Abstract
The genetic relationship between 197 vancomycin-resistant Enterococcus faecium (VREF) isolates and 21 vancomycin-susceptible E. faecium isolates from Norwegian poultry was analyzed by amplified fragment length polymorphism (AFLP). The isolates were compared to 255 VREF isolates from various sources and countries. The Norwegian isolates constituted a relatively homogeneous population of E. faecium and clustered in a previously defined poultry AFLP genogroup.
Vancomycin-resistant enterococci (VRE) have been of increasing concern during the last 15 years as a cause of nosocomial infections, particularly in the United States (16). In the middle of the 1990s, an agricultural reservoir of the VanA type of VRE associated with the use of avoparcin as a feed additive in livestock and poultry was documented in Europe (1, 3, 13, 14). To understand the epidemiology of VRE, the genetic relationship between isolates derived from human sources and isolates derived from animal sources has been investigated in several studies. Fingerprinting analyses have generally shown that the population of VRE outside hospitals is heterogeneous, although indistinguishable or closely related isolates from humans and animals have been described (9, 17, 18, 19). VRE isolated during hospital outbreaks and from clinical specimens have revealed a more homogeneous population, and both interhospital clonal dissemination of VRE and intrahospital clonal dissemination of VRE have been reported (7, 10). Pulse-field gel electrophoresis (PFGE) has been regarded as the “gold standard” for VRE typing in hospital epidemics (15), but this typing method may be too discriminatory to describe the genetic relatedness of epidemiologically unrelated VRE (21). A novel fingerprinting method, amplified fragment length polymorphism (AFLP) analysis, has recently been applied to enterococci and has been suggested as a new gold standard for fingerprinting enterococci from nosocomial outbreaks and in epidemiological studies (2, 21).
In Norway, VanA-type VRE have been shown to persist in poultry production and to be present on broiler farms 4 years after avoparcin was banned (4, 5, 6). The aim of the present work was to study the genetic relationship between vancomycin-resistant Enterococcus faecium (VREF) and vancomycin-susceptible E. faecium (VSEF) isolates recovered from Norwegian poultry production between 1995 and 1999 by using AFLP and to compare these isolates with VREF isolates from both animal and human sources from other countries.
A selection of 197 VREF isolates (MICs of vancomycin, ≥256 μg/ml; vanA gene present) recovered from various poultry sources (4, 5, 6, 14) from 1995 to 1999, as well as 21 VSEF isolates (MICs of vancomycin, 0.38 to 1.0 μg/ml) recovered from poultry carcasses in 1998 (unpublished data), were studied (Table 1). Most isolates originated from different poultry flocks; the exceptions were nine carcass samples which each gave rise to one VREF isolate and one VSEF isolate. The VREF and VSEF isolates were in general susceptible to other antimicrobial agents, although some showed reduced susceptibility to tetracycline and/or erythromycin (4, 5, 6; unpublished data).
TABLE 1.
Numbers of VREF and VSEF isolates recovered from various poultry sources in Norway at different times and analyzed by the AFLP method
| Source of isolates | No. of isolates
|
Reference(s) | |||
|---|---|---|---|---|---|
| 1995 | 1998 | 1999 | Total | ||
| Broiler or turkey feces | 15 | 20 | 14 | 49 | 4, 5, 14 |
| Broiler or turkey carcass | |||||
| VREF | 15 | 20 | 0 | 35 | 6, 14 |
| VSEF | 0 | 21 | 0 | 21 | Unpublished data |
| Total | 15 | 41 | 0 | 56 | |
| Broiler farm environment | 5 | ||||
| Farm 1 | 0 | 0 | 46 | 46 | |
| Farm 2 | 0 | 0 | 45 | 45 | |
| Farm 3 | 0 | 0 | 11 | 11 | |
| Farm 4 | 0 | 0 | 3 | 3 | |
| Farm 5 | 0 | 0 | 8 | 8 | |
| Total | 0 | 0 | 113 | 113 | |
| Total | 30 | 61 | 127 | 218 | |
AFLP analysis was performed as described by Willems et al. (21). GeneScan software (Applied Biosystems) was used for collection of the data, which subsequently were exported into BioNumerics (version 1.5; Applied Maths, St. Martens-Latem, Belgium) for further analysis. The AFLP profiles of the 218 Norwegian isolates were compared to each other, as well as to the AFLP profiles of 255 VREF isolates from poultry, pigs, veal calves, dogs, cats, hospitalized patients, and nonhospitalized persons from other countries (21). The effects of time period, source, and vancomycin susceptibility on clustering of AFLP patterns in the dendrogram were determined by using group statistics (K-means partitioning) and the Jack-knife method (available in the BioNumerics software) based upon maximal similarities between the isolates (BioNumerics Manual, version 1.5; Applied Maths). This method determined the internal stability of the following predefined groups: time period (1995, 1998, and 1999), source (feces, carcasses, and environment), vancomycin susceptibility (resistant and susceptible), and farm (farms 1 to 3 and 5).
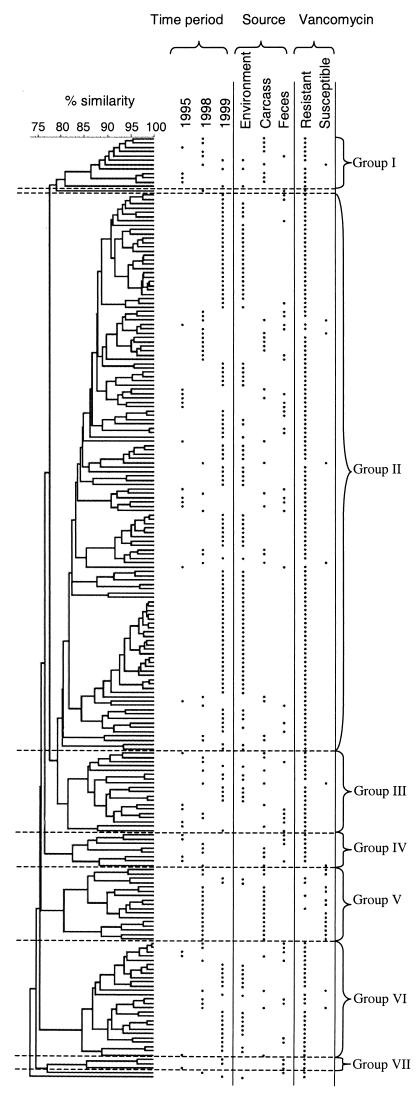
AFLP typing of the 218 Norwegian E. faecium isolates revealed a homogeneous population that shared at least 75% of the restriction fragments. However, all but three isolates originated from a restricted area, the southeastern part of Norway, and the majority originated from only five broiler farms, which may have contributed to the genetic homogeneity observed. Grouping of isolates that shared ≥80% of the restriction fragments revealed seven AFLP groups (groups I to VII) (Fig. 1). The results of the grouping analysis suggested that clustering according to time of isolation occurred. Group separation statistics confirmed that specific clustering of isolates in time also occurred when the sources of isolation during the different time periods were taken into account (Table 2).
FIG.1.
Dendrogram of 218 E. faecium isolates recovered from various Norwegian poultry sources at different times. The levels of similarity were determined by using the Pearson product-moment correlation coefficient. Seven AFLP groups (groups I to VI) were formed at the ≥80% similarity level. The distributions of the isolates according to time and source of isolation, as well as vancomycin susceptibility, are shown.
TABLE 2.
Group separation statistics for distribution based on time period-, source-, vancomycin susceptibility-, and farm-specific isolates among the AFLP clusters in Fig. 1a
| Groups of isolates | % of isolates in groups
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time periodb
|
Sourcesc
|
Vancomycin susceptibilityd
|
Farm of origine
|
|||||||||||||
| 1995
|
1998
|
1999
|
Carcasses
|
Feces
|
Resistant (1998) | Susceptible (1998) | Farm 1 | Farm 2 | Farm 3 | Farm 5 | ||||||
| Feces | Carcasses | Feces | Carcasses | Feces | Carcasses | 1995 | 1998 | 1995 | 1998 | |||||||
| 1995 | ||||||||||||||||
| Feces | 60 | 20 | 0 | |||||||||||||
| Carcasses | 87 | 15 | NDf | |||||||||||||
| 1998 | ||||||||||||||||
| Feces | 33 | 65 | 7 | |||||||||||||
| Carcasses | 23 | 85 | ND | |||||||||||||
| 1999 | ||||||||||||||||
| Feces | 7 | 15 | 93 | |||||||||||||
| Carcasses | ND | ND | ND | |||||||||||||
| Carcasses | ||||||||||||||||
| 1995 | 47 | 67 | ||||||||||||||
| 1998 | 85 | 33 | ||||||||||||||
| Feces | ||||||||||||||||
| 1995 | 53 | 33 | ||||||||||||||
| 1998 | 15 | 67 | ||||||||||||||
| Resistant | 85 | 19 | ||||||||||||||
| Susceptible | 15 | 81 | ||||||||||||||
| Farm 1 | 98 | 4 | 9 | 12 | ||||||||||||
| Farm 2 | 0 | 96 | 9 | 50 | ||||||||||||
| Farm 3 | 0 | 0 | 82 | 0 | ||||||||||||
| Farm 5 | 2 | 0 | 0 | 38 | ||||||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
The values are the percentages of isolates that were placed in the different groups. A higher percentage for identification to the group to which isolates were assigned to (e.g., 1995) than to the other groups (e.g., 1995 and 1998 or 1995 and 1999) is an indication of the likelihood that that group was specific and thus an indication of group-specific (e.g., 1995 isolates-specific) AFLP band pattern clustering.
Calculated for feces and carcass isolates in 1995 and 1998 and feces isolates in 1999.
Calculated only for isolates obtained in 1995 and 1998.
Calculated only for the 41 carcass isolates recovered in 1998.
Calculated only for the 134 isolates obtained from farms 1 to 3 and 5.
ND, not determined.
The majority of carcass and fecal isolates clustered in a restricted number of AFLP groups (Fig. 1). Group separation statistics showed a weak association between AFLP patterns and isolates of carcass or fecal origin for 1998, while for 1995 no separation between the carcass and feces groups was found. This supports the assumption that poultry carcass isolates often are of fecal origin. Of the 21 VSEF isolates from poultry carcasses, 62% clustered in AFLP group V, while only two (10%) of the VREF isolates recovered from poultry carcasses in the same period of time (1998) clustered in this genogroup (Fig. 1). Group separation statistics confirmed this genotypic difference between the VREF and VSEF isolates included in the analysis (Table 2).
For farms 1, 2, and 3, AFLP typing revealed an association between genotype and farm of origin (Table 2), which suggests the presence of a farm-adapted E. faecium population. This is in concordance with results from Italy and Denmark showing clonal spread of VRE within a poultry flock or a pig herd at the same farm obtained by the use of PFGE (11, 12). The absence of an association between AFLP profiles and farm of origin for farms 4 and 5 is most probably due to the low number of isolates included. The homogeneity of the Norwegian AFLP profiles indicates that the VREF isolates recovered from farms in the absence of poultry are genetically related to VREF isolates recovered from poultry feces and carcasses. Consequently, these results support the hypothesis that recycling of VRE in the broiler farm environment is the most likely explanation for the continuing high prevalence of VRE seen in Norwegian poultry production (5).
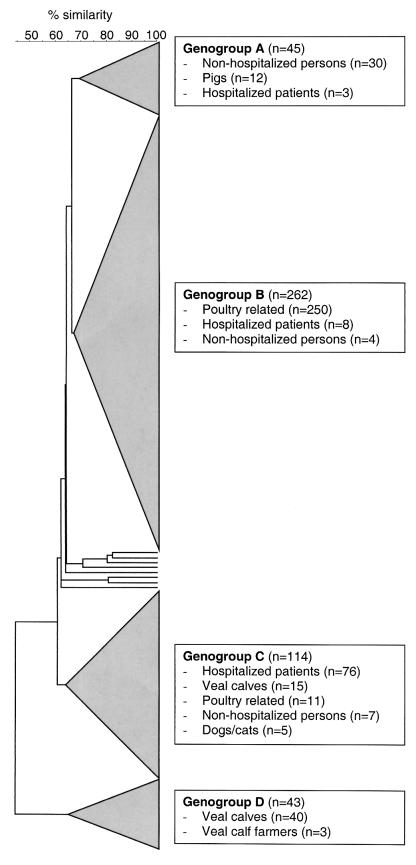
When the AFLP profiles of the Norwegian isolates were compared to the AFLP profiles of 255 VREF isolates from other sources and countries (21), all the Norwegian isolates clustered in the previously defined poultry genogroup, genogroup B (Fig. 2). The finding that the Norwegian isolates clustered with poultry-associated isolates from The Netherlands and not with porcine or veal calf-associated isolates from The Netherlands strengthens the suggestion that clustering of poultry isolates is not a result of geographical isolation but is a result of adaptation of VREF to specific hosts (21). Most of the isolates of human origin in poultry genogroup B originated from humans with a history of poultry exposure. Carriage of VRE in healthy poultry slaughterers and farmers has previously been described (4, 14, 19), and transmission of VREF is likely to occur between poultry and humans in these settings. In contrast, infections with animal-derived VRE have rarely been reported (8), and it has been hypothesized that E. faecium strains causing infections or hospital epidemics are genetically distinct from animal-derived strains (20, 21). Further studies are needed to elucidate the differences and communication between various VRE populations.
FIG. 2.
Abridged dendrogram of the 218 Norwegian poultry-associated E. faecium isolates compared with 255 VREF isolates from various sources and countries.
Acknowledgments
We thank Marga van Santen-Verheuvel and Marit Sørum for excellent laboratory assistance.
The majority of this work was performed at the Research Laboratory for Infectious Diseases, National Institute of Public Health and the Environment, Bilthoven, The Netherlands, and was financially supported by FEMS fellowship 2000-2, as well as by grant 117130/112 from the Research Council of Norway.
REFERENCES
- 1.Aarestrup, F. M. 1995. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb. Drug Resist. 1:255-257. [DOI] [PubMed] [Google Scholar]
- 2.Antonishyn, N. A., R. R. McDonald, E. L. Chan, G. Horsman, C. E. Woodmansee, P. S. Falk, and C. G. Mayhall. 2000. Evaluation of fluorescence-based amplified fragment length polymorphism analysis for molecular typing in hospital epidemiology: comparison with pulsed-field gel electrophoresis for typing strains of vancomycin-resistant Enterococcus faecium. J. Clin. Microbiol. 38:4058-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bager, F., M. Madsen, J. Christensen, and F. M. Aarestrup. 1997. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 31:95-112. [DOI] [PubMed] [Google Scholar]
- 4.Borgen, K., G. S. Simonsen, A. Sundsfjord, Y. Wasteson, O. Olsvik, and H. Kruse. 2000. Continuing high prevalence of VanA-type vancomycin-resistant enterococci on Norwegian poultry farms three years after avoparcin was banned. J. Appl. Microbiol. 89:478-485. [DOI] [PubMed] [Google Scholar]
- 5.Borgen, K., M. Sorum, H. Kruse, and Y. Wasteson. 2000. Persistence of vancomycin-resistant enterococci (VRE) on Norwegian broiler farms. FEMS Microbiol. Lett. 191:255-258. [DOI] [PubMed] [Google Scholar]
- 6.Borgen, K., M. Sorum, Y. Wasteson, and H. Kruse. 2001. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int. J. Food Microbiol. 64:89-94. [DOI] [PubMed] [Google Scholar]
- 7.Chow, J. W., A. Kuritza, D. M. Shlaes, M. Green, D. F. Sahm, and M. J. Zervos. 1993. Clonal spread of vancomycin-resistant Enterococcus faecium between patients in three hospitals in two states. J. Clin. Microbiol. 31:1609-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das, I., A. Fraise, and R. Wise. 1997. Are glycopeptide-resistant enterococci in animals a threat to human beings? Lancet 349:997-998. [DOI] [PubMed] [Google Scholar]
- 9.Descheemaeker, P. R., S. Chapelle, L. A. Devriese, P. Butaye, P. Vandamme, and H. Goossens. 1999. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob. Agents Chemother. 43:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne, W. M., Jr., and W. Wang. 1997. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J. Clin. Microbiol. 35:388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosso, M. D., A. Caprioli, P. Chinzari, M. C. Fontana, G. Pezzotti, A. Manfrin, E. D. Giannatale, E. Goffredo, and A. Pantosti. 2000. Detection and characterization of vancomycin-resistant enterococci in farm animals and raw meat products in Italy. Microb. Drug Resist. 6:313-318. [DOI] [PubMed] [Google Scholar]
- 12.Hammerum, A. M., V. Fussing, F. M. Aarestrup, and H. C. Wegener. 2000. Characterization of vancomycin-resistant and vancomycin-susceptible Enterococcus faecium isolates from humans, chickens and pigs by RiboPrinting and pulsed-field gel electrophoresis. J. Antimicrob. Chemother. 45:677-680. [DOI] [PubMed] [Google Scholar]
- 13.Klare, I., H. Heier, H. Claus, R. Reissbrodt, and W. Witte. 1995. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol. Lett. 125:165-171. [DOI] [PubMed] [Google Scholar]
- 14.Kruse, H., B. K. Johansen, L. M. Rorvik, and G. Schaller. 1999. The use of avoparcin as a growth promoter and the occurrence of vancomycin-resistant Enterococcus species in Norwegian poultry and swine production. Microb. Drug Resist. 5:135-139. [DOI] [PubMed] [Google Scholar]
- 15.Morrison, D., N. Woodford, S. P. Barrett, P. Sisson, and B. D. Cookson. 1999. DNA banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria for defining strains. J. Clin. Microbiol. 37:1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 17.Robredo, B., K. V. Singh, C. Torres, and B. E. Murray. 2000. Streptogramin resistance and shared pulsed-field gel electrophoresis patterns in vanA-containing Enterococcus faecium and Enterococcus hirae isolated from humans and animals in Spain. Microb. Drug Resist. 6:305-311. [DOI] [PubMed] [Google Scholar]
- 18.Simonsen, G. S., H. Haaheim, K. H. Dahl, H. Kruse, A. Lovseth, O. Olsvik, and A. Sundsfjord. 1998. Transmission of VanA-type vancomycin-resistant enterococci and vanA resistance elements between chicken and humans at avoparcin- exposed farms. Microb. Drug Resist. 4:313-318. [DOI] [PubMed] [Google Scholar]
- 19.Stobberingh, E., A. van den Bogaard, N. London, C. Driessen, J. Top, and R. Willems. 1999. Enterococci with glycopeptide resistance in turkeys, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of The Netherlands: evidence for transmission of vancomycin resistance from animals to humans? Antimicrob. Agents Chemother. 43:2215-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willems, R. J., W. Homan, J. Top, M. Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. van Embden, and M. J. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed] [Google Scholar]
- 21.Willems, R. J., J. Top, B. N. van Den, A. Van Belkum, H. Endtz, D. Mevius, E. Stobberingh, B. A. van Den, and J. D. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]