Abstract
We have developed a microtiter plate method for screening a large number of bacterial isolates for the ability to grow on different crystalline polycyclic aromatic hydrocarbons (PAHs). Growth on PAHs cannot easily be determined with standard growth assays because of the very low aqueous solubility and bioavailability of the PAHs. Our microtiter plate assay utilizes a new water-soluble respiration indicator, WST-1 {4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate}, in combination with easily degradable carbon sources. PAH-mineralizing strains were grown on PAHs in microtiter plates for 7 to 10 days. The tetrazolium dye WST-1 was added after incubation. Dehydrogenases in growing cells reduced WST-1 to a water-soluble colored formazan, and the intensity of the color was a measure of the respiration rate. Addition of easily degradable carbon to the wells along with WST-1 resulted in a 3- to 40-fold increase in the absorbance of positive wells within 90 min, which made it possible to detect growth on fluorene, phenanthrene, anthracene, fluoranthene, and pyrene. Addition of the electron transport blocker sodium azide unexpectedly decreased formazan formation. The method was adapted for most-probable-number enumeration of PAH degraders in soil.
Polycyclic aromatic hydrocarbons (PAHs) are considered ubiquitous pollutants of the environment in industrialized areas. Microbial degradation of PAHs has received much attention as a possible strategy for bioremediation of PAH-contaminated soils. Numerous bacterial strains able to grow on three- and four-ring PAHs have been isolated (3, 9, 12, 18). Assessing the range of different PAHs mineralized by a specific strain is desirable in the characterization of such bacterial isolates. Also, when the degradative community of an environmental sample is described, enumeration of the cells degrading specific PAHs is important.
Growth on various PAHs as sole sources of carbon and energy cannot easily be determined with standard growth assays because of the low aqueous solubility and bioavailability of the PAHs, which lead to slow bacterial growth and low cell yields. Traditionally, degradation tests have been done in three ways: (i) by mineralization of radioactive tracers (26), (ii) by formation of clearing zones around colonies growing on agar plates covered with an opaque layer of PAH crystals (12, 15), and (iii) by detection of accumulated colored PAH metabolites (23, 27). In this paper we present a new and sensitive microtiter plate method for detection of bacterial growth on crystalline PAHs. The method is based on respiratory reduction of the tetrazolium salt WST-1 {4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate} to a colored formazan by active cells (10). Tetrazolium salts, like WST-1, serve as indicators of dehydrogenase activity as they shift color upon reduction by electrons flowing through the electron transport system and by superoxide radicals produced (20). The principle of our method is as follows. Microtiter plate wells containing mineral salt medium and crystals of a PAH are inoculated with a dilute suspension of the strain to be tested. The cells proliferate only in wells amended with the PAHs which they can metabolize. After incubation, the metabolic potential of the cells in each well is assayed by adding WST-1 to the wells. We show in this paper that it was possible to boost the flow of electrons, and thus the amount of WST-1 reduced, by adding electron donors (easily degradable carbon) along with WST-1. In this way, the sensitivity of the assay was increased 3- to 40-fold, and the detection limit was lowered accordingly.
Addition of electron transport blockers reduces the flow of electrons to the terminal electron acceptor O2. With some tetrazolium salts, this dramatically increases the production of colored formazans (21). Addition of electron transport blockers may increase the signal-to-noise ratio in assays in which tetrazolium salts are used as growth indicators. We evaluated the effect of the electron transport blocker sodium azide on the reduction of WST-1 by PAH degraders. We also evaluated the applicability of the method for most-probable-number (MPN) enumeration of PAH degraders in soil.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals were analytical grade. The purity of each of the unlabeled PAHs was 98% or better. Radiochemicals were obtained from the following sources: [9-14C]phenanthrene (>98% pure), [3-14C]fluoranthene (>95% pure), and [9-14C]fluorene (98% pure) were obtained from Sigma-Aldrich, and [4,5,9,10-14C]pyrene (95% pure) and [side ring-U-14C]anthracene (89% pure) were obtained from Amersham. Cell proliferation reagent WST-1 was obtained from Roche Molecular Biochemicals, Mannheim, Germany. Sterile, 96-well, flat-bottom microtiter plates were obtained from Nalge Nunc International, Roskilde, Denmark (product no. 269787).
Bacterial strains and culture media.
Sphingomonas sp. strains LH128 and LB126 and Mycobacterium sp. strain LB501T were obtained from L. Bastiaens (3). Mycobacterium sp. strain VM455, Mycobacterium gilvum VM552, and Nocardia asteroides VM451 were obtained from D. Springael. Sphingomonas sp. strain EPA505 (17) and Burkholderia cepacia CRE7 (18) were obtained from P. H. Pritchard. All strains were stored in 40% (vol/vol) glycerol at −80°C. Each inoculum was grown to the late exponential phase at room temperature in phosphate minimal medium (PMM) supplemented with glucose and glycerol (1 g liter−1) as the sole carbon sources (11).
Growth on PAH crystals in microtiter plates.
The PAHs fluorene, phenanthrene, fluoranthene, and pyrene were dissolved in hexane at a concentration of 5 mg ml−1. Anthracene crystals were added to hexane to give a saturated solution. In most cases 20 μl of a PAH solution was added to each microtiter plate well; for anthracene 40 μl was added twice. The hexane was evaporated in a sterile flow cabinet, which resulted in a coat of PAH crystals on the well walls. Wells containing only hexane were used as controls. Two hundred microliters of PMM was added to the wells. Each inoculum was washed once in PMM and diluted to optical density at 540 nm of 0.4 with PMM. Then 10 μl of the diluted inoculum was added to the wells. One microliter of the inoculum was streaked on tryptic soy broth (Difco, Detroit, Mich.) solidified with 15 g of agar per liter to check for contamination. The microtiter plates were wrapped in plastic bags and incubated for 7 or 10 days at room temperature (20 to 22°C) in a fume hood. All treatments were done in quadruplicate. To minimize edge effects, wells at the edges were filled with PMM during incubation and not used for WST-1 assays.
Respiration measurement with WST-1.
Cells were grown on PAHs in mirotiter plates as described above. An electron donor (e-donor) solution was prepared by dissolving glucose, pyruvate, and succinate (16.6 mM each) in Tris buffer (40 mM) and adjusting the pH to 6.5 for maximum reduction of WST-1 (19). Fifty microliters of the e-donor solution was added to the microtiter plate wells along with 10 μl of WST-1 reagent. The plates were incubated on a shaker table (300 rpm) at room temperature (20 to 22°C) for 3 h (pure cultures) or 5 h (MPN enumerations). Absorbance was measured with a microplate autoreader (Bio-Tek Instruments EL311; Struers, Viby, Denmark) at 450 nm with a reference wavelength of 630 nm immediately after the addition of WST-1 and again after 90 min (pure cultures) or 5 h (MPN enumerations). The use of a reference wavelength reduced the noise from PAH crystals and soil particles considerably. The absorbance at time zero was subtracted from the subsequent readings to obtain the change in absorbance.
Effects of various electron donors and azide on the reduction of WST-1.
Four strains were grown on PAHs for 7 days in microtiter plates as described above. Sphingomonas sp. strain EPA505 was grown on fluoranthene, Sphingomonas sp. strain LH128 was grown on phenanthrene, M. gilvum VM552 was grown on pyrene, and Mycobacterium sp. strain LB501T was grown on anthracene. The number of CFU added to the wells was determined by drop plating the inoculum on Luria-Bertani medium plates. The effects on WST-1 reduction of the e-donors salicylate, glucose, succinate, and pyruvate, individually (50 mM) or in combination (16.6 mM each), were examined. The e-donors were dissolved in Tris buffer (40 mM), and the pH was adjusted to 6.5. The WST-1 respiration assay was done as described above by using the five different e-donor solutions. Wells with no e-donors (Tris buffer only) served as controls for the effects of e-donors. Other controls included wells with no PAH but with the combined e-donors to test cell survival without PAH and wells without bacteria but with the combined e-donors to test the nonbiological transformation of WST-1. All treatments were done with and without addition of sodium azide (final concentration, 50 mM [21]) to test the effect of electron transport blockage.
Comparison of WST-1 reduction and 14C-PAH mineralization.
Five PAH-degrading strains (Table 1) were tested for respiration of fluorene, phenanthrene, anthracene, fluoranthene, and pyrene by using the WST-1 microtiter plate method as described above and a 14CO2 production assay. Glucose, succinate, and pyruvate (in combination, 16.6 mM each) were used as e-donors in the WST-1 assay. Fluorene was applied to separate plates to avoid cross-contamination of the wells through the gas phase. Negative controls without PAHs were included. The plates were incubated for 10 days before WST-1 and e-donors were applied. Changes in absorbance were measured after 90 min of incubation with WST-1 and e-donors. Strains were scored positive when the absorbance changes were significantly larger than those of controls without PAH (one-tailed t test). Mineralization of 14C-labeled fluorene, phenanthrene, anthracene, fluoranthene, and pyrene was measured in parallel experiments by using the same inoculum as that used in the WST-1 assays. One-milligram portions of radioactively labeled PAHs (600 to 1,000 Bq) in acetone solutions were added to sterile 250-ml Erlenmeyer flasks. The acetone was evaporated, and 50 ml of PMM was added to each flask to obtain a final PAH concentration of 20 mg liter−1; this was followed by 10 min of sonication at 140 W to increase the surface area of the PAH crystals. After inoculation, the flasks were sealed with sterile silicone stoppers and incubated on a shaker table (150 rpm) at room temperature for 10 days. Noninoculated flasks were used as controls. The 14CO2 produced was collected in 400 μl of KOH in glass vials suspended from the silicone stoppers by steel hooks. The amount of 14CO2 was quantified by mixing the KOH with 1 ml of water and 1.5 ml of Ready Gel scintillation cocktail (Beckman). The samples were counted with an LS1801 Beckman scintillation counter. The counts for the controls were never above the background level. Only 14CO2 production exceeding the radiochemical impurity was considered significant.
TABLE 1.
Comparison of PAH mineralization tests when the WST-1 microtiter plate method and a 14C-PAH mineralization method were used
| Strain | No PAH: WST-1 absorbancea | Fluorene
|
Phenanthrene
|
Anthracene
|
Fluoranthene
|
Pyrene
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 14C %b | WST-1 absorbancea | 14C %b | WST-1 absorbancea | 14C %b | WST-1 absorbancea | 14C %b | WST-1 absorbancea | 14C %b | WST-1 absorbancea | ||
| EPA505 | 0.00 ± 0.00 | 0.1 | 0.00 ± 0.00 | 62.2 | 0.88 ± 0.25 | 11.1 | 0.00 ± 0.01 | 62.0 | 2.07 ± 0.24 | 0.2 | 0.00 ± 0.02 |
| LB126 | 0.00 ± 0.00 | 79.2 | 0.96 ± 0.13 | 0.0 | 0.00 ± 0.00 | 0.2 | 0.00 ± 0.00 | 0.0 | 0.00 ± 0.00 | 0.1 | 0.01 ± 0.00 |
| CRE7 | 0.00 ± 0.00 | 0.1 | 0.00 ± 0.00 | 13.4c | 0.00 ± 0.00c | 0.5 | 0.00 ± 0.00 | 0.0 | 0.00 ± 0.00 | 0.3 | 0.00 ± 0.02 |
| LB501T | 0.14 ± 0.01 | 0.2 | 0.27 ± 0.09 | 45.3 | 2.09 ± 0.11 | 46.4 | 2.19 ± 0.29 | 52.6 | 2.03 ± 0.09 | 0.3c | 2.02 ± 0.48c |
| VM451 | 0.10 ± 0.03 | 0.1 | 0.05 ± 0.01 | 44.1 | 1.36 ± 0.48 | 1.2 | 0.08 ± 0.01 | 44.7 | 1.40 ± 0.08 | 0.2c | 1.58 ± 0.19c |
| VM455 | 0.06 ± 0.02 | 0.1 | 0.05 ± 0.00 | 36.0 | 0.76 ± 0.23 | 4.4 | 0.05 ± 0.01 | 54.3 | 1.96 ± 0.06 | 51.3 | 1.38 ± 0.13 |
Change in WST-1 formazan absorbance measured after 90 min (average ± 1 standard deviation; n = 4).
Percentage of 14C-labeled PAH mineralized to 14CO2 (average of duplicate samples).
The results obtained with the two assays are not consistent.
Effect of inoculum size.
Sphingomonas sp. strain LH128 was grown in PMM amended with 200 mg of sterile phenanthrene per liter until the crystals disappeared (200 rpm, 8 days). From dilution series (10-fold to 10−4-fold and thereafter 5-fold) in PMM, four 200-μl portions of each dilution were transferred to microtiter plate wells with phenanthrene and to control wells without phenanthrene. The number of culturable cells in the inoculum was assayed by drop plating eight 15-μl portions of each dilution on 10% tryptic soy broth plates. Respiration was assayed with WST-1 plus glucose, succinate, and pyruvate after 7 days.
MPN enumeration of PAH-degrading bacteria in soil.
The two soil samples used in this experiment, B&W and Ringe, were collected in October from piles of PAH-polluted soil. Both samples originated from PAH-contaminated industrial sites in Denmark. The Ringe soil is polluted with hydrocarbons, and the B&W soil is polluted with mercury (14.6 mg kg−1 [dry weight]) and hydrocarbons.
The soils were sieved (4-mm mesh) and assayed in triplicate. Cells from 10 g (dry weight) of soil were extracted in 90 ml of tetrasodium pyrophosphate buffer (1.2 mM, pH 7.0) with a wrist shaker for 5 min, and large particles were sedimented for 5 min. Microtiter plate wells were coated with phenanthrene, fluoranthene, or pyrene as described above. Pure hexane was evaporated in negative control wells. Fourfold dilution series were prepared from the soil extracts, and six 200-μl portions of each dilution were transferred to each PAH plate (six-row MPN). A piece of wet filter paper was placed on top of each microtiter plate lid to reduce water loss from the wells. The plates were wrapped in plastic bags and incubated for 21 days at room temperature (20 to 22°C). The potential respiration of the wells was assayed with WST-1 plus glucose, succinate, and pyruvate for 5 h. The MPN was calculated by using a computer program developed by A. J. Klee (16). In parallel, the numbers of phenanthrene-, fluoranthene-, and pyrene-degrading bacteria were estimated by using a three-row radiotracer MPN approach. The 14C-PAH mineralization assay described above was scaled down to one-fifth scale (50-ml glass tubes, 10 ml of PMM containing 0.2 mg of radioactively labeled PAH corresponding to 120 to 200 Bq), but otherwise it was identical. The tubes were inoculated in triplicate with 200 μl of each soil dilution and incubated at room temperature. The amount of 14CO2 produced was determined after 3 weeks and then weekly. Only 14CO2 production exceeding the radiochemical purity was considered significant.
Protocols.
Complete protocols for MPN enumeration of PAH-degrading bacteria and determination of the PAH substrate specificity of pure cultures can be found at http://microtitermethods.dmu.dk.
RESULTS AND DISCUSSION
Tetrazolium salts like WST-1 are converted to colored formazans by all reducing systems possessing redox potentials more negative than that of the tetrazolium-formazan system (20). Our microtiter plate method is based on respiratory reduction of WST-1 to a colored formazan by active cells. Microtiter plate wells with mineral salt medium and crystals of different PAHs were inoculated with a dilute suspension of the strain to be tested. The cells proliferated in wells amended with PAHs that could be metabolized by that strain. After 7 to 10 days of incubation, the metabolic potential of each well was assayed by adding WST-1 to the wells. The metabolic activity of cells growing on PAHs was low, but addition of suitable electron donors (easily degradable carbon) along with WST-1 boosted the flow of electrons and thus the reduction of WST-1. We primarily used strains belonging to the genera Sphingomonas and Mycobacterium, since PAH degraders isolated from soil often belong to these genera (3, 9, 12, 18). These two genera represent very different growth habits; Sphingomonas strains are gram negative, fast growing, and hydrophilic, and Mycobacterium strains are gram positive, slow growing, and hydrophobic. The number of CFU added to each well ranged from 1 × 105 to 6 × 105; the mycobacteria generally showed higher numbers than the sphingomonads.
Effects of e-donors.
The e-donors tested in this study were chosen on the basis of their central role in the metabolism of some PAH-degrading bacteria or in the general metabolic cycles. Salicylic acid is an intermediate in the NAH-type catabolic pathways of some PAH-degrading bacteria (2, 6, 7, 22). Thus, all the enzymes needed for degradation of salicylate should already be present when these bacteria grow on PAHs, and induction of specific enzymes should not be needed. Glucose was chosen because all of the strains tested grow on glucose as a sole source of carbon and energy. The tricarboxylic acid cycle has major biosynthetic as well as energetic functions, and the complete cycle or major parts of it are nearly universal in microbes, with pyruvate and succinate playing a central role. Furthermore, succinate dehydrogenase is known to reduce the tetrazolium salts INT [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride] and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) in E. coli (21) and WST-1 in mitochondria (WST-1 product information sheet; Roche).
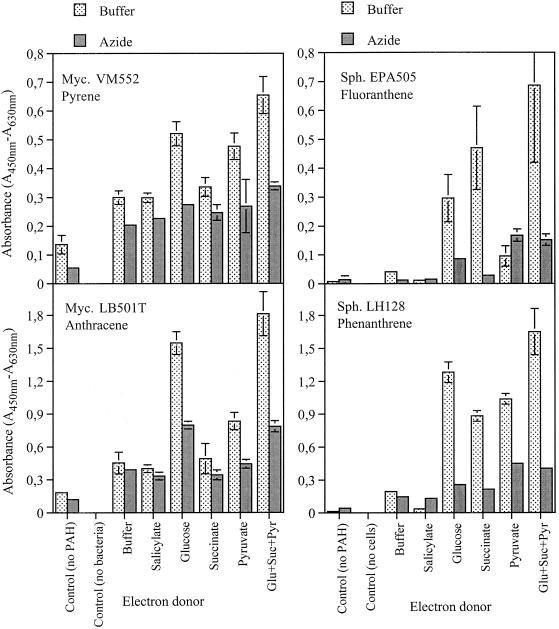
Reduction of WST-1 in the absence of electron donors was low (Fig. 1 and 2), suggesting that WST-1 alone was insufficient to demonstrate growth on PAHs. Glucose, succinate, and pyruvate all increased formazan production, but to different extents for the different strains (Fig. 1). Glucose and pyruvate increased the respiration of strain VM552, pyruvate and glucose in particular increased the respiration of strain LB501T, glucose and succinate increased the respiration of strain EPA505, and all three carbohydrates increased the respiration of strain LH128. Glucose, succinate, and pyruvate in combination resulted in the greatest response from all four strains, suggesting that there was concerted action of several dehydrogenases. Reduction of WST-1 was increased 3- to 40-fold by addition of the e-donors. The three e-donors in combination were used in all subsequent experiments. The method requires uptake and metabolism of at least one of the e-donors during the short incubation with WST-1. This may not be possible in all strains, and hence e-donors like ethanol, glycerol, or citrate may be more efficient when the abilities of some strains to grow on PAHs are assayed. Addition of salicylate did not increase WST-1 reduction, suggesting that salicylate either is not an intermediate in the mineralization pathways of the bacteria used (when they are grown on the specific PAHs examined), could not be taken up by the bacteria during the 3-h incubation, or was toxic at the concentration used.
FIG. 1.
Effects of various electron donors and azide on the respiratory reduction of WST-1 by mycobacteria (Myc.) and sphingomonads (Sph.) grown for 7 days in microtiter plates with PAHs as sole sources of carbon and energy. The plates were incubated with WST-1 for 90 min. The controls included wells with no PAH but with inoculum and the combined e-donors and wells without bacteria but with PAHs and the combined e-donors. The error bars represent ±1 standard deviation.
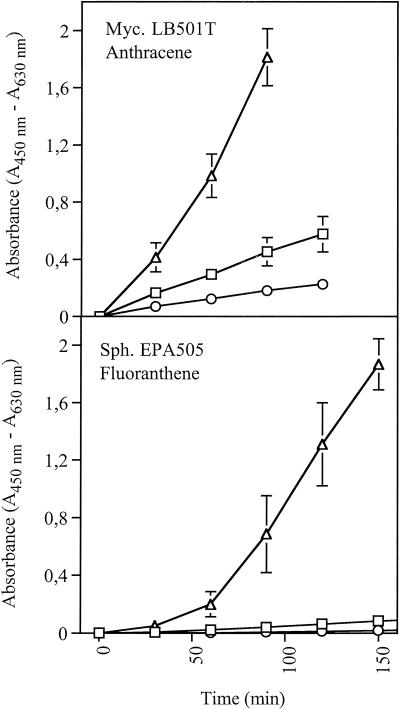
FIG. 2.
Time course experiment to determine WST-1 reduction in the presence and absence of the electron donors glucose, succinate, and pyruvate (16.6 mM each). Mycobacterium (Myc.) and Sphingomonas (Sph.) cells were grown in microtiter plates with PAHs as sole sources of carbon and energy. The error bars represent ±1 standard deviation. Symbols: ▵, PAH plus e-donors; □, PAH with no e-donors; ○, no PAH but with e-donors.
Time course plots of WST-1 reduction characteristic of sphingomonads and mycobacteria are shown in Fig. 2. Sphingomonads typically had a lag phase followed by a linear increase in WST-1 reduction. The values for negative controls with e-donors but without PAHs were close to the detection limit. Mycobacteria reduced WST-1 immediately and at an increasing rate. The values for negative controls with e-donors but without PAHs were quite high compared to the values for sphingomonads. The high levels of survival and activity of Mycobacterium cells starved for 7 days may have been due to cryptic growth on the energy-rich mycolic acid wax surrounding the cells. Cryptic growth is expected to decline over time, and increasing the incubation time from 7 to 10 days did result in lower control values for the mycobacteria (Table 1). For growth on crystalline PAHs, the respiration rate is determined primarily by the rate of dissolution of the PAHs. In the linear and quasi-stationary growth phases, this leads to a low and over time almost constant respiration rate (24), making the method robust for changes in the incubation time.
Azide.
In every case, WST-1 reduction was affected negatively by sodium azide (Fig 1). Addition of the electron transport blocker azide reduces the flow of electrons to the terminal electron acceptor O2. In the case of INT, this led to a 20-fold increase in the transformation of the tetrazolium salt by E. coli cells (21), probably because the electron transport chain was blocked after the site of INT reduction, leading to low competition between INT and O2 for electrons. In the case of CTC the tetrazolium reduction was decreased by azide (21). An electron transport mediator is present in the WST-1 reaction mixture (WST-1 product information sheet). The electron transport mediator catalyzes the direct transfer of electrons or hydrogen or both from NAD(P)H formed by dehydrogenases to the tetrazolium salt (20), circumventing the electron transport chain. This might reduce the effect of azide, but it should not lead to the observed negative effects. At present, no good explanation is available for the negative effects of azide on the cellular reduction of WST-1.
Comparison of WST-1 reduction and 14C-PAH mineralization.
We generally found good agreement between the WST-1 microtiter plate method and the mineralization of 14C-labeled PAHs in liquid shaken cultures (Table 1). Only in a few cases were the results inconsistent. Strain EPA505 mineralized 11.1% of [14C]anthracene, but this was not considered a positive result since the radiochemical purity of anthracene was 89%. Strain CRE7 was [14C]phenanthrene positive (13.4%) but WST-1 negative (0.00), which could be explained by (i) CRE7 cometabolizing phenanthrene without obtaining enough energy for growth, (ii) the microtiter plates not having been incubated for long enough, or (iii) CRE7 not taking up or metabolizing any of the external e-donors added along with WST-1. Strain VM451 was [14C]pyrene negative (0.2%) but WST-1 positive (1.58). Extending the incubation period to 16 days did not produce more 14CO2, so slow growth was not the reason. A possible explanation could be an incomplete pyrene mineralization pathway, leading to partial mineralization of the pyrene molecules. Pyrene was labeled at only two of the four rings ([4,5,9,10-14C]pyrene). Ring opening followed by mineralization of unlabeled carbon atoms may have yielded sufficient energy for proliferation of VM451. LB501T was [14C]pyrene negative (0.2%) but WST-1 positive. Extending the [14C]pyrene incubation period to 16 days gave a 14CO2 recovery value of 31.2%, suggesting that 10 days of incubation was insufficient. Growth on fluoranthene and pyrene has not previously been reported for LB501T.
Microtiter plates in combination with redox dyes are often used to detect microbial growth on various carbon sources (14). However, growth on PAHs as sole sources of carbon and energy cannot easily be determined with standard growth assays because of the low bioavailability of the PAHs, which leads to slow growth and low cell yields. The Biolog MT plate approach leads to false negatives because of the low growth rates, as well as to false positives when plates are incubated for a prolonged period to compensate for the slow growth (data not shown). Tetrazolium salts replace the final electron acceptor (oxygen) and reveal dehydrogenase activity and the flow of electrons through the electron transport chain (20). The Biolog MT system is based on the reduction of tetrazolium violet (4), which is a tetrazolium salt with low reducibility. Reduction of tetrazolium violet requires anaerobic conditions, as oxygen is the preferred electron acceptor (20). Since growth on crystalline PAHs is slow, ample oxygen may diffuse into the wells of the Biolog MT plates, preventing the maintenance of anaerobic conditions and thus the color change in positive wells. WST-1, on the other hand, is readily reduced in the presence of oxygen.
Toxic effects of some monoaromatic compounds (vanillin, phenol, chlorophenols, 5-chlorovanillin, and 3-chlorobenzoate) have been reported for the Biolog MT assay (8). In contrast, toxicity of three- and four-ring PAHs is generally not a problem as the aqueous solubilities of these compounds are 3 to 5 orders of magnitude lower.
Wrenn and Venosa (27) investigated the possible use of INT in microtiter plate MPN enumeration of PAH degraders but concluded that INT could not be used because it was poorly reduced in positive wells of PAH degrader MPN plates. We tested INT and CTC in combination with electron donors as described above for WST-1. INT reduction was estimated visually on the basis of formation of purple crystals in positive wells, but different persons examining the same plates came to very different conclusions (data not shown). Reduction of CTC in positive wells was measured with a microtiter plate fluorimeter, but replicates varied greatly and the fluorescence from the CTC-formazan was low, despite the formation of orange formazan crystals (data not shown). This agrees with the findings of Smith and McFeeters (21) showing that fluorescence from CTC-formazan crystals was depressed at redox potentials higher than −200 mV.
Effect of inoculum size.
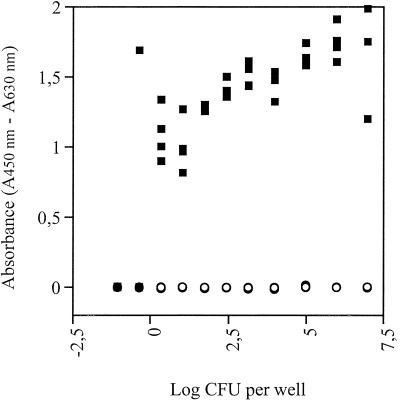
When the wells were inoculated with, on average, 2.22 cells per well, four of four wells were positive (Fig. 3). Inoculation with 0.44 cell per well resulted in one positive well out of four, and inoculation with 0.09 cell per well resulted in four negative wells. We concluded that approximately one culturable cell of Sphingomonas sp. strain LH128 per well is needed for a positive response. This suggested that the method may be suitable for MPN enumeration of PAH degraders.
FIG. 3.
Effect of inoculum size on the WST-1 assay for detection of growth on phenanthrene (▪). Negative controls were incubated without phenanthrene (○). The inoculum was Sphingomonas sp. strain LH128 grown on phenanthrene. All experiments were done in quadruplicate.
MPN enumeration of PAH-degrading bacteria in soil.
The microbial population tested must be able to bring about some readily recognizable transformation of the medium. The MPN technique is based on determination of the presence or absence of microorganisms in several individual portions of each of several consecutive dilutions of soil or other material (1). The observed changes in WST-1 absorbance after 5 h were smaller than the changes observed for pure cultures. The changes for positive phenanthrene wells were typically in the range from 0.200 to 1.500; the changes for positive fluoranthene wells ranged from 0.040 to 0.300; and the changes for positive pyrene wells ranged from 0.070 to 1.000. The threshold value for positive wells was set arbitrarily at 0.030. The values for controls without PAHs were always less than 0.015, showing that reducing substances from the soil and cryptic growth on dead organisms that did not degrade PAH were negligible. Small edge effects were observed, but they were acceptable as each well was only scored positive or negative. The low respiration in positive fluoranthene wells may have been due to accumulation of toxic metabolites, as observed in pure cultures (13, 25).
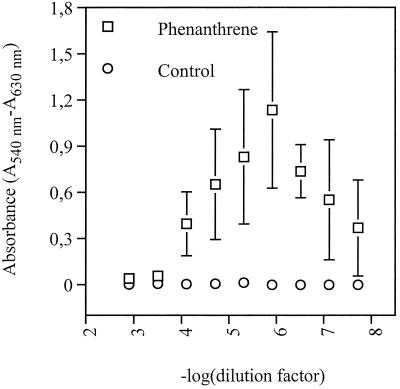
For phenanthrene (Ringe soil), the relationship between the dilution factor and the response of positive wells after 21 days of incubation is presented in Fig. 4. Low dilutions gave a low signal, possibly due to depletion of substrate and accumulation of toxic PAH metabolites caused by a high number of PAH-degrading cells. Negative wells at the lowest dilutions were considered false negatives when wells at the two subsequent dilutions were all positive.
FIG. 4.
MPN enumeration of phenanthrene degraders from PAH-polluted soil: change in absorbance of positive wells as a function of the dilution factor. The error bars represent ±1 standard deviation. Only the solvent was added to the control wells.
The MPN detection limits were calculated as the MPN by assuming one positive well at the lowest dilution. The detection limit for the radiotracer MPN was 39 cells per g (dry weight) of soil (95% confidence limits, 0 to 218 cells per g [dry weight] of soil). The WST-1 MPN detection limit was 92 cells per g (dry weight) of soil (95% confidence limits, 0 to 450 cells per g [dry weight] of soil); the higher detection limit was caused by interference from humic substances and soil particles at the 1:40 dilution.
The WST-1 microtiter plate MPN estimates and the corresponding radiotracer MPN estimates are given in Table 2. For each dilution series, there was no significant difference when the two estimates were compared by using a t test (5). When all the estimates were compared, the WST-1 microtiter plate method seemed to give slightly higher estimates than the radiotracer method.
TABLE 2.
MPN counts of phenanthrene-, fluoranthene-, and pyrene-degrading bacteria from two PAH-contaminated soils
| PAH | Soil | Replicatea | MPN (95% confidence limits) (103 cells g−1)
|
|
|---|---|---|---|---|
| WST-1b | 14C-PAHc | |||
| Phenanthrene | B&W | 1 | 1,700 (240-5,700) | 270 (28-1,300) |
| B&W | 2 | 460 (79-1,500) | 140 (22-680) | |
| B&W | 3 | 560 (120-1,800) | 270 (28-1,300) | |
| Ringe | 1 | 6,400 (1,100-20,000) | 1,300 (120-6,500) | |
| Ringe | 2 | 16,000 (1,700-48,000) | 4,300 (420-22,000) | |
| Ringe | 3 | 22,000 (3,000-68,000) | 3,600 (380-18,000) | |
| Fluoranthene | B&W | 1 | 3.4 (0.33-10) | 9.3 (1.4-44) |
| B&W | 2 | 2.5 (0.26-7.8) | 3.5 (0.39-16) | |
| B&W | 3 | 6.9 (0.84-23) | 2.6 (0.35-12) | |
| Ringe | 1 | 600 (60-1,900) | 840 (90-4,100) | |
| Ringe | 2 | 950 (98-2,900) | 220 (23-1,000) | |
| Ringe | 3 | 510 (54-1,600) | 300 (28-1,600) | |
| Pyrene | B&W | 1 | 56 (5.4-170) | 150 (22-700) |
| B&W | 2 | 55 (5.3-160) | 36 (5.4-170) | |
| B&W | 3 | 93 (13-280) | 67 (7.0-320) | |
| Ringe | 1 | 4,700 (550-14,000) | 1,300 (120-6,500) | |
| Ringe | 2 | 6,300 (770-22,000) | 1,600 (140-7,900) | |
| Ringe | 3 | 4,900 (600-15,000) | 1,100 (110-5,600) | |
The two soil samples (B&W and Ringe) were MPN enumerated three times for each PAH.
MPN estimates were obtained by using a WST-1 microtiter plate MPN method developed in this study (fourfold dilution series, six-row MPN).
MPN estimates were obtained by using a radiotracer method (fourfold dilution series, three-row MPN).
Previous microtiter plate MPN methods for enumeration of PAH degraders have been based on the formation of colored metabolites from single PAHs (23) or the formation of colored cometabolites from fluorene and dibenzothiophene in PAH mixtures (27). The formation of colored metabolites is not needed when the WST-1 method is used.
Acknowledgments
We thank Lone Frette and two anonymous reviewers for critically reading the manuscript and for suggestions.
This work was supported by the European Commission (contract BIO4-CT97-2015) and the Danish Strategic Environmental Research Program (BIOPRO).
REFERENCES
- 1.Alexander, M. 1982. Most probable number method for microbial populations, p. 25-29. In Methods of soil analysis, part 2. Chemical and microbiological properties, 2nd ed. Agronomy monograph no. 9. ASA-SSSA, Madison, Wis.
- 2.Arino, S., R. Marchal, and J.-P. Vandecasteele. 1998. Involvement of a rhamnolipid-producing strain of Pseudomonas aeruginosa in the degradation of polycyclic aromatic hydrocarbons by a bacterial community. J. Appl. Microbiol. 84:769-776. [DOI] [PubMed] [Google Scholar]
- 3.Bastiaens, L., D. Springael, P. Wattiau, H. Harms, R. deWachter, H. Verachtert, and L. Diels. 2000. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 66:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochner, B. 1989. “Breathprints” at the microbial level. An automated redox-based technology quickly identifies bacteria according to their metabolic capacities. ASM News 55:536-539. [Google Scholar]
- 5.Cochran, W. G. 1950. Estimation of bacterial density by means of the ‘most probable number.' Biometrics 6:105-116. [PubMed] [Google Scholar]
- 6.Dagher, F., E. Deziel, P. Lirette, G. Paquette, J. G. Bisaillon, and R. Villemur. 1997. Comparative study of five polycyclic aromatic hydrocarbon degrading bacterial strains isolated from contaminated soils. Can. J. Microbiol. 43:368-377. [DOI] [PubMed] [Google Scholar]
- 7.Déziel, E., G. Paquette, R. Villemur, F. Lepine, and J.-G. Bisaillon. 1996. Biosurfactant production by a soil Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 62:1908-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulthorpe, R. R., and D. G. Allan. 1994. Evaluation of Biolog MT plates for aromatic and chloroaromatic substrate utilization tests. Can. J. Microbiol. 40:1067-1071. [Google Scholar]
- 9.Ho, Y., M. Jackson, Y. Yang, J. Mueller, and P. Pritchard. 2000. Characterization of fluoranthene- and pyrene-degrading bacteria isolated from PAH-contaminated soils and sediments and comparison of several Sphingomonas spp. J. Ind. Microbiol. Biotechnol. 2:100-112. [Google Scholar]
- 10.Ishiyama, M., M. Shiga, K. Sasamoto, M. Mizoguchi, and P. He. 1993. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull. 41:1118-1122. [Google Scholar]
- 11.Johnsen, A. R., M. Hausner, A. Schnell, and S. Wuertz. 2000. Evaluation of fluorescently labeled lectins for noninvasive localization of extracellular polymeric substances in Sphingomonas biofilms. Appl. Environ. Microbiol. 66:3487-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kästner, M., M. Breuer-Jammali, and B. Mahro. 1994. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic hydrocarbons (PAH). Appl. Microbiol. Biotechnol. 41:267-273. [Google Scholar]
- 13.Kazunga, C., M. D. Aitken, A. Gold, and R. Sangaiah. 2001. Fluoranthene-2,3- and -1,5-diones are novel products from the bacterial transformation of fluoranthene. Environ. Sci. Technol. 35:917-922. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, A. C. 1994. Carbon utilization and fatty acid profiles for characterisation of bacteria, p. 543-556. In R. W. Weaver, S. Angle, P. Bottomley, D. Bezdicek, S. Smith, A. Tabatabai, and A. Wollum (ed.), Methods of soil analysis, part 2. Microbiological and biochemical properties. Soil Science Society of America Inc., Madison, Wis.
- 15.Kiyohara, H., K. Nagao, and K. Yana. 1982. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl. Environ. Microbiol. 43:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klee, A. J. 1993. A computer program for the determination of most probable number and its confidence limits. J. Microbiol. Methods 18:91-98. [Google Scholar]
- 17.Mueller, J. G., P. J. Chapman, B. O. Blattmann, and P. H. Pritchard. 1990. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl. Environ. Microbiol. 56:1079-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller, J. G., R. Devereux, D. L. Santavy, S. E. Lantz, S. G. Willis, and P. H. Pritchard. 1997. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek 71:329-343. [DOI] [PubMed] [Google Scholar]
- 19.Pyle, B., S. Broadaway, and G. McFeters. 1995. Factors affecting the determination of respiratory activity on the basis of cyanoditolyl tetrazolium chloride reduction with membrane filtration. Appl. Environ. Microbiol. 61:4304-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidler, E. 1991. The tetrazolium formazan system: design and histochemistry. Prog. Histochem. Cytochem. 24:45-77. [DOI] [PubMed] [Google Scholar]
- 21.Smith, J. J., and G. A. McFeters. 1997. Mechanisms of INT (2-(4-iodophenyl)-3(-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J. Microbiol. Methods 29:161-175. [DOI] [PubMed] [Google Scholar]
- 22.Smith, M. R. 1990. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation 1:191-206. [DOI] [PubMed] [Google Scholar]
- 23.Stieber, M., F. Haeseler, P. Werner, and F. H. Frimmel. 1994. A rapid screening method for micro-organisms degrading polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol. 40:753-755. [Google Scholar]
- 24.Wick, L., T. Colangelo, and H. Harms. 2001. Kinetics of mass-transfer limited bacterial growth on solid PAHs. Environ. Sci. Technol. 35:354-361. [DOI] [PubMed] [Google Scholar]
- 25.Willumsen, P. A., and E. Arvin. 1999. Kinetics of degradation of surfactant-solubilized fluoranthene by a Sphingomonas paucimobilis. Environ. Sci. Technol. 33:2571-2578. [Google Scholar]
- 26.Willumsen, P. A., and U. Karlson. 1997. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 7:415-423. [Google Scholar]
- 27.Wrenn, B. A., and A. D. Venosa. 1996. Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number method. Can. J. Microbiol. 42:252-258. [DOI] [PubMed] [Google Scholar]