Abstract
Experiments were carried out to develop methods to generate well-characterized, polycyclic aromatic hydrocarbon (PAH)-spiked, aged but minimally altered sediments for fate, biodegradation, and bioavailability experiments. Changes in indigenous bacterial populations were monitored in mesocosms constructed of relatively clean San Diego Bay sediments, with and without exposure to gamma radiation, and then spiked with five different PAHs and hexadecane. While phenanthrene and chrysene degraders were present in the unspiked sediments and increased during handling, PAH spiking of nonirradiated sediments led to dramatic increases in their numbers. Phenotypic characterization of isolates able to grow on phenanthrene or chrysene placed them in several genera of marine bacteria: Vibrio, Marinobacter or Cycloclasticus, Pseudoalteromonas, Marinomonas, and Halomonas. This is the first time that marine PAH degraders have been identified as the latter two genera, expanding the diversity of marine bacteria with this ability. Even at the highest irradiation dose (10 megarads), heterotrophs and endospore formers reappeared within weeks. However, while bacteria from the unirradiated sediments had the capacity to both grow on and mineralize 14C-labeled phenanthrene and chrysene, irradiation prevented the reappearance of PAH degraders for up to 4 months, allowing spikes to age onto the sediments, which can be used to model biodegradation in marine sediments.
Prodigious amounts of crude oil are extracted from the earth every year and moved across the ocean. It is estimated that about 0.1% of the total each year ends up in marine systems (1). While accidental spills are the most spectacular, petroleum hydrocarbons also enter the coastal marine environment as exhaust particulates, fuel spills, urban runoff contaminated with crankcase oil, and the by-products of biomass combustion. The more volatile components evaporate, and some are photooxidized, but the less volatile, poorly water-soluble hydrocarbons end up as weathered material sorbed onto coastal sediments. Of this class, the PAHs are of most concern because of their toxicity (11) and carcinogenicity (19), and 16 are listed as priority pollutants by the Environmental Protection Agency (EPA) (26).
PAH-contaminated soil can be cleaned by the use of such abiotic methods as incineration or by such biotic ones as land farming. However, the large amounts of saturated marine sediments make these procedures prohibitively expensive. PAHs are natural organic molecules, many of which are biodegradable by a variety of microorganisms; thus, bioremediation—the act of encouraging the natural process of contaminant biodegradation—is possible in sediments, provided that we gain an understanding of the interaction of PAHs with sediments, the responses of the microbial population to the presence of weathered PAHs, and the factors that limit the microbes' ability to degrade the PAHs to carbon dioxide and water. Only then can rational bioremediation protocols be designed and implemented in situ or the potential for natural attenuation be predicted.
Experimental studies of PAH degradation in both field and artificially contaminated marine sediments are made equivocal by a host of difficulties described in detail by Apitz et al. (3). For example, the heterogeneity inherent in field-contaminated sediments precludes the establishment of replicate mesocosms with relatively undisturbed sediment and also makes it difficult to obtain reliable subsamples for time course measurements. For critical studies of the biodegradation of PAHs, it is useful to add spikes of known concentrations, preferably 14C labeled. Yet, addition of such spikes to uncontaminated or contaminated sediment is very problematic, because standard methods cause considerable alteration of the sediment and because indigenous PAH-degrading microorganisms may immediately begin to degrade the newly added compounds before the PAHs can equilibrate. It is well known that PAHs, and other contaminants, are sorbed to sediment particles over time and become less extractable by chemists and less amenable to biodegradation (36, 42), although biosurfactants produced by certain bacteria (24) may increase extractability of the material over time, further complicating the analysis. For these reasons, sediments spiked by standard methods may not adequately replicate the “natural” behavior being examined.
Given these difficulties, it is important that experiments designed to determine PAH biodegradation rates, fates, and bioavailability and to close mass balances use homogeneous uncontaminated sediments spiked with known amounts of labeled PAHs. Our previous work (3) described the use of a novel sand-PAH mixture that allowed the PAHs to be added in a minimally intrusive manner, without the need for solvents that may alter the chemistry or microbiology of the sediments. To simulate the natural weathering of PAHs in marine sediments, these spiked uncontaminated sediments must be aged for at least 6 months (reference 2 and references therein) before experimentation begins. However, so that this aging process may occur in the absence of indigenous PAH-degrading bacteria, the sediments must be sterile during the aging process. We chose gamma irradiation as the least intrusive method to sterilize the sediments based on earlier soil studies (10, 13, 35) that reported 2.5 to 3.5 megarads as being adequate to sterilize soils; presumably such doses would also sterilize saturated sediments, as the radiation would likely generate more oxygen radicals in such water-saturated materials (30). However, a review of these and similar papers suggested that the claims of soil sterility were based on inadequate data. Usually only one or two physiological types of bacteria were enumerated on nutrient-rich agar plates, and the plates were incubated for very short times, usually 1 to 2 days, before colonies were counted. These conditions are inappropriate for the detection of the many environmental bacteria that require low concentrations of nutrients and considerably longer incubation times to form colonies on plates or turbidity in liquid media (reference 28 and references therein). More importantly, the irradiated soils were sampled immediately after irradiation and then only a few days or weeks postirradiation. These short postirradiation times might not allow survivors to reach detectable numbers, and thus, the studies did not adequately document sterility for our purposes.
To resolve these issues, two experiments were carried out. In the first (ONR-1), sediment mesocosms were irradiated at four levels (0, 2.5, 3.5, and 5 megarads) and spiked with a mixture of PAHs. As described in a preliminary report (3) and in detail in this paper, sterility was not achieved in these sediments, even at 5 megarads. To achieve sterility and to assess the impacts of sample handling without PAH addition, a second experiment (ONR-2) was carried out using higher irradiation levels and repeat irradiation to kill endospores.
The present paper describes the impact on indigenous bacteria in marine sediments following the introduction of PAHs and our attempts to use gamma radiation to sterilize the sediments before PAH addition in these two experiments.
MATERIALS AND METHODS
Sediment preparation.
The two separate mesocosm experiments used the upper 10 cm of sediment (under approximately 1.25 m of water) collected by a Van Veen grab near Coronado Cayes (latitude 32°38′268″N, longitude 117°08′165″W) in San Diego Bay on 19 June 1997 (ONR-1) and on 6 February 1998 (ONR-2). The sediment was sieved through no. 10 mesh (2-mm pore size) and tumbled using bleach-sanitized small (ONR-1) or large (ONR-2) cement mixers for 30 min to ensure homogeneity. About 1,100 g of seawater-saturated sediment was placed into each of four sterile 1-liter glass jars (ONR-1), or about 2,200 g was placed into each of 18 sterile 2-liter glass jars (ONR-2); each mesocosm was sealed with a Teflon-lined lid after subsamples were aseptically removed for microbiological and sediment chemistry measurements. The jars were then packaged individually in cardboard boxes (approximately 30.5 cm square) (ONR-1) or three to a box (approximately 25.5 by 91.5 by 30.5 cm) separated by 4 to 8 cm of plastic bubble wrap (ONR-2) according to the irradiation levels to be received. All mesocosms were transported on water ice to SteriGenics International, Inc. (Tustin, Calif.), to receive gamma radiation treatments from a 60Co source. Mesocosms were refrigerated at the facility between irradiation doses. The ONR-1 mesocosms were returned to the Sediment Management Laboratory after 10 days. Three of the ONR-1 mesocosms and six of the ONR-2 jars were not irradiated but were otherwise treated identically. Three of the ONR-2 jars received 5 megarads and were then tumbled for 2 days and returned to the facility for another 5 megarads. All dosimeters registered radiation doses close to those requested (25), but they were placed only on the six outer sides of the boxes rather than inside the boxes and in the sediment itself. Although all boxes were rotated between irradiation doses, the different packaging of the ONR-2 mesocosms or, more likely, their larger volumes could have shielded parts of the sediment, resulting in uneven killing of bacteria (see Results for a description).
Upon return to the Sediment Management Laboratory, each jar was subsampled for chemical and microbial analysis; the ONR-2 mesocosms were held in the dark at room temperature (20 to 22°C) until irradiation on all mesocosms had been completed (23 February 1998). All of the ONR-1 jars and 15 of the ONR-2 jars were spiked with a sterile PAH-sand mixture that added approximately 30 μg each of phenanthrene, fluoranthene, pyrene, chrysene, and benzo(a)pyrene per g of wet sediment (3). Because planned future experiments included it, hexadecane was also added (0.17 ml) to each mesocosm as a representative alkane; however, its volatility made it difficult to detect by the extraction methods used to track PAHs. Therefore, hexadecane behavior was not monitored in the present experiments. The mesocosms were divided into groups of three and tumbled at 33 rpm, with some alternating time at 161 rpm, until each mesocosm had been tumbled for 15 days (taking a total of 46 days) in a shaded area outside the building (12 to 23°C). The jars were then stored upright in the dark at 13°C.
Sediment chemistry.
Total organic carbon and dissolved organic carbon (DOC) in subsamples of each mesocosm were measured as described in the work of Apitz et al. (3). PAHs were extracted with a dichloromethane-acetone mixture with sonication and analyzed after cleanup with a 5972 series Mass Selective Detector in ion-selective mode (3). Percent solids were found by drying to constant weight duplicate 5-g subsamples at 80°C (microbiology samples) or 120°C (chemistry samples). Sediment pH was determined in sediment pore water, as was phosphate by using a Hach Phos Ver 3 Phosphate Reagent Powder Pillow and reading the color reaction at 820 nm. Ammonia concentrations were found by adding 100 μl of pore water to 1 ml of distilled water, 500 μl of phenol-ethanol solution (8 mg/ml), 500 μl of sodium nitroprusside solution (7.5 mg/ml), and 1 ml of oxidizing solution (0.03 ml of bleach/ml of alkaline solution [7.5 g of sodium citrate and 0.4 g of sodium hydroxide/500 ml of water]) and reading the color intensity at 640 nm after 1 h (M. Stallard, personal communication).
Bacterial enumerations.
Bacterial populations monitored included total, heterotrophs able to grow aerobically and anaerobically, endospore formers, sulfate-reducing bacteria (SRBs), and phenanthrene and chrysene degraders. All populations were enumerated from ONR-1 and ONR-2 mesocosms, except that in the latter heterotophs able to grow anaerobically and the SRBs were enumerated in only one mesocosm from each treatment.
Bacteria were extracted by placing 10 g of wet sediment in 45 ml of sterile, particle-free one-half-strength artificial seawater (ASW) (11.7 g of NaCl, 0.75 g of KCl, 12.35 g of MgSO4·7H2O, 1.45 g of CaCl2 per liter; pH 7.5), shaking the mixture by hand for 1 min, blending it in a sterile microblender (Eberbach, Inc., Ann Arbor, Mich.) on power level 6 for 30 s, and cooling it on ice for 30 s. After two repetitions, the mixture was put back into the bottle and the sediment was allowed to settle for 1 min. An additional 45 ml of diluent was then added, and the blending process continued for a total of 3 min. After blending, the sediment was allowed to settle for 1 min before the upper 10 ml was removed for serial 10-fold dilutions in one-half-strength ASW.
Total bacteria were counted by the acridine orange direct count method (21) modified by allowing the dye to stain the cells for 3 min (23). The black polycarbonate filters (0.22-μm pores) (Poretics Corporation, Livermore, Calif.) were counted within 5 min of preparation at ×1,000 using an American Optical One-Ten Epifluorescent microscope with a BG14+KV418 dichroic filter at 500 nm as an exciter filter, the OG515 barrier filter set, and a 50-W mercury vapor lamp. At least 20 fields were counted on each filter; duplicate filters were prepared from a separate dilution series. If the second count was more than 10% higher or lower than the first, a third filter was prepared from a separate dilution.
Heterotrophs able to grow anaerobically were enumerated on spread plates of glucose and yeast extract marine mineral agar (34) incubated in a BBL Gas Pak 150 System anaerobic jar (Becton Dickinson Microbiology Systems, Sparks, Md.). The most probable numbers (MPNs) of SRBs were enumerated by the five-tube method in 4.5 ml of marine Butlin's medium (40) with sodium lactate as the carbon and energy source. Tubes were scored positive for SRBs if a black precipitate appeared.
MPNs of heterotrophs able to grow aerobically were determined by adding 200 μl of one-half-strength ASW mineral medium (34) supplemented with 0.1% (each) succinate, acetate, glycerol, glucose, and yeast extract (SAGGYE) to each well of a 92-well microtiter plate prior to inoculation of duplicate series of five wells each with 20 μl of each dilution.
Phenanthrene-degrading bacteria were enumerated by two different methods: (i) the phenanthrene overlayer method (34) and (ii) a modification of the microtiter MPN procedure of Stieber et al. (38). Phenanthrene (8 g/liter) was dissolved in isopropanol and pentane (3:1), and 100 μl was added to each well of a sterile microtiter plate. The solvent was allowed to evaporate overnight in an operating fume hood by raising the lid on sterile pipette tips. Plates were stored sealed in Parafilm at −20°C until use. One-half-strength ASW mineral medium (200 μl) (34) was added to each microtiter well prior to inoculation of duplicate series of five wells each with 20 μl of each dilution. Chrysene degraders were enumerated only in the MPN format with microtiter plates with wells precharged with 200 μl of a solution of approximately 1.5 g of chrysene/liter of near-boiling hexane.
Microtiter MPNs included the following controls: one column of wells was inoculated with the appropriate sample dilution but did not contain an added carbon source, and one column contained a carbon source but was not inoculated with dilutions of the sediment. Turbidity was not observed in these control wells, indicating that bacteria were not moving from one well to another and that insufficient organic material was present in the inoculum to support enough growth to cause turbidity. Occasionally fungi would appear in individual wells with no particular pattern; they were easily differentiated from bacterial growth. The consistent absence of bacterial growth in wells without added carbon suggested that any growth observed was due to the metabolism of the provided carbon source.
Growth in each well was judged by turbidity and confirmed by the appearance of a purple color after the addition of 50 μl of iodonitro-tetrazolium violet (INT) (0.3 g/100 ml) and incubation for 18 to 24 h at room temperature (18); the color change indicated reduction of INT by respiring bacteria. INT was not reduced in wells devoid of added carbon, indicating the presence of very low numbers of bacteria able to respire residual organics in the sediment dilutions. MPNs were calculated by using tables for the five-tube format (12). Positive INT results matched well with observed turbidity in the case of heterotrophs and phenanthrene-degrading populations, but because chrysene crystals interfered with turbidity judgments, adding INT to these wells usually produced more wells positive for chrysene-degrading bacteria than would be suggested by turbidity alone.
After inoculation with all other media, dilution tubes were pasteurized at 80°C for 15 min to kill vegetative bacteria. Pasteurized dilutions were spread plated on 1/4-strength ASW-1/10-strength tryptic soy broth (BBL)-2% (wt/vol) agar to determine CFU of endospore-forming bacteria in the sediment.
All plates and tubes were incubated at 15°C for 4 weeks.
Isolation and characterization of PAH-degrading bacteria.
Colonies that cleared phenanthrene on overlayer plates and turbid liquid from microtiter wells with either phenanthrene or chrysene were struck for isolation on nonselective marine agar containing phenanthrene overlayers until pure cultures were obtained. The bacteria were identified by using such phenotypic properties as Gram stain reaction; sodium and oxygen requirements for growth; motility; catalase; nitrate reduction; production of hydrolytic enzymes; utilization of glucose, sucrose, mannitol, gluconate, glycerol, and cellobiose as sole sources of carbon and energy; and growth at 4°C (6, 25). All tests were performed at room temperature (20 to 22°C) except those that required different temperatures. While single-subunit ribosomal DNA and other molecular methods are useful for identification, only the traditional methods were within the scope and resources of the project.
Mineralization of phenanthrene and chrysene.
Certain isolates were tested in triplicate for their ability to degrade [14C]glucose (specific activity, 210 mCi/mmol); [9,10-14C]phenanthrene, 98% purity (specific activity, 55.4 mCi/mmol); or [5,6,11,12-14C]chrysene, 98% purity (specific activity, 48.0 mCi/mmol), the latter two originating from the National Cancer Institute Chemical Carcinogen Reference Standard Repository, Kansas City, Mo. Isolates were grown for 24 h with shaking (150 rpm) at 25°C in SAGGYE. For each test substrate, 5 ml of one-half-strength ASW mineral medium (unsupplemented) was placed in 10 sterile 40-ml EPA vials (I-Chem, Newcastle, Del.) that contained an inner 12- by 75-mm glass test tube with 1 ml of 5 N NaOH as the 14CO2 trap. The EPA vials were then inoculated with 100 to 200 μl of turbid culture and sealed tightly. For killed controls, 0.27 μl of a 37% formaldehyde solution was added prior to capping. The labeled glucose, phenanthrene, or chrysene was injected through the septa into 10 vials each to a final value of 50,000 dpm; no unlabeled compounds were added. All vials were incubated on an orbital shaker at 150 rpm at room temperature. Utilization of each labeled substrate was monitored in triplicate (except for day 0, when one tube was read) at 0, 5, 11, and 26 days by adding 200 μl of 4 N H2SO4 to the culture and shaking overnight to volatilize the 14CO2; 100 μl of each NaOH solution was added to 5 ml of scintillation cocktail (Scintisafe Plus 50%; Fisher Scientific, Pittsburgh, Pa.), mixed, and counted using a Brinkmann LS6000 scintillation counter (Brinkmann Instruments, Inc., Westbury, N.Y.).
Controls included uninoculated vials as well as formaldehyde-killed Escherichia coli and live E. coli from 24-h cultures grown in Luria-Bertani broth at 25°C with shaking. The ability to mineralize [14C]glucose showed that this system could detect 14CO2 production from labeled compounds.
RESULTS
For clarity, mesocosms from the first and second irradiation experiments are designated ONR-1 and ONR-2, respectively. Where appropriate, irradiation levels, 0, 2.5, 3.5, 5, 7, or 10 megarads, are indicated in the mesocosm descriptions. The ONR-2 mesocosms that received irradiation on two separate occasions are designated RP (repeat). The unirradiated mesocosms from both experiments which had PAHs added are designated OH, while the unspiked, unirradiated mesocosms from ONR-2 are designated OU.
Sediment chemistry.
The sediments used in ONR-1 and ONR-2 mesocosms were quite similar in the pHs and salinities of their pore waters, percent solids, and total organic carbon values (Table 1). Irradiation did not appreciably affect these values. DOC in the irradiated sediments was about 10-fold higher than that in the unirradiated sediments. The content of PAHs in the ONR-1 sediment before spiking was below detection levels (<0.09 μg/g); the ONR-2 sediment contained phenanthrene (0.06 ± 0.07 μg/g), fluoranthene (0.02 ± 0.02 μg/g), pyrene (0.02 ± 0.02 μg/g), chrysene (0.007 ± 0.005 μg/g), and benzo(a)pyrene (0.007 ± 0.005 μg/g). The distribution of the PAHs added to the ONR-1 mesocosms was quite homogeneous (3); a similar homogeneous distribution was also obtained in the ONR-2 mesocosms (data not shown).
TABLE 1.
Physical and chemical properties of mesocosm sediments before and immediately after irradiation but before spikinga
| Expt and sample | Value for property
|
|||||
|---|---|---|---|---|---|---|
| pH | Salinity (S‰)g | % Solids | Phosphate (μM) | Ammonia (μM) | DOC (mg/liter)g | |
| ONR-1 | ||||||
| Preirradiationb | 8.05 ± 0.02 | 34 ± 0.0 | 61.9 ± 0.02 | ND | ND | 16 ± 3.6 |
| Not irradiated (OHe) | 7.9 ± 0.03 | NDd | 62.3 ± 0.31 | ND | ND | 27 ± 21 |
| Postirradiation | ||||||
| 2.5 megarads | 8.2 ± 0.06 | ND | 62.4 ± 0.04 | ND | ND | 150 ± 3.1 |
| 3.5 megarads | 8.19 ± 0.01 | ND | 63.2 ± 0.03 | ND | ND | 186 ± 6.4 |
| 5 megarads | 8.08 ± 0.01 | ND | 62.5 ± 0.15 | ND | ND | 212 ± 2.3 |
| ONR-2 | ||||||
| Preirradiationb | 7.51 ± 0.01 | 34.5 | 59 ± 0.1 | 14.6 ± 3.1 | 151.3 ± 4.5 | BDh |
| Not irradiated | ||||||
| OUe | 7.7 ± 0.1 | 35 | 58 ± 0.7 | 30 ± 6 | 247 ± 29 | 22.4 |
| OHe | 7.8 ± 0.1 | 35 | 58 ± 1.0 | 27 ± 15 | 253 ± 25 | 30.6 |
| Postirradiationc | ||||||
| 5 megarads | 7.8 ± 0.0 | 35 | 61 ± 0.7 | 28 ± 2 | 481 ± 13 | 203.6 |
| 7 megarads | 7.8 ± 0.0 | 35 | 62 ± 1.7 | 23 ± 8 | 530 ± 12 | 204.8 |
| 10 megarads | 7.7 ± 0.1 | 35 | 62 ± 0.8 | 20 ± 4 | 575 ± 27 | 278.4 |
| RPf | 7.8 ± 0.1 | 35 | 61 ± 0.2 | 24 ± 11 | 617 ± 38 | 276.7 |
ONR-1 data are arithmetic means (and standard deviations) of triplicate subsamples from each mesocosm. ONR-2 data are arithmetic means (and standard deviations) of single subsamples from each of the triplicate mesocosms. Total organic carbon ranged from 4.5 to 6 mg/g of dry sediment and varied little over time or between treatments; it increased about 5% after addition of the PAHs.
Subsamples of the homogenized sediment taken before separation into the mesocosms.
Many of the ONR-2 mesocosms did not receive the intended radiation exposure (see text).
ND, not determined.
OU and OH were not irradiated but were handled like the mesocosms that were. OU was not spiked with PAHs, whereas OH and the other mesocosms were spiked.
RP, 5 megarads followed by tumbling of mesocosms and exposure to an additional 5 megarads.
For ONR-2, S‰ and DOC were determined in pore water from single mesocosms.
BD, below detection limit of 29.0 mg/liter.
The handling of the sediments during the irradiation process released both ammonia and phosphate into the pore waters of the ONR-2 sediments (compare preirradiation values to those for the nonirradiated OH and OU controls). Irradiation further solubilized ammonia but not phosphate (Table 1).
Bacterial populations in mesocosms before treatment.
Following separation of the sediments into mesocosms, subsamples were examined for their content of total and culturable bacteria (Table 2). The narrow ranges of values and the small standard deviations about the means suggest that both the ONR-1 and the ONR-2 sediment batches were well mixed and that each mesocosm contained reasonably similar populations of bacteria.
TABLE 2.
Bacterial numbers in subsamples of mesocosms collected before irradiation
| Population | No. of bacteria/g of dry sediment
|
|||
|---|---|---|---|---|
| ONR-1 (4 mesocosms)
|
ONR-2 (18 mesocosms)
|
|||
| Mean (SD)a | Range | Mean (SD)b | Range | |
| Total bacteria (AODCc) | 4 (0.5) × 108 | 3 × 108-4.5 × 108 | 7 (1) × 108 | 1 × 108-9 × 108 |
| Heterotrophs able to grow | ||||
| Aerobically (MPN) | 6 (3) × 104 | 4 × 104-10 × 104 | 20 (10) × 104 | 10 × 104-50 × 104 |
| Anaerobically (CFU) | 0.4 (0.2) × 104 | 0.2 × 104-0.5 × 104 | 4 (1) × 104 | 2 × 104-5 × 104 |
| Endospore formers (CFU) | 0.7 (0.4) × 104 | 0.2 × 104-1 × 104 | 2.5 (0.5) × 104 | 1 × 104-3 × 104 |
| Sulfate reducers (MPN) | 0.1 (0.04) × 104 | 0.06 × 104-0.15 × 104 | 2 (0.9) × 104 | 0.6 × 104-3 × 104 |
| Phenanthrene degraders | ||||
| Liquid medium (MPN) | 0.3 (0.2) × 104 | 0.2 × 104-0.5 × 104 | 0.4 (0.1) × 104 | 0.3 × 104-0.7 × 104 |
| Overlayer (CFU) | BDd | BD | BD | BD |
| Chrysene degraders (MPN) | 0.4 (0.1) × 104 | 0.2 × 104-0.5 × 104 | 0.3 (0.2) × 104 | BD-104 |
Arithmetic mean and standard deviation about the mean.
Anaerobic incubation for heterotrophs and determination of SRBs was carried out on only one mesocosm from each triplicate set, six in all.
AODC, acridine orange direct count.
BD, below detection limits of about 60 CFU/g of dry sediment.
Bacterial populations in nonirradiated, unspiked mesocosms.
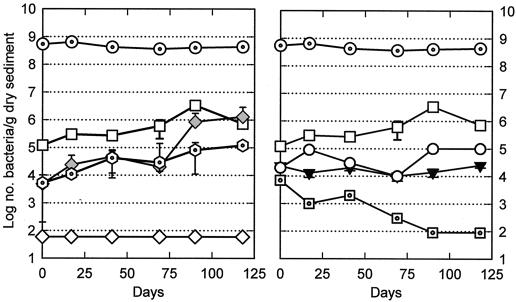
Changes in bacterial populations over time were monitored in three ONR-2 mesocosms that were neither irradiated nor spiked in order to judge the overall effect of the aeration and redistribution of organic compounds resulting from the sieving and mixing prior to separation, as well as the effect of the increase in phosphate and ammonia in pore water during handling. These three mesocosms (OU) had stable numbers of total bacteria and about a 20-fold increase in heterotrophs (aerobic incubation) over 118 days (Fig. 1). Heterotrophs able to grow anaerobically and endospore-forming bacteria were initially present in the sediments (Table 2), and their populations remained reasonably stable. SRBs were also present initially (Table 2), but their numbers steadily declined to about 100/g of dry sediment by day 118. Phenanthrene-degrading bacteria enumerated by the overlayer technique were below detection limits (<60 CFU/g of dry sediment); phenanthrene- and chrysene-degrading bacteria enumerated by liquid MPN were present in low numbers but increased by about 200- and about 20-fold, respectively, by day 118, even though negligible levels of PAHs were present.
FIG. 1.
Mean numbers of total bacteria (dotted circles), MPNs of heterotrophs under aerobic incubation (open squares), CFU of endospores (inverted solid triangles), MPNs (shaded diamonds) and CFU (open diamonds) of phenanthrene degraders, MPNs of chrysene degraders (dotted hexagons), CFU of heterotrophs under anaerobic incubation (open circles), and MPN of SRBs (dotted squares) in nonirradiated unspiked mesocosms over time. Some error bars are not visible because they are close to the data points. The limits of detection of each culturable population are approximately 80, 40, 80, 60, 80, 40, and 12/g of dry sediment, respectively.
Bacterial populations and PAH concentrations in nonirradiated spiked sediment (OH).
Total bacteria determined by acridine orange direct counts remained constant in the nonirradiated spiked mesocosms over time during both the ONR-1 (Fig. 2B) and ONR-2 (Fig. 3B) experiments. Numbers of heterotrophs (aerobic incubation) increased 1,000-fold for ONR-1 and 4,500-fold for ONR-2. In contrast, there was no or very little increase in the numbers of endospore-forming bacteria, heterotrophs able to grow anaerobically, or SRBs.
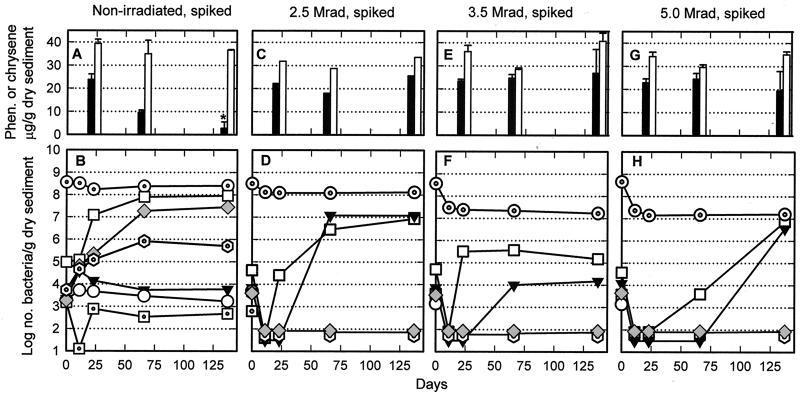
FIG. 2.
Phenanthrene (closed bars) and chrysene (open bars) concentrations and total bacteria and culturable bacteria (for explanations of symbols, see legend to Fig. 1) in nonirradiated (A and B) and irradiated (C to H) ONR-1 mesocosms spiked on day 11 with PAHs. Data points are the means of two (B to D, F, and H) or three (A, E, and G) subsamples, the latter with bars showing the standard deviations about the means. Heterotrophs able to grow anaerobically as well as SRBs were present in all mesocosms prior to irradiation (Table 2) but were undetectable after irradiation and did not reappear on incubation; only their zero time values are plotted. Phenanthrene degraders were not detected by the overlayer technique in any of these mesocosms. The asterisk above the phenanthrene bar in panel A indicates that this value is significantly different from the initial value (P = 0.001 by Student's t test).
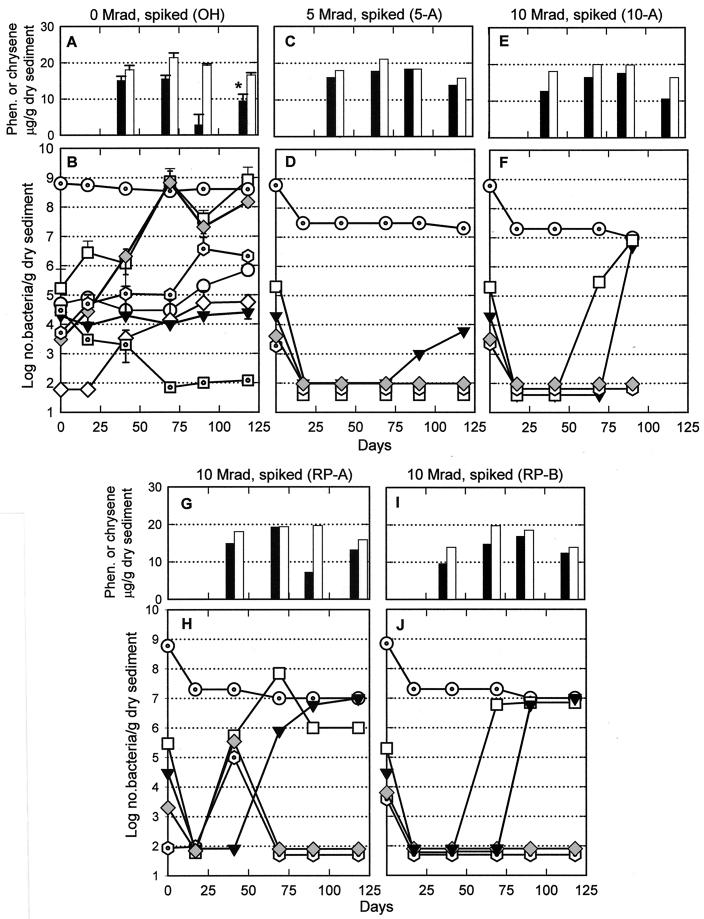
FIG. 3.
Phenanthrene (closed bars) and chrysene (open bars) concentrations and total (dotted circles) and culturable (for explanations of symbols, see legend to Fig. 1) bacteria in three unirradiated (A and B) and in individual successfully irradiated ONR-2 (see text) (C to J) mesocosms spiked on day 17. Some error bars in panels A and B are not visible because they are close to the data points. The asterisk above the phenanthrene bar in panel A indicates that this value is significantly different from the initial value (P = 0.013 by Student's t test).
The added PAHs clearly provided a carbon source for those heterotrophic bacteria able to use them as can be seen by comparing data in Fig 2B and 3B with those in Fig. 1. Populations of phenanthrene and chrysene degraders dramatically increased after PAH addition: 15,000- and 160-fold, respectively, for ONR-1 (Fig. 2B) and 22,000- and 400-fold, respectively, for ONR-2 (Fig. 3B). The increase in numbers of phenanthrene and chrysene degraders corresponded to a decline in phenanthrene concentration but not in chrysene concentration (Fig. 2A and 3A). The ONR-1 mesocosm was examined for PAH content several times after the last sampling date for microbiology (week 19.6). At week 53.7, phenanthrene had declined to 0.4 μg/g of dry sediment (from 26.4 μg/g), chrysene had declined to 27 μg/g (from 37.5 μg/g), fluoranthene had declined to 11.9 μg/g (from 41.4 μg/g), pyrene had declined to 6.2 μg/g (from 36 μg/g), and benzo(a)pyrene had declined to 19.4 μg/g (from 33.6 μg/g) (3).
It is unclear why no phenanthrene degraders from ONR-1 were able to form colonies that could clear phenanthrene overlayers, whereas such degraders did appear in unirradiated, spiked ONR-2 mesocosms (Fig. 3B), although at numbers about 400-fold lower than those detected by the MPN liquid technique. Clearly the latter technique enumerated more degraders, possibly because it allowed the formation of consortia of bacteria; in addition it was less time-consuming to set up and read. Similar results have been obtained in the enumeration of phenanthrene degraders from contaminated soil (37). It should be noted that, although we attempted to collect sediments for the two experiments in the same way and from the same spot (according to global positioning system data), PAH-degrader activity in San Diego Bay sediments is notoriously variable, and microbial populations observed in one set of sediments can be difficult to replicate in subsequent sets, an indication of the complexity of marine sediments (unpublished results of Office of Naval Research workshop on San Diego Bay sediment research).
Bacterial populations in irradiated spiked sediments.
Total bacteria in irradiated ONR-1 mesocosms declined 60% after exposure to 2.5 megarads (Fig. 2D) and about 90% after exposure to 3.5 and 5 megarads (Fig. 2F and H); these numbers remained fairly constant during the postirradiation incubation period. All culturable populations of bacteria were below detection levels in each irradiated mesocosm at the first sample taken after irradiation, and SRBs and heterotrophic bacteria able to grow anaerobically (data not shown) as well as the phenanthrene and chrysene degraders remained undetectable even at 133 days postirradiation. In contrast, heterotrophs able to grow aerobically and endospore formers reappeared. The heterotrophs were detected on day 22 in the 2.5- and 3.5-megarad-irradiated mesocosms (Fig. 2D and F) and on day 66 in the 5-megarad-irradiated mesocosms (Fig. 2H); their numbers were consistently lower, sometimes by 1,000-fold, than those from the unirradiated mesocosm incubated for the same length of time. The failure of phenanthrene- and chrysene-degrading populations to reappear in these irradiated spiked sediments was reflected in the stability of phenanthrene and chrysene concentrations over the same time period (Fig. 2C, E, and G) and after 53.7 weeks (3).
At day 161, fungi overgrew all the media (except for the plates inoculated with pasteurized sediments) that were inoculated with 3.5- and 5-megarad-irradiated sediments but not those inoculated with unirradiated or 2.5-megarad-irradiated sediments. This pattern suggested that fungal survivors were able to proliferate in the former sediments because of lowered bacterial numbers but that the generally higher numbers and earlier appearance of bacteria in the latter sediments restrained fungal growth. These fungi were not PAH degraders, as indicated by the stable levels of PAHs in these mesocosms (3). The fungi could seriously interfere with subsequent studies of the spiked aged sediments after reinoculation with PAH-degrading bacteria. Indeed, Sandoli et al. (36) ascribed such lack of PAH biodegradation to the toxic effects of 2.5-megarad irradiation on 50 g of dried aquifer sediment. However, because these authors reported no microbiological testing in the days following irradiation, it cannot be ruled out that fungal overgrowth was responsible, rather than the buildup of toxic radiation compounds.
While the details will be the subject of another paper, we found that the fungi were killed in the aged ONR-1 sediments by reirradiation at the 5-megarad level. Upon reinoculation with small amounts of freshly collected PAH-contaminated sediment, heterotrophic bacteria reappeared in just 11 days, as did some phenanthrene degraders (based on the overlayer method) together with a significant decrease in phenanthrene, fluoranthrene, and pyrene concentrations (E. W. Lin, E. Arias, S. A. Clawson, and S. E. Apitz, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. Q-183, p. 568, 1999).
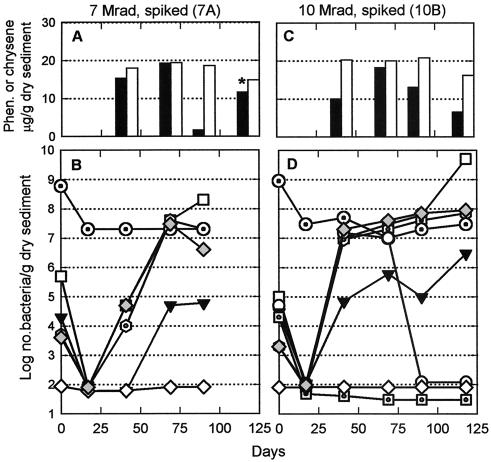
ONR-2 mesocosms were irradiated at higher doses in an attempt to sterilize them, i.e., to kill all microorganisms. While fungi never became numerous, postirradiation numbers of culturable bacteria suggested either that most of the ONR-2 mesocosms did not receive the intended doses at the radiation facility or that the radiation did not uniformly penetrate the sediment jars, which were two times the volume of those used for ONR-1. The latter possibility is supported by two lines of evidence: (i) calculations of theoretical penetration depths for 60Co irradiation into a saturated, mineral-rich sediment suggest that the contents of the 2-liter jars were at the theoretical limit of penetration, and (ii) the most successful irradiation, based upon postirradiation microbial populations, was in two of the RP jars, which, due to mixing between doses, may have exposed sediments (and bacteria) from the center of the jar to the second irradiation. Microbial evidence of incomplete irradiation included heterotrophs able to grow aerobically and phenanthrene and chrysene degraders appearing by day 41 or 69 in two of the mesocosms that had supposedly received 5 megarads, three that had received 7 megarads, two that had received 10 megarads, and one (RP) that got 10 megarads in two 5-megarad doses; examples of these data are shown in Fig. 4. These patterns of microbial recovery were very different from those seen in the smaller sediment volumes of the irradiated ONR-1 mesocosms (Fig. 2) and strongly suggested that these eight ONR-2 mesocosms were not successfully irradiated. As a result, their examination was discontinued. However, four ONR-2 mesocosms remained free of phenanthrene and chrysene degraders postirradiation: one that received 5 megarads, one that received 10 megarads, and two that got 10 megarads in two doses of 5 megarads each (RP). Based on these criteria, these four were studied further (Fig. 3).
FIG. 4.
Phenanthrene (closed bars) and chrysene (open bars) concentrations and total (dotted circles) and culturable (for explanations of symbols, see legend to Fig. 1) bacteria in two of the eight unsuccessfully irradiated ONR-2 mesocosms (see text) spiked on day 17. Microbial examination of 7A (panels A and B) ceased with the day 90 sample. While single points cannot be subjected to statistical analysis, a visual inspection of PAH levels suggested that the phenanthrene concentration, at least, was decreasing in these mesocosms.
As in the ONR-1 mesocosms, total bacteria in these four mesocosms from ONR-2 declined 91 to 97% from the unirradiated values; these low numbers remained fairly constant throughout subsequent incubation (Fig. 3D, F, H, and J). Endospore-forming bacteria were below detectable levels immediately after irradiation but reappeared by day 69 in RP-A (Fig. 3H) and by day 90 in the other three mesocosms (Fig. 3D, F, and J). Heterotrophs able to grow in air did not reappear in the mesocosms that received 5 megarads but did eventually proliferate in the other three mesocosms. SRBs and heterotrophs able to grow anaerobically were destroyed by radiation in these four mesocosms and did not reappear. Phenanthrene and chrysene degraders were enumerated only at day 41 in only one of the four irradiated mesocosms from ONR-2 (Fig. 3H) and not in three subsequent subsamples. However, chrysene levels stayed relatively constant in this mesocosm, although phenanthrene levels fluctuated. In the absence of a clear decline in either PAH, which is seen in unirradiated, spiked sediments (Fig. 2A and 3A), we concluded that this elevation of PAH degraders on day 41 was anomalous.
Identification of PAH-degrading bacteria.
All 44 isolates from the PAH-containing media were gram-negative rods with an absolute requirement for sodium for growth and the ability to grow in liquid mineral medium with phenanthrene as the sole source of carbon and energy. Eight isolates were facultative anaerobes and were classified as Vibrio spp. The remaining isolates were strict aerobes with phenotypic characteristics that placed them in the genera Halomonas, Marinomonas, Pseudoalteromonas, and Pseudomonas or as either Marinobacter or Cycloclasticus (25).
Mineralization of PAHs by bacterial isolates.
When bacteria dissolve PAH particles on overlayer plates and form turbidity in liquid mineral medium with a PAH as the sole source of carbon and energy for growth, it is assumed that the cells are mineralizing the PAH in whole or in part (14). We made this assumption in enumerating both phenanthrene- and chrysene-degrading bacteria and then tested the assumption by exposing a subset of isolates to 14C-labeled PAHs. Uniformly labeled PAHs will detect mineralization by pathways both known (17, 22, 31) and unknown (7, 27), but only [9,10-14C]phenanthrene and [5,6,11,12-14C]chrysene were available to us.
The six isolates (in four different taxa) from ONR-1 unirradiated but spiked sediments formed turbidity in liquid mineral medium but could not clear phenanthrene particles in overlayers, nor could they release 14CO2 from the PAHs (Table 3). Perhaps these isolates are degrading only unlabeled parts of the phenanthrene and chrysene molecules. If this proves to be the case, it will greatly expand the number of species of marine bacteria able to degrade these PAHs.
TABLE 3.
Mineralization of [14C]glucose, [14C]phenanthrene, or [14C]chrysene by isolates from unirradiated, spiked San Diego Bay sediment mesocosms as determined by release of 14CO2 after 11 (glucose) or 26 days of incubation
| Isolate source | Identification | Sediment dilutionb | Phenanthrene overlayer cleared | % dpm of 14CO2 releaseda
|
||
|---|---|---|---|---|---|---|
| [14C]glucosec | [14C]phenanthrene | [14C]chrysene | ||||
| Controls | Uninoculated | NAd | NA | 6.6 | 1.9 | 0.8 |
| Killed E. coli | NA | NA | 3.3 | 1.1 | 0.7 | |
| E. coli | NA | NA | 32.7 | 0.9 | 1.3 | |
| ONR-1 | Vibrio anguillarum II | 10−3 phenanthrene | − | 37.5 | 0.5 | 0.3 |
| Halomonas pacifica | 10−4 chrysene | − | 15.5 | 0.4 | 0.2 | |
| H. pacifica | 10−2 chrysene | − | 1.5 | 0.4 | 0.5 | |
| Halomonas halophila | 10−2 phenanthrene | − | 6.0 | 0.2 | 0.2 | |
| Halomonas halophila | 10−5 phenanthrene | − | 1.5 | 0.3 | 0.3 | |
| M/Ce | 10−5 phenanthrene | − | 0.9 | 0.2 | 0.3 | |
| ONR-2 | M/C | 10−3 phenanthrene | + | 1.7 | 46.6 | 2.5 |
| M/C | 10−5 phenanthrene | + | 0.5 | 40.2 | 18.0 | |
| Halomonas elongata | 10−3 phenanthrene | + | 3.3 | 31.5 | 8.3 | |
| M/C | 10−2 phenanthrene | + | 2.1 | 18.9 | 4.3 | |
| M/C | 10−2 phenanthrene | + | 2.0 | 18.0 | 7.3 | |
| M/C | 10−5 phenanthrene | + | 2.5 | 11.3 | 6.9 | |
| V. pelagius I | 10−2 phenanthrene | + | 5.6 | 1.4 | 0.6 | |
| H. elongata | 10−2 phenanthrene | + | 1.9 | 0.9 | 6.8 | |
| Halomonas marina | 10−3 phenanthrene | − | 1.2 | 0.5 | 0.3 | |
% dpm of 14CO2 released = dpm accumulated in NaOH − dpm at time zero/50,000 dpm added × 100.
Except for V. pelagius I, which was isolated from a phenanthrene overlayer plate, all isolates were taken from the MPN liquid medium with the indicated PAH as the sole source of carbon and energy.
Many marine bacteria are incapable of glucose metabolism (6).
NA, not applicable.
M/C, isolate identified on the basis of phenotypic tests as either M. hydrocarbinoclasticus or Cycloclasticus pugettii.
All but one of the nine isolates (in four different taxa) from ONR-2 (unirradiated sediments) were able to clear phenanthrene on overlayers; six of these (five Marinobacter or Cycloclasticus strains and one Halomonas elongata strain) released 14CO2 from labeled phenanthrene, and five also released 14CO2 from labeled chrysene (Table 3). Another isolate identified as H. elongata could clear phenanthrene overlayers and release some 14CO2 from chrysene only. Two isolates (Vibrio pelagius I and H. elongata) released no 14CO2 at all from the PAHs.
DISCUSSION
Marine sediments tend to be heterogeneous, and this heterogeneity often confounds attempts to take replicate subsamples for chemical and microbial analyses (3). Our protocol for collecting, sieving, and homogenizing sediments mobilized organic compounds, ammonia, and phosphate; undoubtedly introduced oxygen; and led to modest increases in the numbers of aerobic and facultative heterotrophic bacteria and of PAH-degrading bacteria. Neither the presence of PAH-degrading bacteria in the clean sediment nor their increase in number is surprising given their ubiquity in the environment (4), and their ability to also grow on a range of simple organic compounds (15, 16, 25). In contrast, the addition of the organic carbon in the five PAHs and of the hexadecane to the nonirradiated sediment mesocosms led to dramatic changes in the numbers of these populations. About 50 days after spiking, populations of the heterotrophs, phenanthrene degraders, and chrysene degraders increased. Over time, the phenanthrene content declined in the spiked, unirradiated sediments; chrysene concentrations declined only over the very long term as measured in the ONR-1 mesocosms (3). The increase in phenanthrene- and chrysene-degrading populations supports the findings of others that numbers of PAH-degrading bacteria increase after PAH contamination (5, 32, 33). Depending upon the relative rates of input and degradation, these sediment microbial populations may actually help “cleanse” overlying waters of ongoing PAH inputs (7a). As these inputs decrease over time, natural recovery may occur. Our observations about population changes provide some insight into this process.
Although there was a general increase with time in the populations of some culturable bacteria enumerated in the clean, nonirradiated, nonspiked sediments and in the nonirradiated, spiked sediments, this was not reflected in the total numbers of bacteria enumerated in these mesocosms. The technique used to count total numbers of bacteria detects those bacteria that contain sufficient amounts of nucleic acids to take up the fluorescent dye acridine orange; such bacteria are assumed to be alive (21, 23) but not necessarily culturable on the medium used. The presence of clay in these sediments also made counting the fluorescent bacteria on the filters difficult; dilutions were used to provide reasonable numbers of cells to count, but often there was enough clay contamination and background fluorescence to mask stained cells. Thus, total bacterial counts are probably underestimates of the total numbers of bacteria actually present in the mesocosms. Despite this difficulty, it is apparent that shifts in the numbers of culturable populations occurred even though the total number of bacteria did not appear to increase.
Since the main purpose of our experiments was to determine whether various irradiation treatments resulted in sterile sediments, and the microbial response to the treatments (other than whether they were present) was a secondary issue, no effort was made to control or monitor redox potential in the sediments during sample manipulation and aging. Clearly, the anoxic sediments were partially oxidized during collection, homogenization, and transfer. Experience tells us that water-saturated, organic-rich sediments, even after extensive stirring and aeration, become anoxic millimeters below the sediment-water interface within days of being placed in jars. The primary mechanism assumed to drive this process is microbial consumption of oxygen. Clearly, the same processes were not at work, or at least not at the same rates, in the irradiated sediments, since microbial activity had, at a minimum, been slowed down by irradiation. Thus, it is possible, if microbial activity in the jars did not remove the introduced oxygen before irradiation, that these sediments retained regions with very different redox conditions than those in the unirradiated sediment, even though we noted no distinct visual differences between the irradiated and unirradiated jars. It should be pointed out that this lack of control of redox conditions may have affected both the mode of PAH sequestration during the aging process and the numbers of culturable bacteria. The potential exists for PAHs to be sequestered in nanopores as a result of iron oxidation and for very different microbial populations to develop in jars that have undergone different treatments. Ideally, the sediment should be collected, homogenized, divided, and placed into jars under strictly anoxic conditions. However, this was not feasible given the large amounts of sediment needed. Since we are not aware of any other published methods for “realistic” sediment spiking in which this variable was controlled, we cannot predict what the effects of such control would be.
In this study, bacteria able to produce turbidity in liquid medium with phenanthrene or chrysene as the sole source of carbon and energy were isolated from the nonirradiated, spiked mesocosms, usually from microtiter wells inoculated with 1:100 to 1:100,000 dilutions of the sediment; thus, they probably represent the dominant culturable PAH degraders in the mesocosms (8). Phenotypic characterization placed them in several genera of marine bacteria: Vibrio, Marinomonas, Pseudomonas nautica (now Marinobacter hydrocarbonoclasticus), Pseudoalteromonas, Marinobacter or Cycloclasticus, and Halomonas. PAH-degrading species of most of these genera have been isolated before from marine sediments (9, 16); this is the first time that marine PAH degraders have been identified as Marinomonas and Halomonas, although species in the latter genus have been reported elsewhere to cleave aromatic rings (41). The addition of these two genera expands the diversity of marine bacteria able to attack PAHs. We did not isolate nonmarine PAH degraders associated with terrestrial environments (15, 29, 39).
About half of the 15 representative isolates that we tested, including two of the seven Halomonas species, were able to release 14CO2 from labeled phenanthrene or chrysene, or both. Those that did not produce 14CO2 could be partially degrading the PAH, perhaps enough to grow on, but not enough to release the 14C from the few labeled sites in each PAH. It seems clear that the nonirradiated, spiked sediments contained several kinds of PAH-degrading marine bacteria able to respond fairly rapidly to PAH addition.
However, this response just days after addition of five different PAHs models only a very recent introduction of PAHs into sediments in the field. While there is ongoing input of PAHs into sediments at various levels and rates, many sites contain sediments that have been contaminated by PAHs for months to years, during which time weathering and sequestration may alter the contaminants, their relationship to sediment constituents, and their availability to microbes. Thus, experimental sediments containing known amounts of PAHs must be aged in the absence of PAH-degrading bacteria (3, 20). We were able to accomplish this with the irradiated ONR-1 and four of the successfully irradiated ONR-2 mesocosms even though the heterotrophs able to grow in air and endospore formers eventually reappeared. The former population usually recovered first, and the endospore formers reappeared later, probably because their detection depended on endospore formation. These populations probably arose from survivors, most likely endospores, that were able to grow on the DOC present in the sediment or on carbon released from cells lysed by the gamma radiation. The absence of PAH-degrading bacteria, none of which are endospore formers, was supported by the apparent inhibition of PAH disappearance in the mesocosms. The small decreases in extractable levels of the heavier PAHs observed can be ascribed to aging of the PAHs and their sequestration onto organic or inorganic components of the sediments, with a concomitant decrease in extractability (3).
In conclusion, the present work suggests that gamma irradiation can be used to prepare marine sediments free of indigenous PAH-degrading bacteria for the construction of a model system to study PAH interactions and later biodegradation in marine sediments. Such a system should use clean sediment divided after homogenization into several 1-liter or smaller mesocosms. After irradiation at 5 megarads, each mesocosm should be spiked with the sterile sand-PAH mixture and tumbled for several days to homogenize the spike. The mesocosms would then be incubated for several months to allow the spike to age into the sediments. During this time, it would be prudent to enumerate several different bacterial populations to evaluate the uniformity of the radiation exposure and to ensure that the PAH-degrading bacteria do not reappear. The mesocosms could then be reirradiated at 5 megarads to kill off any fungi that might have grown (Lin et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.) and reinoculated with any microbial population or isolate of interest. Degradation of the aged PAHs could then be studied by monitoring both the levels of extractable PAHs and the populations of PAH degraders. To monitor PAH degradation more accurately, one radiolabeled PAH should be added per mesocosm so that the fate of the labeled compound can be traced and its mineralization, transformation, and sequestering monitored. Such model sediments will make it possible to analyze the different variables involved in biodegradation in a more controlled system that mimics the dynamics of field biodegradation. This model would advance our knowledge of the processes involved in and important to biodegradation in situ. Furthermore, the same model sediments can be used to study other fate and bioavailability issues as well as the bacteria involved and the genetic processes required in situ. We could then begin to make predictions about PAH-contaminated sites which could be used to create bioremediation protocols that would more rapidly and effectively reduce PAH contamination of these sites, predict availability of residues, and close mass balances. This is eventually the goal of all sediment management research.
Acknowledgments
We thank Eleanor W. Lin for her help with the sediments and their transportation to the sterilizing facility and later tumbling and sampling. Ernest Arias and Sheri A. Clawson performed the analytical chemistry. Martha Stallard supplied the pH and nutrient analyses, and John Trefrey at the Florida Institute of Technology carried out the DOC and total organic carbon measurements.
Funding was provided by the Office of Naval Research (contract no. N0001499WX20091 and N0001498WX20172), Naval Facilities, and the Applied Microbiology Laboratory at San Diego State University.
REFERENCES
- 1.Albaiges, J. 1989. Marine pollution: an introduction, p. 1-10. In J. Albaiges (ed.), Marine pollution. Hemisphere Publishing Corp., New York, N.Y.
- 2.Alexander, M. 1994. Biodegradation and bioremediation. Academic Press, San Diego, Calif.
- 3.Apitz, S. E., E. Arias, S. A. Clawson, E. W. Lin, R. J. Melcher, and B. B. Hemmingsen. 1999. The development of a sterile, PAH-spiked, aged marine sediment for biodegradation experiments: chemical results. Org. Geochem. 30:891-900. [Google Scholar]
- 4.Atlas, R. M. 1995. Petroleum biodegradation and oil spill bioremediation. Mar. Pollut. Bull. 31:178-182. [Google Scholar]
- 5.Azoulay, E., M. Colin, J. Dubreuil, H. Dou, G. Mille, and G. Giusti. 1983. Relationship between hydrocarbons and bacterial activity in Mediterranean sediments. Part 2. Hydrocarbon degrading activity of bacteria from sediments. Mar. Environ. Res. 9:19-36. [Google Scholar]
- 6.Baumann, L., P. Baumann, M. Mandel, and R. D. Allen. 1972. Taxonomy of aerobic marine eubacteria. J. Bacteriol. 110:402-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardesco, G., S. Dyhrman, E. Gallagher, and M. P. Shiaris. 1998. Spatial and temporal variation of phenanthrene-degrading bacteria in intertidal sediments. Appl. Environ. Microbiol. 64:2560-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Boyd, T. J., M. T. Montgomery, B. J. Spargo, R. B. Coffin, J. K. Steele, J. P. Pohlman, and D. Velinsky. 1999. Characterization of intrinsic bioremediation within the Philadelphia Naval Complex Reserve Basin. NRL technical report. NRL/PU/6115-99-374. Naval Research Laboratory, Washington, D.C.
- 8.Button, D. K., R. Schut, P. Quang, R. Martin, and B. R. Robertson. 1993. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl. Environ. Microbiol. 59:881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen, W. R., X.-F. Li, and K. J. Reimer. 1994. Degradation of phenanthrene and pyrene by microorganisms isolated from marine sediment and seawater. Sci. Total Environ. 156:27-37. [Google Scholar]
- 10.Dalton, B. R., U. Blum, and S. B. Weed. 1989. Plant phenolic acids in soils: sorption of ferulic acid by soil and soil components sterilized by different techniques. Soil Biol. Biochem. 21:1011-1018. [Google Scholar]
- 11.DeWitt, T. H., R. C. Swartz, and J. O. Lamberson. 1989. Measuring the acute toxicity of estuarine sediments. Environ. Toxicol. Chem. 8:1035-1048. [Google Scholar]
- 12.Eaton, A., L. S. Clesceri, and A. E. Greenberg (ed.). 1995. Standard methods for the examination of water and wastewater; 19th ed. American Public Health Association, Washington, D.C.
- 13.Eno, C. F., and H. Popenoe. 1964. Gamma radiation compared with steam and methyl bromide as a soil sterilizing agent. Soil Sci. Soc. Am. Proc. 28:533-535. [Google Scholar]
- 14.Foght, J. M., P. M. Fedorak, and D. W. S. Westlake. 1990. Mineralization of [14C]hexadecane and [14C]phenanthrene in crude oil: specificity among bacterial isolates. Can. J. Microbiol. 36:169-175. [DOI] [PubMed] [Google Scholar]
- 15.García-Valdés, E., E. Cozar, R. Rotger, J. Lalucat, and J. Ursing. 1988. New naphthalene-degrading marine Pseudomonas strains. Appl. Environ. Microbiol. 54:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiselbrecht, A. D., R. P. Herwig, J. W. Deming, and J. T. Staley. 1996. Enumeration and phylogenetic analysis of polycyclic aromatic hydrocarbon-degrading marine bacteria from Puget Sound sediments. Appl. Environ. Microbiol. 62:3344-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal, A. K., and G. J. Zylstra. 1996. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni. Appl. Environ. Microbiol. 62:230-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines, J. R., B. A. Wrenn, E. L. Holder, K. L. Strohmeier, R. T. Herrington, and A. D. Venosa. 1996. Measurement of hydrocarbon-degrading microbial populations by a 96-well plate most-probable-number procedure. Ind. Microbiol. 16:36-41. [DOI] [PubMed] [Google Scholar]
- 19.Harvey, R. G. 1996. Mechanisms of carcinogenesis of polycyclic aromatic hydrocarbons. Polycyclic Aromatic Hydrocarbons 9:1-23. [Google Scholar]
- 20.Hatzinger, P. B., and M. Alexander. 1995. Effect of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 29:537-545. [DOI] [PubMed] [Google Scholar]
- 21.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwabuchi, T., and S. Harayama. 1998. Biochemical and genetic characterization of trans-2′-carboxybenzalpyruvate hydratase-aldolase from a phenanthrene-degrading Nocardioides strain. J. Bacteriol. 180:945-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kepner, R. L., Jr., and J. R. Pratt. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol. Rev. 58:603-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang, S., and F. Wagner. 1993. Biosurfactants from marine microorganisms, p. 391-417. In N. Kosaric (ed.), Biosurfactants. Production, properties, applications. Marcel Dekker, Inc., New York, N.Y.
- 25.Melcher, R. J. 1999. Demonstration of biodegradation in polycyclic aromatic hydrocarbon-spiked marine sediments: population changes and characterization of phenanthrene- and chrysene-degrading bacteria. M.S. thesis. San Diego State University, San Diego, Calif.
- 26.Menzie, C. A., B. B. Potockiki, and J. Santodonato. 1992. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 26:1278-1284. [Google Scholar]
- 27.Moody, J. D., J. P. Freeman, D. R. Doerge, and C. E. Cerniglia. 2001. Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 67:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagarkar, P. P., S. D. Ravetkar, and M. G. Watve. 2001. Oligophilic bacteria as tools to monitor aseptic pharmaceutical production units. Appl. Environ. Microbiol. 67:1371-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okpokwasili, G. C., C. C. Somerville, D. J. Grimes, and R. R. Colwell. 1984. Plasmid-associated phenanthrene degradation by Chesapeake Bay sediment bacteria. Actes Colloq. Inst. Fr. Rech. Exploitation Mer 3:601-610. [Google Scholar]
- 30.Östlund, P., R. Carman, U. G. Edvardsson, and L. Hallstadius. 1989. Sterilization of sediments by ionizing radiation. Appl. Geochem. 4:99-103. [Google Scholar]
- 31.Pothuluri, J. V., and C. E. Cerniglia. 1994. Microbial metabolism of polycyclic aromatic hydrocarbons, p. 92-124. In G. S. Chaudhry (ed.), Biological degradation and bioremediation of toxic chemicals. Dioscorides Press, Portland, Oreg.
- 32.Prince, R. C. 1993. Petroleum spill bioremediation in marine environments. Crit. Rev. Microbiol. 19:217-242. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard, P. H., J. G. Mueller, J. C. Rogers, R. V. Kremer, and J. A. Glaser. 1992. Oil spill bioremediation: experiences, lessons and results from the Exxon Valdez oil spill in Alaska. Biodegradation 3:315-335. [Google Scholar]
- 34.Rice, L. E., and B. B. Hemmingsen. 1997. Enumeration of hydrocarbon-degrading bacteria. Methods Biotechnol. 2:99-109. [Google Scholar]
- 35.Salonius, P. O., J. B. Robinson, and F. E. Chase. 1967. A comparison of autoclaved and gamma-irradiated soils as media for microbial colonization experiments. Plant Soil 27:239-248. [Google Scholar]
- 36.Sandoli, R. L., W. C. Ghiorse, and E. L. Madsen. 1996. Regulation of microbial phenanthrene mineralization in sediment samples by sorbent-sorbate contact time, inocula and gamma irradiation-induced sterilization artifacts. Environ. Toxicol. Chem. 15:1901-1907. [Google Scholar]
- 37.Stein, K. O. 1999. Changes in populations of phenanthrene-degrading bacteria during the bioremediation of polycyclic aromatic hydrocarbon-containing soils. M.S. thesis. San Diego State University, San Diego, Calif.
- 38.Stieber, M., F. Haeseler, P. Werner, and F. H. Frimmel. 1994. A rapid screening method for micro-organisms degrading polycyclic aromatic hydrocarbons in microplates. Appl. Microbiol. Biotechnol. 40:753-755. [Google Scholar]
- 39.Tagger, S., N. Truffaut, and J. Le Petit. 1990. Preliminary study on relationships among strains forming a bacterial community selected on naphthalene from a marine sediment. Can. J. Microbiol. 36:676-681. [DOI] [PubMed] [Google Scholar]
- 40.Tanner, R. S. 1989. Monitoring sulfate-reducing bacteria: comparison of enumeration media. J. Microbiol. Methods 10:83-90. [Google Scholar]
- 41.Ventosa, A., J. J. Nieto, and A. Oren. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62:504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissenfels, W. D., H.-J. Klewer, and J. Langhoff. 1992. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity. Appl. Microbiol. Biotechnol. 36:689-696. [DOI] [PubMed] [Google Scholar]