Abstract
Purpose
Molecular subtype of hepatocellular carcinoma (HCC) is primarily identified via high throughput expression profiles, largely ignoring the dynamic changes of gene expressions. Yet, biological networks remain steadily characterize disease state irrespective of time and conditions. We aim to utilize a metabolic genes interaction perturbation network-based approach to facilitate the subtyping and precision treatment of HCC patients.
Methods
We employed the metabolic genes interaction perturbation network-based approach to identify metabolic reprogramming (MR) subtypes in 922 HCC samples from four independent public datasets and further investigated their clinical and biofunctional implications, immune landscape, multi-omics features and biomarker.
Results
We stratified patients into three unique MR subtypes: (i) MR1 (“immune-deficiency”), frequent CTNNB1 mutation, and moderate prognosis; (ii) MR2 (“immune-activated”), advanced pathological staging and histological grading, frequent TP53 mutation, response to anti-PD-1 therapy, and the worst prognosis; (iii) MR3 (high metabolic activity), low-grade pathological staging and histological grading, fewer mutations and copy number variations, and the best prognosis. Besides, CD24 was identified and validated as a biomarker for MR2 which indicated a poor prognosis with higher expression.
Conclusion
Taken together, the interactome taxonomy could effectively facilitate the stratified management and precise treatment of heterogeneous HCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03232-5.
Keywords: Hepatocellular carcinoma, Gene interaction, Metabolic biological network, Edge perturbation, Nearest template prediction algorithm, Metabolic subtypes
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of death worldwide, characterized by a high mortality rate and recurrent rate [1]. The main therapies for HCC include surgical resection, liver transplantation, image-guided ablation, and chemoembolization, whereas the high recurrence rates persistently plague the clinical management of HCC patients [2]. Although the Barcelona Clinical Liver Cancer Staging (BCLC) system, a traditional staging system, is widely recognized in clinical practice [3], it is insufficient for assessing prognosis and optimizing precise treatment owing to the deficiency of patients’ genomic information. With advances in high-throughput sequencing technology, multiple HCC subtypes with various prognoses and molecular features were identified in terms of expression profiles. Yet they were proved unstable and varied intensively across different conditions in the biological system [2, 4]. Biological networks rise to the occasion with a key contributing factor of stabilization irrespective of time and condition [5–7]. Moreover, gene interactions in biological networks maintain jarless in normal tissues and alter tremendously in disordered tissues, demonstrating its great potential to represent the disease state of organism [7]. Indeed, the superiority and reliability of molecular typing based on gene interaction networks had been demonstrated in breast cancer [7]. To advance precision medicine, it is imperative to seek a novel and more reliable taxonomy with higher potency to stratify HCC.
Dramatic changes in diversiform metabolic processes have been proven to facilitate its proliferation and drug resistance in HCC [8]. These changes lead to the alteration of metabolic patterns in cancer cells known as metabolic reprogramming, which synergistically contributes to cancer initiation and progression from several aspects. For instance, fatty acids as signaling precursors in metabolic regulation contributed to the development of HCC [9]. Besides, excess lactic acid depresses immune cell function and undermines immunosurveillance, eventually leading to the immune escape of tumor cells [10]. Previous evidence has demonstrated the role of the metabolic biological network in liver cancer typing [4, 11]. Unfortunately, these studies which only focused on proteins, metabolites, or the heterogeneity of their networks, still ignored the gene interaction perturbations in metabolic biological networks. Thereby, it’s imperative to comprehensively explore the contribution of metabolic gene interaction perturbations to hepatocellular carcinoma heterogeneity.
In this work, we introduced a rank-based individual-specific method to measure the perturbations of metabolic gene interaction, which characterized perturbations in gene interactions through relative changes of gene expression [7]. Three metabolically-driven HCC subtypes with broadly heterogeneous prognosis, biological processes, immune landscape, genome alterations, and drug sensitivity were identified. In conclusion, this study deepened our understanding of metabolic heterogeneity in HCC from a gene-interacting perspective and enabled individualized therapy for HCC patients with different metabolic patterns.
Materials and methods
Construction of the metabolic gene interaction network
A metabolic gene interaction background network was needed to construct an interaction-perturbation matrix. To gain the background network, metabolic genes were collected by searching metabolism-related gene sets from Molecular Signature Database (MSigDB, https://www.gsea-msigdb.org/gsea/msigdb) and projected them onto the protein interaction network derived from the Search Tool for the Retrieval of Interaction Gene/Proteins (STRING, https://string-db.org/) database. Missing genes in our cohort were wiped off and the remaining gene nodes and interactions were integrated into a background network of metabolic gene interactions. A metabolic gene background network with 20,930 gene interactions between 944 gene nodes was finally obtained.
Measurement of interaction perturbations in the metabolic gene background network
As demonstrated in the flowchart (Fig. 1A), genes were primarily ranked within individuals by expression level and then utilized the discrepancies in the orderings of interacting genes to represent their interactions. Then, the gene expression of normal samples was averaged, ranked, and employed as a benchmark. The interactions of all samples were compared to the benchmark, and the alteration could be used to indicate the disease state of individuals. This approach fully utilized both the expression of specific genes in biological pathways (nodes in a network) and the relative changes of gene interactions in biological networks (edges in a network).
Fig. 1.
Identification and characterization of three metabolic reprogramming subtypes in LIHC through metabolic gene interaction perturbations. A Schematic workflow of the edge-perturbation-based approach for identifying metabolic reprogramming (MR) subtypes in LIHC. B Consensus matrix heatmap demonstrating optimal clustering of TCGA-LIHC samples (k = 3). C Proportion of ambiguous clustering measurements supporting the optimal cluster number (k = 3). D Principal component analysis validating the optimal cluster number (k = 3). E and F)Kaplan-Meier survival analysis of the discovery cohort (TCGA-LIHC) stratified by MR subtypes. G Association between MR subtypes and clinical features, along with their correlation with established classification systems. Missing values are represented by white areas. Statistical significance: ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001
Data resource and processing
Samples were enrolled using the following criteria for further analysis: (1) primary hepatocellular carcinoma; (2) untreated; (3) cohorts with survival data. A total of 929 HCC samples and 110 normal liver samples were systematically collected. The corresponding expression profile and clinical data were downloaded from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov), Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo), International Cancer Genome Consortium (ICGC, https://dcc.icgc.org/) and Genotype-Tissue Expression (GTEx, https://www.gtexportal.org/) databases. The FPKM normalized data from TCGA-LIHC and ICGC-LIRI datasets were further converted to log2(TPM + 1). GSE109211 and GSE104580 were employed to evaluate the response to sorafenib and tra0000nsarterial chemoembolization (TACE) of each subtype. Two immunotherapy datasets treated with anti-PD-1 therapy (GSE126044 and GSE136961) were enrolled to predict immunotherapy response. More details on the sample size, sources, and clinical data of the cohorts could be accessed in Supplementary Tables 1 and 2.
Discovery of HCC metabolic reprogramming (MR) subtypes by consensus clustering
Consensus clustering algorithm was implemented bias ConsensusClusterPlus package [12]. The top 6000 edges with the largest standard deviation in the tumor samples were also selected to clearly distinguish tumor samples and highlight the heterogeneity of tumor samples. The intersection of these two edge sets included 1262 edges was utilized to perform co0nsensus clustering. The optimal clustering number was determined through the consensus matrix, the proportion of ambiguous clustering (PAC) score, and principal component analysis (PCA). The stability of the metabolic subtype was verified through the Nearest Template Prediction (NTP) algorithm [13]. The classification was identified based on the similarity of its signature gene expression pattern to each subtype (Supplementary Table 3). FDR < 0.2 were deemed favorably classified [14].
Biological behavior in three MR subtypes
The gene set variation analysis (GSVA) and single sample gene set enrichment analysis (ssGSEA) were performed. 84 metabolic pathways and 42 tumoral biological behavior genesets were accessed from the KEGG database and MSigDB database, respectively (Supplementary Tables 4–5). To reveal the tumor immune microenvironment in metabolic subtypes, the infiltration levels of 28 immune cells and the expression of 27 immune checkpoints derived from the TNF superfamily, B7-CD28 family were calculated by the ssGSEA algorithm in the TCGA-LIHC cohort [15]. Additionally, eight previously reported immune indicators were employed to assess the immunogenicity of MR subtypes [16]. Considering the discrepancy of immune landscapes among metabolic subtypes, the immunotherapy response performance of different subtypes was evaluated. Tumor inflammation signature (TIS), antigen processing and presenting scores (APS) were classic immune response evaluation indicators and systematically collected from previous studies for immunotherapy evaluation in diverse subtypes [17–19].
Statistical analysis
R 4.2.0 software was utilized to process, statistically analyze and visualize data. Kaplan-Meier survival analysis was utilized to demonstrate the survival rate and perform the log-rank test. Differences between categorical variables were identified by the chi-square test or Fisher’s exact test. Wilcoxon rank-sum test or T-test was used to compare continuous variables. Two-tailed P < 0.05 was deemed statistically significant.
Results
Metabolism-related edge-perturbation networks
To measure the gene interaction perturbations in the metabolic biological network, the expression data of 369 tumor samples and 110 normal liver tissue together with a metabolism-related background network consisting of 20,930 interactions involving 944 genes were systematically collected. In line with the pipeline mentioned previously, genes and their interactions in metabolic biological networks were systematically integrated into an interaction perturbation network with 20,020 interactions and 913 genes (Fig. 1A, Supplementary Table 6). Intriguingly, the distribution of 1000 randomly selected interactions in the tumor group displayed more variable and loosely compared to the normal group, indicating more intense gene interaction perturbations in tumor tissues (Fig S1A–B).
Metabolic gene interaction perturbations unveiled three metabolic reprogramming subtypes
Considering the broad variation in interactions among tumor samples, the metabolic patterns might change widely in HCC patients with different clinical courses. Indeed, 1262 features were finally recognized when overlapping the top 6000 differential edges between tumor and normal samples and the top 6000 differential edges in tumor samples. Subsequently, the consensus clustering algorithm was performed based on this interaction perturbation matrix (Fig. 1A). Notably, PAC, PCA, and consensus matrix consistently revealed that K = 3 was the optimal clustering number (Fig. 1B–D). Hence, patients in the TCGA-LIHC dataset were classified into three MR subtypes termed MR1 (45.5%), MR2 (24.7%), and MR3 (29.8%). Meanwhile, the MR2 subtype possessed an adverse prognosis and the MR3 subtype portended the best prognosis (P < 0.05, Fig. 1E–F). The NTP algorithm validated the robustness and reproducibility of MR subtypes in other three HCC datasets from ICGC and GEO databases (GSE14520, GSE54236, and ICGC-LIRI) (Fig S2A–D). The patients in both GSE14520 and ICGC-LIRI cohorts displayed concordant prognosis with prior results (Fig S2E–F, H). The prognosis of three subtypes in the GSE54236 cohort remained stable only in short-term survival (Fig S2G). Of interest, MR2 exhibited a much lower rate of response to TACE (Fig S2I). It was noticed that stage, grade, and pT were lower in the MR3 subtype, while reversed results were observed in the MR2 subtype (P < 0.001) (Fig. 1G). To decode the relationship between our metabolic subtypes and published HCC subtypes, eight published subtypes (Supplementary Table 7) were retrieved from previous studies including TCGA [20], Lee et al., [21, 22] Cairo et al. [23], Hoshida et al. [24], Sohn et al. [25], Woo et al. [26], and Thorsson et al. [16]. Intriguingly, a molecular convergence was portended due to the significant correlation between published HCC subtypes and the MR subtypes. The MR1 subtype was associated with iClust3, Lee subclass A/B, Lee HC, Cairo C1, Hoshida S2/3, Sohn AH, Woo HCC, and Thorsson C3/4 mainly. The MR2 subtype corresponded to more aggressive subtypes such as iClust1, Lee subclass A, Lee HB, Cairo C2, Hoshida S2, Sohn SOH, Woo CCL, and Thorsson all four immune subtypes. The MR3 subtype was correlated to subtypes with favorable prognosis including iClust2, Lee subclass B, Lee HC, Cairo C1, Hoshida S1/3, Sohn AH, Woo HCC, and Thorsson C3/4 mainly.
MR2 displayed hypometabolic, immune-activated phenotype
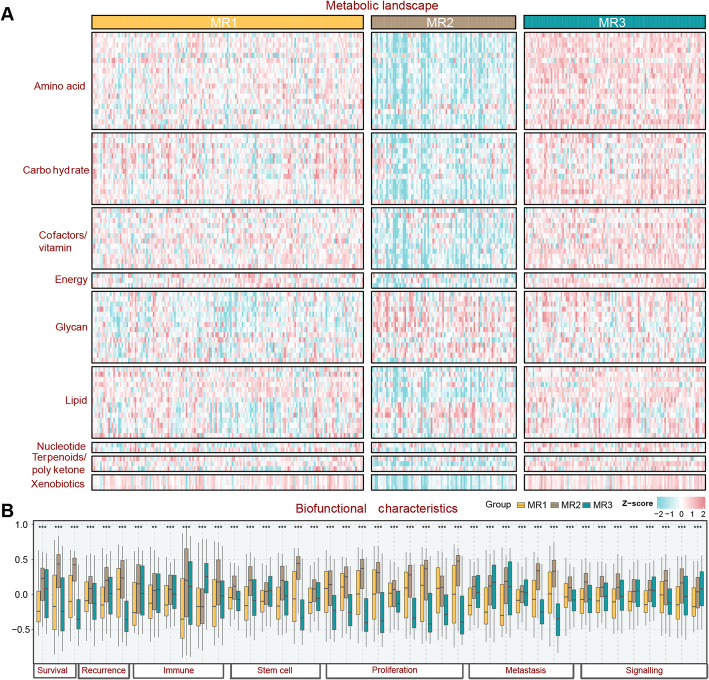
The metabolic landscape demonstrated a considerable difference among the three subtypes. The MR3 subtype displayed the highest metabolic activity, whereas MR2 portended the lowest level in almost all metabolic pathways, and the MR1 subtype was endowed with intermediate metabolic activity (Fig. 2A). Notably, MR2 stood out in glycan metabolism, which was closely related to the progression of HCC [27].Afterward, analysis of tumor biological behavior revealed the aggressiveness of MR2 subtype, together with the activated immune microenvironment. Down-regulated proliferation-related pathways were significantly enriched in MR3, and MR1 was characterized by low levels of tumor signaling-related pathways (Fig. 2B). Altogether, MR2 was prone to be a hypometabolic, immune-activated phenotype, which might facilitate advanced tumor, MR3 exhibited a hypermetabolic, non-proliferative phenotype, and the MR1 subtype was defined as a signaling-suppressed phenotype.
Fig. 2.
Biofunctional characteristics of metabolic reprogramming (MR) subtypes. A Global metabolic landscape across MR subtypes, highlighting distinct metabolic profiles. B Boxplot illustrating differences in tumor biological behaviors among MR subtypes
Immune characteristics and therapeutic implication for MR2 subtype
To further decipher immune characteristics among MR subtypes, immune landscapes containing immune cell infiltration, immune molecules, and signatures were profiled. The MR1 subtype exhibited an “immune-deficiency” feature with low infiltration levels, especially in immune cells characterizing immune responses such as CD8 + T cells, natural killer cells, macrophages (Fig. 3A). On the contrary, the infiltration levels in most immune cells were raised obviously in MR2. The MR3 subtype manifested an intermediate immune infiltration, characterized by high infiltration levels of innate immune cells such as dendritic cells, natural killer cells, neutrophils, and macrophages. These results were further validated by the ESTIMATE, TIMER, quanTIseq, MCP-counter, and EPIC algorithms (Fig. 3A). Notably, the majority of immune checkpoint genes were significantly overexpressed in the MR2 subtype, especially PDCD1 (PD-1) and CTLA4 (Fig. 3B). Overall, MR2 was characterized as an “immune-activated” subtype with favorable infiltration, stronger immunogenicity, and better potential benefit from immunotherapy [28]. As a diverse immune landscape in MRs, immunotherapy efficacy was explored. The higher TIS and APS in MR2 gave us a clue that more benefits from immunotherapy (Fig. 3C, D) [17, 29]. Afterward, the immunogram revealed an immune-active microenvironment for MR2 and an immuno-suppressive microenvironment for MR1 (Fig. 3E). Besides, the CTNNB1 mutation, which reduced the likelihood of immunotherapy benefit, displayed the lowest percentage in MR2 (Fig. 3F) [30]. Consistently, patients in the MR2 subtype displayed effectiveness against the anti-PD-1 therapy (Fig. 3G). Altogether, the above results provided a place to hint at distinct immunotherapy responses.
Fig. 3.
Heterogeneity of the immune landscape across metabolic reprogramming (MR) subtypes. A Heatmap of immune infiltration levels in three MR subtypes, assessed using ssGSEA and five deconvolution algorithms (ESTIMATE, TIMER, quanTIseq, MCP-counter, and EPIC). B Differential expression of immune checkpoint genes among the three MR subtypes. C and D Boxplots comparing tumor inflammation signature (TIS) and antigen processing and presentation (APS) scores across MR subtypes. E Prevalence of CTNNB1 mutations in the three MR subtypes. F Radar plot displaying the expression patterns of 12 immune-related gene signatures in MR subtypes. G SubMap analysis evaluating expression similarities between MR subtypes and patients with different immunotherapy responses. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Underlying molecular mechanisms of MR subtypes
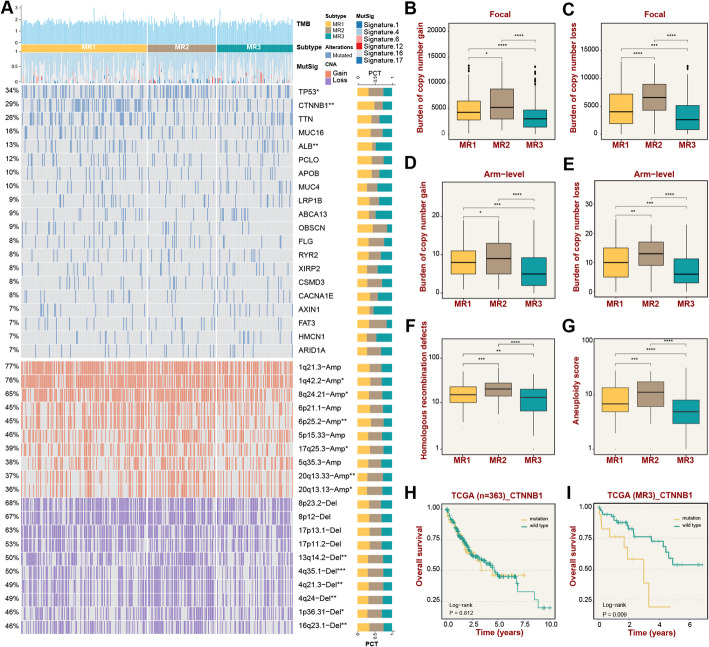
To clarify the potential molecular mechanisms of MR subtypes, multiple genome variations data were comprehensively collected to delineate the multi-omics landscape (Fig. 4A). Firstly, the top 20 frequently mutated genes were investigated, including TP53, CTNNB1, and ALB, which displayed evident differences among the three subtypes. MR2 subtype enriched TP53 mutation, which might drive the dismal prognosis [31]. The highest mutation frequency of CTNNB1 in MR1 subtype portended a worse immune infiltration [32]. Of note, the majority of the top 10 significant CNVs were clustered in the MR2 subtype, revealing its evident genomic instability. The homologous recombination defects, aneuploidy scores, and the total amount of copy number variants for each subtype at the arm and focal levels validated the genomic instability of the MR2 subtype in terms of overall level (Fig. 4B–G). Notably, although CTNNB1 mutation did not affect the prognosis of all patients in the TCGA cohort, the overall survival of MR3 subtype was reduced in patients enriched with CTNNB1 mutation (Fig. 4H–I). In short, the MR2 subtype displayed evident genomic instability, and CTNNB1 mutation could serve as a momentous factor affecting the prognosis of patients in MR3 subtype.
Fig. 4.
Multi-omics landscape of metabolic reprogramming (MR) subtypes in LIHC. A Landscapes of frequently mutated genes and recurrent copy number variations (CNVs), including amplifications and deletions, across the three MR subtypes. B–E CNVs analyzed at both local and chromosomal arm levels. F–G Boxplots illustrating differences in homologous recombination deficiency (HRD) and aneuploidy scores among the three MR subtypes. H and I Kaplan-Meier survival analysis comparing CTNNB1-mutated and wild-type cases within the MR3 subtype and across all three MR subtypes. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Identification of biomarkers for MR2 subtype
Due to the prognostic value of the MR2 subtype in HCC and its favorable response to immunotherapy, we further identified biomarkers to facilitate clinical application. In the TCGA-LIHC cohort, eight genes highly expressed in MR2 were identified (Fig. 5A), among which CD24 and SPHK1 were risk factors associated with poor prognosis (Fig. 5B). Notably, CD24 was significantly overexpressed in MR2 compared to MR1 and MR3, and patients with high CD24 expression showed markedly worse prognosis. To further validate the robustness of CD24 as a characteristic gene for MR2, we analyzed its differential expression, AUC, and prognostic significance across the HCC cohorts used above. Consistent results were observed in the GSE14520 (Fig. 5E–G), GSE54236 (Fig. 5H–J), and ICGC-LIRI (Fig. 5K–M) cohorts. CD24 expression was highest in MR2, with the AUC for identifying MR2 being 0.74, 0.78, and 0.77, respectively, in these cohorts. Most importantly, the prognostic value of CD24 was validated across all cohorts, with patients exhibiting high CD24 expression showing poorer outcomes. In summary, we identified a characteristic gene, CD24, for MR2 that can be used for prognostic evaluation of this subtype.
Fig. 5.
Identification and validation of CD24 as a novel biomarker for the MR2 subtype in HCC. A Volcano plot displaying differential gene expression in the MR2 subtype compared to MR1 and MR3 in the TCGA-LIHC cohort. Eight genes significantly upregulated in MR2 are highlighted. B Hazard ratio analysis of the identified genes in MR2, revealing CD24 and SPHK1 as risk factors for poor prognosis. C Violin plot depicting the expression levels of CD24 across the three MR subtypes (MR1, MR2, MR3) in the TCGA-LIHC cohort. D Kaplan-Meier survival curve showing overall survival of patients stratified by high or low CD24 expression in the TCGA-LIHC cohort. E–G Validation of CD24 expression differences in the GSE14520 cohort, with corresponding ROC curve (AUC = 0.74) and survival analysis. H–J Validation of CD24 expression differences in the ICGC-LIHC cohort, with ROC curve (AUC = 0.78) and survival analysis. K–M Validation of CD24 expression differences in the GSE54236 cohort, with ROC curve (AUC = 0.77) and survival analysis
Discussion
As a highly heterogeneous tumor, broad variation in metabolic patterns portended distinct prognoses in patients with hepatocellular carcinoma [8, 10, 33, 34]. The prior studies investigated the metabolic heterogeneity of HCC mainly based on the gene expression profiles [35, 36], ignoring the gene interactions. Indeed, cellular expression profiles varied broadly under different conditions, whereas gene interactions in biological networks remained relatively constant across time and conditions. Meanwhile, gene interactions were characterized by consistency in normal tissues and heterogeneity in disordered tissues, making it preferable to represent the state of the organism in HCC [36, 37]. Consequently, we constructed a metabolic gene interaction perturbation network, and three metabolic reprogramming subtypes with distinct prognosis and biological characteristics were deciphered.
Distinct clinical and biological features clearly distinguished three MR subtypes. The MR1 subtype occupied the largest proportion of HCC patients and exhibited moderate prognosis and metabolic levels. Moreover, it was observed that all tumor signaling pathways were inhibited in MR1, which might partially explain its general prognosis [38]. MR2 subtype possessed advanced clinical stage and histological grade, lowest metabolic level, and aggressive tumor behaviors. The prior study reported that metabolic levels in HCC were significantly associated with prognosis, with the low metabolic level corresponding to the worst prognosis [34]. In addition, the stronger stemness and proliferative properties also had profound impacts on the tumor phenotype and prognosis of MR2 [39]. Consistent with the best clinical outcome, and lower clinical stage and histological grade, the MR3 subtype was endowed with the highest metabolic level and significantly down-regulated proliferation-related pathways [34].
The multi-omics landscape revealed the intensive genome instability of MR2. Consistent with the previously described poor clinical features, TP53 mutation, which was connected to a terrible prognosis, exhibited the highest frequency in MR2 [31]. Furthermore, multiple genomic variation characteristics such as copy number variations, homologous recombination defects, and aneuploidy scores proved that MR2 possessed intense genomic instability. CTNNB1 mutations were found to be enriched in the MR1 subtype. The previous study had defined Wnt/CTNNB1-mutated liver cancer as “cold tumors” with innate resistance to immune checkpoint inhibitors [32], explaining the non-responsiveness of MR1 in immune response prediction. The frequently mutated gene in MR3 was ALB, which was proposed to genetically regulate the metabolic reprogramming in HCC [20].Notably, CTNNB1 appeared to be a momentous factor for the prognosis of MR3. Nevertheless, the specific intrinsic link between genomic variations and prognosis remained to be further demonstrated.
MR2 is a preferable candidate for immunotherapy. MR2 was characterized by higher levels of immune infiltration and immune checkpoint gene expression, and stronger immunogenicity, indicating its potential benefit from immunotherapy [17, 40]. MR2 may exhibit certain immune evasion characteristics through the high expression of immune checkpoint molecules and inflammatory factors (such as IL-6, TNF-α, etc.) [41].These inflammatory factors not only promote the formation of an immunosuppressive microenvironment but may also further inhibit T cell function by activating immune checkpoints. Immune therapeutic strategies, such as immune checkpoint inhibitors, can effectively enhance its immune response, restore T cell activity, and thereby strengthen the ability to combat tumors [42]. The MR2 demonstrates its immune activity potential through the high expression of immune checkpoint molecules and immune cell infiltration, which may be the main reason for its sensitivity to immunotherapy. These results were further validated in two immunotherapy cohorts with anti-PD-1 therapy that worked on the MR2 subtype. Altogether, our data suggested that MR2 as an “immune-activated” subtype might benefit more from immunotherapy.
In this study, CD24 was identified as a characteristic gene for the MR2 subtype in HCC, with significant implications for prognosis and clinical application. CD24 is highly expressed in various tumors and has been identified as being associated with poor prognostic phenotypes, such as in colorectal cancer [43], bladder cancer [44], and pancreatic cancer [45]. In HCC, studies have shown that high expression of CD24 may be associated with the cytoplasmic and nuclear accumulation of β-catenin, indicating activation of the Wnt/β-catenin pathway, which in turn promotes liver cancer progression and tumor chemoresistance [46, 47]. Furthermore, as a key “don’t eat me” signal molecule, CD24 has emerged as an important immunotherapy target, warranting further investigation in the context of liver cancer [48]. Overall, the identification of CD24 as a hallmark of the MR2 subtype provides a foundation for further research into its biological role and potential as a therapeutic target, potentially paving the way for subtype-specific interventions in HCC. Future studies should focus on elucidating the molecular mechanisms linking CD24 to the aggressive and immune-activated phenotype of MR2 and evaluating its therapeutic targeting in preclinical and clinical settings.
Limitations
Our study shed light on the heterogeneity of the metabolic landscape in HCC patients from an interaction perspective. Since this study was conducted retrospectively, a multicenter prospective study is still needed to further validate our classification of HCC and the feasibility of CD24 as a marker for the MR2 subtype.
Conclusions
Grounded on the metabolic gene interaction perturbation network, we developed three MR subtypes with distinct metabolic patterns, prognostic, clinical, and molecular features. Our work represented an important stride forward in the understanding of the metabolic heterogeneity of HCC, and might provide a promising platform for clinical stratification and targeted therapy of HCC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
H. L., H. Y., and Y. H. conceived the research line. H. L. and H. Y. designed the research methodology. H. Y. and H. X. wrote the main manuscript text. H. Y., Y. Z., and H. X. visualized data and results. L. L. solved software problems and verified results. Y. H., X. M. and Y. H. reviewed the manuscript.
Funding
Not applicable.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Liu and Hongyi Yan have contributed equally to this work and share senior authorship.
References
- 1.Duran SR, Jaquiss RDB. Hepatocellular Carcinoma New Engl J Med. 2019;381(1):e2. [DOI] [PubMed] [Google Scholar]
- 2.Vogel A, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. [DOI] [PubMed] [Google Scholar]
- 3.Reig M, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, et al. Identification and characterization of robust hepatocellular carcinoma prognostic subtypes based on an integrative Metabolite-Protein interaction network. Adv Sci (Weinheim Baden-Wurttemberg Germany). 2021;8(17):e2100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahni N, et al. Widespread macromolecular interaction perturbations in human genetic disorders. Cell. 2015;161(3):647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, et al. A rank-based algorithm of differential expression analysis for small cell line data with statistical control. Brief Bioinform. 2019;20(2):482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y et al. Sample-specific perturbation of gene interactions identifies breast cancer subtypes. Brief Bioinform, 2021. 22(4). [DOI] [PMC free article] [PubMed]
- 8.Bao MH-R, Wong CC-L. Hypoxia, metabolic reprogramming, and drug resistance in liver Cancer. Cells, 2021. 10(7). [DOI] [PMC free article] [PubMed]
- 9.Du D, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sinica B. 2022;12(2):558–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia L, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidkhori G, et al. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc Natl Acad Sci USA. 2018;115(50):E11874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinf (Oxford England). 2010;26(12):1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshida Y. Nearest template prediction: a single-sample-based flexible class prediction with confidence assessment. PLoS ONE. 2010;5(11):e15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, et al. A tumor microenvironment-specific gene expression signature predicts chemotherapy resistance in colorectal cancer patients. NPJ Precision Oncol. 2021;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng S, et al. ALOX12: A novel insight in bevacizumab response, immunotherapy effect, and prognosis of colorectal Cancer. Front Immunol. 2022;13:910582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorsson V et al. The immune landscape of Cancer. Immunity, 2018. 48(4). [DOI] [PMC free article] [PubMed]
- 17.Danaher P, et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from the Cancer genome atlas (TCGA). J Immunother Cancer. 2018;6(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312. [DOI] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Oncology Meets immunology: the cancer-immunity cycle. Immunity, 2013. 39(1). [DOI] [PubMed]
- 20.Comprehensive. and Integrative genomic characterization of hepatocellular carcinoma. Cell, 2017. 169(7). [DOI] [PMC free article] [PubMed]
- 21.Lee J-S, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology (Baltimore MD). 2004;40(3):667–76. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-S, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12(4):410–6. [DOI] [PubMed] [Google Scholar]
- 23.Cairo S, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14(6):471–84. [DOI] [PubMed] [Google Scholar]
- 24.Hoshida Y, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69(18):7385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn BH, et al. Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Research: Official J Am Association Cancer Res. 2016;22(5):1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo HG, et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res. 2010;70(8):3034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu M, et al. The role of N-glycosylation modification in the pathogenesis of liver cancer. Cell Death Dis. 2023;14(3):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. [DOI] [PubMed] [Google Scholar]
- 29.Wang S et al. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. ELife, 2019. 8. [DOI] [PMC free article] [PubMed]
- 30.Chen L, et al. CTNNB1 alternation is a potential biomarker for immunotherapy prognosis in patients with hepatocellular carcinoma. Front Immunol. 2021;12:759565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khemlina G, Ikeda S, Kurzrock R. The biology of hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinyol R, Sia D, Llovet JM. Immune Exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Research: Official J Am Association Cancer Res. 2019;25(7):2021–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinciguerra M, et al. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50(6):1132–41. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, et al. Metabolism-associated molecular classification of hepatocellular carcinoma. Mol Oncol. 2020;14(4):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderaro J, et al. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71(3):616–30. [DOI] [PubMed] [Google Scholar]
- 36.Dai H, et al. Cell-specific network constructed by single-cell RNA sequencing data. Nucleic Acids Res. 2019;47(11):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, et al. Personalized characterization of diseases using sample-specific networks. Nucleic Acids Res. 2016;44(22):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Lezana T, Lopez-Canovas JL, Villanueva A. Signaling pathways in hepatocellular carcinoma. Adv Cancer Res, 2021. 149. [DOI] [PubMed]
- 39.Liang N, et al. Mechanism of cancer stemness maintenance in human liver cancer. Cell Death Dis. 2022;13(4):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu F-F et al. Expression profile of immune checkpoint genes and their roles in predicting immunotherapy response. Brief Bioinform, 2021. 22(3). [DOI] [PubMed]
- 41.Xu H, et al. Immune perturbation network identifies an EMT subtype with chromosomal instability and tumor immune-desert microenvironment. iScience. 2023;26(10):107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, et al. A novel immune classification reveals distinct immune escape mechanism and genomic alterations: implications for immunotherapy in hepatocellular carcinoma. J Transl Med. 2021;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J, et al. An integrated magneto-electrochemical device for the rapid profiling of tumour extracellular vesicles from blood plasma. Nat Biomed Eng. 2021;5(7):678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal N, et al. GON4L drives Cancer growth through a YY1-Androgen Receptor-CD24 Axis. Cancer Res. 2016;76(17):5175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen HD, Lin CC. Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids. Acta Biomater. 2024;177:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, et al. Attenuated Listeria monocytogenes as a cancer vaccine vector for the delivery of CD24, a biomarker for hepatic cancer stem cells. Cell Mol Immunol. 2014;11(2):184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, et al. MiR-125b loss activated HIF1α/pAKT loop, leading to transarterial chemoembolization resistance in hepatocellular carcinoma. Hepatology. 2021;73(4):1381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkal AA, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.