Abstract
Listeriosis is an important food-borne disease that causes high rates of morbidity and mortality. For reasons that are not clear, most large outbreaks of human listeriosis involve Listeria monocytogenes serotype 4b. Relatively little is known about the pathogenesis of listeriosis following gastrointestinal exposure to food-borne disease isolates of L. monocytogenes. In the present study, we investigated the pathogenesis of systemic infection by the food-borne isolate Scott A in an intragastric (i.g.) mouse challenge model. We found that the severity of infection with L. monocytogenes Scott A was increased in mice made neutropenic by administration of monoclonal antibody RB6-8C5. This observation was similar to a previous report on a study with the laboratory strain L. monocytogenes EGD. Prior administration of sodium bicarbonate did not enhance the virulence of L. monocytogenes strain Scott A for i.g. inoculated mice. Following i.g. inoculation of mice, two serotype 4b strains of L. monocytogenes (Scott A and 101M) achieved a greater bacterial burden in the spleen and liver and elicited more severe histopathological damage to those organs than did a serotype 1/2a strain (EGD) and a serotype 1/2b stain (CM). Of the four strains tested, only strain CM exhibited poor survival in synthetic gastric fluid in vitro. The other three strains exhibited similar patterns of survival at pHs of greater than 5 and relatively rapid (<30 min) loss of viability at pHs of less than 5.0. Growth of L. monocytogenes Scott A at temperatures of 12.5 to 37°C did not affect its ability to cause systemic infection in i.g. inoculated mice. These observations suggest that the serotype 4b L. monocytogenes strains Scott A and 101M possess one or more virulence determinants that make them better able to cause systemic infection following inoculation via the g.i. tract than do the serotype 1/2 strains EGD and CM.
Listeriosis is an important food-borne disease that causes considerable morbidity and relatively high mortality rates (15, 18, 25, 33). It is estimated that L. monocytogenes infection causes approximately 2,500 cases of serious illness and as many as 500 deaths per year in the United States (25). Risk factors for listeriosis include age (>65 years), pregnancy, human immunodeficiency virus infection, immunosuppressive therapy, diabetes, kidney disease, and cancer (25). Because of the risk posed to these susceptible individuals, the United States has a very stringent regulation (less than 1 CFU/25 g of product) for L. monocytogenes contamination in ready-to-eat foods. The resulting recalls of L. monocytogenes-contaminated food products result in large costs to the food industry and, ultimately, consumers on an annual basis.
Sporadic cases of listeriosis can be caused by serotype 4b, 1/2a, or 1/2b L. monocytogenes. However, human epidemics of listeriosis in both the United States and Europe are usually caused by serotype 4b (3, 18, 25, 33). The reason for this association is not known. Some of the more prominent serotype 4b epidemics include an outbreak associated with ingestion of contaminated coleslaw in Nova Scotia (25), a major outbreak resulting from ingestion of soft-style Mexican cheese made with nonpasteurized milk in Los Angeles in the mid-1980s (25), and a huge outbreak in 1997 that involved more than 1,700 individuals and resulted in 292 hospitalized people in Italy (1). What is particularly noteworthy about the latter outbreak is that many of those affected had no known risk factor (other than the relative youth of the schoolchildren involved). The source of exposure in this outbreak was a corn salad, distributed at several school cafeterias, that was heavily contaminated with L. monocytogenes (up to 106 CFU/g). More recently, there was a large U.S. outbreak in 1998 and 1999 resulting from ingestion of frankfurters contaminated with a 4b serotype possessing an unusual ribotype of L. monocytogenes (18). Although most of the cases occurred in people for whom some risk factor could be identified, a few cases occurred in individuals with no known risk factors.
Most previous studies of murine listeriosis required relatively high (often 108 CFU or greater) numbers of L. monocytogenes bacteria to cause systemic infection via the gastrointestinal (g.i.) tract in normal mice (2, 8, 11, 14, 19, 22, 24, 30). There is one report (27) that intragastric (i.g.) inoculation had a lower 50% lethal dose than intraperitoneal (i.p.) inoculation, but that has not been confirmed by other investigators and may have been related, in part, to the use of young mice (15 g) in that study. We hypothesized that the observed relationship between serotype 4b L. monocytogenes and large outbreaks of listeriosis is related to the greater ability of serotype 4b strains of L. monocytogenes to translocate across the g.i. mucosa and cause systemic infection. It has been reported that a greater proportion of serotype 4b strains than of other serotypes can cause systemic infection via the g.i. tract in mice (2). However, a difference in the mean numbers of CFU recovered from the spleens and livers of mice inoculated with serotype 4b versus serotype 1/2 strains of L. monocytogenes was not observed in that study. In the present study, we found that L. monocytogenes strain Scott A (serotype 4b) caused a more severe infection in i.g. inoculated outbred (ICR strain) mice than did L. monocytogenes strain EGD (serotype 1/2a). A second serotype 4b strain of L. monocytogenes (101M) was also more virulent than strain EGD or a serotype 1/2b strain (CM). The findings suggest that at least some serotype 4b L. monocytogenes strains possess one or more virulence determinants that make them more effective pathogens in the g.i. tract than serotype 1/2 strains of L. monocytogenes.
MATERIALS AND METHODS
Strains of L. monocytogenes.
One strain of L. monocytogenes from our laboratory (EGD) and three from the culture collection of the Food Research Institute (Madison, Wis.) were used in this study. For serotype 4b isolates, we chose strain Scott A, which was a clinical isolate obtained from a food-borne disease outbreak (32, 36), and strain 101M, which was isolated at the Food Research Institute from ground beef (unpublished observations). For comparison, we chose the serotype 1/2a strain EGD (previously used in g.i. challenge studies described by our laboratory and those of others) (5, 8,11, 18, 21, 30) and a serotype 1/2b strain (hereafter designated CM) isolated from an unusual outbreak in which people developed g.i. symptoms following ingestion of L. monocytogenes-contaminated chocolate milk (28).
Preparation of L. monocytogenes.
L. monocytogenes was inoculated into brain heart infusion (BHI) broth and incubated overnight with shaking at 37°C. Following this, the bacteria were harvested by centrifugation, resuspended in BHI broth containing 20% glycerol, and stored at −70°C as 0.75-ml aliquots. Before each experiment, an aliquot was thawed, inoculated into 50 ml of BHI broth, and incubated at 37°C with shaking until mid-log-phase growth was reached (approximately 4 h). The numbers of CFU of L. monocytogenes were extrapolated from a standard growth curve. To prepare the inoculum for mice, the bacterial cells were harvested by centrifugation (800 × g at room temperature for 20 min). The bacterial pellet was resuspended in the original volume of phosphate-buffered saline, and appropriate dilutions were made in sterile phosphate-buffered saline to achieve the desired bacterial concentration. The actual number of CFU in the inoculum was verified by plating on blood agar.
Inoculation of mice.
Outbred female ICR mice were obtained (Harlan Sprague-Dawley, Indianapolis, Ind.) at 7 to 8 weeks of age and housed under microisolator caps at the School of Veterinary Medicine animal care facility. Mice received food and water ad libitum until 5 h prior to an i.g. inoculation experiment, at which time food was removed from the cage. This prevented physical blockage of the delivery of the L. monocytogenes inoculum into the stomachs of mice that might otherwise be engorged with mouse chow. We were concerned that if this occurred it might lead to aspiration of the inoculum into the lungs. Mice were anesthetized by i.p. injection of sodium pentobarbital (1 mg per mouse). Once mild sedation occurred, the listerial inoculum was introduced (in a total volume of 0.2 ml) via an infant feeding tube (3.5 French) attached to a 1-ml syringe as described previously (8, 11, 30).
In those experiments in which we wanted to investigate the effects of neutrophil depletion on anti-Listeria resistance, neutropenia was achieved by i.p. injection of monoclonal antibody (MAb) RB6-8C5 (11) 24 h before i.g. inoculation of L. monocytogenes. This treatment substantially depletes mice of neutrophils for at least 4 days without adversely affecting the numbers of other leukocytes or causing any obvious adverse effects in the mice (5, 11, 21). In those experiments in which we wished to neutralize gastric acidity, mice were given sodium bicarbonate (50 μl of a 10% [wt/vol] solution in sterile water) 15 min before i.g. inoculation of L. monocytogenes.
Recovery of L. monocytogenes from the tissues of infected mice.
At the desired time points, mice were humanely euthanized by asphyxiation with CO2, followed by cervical dislocation. The abdominal cavity was then aseptically opened, and portions of the spleen and liver were removed, weighed, and placed in sterile tissue grinders that contained 1 ml of cold sterile saline. The above-described tissues were then homogenized, diluted in sterile saline, and plated in duplicate on blood agar. Additional portions of the above-described organs were removed, placed in plastic cassettes, and fixed in 10% buffered formalin. Following fixation, the tissues were cut into thin sections, mounted on glass slides, and stained with hematoxylin-and-eosin stain and a tissue Gram stain.
Survival of L. monocytogenes strains in synthetic gastric fluid in vitro.
To assess in vitro the relative susceptibility of the different strains of L. monocytogenes to inactivation at the low pH found in the stomach, we used a synthetic gastric fluid solution (8.3 g of Proteose Peptone, 3.5 g of d-glucose, 2.05 g of NaCl, 0.6 g of KH2PO4, 0.11 g of CaCl2, 0.37 g of KCl, 0.1 g of lysozyme, 50 mg of bile, and 13.3 mg of pepsin per liter of distilled water) as described previously (7). The synthetic gastric fluid was adjusted to pH 2.5 by addition of HCl, and then various amounts of sodium bicarbonate (10% [wt/vol] solution) were added to achieve final pHs ranging from 2.5 to 7.0. To test the effects of pH on the survival of each of the strains of L. monocytogenes, 1 ml of log-phase organisms (in sterile saline) was added to 9 ml of gastric fluid (prewarmed to 37°C) and incubated at 37°C. At 30-min intervals, samples were removed from the tubes, diluted in 1% sterile peptone broth, and plated on blood agar. The plates were incubated at 37°C for 48 h, and the resulting numbers of CFU of L. monocytogenes were calculated.
Statistical analysis.
Data were analyzed by a repeated-measures analysis of variance. If a significant F value was obtained (P < 0.05), then the Newman-Keuls multiple-comparison test was performed to compare relevant pairs of data. Statistical significance for all comparisons was set at P < 0.05. All calculations were performed with GraphPad Prism version 3.0 (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Because most large outbreaks of listeriosis have been caused by serotype 4b L. monocytogenes (3, 18, 32), in this study, we decided to investigate the virulence of strain Scott A and a second serotype 4b strain (101M) of L. monocytogenes to cause systemic infection in a previously described mouse model of g.i. listeriosis (11, 30). For comparison, we included the well-characterized laboratory strain EGD (serotype 1/2a) and a serotype 1/2b strain (CM) of L. monocytogenes.
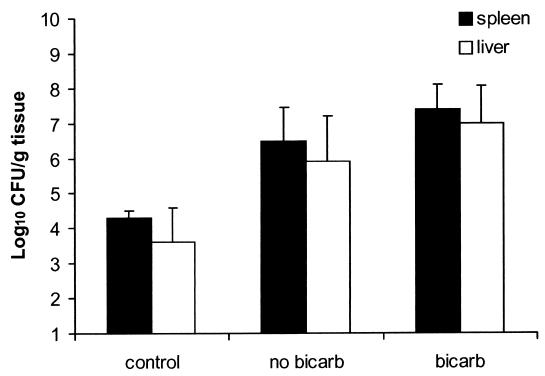
We first tested the serotype 4b food-borne isolate Scott A. We and others have reported previously that normal mice are relatively resistant to i.g. inoculation with L monocytogenes EGD (serotype 1/2a) and that resistance was greatly impaired by injecting mice with a MAb that causes neutropenia (5, 11, 20). As a result, we assumed that rendering mice neutropenic might also be necessary to facilitate invasive systemic infection of mice inoculated i.g. with strain Scott A. However, greater numbers of CFU of strain Scott A were recovered from the spleens and livers of nonneutropenic mice (Fig. 1) than were expected on the basis of our prior experience with L. monocytogenes EGD (30). Neutropenia, resulting from injection of antigranulocyte MAb RB6-8C5, increased the ability of L. monocytogenes Scott A to translocate across the intestinal epithelium and cause systemic infection (Fig. 1). In addition, we found previously that oral administration of sodium bicarbonate shortly before i.g. inoculation enhanced the virulence of L. monocytogenes EGD for mice (9). Although sodium bicarbonate pretreatment resulted in a small additional increase in the numbers of L. monocytogenes Scott A bacteria recovered from the spleens and livers of neutropenic mice, the effect was not statistically significant (P > 0.05). In separate experiments, sodium bicarbonate did not increase the severity of infection of L. monocytogenes Scott A in nonneutropenic mice (data not shown).
FIG. 1.
Effects of neutrophil depletion (MAb RB6-8C5 treatment) and sodium bicarbonate (bicarb) administration on the severity of systemic infection of mice inoculated i.g. with L. monocytogenes strain Scott A. Groups of five mice were injected i.p. with 150 μg of MAb RB6-8C5 or saline (control). Twenty-four hours later, some mice received sodium bicarbonate (50 μl of a 10% solution) 15 min before all of the mice were anesthetized with sodium pentobarbital (10 mg/kg given i.p.). Once the mice were sedated, they received 105 CFU L. monocytogenes Scott A i.g. Three days later, the mice were euthanized and the numbers of CFU of listeriae present in spleen (▪) and liver (□) homogenates were determined as described in Materials and Methods. The data are the mean ± the standard error of the mean of five mice per group.
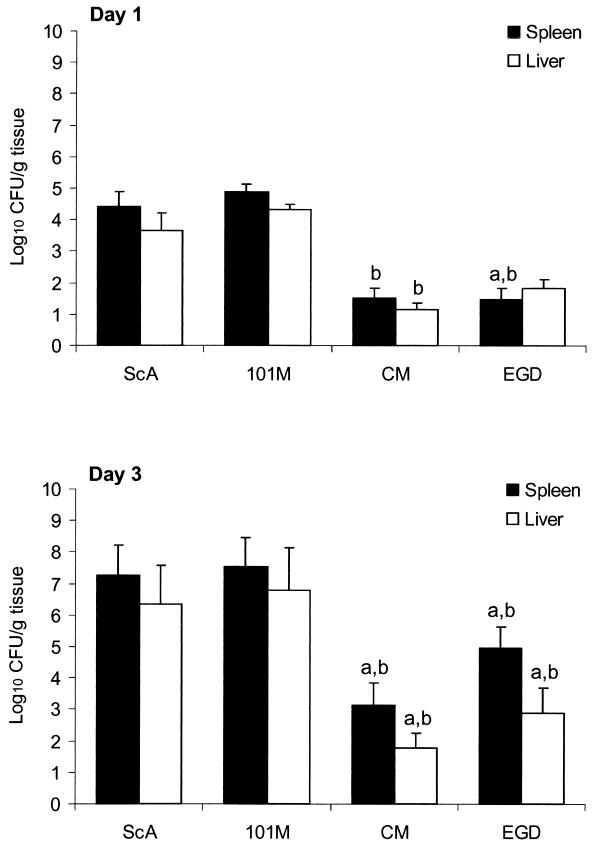
The above-described results suggested that L. monocytogenes strain Scott A (serotype 4b) might be more virulent for i.g. inoculated mice than for the previously studied strain EGD (serotype 1/2a). We therefore decided to directly compare the virulence of strains Scott A and EGD and two other strains (serotypes 4b and 1/2b) of L. monocytogenes following i.g. inoculation of normal (nonneutropenic) mice. As illustrated in Fig. 2, there was no difference in the numbers of CFU of listeriae recovered from the spleens and livers of mice 1 or 3 days after i.g. inoculation with L. monocytogenes strains Scott A and 101M (both serotype 4b). Likewise, there was no significant difference (P > 0.05) between mice infected with strains EGD and CM (serotypes 1/2a and 1/2b, respectively). However, greater numbers of CFU were recovered from the spleens and livers of mice inoculated with L. monocytogenes strains Scott A and 101M (both serotype 4b) than from those inoculated with strains CM (serotype 1/2b) and EGD (serotype 1/2a). Strain CM, in particular, yielded low numbers of CFU from the spleen and liver, whereas strain EGD was somewhat variable and yielded slightly greater numbers of CFU.
FIG. 2.
Systemic infection is more severe in mice inoculated i.g. with L. monocytogenes strains Scott A (ScA) and 101M (both serotype 4b) than in those inoculated with strains CM and EGD (serotypes 1/2b and 1/2a, respectively). Mice were anesthetized with sodium pentobarbital and then inoculated i.g. with 106 CFU of the indicated strains of L. monocytogenes. One and 3 days later, the mice were euthanized and the numbers of CFU of listeriae present in spleen (▪) and liver (□) homogenates were determined as described in Materials and Methods. The data are the mean ± the standard error of the mean of five mice per group. Bars marked with the letter a differ at P < 0.05 from strain Scott A; those marked with the letter b differ at P < 0.05 from strain 101M as determined by analysis of variance, followed by the Newman-Keuls multiple-comparison test.
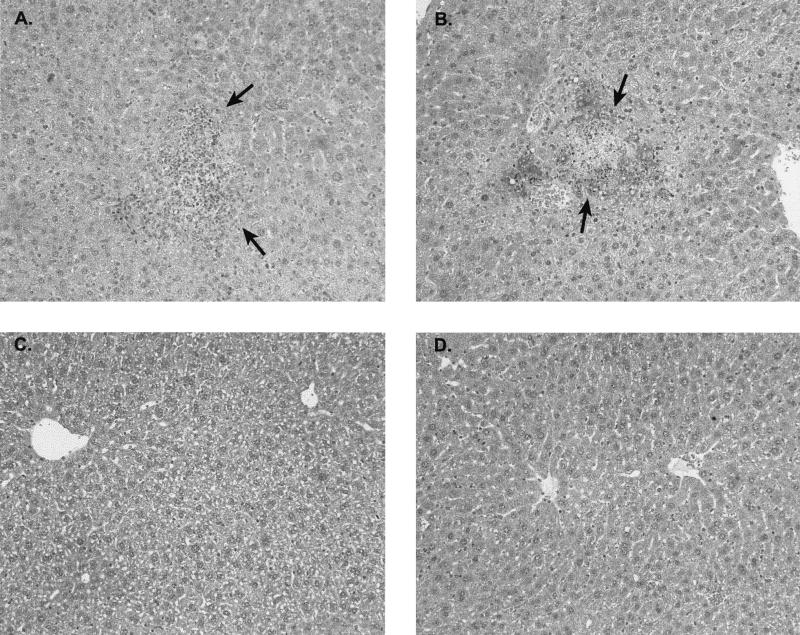
Examination of the histopathological response of spleens and livers from infected mice confirmed the greater virulence of strains Scott A and 101M (serotype 4b) of L. monocytogenes. As summarized in Table 1, generally greater levels of inflammation and necrosis were observed in mice inoculated i.g. with the serovar 4b strain Scott A (Fig. 3A) or 101M (Fig. 3B) than in those infected with the serovar 1/2 strain CM (Fig. 3C) or EGD (Fig. 3D). Likewise, gram-positive rods were observed within hepatocytes from mice infected with the L. monocytogenes serotype 4b strains Scott A and 101M but not in those from mice infected with the serotype 1/2 strains (data not shown).
TABLE 1.
Comparative histopathological evaluation of spleens and livers from mice inoculated i.g. with several strains of L. monocytogenesa
| Listerial strain and mouse no. | Splenic necrosisb | Splenic organismsc | Hepatic inflammation | Hepatic necrosis | Hepatic organisms |
|---|---|---|---|---|---|
| Scott A | |||||
| 1 | 4+ | 1+ | 4+ | 3+ | 3+ |
| 2 | 4+ | 2+ | 3+ | 2+ | 3+ |
| 3 | 2+ | 2+ | 2+ | 2+ | 2+ |
| 4 | 1+ | — | 1+ | — | — |
| 101M | |||||
| 1 | 4+ | 4+ | 4+ | 4+ | 4+ |
| 2 | 4+ | 4+ | 4+ | 4+ | 4+ |
| 3 | 1+ | 1+ | 2+ | 1+ | — |
| 4 | 1+ | — | 1+ | 1+ | — |
| CM | |||||
| 1 | 1+ | — | 1+ | — | — |
| 2 | 1+ | — | 1+ | — | — |
| 3 | 1+ | — | 1+ | 1+ | — |
| 4 | — | — | 1+ | 1+ | — |
| 5 | — | — | 1+ | 1+ | — |
| EGD | |||||
| 1 | — | — | — | — | — |
| 2 | — | — | — | — | — |
| 3 | — | — | — | — | — |
| 4 | — | — | — | — | — |
| 5 | — | — | — | — | — |
Mice were anesthetized and inoculated i.g. with 106 CFU of the indicated strains of L. monocytogenes. Mice were euthanized 3 days after infection, and portions of the spleen and liver were removed to buffered formalin. These were used to prepare slides that were stained with hematoxylin and eosin or a tissue Gram stain and evaluated microscopically by an American College of Veterinary Pathologists board certified pathologist (H.S.). One mouse each died after infection with strain Scott A and strain 101M, and both were lost to histopathological analysis.
Splenic and hepatic necrosis and suppurative inflammation were graded as follows: −, none; 1+, minimal; 2+, mild; 3+, moderate; 4+, severe.
Microscopic semiquantitative evaluation of gram-positive rods at a magnification of ×400 was graded as follows: —, none seen; 1+, rare; 2+, small numbers; 3+, moderate numbers; 4+, large numbers.
FIG. 3.
Representative histopathological lesions in the livers of mice at 3 days after i.g. inoculation with L. monocytogenes strains Scott A (A), 101M (B), CM (C), and EGD (D). Slides were stained with hematoxylin and eosin. Original magnification, ×200.The arrows in panels A and B indicate regions of hepatic necrosis and suppurative inflammation.
Most naturally occurring outbreaks of human listeriosis involve growth of the listeriae in some food product at ambient or refrigerator temperatures rather than at the 37°C commonly used in studies of pathogenesis and host defense (1, 18, 32). We did not observe any difference in the severity of infection among mice inoculated with strain Scott A grown in static BHI broth cultures at temperatures ranging from 12.5 to 37°C (data not shown). This is similar to our previous report that growth of L. monocytogenes EGD at lower temperatures did not enhance its virulence for i.g. inoculated mice (8).
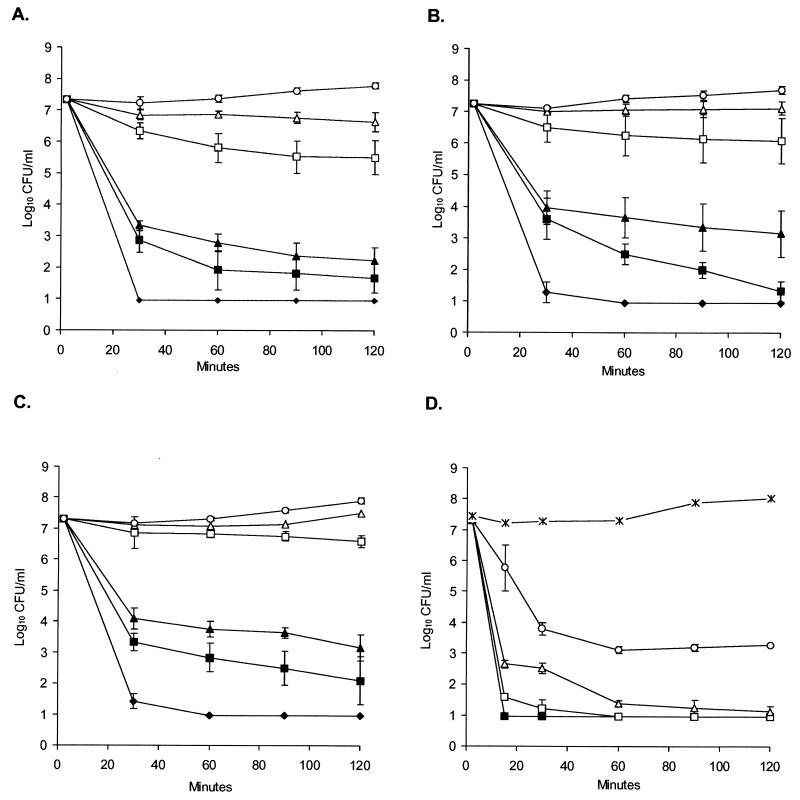
We considered the possibility that the greater virulence of strains Scott A and 101M might reflect their greater resistance to inactivation by the low pH in the stomach. To test this possibility, we compared the survival of the four strains of L. monocytogenes in synthetic gastric fluid as described previously (7). As illustrated in Fig. 4, strains Scott A, 101M, and EGD displayed similar survival patterns in synthetic gastric fluid, being readily inactivated within 30 min at pHs of less than 5. In contrast, strain CM was highly susceptible to inactivation in synthetic gastric fluid, even at pH 7. Although these data may provide some insight into why strain CM is relatively avirulent in our mouse model, they do not explain the relative differences in virulence noted between the serotype 4b strains Scott A and 101M, and the serotype 1/2a strain EGD.
FIG. 4.
Survival curves of L. monocytogenes strains Scott A (A), 101M (B), EGD (C), and CM (D) in synthetic gastric fluid. Approximately 107 CFU of the indicated strains were suspended in synthetic gastric fluid (see Materials and Methods) to which various amounts of sodium bicarbonate were added to adjust the pH to 7.0 (○), 6.0 (▵), 5.0 (□), 4.0 (▴), 3.5 (▪), or 2.5 (⧫) and incubated at 37°C. As an additional control, strain CM was incubated in the same concentration of Proteose Peptone as the synthetic gastric fluid without the other supplements (×). At the indicated time points, samples were removed, diluted in 1% peptone broth, and plated on blood agar. Results are the mean ± the standard error of the mean of three experiments.
DISCUSSION
Previous reports from our laboratory and those of other investigators have generally found that relatively high numbers of L. monocytogenes bacteria (108 CFU or greater) are required to cause significant systemic infection following i.g. inoculation of mice (2, 8,11, 14, 18, 19, 22-24, 30). These inoculation doses are substantially greater than what is generally found in L. monocytogenes-contaminated food products. With one notable exception (27), prior reports indicated that only when the innate defense mechanisms of mice were compromised (e.g., by elimination of neutrophils or T cells) was systemic infection achieved with smaller i.g. challenge doses (5, 10, 11, 16, 24). However, some of these previous studies were performed with serotype 1/2 laboratory strains of L. monocytogenes (i.e., EGD and others) that were not obtained from contaminated food or food-borne disease outbreaks. In contrast, the present study demonstrates that two serotype 4b strains of L. monocytogenes (i.e., Scott A and 101M) can cause significant systemic infection in normal mice following i.g. inoculation with lower numbers of listeriae (104 to 106 CFU). These challenge doses are closer to what one might expect to find within contaminated food products. This finding suggests that perhaps strain Scott A and other serotype 4b strains of L. monocytogenes are better equipped to survive within the g.i. tract or translocate across the intestinal mucosa and multiply within internal organs. This, in turn, would allow the listeriae to multiply more rapidly if there were a concomitant impairment of host defense mechanisms (i.e., neutropenia or immunosuppression). Our observations are consistent with a recent comparison of a number of strains of L. monocytogenes in which serotype 4b strains, as a whole, were consistently able to cause systemic infection in i.g. inoculated mice (2). However, that study used a relatively large i.g. challenge dose (109 CFU) and only examined the mice at a single time point. Furthermore, the mean number of CFU recovered from the spleen did not differ between serotype 4b and 1/2 strains of L. monocytogenes (2)
It has been reported that onset of clinical listeriosis and fecal shedding of L. monocytogenes by humans are exacerbated by antacid treatment (4, 33). In addition, neutralization of the gastric pH has been reported to increase the severity of listeriosis in mice and rats inoculated via the g.i. tract (31, 32). In most cases, this was achieved through inhibition of gastric acid release by H-2 antagonists, such as cimetidine, rather than via neutralization of gastric acid with orally administered sodium bicarbonate. Use of cimetidine in mice is problematic, since experimental studies indicate that cimetidine can impair T-cell production of interleukin-12 and enhance production of interleukin-10 (13). This type of cytokine response would be expected to be detrimental to anti-Listeria resistance (34, 35). We previously reported that oral administration of sodium bicarbonate 15 min before i.g. inoculation with L. monocytogenes EGD enhanced the severity of infection of neutropenic mice (9). However, in the present study, infections with serotype 4b strain Scott A were not significantly affected by sodium bicarbonate administration. The reason for the different effects of sodium bicarbonate on systemic infection of mice by i.g. inoculation with L. monocytogenes strains EGD and Scott A is unclear. Although this finding might suggest that strain Scott A is better able to survive transit through the low-pH environment of the stomach, our in vitro analysis of survival in synthetic gastric fluid does not directly support this inference. Only strain CM was markedly different in its ability to survive in synthetic gastric fluid. Serotype 4b strains Scott A and 101M and serotype 1/2a strain EGD exhibited similar survival patterns in synthetic gastric fluid, being rapidly inactivated in 30 min at a pH of ≤5. In contrast, strain CM was rapidly inactivated in synthetic gastric fluid even at pH 7. The reason for the exquisite susceptibility of strain CM is not clear, but it may reflect inactivation by the bile or enzymes in the synthetic gastric fluid. It is interesting that this particular strain was associated with a large outbreak in which most people developed g.i. illness (i.e., diarrhea) but less than 10% required hospitalization for systemic infection (12) following ingestion of chocolate milk that was stored at an improper temperature and contained large numbers of listeriae (12, 28).
Perhaps there are other explanations for the virulence of L. monocytogenes in the g.i. tract. For example, there is evidence that acid-adapted or acid-resistant mutant strains of L. monocytogenes may be more invasive in vitro (6) and virulent in vivo (26). A two-protein environmental sensing system has been reported in L. monocytogenes (7). Perhaps this or some other transcription regulation system involved in the response of L. monocytogenes to low pH and other environmental conditions in the g.i. tract (3, 14, 29) is more effective in serotype 4b strains like Scott A. Recently, a novel gene cassette for the teichoic acids responsible for the serotype 4b antigen phenotype was described (20). Perhaps these changes in the serotype 4b teichoic acids make L. monocytogenes more resistant to the harsh conditions in the g.i. tract or better able to translocate across the gut mucosa. Likewise, serotype 4b strains of L. monocytogenes are reported to be more resistant to heat or cold stress than serovar 1/2a isolates (3). A recent genomic cross-hybridization study reported that 39 DNA fragments, including 15 with homology to bacterial surface proteins, were upregulated in epidemic-associated isolates of L. monocytogenes serotype 4b (17). Completion of the sequencing and subsequent analysis of the genomes of L. monocytogenes Scott A and EGD may further illuminate virulence determinants between the two strains that might be associated with the ability to cause systemic infection following g.i. listeriosis.
In summary, the results of this study demonstrate that strain Scott A and a second serotype 4b strain (101M) of L. monocytogenes are more virulent when inoculated into the g.i. tracts of normal mice than is the well-characterized laboratory strain EGD (serotype 1/2a) or a serotype 1/2b strain (CM) isolated from an outbreak of human g.i. illness. This finding is consistent with epidemiological evidence that most large outbreaks of listeriosis are caused by serotype 4b strains of L. monocytogenes. The severity of infection by all four strains was enhanced in neutropenic mice (data not shown for strains 101M and CM), suggesting that neutrophils are essential for host defense against L. monocytogenes bacteria that have translocated across the g.i. tract in mice. The mechanism that makes serotype 4b strains more effective at causing systemic infection when they are introduced via the g.i. tract is unknown, but it could reflect greater attachment to or invasion of intestinal epithelial cells (19, 23) or a better ability to respond to the environmental signals that listeriae encounter in the gut.
Acknowledgments
This work was supported by funding from the Food Research Institute, U.S. Department of Agriculture National Alliance for Food Safety, and the National Institutes of Health.
REFERENCES
- 1.Aureli, P., G. C. Fiorucci, D. Caroli, G. Marchiaro, O. Novara, L. Leone, and S. Salmaso. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342:1236-1241. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buncic, S., S. M. Avery, J. Rocourt, and M. Dimitrijevic. 2001. Can food-related environmental factors induce different behaviour in two key serovars, 4b and 1/2a, of Listeria monocytogenes? Int. J. Food Microbiol. 65:201-212. [DOI] [PubMed] [Google Scholar]
- 4.Cobb, C. A., G. D. W. Curtis, D. S. Bansi, E. Slade, W. Mehal, R. G. Mitchell, and R. W. Chapman. 1996. Increased prevalence of Listeria monocytogenes in the faeces of patients receiving long-term H-2-antagonists. Gastroenterol. Hepatol. 8:1071-1074. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W. 1997. Neutrophils and tumour necrosis factor-alpha are important for controlling early gastrointestinal stages of experimental murine listeriosis. J. Med. Microbiol. 46:239-250. [DOI] [PubMed] [Google Scholar]
- 6.Conte, M. P., G. Petrone, A. M. Di Biase, M. G. Ammendolia, F. Superti, and L. Seganti. 1997. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb. Pathog. 29:137-144. [DOI] [PubMed] [Google Scholar]
- 7.Cotter, P. D., N. Emerson, C. G. M. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czuprynski, C. J., J. F. Brown, and J. T. Roll. 1989. Growth at reduced temperatures increases the virulence of Listeria monocytogenes for intravenously, but not intragastrically, inoculated mice. Microb. Pathog. 7:213-223. [DOI] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., and N. G. Faith. 2002. Prior administration of sodium bicarbonate enhances the severity of systemic infection in neutropenic mice intragastrically inoculated with Listeria monocytogenes EGD. Clin. Diagn. Lab. Immunol. 9:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czuprynski, C. J., and M. Haak-Frendscho. 1997. Non-specific resistance mechanisms to listeriosis: implications for experimental and naturally occurring infection. Immunol. Rev. 158:47-56. [DOI] [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., C. Theisen, and J. F. Brown. 1996. Treatment with the antigranulocyte monoclonal antibody RB6-8C5 impairs resistance of mice to gastrointestinal infection with Listeria monocytogenes. Infect. Immun. 64:3946-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffin. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 9:130-132. [DOI] [PubMed] [Google Scholar]
- 13.Elenkov, I. J., E. Webster, D. A. Papanicolaou, T. A. Fleisher, G. P. Chrousos, and R. L. Wilder. 2000. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H-2 receptors. J. Immunol. 161:2586-2593. [PubMed] [Google Scholar]
- 14.Farber, J. M., E. Daley, F. Coates, N. Beausoleil, and J. Fournier. 1991. Feeding trials of Listeria monocytogenes with a nonhuman primate model. J. Clin. Microbiol. 29:2606-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farber, J. M., W. H. Ross, and J. Harwig. 1996. Health risk assessment of Listeria monocytogenes in Canada. Int. J. Food Microbiol. 30:145-156. [DOI] [PubMed] [Google Scholar]
- 16.Golnazarian, C. A., C. W. Donnelly, S. J. Pintauro, and D. B. Howard. 1989. Comparison of infectious dose of Listeria monocytogenes F5817 as determined for normal versus compromised C57BL/6J mice. J. Food Prot. 52:696-701. [DOI] [PubMed] [Google Scholar]
- 17.Herd, M., and C. Kocks. 2001. Gene fragments distinguishing an epidemic-associated strain from a virulent prototype strain of Listeria monocytogenes belong to a distinct functional subset of genes and partially cross-hybridize with other Listeria species. Infect. Immun. 69:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurd, S., et al. 1998. Multistate outbreak of listeriosis—United States, 1998. Morb. Mortal. Wkly. Rep. 47:1085-1086. [PubMed] [Google Scholar]
- 19.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Guonon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1723. [DOI] [PubMed] [Google Scholar]
- 20.Lie, X. H., F. Fiedler, Z. Lam, and S. Kathariou. 2001. A novel serotype-specific gene cassette (gHA-gHB) is required for expression of teichoic acid-associated, surface antigens in Listeria monocytogenes serotype 4b. J. Bacteriol. 183:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez, S., A. J. Marco, N. Prats, and C. J. Czuprynski. 2000. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infect. Immun. 68:4789-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marco, A. J., N. Prats, J. A. Ramos, V. Briones, M. Blanco, L. Dominguez, and M. Domingo. 1992. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J. Comp. Pathol. 107:1-9. [DOI] [PubMed] [Google Scholar]
- 23.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 24.Menudier, A., C. Bosiraud, and J. A. Nicolas. 1991. Virulence of Listeria monocytogenes serovars and Listeria spp. in experimental infection of mice. J. Food Prot. 54:917-921. [DOI] [PubMed] [Google Scholar]
- 25.Notermans, S., J. Dufrenne, P. Teunis, and T. Chackraborty. 1998. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 61:244-248. [DOI] [PubMed] [Google Scholar]
- 26.Odriscoll, B., C. G. M. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes—isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pine, L., G. B. Malcolm, and B. D. Plikaytis. 1990. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect. Immun. 58:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor, M. E., R. Brosch, J. W. Mellen, L. A. Garrett, C. W. Kaspar, and J. B. Luchansky. 1995. Use of pulsed-field gel electrophoresis to link sporadic cases of invasive listeriosis with recalled chocolate milk. Appl. Environ. Microbiol. 61:3177-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripio, M. T., G. Dominguezbernal, M. Suarez, K. Brehm, P. Berche, and J. A. Vazquezboland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 30.Roll, J. T., and C. J. Czuprynski. 1990. Hemolysin is required for extraintestinal dissemination of Listeria monocytogenes in intragastrically inoculated mice. Infect. Immun. 58:3147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saklani-Jusforgues, H., E. Fontan, and P. L. Goosens. 2000. Effect of acid-adaptation on Listeria monocytogenes survival and translocation in a murine intragastric infection model. FEMS Microbiol. Lett. 193:155-159. [DOI] [PubMed] [Google Scholar]
- 32.Schlech, W. F., D. P. Chase, and A. Badley. 1993. A model of food-borne Listeria monocytogenes infection in the Sprague-Dawley rat using gastric inoculation: development and effect of gastric acidity on infective dose. Int. J. Food Microbiol. 18:15-24. [DOI] [PubMed] [Google Scholar]
- 33.Schuchat, A., B. Swaminathan, and C. V. Broome. 1991. Epidemiology of human listeriosis. Clin. Microbiol. Rev. 4:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripp, C. S., M. K. Gately, J. Hakimi, P. Ling, and E. R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C, B-17 mice. Reversal by IFN-γ. J. Immunol. 152:1883-1887. [PubMed] [Google Scholar]
- 35.Wagner, R. D., N. M. Maroushek, J. F. Brown, and C. J. Czuprynski. 1994. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to, but impairs complete clearance of, Listeria monocytogenes infection in mice. Infect. Immun. 62:2345-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]