are represented by interquartile spacing (IQR). The Kruskal-Wallis test was used to compare differences between groups. In addition, counts for which the categorical variable is expressed as a percentage (%) are analyzed using Chi-square tests or Fisher precision tests.
To obtain the relationship between target variables and patient outcomes, Kaplan-Meier (K-M) curves were used in survival analysis to illustrate trends in survival in the ICU and at 28 days for different HbA1c, HGI, SHR, and GV groups. We then performed univariate Cox regression analyses to further clarify the association between HbA1c, HGI, SHR, and GV levels and patients’ all-cause mortality in the ICU and at 28 days. In order to further explain the potential confounders in the Cox regression model, we conducted a multi-factor Cox regression analysis, and adjusted for various covariables, and constructed three models. In addition, smooth fitting curves and threshold effect analysis were used to identify potential inflection points to assess linear or non-linear relationships between clinical outcomes and HbA1c, HGI, SHR, and GV levels as continuous variables. The smoothing method uses a generalized additive model (GAM) combined with penalized splines to generate curves. Core parameters: The smoothing parameter selection is the restricted maximum likelihood method (REML), with a maximum degree of freedom of k = 10 (preset node upper limit). The breakpoint identification method for threshold effect analysis is data-driven and not pre-set by us. It is mainly achieved through three steps: 1 Generate candidate breakpoints within the full data range (5th to 95th percentiles) of exposed variables (such as HbA1c); 2. Fit piecewise Cox models for each candidate point and calculate the log-likelihood values; 3. Select the breakpoint that maximizes the likelihood function. The core statistical method was two-stage linear Cox regression. The likelihood ratio test (LR test) was used to compare the piecewise model with the linear model (P < 0.05 was considered to have a threshold effect).
To assess the effects of HbA1c, HGI, SHR, and GV levels on patients’ survival outcomes in the ICU and at 28 days, as well as to assess the weight of each measure, we performed a Weighted sum model (WQS) analysis, in which 40% of the random sample was used for examination. 60% of the random sample was used to validate the data. To further determine the association between HbA1c, HGI, SHR, and GV levels and whether key outcomes differ across populations, and to identify potential interactions, we performed subgroup analyses and performed sensitivity analyses to demonstrate the robustness of the results. Finally, we plotted time-dependent receiver operating characteristic (ROC) curves to visualize the predictive value of individual HbA1c, HGI, SHR, and GV measures for clinical outcomes. All analyses were performed using version R 4.2.2 and version Stata16. A P value of < 0.05 was considered statistically significant.
Result
Baseline characteristics
A total of 952 patients were included in this study. The baseline characteristics of the study population are shown in Table 1. There were 119 patients who died in the ICU and 208 who died 28 days after the ICU. Compared with survivors, patients who died were older, with significantly higher WBC, Lac, and BUN, and significantly lower levels of HGB, Albumin, Cholesterol, and low-density lipoprotein (LDL). The complications of acute kidney injury and hyperlipidemia were more likely to occur in the patients who died. Analysis of the target variables, HGI, SHR, and GV, found significantly higher mortality in the ICU and at 28 days. Among the 286 patients enrolled in Binhai County People’s Hospital, 32 died in ICU and 53 died 28 days after ICU. Compared with the death group, HGB, Lac, WBC, creatine Kinase (CK) and so on were significantly higher in the survival group. Baseline results for the external dataset can be found in Table S1.
Table 1.
Incorporating basic demographic information and clinical characteristics of the population, based on the MIMIC-IV database.
| Variable | ICU-day | 28-day | ||||
|---|---|---|---|---|---|---|
| Survivors | Non-survivors | p value | Survivors | Non-survivors | p value | |
| N | 833 | 119 | 744 | 208 | ||
| Age (years) | 73.26 ± 11.76 | 75.29 ± 10.40 | 0.074 | 72.66 ± 11.60 | 76.59 ± 11.19 | < 0.001 |
| Gender, (%) | 0.901 | 0.201 | ||||
| Female | 341 (40.94%) | 48 (40.34%) | 296 (39.78%) | 93 (44.71%) | ||
| Male | 492 (59.06%) | 71 (59.66%) | 448 (60.22%) | 115 (55.29%) | ||
| BMI, (kg/m2) | 29.52 ± 8.35 | 29.98 ± 7.96 | 0.568 | 29.76 ± 8.44 | 28.89 ± 7.74 | 0.181 |
| WBC, (K/uL) | 11.93 ± 6.14 | 13.30 ± 5.50 | 0.021 | 11.88 ± 5.77 | 12.88 ± 7.02 | 0.036 |
| PLT, (K/uL) | 204.86 ± 89.04 | 210.61 ± 82.34 | 0.506 | 206.54 ± 89.83 | 202.15 ± 82.25 | 0.526 |
| HGB, (mg/dL) | 11.71 ± 2.32 | 11.04 ± 2.35 | 0.003 | 11.75 ± 2.30 | 11.21 ± 2.39 | 0.004 |
| Albumin, (g/dL) | 3.23 ± 0.54 | 3.09 ± 0.54 | 0.010 | 3.24 ± 0.54 | 3.12 ± 0.51 | 0.006 |
| Sodium, (mEq/L) | 138.53 ± 5.52 | 138.08 ± 6.58 | 0.425 | 138.45 ± 5.58 | 138.55 ± 5.94 | 0.815 |
| Potassium, (mEq/L) | 4.19 ± 0.76 | 4.35 ± 0.96 | 0.036 | 4.18 ± 0.77 | 4.31 ± 0.87 | 0.040 |
| Calcium, (mg/dL) | 8.46 ± 0.88 | 8.46 ± 0.80 | 0.979 | 8.47 ± 0.88 | 8.41 ± 0.82 | 0.406 |
| Chloride, (mEq/L) | 102.78 ± 6.60 | 102.42 ± 7.48 | 0.590 | 102.65 ± 6.62 | 103.00 ± 7.07 | 0.506 |
| Lactate, (mmol/L) | 1.97 ± 1.44 | 2.63 ± 2.05 | < 0.001 | 1.96 ± 1.48 | 2.37 ± 1.71 | < 0.001 |
| TG, (mg/dL) | 149.02 ± 165.83 | 165.87 ± 186.97 | 0.308 | 149.67 ± 163.94 | 156.35 ± 184.66 | 0.614 |
| Cholesterol, (mg/dL) | 137.26 ± 33.62 | 126.80 ± 36.95 | 0.002 | 137.77 ± 33.62 | 129.44 ± 35.55 | 0.002 |
| HDL, (mg/dL) | 41.56 ± 14.60 | 40.23 ± 13.52 | 0.351 | 41.23 ± 14.17 | 41.97 ± 15.54 | 0.516 |
| LDL, (mg/dL) | 72.41 ± 26.59 | 64.15 ± 25.53 | 0.001 | 72.98 ± 26.45 | 65.62 ± 26.34 | < 0.001 |
| ALT, (IU/L) | 104.03 ± 390.68 | 159.10 ± 539.98 | 0.173 | 108.20 ± 412.54 | 120.64 ± 412.59 | 0.701 |
| AST, (IU/L) | 161.30 ± 660.54 | 245.69 ± 737.94 | 0.199 | 171.35 ± 696.43 | 173.63 ± 571.66 | 0.965 |
| BUN, (mEq/L) | 28.23 ± 21.50 | 34.82 ± 21.93 | 0.002 | 27.69 ± 20.74 | 33.93 ± 24.07 | < 0.001 |
| CR, (mg/dL) | 1.45 ± 1.39 | 1.70 ± 1.36 | 0.071 | 1.44 ± 1.40 | 1.62 ± 1.34 | 0.105 |
| LD, (IU/L) | 478.70 ± 916.93 | 612.70 ± 789.00 | 0.130 | 498.51 ± 979.99 | 484.53 ± 543.91 | 0.844 |
| CK, (IU/L) | 1051.65 ± 7407.49 | 1253.17 ± 3076.40 | 0.770 | 1107.54 ± 7824.40 | 967.03 ± 2489.66 | 0.799 |
| Ventilation_hour | 125.38 ± 138.13 | 187.48 ± 191.58 | < 0.001 | 133.79 ± 154.61 | 130.82 ± 117.22 | 0.797 |
| HbA1c, (%) | 6.23 ± 1.32 | 6.31 ± 1.63 | 0.523 | 6.25 ± 1.35 | 6.20 ± 1.42 | 0.694 |
| HGI | 0.01 ± 1.15 | − 0.12 ± 1.35 | 0.261 | 0.04 ± 1.16 | − 0.15 ± 1.23 | 0.038 |
| SHR | 1.17 ± 0.38 | 1.29 ± 0.44 | 0.003 | 1.16 ± 0.35 | 1.27 ± 0.49 | < 0.001 |
| GV | 17.04 ± 10.29 | 20.75 ± 10.63 | < 0.001 | 16.82 ± 10.04 | 19.93 ± 11.30 | < 0.001 |
| Hypertension, (%) | 0.242 | 0.820 | ||||
| No | 485 (58.22%) | 76 (63.87%) | 437 (58.74%) | 124 (59.62%) | ||
| Yes | 348 (41.78%) | 43 (36.13%) | 307 (41.26%) | 84 (40.38%) | ||
| Diabetes, (%) | 0.114 | 0.922 | ||||
| No | 538 (64.59%) | 68 (57.14%) | 473 (63.58%) | 133 (63.94%) | ||
| Yes | 295 (35.41%) | 51 (42.86%) | 271 (36.42%) | 75 (36.06%) | ||
| Heart Failure, (%) | 0.941 | 0.614 | ||||
| No | 416 (49.94%) | 59 (49.58%) | 368 (49.46%) | 107 (51.44%) | ||
| Yes | 417 (50.06%) | 60 (50.42%) | 376 (50.54%) | 101 (48.56%) | ||
| Chronic Kidney Disease, (%) | 0.019 | 0.825 | ||||
| No | 636 (76.35%) | 79 (66.39%) | 560 (75.27%) | 155 (74.52%) | ||
| Yes | 197 (23.65%) | 40 (33.61%) | 184 (24.73%) | 53 (25.48%) | ||
| Acute Kidney Injury, (%) | < 0.001 | 0.001 | ||||
| No | 447 (53.66%) | 40 (33.61%) | 401 (53.90%) | 86 (41.35%) | ||
| Yes | 386 (46.34%) | 79 (66.39%) | 343 (46.10%) | 122 (58.65%) | ||
| Hyperlipidemia, (%) | 0.106 | 0.030 | ||||
| No | 389 (46.70%) | 65 (54.62%) | 341 (45.83%) | 113 (54.33%) | ||
| Yes | 444 (53.30%) | 54 (45.38%) | 403 (54.17%) | 95 (45.67%) | ||
| Ventilation, (%) | 0.474 | 0.904 | ||||
| No | 80 (9.60%) | 9 (7.56%) | 70 (9.41%) | 19 (9.13%) | ||
| Yes | 753 (90.40%) | 110 (92.44%) | 674 (90.59%) | 189 (90.87%) | ||
| HbA1c, group (%) | 0.344 | 0.832 | ||||
| Q1 | 177 (21.25%) | 29 (24.37%) | 158 (21.24%) | 48 (23.08%) | ||
| Q2 | 244 (29.29%) | 26 (21.85%) | 211 (28.36%) | 59 (28.37%) | ||
| Q3 | 182 (21.85%) | 31 (26.05%) | 171 (22.98%) | 42 (20.19%) | ||
| Q4 | 230 (27.61%) | 33 (27.73%) | 204 (27.42%) | 59 (28.37%) | ||
| HGI, group (%) | 0.005 | 0.010 | ||||
| Q1 | 194 (23.29%) | 44 (36.97%) | 170 (22.85%) | 68 (32.69%) | ||
| Q2 | 219 (26.29%) | 19 (15.97%) | 199 (26.75%) | 39 (18.75%) | ||
| Q3 | 211 (25.33%) | 26 (21.85%) | 183 (24.60%) | 54 (25.96%) | ||
| Q4 | 209 (25.09%) | 30 (25.21%) | 192 (25.81%) | 47 (22.60%) | ||
| SHR, group (%) | < 0.001 | < 0.001 | ||||
| Q1 | 212 (25.45%) | 26 (21.85%) | 195 (26.21%) | 43 (20.67%) | ||
| Q2 | 217 (26.05%) | 21 (17.65%) | 198 (26.61%) | 40 (19.23%) | ||
| Q3 | 216 (25.93%) | 22 (18.49%) | 188 (25.27%) | 50 (24.04%) | ||
| Q4 | 188 (22.57%) | 50 (42.02%) | 163 (21.91%) | 75 (36.06%) | ||
| GV, group (%) | < 0.001 | < 0.001 | ||||
| Q1 | 225 (27.01%) | 13 (10.92%) | 209 (28.09%) | 29 (13.94%) | ||
| Q2 | 208 (24.97%) | 30 (25.21%) | 184 (24.73%) | 54 (25.96%) | ||
| Q3 | 205 (24.61%) | 33 (27.73%) | 182 (24.46%) | 56 (26.92%) | ||
| Q4 | 195 (23.41%) | 43 (36.13%) | 169 (22.72%) | 69 (33.17%) | ||
HbA1c: Q1(≤ 5.4), Q2(5.50–5.80), Q3(5.90–6.30), Q4(≥ 6.40);
HGI: Q1(≤-0.60), Q2(-0.60–0.16), Q3(-0.16-0.29), Q4(≥ 0.29);
SHR: Q1(≤ 0.94), Q2(0.94–1.12), Q3(1.12–1.36), Q4(≥ 1.36);
GV: Q1(≤ 10.43), Q2(10.44–15.66), Q3(15.66–21.50), Q4(21.51–83.67);
BMI: body mass index, WBC: white blood cells, PLT: platelets, HGB: hemoglobin, Cre: creatinine, HDL: high-density lipoprotein, LDL: low-density lipoprotein, HbA1c: glycosylated hemoglobin, HGI: hemoglobin glycation index, SHR: stress hyperglycemia ratio, GV: glycemic variability, ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, BUN: Blood Urea Nitrogen, LD: Lactate Dehydrogenase, CK: Creatine kinase.
Association between glycemic control measures and end events
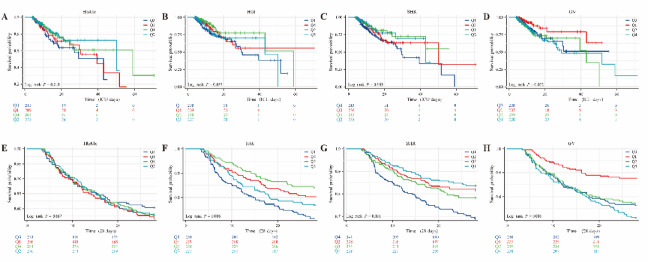
All-cause mortality of AF patients in ICU was analyzed. 119 patients (12.5%) died, and 208 patients (21.8%) died 28 days after ICU. Based on the target variables HbA1c, HGI, SHR and GV groups, the relationship between patient mortality among different groups was compared, and the K-M survival curve was drawn (Fig. 2). When analyzed in the HbA1c group, the Q3 group had the highest mortality for deaths in the ICU and deaths occurring 28 days after the ICU, but there was no statistically significant difference in either outcome (Fig. 2A and E). Analysis of HGI showed that there was no statistical difference in the outcome of death in ICU (Fig. 2B), but in the outcome of death occurring 28 days after ICU (Fig. 2F), it was found that the mortality rate of group Q1 was significantly higher than that of other groups, and the survival rate of group Q2 was obviously the highest, with a significant difference (log-rank P = 0.006). Analysis of SHR showed that it was consistent with HGI, and there was no statistical difference in the outcome of mortality in ICU (Fig. 2C). Analysis of outcomes at 28 days after ICU (Fig. 2G) showed that the mortality rate of Q4 was significantly higher than that of other groups, and the survival rate of patients in Q1 group was the highest, with statistical difference (log-rank P < 0.001). GV levels were positively associated with in-ICU and 28-day post-ICU mortality (Fig. 2D, H), and higher GV levels were associated with poorer survival outcomes (log-rank P < 0.05). However, in the K-M curve of externally validated data, we only observed a significant association between HbA1c and mortality in the ICU (log-rank P = 0.022). For the remaining indicators, although the mortality trends in the HGI, SHR, and GV groups in both outcomes were consistent with the KM curve of the MIMIC-IV dataset, there were no statistically significant differences between them (log-rank P > 0.05). The results are shown in Fig. S1.
Fig. 2.
Relationship between different intervals of the four indicators HbA1c, HGI, SHR, and GV in patients with atrial fibrillation and all-cause mortality in the ICU and 28-day all-cause mortality after ICU. (A–D) are respectively the relationships between HbA1c, HGI, SHR, and GV and all-cause mortality in ICU; (E–H) are respectively the relationships between HbA1c, HGI, SHR, and GV and all-cause mortality in the ICU. (MIMIC Database).
Analysis of patients’ baseline characteristics revealed significant differences between the two outcomes with different HbA1c, HGI, SHR, and GV levels, and further survival analysis (Table 2). The Q1 subgroup of GV had the lowest mortality rate, and high levels of GV were harmful factors. A Cox regression risk model was constructed with the Q1 subgroup as the reference point to analyze the association between HbA1c, HGI, SHR and GV and the two clinical outcomes. In Cox proportional hazard model 1 without adjusting for any variable, HGI Q2 was significantly associated with all-cause mortality in the ICU (HR = 0.5, 95% CI 0.3–0.9; P = 0.012), there was no significant difference in the other groups. Statistical differences in all-cause mortality at 28 days after ICU analysis were observed between the HGI Q2 and Q4 groups (Q1vsQ2: HR = 0.5, 95% CI 0.4–0.8; P = 0.001; Q1vsQ4: HR = 0.6, 95% CI 0.4–0.9; P = 0.022). After adjusting part of confounder model 2, HGI analysis results were consistent with those of model 1. After adjusting the full variable model 3, the results of HGI analysis were basically consistent with those of the other two models, but statistically significant differences were observed only in HGI Q2 group for all-cause mortality outcomes at 28 days after ICU (HR = 0.5, 95% CI 0.3–0.8; P = 0.001). When analyzed in the SHR group, there was no statistical difference in mortality in the ICU, and only statistical difference was observed in the SHR Q4 group for all-cause mortality outcomes after 28 days in the ICU (Model 1: HR = 1.9, 95% CI 1.3–2.7; P < 0.001; Model 2: HR = 1.8, 95% CI 1.2–2.6; P = 0.003; Model 3: HR = 1.6, 95% CI 1.1–2.4; P = 0.022). Subsequently, the GV group analysis of the outcomes of the two groups found that the other three groups in the three groups of models had statistical differences and were risk factors, and were strongly correlated with mortality. No statistical difference was found in HbA1c analysis.
Table 2.
Multivariable Cox regression analysis of ICU and 28-day all-cause mortality in the HbA1c group, HGI group, SHR group, and GV group.
| Outcomes Exposure | Model 1 HR (95% CI, P) |
Model 2 HR (95% CI, P) |
Model 3 HR (95% CI, P) |
|---|---|---|---|
| ICU mortality | |||
| HbA1c group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.7 (0.4, 1.2) 0.222 | 0.7 (0.4, 1.2) 0.159 | 0.7 (0.4, 1.3) 0.264 |
| Q3 | 1.1 (0.7, 1.9) 0.617 | 1.2 (0.7, 2.0) 0.577 | 1.3 (0.7, 2.2) 0.398 |
| Q4 | 0.8 (0.5, 1.2) 0.267 | 0.6 (0.3, 1.1) 0.088 | 0.6 (0.3, 1.1) 0.097 |
| HGI group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.5 (0.3, 0.9) 0.012 | 0.5 (0.3, 0.8) 0.009 | 0.5 (0.3, 0.8) 0.010 |
| Q3 | 0.7 (0.5, 1.2) 0.246 | 0.7 (0.5, 1.2) 0.246 | 0.8 (0.4, 1.3) 0.282 |
| Q4 | 0.7 (0.5, 1.2) 0.171 | 0.6 (0.4, 1.0) 0.055 | 0.7 (0.4, 1.1) 0.147 |
| SHR group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 1.1 (0.5, 1.5) 0.573 | 1.1 (0.5, 1.6) 0.648 | 1.2 (0.5, 1.6) 0.727 |
| Q3 | 1.3 (0.5, 1.6) 0.487 | 1.3 (0.5, 1.6) 0.640 | 1.3 (0.5, 1.6) 0.712 |
| Q4 | 1.5 (0.9, 2.4) 0.116 | 1.5 (0.9, 2.4) 0.090 | 1.4 (0.8, 2.4) 0.193 |
| GV group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 2.3 (1.2, 4.5) 0.010 | 2.3 (1.2, 4.5) 0.012 | 2.4 (1.2, 4.8) 0.010 |
| Q3 | 2.2 (1.2, 4.2) 0.015 | 2.3 (1.2, 4.4) 0.012 | 2.5 (1.3, 4.8) 0.008 |
| Q4 | 2.4 (1.3, 4.5) 0.005 | 2.4 (1.2, 4.5) 0.009 | 2.4 (1.2, 4.7) 0.010 |
| 28-day mortality | |||
| HbA1c group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.9 (0.6, 1.4) 0.710 | 0.9 (0.6, 1.3) 0.516 | 1.0 (0.7, 1.5) 0.940 |
| Q3 | 0.8 (0.6, 1.3) 0.410 | 0.8 (0.5, 1.2) 0.278 | 0.9 (0.6, 1.3) 0.521 |
| Q4 | 1.0 (0.7, 1.4) 0.819 | 1.0 (0.6, 1.5) 0.830 | 1.0 (0.6, 1.6) 0.962 |
| HGI group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.5 (0.4, 0.8) 0.001 | 0.5 (0.3, 0.7) < 0.001 | 0.5 (0.3, 0.8) 0.001 |
| Q3 | 0.8 (0.5, 1.1) 0.145 | 0.8 (0.5, 1.1) 0.176 | 0.9 (0.6, 1.4) 0.749 |
| Q4 | 0.6 (0.4, 0.9) 0.022 | 0.6 (0.4, 0.9) 0.025 | 0.8 (0.5, 1.1) 0.160 |
| SHR group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.9 (0.6, 1.4) 0.695 | 0.9 (0.6, 1.3) 0.500 | 0.9 (0.6, 1.4) 0.580 |
| Q3 | 1.2 (0.8, 1.8) 0.423 | 1.2 (0.8, 1.7) 0.499 | 1.1 (0.7, 1.6) 0.734 |
| Q4 | 1.9 (1.3, 2.7) < 0.001 | 1.8 (1.2, 2.6) 0.003 | 1.6 (1.1, 2.4) 0.022 |
| GV group | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 2.0 (1.3, 3.1) 0.003 | 1.9 (1.2, 3.0) 0.005 | 1.9 (1.2, 3.0) 0.006 |
| Q3 | 2.1 (1.3, 3.3) 0.001 | 2.1 (1.3, 3.3) 0.001 | 2.1 (1.3, 3.3) 0.002 |
| Q4 | 2.6 (1.7, 4.0) < 0.001 | 2.6 (1.7, 4.1) < 0.001 | 2.6 (1.6, 4.1) < 0.001 |
with the outcome and p-value less than 0.05.
Model 1: no covariates were adjusted.
Model 2: adjusted for age, gender (male, female), body mass index (BMI), Hypertension (yes, no), Diabetes (yes, no), Heart Failure (yes, no), Chronic Kidney Disease (yes, no), acute kidney injury (yes, no), Hyperlipidemia (yes, no).
Model 3: adjusted for Model 2 plus Laboratory indicators.
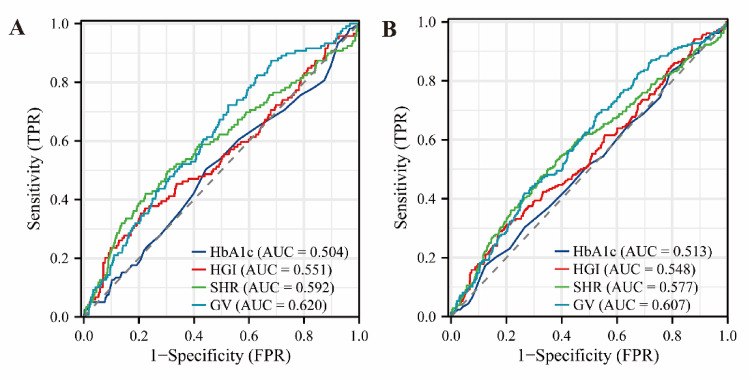
By multivariate cox regression analysis, we found that partial subgroups of HGI, SHR, and GV were significantly associated with the risk of death in the ICU and at 28 days post-ICU in AF patients. To better reflect the predictive value of four single indicators for both outcomes, we plotted a time-dependent ROC curve (Fig. 3). As a single variable, GV was the strongest predictor of both death outcomes (in-ICU: area under curve(AUC) = 0.620; 28-day post-ICU: AUC = 0.607). In the external data, we also performed univariate and multifactor cox regression model analyses, which were consistent with the analyses in the MIMIC-IV data. However, we observed that HbA1c, HGI, SHR, and GV did not show significant associations with in-ICU and 28-day post-ICU mortality, although their overall trends were similar to those obtained in MIMIC-IV (Table S2). Similarly, for the data of Binhai County People’s Hospital, we still drew a time-dependent ROC curve (Fig. S2), and it was also observed that GV, as a single variable, had the strongest predictive ability for the two death outcomes of patients (in-ICU: AUC = 0.593; 28-day post-ICU: AUC = 0.577).
Fig. 3.
ROC curves of HbA1c, HGI, SHR and GV for predicting all-cause mortality in the ICU and all-cause mortality 28 days after ICU: A is the ROC curve of HbA1c, HGI, SHR and GV for predicting all-cause mortality in the ICU; B is the ROC curve of HbA1c, HGI, SHR and GV for predicting all-cause mortality 28 days after ICU.
Smooth fitting curve
To more visually assess the relationship between HbA1c, HGI, SHR, and GV levels and mortality in the ICU and at 28 days after the ICU, we plotted a smooth fitting curve (Fig. 4). For both outcomes, we observed a negative correlation between HbA1c and outcomes, but not a completely linear correlation (Fig. 4A and E). HGI showed a segmentation relationship with all-cause mortality in the ICU but a negative overall trend (Fig. 4B), and an inverted “L-shaped” relationship with 28-day post-ICU mortality (Fig. 4F). SHR and GV levels were positively correlated with both outcomes (Fig. 4C, D and G, and H). External validation also shows a largely consistent trend (Fig. S3).
Fig. 4.
Association between HbA1c, HGI, SHR, and GV and all-cause mortality in the ICU and 28-day all-cause mortality after ICU in patients with atrial fibrillation. (A–D) are respectively the curve relationships between HbA1c, HGI, SHR, and GV and all-cause mortality in ICU; (E–H) are respectively the curve relationships between HbA1c, HGI, SHR, and GV and all-cause mortality in ICU. The solid and dashed lines represent the estimated values and their corresponding 95% confidence intervals. (MIMIC Database).
Threshold effect analysis identified the critical inflection point while adjusting for covariates consistent with model 3 above. First, for the analysis of all-cause mortality in ICU, the inflection point of HbA1c was 9. When < 9, HR = 0.9 (0.7–1.1), p = 0.256; when ≥ 9, HR = 1.1 (0.8–1.4), p = 0.509; and log-likelihood ratio test p = 0.336. The HGI inflection point was − 0.4, the SHR was 0.9 and the GV was 14.7. The relationship between the outcome before and after each inflection point was analyzed, and there was no significant statistical difference. However, the analysis of all-cause mortality after 28 days of ICU found that HbA1c was 9, HGI was 2.2, SHR was 0.8, and GV was 20.9, and it was found that there were some significant statistical differences between the all-cause mortality before and after the inflection point.
Overall relationship between HbA1c, HGI, SHR and GV and outcome (WQS model)
The relationship between estimated chemical quantities of HbA1c, HGI, SHR and GV is shown in Fig. 5. Figure 5A and B show the contribution values of the above four indicators to the occurrence of death in ICU, in which GV is the highest 0.473, HbA1c is the lowest 0.040, HGI is 0.166 and SHR is 0.321. The overall trend is positive correlation. The risk of death in ICU was significantly higher (β = 0.058, p = 0.008), and the trend was consistent, confirming the robustness of the overall positive association with mixed exposure. Figure 5C and D show the contribution value of death after 28 days in ICU, which are GV0.627, SHR0.288, HGI0.064 and HbA1c0.021, respectively. The overall trend is positive, and the risk of death in ICU is significantly increased with each 1-decile increase in WQS index (β = 0.064, p = 0.004).
Fig. 5.
The WQS weighting curves of HbA1c, HGI, SHR and GV with all-cause mortality in ICU and all-cause mortality 28 days after ICU in patients with atrial fibrillation. A and B represent the weight relationship between HbA1c, HGI, SHR, GV and all-cause mortality in the ICU. C and D represent the WQS weight relationship between HbA1c, HGI, SHR, GV and all-cause mortality 28 days after ICU.
Subgroup analysis
In addition, we performed subgroup analyses based on factors such as sex, age (≤ 65 years or > 65 years), and body mass index (BMI) (≤ 25 kg/m or > 25 kg/m) (Table S4, S5). In subgroup analysis of HbA1c and key outcomes, no statistically significant difference in the relationship between HbA1c and any subgroup of factors was observed. In analyzing HGI, we observed that lower HGI levels were significantly associated with an increased risk of death in the ICU. Specifically, this relationship was significant in men, patients > 65 years of age, BMI > 25 kg/m, patients without hypertension or diabetes, and patients with heart failure or kidney damage. In subgroup analyses of HGI and 28-day post-ICU mortality, HGI in both Q2 and Q4 had a risk effect on outcomes, with no significant effect in Q3. For the SHR analysis, higher levels of SHR were found to be significantly associated with an increased risk of death in the ICU for patients > 65 years of age, but other subgroup analyses found no statistical difference. However, in a subgroup analysis of 28-day mortality after ICU, the analysis found that high SHR levels were significantly associated with an increased risk of death, especially in patients > 65 years of age, women, BMI > 25 kg/m, with hypertension or diabetes or hyperlipidemia, or with mechanical ventilation, and without heart failure or kidney injury. In addition, in subgroup analyses of mortality in GV and ICU, statistically significant differences were found among patients > 65 years old, male, BMI > 25 kg/m, with hypertension or diabetes, or with heart failure or kidney injury, or hyperlipidemia, or mechanical ventilation. Most of the subgroup relationships between GV and 28-day post-ICU mortality were statistically different, and the higher the level of GV, the higher the mortality.
Sensitivity analysis
Sensitivity analysis further confirmed the robustness of the results. In a multivariate Cox regression model that investigated the association between HbA1c, HGI, SHR, and GV and the risk of death in the ICU and at 28 days after the ICU, we used a backward and forward data processing approach, and we performed sensitivity analyses on the impact of mortality in diabetic and non-diabetic patients. In our all-cause mortality analysis in the ICU, we still found that patients with high GV levels showed a higher risk of death in the non-diabetic population. However, in the diabetic population, patients with high GV levels show a greater risk of death (Table S3). Similarly, 28 days post-ICU mortality analysis found that patients with high GV levels were consistent with the above results and were more pronounced. For the analysis of other indicators, taking group Q1 as a reference, HGI group Q2 patients were associated with a reduced risk of death, suggesting that patients with low levels of HGI were more likely to die. In addition, for SHR analysis, high levels of SHR were more likely to die, especially in patients with diabetes than those without diabetes.
Discussion
AF is a prevalent cardiac arrhythmia associated with increased morbidity and mortality, often leading to severe complications such as stroke and heart failure. It is characterized by an irregular and often rapid heart rate, which can compromise hemodynamic stability and necessitate intensive medical intervention. Patients with AF are at a heightened risk of developing other cardiovascular diseases, and their management in critical care settings poses unique challenges19. In recent years, researchers have gradually realized that biomarkers such as HbA1c, HGI, SHR and GV may be closely related to the clinical outcomes of patients with atrial fibrillation8,12.
In this study, we examined the relationship between various glycemic control metrics and patient outcomes in critically ill patients diagnosed with AF, utilizing data from the MIMIC-IV database and a cohort from Binhai County People’s Hospital. Our findings revealed significant correlations between HGI, SHR, GV, and all-cause mortality during ICU stay and at 28-day post-ICU. Notably, while previous studies have suggested associations between hyperglycemia and adverse outcomes in critically ill patients20this research uniquely positions these glycemic indices as potential prognostic tools for predicting mortality in AF patients, contributing to the limited literature on this specific patient population. The findings demonstrate that higher levels of GV and abnormal HGI, SHR are predictive of increased mortality risk. The implications of these results suggest that glycemic control plays a critical role in the prognosis of AF patients in critical care settings, warranting further investigation into individualized management strategies.
K-M analysis indicated that GV levels were positively correlated with mortality in the ICU and at 28 days after ICU, and higher GV levels were associated with poorer survival outcomes (log-rank P < 0.05). The Q1 subgroup of GV had the lowest mortality rate, and high levels of GV were harmful factors. Monitoring and management of GV is particularly important in clinical practice, especially in patients with AF, who often have other cardiovascular diseases, and fluctuations in GV can aggravate their condition and lead to higher mortality21. A cohort analysis3 of 8,989 AF patients suggested that a higher quartile of GV was significantly associated with a higher risk of all-cause mortality at 30, 90, and 360 days after ICU admission, with a trend of linear association. GV is an important predictor of short -, medium - and long-term all-cause mortality in the ICU of AF patients. Glucose metabolism plays a crucial role in cardiovascular function, as the heart relies primarily on glucose for energy22. Therefore, the metabolic processing of glucose is essential for maintaining the physiological integrity of the cardiovascular system. This disruption of metabolic balance, especially within diseased heart tissue, can be a key catalyst for the onset and progression of cardiovascular disease. In patients with atrial fibrillation, regular monitoring of GV combined with individualized treatment can significantly improve patient prognosis and reduce in-hospital mortality. Therefore, the effective management of GV not only helps to improve blood glucose control in patients with atrial fibrillation, but also provides a new idea and method to reduce the risk of death.
FPG alone had low predictive value for cardiovascular outcomes. HbA1c is currently the most reliable measure for assessing long-term blood glucose control23. HbA1c, which is used for the diagnosis and management of DM, reflects an individual’s average blood glucose over a three-month period and is currently the most commonly used alternative marker for the effectiveness of hypoglycemic interventions24. In the K-M curve of externally validated data, we observed only a significant association between HbA1c and mortality in the ICU (log-rank P = 0.022). Studies have shown that HGI is associated with a poor prognosis for cardiovascular disease (CVD), particularly in the case of coronary heart disease (CHD) and arrhythmias25. Previous studies have shown that both high and low levels of HGI are strongly associated with adverse cardiovascular events26,27. K-M analysis indicated that death at 28 days post-ICU was significantly higher in the Q1 group than in the other groups (log-rank P = 0.006). The smooth-fitting curve indicated that that HGI had a segmentalized relationship with all-cause mortality in ICU, but a negative correlation with the overall trend, and an inverted “L-shaped” relationship with 28-day post-ICU mortality. Low levels of HGI are a risk factor for death. Studies have shown that for patients with hemorrhagic heart failure (CHF), a higher HGI is more protective than a lower HGI and is directly associated with short-term mortality.
SHR levels were positively correlated with mortality at 28 days post-ICU. SHR can reflect the blood glucose fluctuation of patients in acute pathological state, and then correlate with clinical outcome. Studies have shown that SHR is associated with poor prognosis in a variety of diseases, including heart disease and stroke28. One study shared our findings that in critically ill AF patients, higher levels of the SHR index were significantly associated with increased risk of all-cause death at 30, 90, 180, and 365 days29. SHR index can be used as an effective indicator to evaluate the severity of AF patients in ICU and guide treatment. SHR was developed to mitigate the effects of long-term chronic glycemic factors on stress hyperglycemic levels, which are associated with adverse clinical events, particularly cardiovascular events. Higher SHR has been identified as an independent predictor of adverse cardiovascular outcomes such as heart failure and myocardial infarction30,31. The dynamic changes of SHR can provide a basis for clinical decision making, and help medical personnel to take more effective interventions in the management of patients with hyperglycemia and atrial fibrillation to reduce the incidence of complications and the risk of death.
GV was the dominant factor in both outcomes and had a higher weight for death at 28 days after ICU (62.7% for ICU mortality vs. 47.3% for 28-day post-ICU mortality). The importance of SHR was relatively stable in both outcomes, but the weight decreased slightly (32.1% for ICU mortality vs. 28.8% for 28-day post-ICU mortality). The contribution of HGI and HbA1c was weak and further decreased, especially in the outcome after 28 days of ICU. Integrating HbA1c, HGI, SHR and GV into clinical practice can optimize management strategies for patients with atrial fibrillation.
The clinical implications of this research are profound, as they suggest that monitoring and managing glycemic indices could enhance patient outcomes and inform treatment protocols in critically ill patients with AF. The identification of GV, SHR, and HGI as significant predictors of mortality underscores the need for rigorous glycemic control in these patients, potentially leading to the development of targeted interventions aimed at improving survival rates. This could influence clinical practice guidelines and policies concerning the management of patients with AF in the ICU, as healthcare providers may be prompted to adjust their monitoring and treatment strategies based on these glycemic indicators. Furthermore, the research advocates for a multidisciplinary approach in managing critically ill patients, integrating endocrinological insights into routine ICU care, which could ultimately enhance patient prognostication and treatment outcomes.
This study has several limitations. First, its retrospective design may introduce selection bias. Reliance on electronic health records carries inherent risks of incomplete data, particularly regarding laboratory indicators and comorbidity-related information. Additionally, while the research incorporated diverse clinical variables, it may not have comprehensively accounted for all potential confounders influencing mortality outcomes. Notably, acute cardiovascular complications such as myocardial infarction and stroke were not included, nor were severity assessments of underlying conditions or discrepancies in treatment approaches across medical institutions. Future research should focus on leveraging larger, multicenter datasets and prospective designs to validate these findings and investigate the mechanisms underpinning the observed associations. Moreover, longitudinal studies tracking patients over extended periods may yield deeper insights into the long-term implications of glycemic control in critically ill patients with atrial fibrillation.
Conclusion
In conclusion, our findings highlight the important relationship between glycemic control measures (HbA1c, HGI, SHR, and GV) and mortality outcomes in patients with critical AF. Specifically, high GV emerged as an important risk factor for both in-ICU and 28-day mortality, indicating its potential utility as a target for therapeutic intervention. Integrating HbA1c, HGI, SHR, and GV into clinical practice can optimize management strategies for patients with atrial fibrillation. The study highlights the need for rigorous blood sugar management strategies in these susceptible populations. Future studies should focus on prospective validation of these associations and explore effective interventions aimed at optimizing glycemic control in critically ill patients to improve survival outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are very grateful to the PhysioNet website for making available the MIMIC-IV database.
Author contributions
Author ContributionsAFH and CZ substantially contributed to the study conception and design. HJL and LLZ conducted the research and prepared the figures. AFH and SKY analyzed and interpreted the data and drafted the article. LMX, CLY, and SHL critically revised the important intellectual content of the manuscript and provided final approval. AFH and CZ supervised the study. All authors read and approved the final manuscript.
Funding
This study was sponsored by the Yancheng Science and Technology Bureau (YCBE202365), the Jiangsu Vocational College of Medicine’s School-Local Collaborative Innovation Research Project (202491001).
Data availability
The datasets used for these analyses are publicly available (https://mimic-iv.mit.edu/).
Declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The MIMIC-IV database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Due to the retrospective nature of the study, the People’s Hospital of Binhai County waived the necessity of obtaining informed consent.
Consent for publication
All the authors listed have approved the manuscript that is enclose.
Consent to participate
All the authors of the article agree to participate in the journal submission.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aifeng He, Hongjing Li and Shengkai Yang are contributed equally to this work and share the corresponding authorship.
References
- 1.Burzynska, M. et al. Prevalence of hyperlipoproteinemia(a) in individuals of European ancestry treated at outpatient cardiology clinics: results from a cross-sectional STAR-Lp(a) study. Pol. Arch. Intern. Med.1341–10 (2024). [DOI] [PubMed]
- 2.Chang, C. H. et al. Dialysis mode and associated outcomes in patients with End-Stage renal disease and atrial fibrillation: A 14-Year nationwide cohort study. J. Am. Heart Assoc.10, e019596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Y. et al. Prognostic value of glycaemic variability for mortality in critically ill atrial fibrillation patients and mortality prediction model using machine learning. Cardiovasc. Diabetol.23, 426 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KC, C. Increased glucose variability is associated with atrial fibrillation after coronary artery bypass. J. Card Surg.34, 549–554 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Ludwig, S. et al. Quality of life and metabolic outcomes after total pancreatectomy and simultaneous islet autotransplantation. Commun. Med. (Lond). 2, 24 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li, M., Huang, J., Lu =, W., et al. Serum glycosylated hemoglobin and prostate cancer risk: results from a systematic review and dose-response meta-analysis. Urol. Oncol.43:195-e1 (2025). [DOI] [PubMed]
- 7.Guo, Z. et al. Association between the haemoglobin glycation index and 30-day and 365-day mortality in patients with heart failure admitted to the intensive care unit. Diabetol. Metab. Syndr.17, 87 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia, W. et al. Predictive value of glycemic gap and stress glycemia ratio among critically ill patients with acute kidney injury: a retrospective analysis of the MIMIC-III database. BMC Nephrol.24, 227 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao, L. et al. Stress hyperglycemia ratio and its influence on mortality in elderly patients with severe community-acquired pneumonia: a retrospective study. Aging Clin. Exp. Res.36, 175 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo, Z. et al. Joint association of the triglyceride-glucose index and stress hyperglycemia ratio with incidence and mortality risks of new-onset atrial fibrillation during sepsis: a retrospective cohort study. Cardiovasc. Diabetol.24, 149 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu, J. C. et al. Higher long-term visit-to-visit glycemic variability predicts new-onset atrial fibrillation in patients with diabetes mellitus. Cardiovasc. Diabetol.20, 148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, W., Wang, Y. & Zhong, G. Glycemic variability and the risk of atrial fibrillation: a meta-analysis. Front. Endocrinol. (Lausanne). 14, 1126581 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaver, C. M. et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit. Care Med.43, 2104–2111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll, G. C. & Herbert, D. A. Using population death rate to predict rate of admissions to the intensive care unit. Crit. Care Med.32, 70–76 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data. 10, 1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin, S. et al. Corrigendum: association between hemoglobin glycation index and diabetic kidney disease in type 2 diabetes mellitus in china: a cross-sectional inpatient study. Front. Endocrinol. (Lausanne). 14, 1199643 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, G. W. et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J. Clin. Endocrinol. Metab.100, 4490–4497 (2015). [DOI] [PubMed] [Google Scholar]
- 18.M, L. et al. Key indices of glycaemic variability for application in diabetes clinical practice. Diabetes Metab.49, 101488 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof, P. Can we improve outcomes in AF patients by early therapy? BMC Med.7, 72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan, F. et al. Association between the stress hyperglycemia ratio and 28-day all-cause mortality in critically ill patients with sepsis: a retrospective cohort study and predictive model establishment based on machine learning. Cardiovasc. Diabetol.23, 163 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Z Z, H Z, Y. G. et al. Relationship between glycemic variability and the incidence of postoperative atrial fibrillation following cardiac surgery: A retrospective study from MIMIC-IV database. Diabetes Res. Clin. Pract.219, 111978 (2025). [DOI] [PubMed] [Google Scholar]
- 22.Lopaschuk, G. D. et al. Cardiac energy metabolism in heart failure. Circ. Res.128, 1487–1513 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elder, D. H. et al. Mean HbA1c and mortality in diabetic individuals with heart failure: a population cohort study. Eur. J. Heart Fail.18, 94–102 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Yang, G., Au Yeung, S. L. & Schooling, C. M. Sex differences in the association of fasting glucose with HbA1c, and their consequences for mortality: A Mendelian randomization study. EBioMedicine84, 104259 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing, W. et al. Risk analysis of the association between different hemoglobin glycation index and poor prognosis in critical patients with coronary heart disease-A study based on the MIMIC-IV database. Cardiovasc. Diabetol.23, 0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, S. et al. The hemoglobin glycation index predicts the risk of adverse cardiovascular events in coronary heart disease patients with type 2 diabetes mellitus. Front. Cardiovasc. Med.9, 992252 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng, M. D. et al. Association of hemoglobin glycation index with prognosis of coronary artery disease after percutaneous coronary intervention: A retrospective cohort study. Diab Vasc Dis. Res.20, 14791641231193306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, J. et al. Impact of stress hyperglycemia ratio on mortality in patients with critical acute myocardial infarction: insight from American MIMIC-IV and the Chinese CIN-II study. Cardiovasc. Diabetol.22, 281 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng, S. et al. Association between stress hyperglycemia ratio index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study using the MIMIC-IV database. Cardiovasc. Diabetol.23, 363 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, Y. et al. High triglyceride-glucose index and stress hyperglycemia ratio as predictors of adverse cardiac events in patients with coronary chronic total occlusion: a large-scale prospective cohort study. Cardiovasc. Diabetol.22, 180 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui, K. et al. The impact of fasting stress hyperglycemia ratio, fasting plasma glucose and hemoglobin A1c on in-hospital mortality in patients with and without diabetes: findings from the China acute myocardial infarction registry. Cardiovasc. Diabetol.22, 165 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for these analyses are publicly available (https://mimic-iv.mit.edu/).