Abstract
The present study focuses on improving the flexibility of PLA by reinforcing it with a nanohydroxyapatite (n-HAp) filler. The n-HAp was synthesized via pink perch fish scales (PPFS) using a simple and feasible pyrolysis method and was characterized using XRD, FTIR, Raman spectroscopy, TGA, SEM, and TEM. n-Hap of 0.25, 0.5, 0.75, and 1 wt % was used to reinforce the PLA matrix, and PLA_HAp composite blown films were extruded for further studies. The PLA_HAp composite blown films were characterized by using XRD, FTIR, Raman spectroscopy, and SEM to understand the interaction of the n-HAP in the PLA matrix. The thermal and mechanical analyses of the PLA_HAp blown films reveal that a low concentration of n-HAp can reduce the thermal stability and improve the flexibility of the films. The thermal characterizations infer that n-HAp in PLA acts as a nucleating center in the PLA backbone to improve the flexibility of films. Tensile results show that PLA_HAp 0.5 exhibited maximum flexibility with elongation at break of 20%, which is a 400% enhancement compared to neat PLA. The WVTR analysis of PLA-HAp composites, compared to neat PLA blown films, revealed a reduction in water permeability by 56% for PLA_HAp 0.5 and 66% for PLA_HAp 1 under ambient conditions, making them suitable for food packaging applications.


1. Introduction
By the end of 2030, the use of plant- and animal-based resources in composite material production is expected to increase by 25%, driven by the impact on environmental sustainability. , Biocomposites made from one or more natural resources can naturally decompose at the end of their lifecycle without causing environmental harm. Among various biocomposites, biopolymer composites offer a promising solution to the increasing pollution caused by petroleum-based plastics. One such biodegradable polymer is polylactic acid (PLA), a fully biobased polymer produced through the fermentation of natural sources like sugar cane, cassava, corn, or sugar beet pulp. , It is widely used in making bottles, plastic films, medical implants, drug delivery systems, etc. − PLA is a versatile material recognized by the United States Food and Drug Administration (FDA) and classified as Generally Recognized As Safe (GRAS) for food packaging applications. However, certain drawbacks, including low thermal stability, limited elongation, brittleness, and poor barrier properties, restrict the potential use of PLA in food packaging applications.
Many studies have reported different approaches to overcome the limitations of PLA, especially in improving the mechanical properties, thermal stability, barrier, and antimicrobial properties. , Largely explored techniques to enhance polymer characteristics have been adding fillers such as organics, inorganics, and essential oils or blending them with other polymers. , It has been reported that using natural fillers in polymers can dramatically improve the properties and biodegradation of polymers due to their hydrophilic nature, which can attract microorganisms from the soil. ,
Studies on reinforcing the PLA matrix with natural fillers have shown promising results in improving the thermal and mechanical properties for various applications. , Extracting or isolating biofillers from bioindustry and agricultural waste is an efficient approach to recycling waste sustainably. Among various natural fillers, hydroxyapatite (HAp), a natural mineral form of calcium apatite, has been a standout performer. , Its good biocompatibility, biodegradability, and stability in polymers make it the top choice. Fish scales and bones are abundant sources of hydroxyapatite (HAp), and recycling fish scales offers an efficient way to repurpose waste from the fish scale industry. Studies on HAp-reinforced polymers have revealed that HAp can significantly improve the mechanical properties of the polymer composite by controlling the interfacial bonding between the polymer matrix and filler without any coupling agents. The success of HAp in improving the properties of PLA should instill confidence in its potential for food packaging applications.
PLA_HAp composite films have been studied for food packaging applications utilizing various preparation methods. Thool et al. have reported n-HAp isolated from fish bones using thermal calcination and reinforced in PLA matrix using the solution casting method for food packaging application. PLA_HAp hybrid biodegradable packaging was reported with HAp synthesized through chemical precipitation and reinforced in PLA via melt mixing. Osial et al. have reported PLA/HAp/curcumin films prepared for food packaging application from the solution casting method. However, no studies have been conducted in PLA_HAp composite films prepared using blown film extrusion for food packaging applications.
The current study focuses on developing biopolymer composite films made from PLA and nanohydroxyapatite (n-HAp) extracted from pink perch fish scales (PPFS) for food packaging applications. n-HAp was isolated from PPFS using pyrolysis and characterized through XRD, FTIR, Raman spectroscopy, TGA, SEM, and TEM analyses. The biopolymer composite was prepared by incorporating varying weight percentages (0.25, 0.5, 0.75, and 1) of n-HAp into PLA using solution blending followed by blown film extrusion. The biopolymer composite blown films were investigated for their thermal, mechanical, and barrier properties.
2. Materials and Methods
2.1. Materials
The pink perch fish scales (PPFS) were used as sources of HAp, and these scales were procured from Nizona Inc., Mumbai, India. PLA polymer pellets used in composite preparation were purchased from Natureworks LLC, USA. Chloroform (≥99.8% with 0.5–1% ethanol as a stabilizer), used as a solvent for dissolving PLA pellets, was procured from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Experimental Section
2.2.1. Preparation of Nanohydroxyapatite from Fish Scales
A simple pyrolysis method in a pressure reactor was carried out to synthesize n-HAp from the PPFS powder. First, we cleaned the PPFS with distilled water many times, followed by drying in the oven to remove the moisture from the scales. The cleaned PPFS was made into a fine powder using a 6875D freezer/mill high-capacity cryogenic grinder. The cryogenic grinder pulverized the sample to a fine powder by cooling it to cryogenic temperatures using liquid nitrogen and a magnetically driven impactor. The pyrolysis of the PPFS powder was performed in a superalloy high-pressure hydrothermal reactor (GLX-1100X-RC series) with a 100 mL capacity. Twenty grams of PPFS powder is added to the pressure reactor and heated to 900 °C under a nitrogen atmosphere with a pressure up to 4 MPa, and heating rate of 5 °C/min, and a holding time of 2 h. At 900 °C, it completely eliminates organic components such as proteins from the PPFS and transforms the residual calcium phosphate into the crystalline hydroxyapatite (HAp) phase. The pyrolyzed PPFS powder was then collected from the reactor and dried in an oven to remove the moisture.
2.2.2. Preparation of the PLA_HAp Composite
The solution blending method incorporated synthesized n-HAp into the PLA using chloroform as the solvent. The schematic representation of the solution blending method used for the composite preparation is given in Figure a. 0.25, 0.5, 0.75, and 1 wt % of n-HAp were added to PLA to prepare the PLA_HAp composite. The synthesized n-HAp is first dispersed in chloroform by using probe sonication for 30 min to reduce aggregation and ensure an even distribution of the filler in the composite. PLA pellets are added to the n-HAp/chloroform solution and are magnetically stirred at 600 rpm for 5 h to prepare the PLA_HAp composite. The prepared PLA_HAp solution is dried in an aluminum pan under a fume hood overnight at room temperature to evaporate the chloroform.
1.
Preparation of PLA_HAp composite using (a) the solution blending method, (b) PLA_HAp filament extrusion, and (c) PLA_HAp blown film extrusion.
2.2.3. Preparation of PLA_HAp Composite Filament
The dried PLA_HAp composite sheet from the above step was then chopped into small pieces to further extrude as PLA_HAp filaments for better reinforcement of the filler in the composite. Figure b shows the Filabot EX2 single screw filament extruder, airpath, and spooler used for PLA_HAp filament extrusion, and the composite filaments were extruded into spools. The extruder is heated to 170 °C and the extruder screw speed was set to 55–60 rpm to get a relatively uniform thickness (1.6–1.8 mm) for the composite filaments extruded. The extruded PLA_HAp filaments (0.25, 0.5, 0.75, and 1 wt %) were pelletized using a high-speed Filabot pelletizer for blown film extrusion.
2.2.4. Preparation of PLA_HAp Composite Blown Films
The pelletized PLA_HAp pellets were extruded into blow films having 0.25, 0.5, 0.75, and 1 wt % HAp in PLA using an ultramicro film blowing line type LUMF-150 single screw extruder by Lab Tech Engineering Company LTD (Figure c). The blown film extruder has a conical screw with a diameter of 18 mm at the feed and 8 mm at the end with a screw L/D ratio of 30. During film extrusion, the screw speed was set to 60 rpm with the barrel and die temperatures maintained at 170 °C (340 F) and 163 °C (325 F), respectively. Figure c shows the blown film extruded films with 0.25, 0.5, 0.75, and 1 wt % HAp in PLA extruded through the die and directed through the spring-loaded nip rolls to the winding bobbin at the other end of the machine to roll down the films. It was observed that the transparency of the blown films reduced as the concentration of carbonized HAp in PLA was increased from 0.25 to 1 wt %. At 1 wt % of HAp in the PLA matrix, the films became thicker and stiffer, causing difficulty in film extrusion.
2.3. Characterization Techniques
2.3.1. X-ray Diffraction (XRD) Analysis
The X-ray diffraction analysis of the HAp and PLA_HAp composite films was obtained using a RIGAKU Smart Lab X-ray diffractometer equipped with monochromatic copper Kα1 radiation, with samples scanned at a scan rate of 5 °/min from 5 to 80° Bragg’s angle at 45 kV and 40 mA.
2.3.2. Raman Spectroscopy
The chemical analysis of the HAp and PLA_HAp composite films prepared at different wt % was studied using a Thermo Scientific DXR Raman spectrometer. The Raman spectrum was analyzed from 0 to 3500 cm–1 with a laser power of 5 mW.
2.3.3. FTIR Spectrometer
A Fourier transform infrared (FTIR) spectrometer (IR Tracer-100) with a high resolution at 0.25 cm–1 and high scanning speed was used to identify the functional groups attached to the HAp and PLA_HAp composite.
2.3.4. Scanning Electron Microscopy
The morphology and microstructure of the HAp and PLA_HAp composite films were imaged by using a JEOL JSM-7200F field emission scanning electron microscope. The PLA_HAp composite films were fixed on aluminum stubs using carbon tape and Au–Pd sputtered for 30 s using a Hummer 6.2 vacuum sputter coater before SEM imaging. The HAp powder was imaged at an accelerating voltage of 5 kV, and PLA_HAp composite films were SEM imaged for particle distribution and fracture analysis after tensile testing at an accelerating voltage of 5 kV.
2.3.5. Energy Dispersive X-ray (EDX) Analyzer
The quantitative and chemical composition with elemental mapping of the n-HAp powder was obtained using an energy-dispersive X-ray Analyzer (EDX) JEOL, EX-37001. The EDX was conducted with a working distance of 10 mm and an accelerating voltage of 10 kV.
2.3.6. Thermal Property Analysis
The thermal property analysis of the samples was performed using a thermogravimetric analyzer (TGA) and differential scanning calorimetry (DSC). A TA Q500, Thermogravimetric analyzer (TGA) instrument was used to study the thermal stability and weight change of the n-HAp and PLA_HAp composite films. Each sample with 15–20 mg was placed in platinum pans for the thermal degradation study, and the change in weight of the samples with increasing temperature was observed using TGA under a nitrogen atmosphere. The thermal reactions in the polymer films were analyzed with precision by using a Differential Scanning Calorimetry (DSC) TA-Q series 2000 instrument for each PLA_HAp composite film. The composite film samples of 6–12 mg are placed in hermetic pans and compared with a reference pan for thermal analysis. The DSC thermograms for the PLA_HAp composite films were obtained for heating and cooling cycles at a heating rate of 10 °C/min from −20 to 200 °C under a nitrogen atmosphere.
2.3.7. Mechanical Property Analysis
The tensile testing of the PLA_HAp composite films was conducted using a Zwick/Roell Z2.5 universal testing machine following the ASTM D882–10 standard for polymer films less than 1 mm thick. The average thickness of the films was measured at different points by using a digital Vernier caliper with 0.001 mm resolution for reliable results. Each polymer film sample with a length of 150 mm and a thickness (0.04–0.05) mm was placed between the upper and lower wedge grips of the Zwick/Roell Z2.5 universal testing machine. The tensile testing of different composite polymer samples was analyzed in the universal testing machine with a load cell of 2.5 kN and a constant crosshead speed of 50 mm/min. The obtained stress vs strain curve gives Young’s modulus, elongation at break, ultimate tensile strength, and other parameters of each polymer film sample.
2.3.8. Water Vapor Transmission Rate (WVTR)
The WVTR of the films was studied by using the AMETEK/MOCON AQUATRAN 3/40 water vapor permeation analyzer. This analyzer is a four-cell cartridge system for package testing and complies with ASTM F1249 for analysis, making it one of the most accurate modulated sensor systems. The neat PLA films and composite PLA films containing 0.5 and 1 wt % of HAp in PLA were tested for WVTR under ambient conditions (23 °C/50% RH) and tropical conditions (35 °C/85% RH).
3. Results and Discussion
3.1. Characterization of n-HAp Powder
XRD of the neat PPFS powder and the n-HAp synthesized from pyrolysis of PPFS at 900 °C is given in Figure a. In the XRD spectrum of neat PPFS, no distinct peaks were observed because of the presence of embodied organic matter, such as protein, fat, collagen, minerals, and other compounds. Meanwhile, the pyrolysis of PPFS powder removed the organic compounds and retained hydroxyapatite as ash with calcium phosphates in mineral form with molecular formula Ca5P3O13H. The XRD spectrum of the pyrolyzed PPFS-derived n-HAp showed evident peaks of standard HAp in good agreement with the JCPDS PDF # 98–000–0251 that corresponds to polycrystalline hexagonal lattice cells. , The XRD spectrum of n-HAp exhibited strong characteristic diffraction peaks with 2θ values at 25.935, 31.73, 32.202, 34.05, 46.84, and 53.5 corresponding to (002), (211), (300), (202), (222), and (104) planes, respectively.
2.
(a) XRD spectrum obtained for the neat PPFS and hydroxyapatite powder from pyrolysis of PPFS. (b) Raman spectrum obtained for the hydroxyapatite powder from pyrolysis of PPFS.
The crystallite particle size of the n-HAp synthesized was calculated using the Debye–Scherrer equation given in eq .
| 1 |
where D is the crystalline size in nm, K denotes the Scherrer constant, usually 0.94, λ is the diffraction wavelength of 1.54 Å, β denotes the full width at half-maximum of the considered peaks, and θ is the diffraction angle. The equation calculates the average particle size obtained by evaluating the distinct peaks from the XRD spectrum. The average crystalline size of HAp synthesized from pyrolyzed PPFS, determined from the Scherrer equation, was 18–32 nm, confirming the presence of nano-HAp.
The Raman spectrum of the pyrolyzed PPFS-derived n-HAp is shown in Figure b. The carbon formed during the synthesis of n-HAp from pyrolysis exhibited broad peaks, indicating the presence of amorphous carbon particles. The spectrum’s broad peaks at 1319.8 and 1593.6 cm–1 correspond to the D and G bands, respectively. The carbon in the n-HAp sample shadows most of the phosphate functional group in the n-HAp sample. The Raman spectrum reveals only the high intensity hydroxyapatite peak on the shoulder of the D peak at 960 cm–1 that corresponds to the symmetric stretching of P–O and originates from the vibrational phosphate functional group PO4 3– in the n-HAp. ,
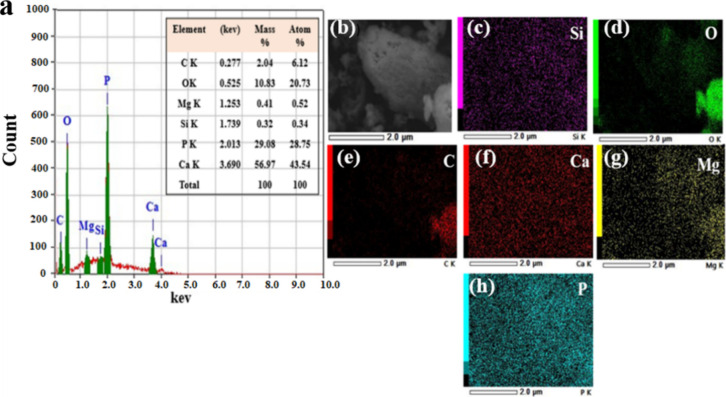
The elemental composition of n-HAp synthesized from the pyrolysis of PPFS was analyzed using SEM-EDX. Figure a shows the EDX spectrum obtained for the n-HAp sample, and Figure b–h shows the elemental mapping of the different elements possessed by the sample. The elemental analysis of the n-HAp sample indicates the presence of calcium (Ca), phosphorus (P), and oxygen (O) in higher quantities of 43.54, 28.75, and 20.73% atomic percentages, respectively. Along with Ca, P, and O, the sample indicated the presence of carbon (C), magnesium (Mg), and Silicon with 6.12, 0.52, and 0.34%, respectively. The fish scales possess a multilayer structure with collagen and HAp overlapping with other organic compounds. During pyrolysis, the organic compounds and carbon decompose, forming a carbon/HAp composite. It has been reported that the pyrolysis process at high temperatures can significantly reduce the carbon content in the carbon/HAp composite formed during the pyrolysis of PPFS.
3.
(a) EDX spectrum and (b) SEM image of the sample elemental mapping. (c) Si, (d) O, (e) C, (f) Ca, (g) Mg, and (h) P of the n-HAp powder from pyrolysis of PPFS.
The morphology of the neat PPFS powder and the n-HAp synthesized from the pyrolysis of the PPFS is imaged by using SEM. Figure a shows the SEM image of the PPFS with large, irregular, and agglomerated particles. The SEM image of n-HAp synthesized from pyrolysis of PPFS in Figure b confirms the presence of n-HAp particles, showing good agreement with the average particle size calculated from the Debye–Scherrer equation. It has been observed that the n-HAp was seen agglomerated in the SEM image with a large mean size, probably because of the significant improvement in nucleation at high temperatures. TEM image of the n-HAp synthesized from the pyrolysis of PPFS in Figure c also confirms the nanoparticle size and aggregation of particles in the sample.
4.
(a) SEM image obtained for neat PPFS powder, (b) n-HAp powder from pyrolysis of PPFS, and (c) TEM image of the n-HAp powder from pyrolysis of PPFS.
Figure a shows the thermal degradation behavior of the n-HAp synthesized using TGA under air and N2 atmosphere, heated from 30 −900 °C. The final weight loss obtained for the n-HAp samples in N2 and air was almost the same, with nearly 11.5% of the initial weight. This slight weight loss from the n-HAp sample might be the loss of residue formed during pyrolysis from burning carbon and other organic compounds in PPFS. It was observed that the TGA curves of n-HAp under N2 exhibited a three-step weight loss, whereas in air, they exhibited a four-step weight loss. Both the n-HAp samples showed an initial weight loss in the TGA curve due to the water loss and dehydration of the HAp. In the TGA curve obtained for the n-HAp in N2, from 350–675 °C, a significant weight loss of nearly 7.5% was measured, with an initial decomposition temperature of 604.2 °C. The TGA curve for n-HAp in the air exhibited initial decomposition temperatures at 393.25, 646.60, and 823.38 °C attributed to the weight loss of carbonized organic compounds, dehydroxylation, loss of hydroxyl group, and minor decomposition, respectively. Figure b gives the FTIR spectrum obtained for HAp, which helps identify the functional groups in the sample. The ion stretching vibration of the phosphate group (PO4 3–) around 985–1080 cm–1 and the carbonyl group (CO3 2–) around 1415–1500 cm–1 were observed in the FTIR spectrum. The vibration mode of hydroxyl groups (OH−) was also observed around 2500–3600 cm–1, representing n-HAp already reported. ,
5.

(a) TGA in air and N2 atmosphere and (b) FTIR spectrum of n-HAp powder from the pyrolysis of PPFS.
3.2. Characterization of PLA_HAp Films
The blown film extruded films of neat PLA and PLA_HAp composite with different n-HAp wt % of 0.25, 0.5, 0.75, and 1 were analyzed using XRD, as shown in Figure . The neat PLA films exhibited a broad diffraction peak centered at 2θ approximately 16°, which indicates the structure is amorphous.
6.

XRD analysis of the PLA_HAp composite blown films at different wt % of hydroxyapatite.
It was observed that for the PLA_HAp composite films, the diffraction peaks of the HAp were masked by the PLA crystalline diffraction at lower concentrations of n-HAp. A characteristic peak of HAp at 2θ = 21.86° associated with (200) was exhibited by the PLA_HAp 0.75% and PLA_HAp 1% composite films in the XRD spectrum. The broadening of the PLA peak was observed with an increase in HAp concentration, showing reduced crystallinity. The spectrum does not exhibit any additional diffraction peaks or peak shifts in the XRD of PLA composite films, probably due to the low concentration of n-HAp, suggesting that no structural modification took place in the PLA matrix while incorporating HAp. ,
To investigate the interaction of n-HAp in the PLA composite film, the FTIR spectrum (Figure a) was obtained for neat PLA,n-HAp, and PLA_HAp composite films. The FTIR results obtained showed good agreement with the FTIR spectrum reported for PLA_HAp 3D filaments and PLA_HAp solution-casted films. The IR spectrum of the Neat PLA films showed strong characteristic stretching peaks at 1743.2 cm–1 corresponding to the CO bond. The peak at 1452.3 cm–1 is attributed to deformation of the CH3 group. The peaks at 2995.4, 2916.2, and 2848.8 cm–1 correspond to the C–H symmetric and asymmetric stretching of −CH3 are shown as 1, 2, and 3 peaks in Figure b, respectively. The most characteristic absorption peaks at 1184.2 and 1080.6 cm–1 are identified as the backbone of ester groups in PLA. The peaks at 1045 cm–1 refer to the OH bending, and 1200–1000 cm–1 refers to the C–O stretching. The prominent phosphate peaks (PO4) of n-HAp were found to overlap with the PLA.
7.
PLA_HAp composite blown films at different wt % of hydroxyapatite (a) FTIR spectrum, (b) FTIR spectrum from 2700 to 3100 cm–1, and (c) Raman spectrum.
The polymeric composite film spectra exhibited a similar structure of the neat PLA. However, it was interesting to observe that PLA_HAp 0.5% (Figure b) was distinct from neat PLA and other composite samples. PLA_HAp 0.5% exhibited high absorption intensities at 1743.2 cm–1 (CO stretching), 1452.3 cm–1 (asymmetric bending of CH3), 1184.2 cm–1, and 1080.6 cm–1 (asymmetric C–O–C asymmetric rocking CH3) peaks and very low absorption intensity at 2995.4, 2916.2, and 2848.8 cm–1 (−CH symmetric and asymmetric stretching) with two additional peaks formed at 2945.3 and 2881.6 cm–1 in the −CH stretching region. It has been reported that the peaks at 2945.3 and 2881.6 cm–1 correspond to the asymmetric stretching of CH3 and symmetric stretching of CH3, respectively. The FTIR spectrum of the PLA_HAp films indicates that the HAp filler is mechanically embedded with the PLA matrix rather than through chemical bonding. This can be attributed to the uneven distribution of the HAp particles in the PLA matrix.
Figure b illustrates the Raman spectrum obtained for the PLA_HAp composite films. The characteristic peaks of PLA at 2997.5, 2953.2, and 2884.7 cm–1 correspond to the stretching modes of the CH3 and CH of the main and side chains in PLA. The easter group of the PLA with stretching modes of CO appeared at 1769.5 cm–1. The Raman peaks obtained at 1128, 1052, 740, and 658 cm–1 correspond to the stretching modes of C–C, and the characteristic peak at 875 cm–1 corresponds to the stretching vibration of C–COO groups. , These characteristic peaks of the neat PLA were retained in all of the PLA_HAp film samples. Raman spectrum obtained for HAp powder (Figure c) with a D peak at 1319.8 cm–1, G peak at 1593.6 cm–1, and a prominent HAp peak at 960 cm–1 was not identified in the PLA_HAp films, probably due to the low concentration of HAp in the PLA_HAp film samples. Raman spectrum also confirms that the HAp has not chemically bonded to the PLA backbone.
For the analysis of HAp filler dispersion in the PLA composite blown films, the surface morphology of the films using SEM was imaged. Figure a–c compares the SEM image of the neat PLA film with PLA_HAp 0.25% and PLA_HAp 1% films. The neat PLA film exhibited a clear surface, while the PLA_HAp composite films showed HAp particle distribution on the surface. Notably, the particle distribution in the composite film increased with the concentration of filler, with the 1 wt % of HAp filler showing a distribution of the filler with smaller and larger particles of HAp embedded in the surface of the PLA_HAp composite film.
8.
SEM image of the PLA_HAp composite blown films showing particle distribution in film (a) neat PLA, (b) PLA_HAp 0.25, and (c) PLA_HAp 1.
3.3. Thermal Property Analysis of PLA_HAp Films
The TGA and DTG thermograms obtained for the neat PLA and PLA_HAp composite films are shown in Figure a,b, respectively. The TGA and DTG curves of neat PLA films were compared with those of the PLA_HAp composite films, highlighting the impact of the HAp filler on the thermal degradation behavior. The thermal degradation values obtained from the TGA and DTG thermograms are summarized in Table . The TGA analysis of the films infers that the thermal decomposition resistance of the neat PLA film is higher than that of the PLA_HAp composite films, exhibiting consistency with the findings from prior studies. , Reinforcing the idea that the presence of a HAp filler adversely affects the thermal stability of the composites. The initial degradation temperature of the composite film decreased as filler concentration increased, and PLA_HAp 1% films exhibited the least thermal stability with a temperature difference of 17.7 °C from the neat PLA film. It has been reported that the interfacial interaction of the HAp filler with the PLA is influenced by the electrostatic attraction of the Ca2+ ions in the HAp and the carboxylate group in the PLA polymer that resulted in the reduction in thermal stability of the composite film compared to neat PLA. , Laput et al. have reported the XPS analysis of the PLA/HAp composite prepared using solution mixing showing changes in peak intensities of C, O, Ca2+, and P. The final degradation and major degradation temperatures of the PLA_HAp composite showed variation in temperature with increasing filler concentration, which was attributed to the uneven distribution of particles or agglomerated particles in the composite film.
9.
PLA_HAp composite blown films at different wt % of hydroxyapatite (a) TGA curves and (b) DTG curves.
1. Summary of the TGA Data Obtained for the PLA_HAp Composite Films.
| samples | initial degradation temp (°C) | final degradation temp (°C) | major degradation temp (°C) |
|---|---|---|---|
| Neat PLA | 344.10 | 381.80 | 373.19 |
| PLA_HAp 0.25 | 332.07 | 372.10 | 361.15 |
| PLA_HAp 0.5 | 333.55 | 368.12 | 358.55 |
| PLA_HAp 0.75 | 332.85 | 374.71 | 363.89 |
| PLA_HAp 1 | 326.46 | 363.68 | 353.06 |
The thermal behavior of the PLA composite films was further analyzed using DSC, and the DSC curves obtained for the first heating and cooling cycles of the polymer film samples are shown in Figure a,b. The glass transition temperature (T g), crystallization peak (T c), melting peak (T m), enthalpy, and calculated degree of crystallinity (χc) values obtained for the PLA composite film samples from DSC are summarized in Table .
10.
PLA_HAp composite blown films at different wt % of hydroxyapatite (a) DSC heating curves and (b) DSC cooling curves.
2. Summary of the DSC Data Obtained for PLA_HAP Films.
| first
heating cycle |
crystallinity |
|||||
|---|---|---|---|---|---|---|
| samples | glass transition temperature T g (°C) | Tc (°C) | ΔH c (J/g) | TM(°C) | ΔH m(J/g) | χc (%) |
| Neat PLA | 61.91 | 94.52 | 17.93 | 169.33 | 36.46 | 19.7 |
| PLA_HAp 0.25 | 55.08 | 94.51 | 16.27 | 169.93 | 38.32 | 23.5 |
| PLA_HAp 0.5 | 56.01 | 92.39 | 19.27 | 169.78 | 40.85 | 23.0 |
| PLA_HAp 0.75 | 53.13 | 91.25 | 16.99 | 169.17 | 36.72 | 21.0 |
| PLA_HAp 1 | 55.61 | 92.50 | 17.93 | 169.46 | 37.90 | 21.3 |
Compared to the neat PLA film, the PLA_HAp film exhibited a decrease in the T g value, probably due to the interaction of the HAp filler with the composite film. It has been reported that a reduction in T g might result from an increase in the interfacial area between the HAp and PLA, which, in turn, obstructs the molecular mobility of the polymer molecule. The differences in the T g values of the PLA composite films with increasing HAp filler concentration might be due to the nonuniform distribution or aggregation of the filler in PLA composite films. The peaks around 85–95 °C and 165–175 °C correspond to the crystallization and melting temperatures of the polymer films. A slight variation in T c with a 2–3 °C shift toward lower temperature was observed in PLA_HAp composite films compared to neat PLA, indicating a faster rate of crystallization attributed to the HAp acting as a nucleation site in the PLA matrix. , Numerous studies have acknowledged that the incorporation of fillers in polymer composites can significantly affect the crystallization of the polymer matrix, either by increasing or decreasing the crystallization rate. No significant variation was observed in the melting peaks of the polymer composite films compared to the neat PLA film with an increasing concentration of HAp filler. The cooling curve obtained for the polymer samples in Figure b exhibited broad melt crystallization peaks for the PLA_HAp composite films, whereas a faint peak was observed for the neat PLA.
It has been reported that the faint melt crystallization obtained for the neat PLA film is due to the poor crystalline ability of the PLA during the cooling cycle at 10 °C/min. However, the composite films exhibited significantly large and broad melt crystalline peaks during the cooling cycle. The cold crystallization peak shifted to a lower temperature of 98–102 °C with increasing filler concentrations in the PLA matrix.
DSC curves were used to investigate the crystallinity behavior of PLA_HAp composite films. Based on the first heating cycle, the crystallinity (χc) of the composite film samples was calculated using the eq below.
| 2 |
where ΔH m denotes the crystallization enthalpy of the samples in (J/g), ΔH c is the cold crystallization enthalpy, ΔH°m represents the melting enthalpy of 100% crystalline PLA that is 93.7 J/g. ,
The crystallinity obtained for the different PLA_HAp blown films is given in the last column of Table . The crystallinity values increased with the addition of n-HAp in the PLA composite films compared with neat PLA films. However, the crystallinity decreased with increasing concentrations of n-HAp in the films, attributed to the reduction in the orientation of the PLA polymer chain in the presence of n-HAP. It can be observed that there is hardly any change in the melting point of the PLA_HAp composite films compared to neat PLA. The DSC analysis reveals that adding n-HAp has influenced the PLA polymer matrix, causing changes in polymer chain arrangements while heating. That confirms that n-HAp has acted as a nucleating center in the PLA matrix.
3.4. Mechanical Property Analysis of PLA_HAp Films
The PLA_HAp blown films were tested for their mechanical properties. The tensile stress versus strain plot for the PLA_HAp blown films is shown in Figure a, and the bar plot with error bars for the tensile parameters is given in Figure b. Tensile parameters obtained during tensile testing are summarized in Table . As usual, the neat PLA exhibited high Young’s modulus and tensile strength of 2286 and 62.5 MPa, respectively. The low concentrations of the n-HAp (0.25, 0.5, 0.75, and 1 wt %) in the PLA matrix have significantly reduced the tensile strength, probably due to the reduced crystallinity. As the concentration of n-HAp increased from 0.25 to 1 wt %, the tensile strength of the polymer gradually increased. Most of the reported studies on PLA_HAp composite with very high concentrations of HAp (more than 1% up to 20%) exhibited a significant increase in tensile strength. , However, the present studies show that a low concentration of n-HAp, less than 0.75 wt % in the PLA matrix, can significantly increase the elongation at break, making it more flexible. PLA_HAp with 0.5 wt % of n-HAp exhibited high elongation at a break of nearly 20%, which is a 400% enhancement compared to neat PLA. It is understood that 0.5 wt % n-HAp made a perfect interface with the PLA matrix, improving structural integrity and resisting mechanical deformation. As the filler concentration increased beyond 0.5 wt %, the PLA exhibited reduced flexibility, likely due to particle aggregation, which restricted polymer mobility within the PLA matrix. ,
11.

Tensile results were obtained for the PLA_HAp composite blown films at different wt % of HAp in PLA. (a) Strain–Stress curve and (b) bar plot with error bars for tensile analysis.
3. Summary of Tensile Study of Blown Film Samples.
| sample | Young’s modulus MPa | tensile, F max MPa | elongation at break (%) |
|---|---|---|---|
| Neat PLA | 2286.0 ± 110 | 62.5 ± 8.4 | 4.41 ± 2.5 |
| PLA_HAp 0.25 | 1361.3 ± 127 | 43.5 ± 1.7 | 7.21 ± 2.0 |
| PLA_HAp 0.5 | 1427.2 ± 64 | 42.7 ± 2.4 | 19.21 ± 1.7 |
| PLA_HAp 0.75 | 1382.6 ± 85 | 42.2 ± 2.5 | 12.4 ± 1.8 |
| PLA_HAp 1 | 1660.0 ± 110.2 | 50.3 ± 2.4 | 4.03 ± 1.2 |
Figure a–d illustrates the SEM image of the fracture analysis of the neat PLA and PLA_HAp 0.5% composite films after the tensile test, respectively. The fracture surface of the neat PLA films exhibited relatively smooth surfaces with minimum defects on the surface, as shown in Figure a,b, inferring to be a brittle fracture. Meanwhile, the PLA_HAp 0.5% composite sample exhibited intensive deformation on the surface, indicating a ductile surface. The surface reveals the synergic effect of the HAp filler with the polymer matrix, which transforms it from brittle to a plastic deformation.
12.
Fracture analysis of the PLA_HAp composite blown films after tensile testing. (a, b) Neat PLA film and (c, d) PLA_HAp 0.5 films.
3.5. Water Vapor Permeability Studies of the Films
Neat PLA and PLA_HAp composite film samples were tested for water vapor permeability by using ASTM F-1249. The samples were tested under ambient and tropical conditions, with 23 °C/50% RH and 35 °C/85% RH, respectively. Table summarizes the WVTR results obtained for the PLA blown film samples. The results show that the addition of HAp to PLA significantly reduces water vapor permeability with an increasing concentration. PLA_HAp 0.5 and PLA_HAp 1 composite films exhibited decreases in water permeability of 56 and 66%, respectively, under ambient conditions. In contrast, PLA_HAp 0.5 and PLA_HAp 1 composite films exhibited decreases in water permeability of 64 and 72%, respectively, under tropical conditions. Thool et al. have reported that n-HAp in cassava starch composite films results in reduced WVTR as the concentration of n-HAp increases. It is understood that the HAp in the PLA matrix improves the interfacial interaction, thereby decreasing the porosity and free space in the matrix, which in turn reduces water vapor permeability through the films.
4. Summary of the WVTR Results Obtained for the PLA Blown Films.
| samples | ambient condition (23 °C/50% RH) average WVTR | tropical condition (35 °C/85% RH) average WVTR |
|---|---|---|
| neat PLA | 143.5 ± 19.2 | 580.5 ± 25.4 |
| PLA_0.5HAp | 62.8 ± 1.1 | 210.5 ± 3.5 |
| PLA_1HAp | 49.4 ± 1.2 | 160 ± 5.3 |
4. Conclusions
Nanohydroxyapatite was synthesized from the pyrolysis of the PPFS and effectively used as a filler in the PLA matrix to enhance the thermal and mechanical properties of PLA_HAp composite films. The PLA_HAp composite films have different wt % (0.25, 0.5, 0.75, and 1) of n-HAP that were blown film-extruded. The blown films of PLA_HAp 0.25, PLA_HAp 0.5, PLA_HAp 0.75, and PLA_HAp 1 were characterized by using FTIR, XRD, and Raman spectroscopy to find the interaction of n-HAp in the PLA matrix. Thermal properties of the PLA_HAp films were analyzed by using TGA and DSC. The thermal behavior of the PLA_HAp films confirms that the n-HAp has acted as a nucleating center in the PLA matrix. The tensile studies have shown that 0.5 wt % of n-HAp in PLA exhibited a maximum elongation at a break of 20%. The WVTR shows that increasing the concentration of HAp in PLA reduces the water permeability for PLA_HAp composite films. The study on thermal, mechanical, and barrier properties indicates that a low concentration of n-HAp reinforced PLA composite films can significantly enhance the flexibility, crystallinity, and barrier performance of PLA_HAp blown films, making them highly suitable for food packaging applications. Future research will explore the impact of n-HAp on the biodegradability of PLA composite films in soil.
Acknowledgments
The authors would like to acknowledge the financial support from MARS Wrigley, Global Innovation Center, Chicago, IL., NSF CREST #1735971, and DMR# 2117242.
The authors declare no competing financial interest.
References
- Li S., Ciardullo K., Donner E., Thompson M. R., Rempel C., Liu Q.. Reactive Extrusion Preparation and Characterization of Canola Meal Composites Reinforced by a Novel Polymeric Chain Extender. Mater. Des. 2018;138:1–10. doi: 10.1016/j.matdes.2017.10.053. [DOI] [Google Scholar]
- Weal S., Shah S., Parker K., Vaidya A.. Incorporation of Canola Meal as a Sustainable Natural Filler in PLA Foams. Bioresour. Bioprocess. 2024;11(1):57. doi: 10.1186/s40643-024-00773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikiaris N. D., Koumentakou I., Samiotaki C., Meimaroglou D., Varytimidou D., Karatza A., Kalantzis Z., Roussou M., Bikiaris R. D., Papageorgiou G. Z.. Recent Advances in the Investigation of Poly(Lactic Acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and Their Properties and Applications. Polymers. 2023;15:1196. doi: 10.3390/polym15051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeen, L. 12 - Renewable Resource and Biodegradable Polymers. In Plastics Design Library; McKeen, L. ; Third Ed.; William Andrew Publishing: Boston, 2012; 305–317. [Google Scholar]
- Khouri N. G., Bahú J. O., Blanco-Llamero C., Severino P., Concha V. O. C., Souto E. B.. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications–A Review of the Literature. J. Mol. Struct. 2024;1309:138243. doi: 10.1016/j.molstruc.2024.138243. [DOI] [Google Scholar]
- Sahini M. G.. Polylactic Acid (PLA)-Based Materials: A Review on the Synthesis and Drug Delivery Applications. Emergent Mater. 2023;6(5):1461–1479. doi: 10.1007/s42247-023-00551-7. [DOI] [Google Scholar]
- Swetha T. A., Bora A., Mohanrasu K., Balaji P., Raja R., Ponnuchamy K., Muthusamy G., Arun A.. A Comprehensive Review on Polylactic Acid (PLA)–Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023;234:123715. doi: 10.1016/j.ijbiomac.2023.123715. [DOI] [PubMed] [Google Scholar]
- Fiori, S. Industrial Uses of PLA. Polymer Chemistry Series; Royal Society of Chemistry, 2014, 315–333. [Google Scholar]
- Cheng Y., Deng S., Chen P., Ruan R.. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China. 2009;4(3):259–264. doi: 10.1007/s11458-009-0092-x. [DOI] [Google Scholar]
- Murariu M., Dechief A.-L., Ramy-Ratiarison R., Paint Y., Raquez J.-M., Dubois P.. Recent Advances in Production of Poly(Lactic Acid) (PLA) Nanocomposites: A Versatile Method to Tune Crystallization Properties of PLA. Nanocomposites. 2015;1(2):71–82. doi: 10.1179/2055033214Y.0000000008. [DOI] [Google Scholar]
- Zhao X., Liu J., Li J., Liang X., Zhou W., Peng S.. Strategies and Techniques for Improving Heat Resistance and Mechanical Performances of Poly(Lactic Acid) (PLA) Biodegradable Materials. Int. J. Biol. Macromol. 2022;218:115–134. doi: 10.1016/j.ijbiomac.2022.07.091. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Ghalsasi P., Radha P.. Insight into Nano-Fillers and Their Reinforcement onto Polylactic Acid. J. Inorg. Organomet. Polym. Mater. 2023;33(5):1119–1133. doi: 10.1007/s10904-023-02605-z. [DOI] [Google Scholar]
- Syuhada D. N., Azura A. R.. Waste Natural Polymers as Potential Fillers for Biodegradable Latex-Based Composites: A Review. Polymers. 2021;13:3600. doi: 10.3390/polym13203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahdan D., Rosli N. A., Chen R. S., Ahmad S., Gan S.. Strategies for Strengthening Toughened Poly(Lactic Acid) Blend via Natural Reinforcement with Enhanced Biodegradability: A Review. Int. J. Biol. Macromol. 2023;251:126214. doi: 10.1016/j.ijbiomac.2023.126214. [DOI] [PubMed] [Google Scholar]
- Li G., Zhao M., Xu F., Yang B., Li X., Meng X., Teng L., Sun F., Li Y.. Synthesis and Biological Application of Polylactic Acid. Molecules. 2020;25(21):5023. doi: 10.3390/molecules25215023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano V., Khan S., Tabada A.. Applications of PLA in Modern Medicine. Eng. Regen. 2020;1:76–87. doi: 10.1016/j.engreg.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore T. H. M., Patil A. Y., Hegde C., Sudeept M. A., Kumar R., Soudagar M. E. M., Fattah I. M. R.. Apatite Insights: From Synthesis to Biomedical Applications. Eur. Polym. J. 2024;209:112842. doi: 10.1016/j.eurpolymj.2024.112842. [DOI] [Google Scholar]
- Varadavenkatesan T., Vinayagam R., Pai S., Kathirvel B., Pugazhendhi A., Selvaraj R.. Synthesis, Biological and Environmental Applications of Hydroxyapatite and Its Composites with Organic and Inorganic Coatings. Prog. Org. Coatings. 2021;151:106056. doi: 10.1016/j.porgcoat.2020.106056. [DOI] [Google Scholar]
- Manjudevi M., Kamaraj M., Aravind J., Wong L. S.. Application of the Circular Economy to Fish Scale Waste. Sustain. Chem. Environ. 2024;8:100170. doi: 10.1016/j.scenv.2024.100170. [DOI] [Google Scholar]
- Nihmath A., Ramesan M. T.. Hydroxyapatite as a Potential Nanofiller in Technologically Useful Chlorinated Acrylonitrile Butadiene Rubber. Polym. Test. 2020;91:106837. doi: 10.1016/j.polymertesting.2020.106837. [DOI] [Google Scholar]
- Thool O. K., Sasidharan A., Krishna B. M., Sabu S., Navaf M., Sunooj K. V.. Nano-Hydroxyapatite (n-HAP) from Pangasius Bone Side Streams and Its Application as a Reinforcing Agent in Biodegradable Food Packaging Films. Sustain. Food Technol. 2025;3:227–238. doi: 10.1039/D4FB00264D. [DOI] [Google Scholar]
- Zaharescu T., Tardei C., Râpă M., Iordoc M.. Size Particle Effects on the Thermal Stability of Poly(Lactic Acid)/Hydroxyapatite Hybrids for Biodegradable Package. Ceram. Int. 2020;46(6):7288–7297. doi: 10.1016/j.ceramint.2019.11.223. [DOI] [Google Scholar]
- Osial M., Wilczewski S., Godlewska U., Skórczewska K., Hilus J., Szulc J., Roszkiewicz A., Dąbrowska A., Moazzami Goudarzi Z., Lewandowski K., Wypych T. P., Nguyen P. T., Sumara G., Giersig M.. Incorporation of Nanostructural Hydroxyapatite and Curcumin Extract from Curcuma Longa L. Rhizome into Polylactide to Obtain Green Composite. Polymers (Basel) 2024;16(15):2169. doi: 10.3390/polym16152169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Pu’ad N. A. S., Koshy P., Abdullah H. Z., Idris M. I., Lee T. C.. Syntheses of Hydroxyapatite from Natural Sources. Heliyon. 2019;5(5):e01588. doi: 10.1016/j.heliyon.2019.e01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali D., Hembrick-Holloman V., Gunturu D. R., Samuel T., Jeelani S., Rangari V. K.. Influence of Fish Scale-Based Hydroxyapatite on Forcespun Polycaprolactone Fiber Scaffolds. ACS Omega. 2022;7(10):8323–8335. doi: 10.1021/acsomega.1c05593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sricharoen P., Kongsri S., Kukusamude C., Areerob Y., Nuengmatcha P., Chanthai S., Limchoowong N.. Ultrasound-Irradiated Synthesis of 3-Mercaptopropyl Trimethoxysilane-Modified Hydroxyapatite Derived from Fish-Scale Residues Followed by Ultrasound-Assisted Organic Dyes Removal. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-85206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panickar R., Sobhan C. B., Chakravorti S.. Chemical Vapor Deposition Synthesis of Carbon Spheres: Effects of Temperature and Hydrogen. Vacuum. 2020;172:109108. doi: 10.1016/j.vacuum.2019.109108. [DOI] [Google Scholar]
- Vasconcellos L., Dias R., Cairo C., Leite D., Santos E., Campos G., Prado R., Jardini M., Vasconcellos L. G., Carvalho Y.. Porous Titanium Associated with CaP Coating: In Vivo and In Vitro Osteogenic Performance. Mater. Res. 2017;21:e20170557. doi: 10.1590/1980-5373-mr-2017-0557. [DOI] [Google Scholar]
- Padmanabhan V. P., Kulandaivelu R., Panneer D., Vivekananthan S., Suresh S., Lett A.. Microwave Synthesis of Hydroxyapatite Encumbered with Ascorbic Acid Intended for Drug Leaching Studies. Mater. Res. Innov. 2019;24:171–178. doi: 10.1080/14328917.2019.1624940. [DOI] [Google Scholar]
- Harikrishna N., Mahalakshmi S., Kiran Kumar K., Reddy G.. Fish Scales as Potential Substrate for Production of Alkaline Protease and Amino Acid Rich Aqua Hydrolyzate by Bacillus Altitudinis GVC11. Indian J. Microbiol. 2017;57(3):339–343. doi: 10.1007/s12088-017-0664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittitut U., Jettanasen J., Supothina S., Rattanakam R.. Dissolution Performance of Carbon/Hydroxyapatite Nanocomposite Prepared from Fish Scales. Inorganics. 2022;10(12):242. doi: 10.3390/inorganics10120242. [DOI] [Google Scholar]
- Tourbin M., Brouillet F., Galey B., Rouquet N., Gras P., Abi Chebel N., Grossin D., Frances C.. Agglomeration of Stoichiometric Hydroxyapatite: Impact on Particle Size Distribution and Purity in the Precipitation and Maturation Steps. Powder Technol. 2020;360:977–988. doi: 10.1016/j.powtec.2019.10.050. [DOI] [Google Scholar]
- Sathiskumar S., Vanaraj S., Sabarinathan D., Bharath S., Sivarasan G., Arulmani S., Preethi K., Ponnusamy V. K.. Green Synthesis of Biocompatible Nanostructured Hydroxyapatite from Cirrhinus Mrigala Fish Scale–A Biowaste to Biomaterial. Ceram. Int. 2019;45(6):7804–7810. doi: 10.1016/j.ceramint.2019.01.086. [DOI] [Google Scholar]
- Muhammad N., Gao Y., Iqbal F., Ahmad P., Ge R., Nishan U., Rahim A., Gonfa G., Ullah Z.. Extraction of Biocompatible Hydroxyapatite from Fish Scales Using Novel Approach of Ionic Liquid Pretreatment. Sep. Purif. Technol. 2016;161:129–135. doi: 10.1016/j.seppur.2016.01.047. [DOI] [Google Scholar]
- Liu Y., Liu M., Ji S., Zhang L., Cao W., Wang H., Wang S.. Preparation and Application of Hydroxyapatite Extracted from Fish Scale Waste Using Deep Eutectic Solvents. Ceram. Int. 2021;47(7):9366–9372. doi: 10.1016/j.ceramint.2020.12.067. [DOI] [Google Scholar]
- Sirisoam T., Saelee C., Thiansem S., Punyanitya S.. Characteristic, Microstructure and Properties of Dense Hydroxyapatite Ceramic from Cockle Shell for Biomaterials. Mater. Sci. Forum. 2018;940:3–7. doi: 10.4028/www.scientific.net/MSF.940.3. [DOI] [Google Scholar]
- Gheisari H., Karamian E., Abdellahi M.. A Novel Hydroxyapatite–Hardystonite Nanocomposite Ceramic. Ceram. Int. 2015;41:5967–5975. doi: 10.1016/j.ceramint.2015.01.033. [DOI] [Google Scholar]
- Ramos M., Beltran A., Fortunati E., Peltzer M. A., Cristofaro F., Visai L., Valente A. J. M., Jiménez A., Kenny J. M., Garrigós M. C.. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants. 2020;9(5):395. doi: 10.3390/antiox9050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B. W., Ibrahim N. A., Yunus W. M. Z. W., Hussein M. Z., Then Y. Y., Loo Y. Y.. Effects of Graphene Nanoplatelets and Reduced Graphene Oxide on Poly(Lactic Acid) and Plasticized Poly(Lactic Acid): A Comparative Study. Polymers (Basel). 2014;6(8):2232–2246. doi: 10.3390/polym6082232. [DOI] [Google Scholar]
- Lu F., Yu H., Yan C., Yao J.. Polylactic Acid Nanocomposite Films with Spherical Nanocelluloses as Efficient Nucleation Agents: Effects on Crystallization. Mechanical and Thermal Properties. RSC Adv. 2016;6(51):46008–46018. doi: 10.1039/C6RA02768G. [DOI] [Google Scholar]
- Custodio C., Broñola P., Cayabyab S. R., Lagura V., Celorico J., Basilia B.. Powder Loading Effects on the Physicochemical and Mechanical Properties of 3D Printed Poly Lactic Acid/Hydroxyapatite Biocomposites. Int. J. Bioprint. 2021;7:326. doi: 10.18063/ijb.v7i1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandele A. M., Constantinescu A., Radu I. C., Miculescu F., Ioan Voicu S., Ciocan L. T.. Synthesis and Characterization of PLA-Micro-Structured Hydroxyapatite Composite Films. Materials (Basel) 2020;13(2):274. doi: 10.3390/ma13020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla P., Mehta R., Berek D., Upadhyay S.. Microwave Assisted Synthesis of Poly(Lactic Acid) and Its Characterization Using Size Exclusion Chromatography. J. Macromol. Sci. Part A Pure Appl. Chem. 2012;A49:963–970. doi: 10.1080/10601325.2012.722858. [DOI] [Google Scholar]
- Monika, Pal A. K., Bhasney S. M., Bhagabati P., Katiyar V.. Effect of Dicumyl Peroxide on a Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate) (PBS)/Functionalized Chitosan-Based Nanobiocomposite for Packaging: A Reactive Extrusion Study. ACS Omega. 2018;3(10):13298–13312. doi: 10.1021/acsomega.8b00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal A. K., Usmani M., Ahammad S. Z., Saha S.. Unveiling the Slow Release Behavior of Hollow Particles with Prolonged Antibacterial Activity. J. Mater. Sci. 2018;53(8):5942–5957. doi: 10.1007/s10853-018-1991-3. [DOI] [Google Scholar]
- Rakmae S., Ruksakulpiwat Y., Sutapun W., Suppakarn N.. Effects of Mixing Technique and Filler Content on Physical Properties of Bovine Bone-Based CHA/PLA Composites. J. Appl. Polym. Sci. 2011;122:2433–2441. doi: 10.1002/app.34422. [DOI] [Google Scholar]
- Aslan M., YERLİ H., Çava K.. Characterisation of 3D Printed Hydroxyapitate Powder (HAp) Filled Polylacticacid (PLA) Composites. Int. J. 3D Print. Technol. Digit. Ind. 2022;6:540–547. doi: 10.46519/ij3dptdi.1172937. [DOI] [Google Scholar]
- Akindoyo J. O., Beg M. D. H., Ghazali S., Heim H. P., Feldmann M.. Impact Modified PLA-Hydroxyapatite Composites–Thermo-Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2017;2018(107):326–333. doi: 10.1016/j.compositesa.2018.01.017. [DOI] [Google Scholar]
- Cucuruz A. T., Andronescu E., Ficai A., Ilie A., Iordache F.. Synthesis and Characterization of New Composite Materials Based on Poly(Methacrylic Acid) and Hydroxyapatite with Applications in Dentistry. Int. J. Pharm. 2016;510(2):516–523. doi: 10.1016/j.ijpharm.2016.01.061. [DOI] [PubMed] [Google Scholar]
- Laput O., Vasenina I., Salvadori M. C., Savkin K., Zuza D., Kurzina I.. Low-Temperature Plasma Treatment of Polylactic Acid and PLA/HA Composite Material. J. Mater. Sci. 2019;54(17):11726–11738. doi: 10.1007/s10853-019-03693-4. [DOI] [Google Scholar]
- Bernardo M. P., da Silva B. C. R., Hamouda A. E. I., de Toledo M. A. S., Schalla C., Rütten S., Goetzke R., Mattoso L. H. C., Zenke M., Sechi A.. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022;12(1):2333. doi: 10.1038/s41598-022-05207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akindoyo J. O., Beg M. D. H., Ghazali S., Heim H. P., Feldmann M.. Effects of Surface Modification on Dispersion, Mechanical, Thermal and Dynamic Mechanical Properties of Injection Molded PLA-Hydroxyapatite Composites. Compos. Part A Appl. Sci. Manuf. 2017;103:96–105. doi: 10.1016/j.compositesa.2017.09.013. [DOI] [Google Scholar]
- Ren Z., Dong L., Yang Y.. Dynamic Mechanical and Thermal Properties of Plasticized Poly(Lactic Acid) J. Appl. Polym. Sci. 2006;101(3):1583–1590. doi: 10.1002/app.23549. [DOI] [Google Scholar]
- Song L., Li Y., Meng X., Wang T., Shi Y., Wang Y., Shi S., Liu L.-Z.. Crystallization, Structure and Significantly Improved Mechanical Properties of PLA/PPC Blends Compatibilized with PLA-PPC Copolymers Produced by Reactions Initiated with TBT or TDI. Polymers (Basel) 2021;13(19):3245. doi: 10.3390/polym13193245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Hou A., Qu J. P.. Phase Morphology and Performance of Supertough PLA/EMA-GMA/ZrP Nanocomposites Prepared through Reactive Melt-Blending. ACS Omega. 2019;4(21):19046–19053. doi: 10.1021/acsomega.9b02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserat B., Joshee N., Mahapatra A., Selling G., Finkenstadt V.. Physical and Mechanical Properties of Extruded Poly(Lactic Acid)-Based Paulownia Elongata Biocomposites. Ind. Crops Prod. 2013;44:88–96. doi: 10.1016/j.indcrop.2012.10.030. [DOI] [Google Scholar]
- Panickar R., Rangari V. K.. Investigation of the Thermal and Mechanical Properties of Hydrolyzed-Collagen-Reinforced Poly(Lactic Acid) Composite Blown Films. ACS Sustain. Resour. Manag. 2025;2(1):62–71. doi: 10.1021/acssusresmgt.4c00282. [DOI] [Google Scholar]
- Gendviliene I., Simoliunas E., Rekstyte S., Malinauskas M., Zaleckas L., Jegelevicius D., Bukelskiene V., Rutkunas V.. Assessment of the Morphology and Dimensional Accuracy of 3D Printed PLA and PLA/HAp Scaffolds. J. Mech. Behav. Biomed. Mater. 2020;104:103616. doi: 10.1016/j.jmbbm.2020.103616. [DOI] [PubMed] [Google Scholar]
- Tazibt N., Kaci M., Dehouche N., Ragoubi M., Atanase L.. Effect of Filler Content on the Morphology and Physical Properties of Poly(Lactic Acid)-Hydroxyapatite Composites. Materials (Basel). 2023;16:809. doi: 10.3390/ma16020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidlou S., Huneault M. A., Li H., Park C. B.. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012;37:1657. doi: 10.1016/j.progpolymsci.2012.07.005. [DOI] [Google Scholar]
- Martin P., Devaux J., Legras R., van Gurp M., van Duin M.. Competitive Reactions during Compatibilization of Blends of Polybutyleneterephthalate with Epoxide-Containing Rubber. Polymer (Guildf). 2001;42:2463. doi: 10.1016/S0032-3861(00)00496-1. [DOI] [Google Scholar]
- Gökmen F. Ö.. Hydroxyapatite-Doped Polyhydroxyethylmethacrylate Hydrogels as Smart Porous Packaging Materials. Food Bioprocess Technol. 2023;16(11):2692–2704. doi: 10.1007/s11947-023-03097-y. [DOI] [Google Scholar]